Gynecological qianjin capsule and preparation method thereof
A gynecological Qianjin Capsule and Qianjinpu technology, which can be used in capsule delivery, scientific instruments, pharmaceutical formulations, etc., can solve problems such as difficulty in consistency of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
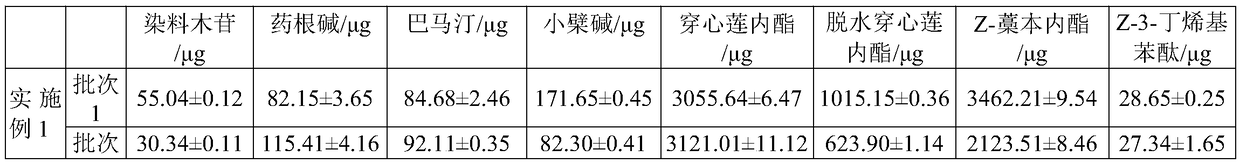
[0051] Example 1 Fuke Qianjin Capsules
[0052] The formula of Fuke Qianjin Capsules: Angelica, Codonopsis, Andrographis paniculata, and Shuangmianzhen each account for 9% of the total amount of medicinal materials; the amount of golden cherry root, Caulis Spatholobus, Gongluomu, and Qianjinba each accounts for 16% of the total amount of medicinal materials. The total amount of medicinal materials is 500kg. The product is prepared by:
[0053] (1) First, extract Angelica sinensis with ethanol percolation method to make clear paste: Angelica sinensis is crushed into coarse powder, passed through an 8-mesh sieve, infiltrated with ethanol with a mass concentration of 70%, put into a percolation cylinder, soaked for 48 hours, and mixed with 0.2mL Percolation at a speed of 1 / min, collecting 10 times the amount of percolation liquid, and concentrating to obtain a relative density of 1.1 (80°C), which is Angelica Clear Paste;
[0054] (2) Extract Andrographis paniculata with ethano...
Embodiment 2
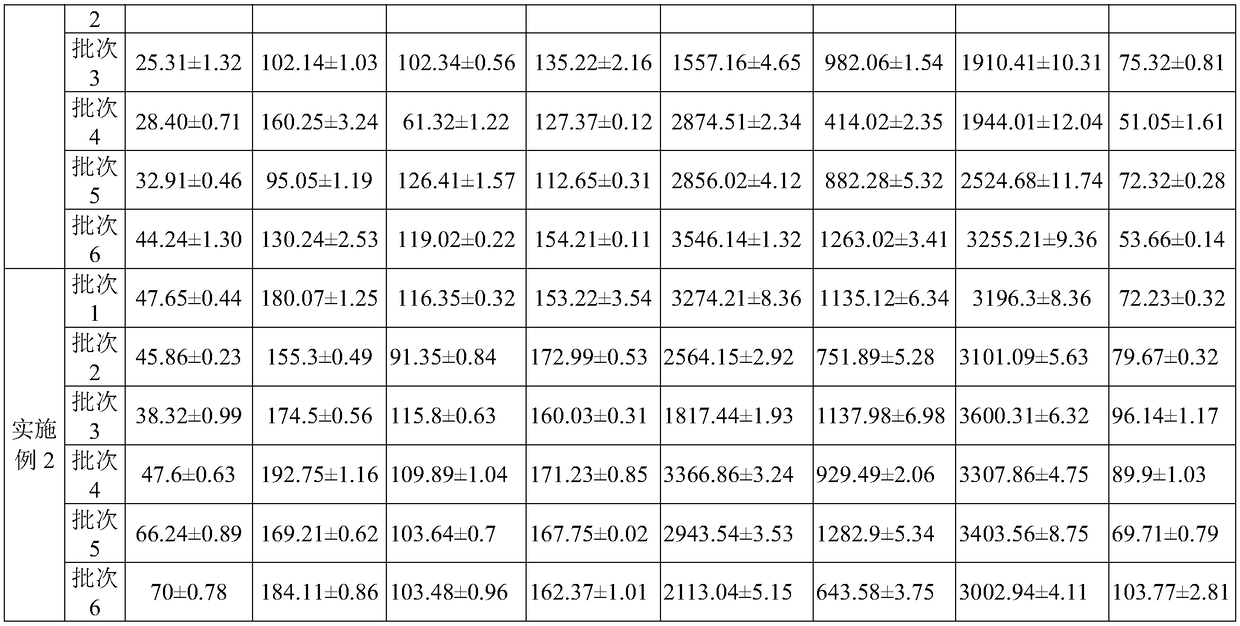
[0065] A Fuke Qianjin Capsule, the steps (1) to (4) in the preparation process are the same as those in Example 1, the difference is that in step (5), the content of Z-ligustilide in each milligram of Qing Gao is controlled to be quite a lot less than 0.015mg, and / or the content of Z-3-butenylphthalide is not less than 0.00025mg, and / or the content of genistin is not less than 0.002mg, and / or the content of jatrorrhizine is not less than 0.006 mg, and / or the content of palmatine is not less than 0.006mg, and / or the content of berberine is not less than 0.006mg, and the total amount of andrographolide and dehydroandrographolide is not less than 0.015mg, spray Dried, then granulated and canned to obtain the Fukeqianjin Capsules, according to the 2015 edition of the "Pharmacopoeia of the People's Republic of China", the content of each Fukeqianjin Capsules is 0.4g.
[0066]Prepare Fuke Qianjin Capsules according to the above method, randomly select multiple batches of products fr...
Embodiment 3
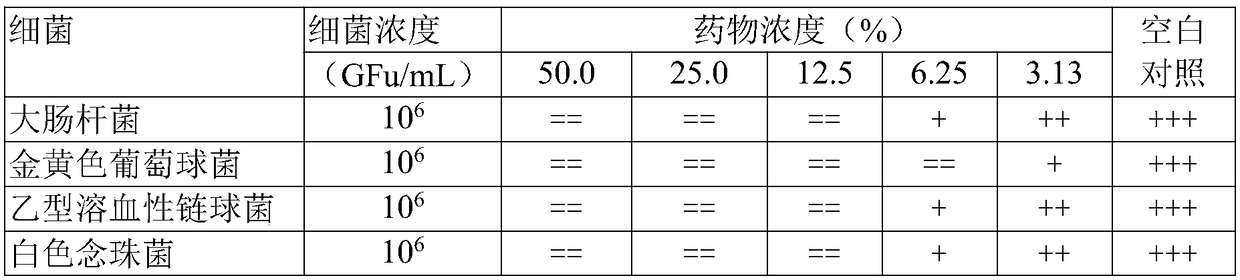
[0077] Embodiment 3 in vitro drug efficacy test
[0078] Drugs or materials used: croton oil, provided by Nanjing Institute of Dermatology; carrageenan, produced by Japan Wako Pure Chemical Industry Co., Ltd.; nutrient broth medium, product of Guangdong Huankai Microbial Technology Co., Ltd.; mold medium, Provided by the China Institute for the Control of Pharmaceutical and Biological Products.
[0079] The Qianjin capsules prepared in Example 1 (batch 1 and batch 6) and comparative example 1 (batch 1) were used as samples respectively. The clean-grade Kunming mice and SD rats used were all provided by the Hunan Drug Inspection Institute; Escherichia coli ATCC25922, Staphylococcus aureus ATCC25923, and beta-hemolytic streptobacter ATCC32172 were all provided by the Hunan Provincial Health and Epidemic Prevention Station, white Candida, isolated clinically, was provided by the Bacteria Laboratory of the Clinical Laboratory Department of the Third Affiliated Hospital of Hunan M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com