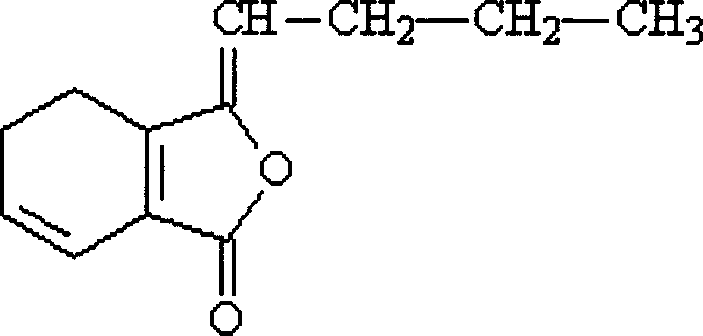
Ligustilide cyclodextrin or its derivatives clathrate, its preparation method and pharmaceutical formulation
A technology of ligustilide and pharmaceutical preparations, which is applied in drug delivery, drug combination, and pharmaceutical formulations to achieve good dissolution, increased stability, and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Preparation of ligustilide hydroxypropyl-β-cyclodextrin inclusion compound
[0033] Weigh 40g (0.0258mol) of hydroxypropyl-β-cyclodextrin into 100ml of distilled water, fully stir to dissolve; take 1g (0.0052mol) of cis-ligustilide and dilute with 2ml of absolute ethanol, add the above-mentioned hydroxypropyl Base-β-cyclodextrin solution, magnetically stirred for 30 minutes, the stirring speed should be such that the liquid does not splash out, observe the solution until it is clear and transparent, and the liquid ligustilide hydroxypropyl-β-cyclodextrin inclusion compound is obtained . The liquid can be made into liquid dosage forms such as water injection, oral liquid, syrup and the like. Another part of the above-mentioned liquid ligustilide hydroxypropyl-β-cyclodextrin inclusion compound was taken and packed into vials, and freeze-dried to obtain a white freeze-dried powder for future use.
[0034] (2) Detection of inclusion degree
[0035] Since the inclusio...
Embodiment 2
[0044] Example 2: Weigh 100 g (0.0646 mol) of hydroxypropyl-β-cyclodextrin, add 150 ml of distilled water, and stir to dissolve. In addition, weigh 5 g (0.0263 mol) of ligustilide and add 10 ml of 95% ethanol to dilute it, pour it into the above-mentioned hydroxypropyl-β-cyclodextrin solution, and stir the mixture with magnetic force for 30 minutes, and the stirring speed is such that the liquid does not splash. Preferably, observe the solution until it is clear and transparent, concentrate, and dry under reduced pressure to obtain solid ligustilide hydroxypropyl-β-cyclodextrin inclusion compound. The solid clathrate can be prepared into solid dosage forms such as tablets and capsules according to the solid preparation method.
[0045] According to the method described in Example 1, the clathrate degree, water solubility and stability of the clathrate obtained were detected. The measurement results show that the inclusion complex is complete; 1g of the inclusion complex freez...
Embodiment 3
[0046] Example 3: Weigh 40g (0.0258mol) of hydroxypropyl-β-cyclodextrin, pour it into 100ml of distilled water, stir to dissolve; take another 0.5g (0.0026mol) of cis-ligustilide, and pour it into the above-mentioned hydroxypropyl-β-cyclodextrin Propyl-β-cyclodextrin solution; placed in an ultrasonic generator, ultrasonically oscillating at 40KHz for 20 minutes, observe the solution until it is clear and transparent, pour it out and freeze-dry to obtain solid cis-ligustilide hydroxypropyl - β-cyclodextrin inclusion complex. The solid clathrate can be prepared into solid dosage forms such as granules and dispersible tablets according to the solid preparation method.
[0047] According to the method described in Example 1, the clathrate degree, water solubility and stability of the clathrate obtained were detected. The measured results show that the inclusion complex is complete; 1g of the inclusion compound freeze-dried powder can be completely dissolved by gradually adding 1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com