Fingerprint of Jialong capsules and its application in quality control and component analysis
A technique for component analysis, capsules, applied in the field of pharmaceutical analysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
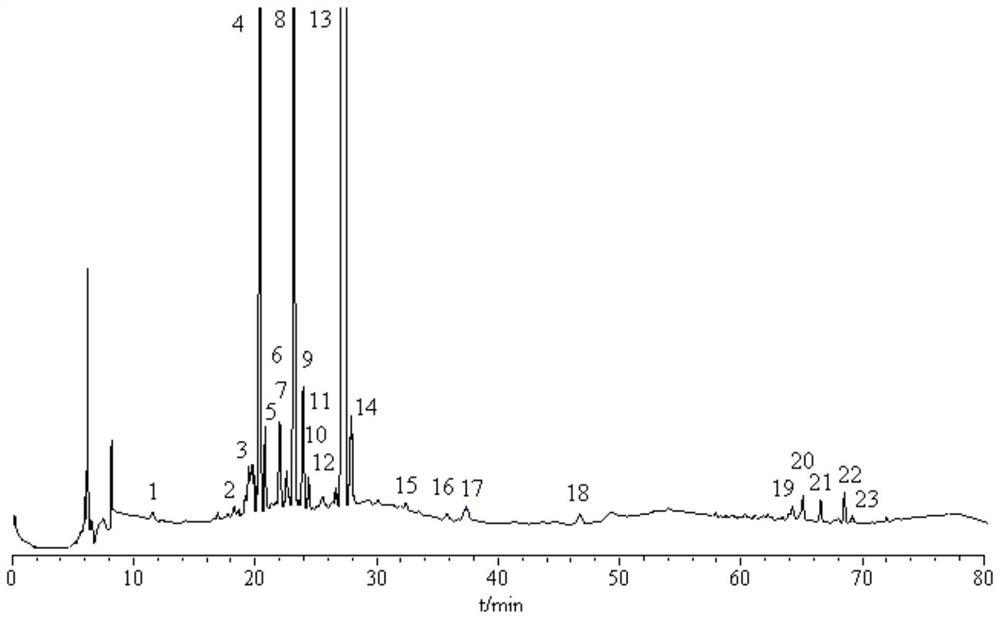
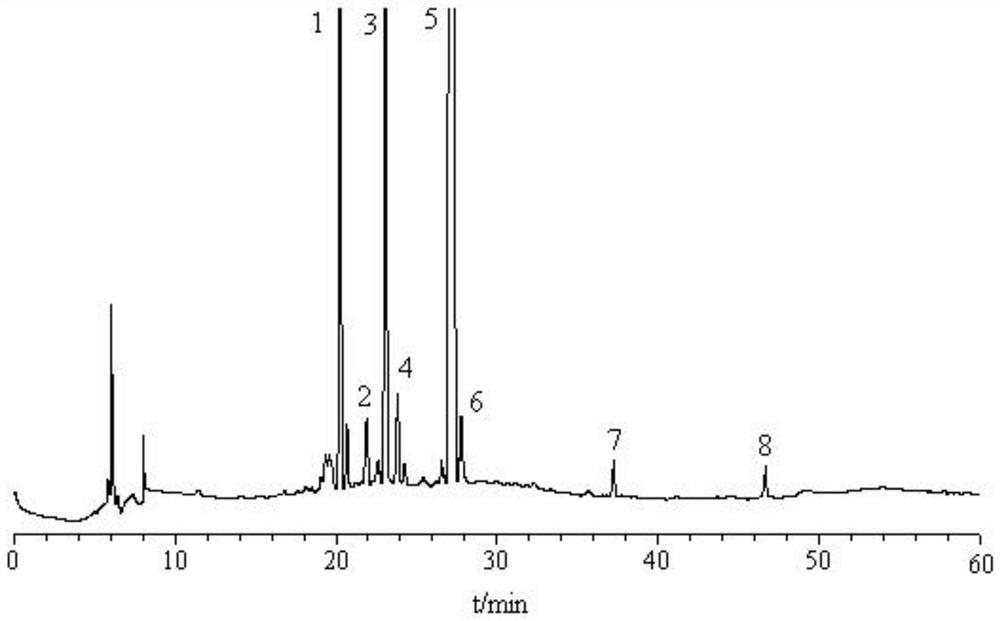
[0040] The establishment of embodiment 1 fingerprint
[0041] 1 Instruments and reagents
[0042] Shimadzu high performance liquid chromatography (LC-2010C HT , LC / Labsoluion chromatographic workstation), Japan Shimadzu Corporation; German sartorius electronic balance, model CPA225D, Germany Sartorius Company; ultrasonic generator, model KQ-5200DE numerical control, Kunshan Ultrasonic Instrument Co., Ltd. Acetonitrile, methanol, chromatographic grade, U.S. Honeywell Company; Ultrapure water, is made by U.S. Millipore pure water instrument, U.S. Millipore Company; 111873-201103, the content is 98.3%), swerticin (batch number 110785-201404, the content is 98.3%), gentiopicroside (batch number 110770-201716, the content is 99.1%), isoorientin (batch number 111974 -201401, with a content of 94.0%) and other reference substances were purchased from China National Institute for the Control of Pharmaceutical and Biological Products; Reference substances such as glucoside (batch nu...
Embodiment 2
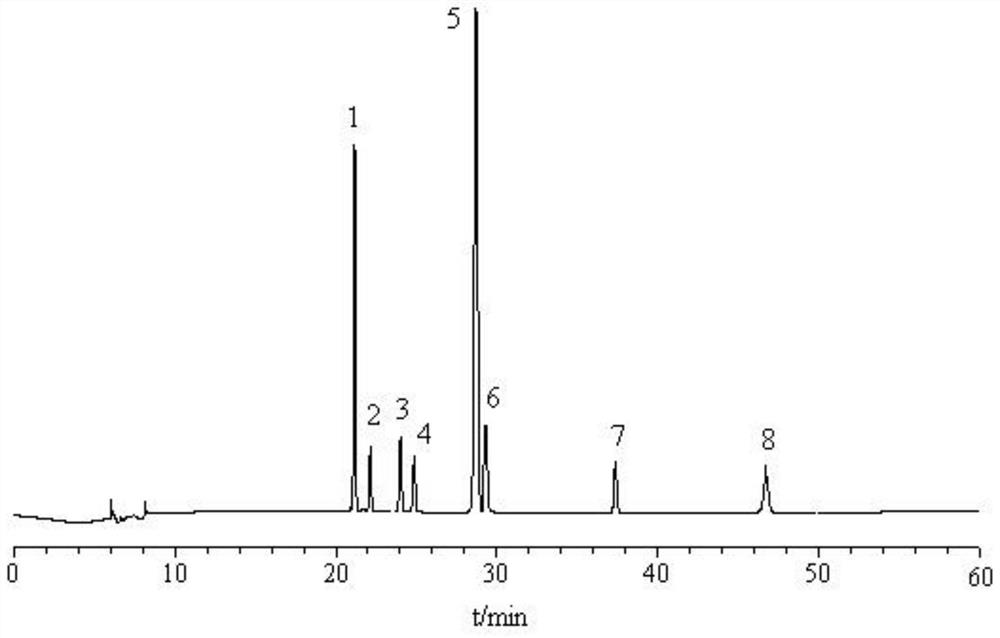
[0052] The content determination of each component of embodiment 2 Geranium Capsules
[0053] 3.1 Chromatographic conditions The recording time is 60 minutes, and the rest are the same as 2.1 in Example 1.
[0054] 3.2 Preparation of reference substance solution Accurately weigh a certain amount of shanzhiside methyl ester, swerticroside, swertigin, isoorientin, isovitexin, loganinic acid, 6′-O -β-D-glucosylgentiopicroside, gentiopicroside, configured as a reference substance mixed solution, loganinic acid, shanzhiside methyl ester, 6′-O-β-D- The concentrations of glucosylgentiopicroside, swertiamarin, gentiopicroside, swertiin, isoorientin, and isovitetin reference substances were 2100.00, 320.00, 1500.00, 510.00, 3900.00, 420.00, 220.00, 110μg·mL -1 .
[0055] 3.3 The preparation of the test solution is the same as 2.2.2 in Example 1.
[0056] 3.4 Linear relationship Precisely measure 0.01, 0.05, 0.10, 0.25, 0.50 and 1.00mL of the reference substance mixture prepared in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| percent by volume | aaaaa | aaaaa |
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com