Fingerprint of Fushiming capsules and its application in quality control and component analysis
A fingerprint and capsule technology, which is applied in the field of drug analysis, can solve the problems of complex components, low separation degree of chemical components, and lack of quality control standards and component analysis methods for dipilide capsules, and achieves the treatment of diabetic retina. Lesion, significant curative effect, easy quality control and application effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
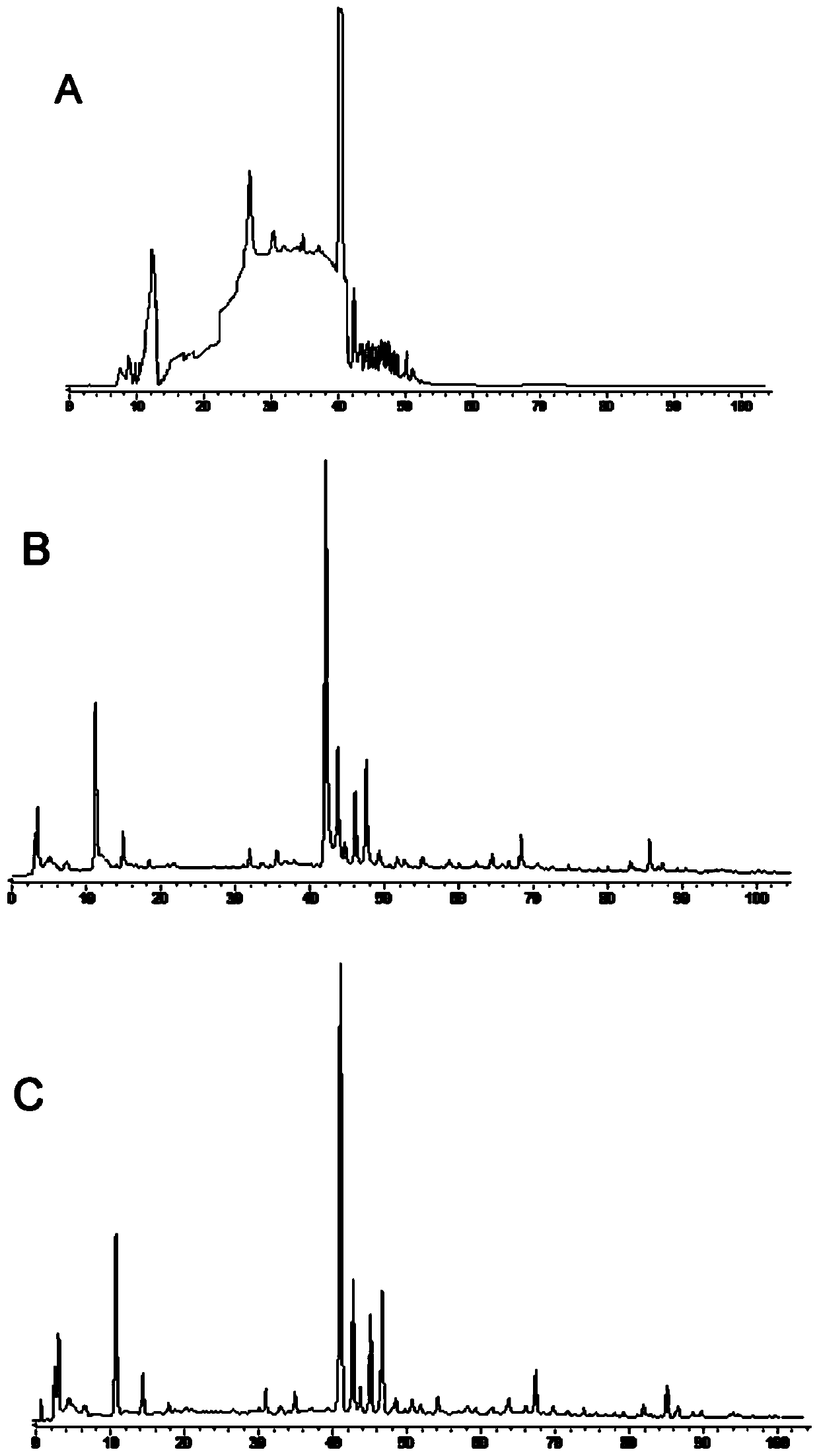
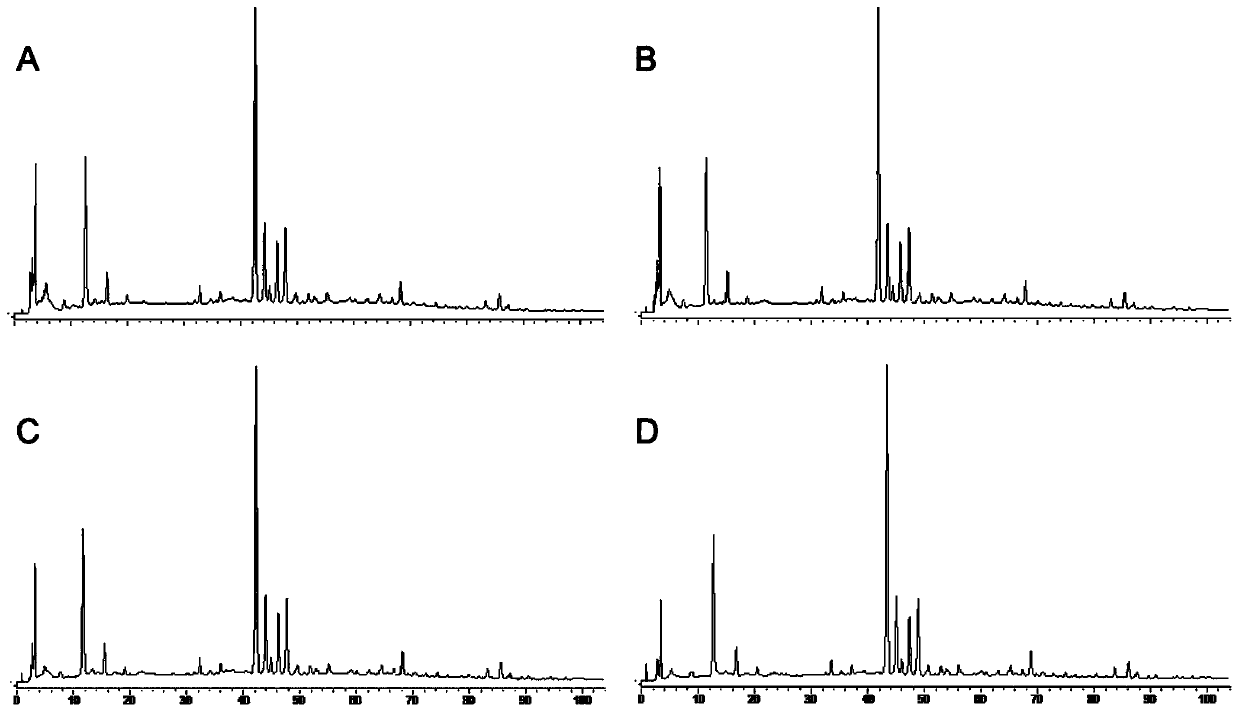
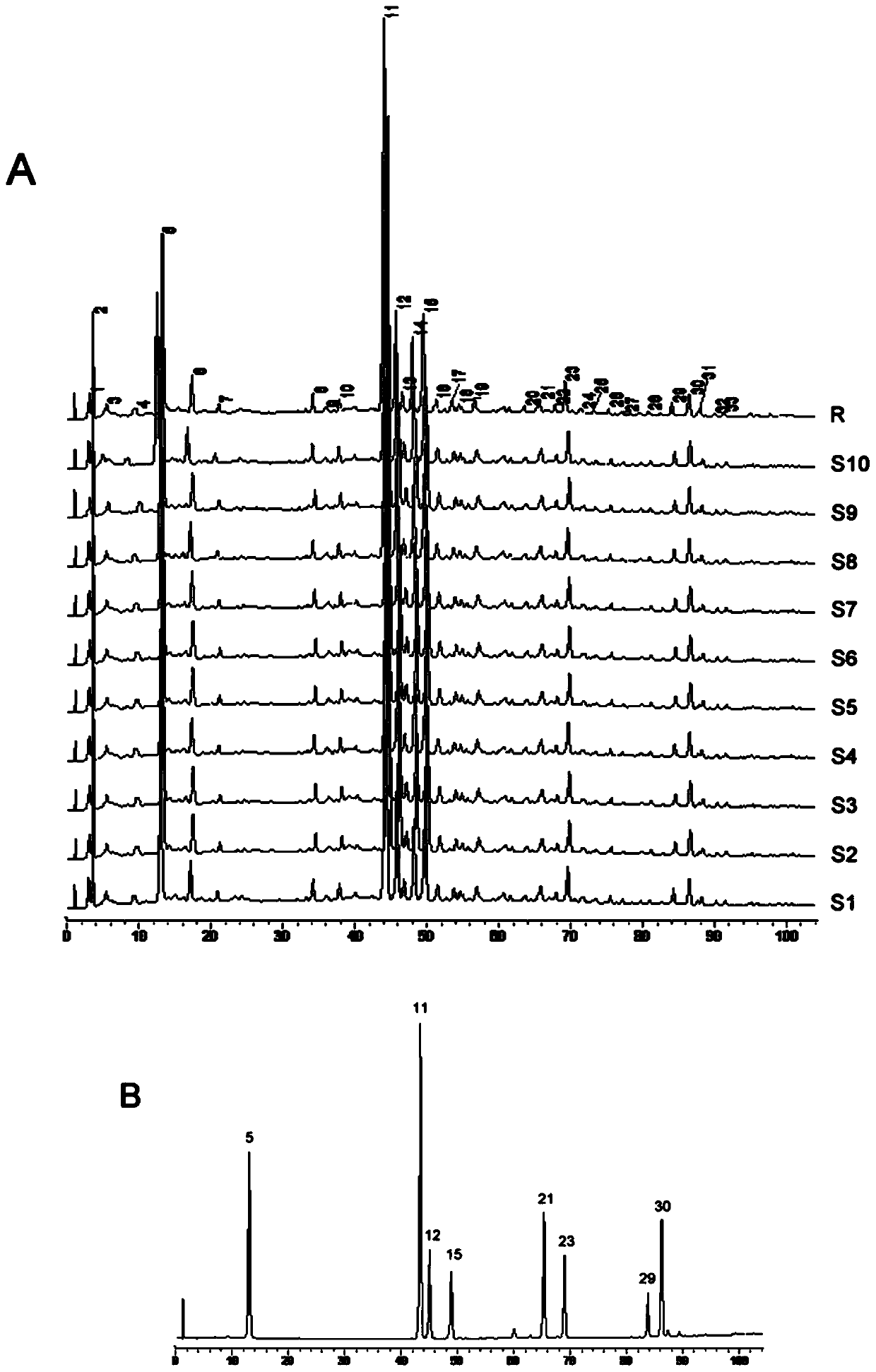
[0054] The establishment of embodiment 1 fingerprint
[0055] 1. Instruments, materials and reagents
[0056] 1.1 Instruments and materials
[0057]
[0058] 1.2 Drugs and reagents
[0059] Fushiming Capsules (Xi'an Lejian Biotechnology Co., Ltd., batch number: 20180506, 20180507, 20180508, 20180605, 20180508, 20180509, 20180607, 20180608, 20180609, 20180610, specification: 400mg / capsule) (S1~S10); Methylfurfural reference substance (batch number: 111626-201610, purity: 99.2%), puerarin reference substance (batch number: 110752-201615, purity: 95.4%), daidzin reference substance (batch number: 111738-201603, purity: 93.3%) ), orange cassiatin reference substance (batch number: 111900-201605, purity: 98.3%), and ligustilide reference substance (batch number: 111737-201608, purity: 99.2%) were purchased from China National Institutes for Food and Drug Control. Formononetin reference substance (batch number: 486-62-4, purity: 98.0%), 3'-methoxypuerarin reference substance (...
Embodiment 2
[0102] Example 2 Pharmacodynamic studies of 8 kinds of component pharmaceutical compositions
[0103] 1. Instruments, materials and reagents
[0104] 1.1 Instruments and materials
[0105]
[0106]
[0107] 1.2 Drugs and reagents
[0108] Fushiming Capsule Powder, provided by the Institute of Drug Research, Air Force Military Medical University, batch number 20170301. Calcium dobesilate capsules are commercial products, batch number: 20161104, produced by Guizhou Tianan Pharmaceutical Co., Ltd., approval number: Guoyao Zhunzi H20010481. 5-Hydroxymethylfurfural reference substance (batch number: 111626-201610, purity: 99.2%), puerarin reference substance (batch number: 110752-201615, purity: 95.4%), daidzein reference substance (batch number: 111738-201603, purity: 93.3%), orange cassiacin reference substance (batch number: 111900-201605 , purity: 98.3%), ligustilide reference substance (batch number: 111737-201608, purity: 99.2%) were purchased from China National Ins...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com