Quality control method for gynecological Qianjin tablets
A quality control method, the technology of Fukeqianjin Tablets, is applied in the field of quality control of Fukeqianjin Tablets, which can solve problems such as difficulty in consistency of effects, and achieve good clinical treatment effects, stable drug effect consistency, and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Fuke Qianjin Tablets
[0034] The formula of Fukeqianjin Tablets: Angelica, Codonopsis, Andrographis paniculata and Shuangmianzhen each account for 9% of the total amount of medicinal materials; the dosages of Rosa japonica root, Caulis Spatholobus, Gongluomu and Qianjinba each account for 16% of the total amount of medicinal materials. The total amount of medicinal materials is 500kg. The product is prepared by:
[0035] (1) select Chinese angelica, Codonopsis pilosula, and Andrographis paniculata, grind them into fine powders of more than 100 meshes, and reserve them for later use according to a powder yield of at least 93.3%;
[0036] (2) Select single-faced needle, golden cherry root, Caulis Spatholobus, Gonglaomu and Qianjinba, add water to extract twice, add 10 times the total weight of five-flavored traditional Chinese medicine for the first time, and add 10 times the total weight of five-flavored traditional Chinese medicine for the second time water, ...
Embodiment 2
[0044] A Fuke Qianjin Tablet, in which steps (1) and (2) are the same as in Example 1 during its preparation process, and the difference from Example 1 is that the content of genistin in each mg of mixture is controlled in step S3 to be no less than 0.00007mg, the content of Z-ligustilide not less than 0.0045mg and / or the content of Z-3-butenylphthalide not less than 0.00011mg, the content of andrographolide and dehydroandrographolide not less than 0.00788 mg; then dried, compressed into tablets, and packaged into tablets to obtain the Fukeqianjin Tablets. According to existing products and production indicators as a reference, the quality of each Fukeqianjin Tablets is 0.32g.
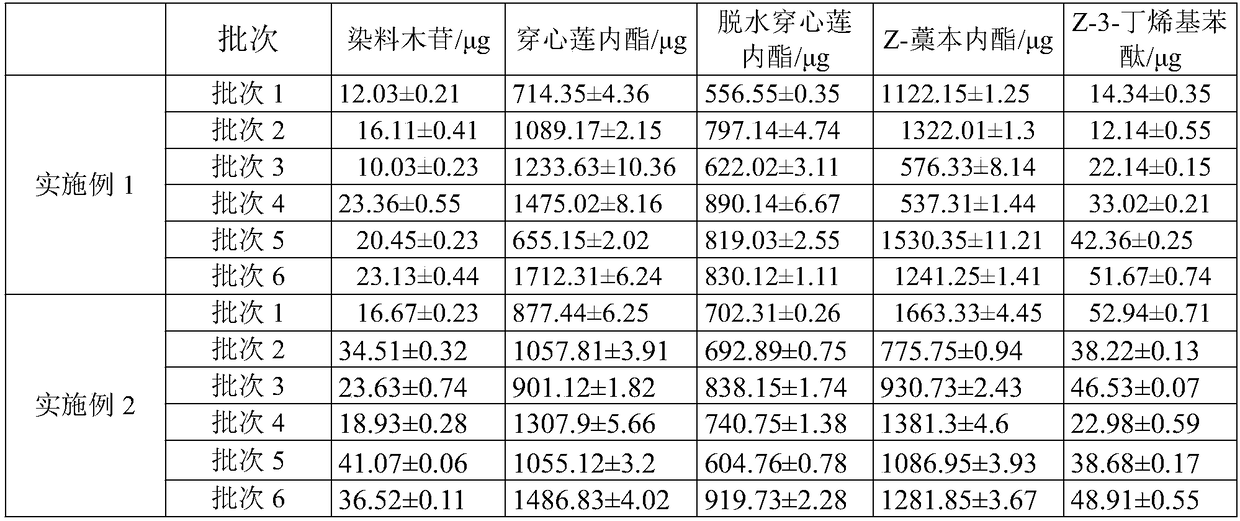
[0045] Fuke Qianjin Tablets were prepared according to the above method, and three batches of products were randomly selected from long-term and large quantities of products, and each batch of Fuke Qianjin Tablets was tested referring to the HPLC detection method in Example 1.
[0046] The HPLC detecti...
Embodiment 3
[0055] Embodiment 3 in vitro drug efficacy test
[0056] Drugs or materials used: croton oil, provided by Nanjing Institute of Dermatology; carrageenan, produced by Japan Wako Pure Chemical Industry Co., Ltd.; nutrient broth medium, product of Guangdong Huankai Microbial Technology Co., Ltd.; mold medium, Provided by the China Institute for the Control of Pharmaceutical and Biological Products.
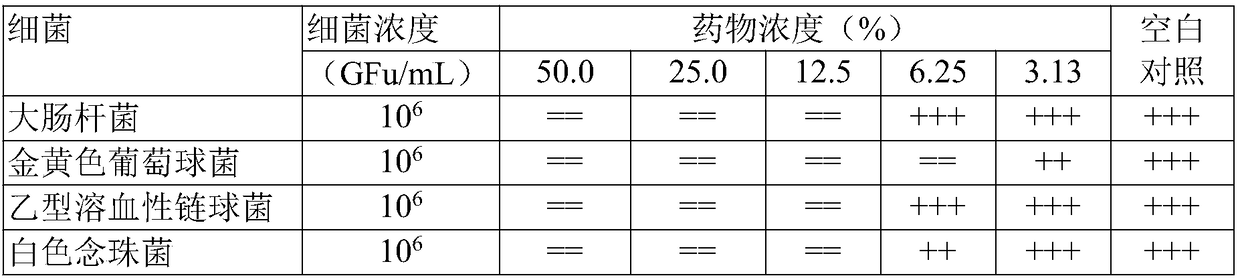
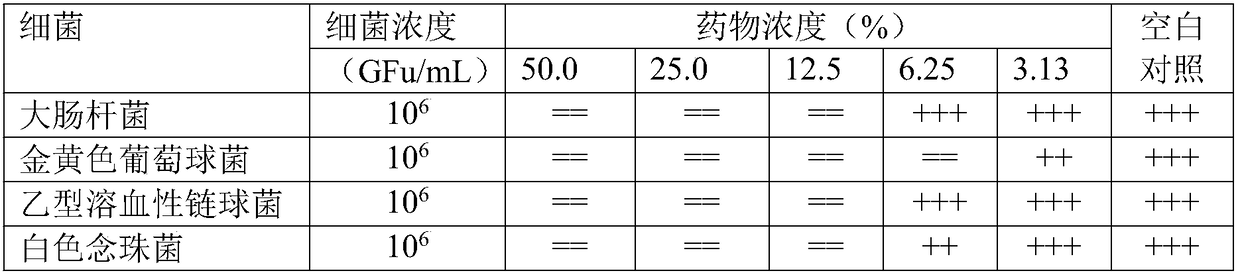
[0057] The Fukeqianjin Tablets prepared in Example 1 (batch 1 and batch 6) and Comparative Example 1 (batch 1) were used as samples respectively. The clean-grade Kunming mice and SD rats used were all provided by the Hunan Drug Inspection Institute; Escherichia coli ATCC25922, Staphylococcus aureus ATCC25923, and beta-hemolytic streptobacter ATCC32172 were all provided by the Provincial Health and Epidemic Prevention Station. Bacteria were isolated clinically and provided by the Bacteriology Laboratory of the Clinical Laboratory Department of the Third Affiliated Hospital of Hunan Me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com