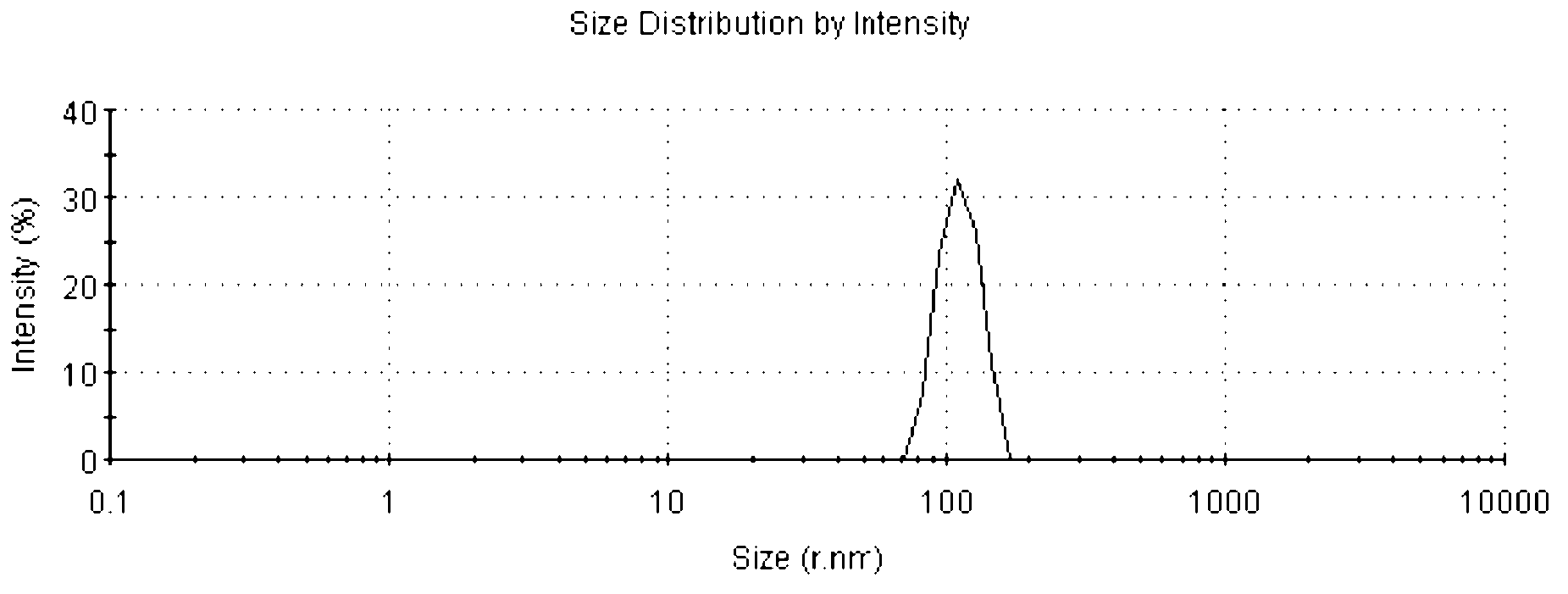
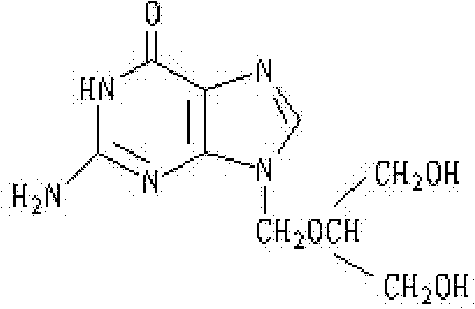
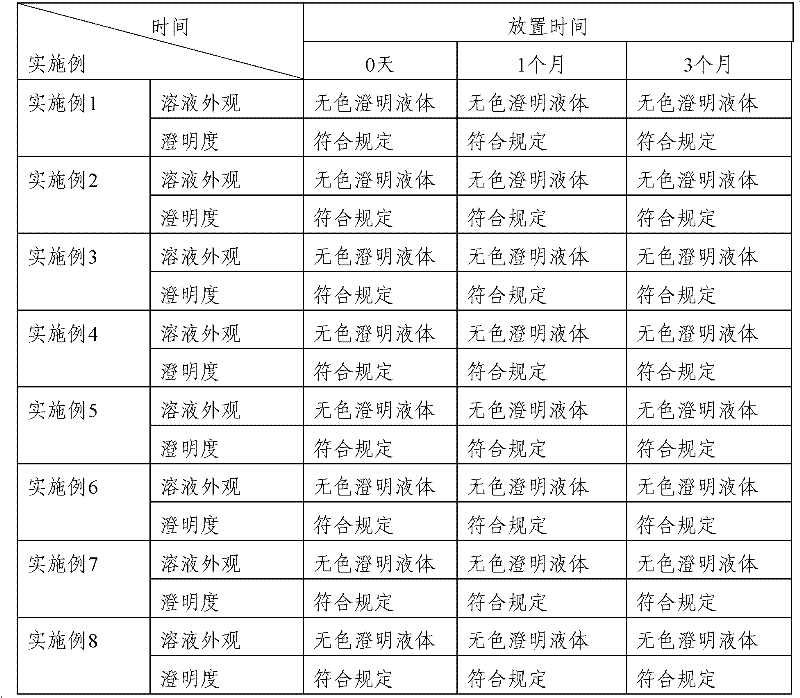
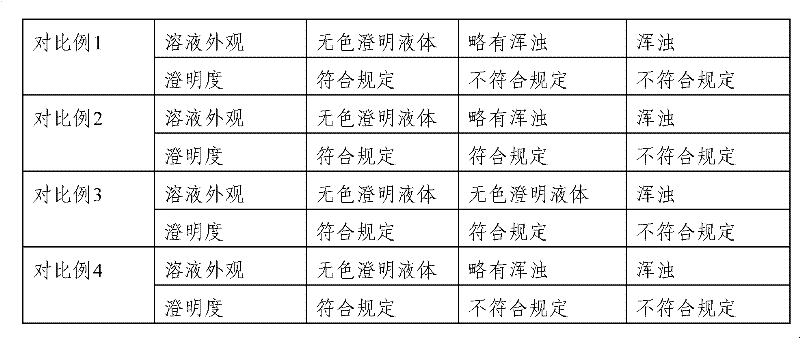
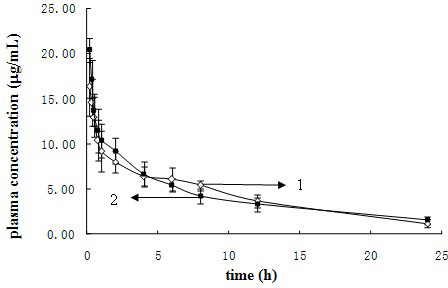
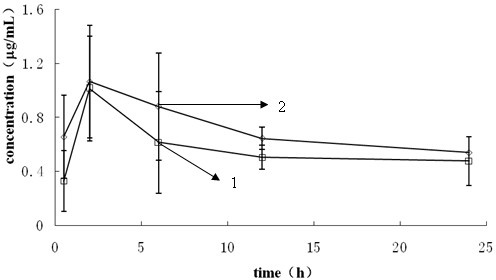
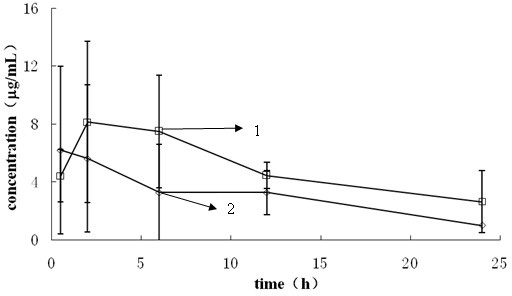
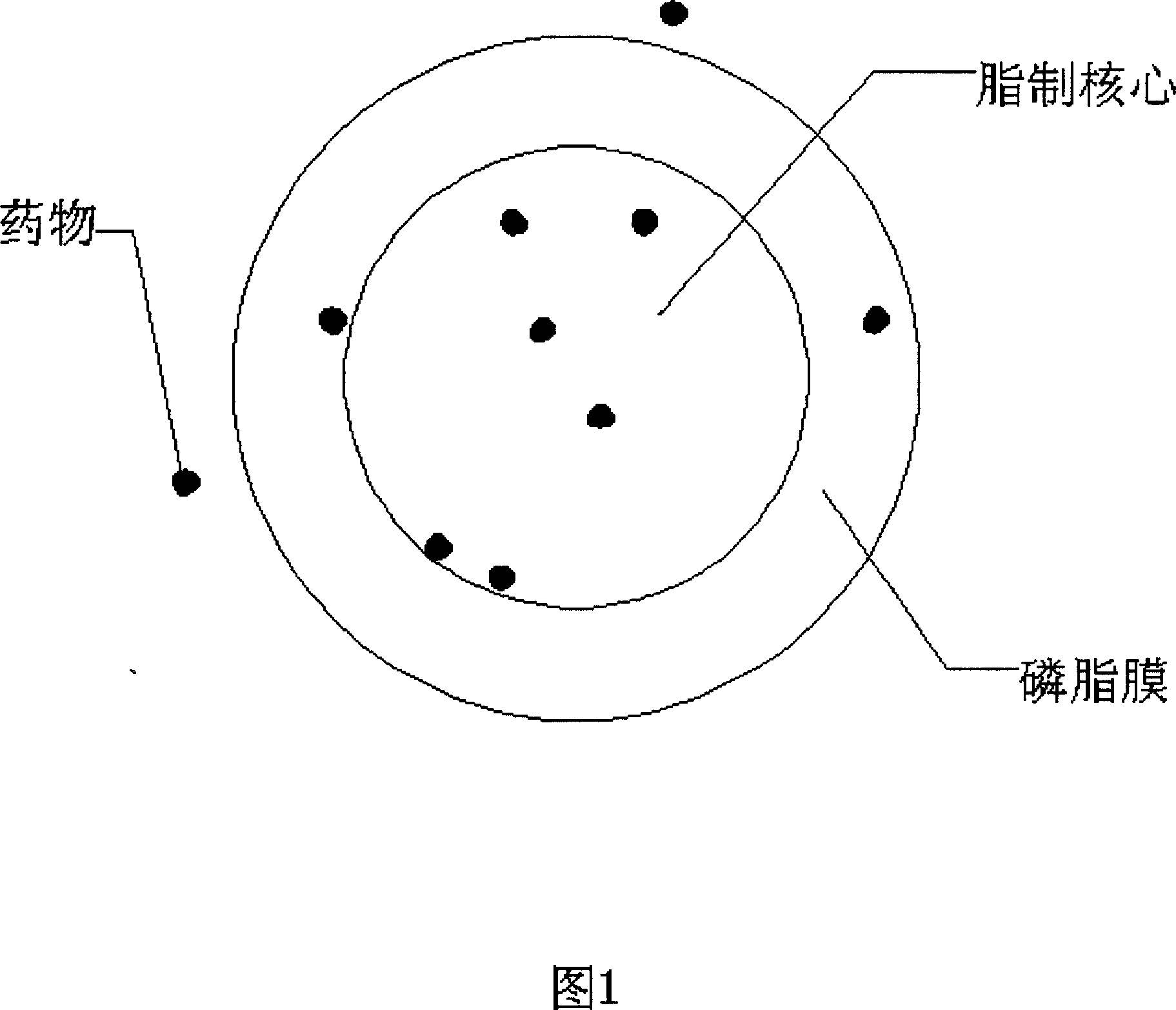
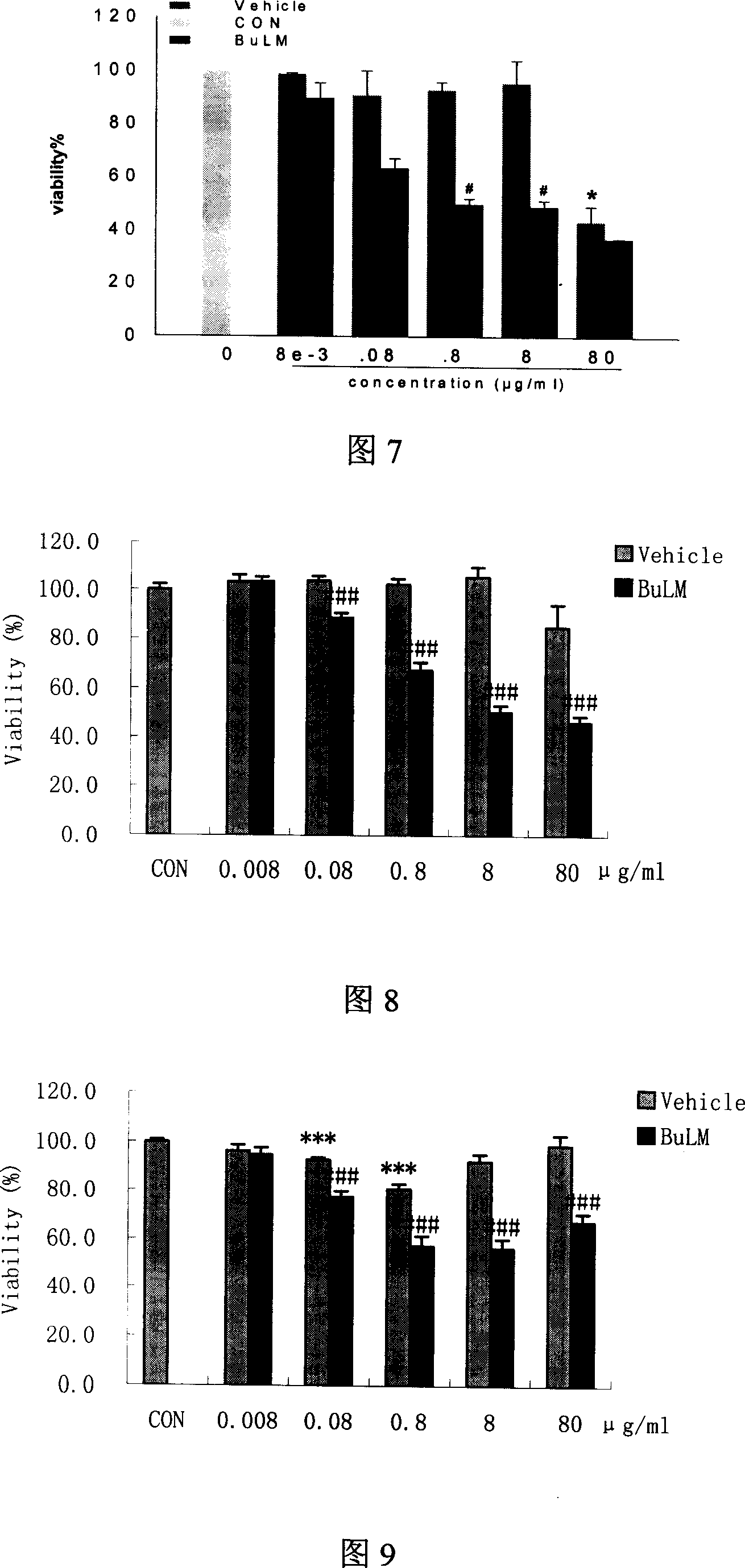
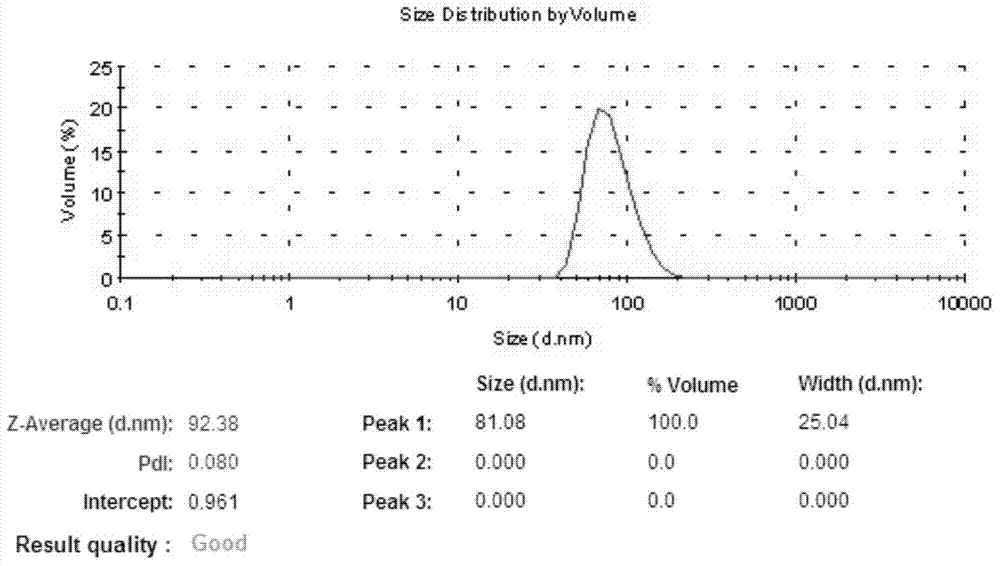
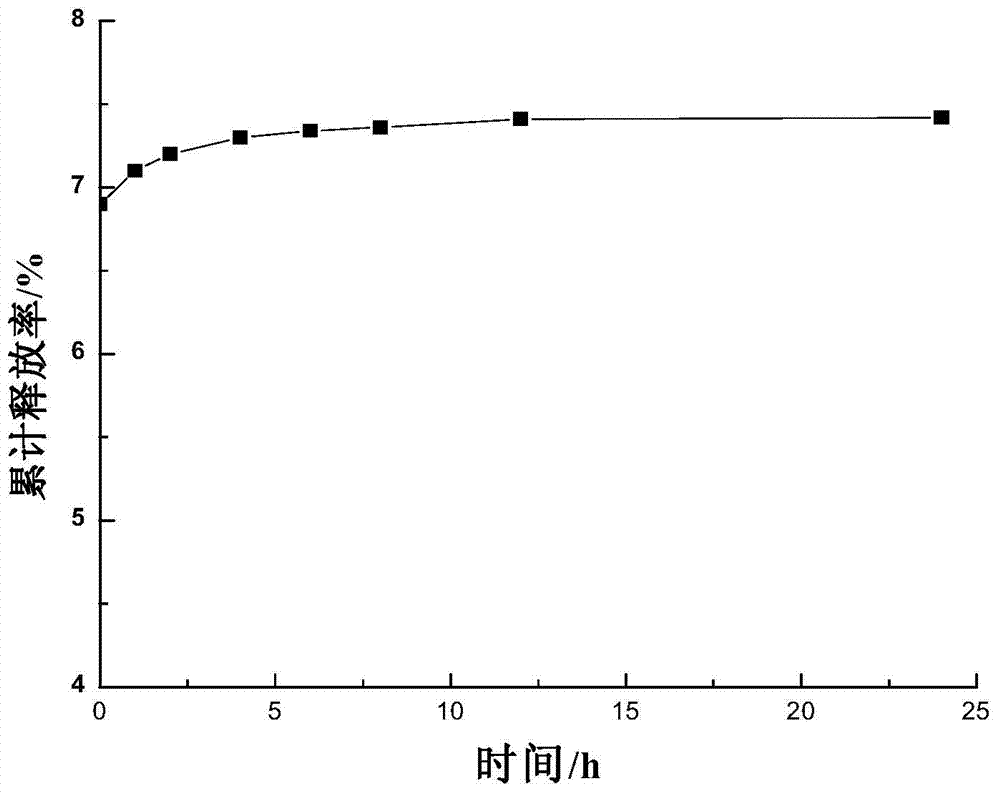
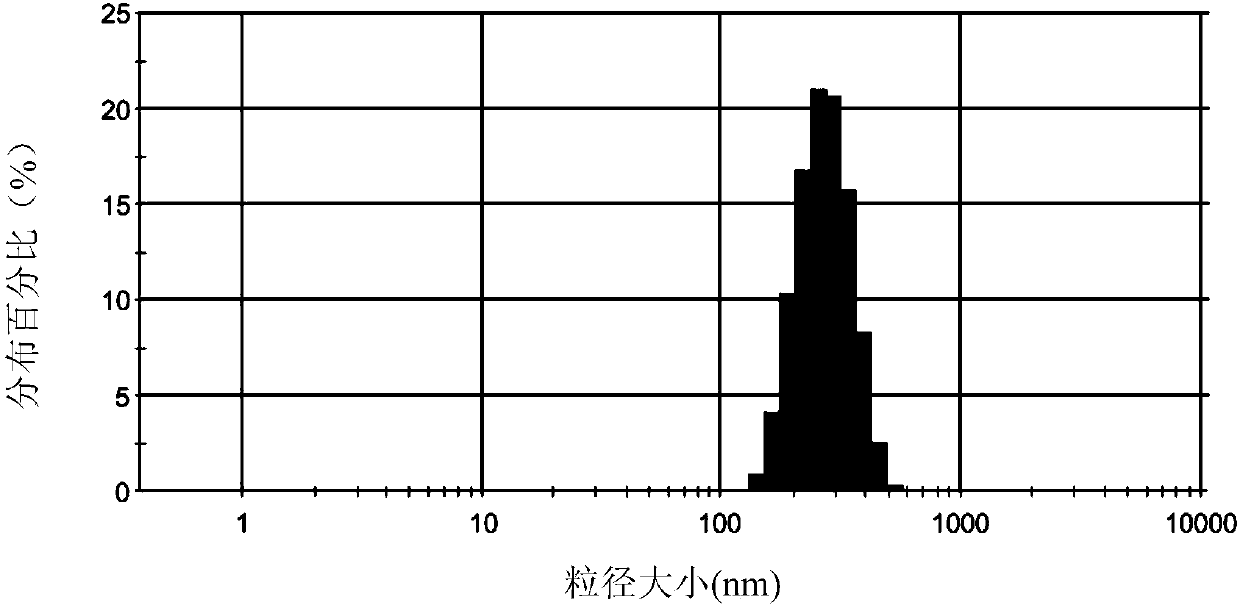
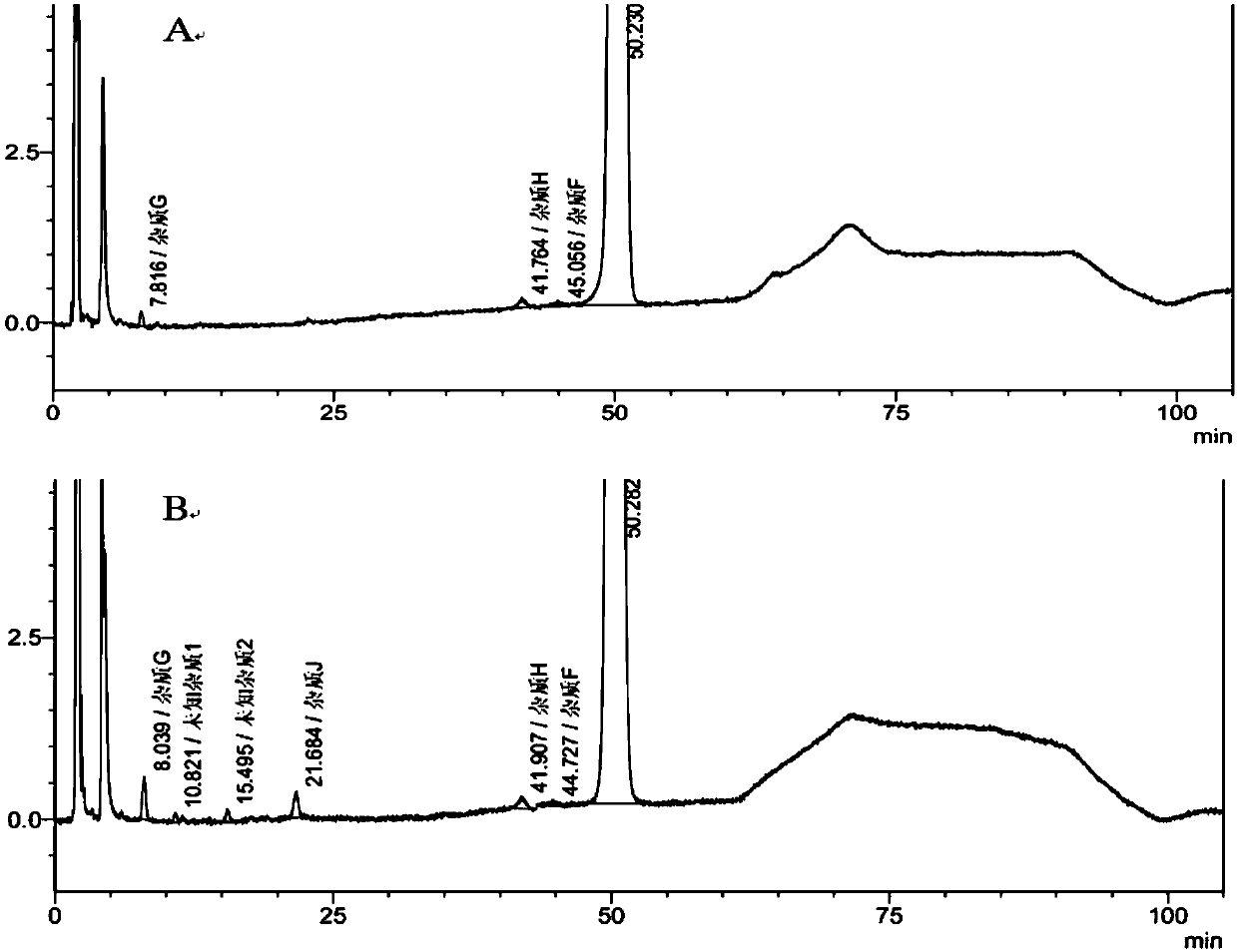
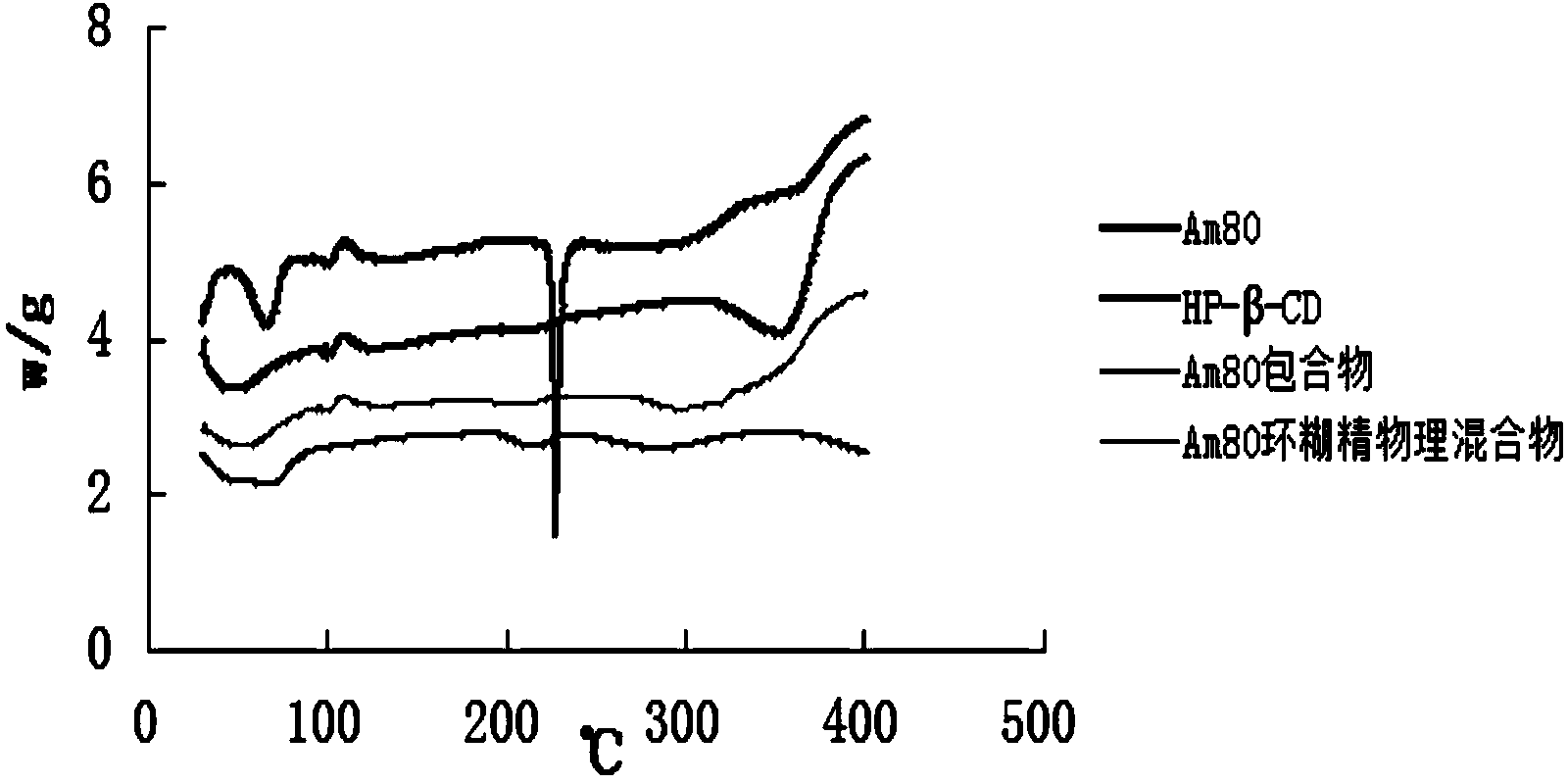
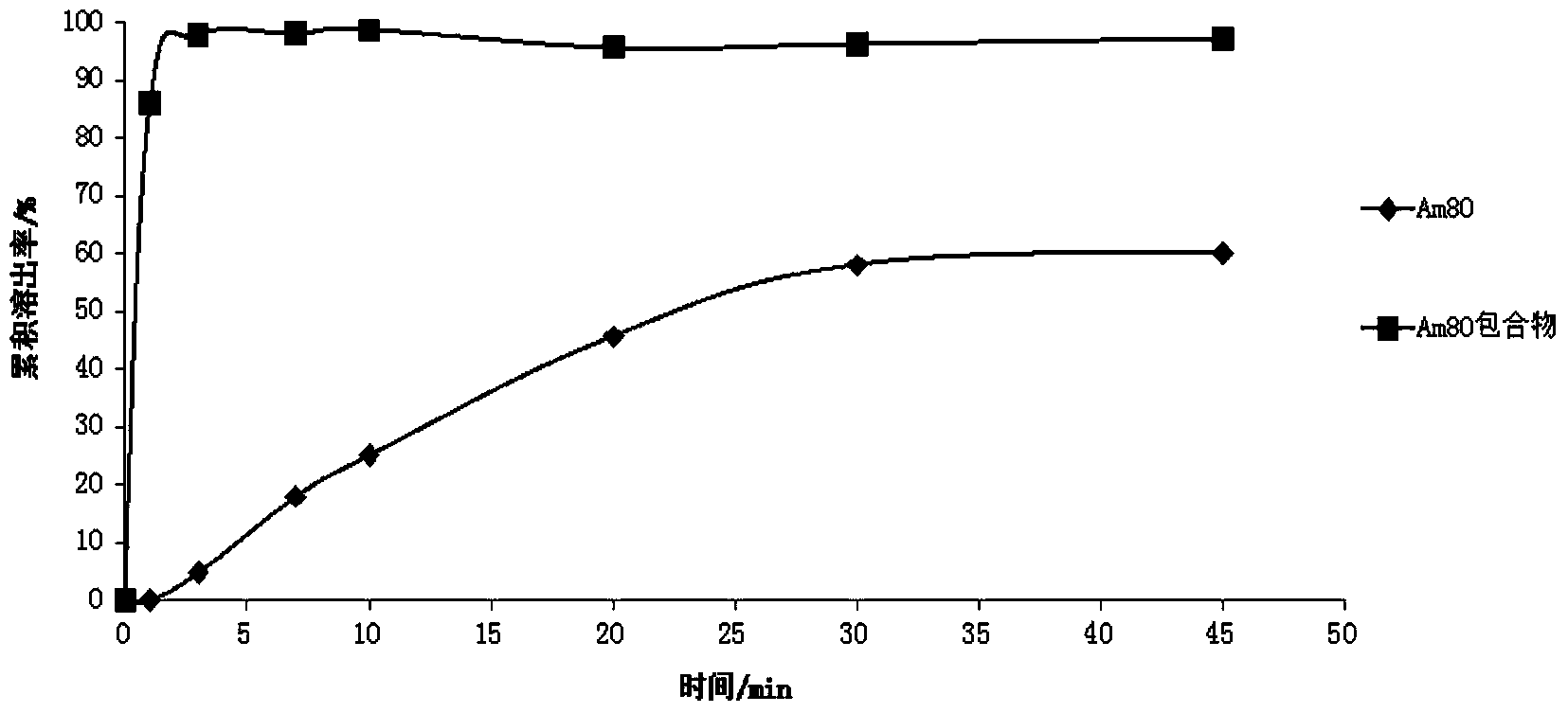
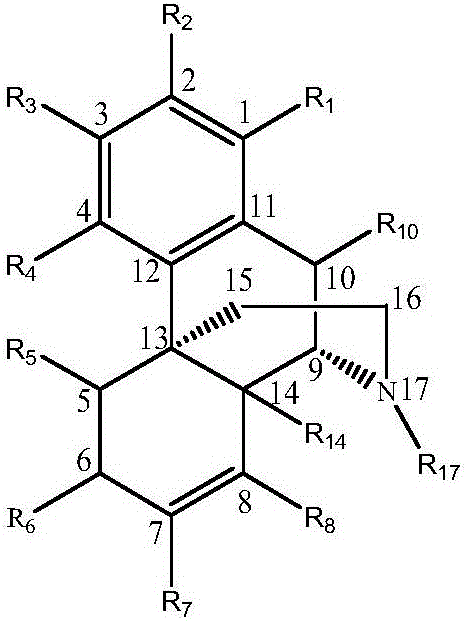
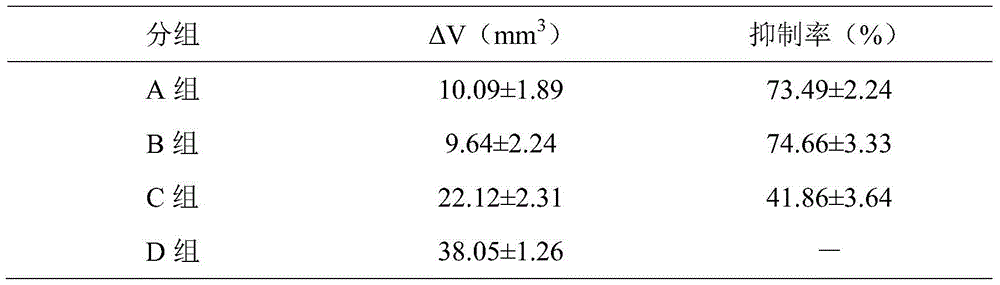
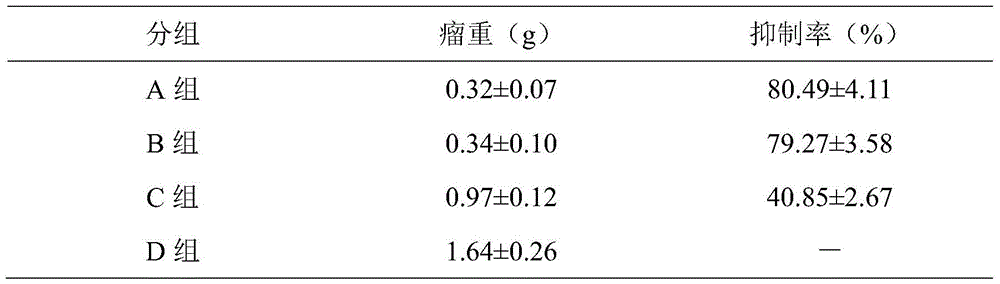
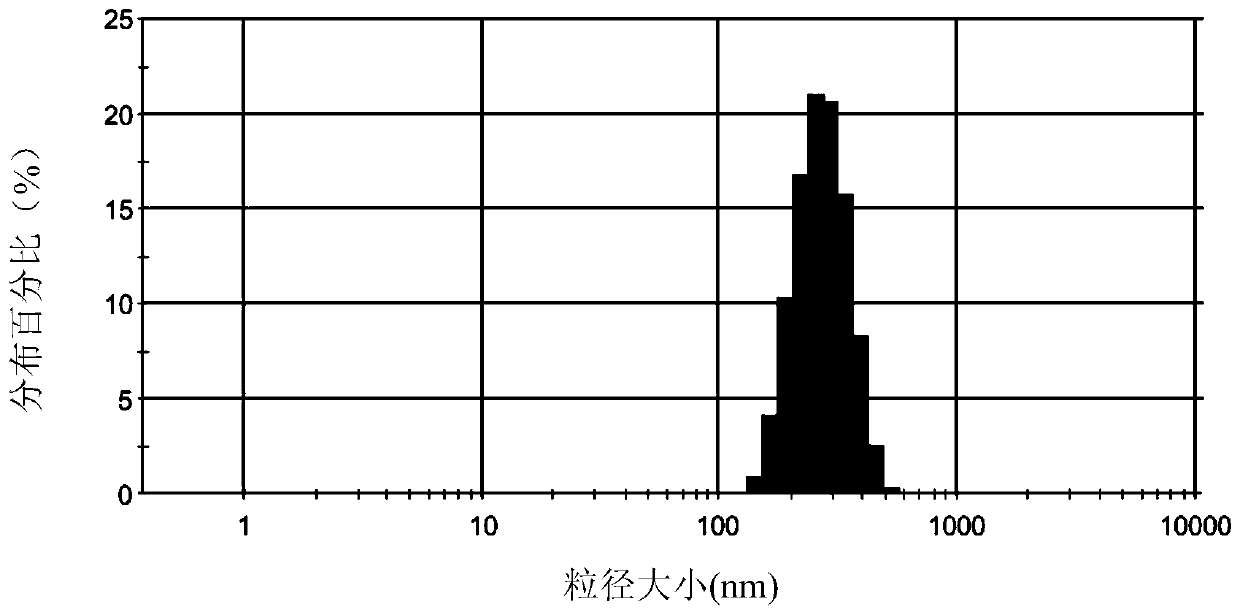
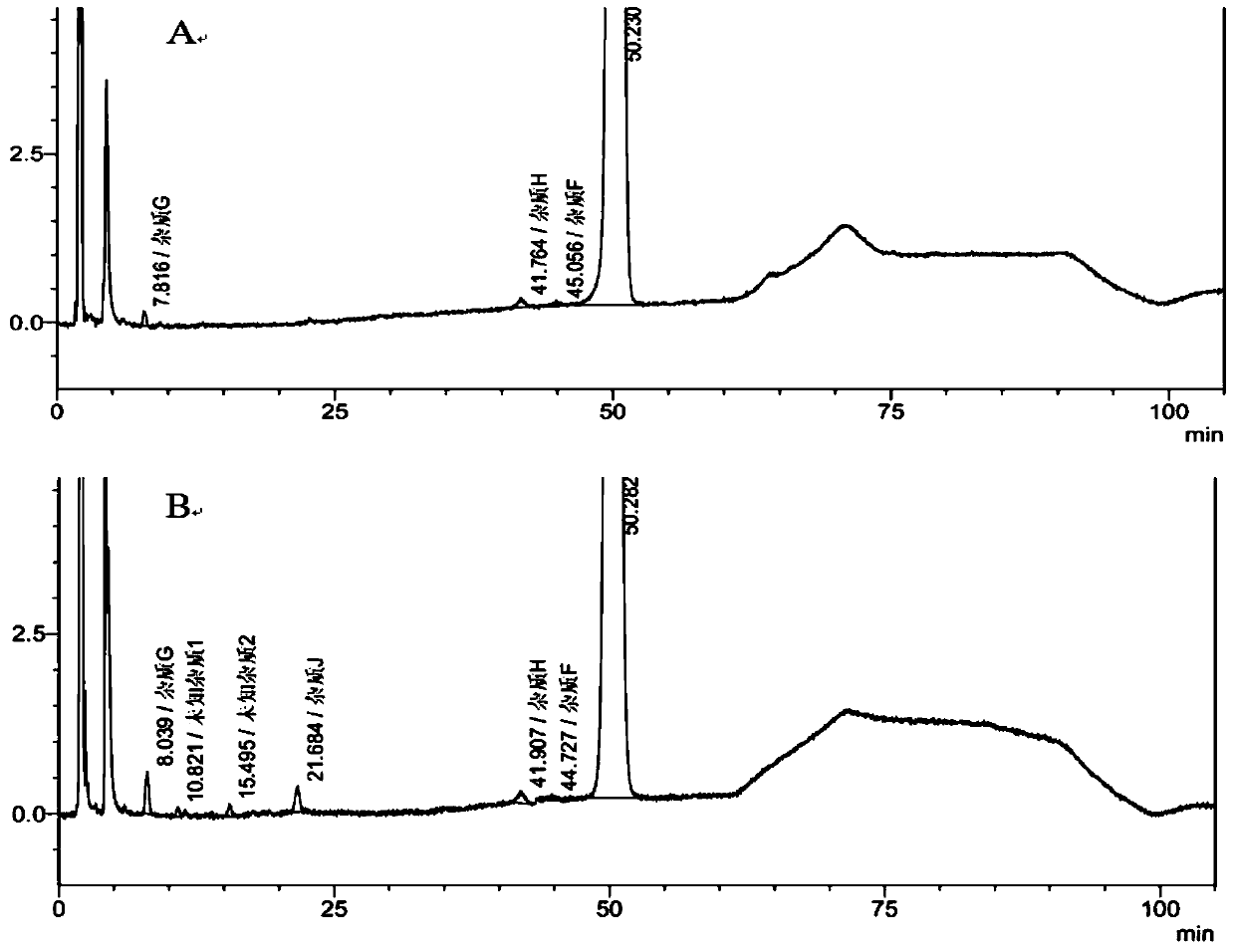
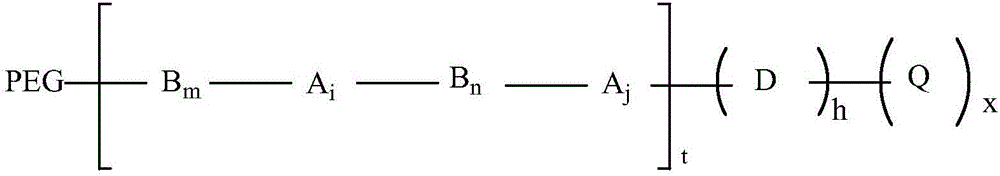Patents
Literature
35results about How to "Less irritating to blood vessels" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
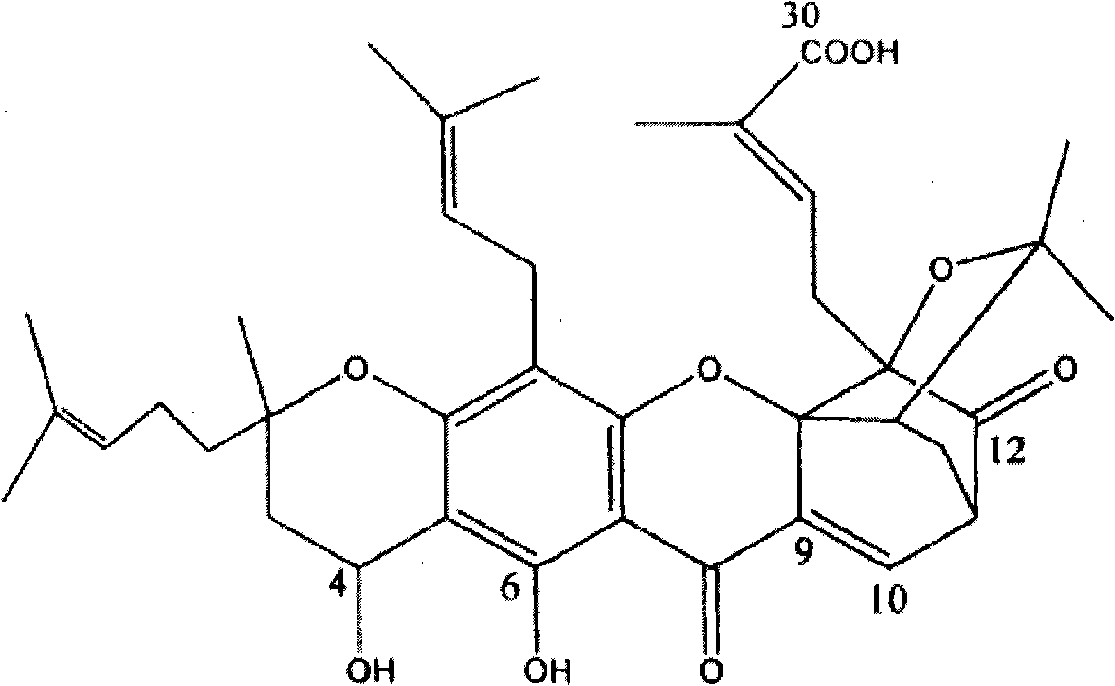
Neo-gambogic acid SLN (solid lipid nanoparticle) and preparation method thereof
InactiveCN101947204ALow toxicityImprove tolerancePowder deliveryOrganic non-active ingredientsLipid formationSolubility
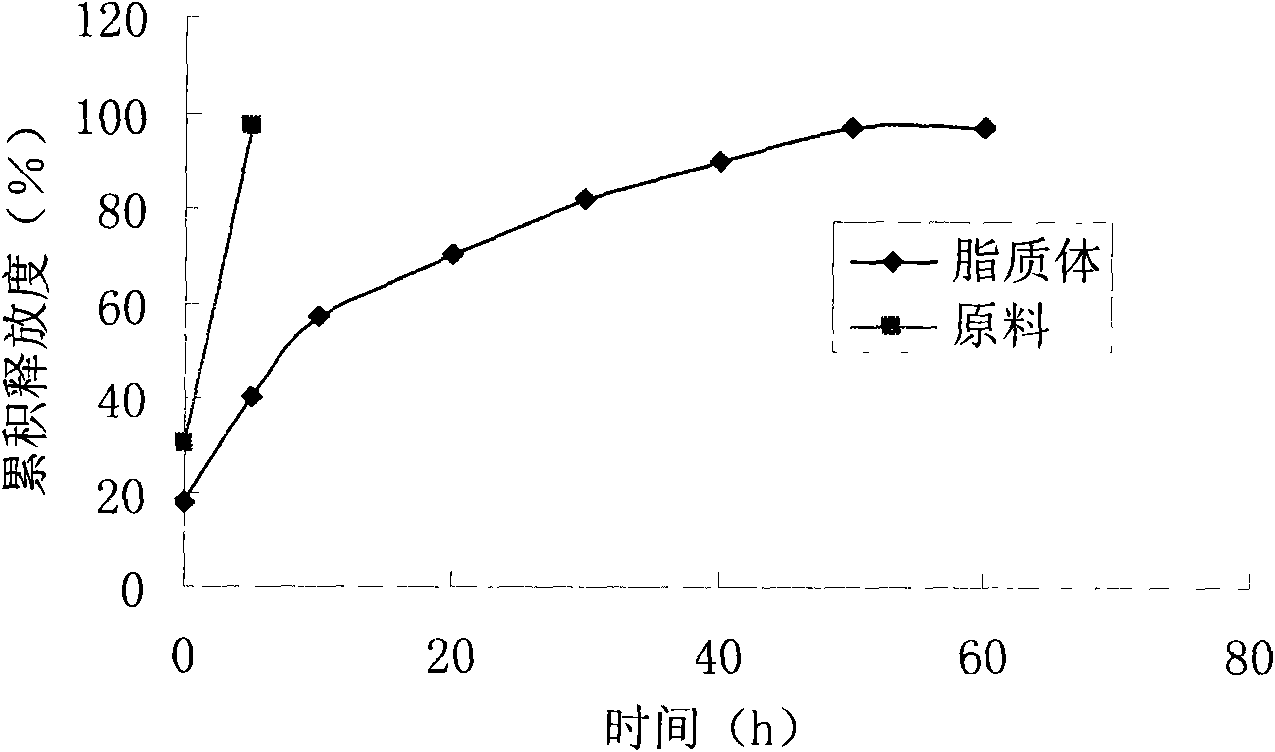
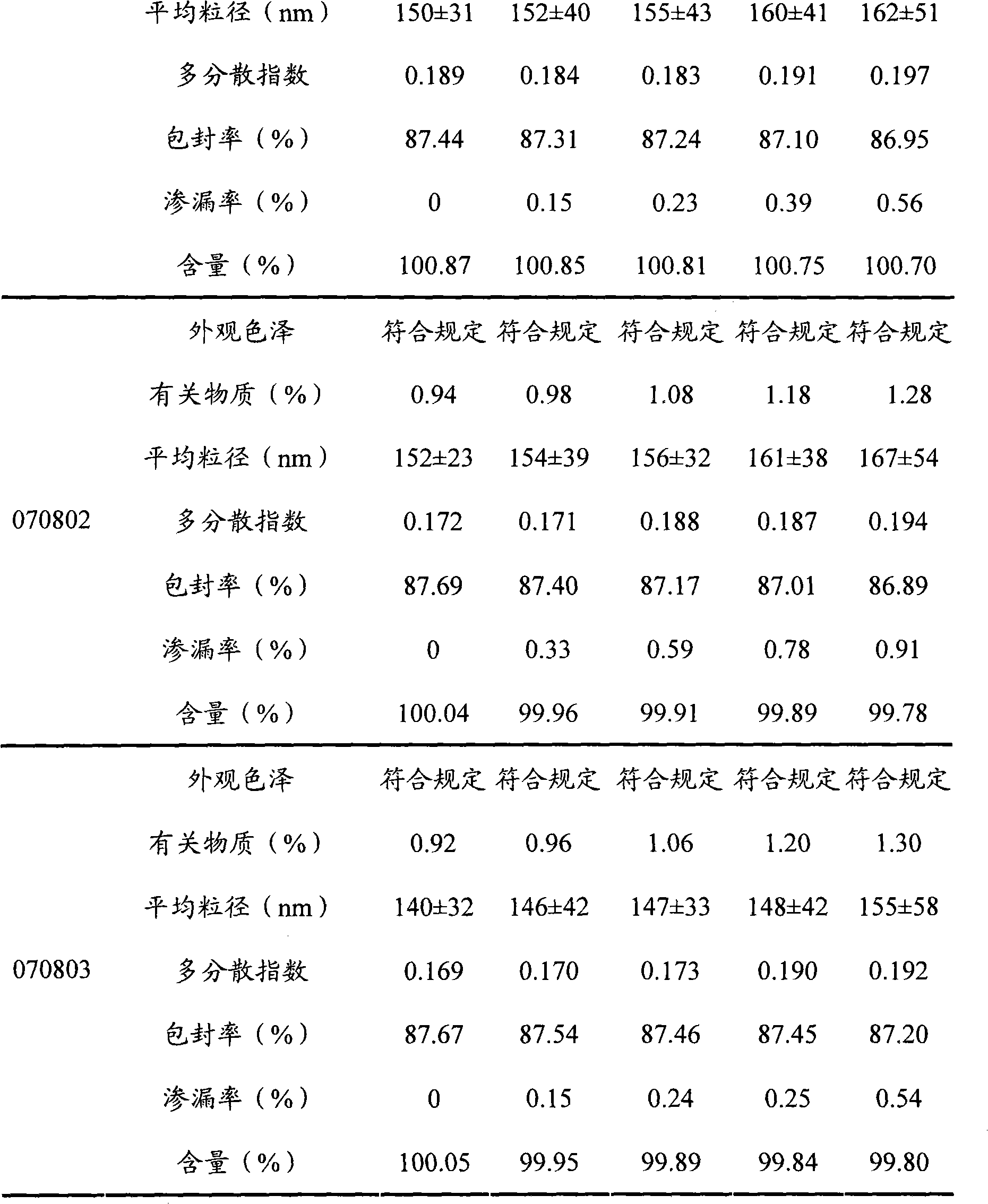
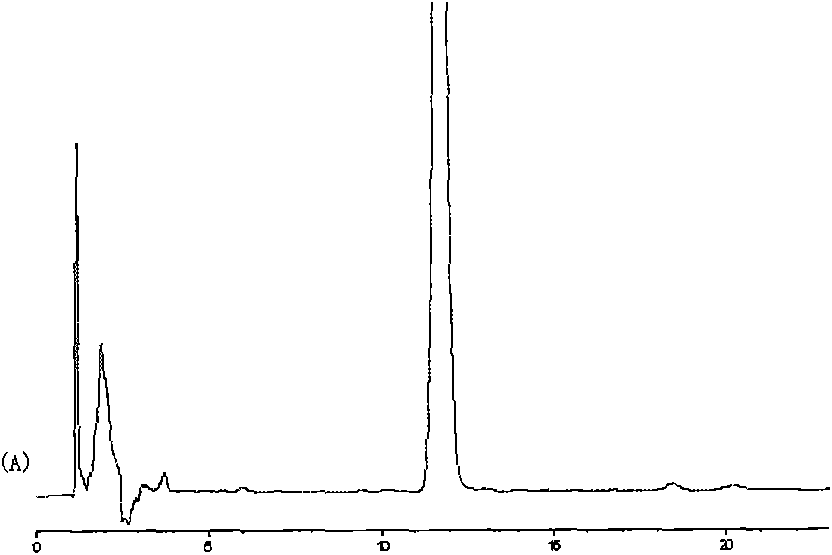
The invention relates to a neo-gambogic acid SLN (solid lipid nanoparticle) and a preparation method thereof. The neo-gambogic acid SLN comprises a therapeutically effective amount of neo-gambogic acid, medicinal phosphatide, a surfactant and a lipid material. In the invention, the neo-gambogic acid is prepared into SLNs (solid lipid nanoparticles), thereby improving the solubility of the neo-gambogic acid, reducing the irritability, improving the bioavailability, and prolonging the action time of medicaments in a human body, in addition, the neo-gambogic acid SLNs can be gathered partially in the human body so as to play the targeted action of the SLNs and exert the anti-cancer therapeutic action of the SLNs better.
Owner:彭代银 +4
Etoposide lipidosome and preparation method thereof
InactiveCN101584662ATargetedGood curative effectOrganic active ingredientsPharmaceutical non-active ingredientsSolubilitySide effect
The invention provides an etoposide lipidosome used for injection or oral administration. The etoposide lipidosome is characterized in that etoposide is enveloped with phospholipid type substance, and the etoposide lipidosome with small grain size, high entrapment efficiency, good stability and low toxic side effect is prepared. The prepared etoposide lipidosome enhances the solubility and the stability of the etoposide, reduces the toxicity of the etoposide and prolongs the cycling time of the medicine in the blood, thereby improving the therapeutic effect of the medicine; and the preparation prepared by the etoposide lipidosome has the characteristics of low toxicity, low sensitivity and high efficiency on the clinical application. The invention also relates to a preparation method of the etoposide lipidosome, which has simple preparation process and low cost and is suitable for the industrial production.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Taxol submicroemulsion taking cholesterol complex as intermediate carrier
ActiveCN102048688AReduce intakeReduce manufacturing costOrganic active ingredientsEmulsion deliverySolubilityCholesterol
The invention discloses a taxol submicroemulsion. The submicroemulsion comprises taxol-cholesterol complex, injection oil, injection water, emulsifier, assistant emulsifier and isosmotic agent, wherein the weight ratio of taxol to cholesterol in the taxol-cholesterol complex is (1: 0.09) to (1: 0.90). The invention also discloses a preparation method and application of the taxol submicroemulsion. The oil-in-water taxol submicroemulsion, the pH value of which is 4 to 6 and the average particle size of which is less than 600nm, is prepared by taking the cholesterol complex as an intermediate carrier through the step of dissolving the cholesterol complex into an oil phase by using the improvement of the drug oil solubility by the cholesterol complex. The taxol submicroemulsion is used for treating malignant tumors and has higher safety and efficiency.
Owner:BEIJING WEHAND BIO PHARMA CO LTD
Flurbiprofen acetaminophen resin lipid microsphere injection, freeze-drying lipid microsphere injection and preparation methods
ActiveCN103054800AImprove solubilityImprove physical stabilityPowder deliveryOrganic active ingredientsSolubilityTreatment effect
The invention relates to a flurbiprofen acetaminophen resin lipid microsphere injection, a freeze-drying lipid microsphere injection and a preparation method. Through a reasonable composition proportion and a preparation process with controllable parameters, good stability and strong operability, the flurbiprofen acetaminophen resin which is an indissolvable drug is wrapped in an oil phase and an interfacial film of a lipid microsphere, so that not only is the solubility of the drug remarkably strengthened, but also precipitation and oxidation do not occur easily, the physical and chemical stabilities of the drug are greatly improved, the devitrification phenomenon is avoided in the use and storage processes, a slow release effect is exerted and the action time of the drug in the blood plasma is prolonged, meanwhile, as the lipid microsphere targets to an inflammation part, the toxicity and the vessel stimulation during injection are remarkably reduced, the clinical application of the injections is safer, the treatment effect is more remarkable, and the injections and the methods are suitable for industrialized production and clinical application.
Owner:WUXI ERYUN TECH CO LTD
Pharmaceutical composition containing ganciclovir compound, and preparation method thereof
InactiveCN102210686APromote dissolutionReduce the amount addedPowder deliveryInorganic non-active ingredientsInjection siteGanciclovir
The invention discloses a pharmaceutical composition containing a ganciclovir compound, and a preparation method thereof. The pharmaceutical composition is composed of ganciclovir, polysorbate 80, sodium hydroxide and dextran. The ganciclovir pharmaceutical composition overcomes the irritation of the existing ganciclovir lyophilized powder injections on an injection site, and improves the stability of the ganciclovir lyophilized powder injection and the compliance of patients.
Owner:罗诚
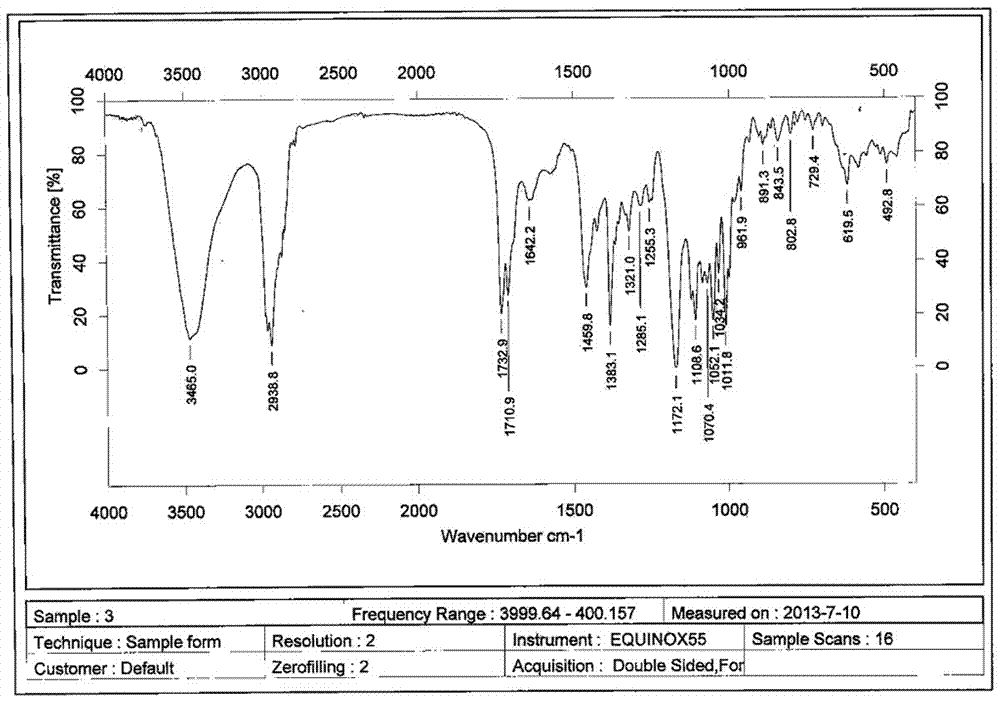
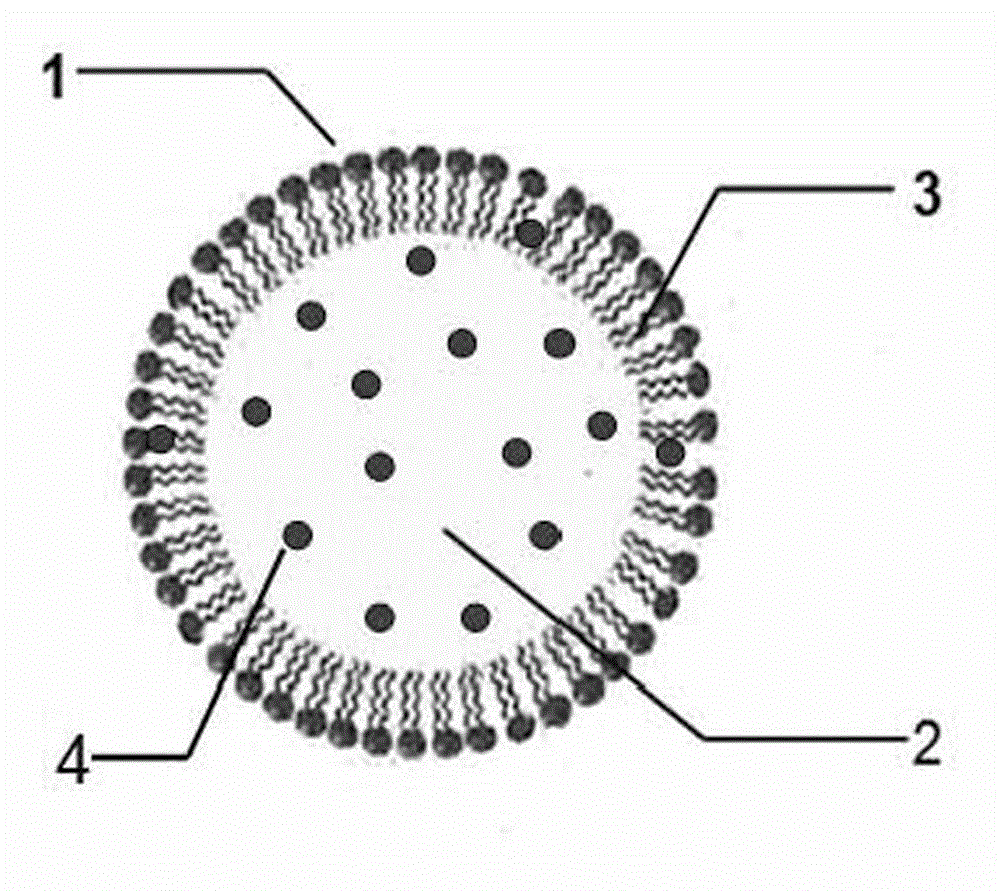
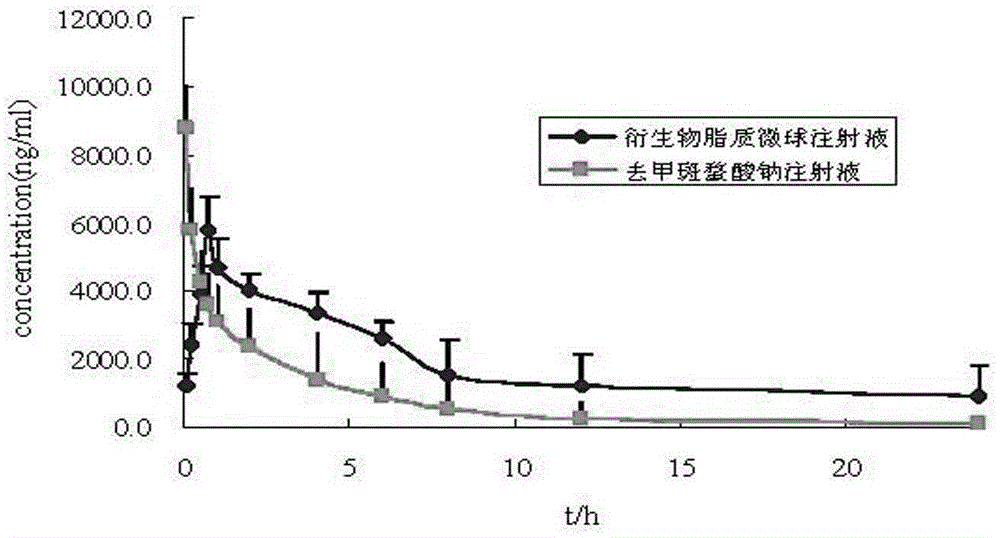
Lipid microsphere injection containing sodium demethyl cantharidate-phosphatide complex and preparation method thereof
The invention discloses a lipid microsphere injection containing sodium demethyl cantharidate-phosphatide complex and preparation method thereof. The injection comprises the sodium demethyl cantharidate-phosphatide complex, fat-soluble medium, surfactant and other ingredients. In the sodium demethyl cantharidate-phosphatide complex, the molar ratio of sodium demethyl cantharidate to phosphatide is 1-1:10. The prepared sodium demethyl cantharidate-phosphatide complex improves the distribution of sodium demethyl cantharidate in oil-water 2-phase interfacial film and oil phase greatly, increasesthe entrapment efficiency of the medicine in preparation, realizes the interfacial film loading medicine, reduces the toxicity, and can carry out targeting drug release in vivo. The prepared lipid microsphere injection reduces the vascular stimulation of sodium demethyl cantharidate, improves the curative effect, and reduces toxic and side effect.
Owner:SHENYANG PHARMA UNIVERSITY
Injection containing lipoid microsphere of etoposide and its prepn process
InactiveCN1973826ALess irritating to blood vesselsGood chemical stabilityOrganic active ingredientsAntineoplastic agentsMicrosphereIrritation
The present invention belongs to the field of medicine technology, and is especially lipoid microsphere injection containing etoposide and its preparation process. The injection contains etoposide, fat soluble medium, water and surfactant; and consists of oil phase 5-30 wt%, etoposide 0.001-0.5 wt%, surfactant 0.5-5 wt%, osmotic regulator 0.5-5 wt%, and water for injection the rest. Etoposide is high pressure homogenized under the action of high speed airflow to form ultrasonic stirring, so as to be dissolved fast and permeated in molecular form into oil / water interface film. By means of medicine carrying interface film principle, the present invention raises the medicine carrying amount of insoluble etoposide and the stability while lowering the toxicity and blood vessel irritation. The preparation of the present invention has low toxicity, low irritation and high curative effect in clinical application.
Owner:SHENYANG PHARMA UNIVERSITY
Lipoid microsphere injection containing toad cake extract and its preparing method
InactiveCN1985851ALess irritating to blood vesselsAvoid ingestionAmphibian material medical ingredientsEmulsion deliveryMicrosphereIrritation
The present invention relates to lipoid microspere injection composition containing liposoluble toad cake extract and its preparation process. The injection composition contains liposoluble toad cake extract 0.0001-5 wt%, liposoluble medium 1-30 wt%, surfactant 0.5-10 wt%, isoosmotic regulator 0.5-10 wt%, and water for the rest. The injection has anticancer and analgetic effects, less blood vessel irritation caused by toad cake, no toxicity on heart and capacity of raising the medicine concentration inside tumor.
Owner:SHENYANG YAODA MEDICINE DEV
Alprostadil medium-and-long-chain lipid emulsion for injection and preparation method thereof
ActiveCN103610640AExtensive developmentWide range of applicationsOrganic active ingredientsPharmaceutical non-active ingredientsTG - TriglycerideGlycerol
The invention discloses an alprostadil medium-and-long-chain lipid emulsion for injection and a preparation method thereof, belonging to the technical field of biological medicines. The lipid emulsion is composed of the following components in parts by weight: 0.01-0.1 part of alprostadil, 10-100 parts of long-chain triglycerides, 10-100 parts of medium-chain triglycerides, 1-20 parts of phospholipids, 0.01-1 part of oleic acid, 5-20 parts of glycerol and 300-2500 parts of water for injection. The particle size of the lipid emulsion is 50-200 nm, the lipid emulsion has good stability while the pH value is 5.0-8.0, and the contents of degradation products including prostaglandin A1 and prostaglandin B1 of alprostadil measured by using an HPLC (High Performance Liquid Chromatography) method are respectively below 1%. Compared with traditional alprostadil long-chain lipid emulsions, aqueous-phase alprostadil is significantly decreased, so that the vascular stimulation is reduced, and the liver load is reduced, therefore, the lipid metabolism can be accelerated, and the triglyceride level of plasma can be reduced.
Owner:JILIN UNIV
Antihypertensive drug fat milk injection and preparation method thereof
ActiveCN107661294AQuality improvementImprove securityOrganic active ingredientsEmulsion deliveryClevidipineOil phase
The invention discloses an antihypertensive drug fat milk injection and a preparation method thereof. The antihypertensive drug fat milk injection is a clevidipine butyrate fat milk injection and contains clevidipine butyrate and a pharmacologically acceptable medical excipient; the medical excipient comprises an oil-phase medium, an emulsifier, an osmotic pressure regulator, a stabilizer, a metalcheating agent, a pH value regulator and injection water. The antihypertensive drug fat milk injection has stable quality and high safety and is not irritant to blood vessels.
Owner:WUHAN CONFORM PHARMA CO LTD
Red sage root A magnesium injection preparation and preparation method thereof
ActiveCN101125123AReduced total impurity contentImprove securityPowder deliveryOrganic active ingredientsVitamin CSalvianolic acid A
The present invention discloses a salvianolic acid A injection preparation and the preparation method, the components are salvianolic acid A and pharmaceutical excipients, the present invention is characterized in that the pharmaceutical excipients are vitamin C, the weight ratio of the salvianolic acid A and the vitamin C is 16 to 25 :1, the preferential weight ratio of the salvianolic acid A and the vitamin C is 20:1; the toxicological experiments show that the salvianolic acid A injection preparation of the application has better safety; and the pharmacological experiments indicate that all the groups of the preparation of the application have great pharmacological effects.
Owner:CHIATAI QINGCHUNBAO PHARMA
Tamibarotene cyclodextrin or cyclodextrin derivative clathrate and preparation method thereof
InactiveCN104162167AImprove solubilityIncrease dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityFreeze-drying
The invention discloses a tamibarotene cyclodextrin or cyclodextrin derivative clathrate which is prepared from tamibarotene and cyclodextrin or a cyclodextrin derivative, the molar ratio of tamibarotene to cyclodextrin or cyclodextrin derivative is 1:1-1:100, and the preparation method is as follows: the cyclodextrin or cyclodextrin derivative is added to a solvent to produce a solution or suspension, and then the tamibarotene is added for stirring, grinding or ultrasonic mixing to obtain the tamibarotene cyclodextrin or cyclodextrin derivative clathrate; or the cyclodextrin or cyclodextrin derivative is put in a colloid mill or mortar, a solvent is added for stirring to make a paste, then the tamibarotene is added into the paste for grinding for 1-5 hours to obtain a homogeneous thick paste, and the tamibarotene cyclodextrin or cyclodextrin derivative clathrate is obtained by filtration, concentration or freeze drying. The invention also discloses a tamibarotene-containing composition. The tamibarotene cyclodextrin or cyclodextrin derivative clathrate improves the solubility and dissolution rate of the tamibarotene, has good water solubility, less vascular stimulation, quick disintegration, higher bioavailability and other characteristics.
Owner:SHANDONG UNIV
Total bufadienolides solid lipid nanoparticle drug delivery system for injection and preparation method thereof
ActiveCN104997759AEase of industrial productionLow toxicityAmphibian material medical ingredientsDigestive systemSolubilityControl release
The invention belongs to the field of pharmaceutical preparation, and relates to a total bufadienolides solid lipid nanoparticle drug delivery system for injection and a preparation method thereof. Total bufadienolides solid lipid nanoparticles are composed of the components in parts by weight: 0.1-2 parts of total bufadienolides with therapeutically effective amount, 0.5-20 parts of a lipid material, 0.5-15 parts of a lipid soluble emulsifier, 0.5-10 parts of a water soluble emulsifier, 0.002-2.5 part of an antioxidant, 0.1-25 parts of sugar and a proper amount of water for injection. The total bufadienolides solid lipid nanoparticles for injection allow the total bufadienolides to be wrapped in a lipoid nucleus, increase the solubility of the total bufadienolides, reduce the irritation, avoid degradation or leakage of the drug, can achieve targeting effect, controlled release and other effects, and have the advantages of stable physical and chemical properties and the like.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
Cyclodextrin inclusion compound containing resibufogenin and preparation and application
InactiveCN107296959AGood water solubilitySolve the problem of insoluble in waterOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityVein
The invention relates to a cyclodextrin inclusion compound containing resibufogenin and a preparation method of the cyclodextrin inclusion compound. The cyclodextrin inclusion compound consists of resibufogenin and an inclusion material at a mole ratio of 1:(1-100), and the preparation method comprises the following steps: dissolving resibufogenin, cyclodextrin and derivatives thereof into a proper amount of a solvent, stirring, and drying, thereby obtaining the cyclodextrin inclusion compound containing resibufogenin. The cyclodextrin inclusion compound containing resibufogenin, provided by the invention, is high in water solubility, low in toxicity, small in blood vessel irritation and high in stability, is capable of ensuring uniformity of preparations, has remarkable anti-tumor functions, is expected to be made into oral tablets, capsules or vein injections, and has great clinical application values.
Owner:SHENYANG PHARMA UNIVERSITY
Artemether liposome for injection and preparation method and application thereof
ActiveCN107669637ASmall particle sizeUniform particle size distributionOrganic active ingredientsPharmaceutical non-active ingredientsHigh concentrationEthanol Injection
The invention provides an artemether liposome for injection. The artemether liposome is prepared by adopting an ethanol injection method. The method is simple in preparation process and can be appliedto industrial production. According to the invention, by selecting appropriate material components and adopting appropriate preparation processes, the prepared artemether liposome is small in particle size (150-200nm) and uniform in particle size distribution and has the encapsulation efficiency greater than 90%. The artemether liposome is slowly released in vivo to avoid adverse reactions causedby excessively high concentration of drugs at the initial stage of the injection. Furthermore, the inventor finds that the artemether liposome prepared with the method has high stability, high bioavailability, good resolubility and long storage time, and meets needs of drug use.
Owner:SHANDONG UNIV
Magnetic sodium cantharidinate vitamin B6 compound preparation and preparation method thereof
ActiveCN104814959ALess irritating to blood vesselsImprove medication safetyPharmaceutical non-active ingredientsAntineoplastic agentsVitamin b6Cholesterol
The present invention discloses a magnetic sodium cantharidinate vitamin B6 compound preparation and a preparation method thereof, wherein the magnetic sodium cantharidinate vitamin B6 compound preparation is prepared from 50-300 parts by weight of magnetic sodium cantharidinate liposome and 1-10 parts by weight of vitamin B6, wherein the magnetic sodium cantharidinate liposome is prepared from 1-20 parts of sodium cantharidinate, 1-5 parts of magnetic Fe3O4, 5-20 parts of phospholipid, 1-5 parts of cholesterol, 0-2 parts of sodium deoxycholate, and 50-300 parts of water. The preparation method comprises: preparing magnetic Fe3O4, preparing a magnetic sodium cantharidinate solution, preparing a magnetic liposome suspension, adding vitamin B6, dissolving, adding water for injection to carry out volume metering, and finally preparing the injection or freeze-drying powder. The prepared magnetic sodium cantharidinate vitamin B6 compound preparation of the present invention has effects of reduction of irritation on blood vessels, improvement of medication safety, reduction of toxic-side effect, and improvement of drug stability.
Owner:GUIZHOU BAIQIANG PHARMA
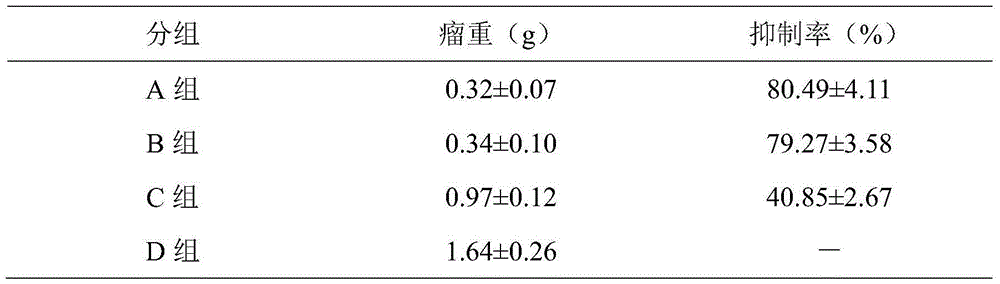
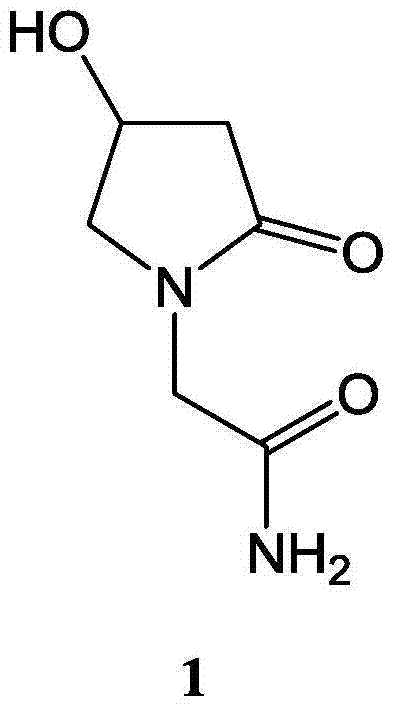
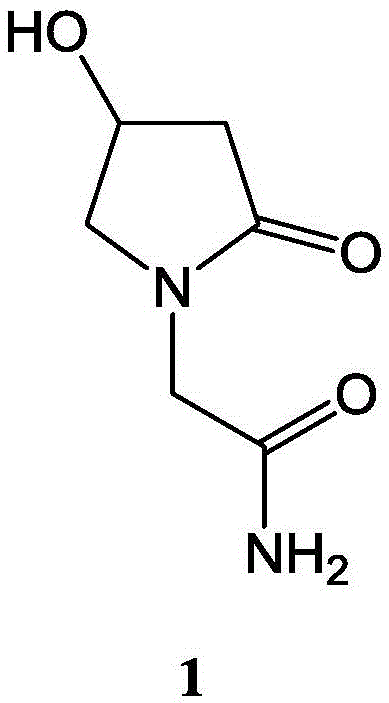
Medicinal composition containing 4-hydroxy-2-oxo-1-pyrrolidine acetamide and application thereof
ActiveCN103877081AGood treatment effectReduce the effective doseNervous disorderHydroxy compound active ingredientsTreatment effectPharmacy medicine
The invention belongs to the technical field of medicinal products, and particularly relates to a medicinal composition containing 4-hydroxy-2-oxo-1-pyrrolidine acetamide and application thereof. A 4-hydroxy-2-oxo-1-pyrrolidine acetamide-borneol medicinal composition provided by the invention has a synergistic treatment effect on neuromotor dysfunction and impaired memory dysfunction vascular dementia and senile dementia, and the treatment effect of oxiracetam can be improved remarkably by using borneol, so that the acting dose of oxiracetam is reduced, the dosage is reduced, and adverse reactions are reduced after long-time application. By adopting the medicinal composition, intelligence disturbance of a dementia patient can be treated more effectively, and a treatment effect can be achieved with a small dosage. The medicinal composition is suitable for clinical use.
Owner:CSPC OUYI PHARM CO LTD +1
Pinocembrin and cyclodextrin or cyclodextrin derivative clathrate compound
InactiveCN102716070ALess irritating to blood vesselsDisintegrates quicklyAntibacterial agentsOrganic active ingredientsSolubilityWater soluble
The invention discloses a pinocembrin and cyclodextrin or cyclodextrin derivative clathrate compound. Cyclodextrin or cyclodextrin derivatives are used for realizing the clathration on pinocembrin, the clathrate compound comprises the pinocembrin and cyclodextrin or cyclodextrin derivatives according to a mol ratio being 1:(1-100). The water solubility of the pinocembrin is improved, the treatment effect of the pinocembrin is more perfectly realized, the clathrate compound is suitable for being used for preparing solid preparations and liquid preparations required in clinics, and the preparations include transfusion, water injection, powder injection, oral liquid, syrup, tablets, capsules, granules and dispersible tablets. The clathrate compound can be used for preparing medicine for preventing and / or treating cardiovascular and cerebrovascular diseases, particularly cerebral apoplexy, and can also be used for preparing medicine for preventing and / or treating germ and / or fungal infection.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
PEG-modified compounds of sinomenine and its derivatives and preparation method thereof
ActiveCN106478938AReduced immunogenic responseReduce allergic reactionsOrganic chemistryAntipyreticPolyethylene glycolDegree of polymerization
The invention relates to PEG-modified compounds of sinomenine and its derivatives and a preparation method thereof. The PEG-modified compounds of sinomenine and its derivatives have structures shown in the general formula (I), wherein PEG represents polyethylene glycol polymers with different polymerization degrees and molecular weight of 100 to 60000 daltons.
Owner:CHENGDU YIPING MEDICAL SCI & TECH
A kind of pharmaceutical composition containing 4-hydroxyl-2-oxo-1-pyrrolidineacetamide and its application
ActiveCN103877081BReduced activityElevated MDA contentNervous disorderHydroxy compound active ingredientsPharmacy medicineTherapeutic effect
The invention belongs to the technical field of medicinal products, and particularly relates to a medicinal composition containing 4-hydroxy-2-oxo-1-pyrrolidine acetamide and application thereof. A 4-hydroxy-2-oxo-1-pyrrolidine acetamide-borneol medicinal composition provided by the invention has a synergistic treatment effect on neuromotor dysfunction and impaired memory dysfunction vascular dementia and senile dementia, and the treatment effect of oxiracetam can be improved remarkably by using borneol, so that the acting dose of oxiracetam is reduced, the dosage is reduced, and adverse reactions are reduced after long-time application. By adopting the medicinal composition, intelligence disturbance of a dementia patient can be treated more effectively, and a treatment effect can be achieved with a small dosage. The medicinal composition is suitable for clinical use.
Owner:CSPC OUYI PHARM CO LTD +1
Alprostadil medium-and-long-chain lipid emulsion for injection and preparation method thereof
ActiveCN103610640BLess irritatingReduce loadOrganic active ingredientsPharmaceutical non-active ingredientsGlycerolPhospholipid
The invention discloses an alprostadil medium-and-long-chain lipid emulsion for injection and a preparation method thereof, belonging to the technical field of biological medicines. The lipid emulsion is composed of the following components in parts by weight: 0.01-0.1 part of alprostadil, 10-100 parts of long-chain triglycerides, 10-100 parts of medium-chain triglycerides, 1-20 parts of phospholipids, 0.01-1 part of oleic acid, 5-20 parts of glycerol and 300-2500 parts of water for injection. The particle size of the lipid emulsion is 50-200 nm, the lipid emulsion has good stability while the pH value is 5.0-8.0, and the contents of degradation products including prostaglandin A1 and prostaglandin B1 of alprostadil measured by using an HPLC (High Performance Liquid Chromatography) method are respectively below 1%. Compared with traditional alprostadil long-chain lipid emulsions, aqueous-phase alprostadil is significantly decreased, so that the vascular stimulation is reduced, and the liver load is reduced, therefore, the lipid metabolism can be accelerated, and the triglyceride level of plasma can be reduced.
Owner:JILIN UNIV
A kind of alprostadil freeze-dried microemulsion composition and preparation method thereof
ActiveCN105748415BHigh encapsulation efficiencyImprove stabilityOrganic active ingredientsPowder deliveryFreeze-dryingIrritation
The invention provides PGE1 (prostaglandin E1) freeze-dried microemulsion composition. The composition is prepared from PGE1, a non-ionic emulsifier, chondroitin sulfate, anionic phospholipid and a freeze-drying protective agent, and raw materials and components comprise, in percentage by mass, 0.001%-0.04% of PGE1, 1%-55% of the non-ionic emulsifier, 0.1%-20% of anionic phospholipid, 0.6%-28% of an oil phase and 5%-98% of the freeze-drying protective agent. The encapsulation efficiency and the stability of the composition are significantly improved, reduction of free drug concentration is facilitated, vascular irritation of a preparation is reduced, and the composition is safer and more stable.
Owner:SHANGHAI FANGYU HEALTH PHARMA TECH CO LTD
A clarithromycin ion-pair lipid microsphere injection and preparation method thereof
ActiveCN104771362BHigh antibacterial activityAddressing drug resistanceAntibacterial agentsOrganic active ingredientsYolkPatient compliance
The invention relates to a method for preparing clarithromycin ion-pair lipid microsphere injection by using cholesterol succinate monoester (CHEMS). Based on 100ml injection, it contains: clarithromycin 0.05g-0.5g; cholesterol succinate monoester 0.3g-0.6g; medium-chain fatty acid triglyceride 10g-20g; soybean oil for injection 0-10g; egg yolk lecithin 0.2g~4g; soybean lecithin 0g~4g; Pluronic F‑68 0.2g~1g; glycerin 2g~5g; water for injection 70g~90g. The physical and chemical properties of the clarithromycin ion-pair lipid microsphere injection of the present invention meet the requirements for intravenous administration, and can withstand high-pressure steam sterilization at 121° C. for 10 minutes. At the same time, this study is the first in the world to apply ion-pairing technology to the preparation of nano-preparations, which improves the transmembrane ability of clarithromycin, reduces bacterial drug resistance, and improves drug efficacy. The samples prepared in this study have good physical and chemical stability in long-term storage, and have little vascular irritation, which can improve patient compliance and curative effect.
Owner:SHENYANG PHARMA UNIVERSITY
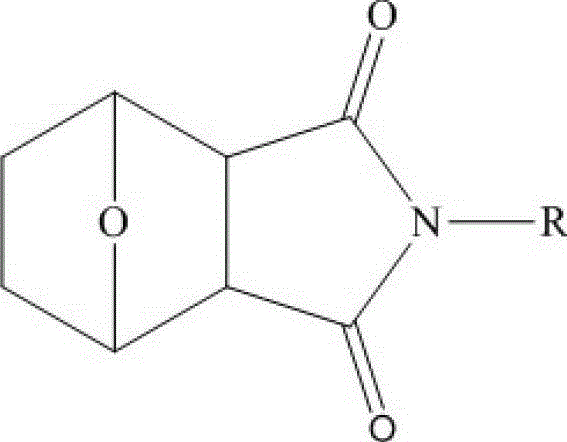
Norcantharidin derivative lipid microsphere injection and preparation method thereof
InactiveCN102973503BHigh encapsulation efficiencyHigh drug loadingOrganic active ingredientsSolution deliveryAlkaneActive agent
The invention relates to norcantharidin derivative lipid microsphere injection and a preparation method thereof. The injection contains norcantharidin imide derivative, oil phase, water and surfactant; and the formula of the injection contains the following components by mass: 5 to 30 percent of oil phase, 0.001 to 1 percent of norcantharidin imide N-alkyl derivative, 0.5 to 7 percent of surfactant, 1 to 5 percent of osmotic pressure regulator, and the balance of injection water. Norcantharidin is connected with a long-chain saturated alkane group through amino, so that the lipid solubility of the injection is greatly improved, and the lipid solubility of the injection is improved together with increase of the chain length of the saturated alkane group. Lipid microspheres can carry medicaments with the granularity of more than 90 percent into lipid cores and (or) an interfacial film, so that the medicament loading capacity and the encapsulation efficiency are greatly improved, the vascular stimulation during injection is reduced, the physical and chemical stability of the medicaments and the lipid microsphere injection is improved, the in-vivo acting time of the medicaments is prolonged, the treatment effect is improved, and the toxic or side effect is reduced.
Owner:辽宁正鑫药物研究有限公司
A kind of artemether liposome for injection and its preparation method and application
ActiveCN107669637BSmall particle sizeUniform particle size distributionOrganic active ingredientsPharmaceutical non-active ingredientsHigh concentrationEthanol Injection
The invention provides an artemether liposome for injection, and the artemether liposome is prepared by an ethanol injection method. The preparation process of the method is simple and can be applied to industrial production. With a suitable preparation process, the prepared artemether liposome has a small particle size (150-200nm), uniform particle size distribution, and an encapsulation efficiency greater than 90%. Artemether liposomes are released slowly in the body to avoid adverse reactions caused by excessive drug concentration at the initial stage of injection. At the same time, the inventors found that the artemether liposome prepared by this method has high stability, high bioavailability, good resolubility, and long storage time, which meets its medication requirements.
Owner:SHANDONG UNIV
A kind of clarithromycin ion pair liposome injection and preparation method thereof
ActiveCN104173288BHigh antibacterial activityStable contentAntibacterial agentsOrganic active ingredientsVeinPatient compliance
The invention relates to a method for preparing clarithromycin ion pair lipidosome injection from cholesteryl hemisuccinate (CHEMS). Every 100ml of injection comprises 0.05-0.8g of clarithromycin, 0.3-1.0g of cholesteryl hemisuccinate, 0.8-5g of high-purity yolk lecithin, 0.05-0.5g of MPEG-DSPE, 0.2-0.4g of Na2HPO4.12H2O, 0.1-0.2g of NaH2PO4.2H2O, 0.01-0.03g of KH2PO4, 0.7-0.9g of NaCl, 0.01-0.03g of KCl and 70-90g of injection water. The physicochemical property of clarithromycin ion pair lipidosome injection meets the vein medicine requirements. Meanwhile the method adopts an ion pair technique to prepare nano preparations in the world for the first time, the transmembrane capability of clarithromycin is improved, bacterial drug resistance is degraded, and the medicine effect is improved. A sample prepared by using the method is good in physical and chemical stability after being stored for a long time, irritation to blood vessels is small, the patient compliance is improved, and the curative effect is improved.
Owner:SHENYANG PHARMA UNIVERSITY
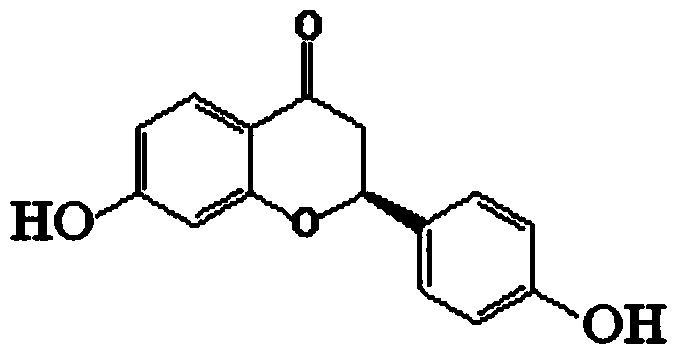
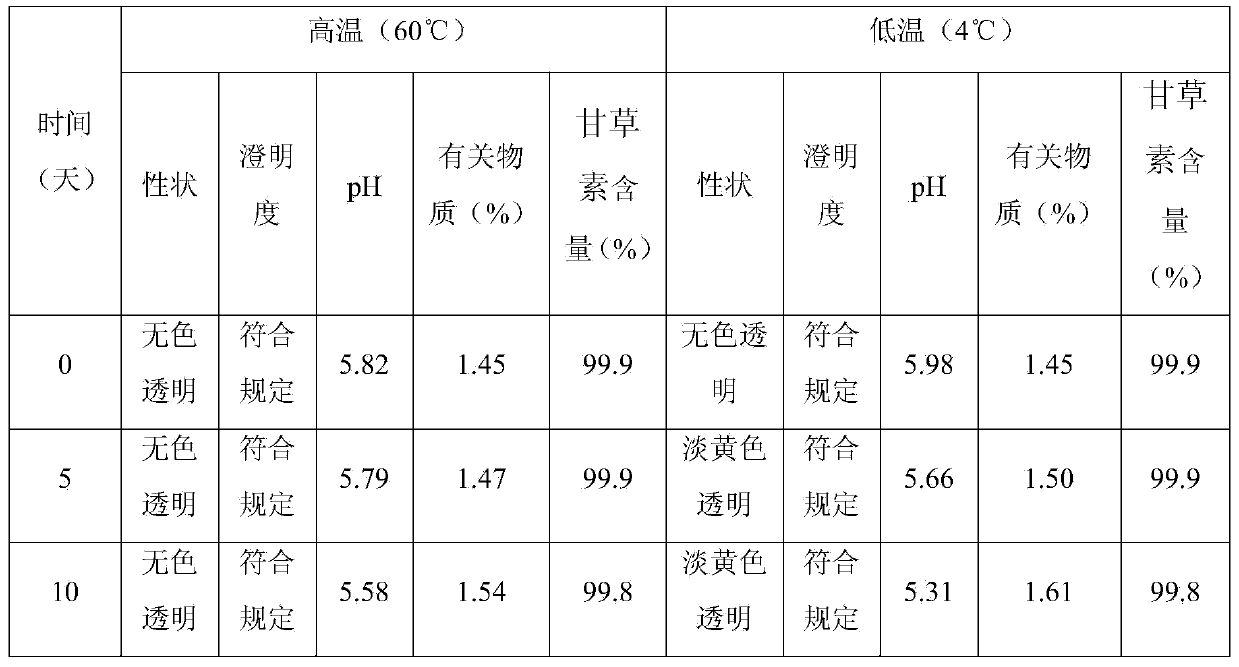
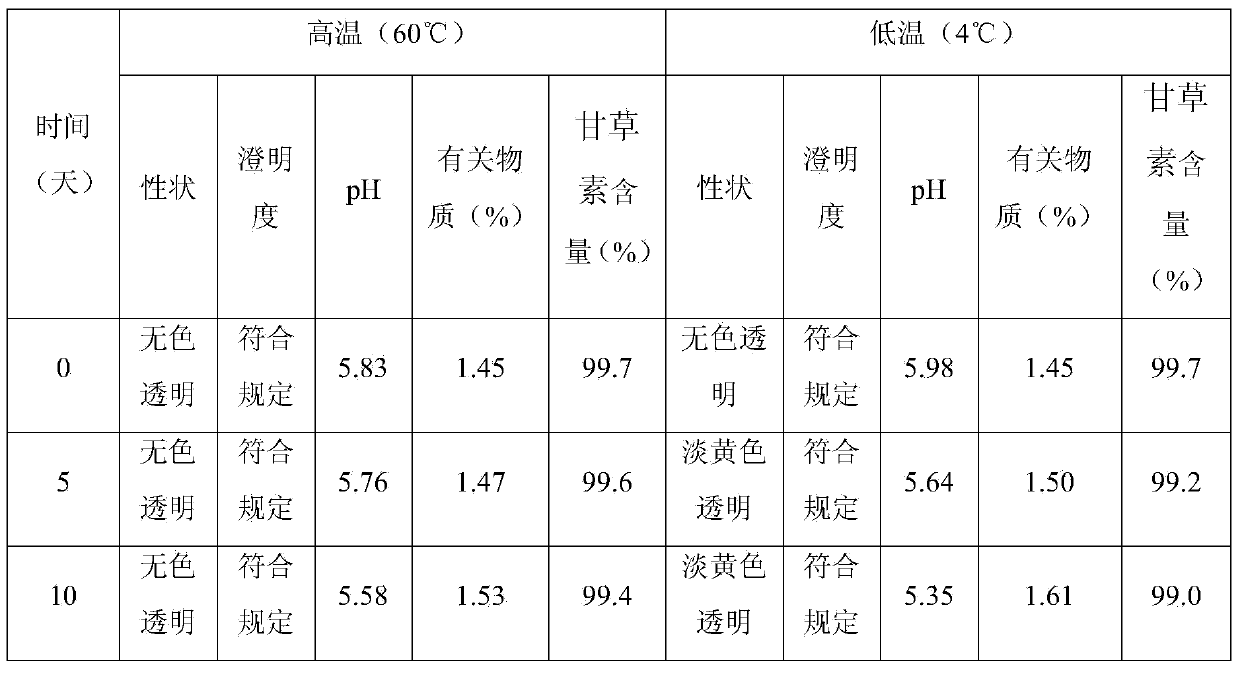
Liquiritigenin injection and preparation method and application thereof
ActiveCN103735497AImprove stabilityLess irritating to blood vesselsOrganic active ingredientsPharmaceutical delivery mechanismHemolysisIrritation
The invention discloses a liquiritigenin injection and a preparation method and application thereof. Each 100mL of liquiritigenin injection provided by the invention contains 10mg-1000mg of liquiritigenin, 20-50mL of propylene glycol and the balance of injection water; the liquiritigenin injection has a pH value of 4.0-6.0. According to the liquiritigenin injection, through a plenty of experiments, the dissolving solvent and the consumption thereof of the liquiritigenin injection, the concentration of liquiritigenin, and the pH value of the injection are screened, and thus an optimal injection formula is obtained; meanwhile, according to the solubility, the stability, the vascular stimulation, the hemolysis and the like, a preparation process of the liquiritigenin injection is screened. Experiments indicate that the liquiritigenin injection prepared by adopting the preparation method is small in irritation, free from hemolytic activity, high in safety, especially better in stability, strong in anti-tumor activity, capable of overcoming the defects of serious first-pass effect, low bioavailability and the like of an existing preparation, and better in clinical effect.
Owner:NANJING HAICHANG CHINESE MEDICINE GRPCO LTD
Lipid microsphere injection containing sodium demethyl cantharidate-phosphatide complex and preparation method thereof
The invention discloses a lipid microsphere injection containing sodium demethyl cantharidate-phosphatide complex and preparation method thereof. The injection comprises the sodium demethyl cantharidate-phosphatide complex, fat-soluble medium, surfactant and other ingredients. In the sodium demethyl cantharidate-phosphatide complex, the molar ratio of sodium demethyl cantharidate to phosphatide is 1-1:10. The prepared sodium demethyl cantharidate-phosphatide complex improves the distribution of sodium demethyl cantharidate in oil-water 2-phase interfacial film and oil phase greatly, increasesthe entrapment efficiency of the medicine in preparation, realizes the interfacial film loading medicine, reduces the toxicity, and can carry out targeting drug release in vivo. The prepared lipid microsphere injection reduces the vascular stimulation of sodium demethyl cantharidate, improves the curative effect, and reduces toxic and side effect.
Owner:SHENYANG PHARMA UNIVERSITY
Magnetic sodium cantharidinate vitamin b6 compound preparation and preparation method thereof
ActiveCN104814959BLess irritating to blood vesselsImprove medication safetyPharmaceutical non-active ingredientsAntineoplastic agentsSide effectCholesterol
The invention discloses a magnetic sodium cantharidate vitamin B6 compound preparation and a preparation method thereof. In parts by weight, the compound preparation is prepared from 50-300 parts of magnetic sodium cantharidate liposome and 61-10 parts of vitamin B, wherein The magnetic sodium cantharidate liposome is prepared from 1-20 parts of sodium cantharidate, 1-5 parts of magnetic Fe3O4, 5-20 parts of phospholipids, 1-5 parts of cholesterol, 0-2 parts of sodium deoxycholate and 50-300 parts of water. to make. The preparation method of magnetic sodium cantharidate vitamin B6 compound preparation includes the preparation of magnetic Fe3O4, the preparation of sodium cantharidate magnetic liquid, the preparation of magnetic liposome suspension, adding vitamin B6 to dissolve and then adding water for injection to make up the volume, and finally preparing an injection Or freeze-dried powder. The magnetic sodium cantharidinate vitamin B6 compound preparation prepared by the invention reduces vascular irritation, improves drug safety, reduces toxic and side effects, and also improves drug stability.
Owner:GUIZHOU BAIQIANG PHARMA
Antihypertensive drug fat emulsion injection and preparation method thereof
ActiveCN107661294BNot easily oxidizedPrevent oxidationOrganic active ingredientsEmulsion deliveryOfficinalClevidipine
Owner:WUHAN CONFORM PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com