Fingerprint of Fushiming Capsules and Its Application in Quality Control and Component Analysis
A fingerprint and component analysis technology, which is applied in the field of drug analysis, can solve the problems of Fushiming capsules with complex components, low separation of chemical components, and no quality control standards and component analysis methods for Fushiming capsules, so as to achieve the goal of treating diabetes Retinopathy, significant curative effect, easy quality control and application effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
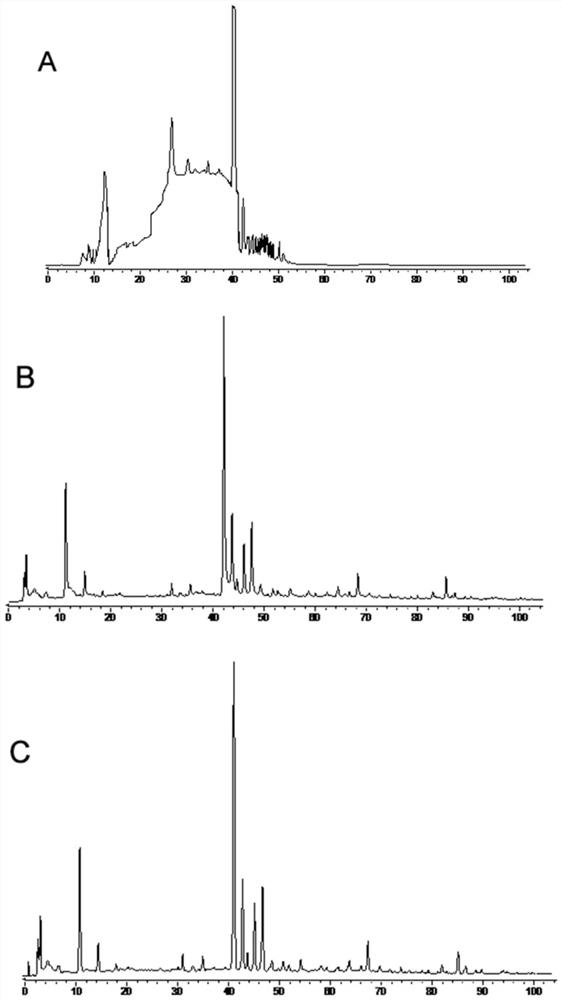
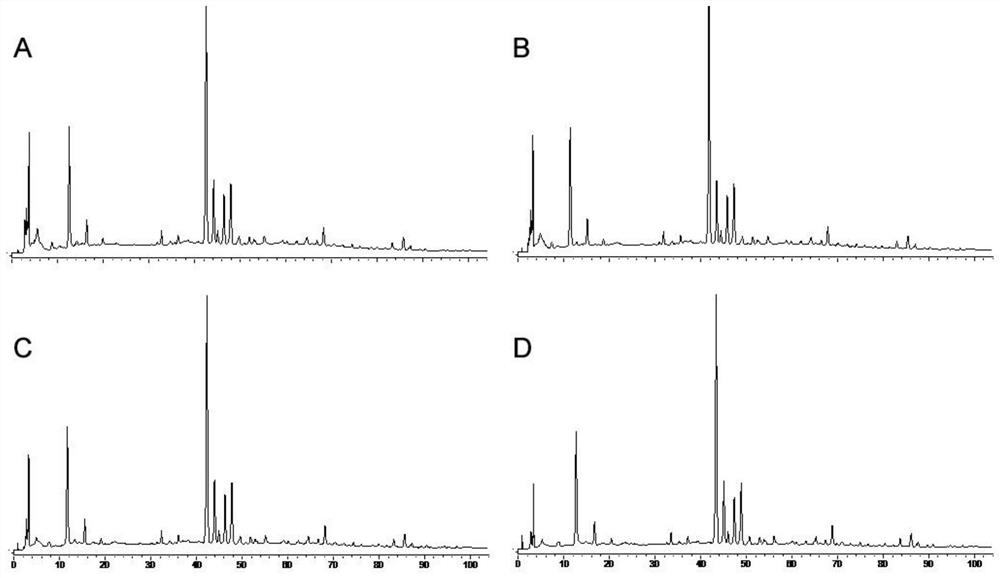
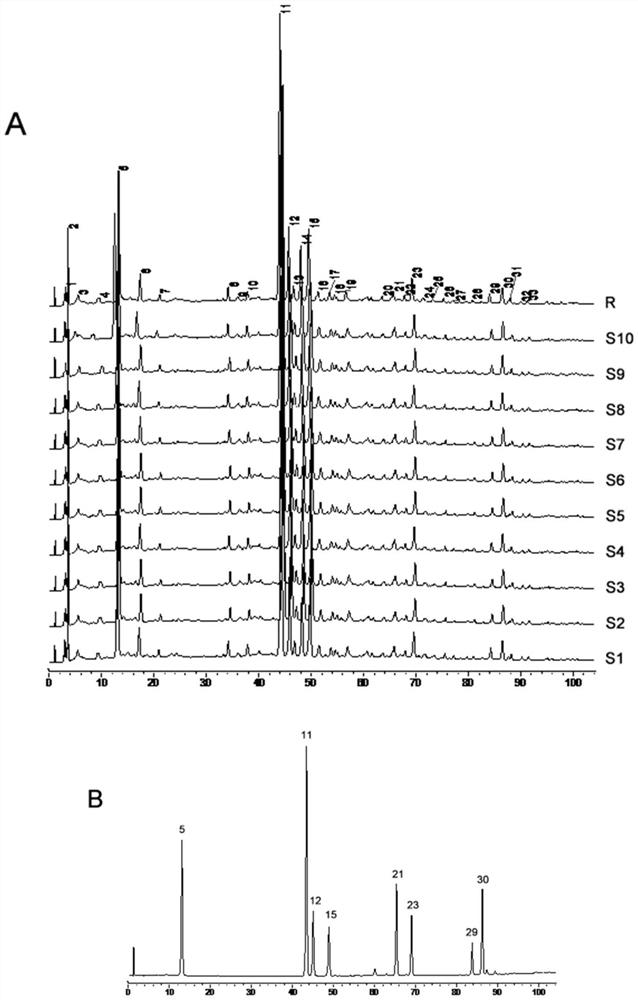
[0054] The establishment of embodiment 1 fingerprint spectrum
[0055] 1. Instruments, materials and reagents
[0056] 1.1 Instruments and Materials
[0057]
[0058] 1.2 Drugs and Reagents
[0059] Di Shiming Capsules (Xi'an Lejian Biotechnology Co., Ltd., batch number: 20180506, 20180507, 20180508, 20180605, 20180508, 20180509, 20180607, 20180608, 20180609, 20180610, specification: 400mg / capsule) (S1~S10); Methyl furfural reference substance (batch number: 111626-201610, purity: 99.2%), puerarin reference substance (batch number: 110752-201615, purity: 95.4%), daidzein reference substance (batch number: 111738-201603, purity: 93.3%) ), Cassia orange reference substance (batch number: 111900-201605, purity: 98.3%), ligustilide reference substance (batch number: 111737-201608, purity: 99.2%) were purchased from China National Institute for Food and Drug Control. Formononetin reference substance (lot number: 486-62-4, purity: 98.0%), 3'-methoxypuerarin reference substance...
Embodiment 2
[0102] Example 2 Pharmacodynamic study of 8-component pharmaceutical composition
[0103] 1. Instruments, materials and reagents
[0104] 1.1 Instruments and Materials
[0105]
[0106]
[0107] 1.2 Drugs and Reagents
[0108] Di Shiming capsule powder, provided by the Institute of Materia Medica, Air Force Military Medical University, batch number 20170301. Calcium dobesilate capsules are commercially available products, batch number: 20161104, produced by Guizhou Tianan Pharmaceutical Co., Ltd., approval number: Guoyao Zhunzi H20010481. 5-Hydroxymethylfurfural reference substance (batch number: 111626-201610, purity: 99.2%), puerarin reference substance (batch number: 110752-201615, purity: 95.4%), daidzein reference substance (batch number: 111738-201603, purity: 93.3%), cassia orange reference substance (batch number: 111900-201605 , purity: 98.3%), ligustilide reference substance (batch number: 111737-201608, purity: 99.2%) were purchased from China National Inst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com