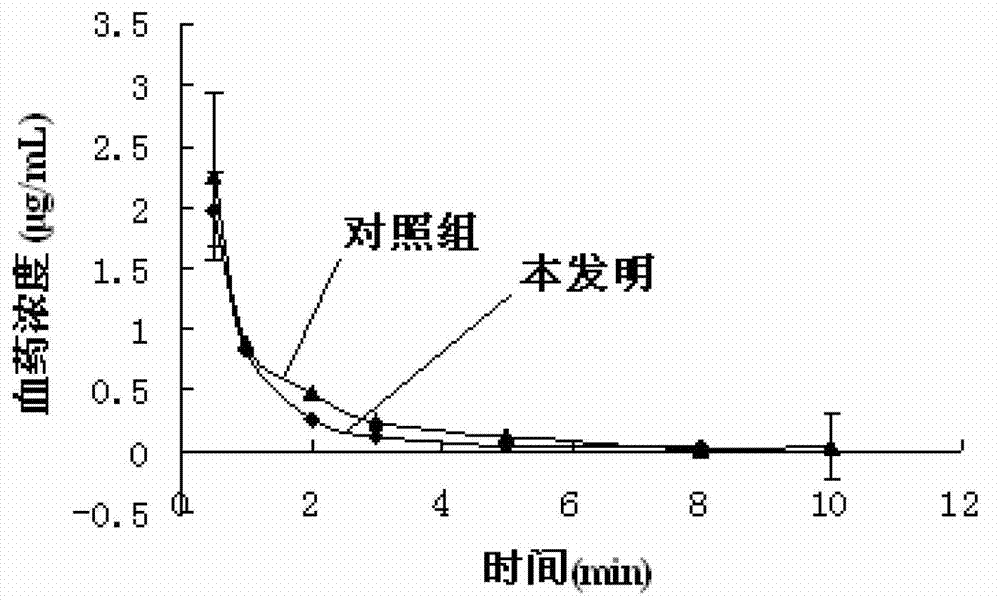
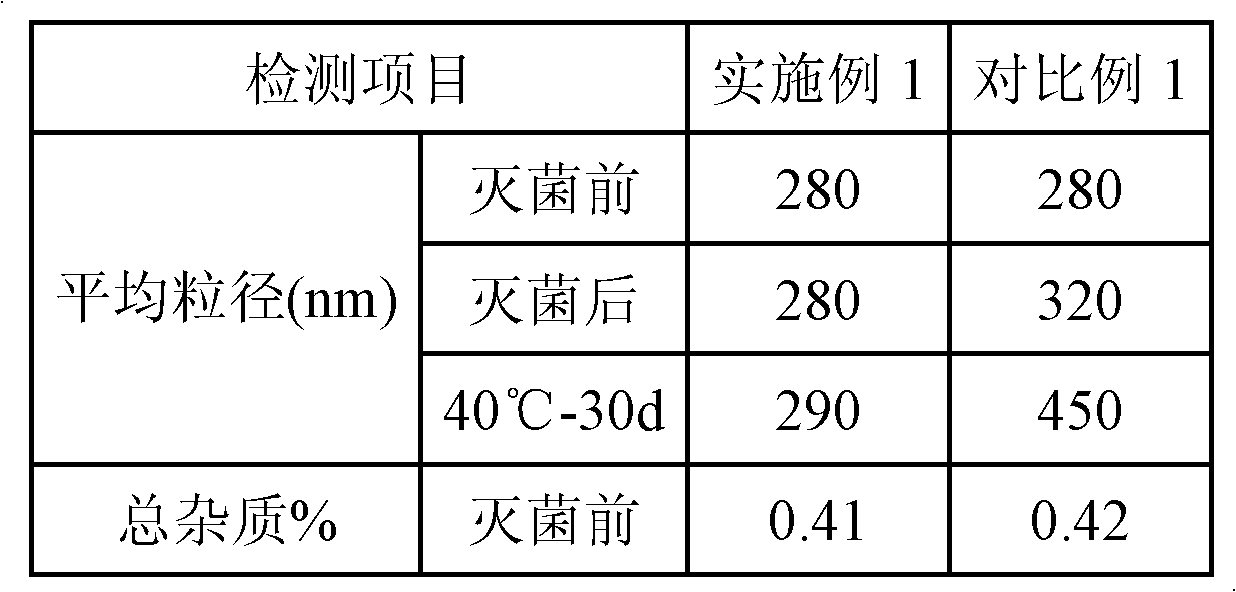
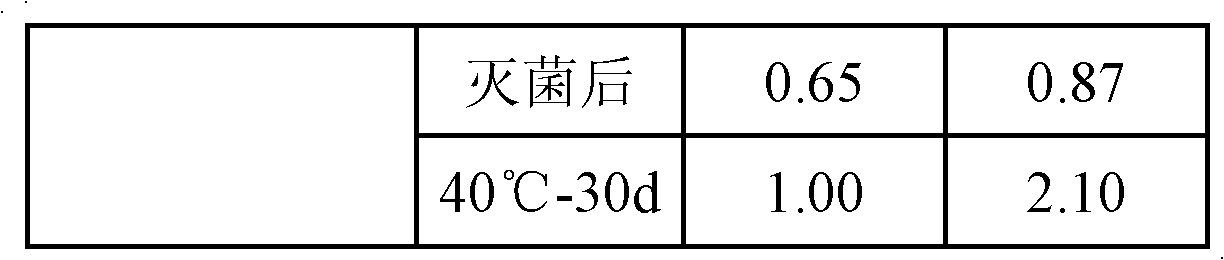
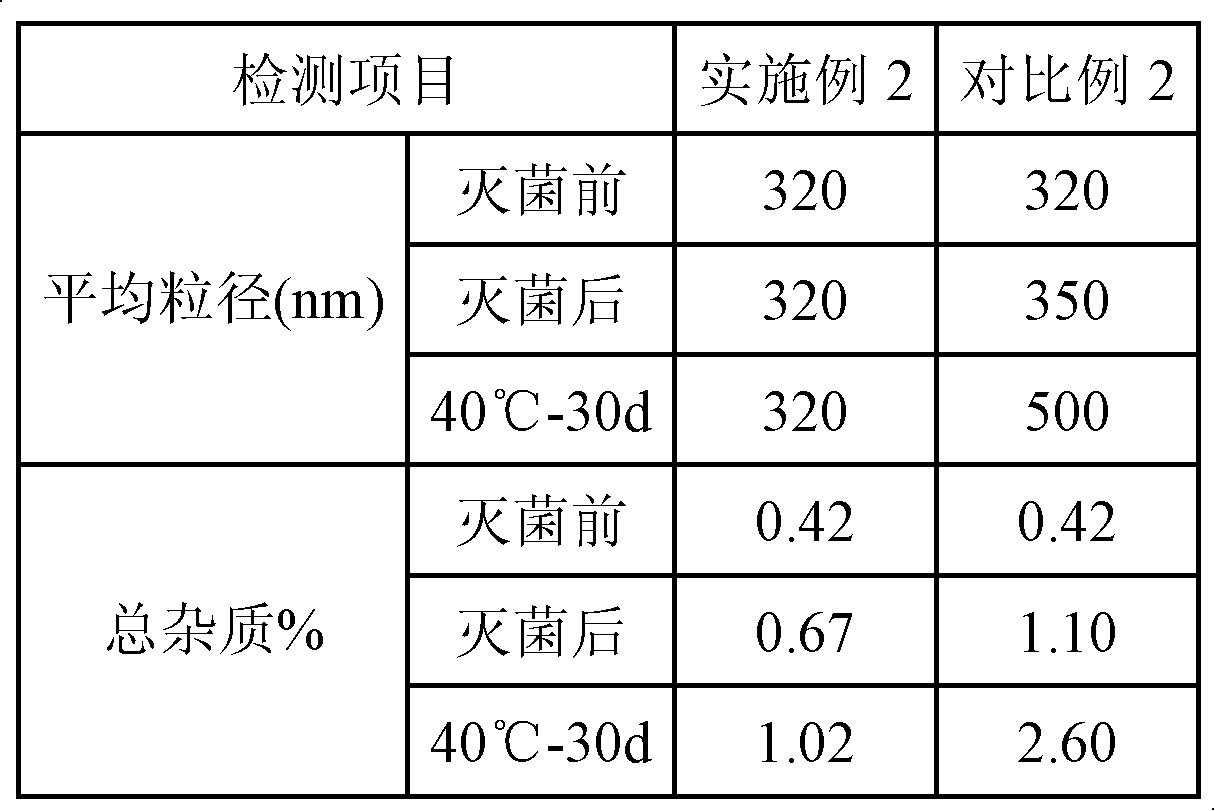
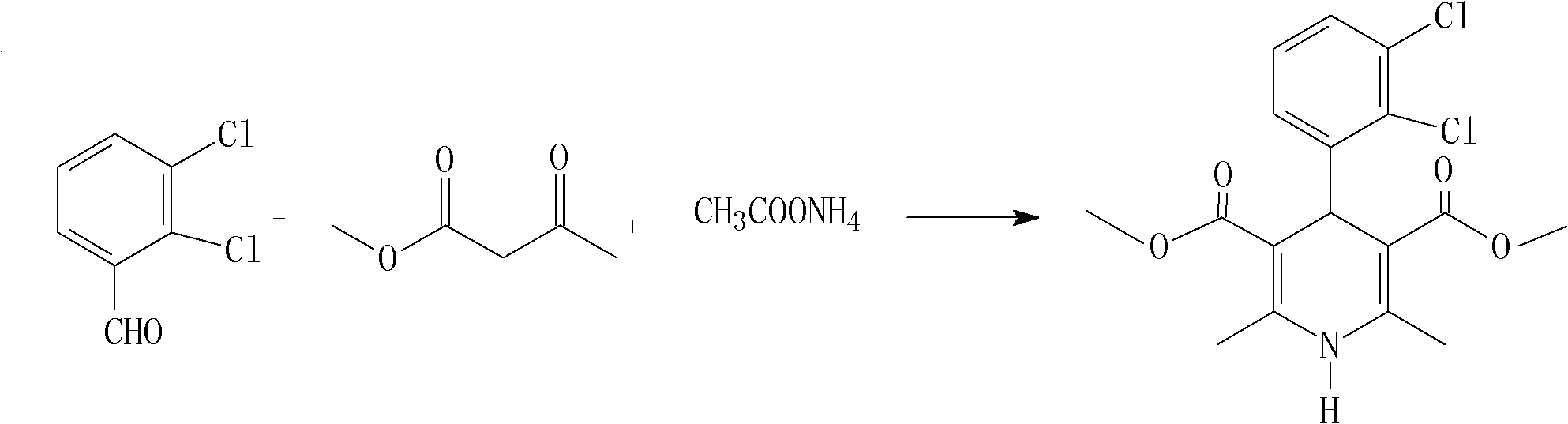Patents
Literature
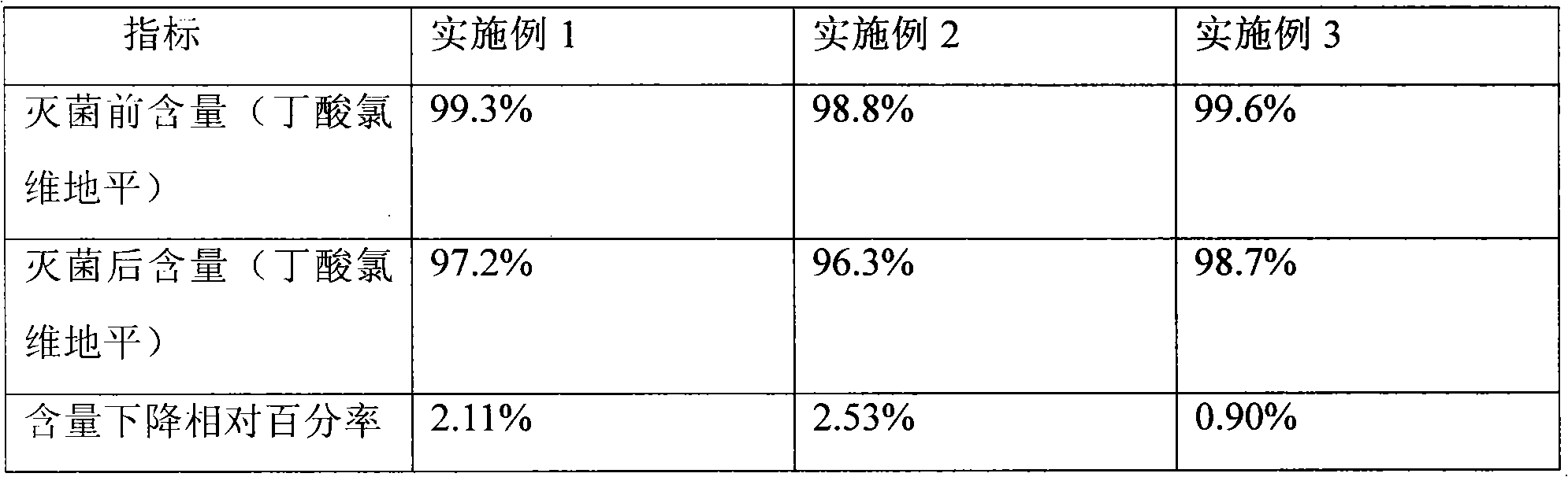
104 results about "Clevidipine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
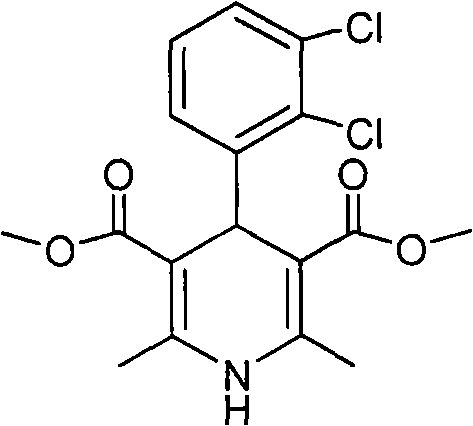
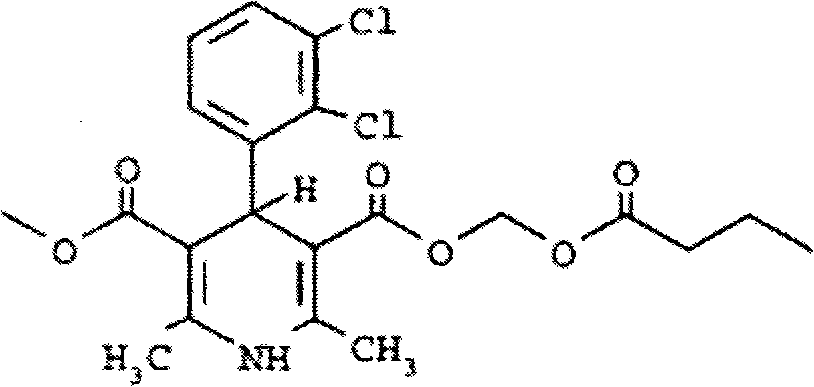
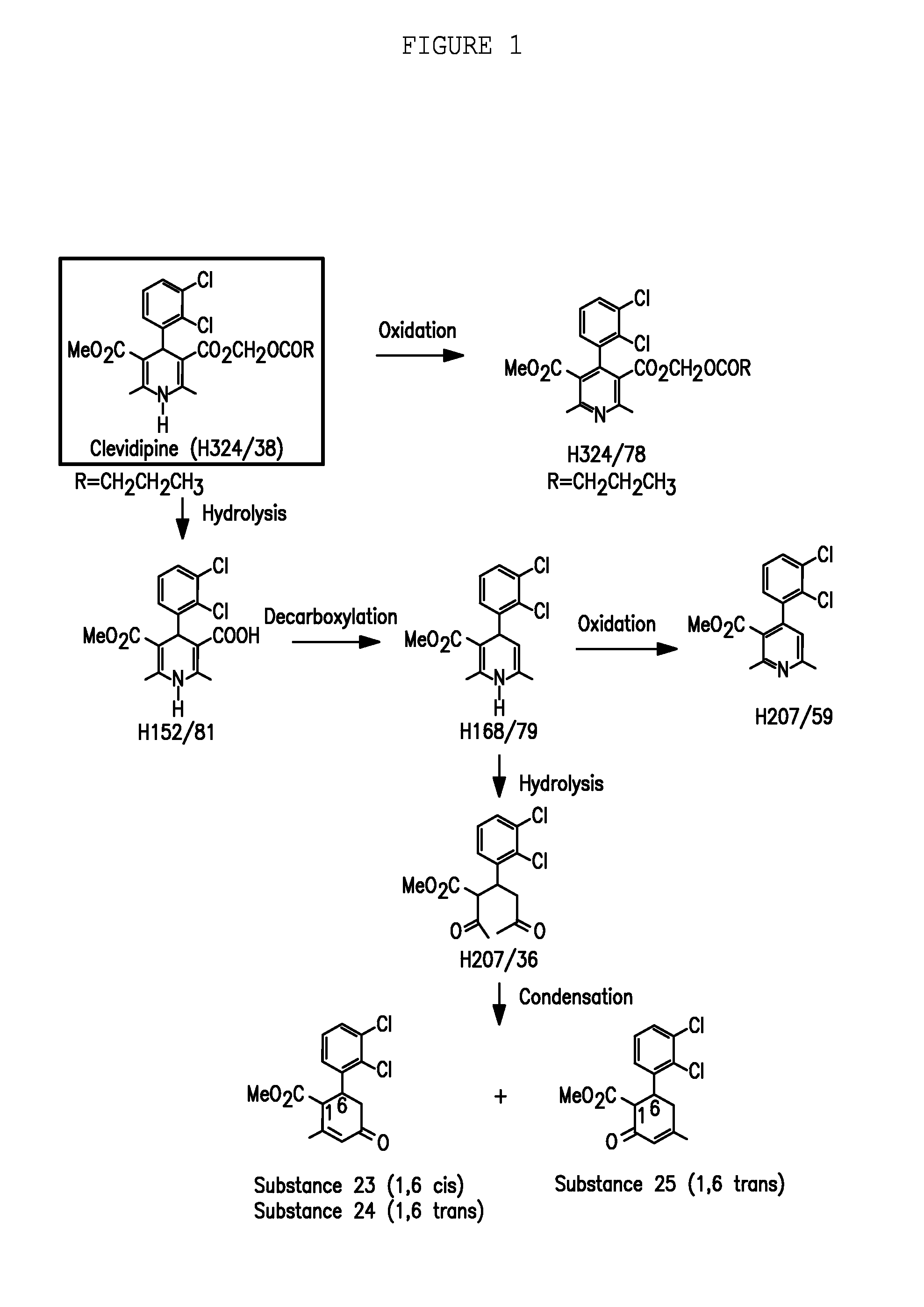
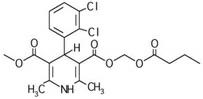
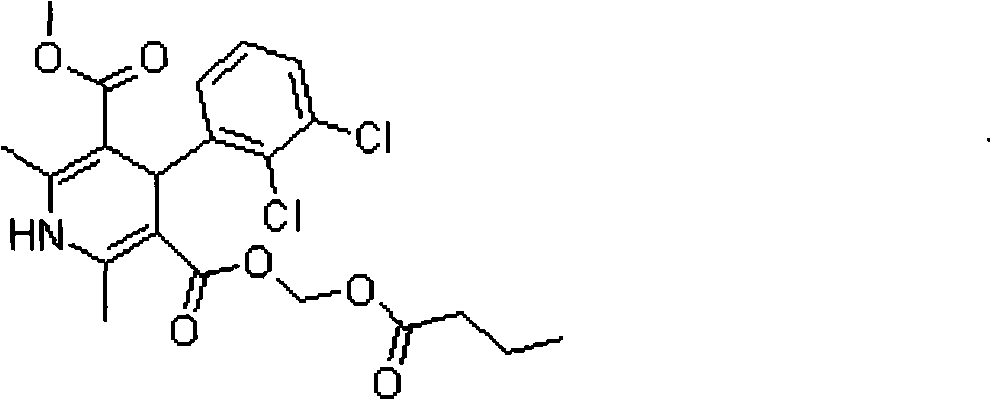
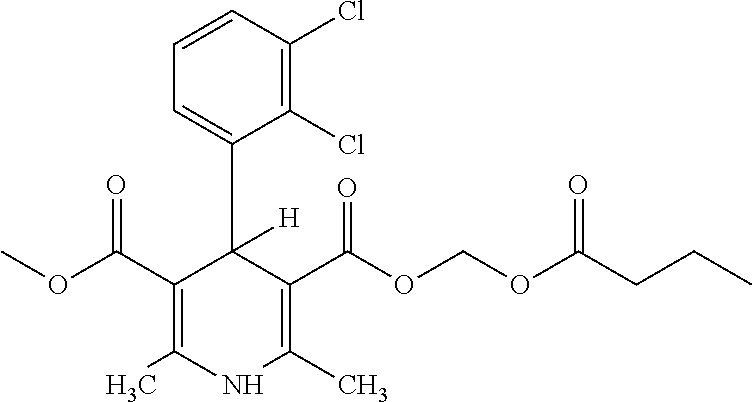
Clevidipine (INN, trade name Cleviprex) is a dihydropyridine calcium channel blocker indicated for the reduction of blood pressure when oral therapy is not feasible or not desirable. It was approved by the United States Food and Drug Administration on August 1, 2008.
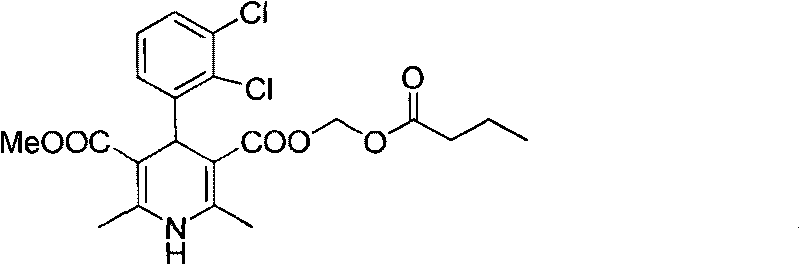
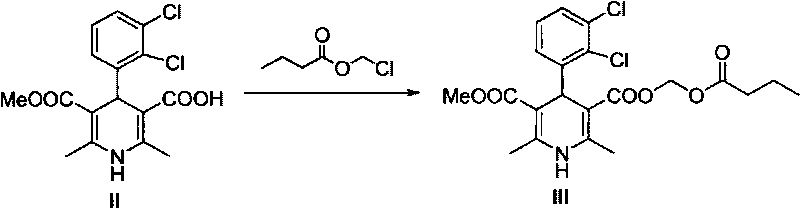
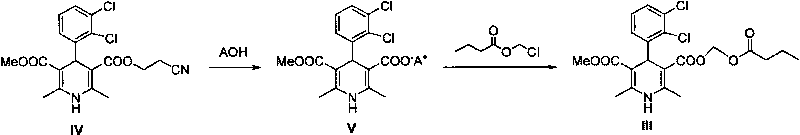
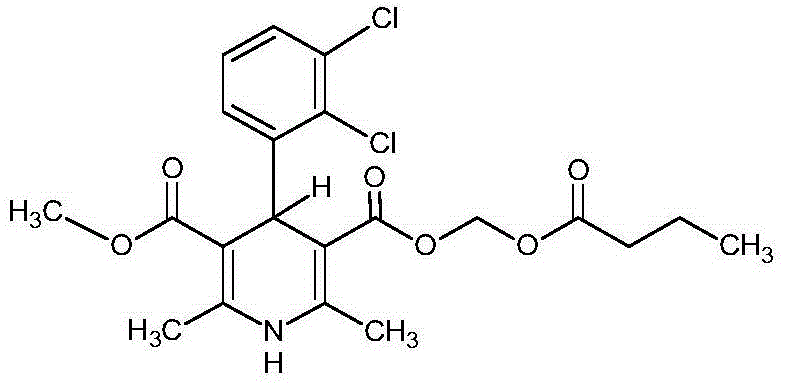
Method for preparing butyrate clevidipine
Owner:SUN YAT SEN UNIV +1
New preparation method of key intermediate of clevidipine butyrate
InactiveCN101602710AShort reaction stepsMild reaction conditionsOrganic chemistryAcetic acidClevidipine
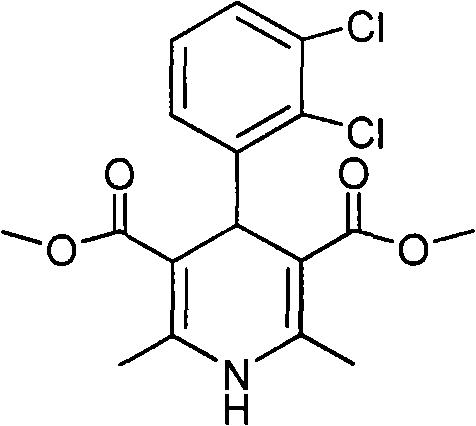
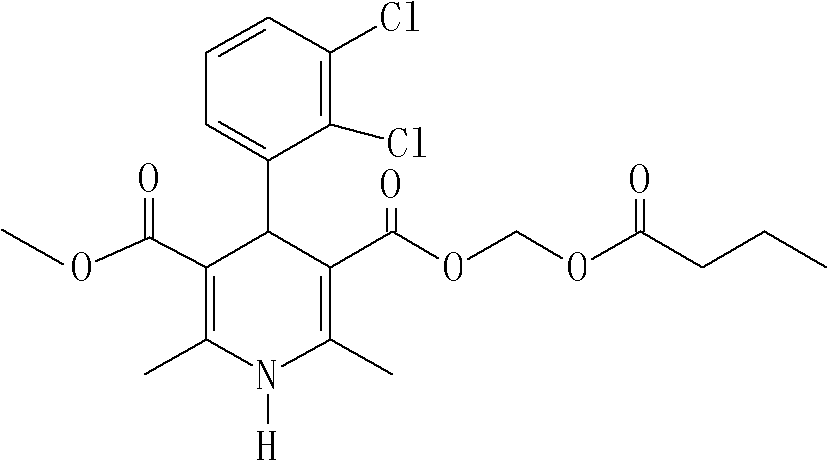
The invention relates to a preparation method of 4-(2, 3-dichlorophenyl)-2, 6-dimethyl-1, 4-dihydro-5-methoxylcarbonyl-3-pyridinecarboxylic acid (I). The method is characterized in that methyl acetoacetate, stronger ammonia water and 2, 3-dichlorobenzaldehyde are used as raw materials for direct cyclic condensation to obtain 4-(2, 3-dichlorophenyl)-2, 6-dimethyl-1, 4-dihydro-3, 5-pyridinedicarboxylic acid methyl ester (II); part of the compound II is hydrolyzed under alkaline condition to obtain the product 4-(2, 3-dichlorophenyl)-2, 6-dimethyl-1, 4-dihydro-5-methoxylcarbonyl-3-pyridinecarboxylic acid (I). The method has the advantages that the starting materials are cheap and easy to obtain, the production cost is lowered, the reaction is easy to operate and industrialization is easy to realize.
Owner:CHINA PHARM UNIV
Clevidipine emulsion formulations containing antimicrobial agents
ActiveUS20120088804A1Maintain stabilityReduction tendencyBiocideAntiinfectivesEmulsionMicrobial agent
Pharmaceutical formulations comprising clevidipine and an antimicrobial agent exhibit a reduced propensity for microbial growth and provide increased convenience to health care workers administering clevidipine-containing formulations to patients.
Owner:CHIESI FARM SPA
Emulsion containing clevidipine and preparation process and application thereof
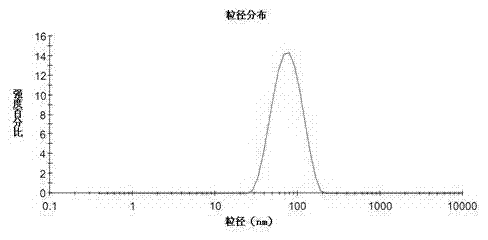
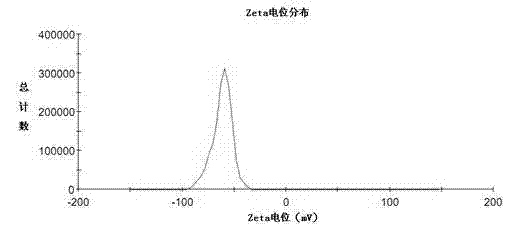
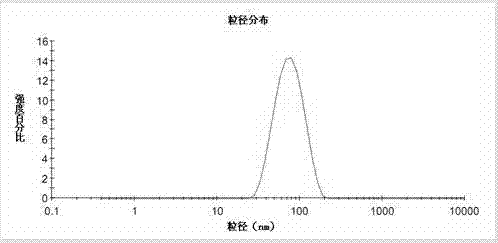
InactiveCN101766568AUniform particle size distributionQuality improvementOrganic active ingredientsEmulsion deliveryEmulsionAdditive ingredient
The invention discloses to an emulsion containing clevidipine and a preparation process and application thereof, relating to the emulsion utilizing the clevidipine and pharmaceutically acceptable salt or hydrate as active ingredients and the preparation process and the application thereof. The emulsion used for vein injection, which utilizes the clevidipine and the pharmaceutically acceptable salt or hydrate as the active ingredients, is formed by carrying out a certain preparation process on the active ingredients and pharmaceutically acceptable auxiliary materials, and can be used for rapid depressurization during and after a surgery. In the invention, the clevidipine and the pharmaceutically acceptable salt are taken as the active ingredients and some auxiliary materials with specific varieties and ratios are added, thus the emulsion for vein injection is developed according to the preparation process explained by the patent.
Owner:北京利乐生制药科技有限公司
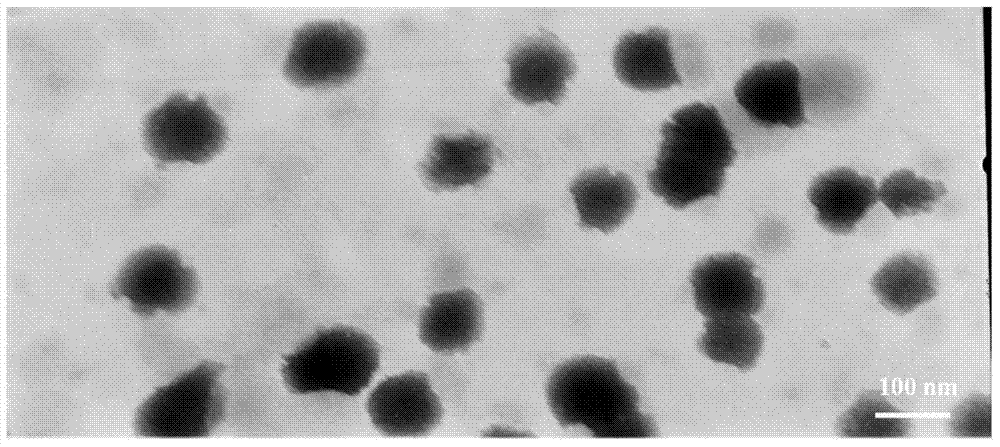
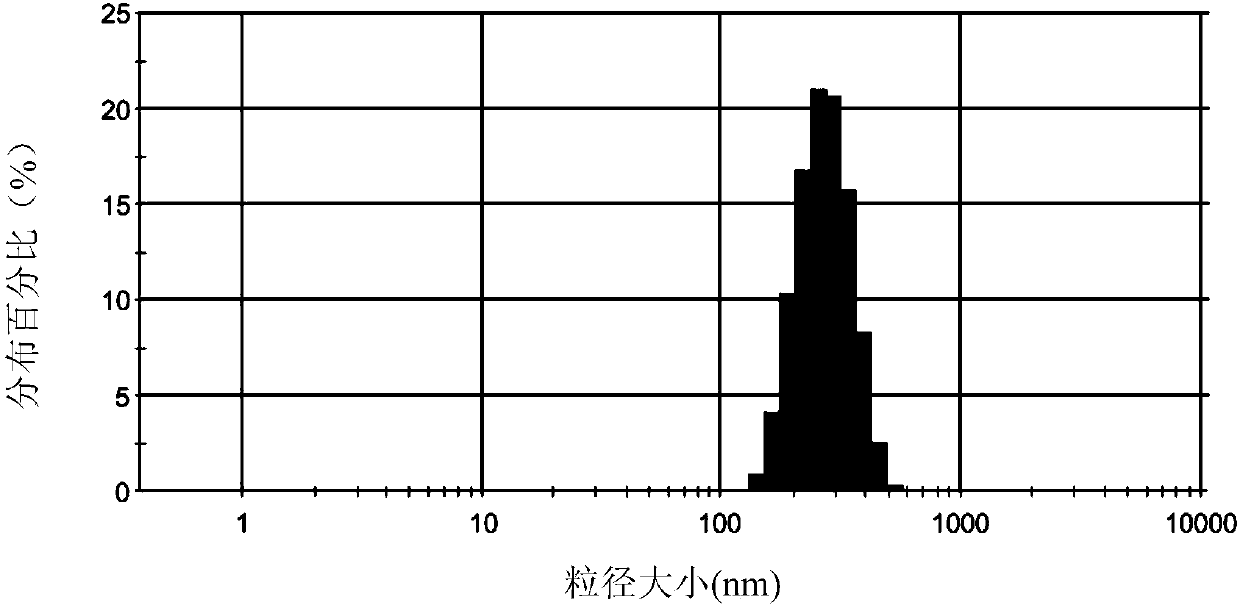
Lipid nanosuspension containing clevidipine butyrate, and freeze-drying preparation of lipid nanosuspension
InactiveCN103479577AIncrease contentImprove solubilityPowder deliveryOrganic active ingredientsLipid formationSide effect
The invention discloses a lipid nanosuspension containing clevidipine butyrate, and a freeze-drying preparation of the lipid nanosuspension. A preparation method comprises the following steps that (1) a surfactant is dispersed in an aqueous solution to form a solution A; (2) the clevidipine butyrate is dispersed in the solution A obtained in Step (1) to form a suspension solution B; (3) the suspension solution B is cut for 1-5min at a high speed to form a first emulsion C; (4) the first emulsion C is subjected to high-pressure homogenization treatment, and the clevidipine butyrate lipid nanosuspension is obtained; (5) a freeze-drying protective agent is added to the nanosuspension, and filtration and sterilization are performed; (6) freeze-drying is performed, moisture is removed, and the freeze-drying preparation of the clevidipine butyrate lipid nanosuspension is obtained. The lipid nanosuspension and the freeze-drying preparation are free of any organic solvent and long chain triglyceride or medium chain triglyceride, high in safety, low in toxic and side effects, high in drug loading capacity, good in stability and free from drug leakage; and meanwhile, a preparation technology is simple, and the repeatability is good, and the industrial production can be realized.
Owner:SHANDONG UNIV
Clevidipine butyrate medium-length chain fat emulsion and preparation method thereof
InactiveCN102000027AGood quality indexSolve the real problemOrganic active ingredientsEmulsion deliveryClevidipineFat emulsions
The invention relates to a clevidipine butyrate medium-length chain fat emulsion comprising the following components: 0.05-0.1% (w / v) of clevidipine butyrate, 0.5-1.0% (w / v) of long chain oil for injection, 0.5-1.0% (w / v) of medium-chain oil for the injection, 1.0-2.0% (w / v) of emulsifying agents, 1.0-5.0% (w / v) of isotonic agents, pH regulating agents and water for the injection.
Owner:辽宁中海康生物制药股份有限公司
Pharmaceutical composition with short pressure-reducing function
InactiveCN101791311AHas a short-acting antihypertensive effectHas anti-lipid peroxidation effectOrganic active ingredientsAntinoxious agentsHypertriglyceridemiaEmulsion
The invention discloses a pharmaceutical composition with short pressure-reducing function and a preparation method of emulsion injection thereof. The pharmaceutical composition comprise: 1) dihydropyridine medicine clevidipine butyrate with the function of reducing pressure in short period; 2) alpha-DL-vitamin E; 3) one or more kinds of injection oil; 4) one or more kinds of emulsifying agents; 5) injection water or buffer solution. The pharmaceutical composition not only can reduce blood pressure but also can improve damage to tissue and organs of the user caused by lipid peroxidation, thereby obviously reducing load of liver and reducing incidence rate of hypertriglyceridemia.
Owner:广州中大创新药物研究与开发中心有限公司 +1
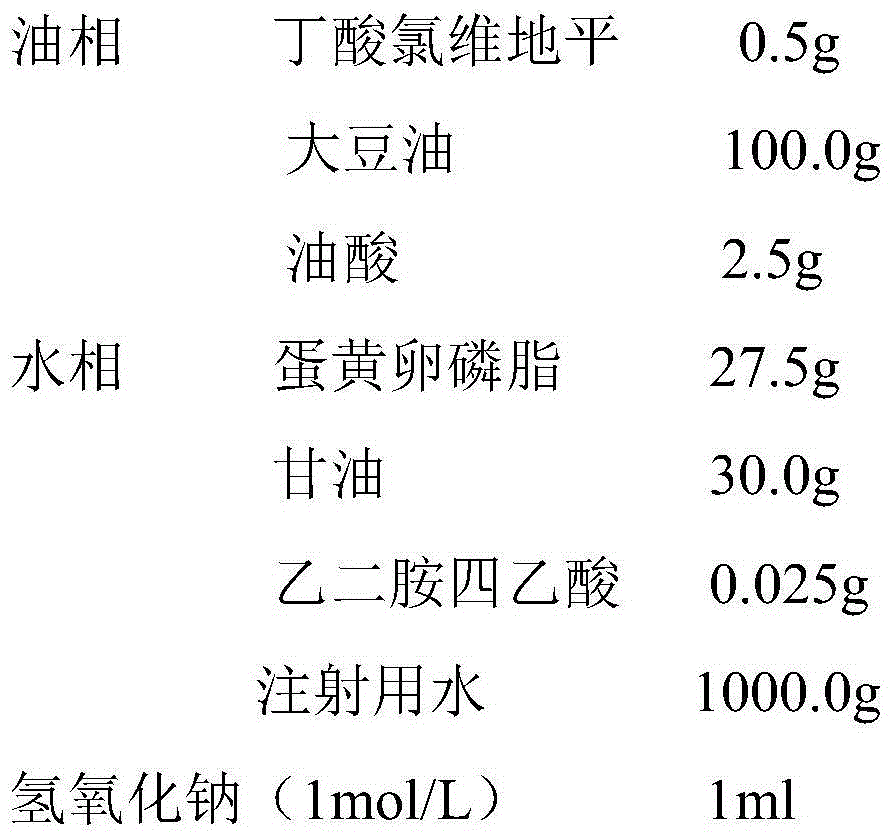
Clevidipine butyrate fat emulsion injection and preparation process thereof
The invention provides a clevidipine butyrate fat emulsion injection for intravenous injection. The clevidipine butyrate fat emulsion injection is prepared from an active ingredient, namely clevidipine butyrate and excipients, namely oil for injection, an emulsifier, an isoosmotic adjusting agent, a pH value regulator and water for injection. The fat emulsion injection for the intravenous injection is developed by taking the clevidipine butyrate as the active ingredient and adding some specific kinds of excipients in a specific proportion by the preparation process; and each milliliter of clevidipine butyrate fat emulsion injection contains 0.5 or 1.0mg of the clevidipine butyrate.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Pharmaceutical Compositions And Methods For Producing Low Impurity Concentrations Of The Same
InactiveUS20100113534A1Level of certain is minimized and reducedLower Level RequirementsBiocideComponent separationMedicineClevidipine
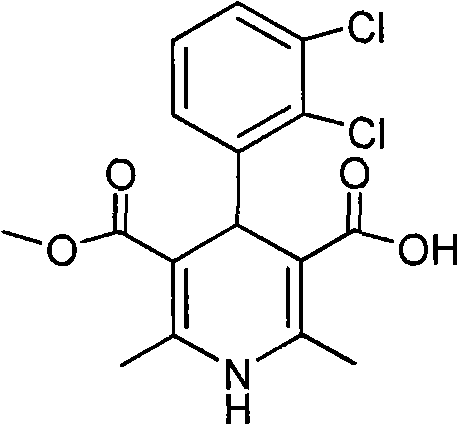
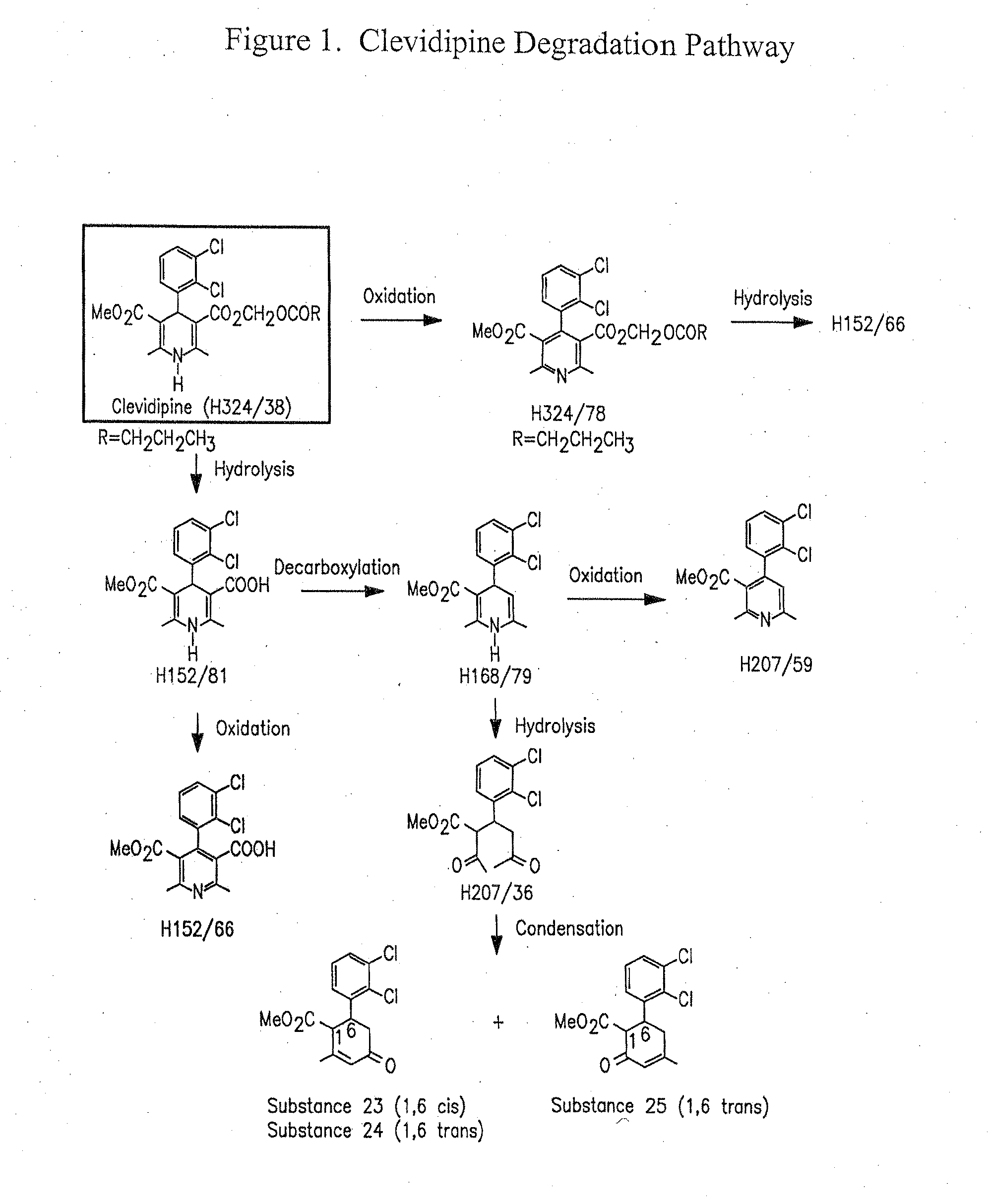
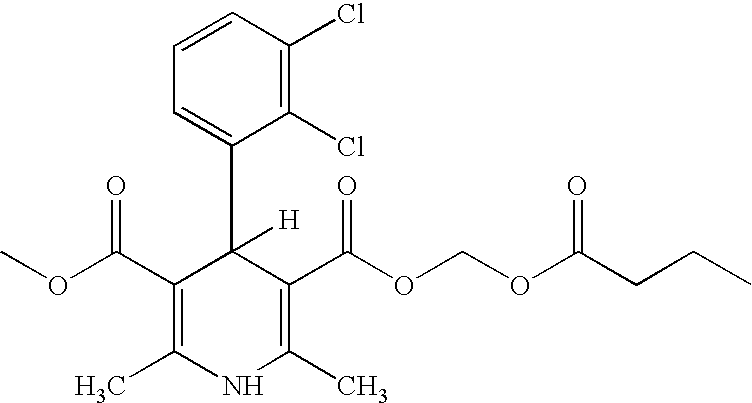
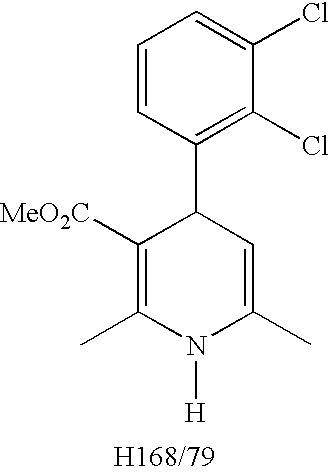
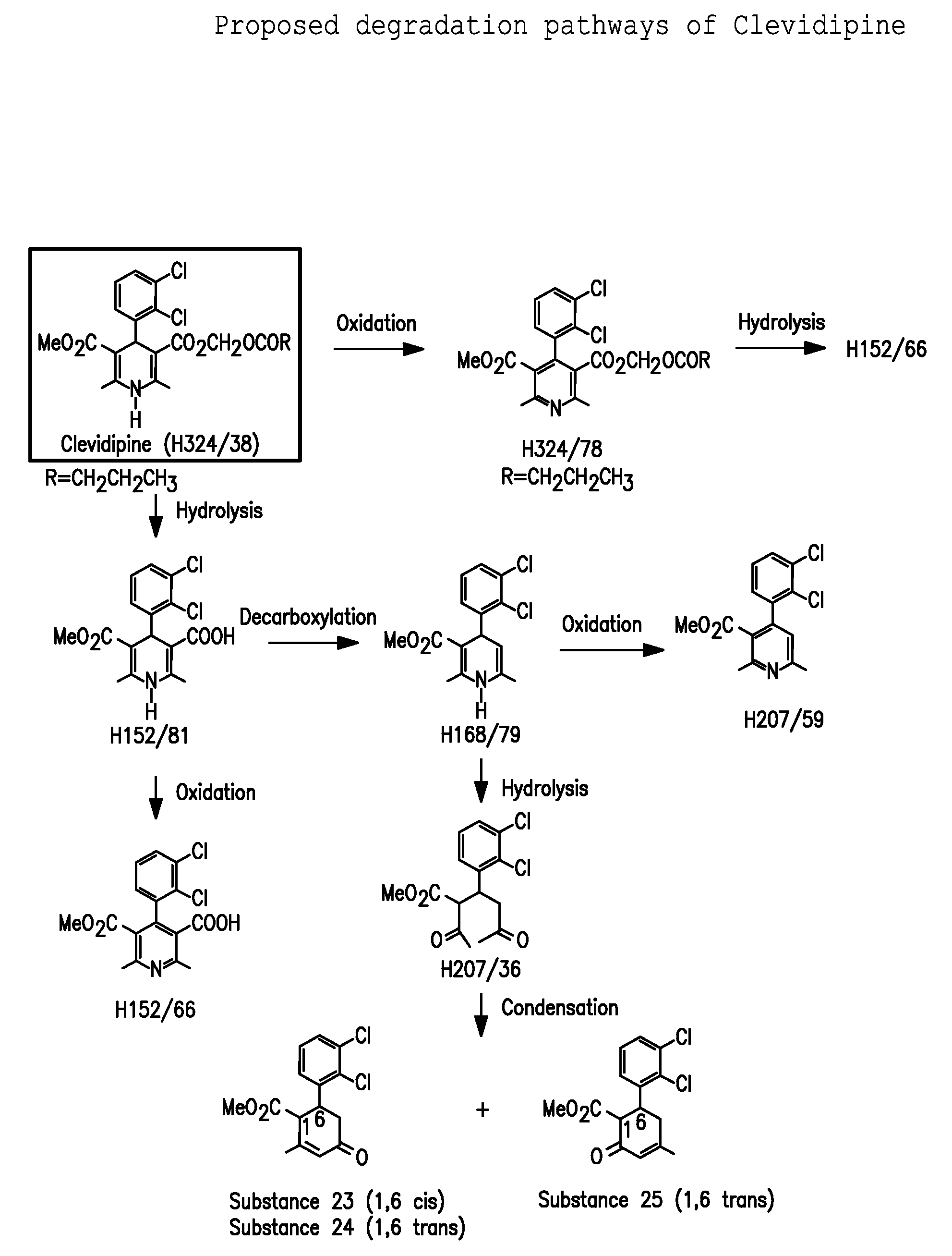
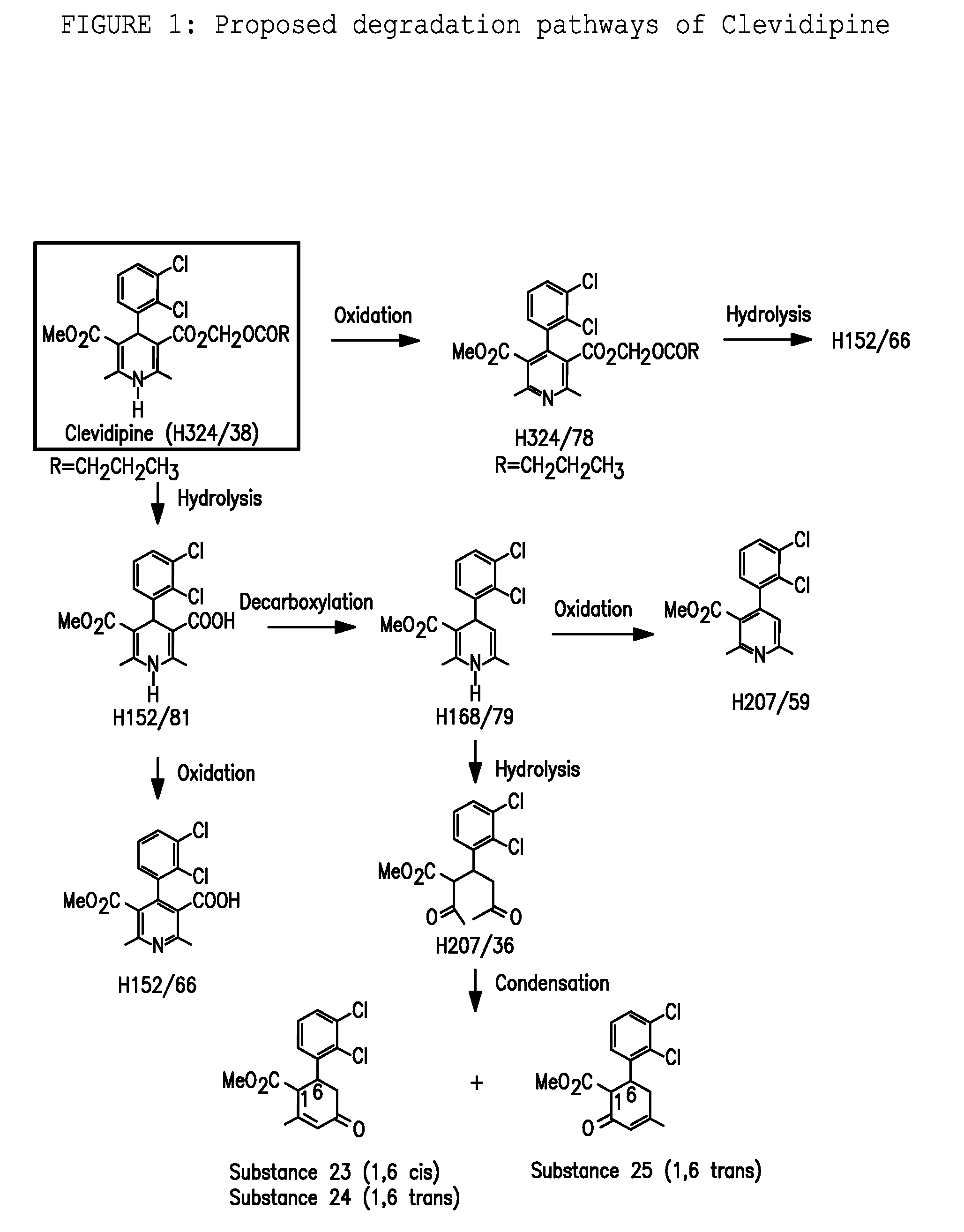
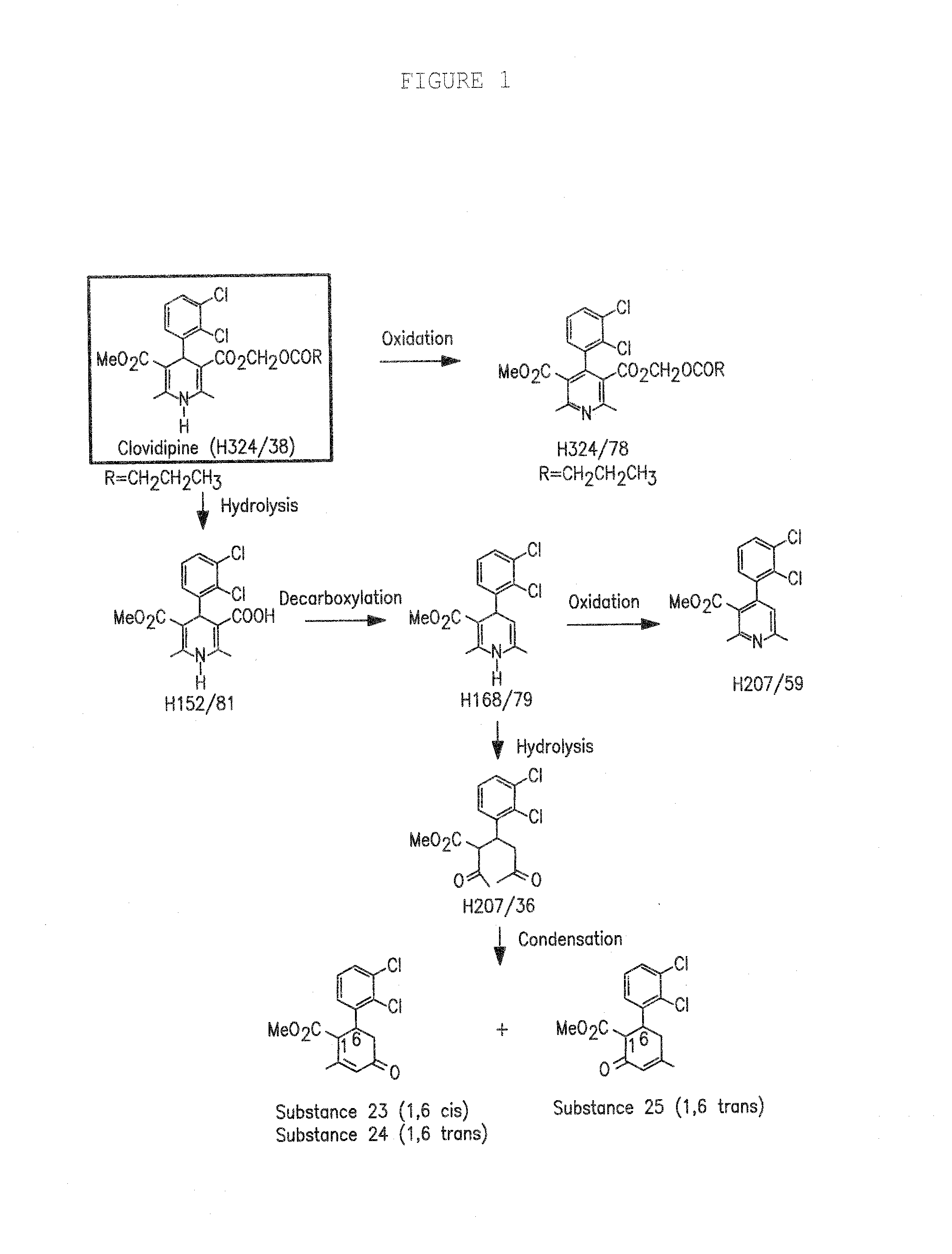
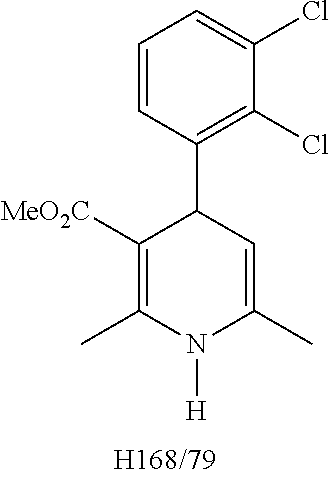
A composition having clevidipine as an active ingredient is described. The composition includes clevidipine as an active ingredient and an amount of the impurity H168 / 79 that is no greater than about 1.5%, or where the ratio between clevidipine and H168 / 79 is equal or above 60 to 1.
Owner:HOSPIRA +1
Clevidipine butyrate emulsion, preparation method thereof and purpose thereof
InactiveCN102228434AOrganic active ingredientsPharmaceutical non-active ingredientsEmulsionClevidipine
The invention relates to a therapeutic emulsion of clevidipine butyrate. The emulsion comprises clevidipine butyrate, a lipid phase, an emulsifier, and water or a buffer. The invention also relates to a purpose and a preparation method of the emulsion.
Owner:SICHUAN UNIV
Pharmaceutical compositions and methods for stabilizing the same
InactiveUS20100105743A1Minimized impurityReducing and preventing reactionBiocideNervous disorderLight energyClevidipine
Pharmaceutical compositions, and a method of stabilizing pharmaceutical compositions having clevidipine, or any pharmaceutically acceptable salt thereof, as the active ingredient is described. The method includes the slowing down or inhibiting of the oxidation pathway of clevidipine. This can be accomplished by reducing the amount the pharmaceutical composition is exposed to oxygen and / or light during the manufacturing and storing processes. According to this method, oxygen must be removed or replaced, or light must be sufficiently blocked such that light energy cannot reach the active ingredient of the composition, or is reduced to a level that the light-induced oxidation reaction converting clevidipine to H324 / 78 is minimized, such that the total detectable level of H324 / 78 in a given composition sample does not exceed about 0.2% on a weight-by-weight basis, or the ratio of clevidipine to H324 / 78 is equal to or greater than about 450 to 1 on a weight-to-weight basis.
Owner:CHIESI FARM SPA
Clevidipine butyrate structured lipid emulsion and preparation method thereof
InactiveCN102319212ATo promote metabolismImprove technical effectOrganic active ingredientsEmulsion deliveryEmulsionMedicine
The invention relates to a clevidipine butyrate structured lipid emulsion which comprises clevidipine butyrate, structured triglyceride, an emulsifier, an isotonic agent, a pH regulator, and injection water, wherein the weight volume percentage of clevidipine butyrate is 0.05-0.1% (w / v); the weight volume percentage of structured triglyceride is 5-30% (w / v); the weight volume percentage of the emulsifier is 1.0-2.0% (w / v); and the weight volume percentage of the isotonic agent is 1.0-5.0% (w / v).
Owner:辽宁中海康生物制药股份有限公司
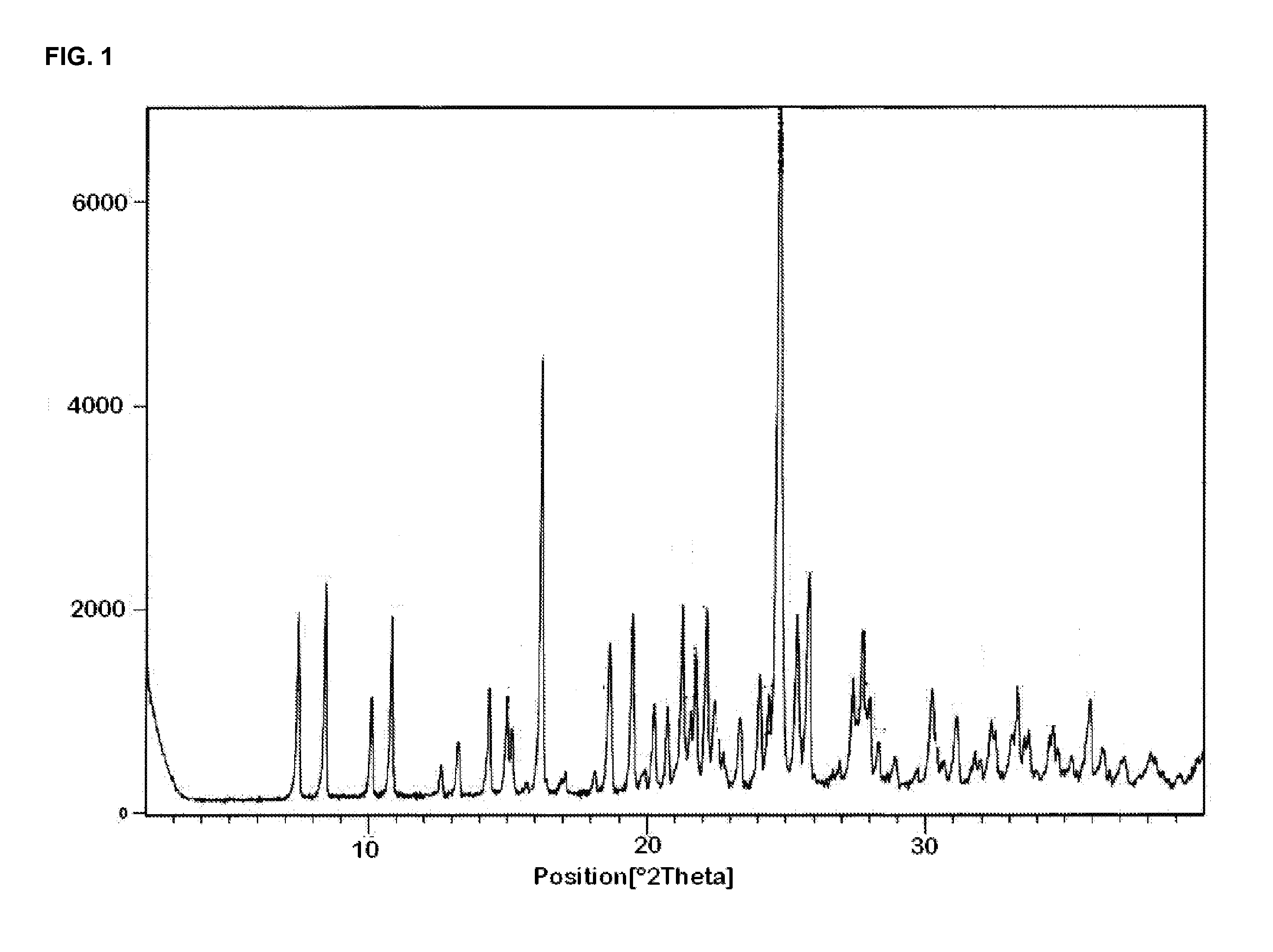
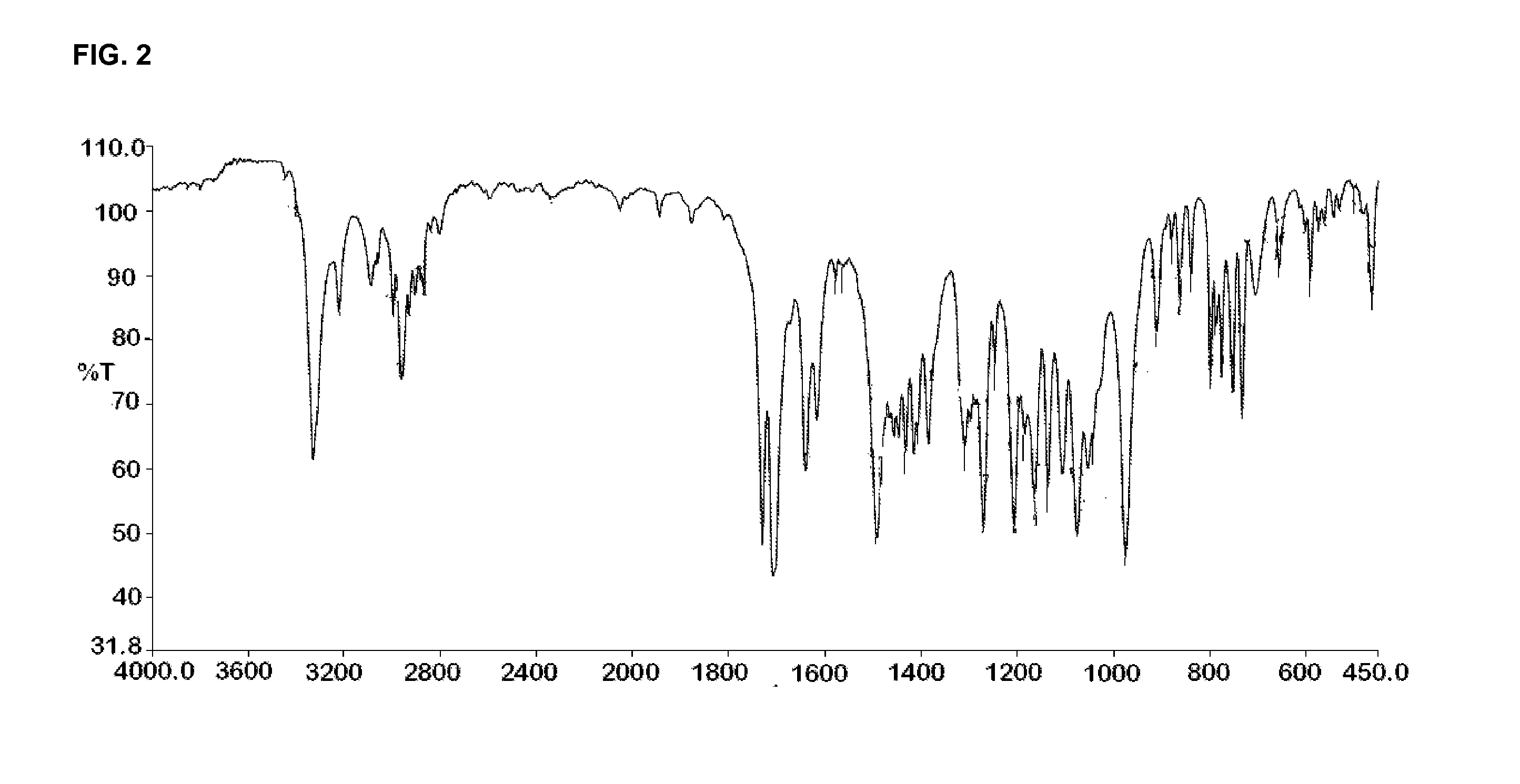
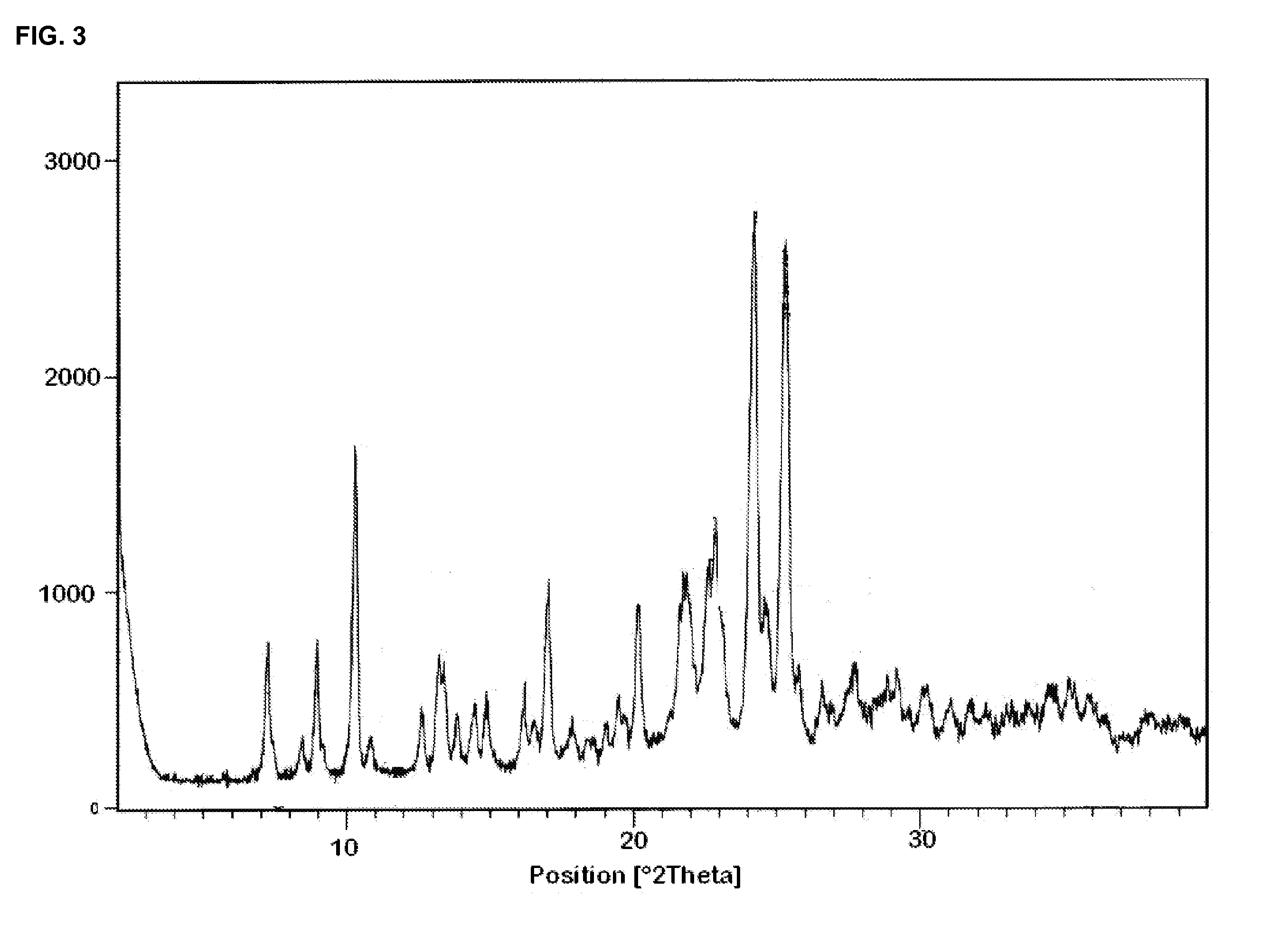
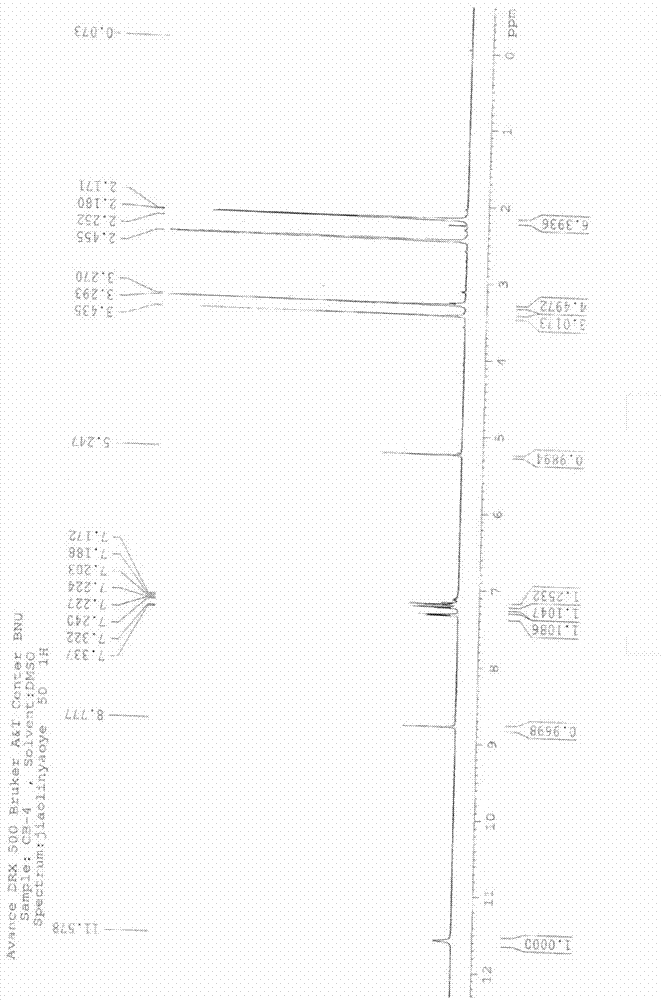
Crystal form of butyric acid clevidipine
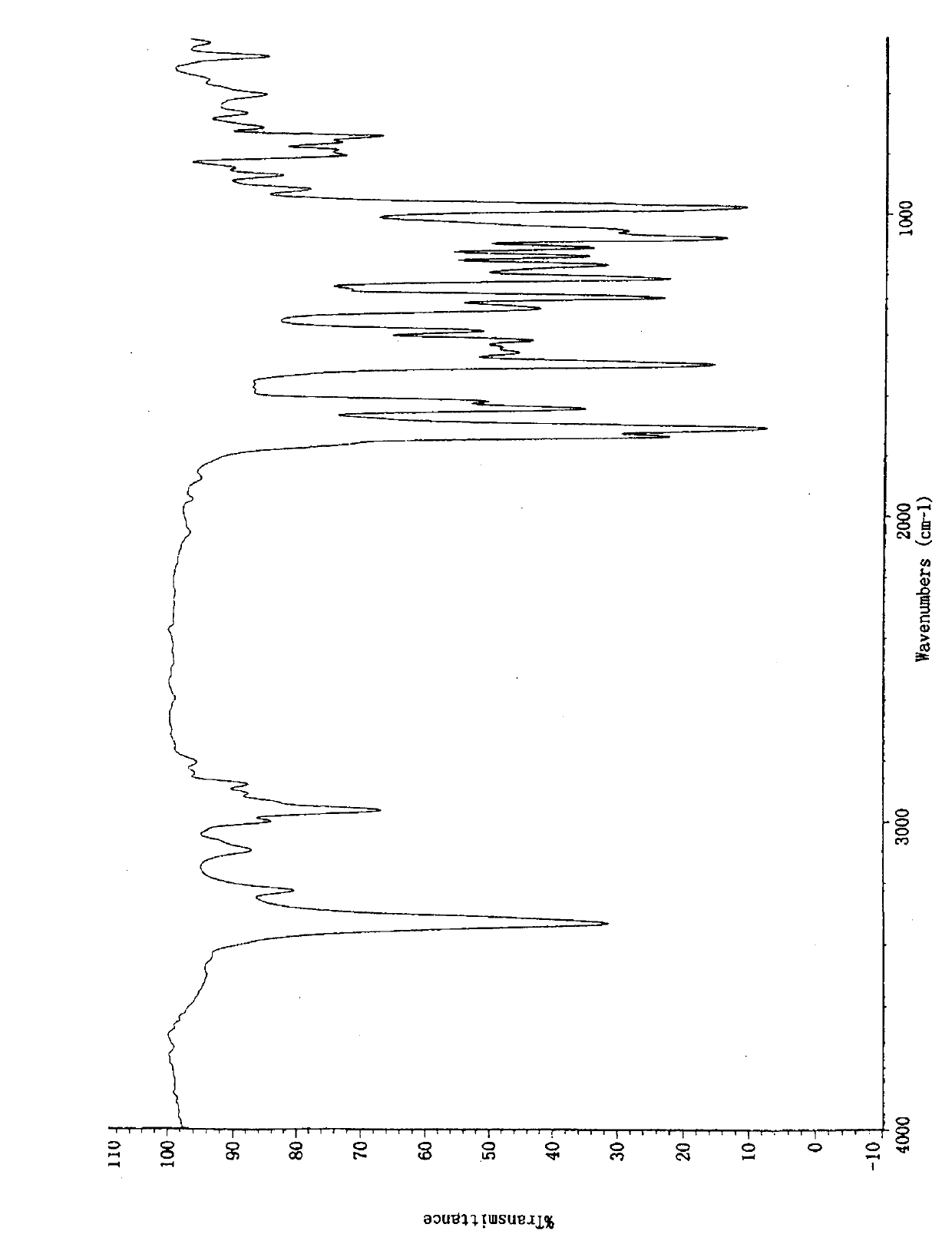
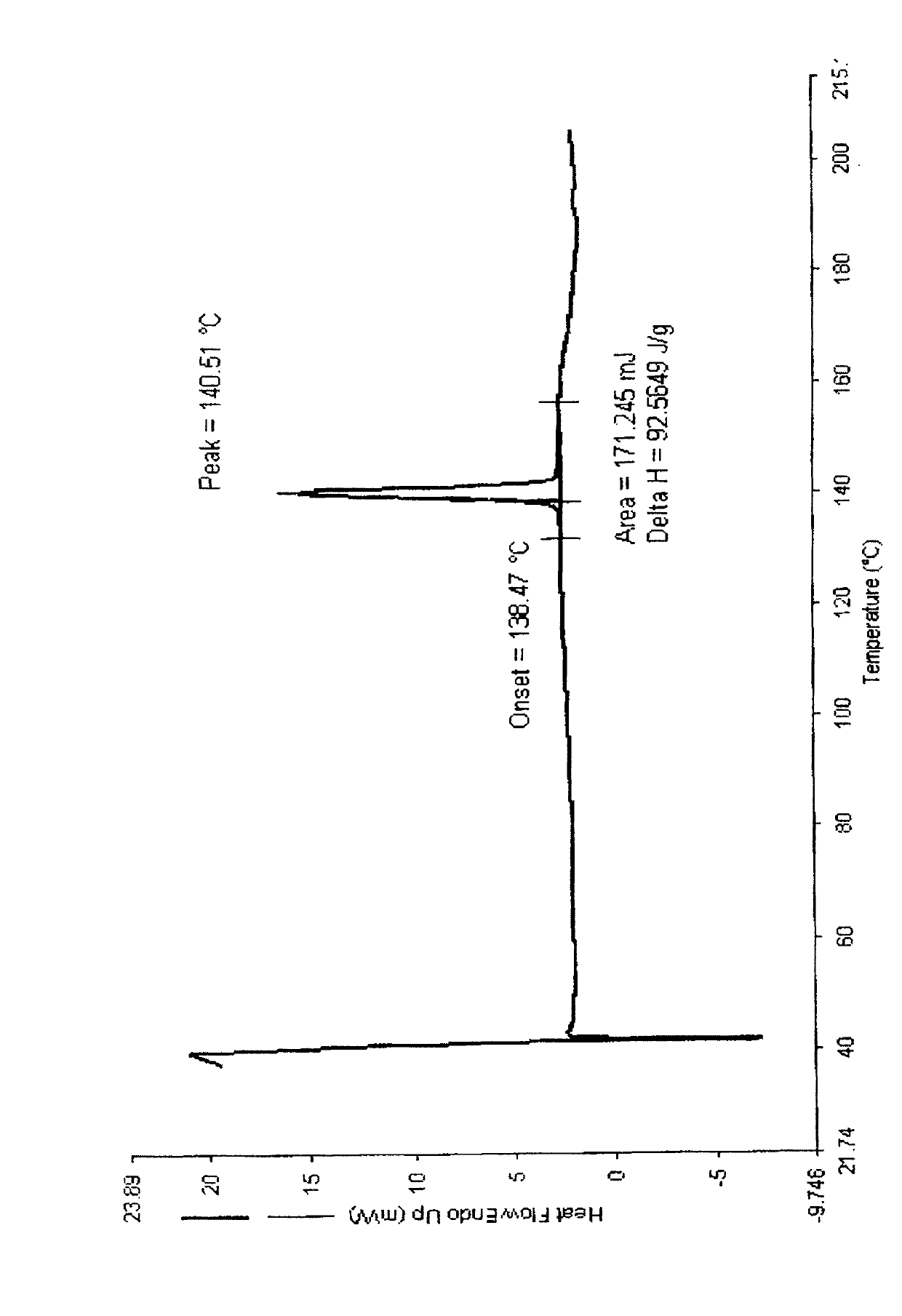
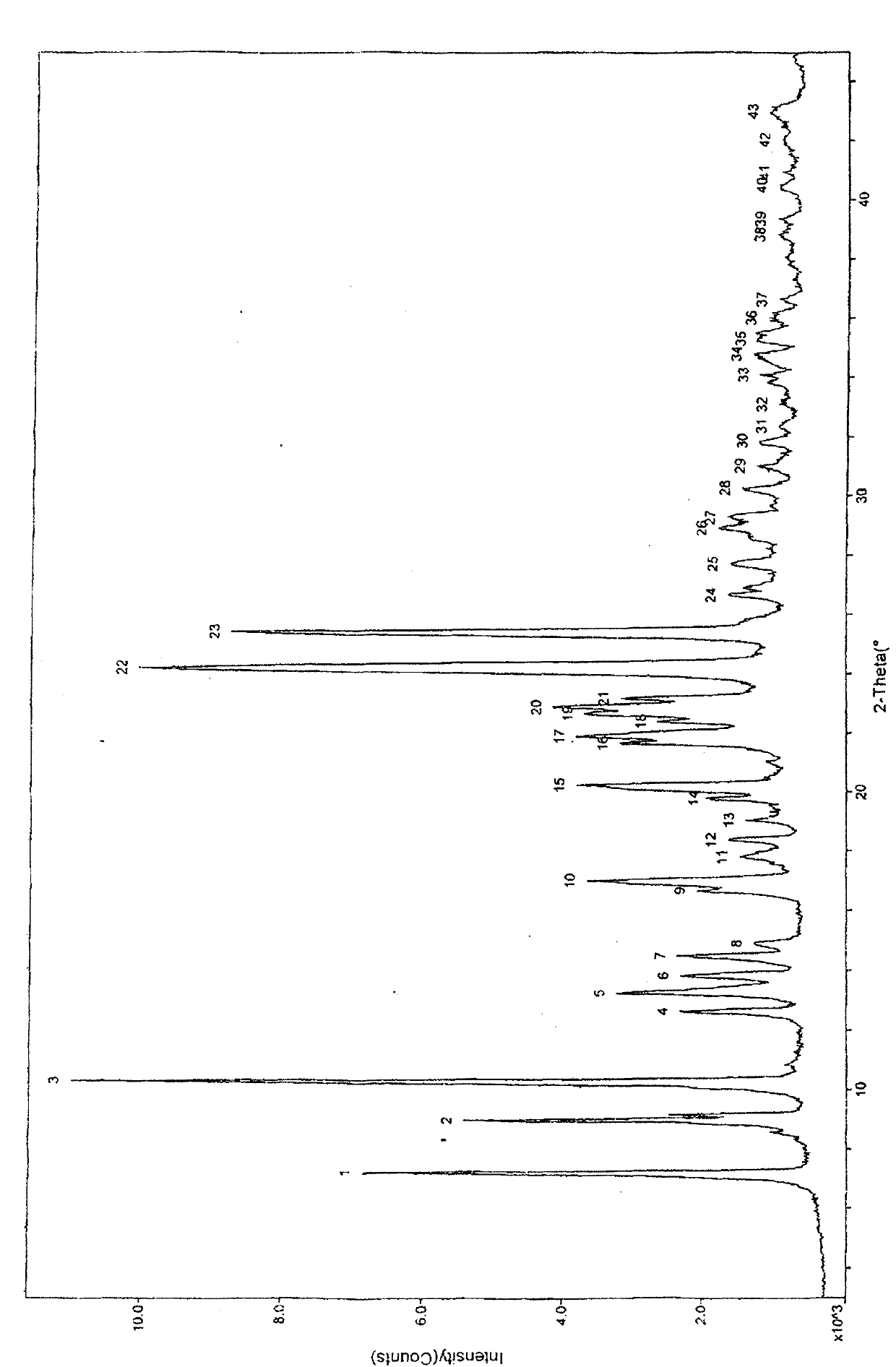
The invention belongs to the technical field of medicines, relating to a chemical drug and a preparation method thereof, in particular to a crystal form of butyric acid clevidipine and a preparation method thereof. The position of powder X-ray diffraction pattern peak of the crystal is at 7.5 degrees, 8.5degrees, 10.1 degrees, 16.3 degrees, 20.3 degrees, and 24.8 Ddegrees+ / -0.2degrees 2 theta, the absorption peak of a differential scanning calorimetry pattern is at 140.5 DEG C, and the position of the characteristic peak of an infrared pattern is at 3332, 1732 and 1707 cm<1>. The crystal form of butyric acid clevidipine is prepared by adding a solvent which can dissolve the solid to the butyric acid clevidipine, heating and dissolving, then adding activated carbon for decoloration, filtering during heating, cooling the filtered liquid so as to crystallize butyric acid clevidipine crystal, and then filtering and drying. The physical and chemical properties of the invention are stable, and the invention is convenient for being made to stable pharmaceutical preparations in large scale.
Owner:WUHAN WUYAO SCI & TECH
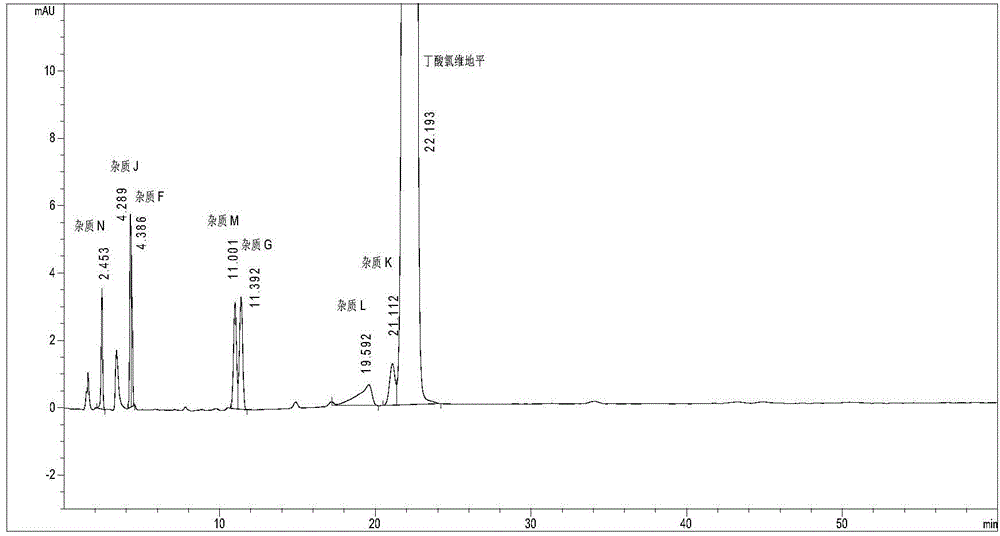
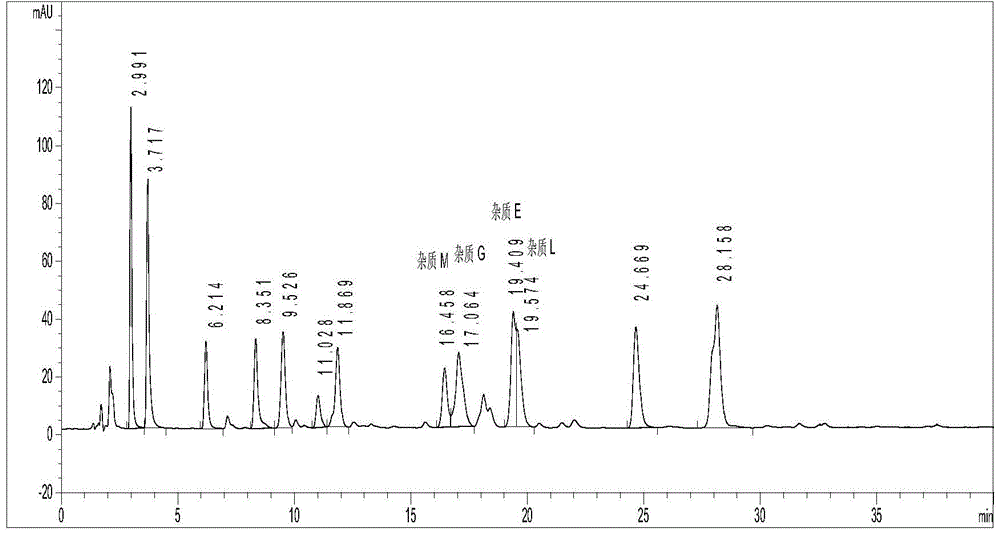
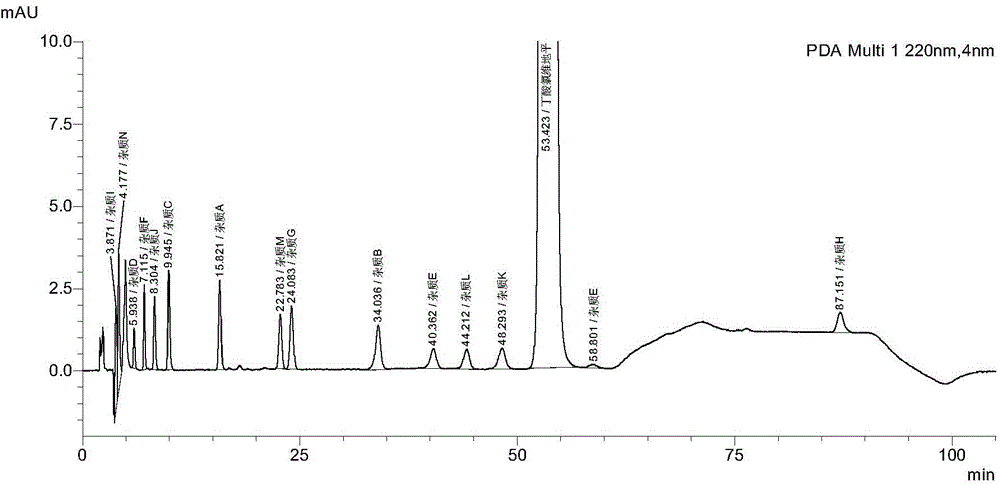
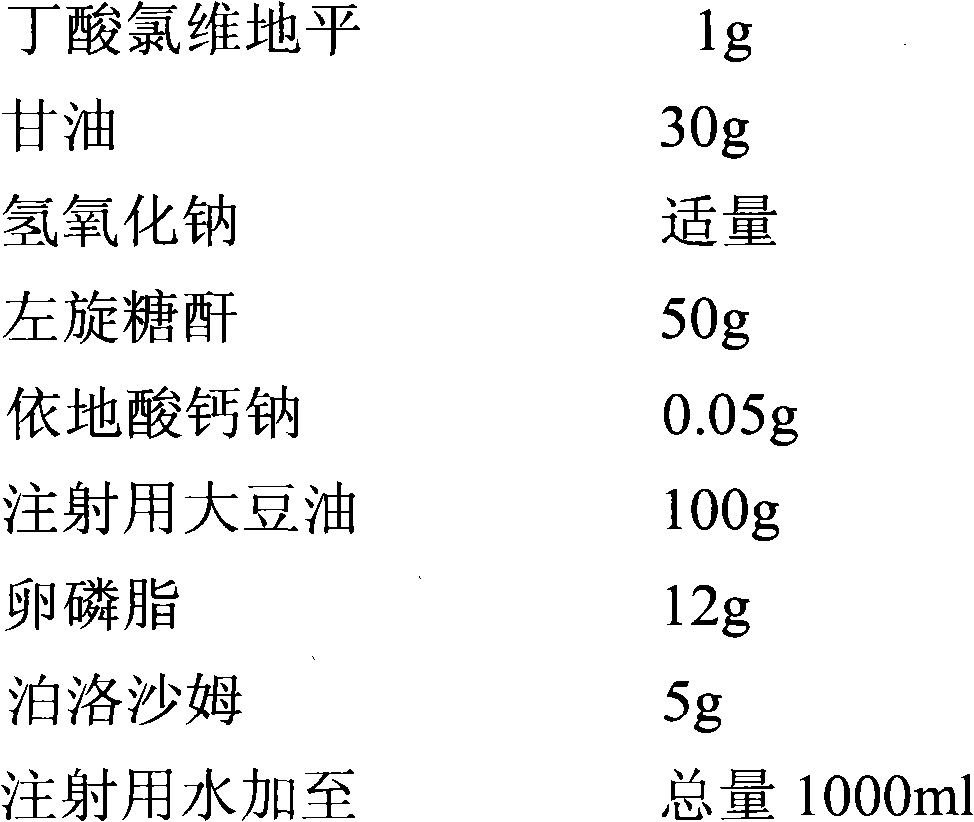
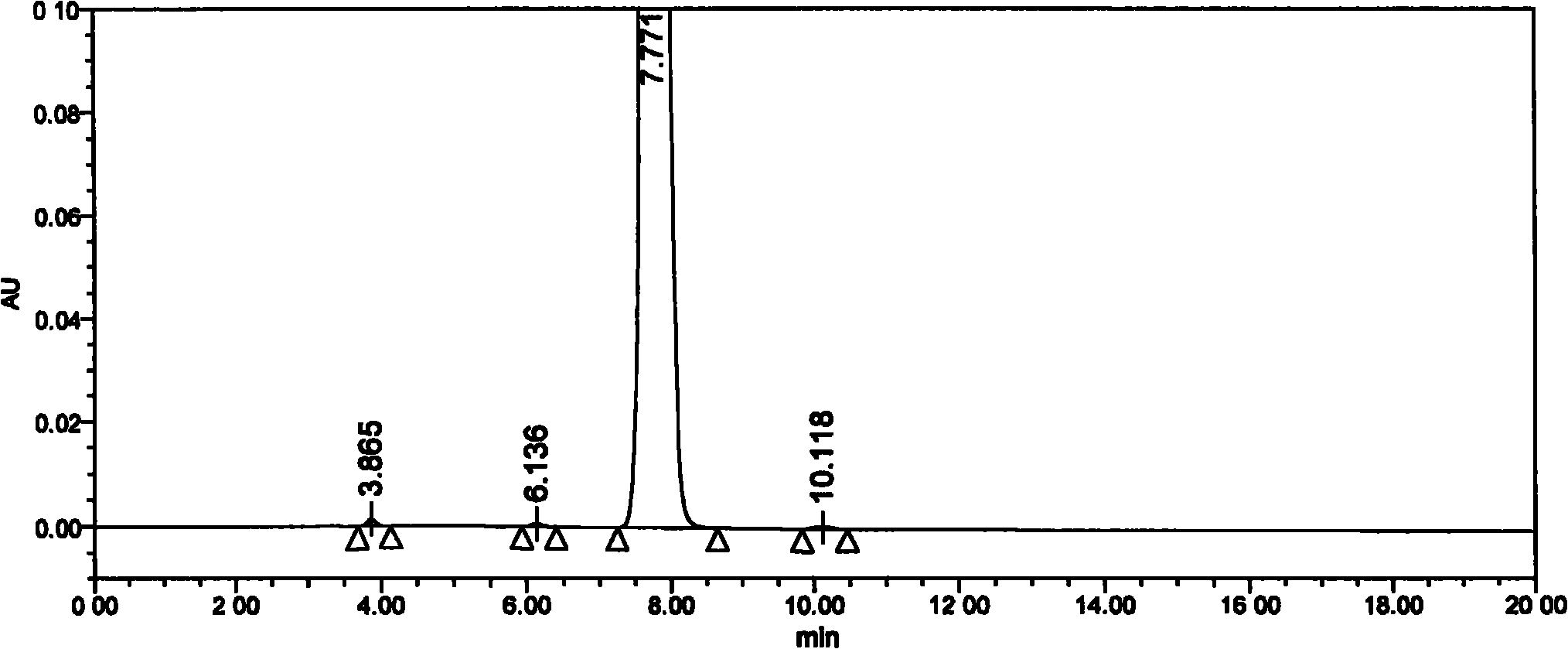
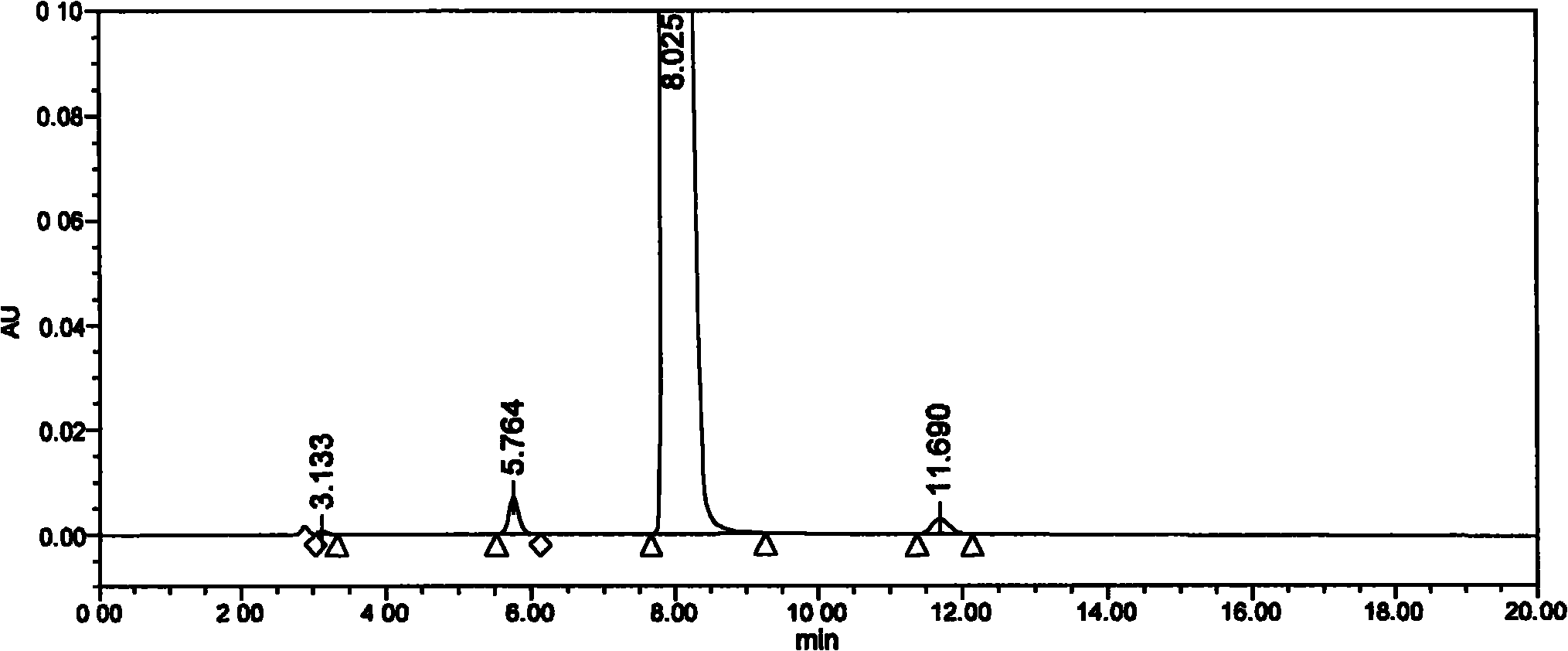
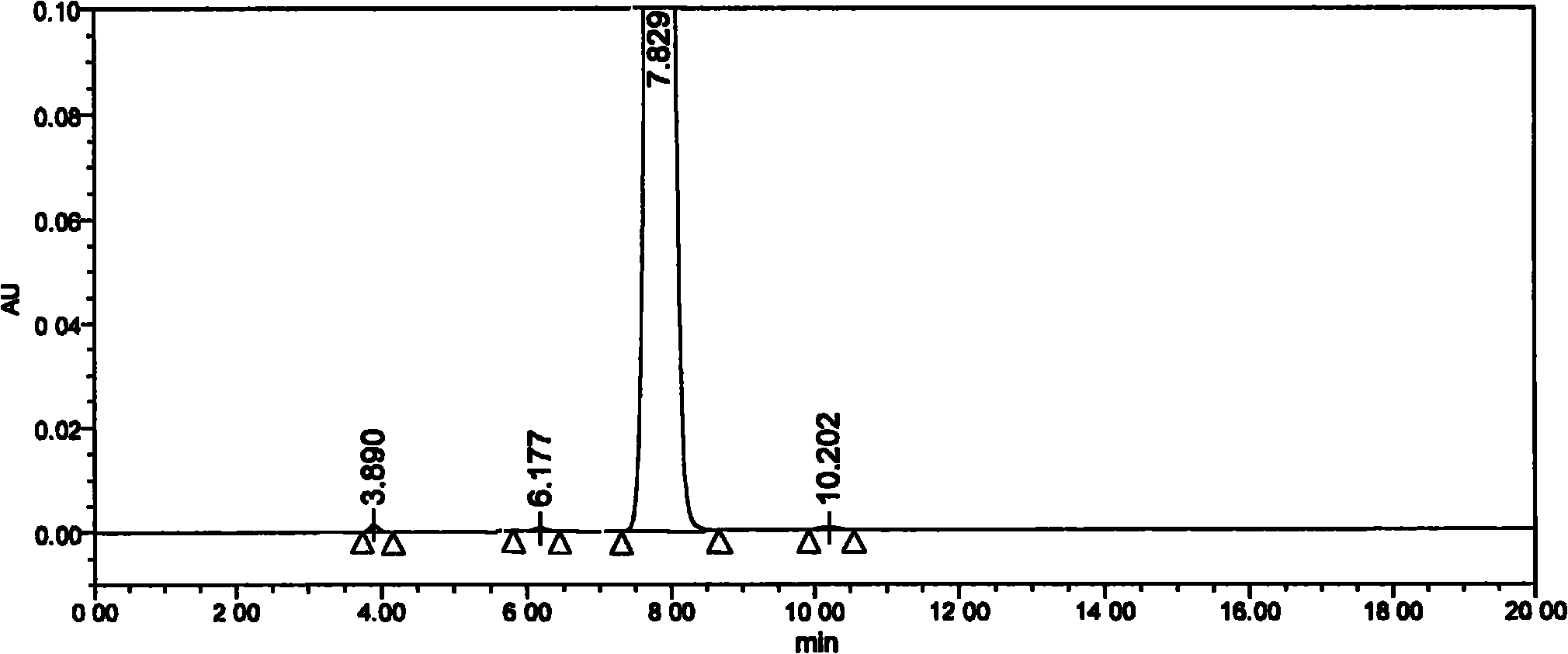
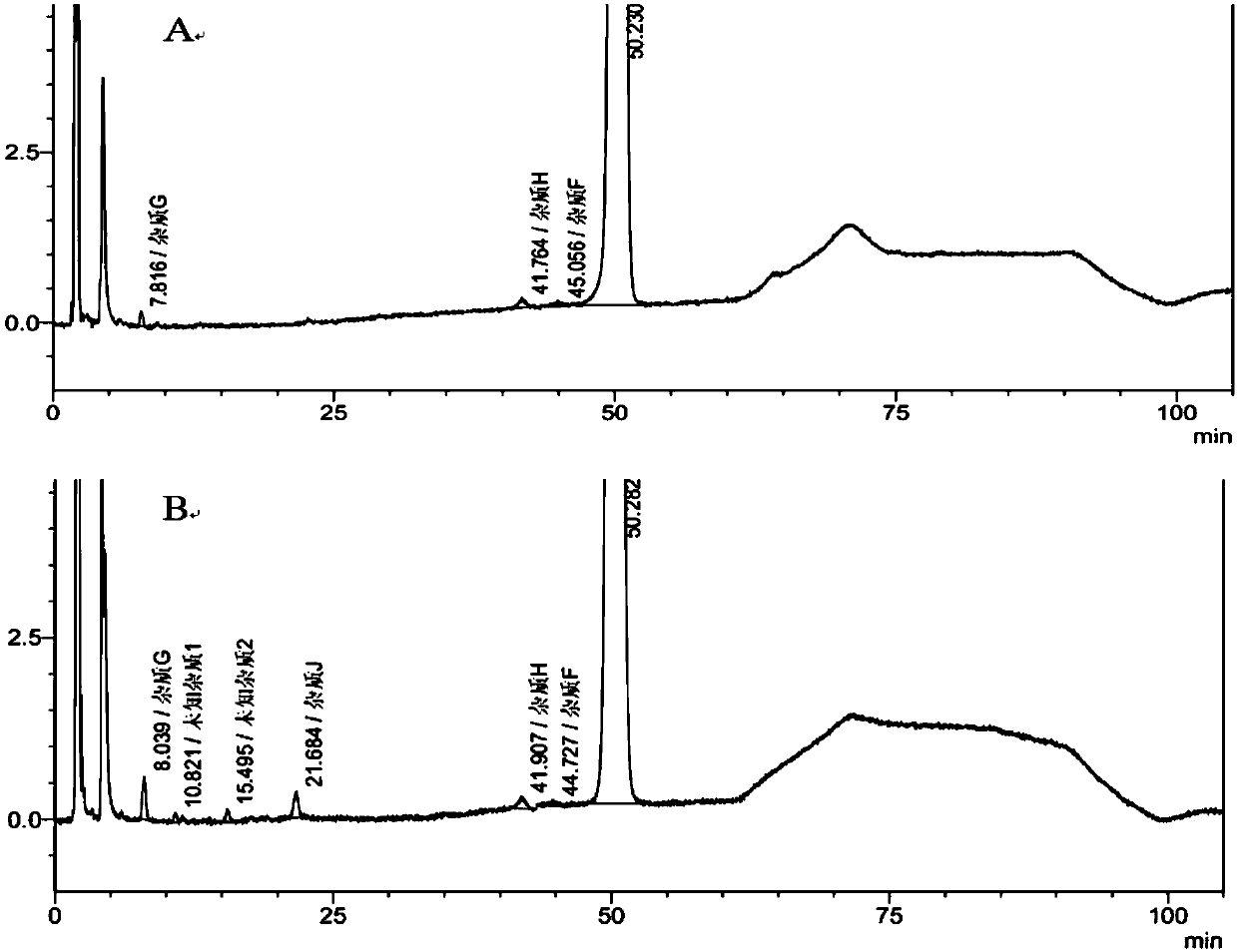
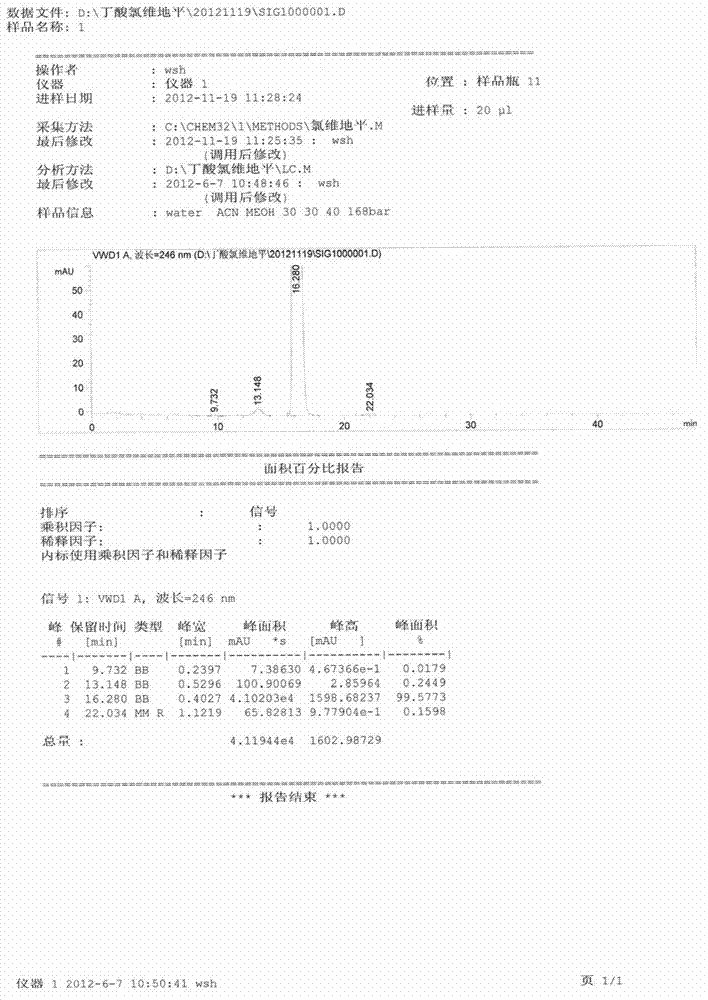
Method for detecting clevidipine butyrate and related substances in preparations of clevidipine butyrate
ActiveCN104597192AAchieve separationEasy to controlComponent separationTesting medicinal preparationsPhosphateClevidipine
The invention discloses a method for detecting clevidipine butyrate and related substances in preparations of the clevidipine butyrate. The method is characterized in that gradient elution is carried out by virtue of a C18 chromatographic column by taking methanol-acetonitrile-phosphate buffer salt solution as a mobile phase according to a reversed-phase high-performance liquid chromatography and an ultraviolet detector is used. By means of the method, effective separation of the clevidipine butyrate from all known impurities can be realized; interference of various impurities generated in the synthesis and preparation production processes to the purity of products can be avoided; the quantity of the clevidipine butyrate or preparations can be controlled comprehensively and effectively; the method is simple to operate and high in specificity and sensitivity and is capable of well detecting the clevidipine butyrate and related substances in the preparations; and an effective and reliable analysis method is provided for quality control of research, development and production processes.
Owner:WUHAN CONFORM PHARMA CO LTD
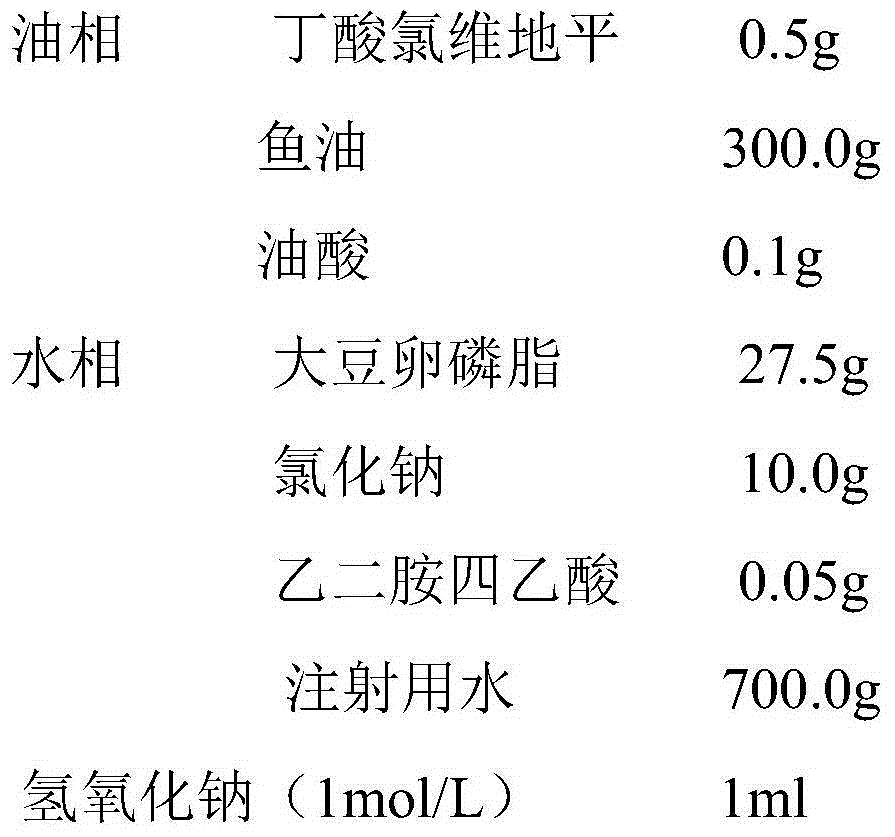
Clevidipine butyrate emulsion and preparation method thereof
ActiveCN104146958AGuaranteed emulsification efficiencyGuaranteed stabilityOrganic active ingredientsEmulsion deliveryClevidipineOil phase
The invention discloses a clevidipine butyrate emulsion and a preparation method thereof. The clevidipine butyrate emulsion includes an oil phase and a water phase. The oil phase comprises clevidipine butyrate, long-chain oil and a stabilizer. The water phase comprises an emulsifier and water. A mass ratio of the oil phase and the water phase is 1:11-1:1. The clevidipine butyrate accounts for 0.05-0.5% of the total mass of the clevidipine butyrate emulsion; the long-chain oil accounts for 10-50% of the total mass of the clevidipine butyrate emulsion; the stabilizer accounts for 0.01-0.5% of the total mass of the clevidipine butyrate emulsion; and the emulsifier accounts for 0.5-5.0% of the total mass of the clevidipine butyrate emulsion. The water phase can also include a metal ion chelating agent and / or an isotonic agent. The emulsion can also include a pH adjusting agent. The clevidipine butyrate emulsion is safe and effective, is less in adverse reactions, is high in drug encapsulation efficiency, is good in storage stability, is controllable in preparation processes and is low in contents of impurities. The method is easy to industrially control.
Owner:SHANGHAI SINE PHARMA LAB
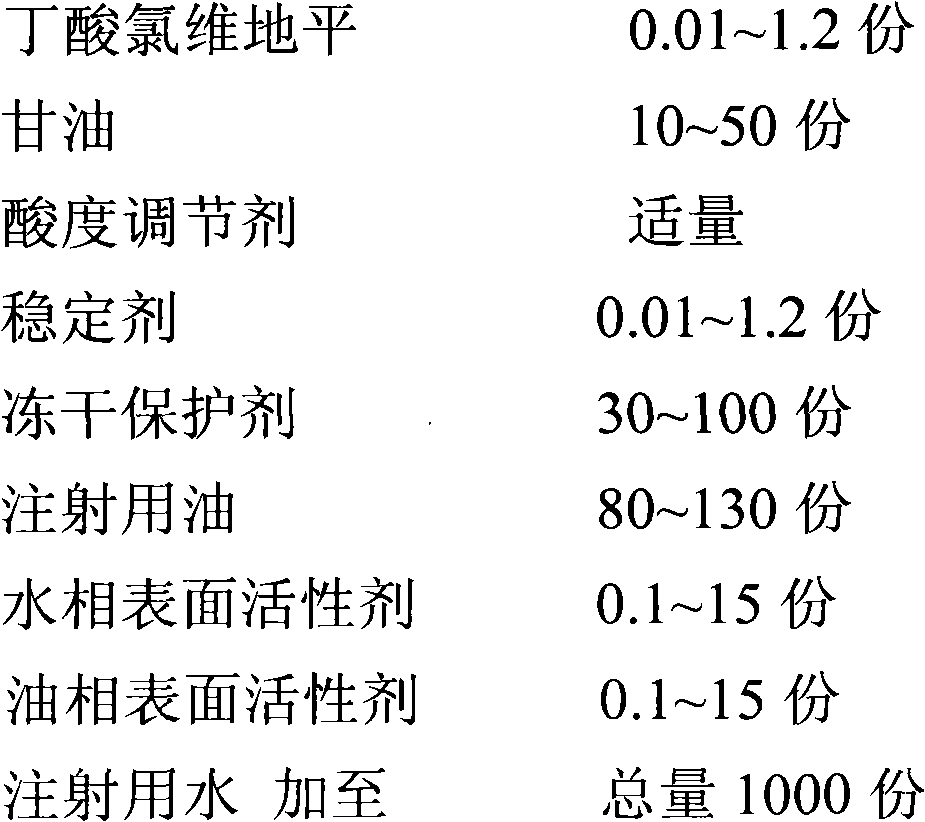
Clevidipine butyrate freeze-dried emulsion
InactiveCN103169672AImprove securityImprove stabilityPowder deliveryOrganic active ingredientsSide effectFreeze-drying
The invention discloses a clevidipine butyrate freeze-dried emulsion, and belongs to the field of a pharmaceutical preparation. The freeze-dried emulsion is prepared from clevidipine butyrate, glycerol, an acidity regulator, a stabilizing agent, a freeze-drying protecting agent, injection oil, a water-phase surfactant and an oil-phase surfactant. The clevidipine butyrate freeze-dried emulsion disclosed by the invention is free from high-temperature sterilization, and is under a dry state in the storage process; compared with other clevidipine butyrate dosage forms, the clevidipine butyrate freeze-dried emulsion has characteristics of good stability, low toxic and side effects, good curative effect, and the like, and is better applicable to critical injection; and the emulsion has great popularization and application values.
Owner:CISEN PHARMA
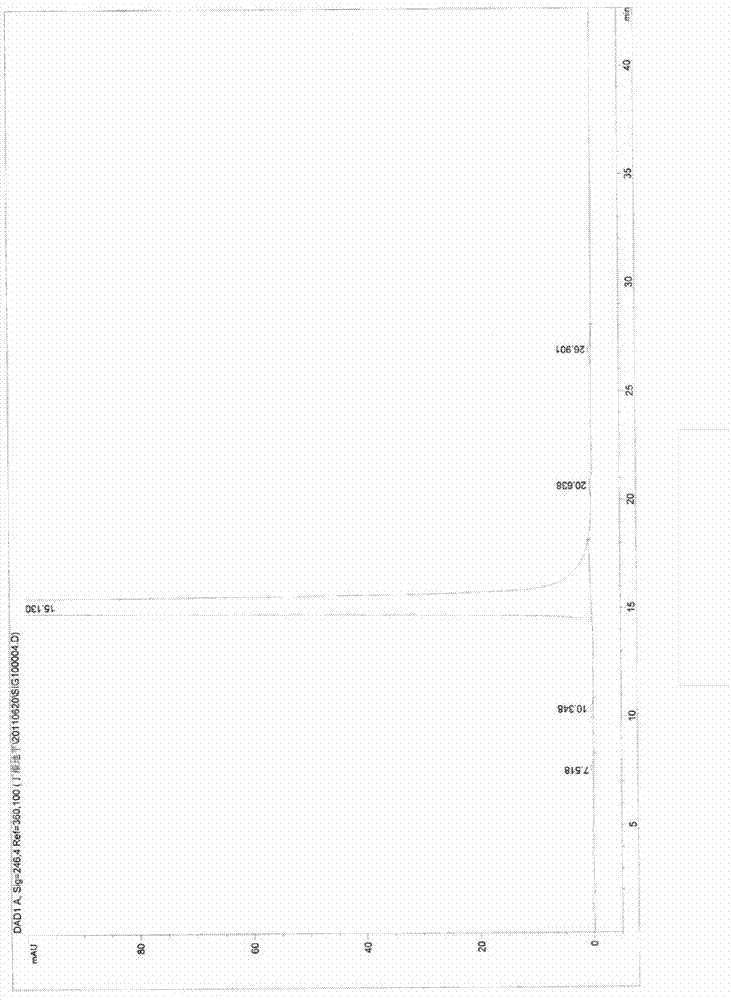
Measuring method of content of butyric acid clevidipine butyrate and content of related substances
InactiveCN103134891ALow equipment requirementsCommonly available mediaComponent separationClevidipineWavelength
Disclosed is a measuring method of the content of butyric acid clevidipine butyrate and the content of related substances. A high performance liquid chromatography is adopted, and octadecyl silane bonded silica gel is used as a reversed phase column of filler; ultraviolet detection is adopted, and detection wavelength is 230-250 nm; and a mobile phase A is acetonitrile, a mobile phase B is water, and the volume of the phase A accounts for 50%-90% of that of the mobile phases.
Owner:TIANJIN JINYAO GRP
Preparation of dihydropyridines
ActiveUS20110275825A1High purityHigh yieldPreparation by ester-hydroxy reactionOrganic compound preparationClevidipineMedicinal chemistry
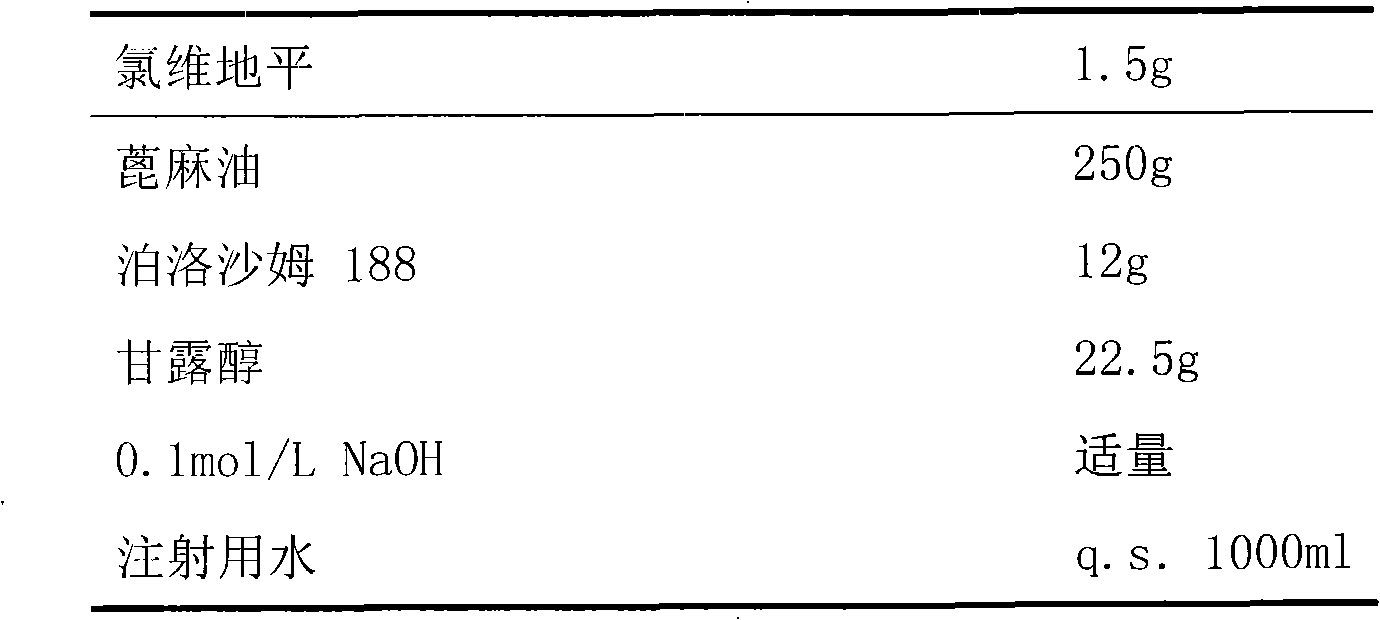
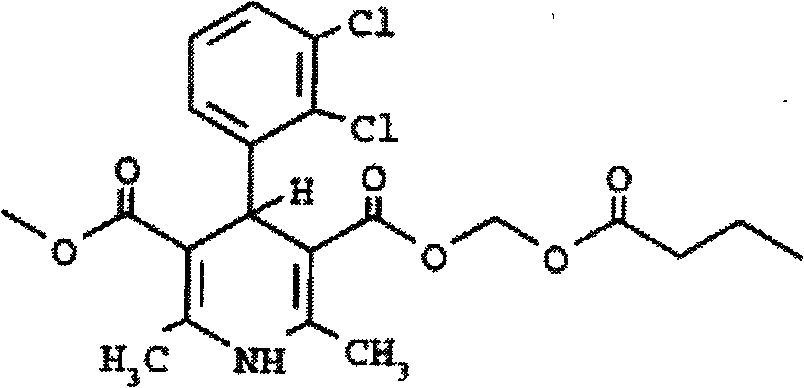
The invention relates to a method and compounds for the preparation of clevidipine butyrate, a very short acting hypertensive calcium antagonist, as well as the synthesis of these compounds useful for the preparation of clevidipine (also known as clevidipine butyrate). Moreover the invention also discloses polymorphic forms of clevidipine butyrate, useful for the preparation of pharmaceutical compositions, and processes to prepare them.
Owner:INKE SA (ES)
Method for preparing clevidipine butyrate
ActiveCN102432527AHigh selectivityReduce generationOrganic chemistryFermentationClevidipineCarboxylic acid
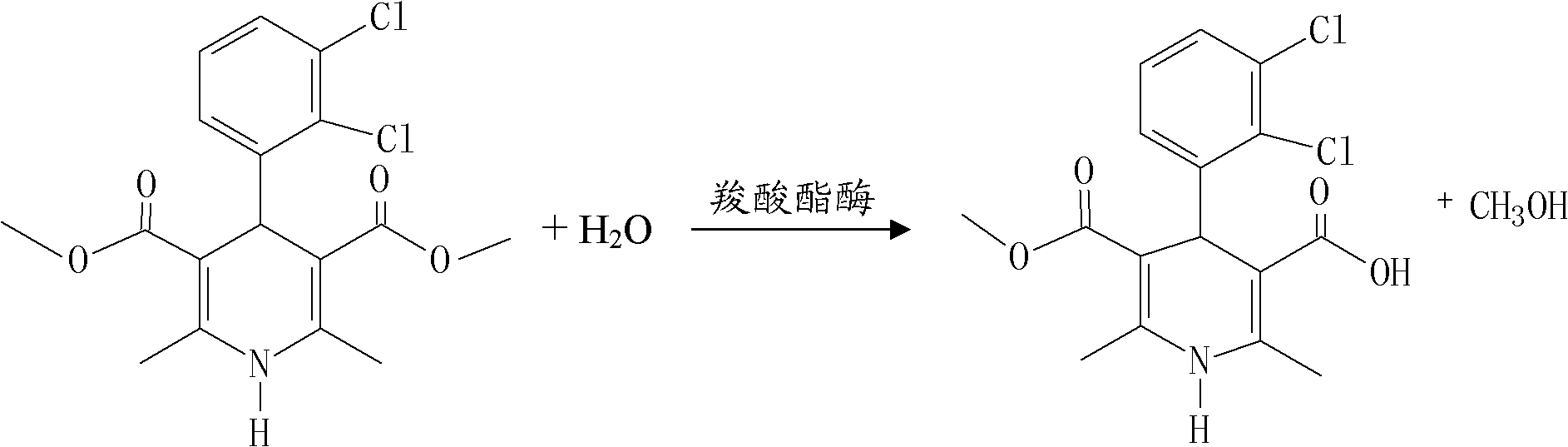
The invention relates to a method for preparing clevidipine butyrate and also relates to a method for preparing 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-methoxycarbonyl-3-picolinic acid methyl ester (an intermediate I) and 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-methoxycarbonyl-3-pyridine carboxylic acid (an intermediate II) which are used as intermediates of the clevidipine butyrate. The intermediate I is prepared by using 2,3-dichlorobenzaldehyde, ammonium acetate and methyl acetoacetate as raw materials and performing a reaction by using ultrasound. The intermediate II is prepared by carrying out enzyme hydrolysis on the intermediate I. When the clevidipine butyrate is prepared by using the method disclosed by the invention, ammonia water is not used as the raw material, so that no irritant gas is generated. Moreover, the cyclizative condensation is carried out by using ultrasonic waves and the hydrolysis is carried out by using carboxy lesterase, so that the reaction time can be shortened and the reaction yield is increased.
Owner:ZHEJIANG JIUXU PHARMA
Clevidipine butyrate injection
ActiveCN103110580AImprove stabilityAvoid oxidation reactionsOrganic active ingredientsEmulsion deliveryOrganic solventClevidipine
The invention belongs to the field of a pharmaceutical preparation, and in particular relates to a clevidipine butyrate injection. The injection comprises 0.01-10% of clevidipine butyrate, 0.1-10% of oil phase, 5-50% of phospholipid and 40-90% of organic solvent in percentage by weight. According to the invention, the clevidipine butyrate injection with good stability can be obtained, and the clevidipine butyrate can be used for lowering blood pressure during a surgery and after the surgery and when an oral therapy is not feasible or desirable. In comparison with the prior art, the clevidipine butyrate injection is always an anhydrous system in a storage process of the injection, the injection can be prepared on spot during use, thereby being capable of preventing an oxidation reaction of phospholipids and a degradation reaction of medicines, prolonging the preservation time of the preparation and improving the stability of the injection provided by the invention.
Owner:BEIJING DELI FURUI MEDICAL SCI & TECH
Emulsion for clevidipine butyrate intravenous injection and preparation method thereof
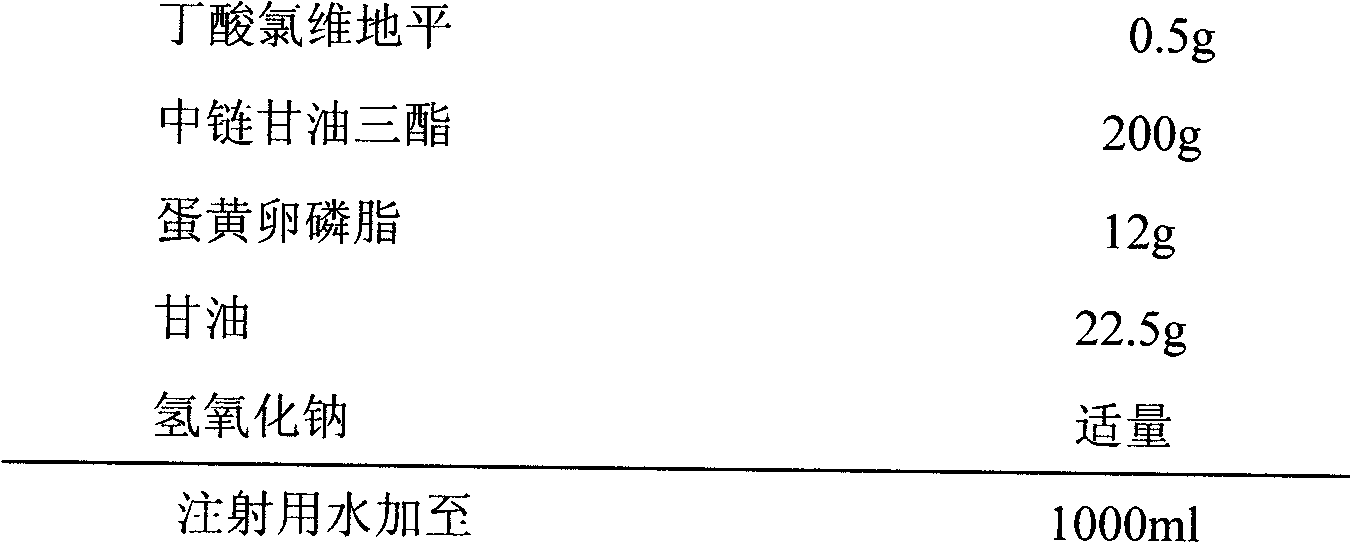
InactiveCN103126986AEasy to prepareLow costOrganic active ingredientsEmulsion deliveryEthylene diamineEmulsion
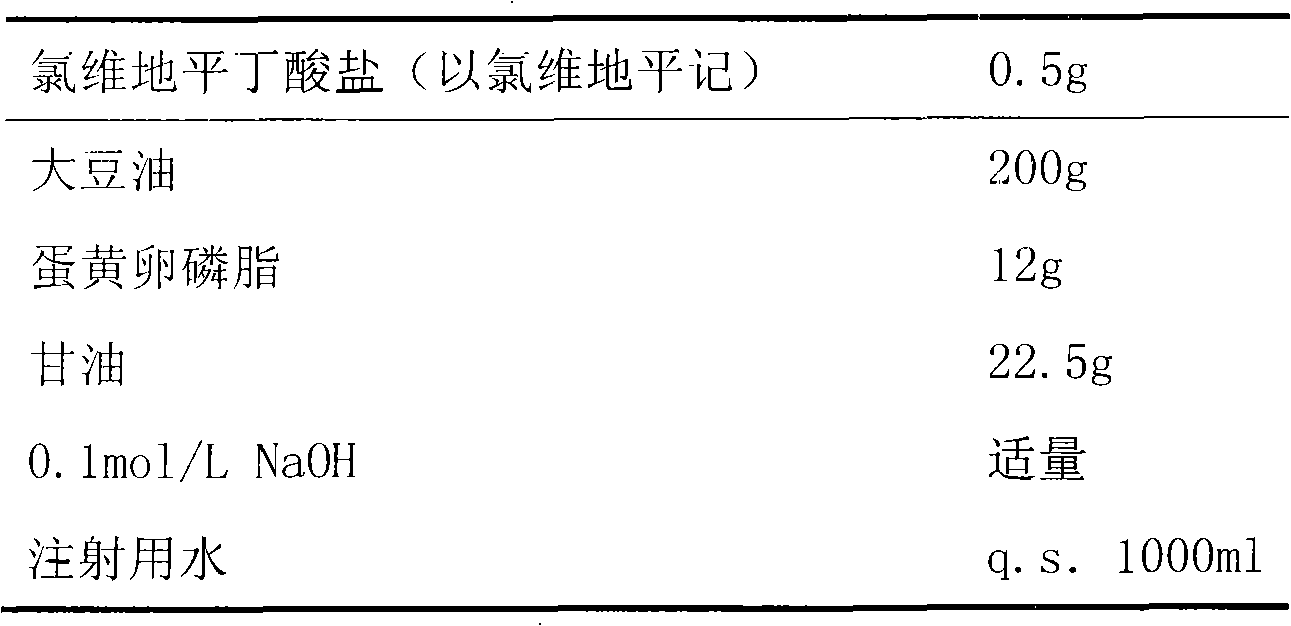
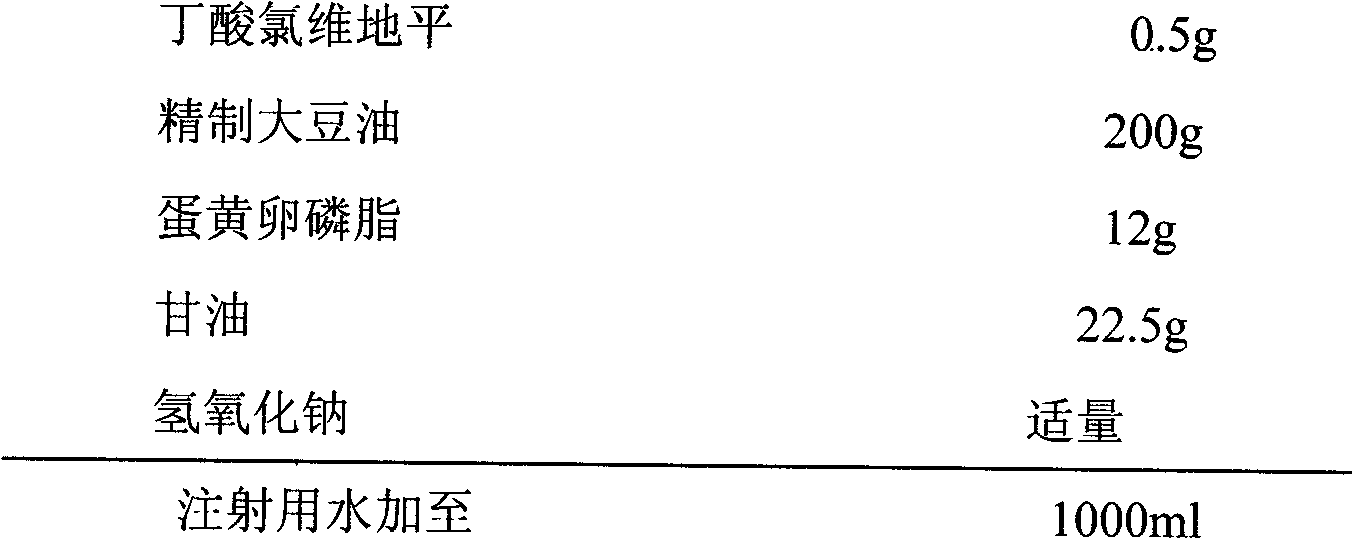
The invention discloses an emulsion for clevidipine butyrate intravenous injection and a preparation method thereof. The emulsion comprises the following components: 0.5mg / mL of clevidipine butyrate, 200mg / mL of refined soybean oil, 12mg / mL of high-purity lecithin, 0.3mg / mL of oleic acid, 22.5mg / mL of glycerol and 0.05mg / mL of ethylene diamine tetraacetic acid (EDTA)-2Na. Each bottle of emulsion contains 0.5mg / mL or 1.0mg / ml of an active ingredient of clevidipine butyrate.
Owner:董慧芳
Clevidipine butyrate intralipid and preparation method thereof
ActiveCN103211760ALow biological toxicityGood biocompatibilityOrganic active ingredientsEmulsion deliveryActive componentClevidipine
The invention discloses a clevidipine butyrate intralipid and a preparation method thereof. The intralipid comprises clevidipine butyrate active components, injection oil, an emulsifier, an isoosmotic adjusting agent, a pH adjusting agent and injection water, and also comprises a stabilizer, and the stabilizer is an electronegative phosphatide compound. The preparation method of the intralipid comprises the following steps: preparing an oil phase, preparing a water phase, mixing the prepared oil phase with the prepared water phase, shearing to form an crude intralipid, adding water to a constant volume, carrying out high pressure homogenization to obtain a finished intralipid, adjusting the pH value, filtering, carrying out nitrogen loading, and disinfecting. The addition of the electronegative phosphatide compound as the stabilizer in the invention improves the stability of the clevidipine butyrate intralipid, reduces the toxic side effects, ensures the use safety of the clevidipine butyrate intralipid, and has practical values.
Owner:SHANGHAI ACEBRIGHT PHARMA CO LTD
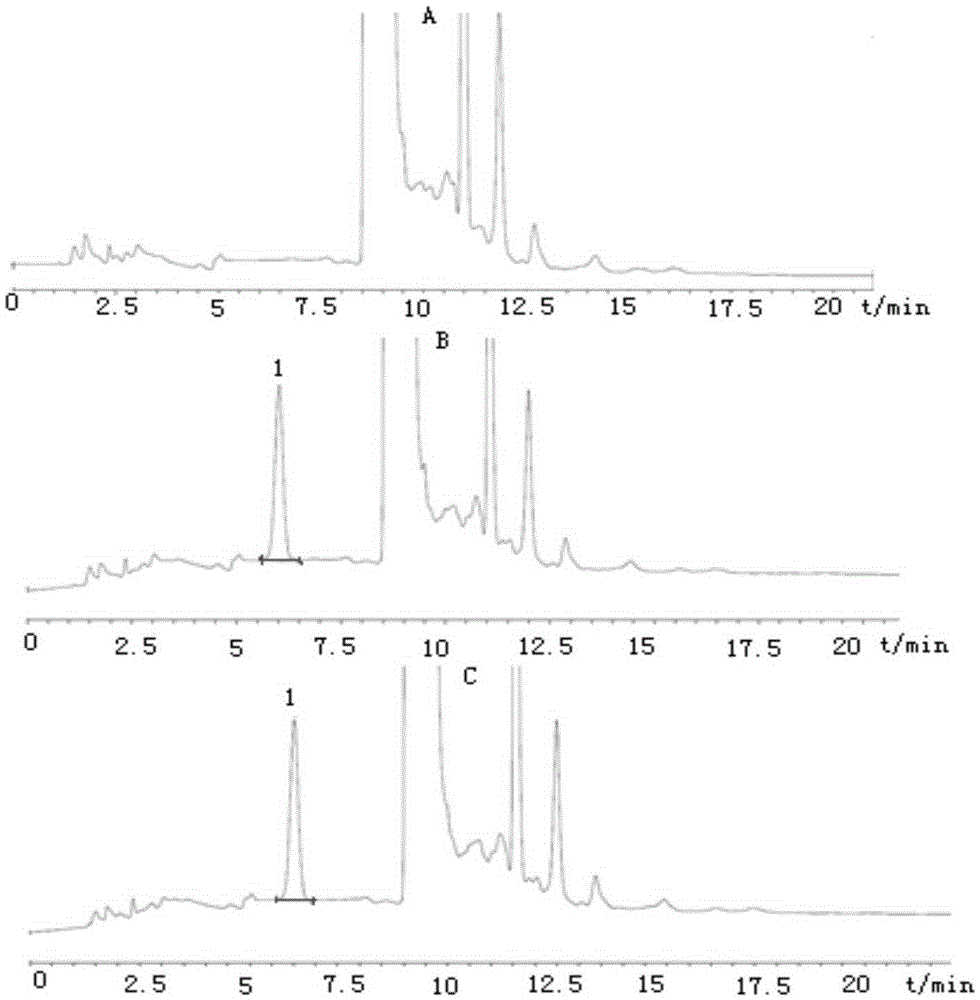
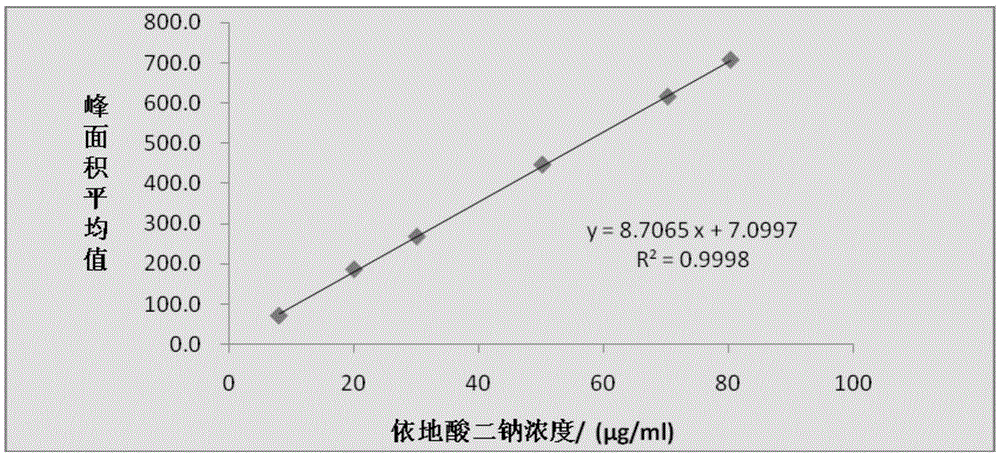
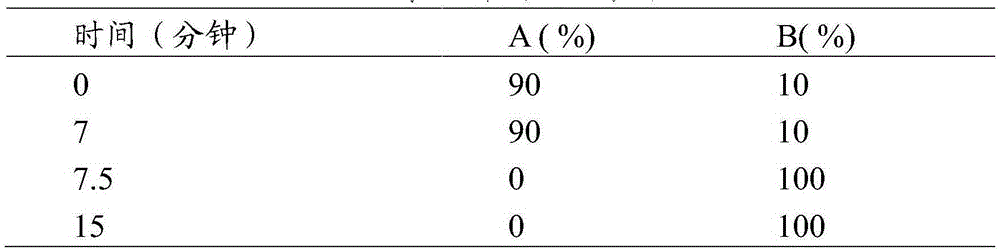
Method for detecting disodium edetate in clevidipine butyrate injection emulsion
The invention provides a preparation method of a sample used for HPLC detection of disodium edetate in a clevidipine butyrate injection emulsion. The preparation method comprises the following steps: adding an isopropanol and n-hexane mixed liquid into the clevidipine butyrate injection emulsion, shaking the mixed liquid and the emulsion, centrifuging the obtained solution, taking obtained water phase, and adding a metal ion salt solution able to complex edetic acid to form a complex in order to form a sample solution. The invention also provides a method for detecting disodium edetate in the clevidipine butyrate injection emulsion. The detection method is an HPLC method. An oil phase and a water phase in the clevidipine butyrate injection emulsion are effectively separated to make disodium edetate dissolved in the water phase be accurately detected. The detection method has the advantages of high detection sensitivity, good precision and high specificity, and is an effective method for strictly controlling the content of disodium edetate in the clevidipine butyrate injection emulsion.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Antihypertensive drug fat milk injection and preparation method thereof
ActiveCN107661294AQuality improvementImprove securityOrganic active ingredientsEmulsion deliveryClevidipineOil phase
The invention discloses an antihypertensive drug fat milk injection and a preparation method thereof. The antihypertensive drug fat milk injection is a clevidipine butyrate fat milk injection and contains clevidipine butyrate and a pharmacologically acceptable medical excipient; the medical excipient comprises an oil-phase medium, an emulsifier, an osmotic pressure regulator, a stabilizer, a metalcheating agent, a pH value regulator and injection water. The antihypertensive drug fat milk injection has stable quality and high safety and is not irritant to blood vessels.
Owner:WUHAN CONFORM PHARMA CO LTD
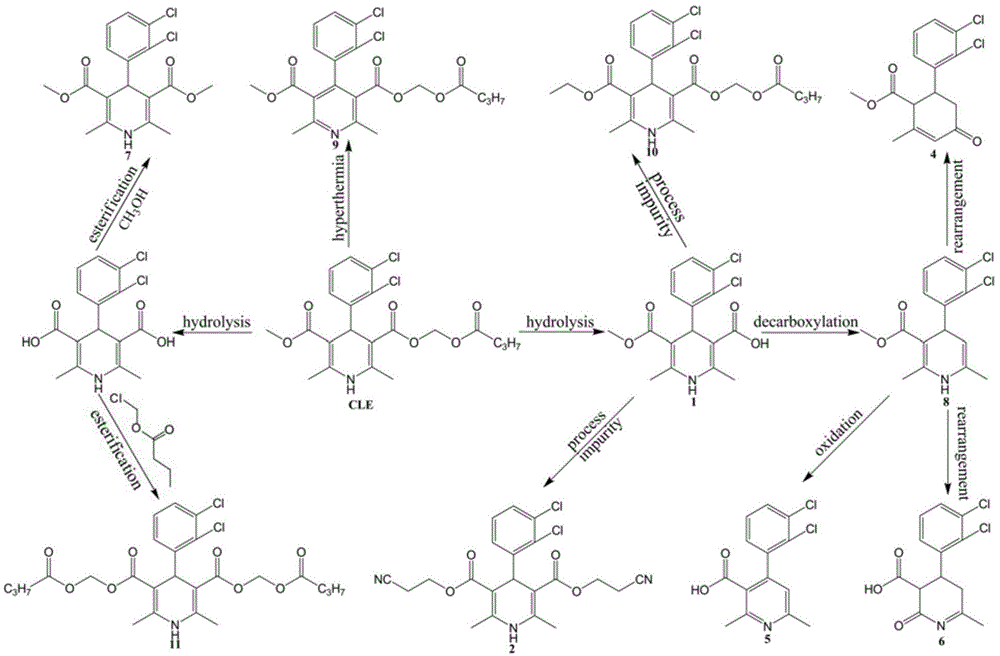
Purification method of clevidipine butyrate intermediate
The invention discloses a purification method of 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3-methyl carboxylate-5-carboxylic acid as an import intermediate for synthesizing clevidipine butyrate. The purification method comprises the following steps of: reacting the 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3-methyl carboxylate-5-carboxylic acid with alkali to generate alkali metal salt; separating out the alkali metal salt from a solvent to achieve the impurity removal purpose; acidifying the alkali metal salt, and then reacting with n-butyric acid chloroformate to prepare the clevidipine butyrate. The purification method disclosed by the invention obtains high-purity high-yield monocarboxylic acid by effectively removing dicarboxylic acid impurities contained in the monocarboxylic acid intermediate through operation steps which are easy to operate and can obviously reduce the content of principal impurities contained in the clevidipine butyrate by adopting the prepared high-purity monocarboxylic acid intermediate, simplify the purification procedure of the clevidipine butyrate and increase the total yield.
Owner:BEIJING JIALIN PHARM INC
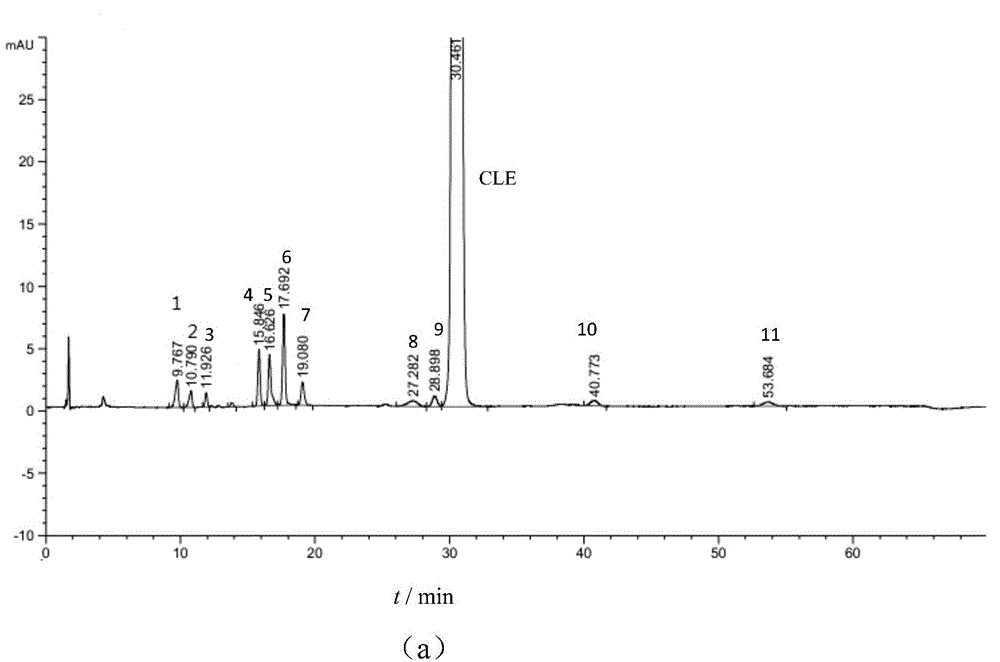
Method for simultaneously detecting clevidipine butyrate and related substances
InactiveCN105092769AHigh precisionGood reproducibilityComponent separationAdditive ingredientClevidipine
The invention provides a method for simultaneously detecting clevidipine butyrate and eleven related substances and a method for determining clevidipine butyrate content. The methods utilize a reversed phase high-performance liquid chromatography method. Through a lot of repeated experiments, chromatographic conditions for simultaneously separating clevidipine butyrate and eleven related substances and conditions for determining clevidipine butyrate content are acquired, and the chromatographic conditions are suitable for analysis of clevidipine butyrate drug ingredients and content in different environments so that the problem that in analysis of clevidipine butyrate drug ingredients in different environments, the prior art needs respective exploration of corresponding chromatographic conditions. The chromatographic conditions have the advantages of high precision, good reappearance, good durability and mastering easiness.
Owner:SHANGHAI XINPING MEDICALSCI&TECH CO LTD
Clevidipine butyrate liquid liposome preparation
ActiveCN102335134AAvoid time costReduce economic costsOrganic active ingredientsPharmaceutical non-active ingredientsSterolClevidipine
The invention discloses a clevidipine butyrate liposome preparation and a preparation method thereof. The preparation includes 0.05wt%(wt%)-0.1%(wt%) of clevidipine butyrate, 40%(wt%) -70%(wt%) of phosphatidylcholine, 10%(wt%)-40%(wt%) of phosphatidyl glycerol, 10%(wt%)-30%(wt%) of sterol, and 0.55%(wt%)-3.3%(wt%) of stabilizer. The stabilizer contains a component A and a component B, wherein the component A is selected from one or more of oleic acid, sodium oleate, linoleic acid and sodium linoleate; and the component B is selected from one or more of vitamin E, coenzyme Q10, propyl gallate and ascorbyl palmitate. The liposome prepared by the method is in the form of liquid, and can be stored for a long time, therefore not only the potential safety hazard due to use of middle-chain and long-chain triglyceride in the traditional clevidipine butyrate emulsion is thoroughly solved, but also the freeze-drying operation in the traditional liposome preparation is omitted and the production cost is reduced; moreover, the liposome preparation is convenient for clinical application, overcomes the defect of uneven quality of redissolved freeze-drying liposome preparation and is beneficial to increase of the compliance and the medication safety of a patient.
Owner:BEIJING TIDE PHARMA
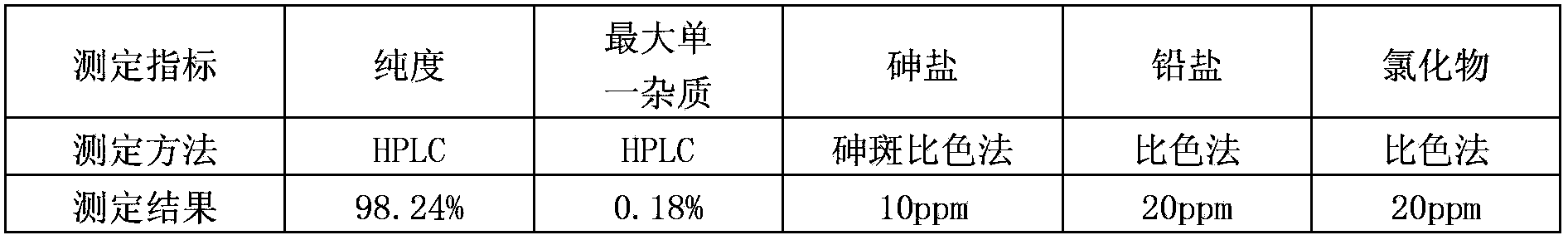
Purification method of clevidipine butyrate
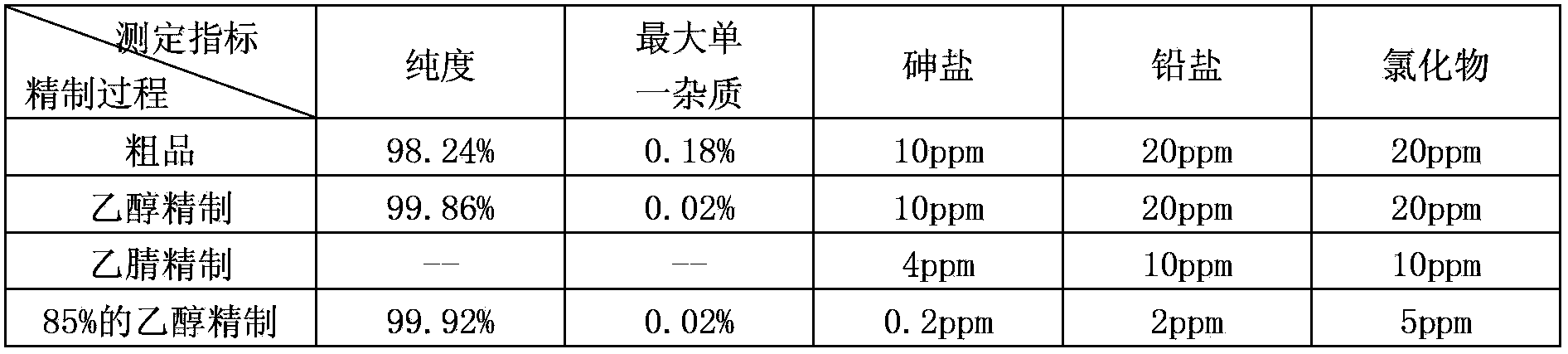
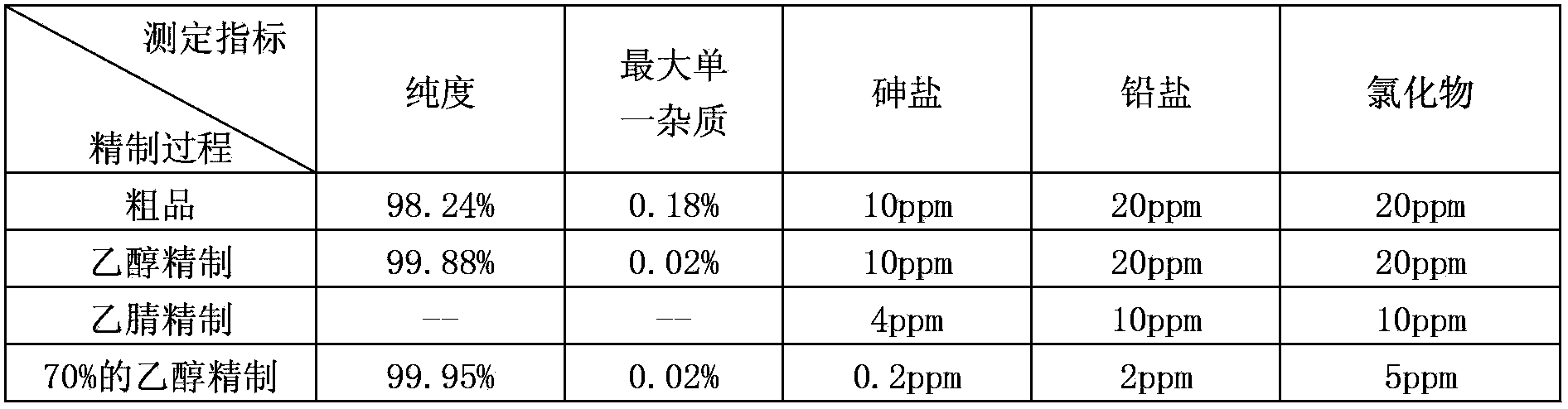
The invention discloses a purification method of clevidipine butyrate The purification method comprises following steps: step A, an ethanol solution of clevidipine butyrate is prepared, and organic impurities are removed by crystallization purification; step B, crystals purified by ethanol are dissolved in acetonitrile, and then are precipitated by adding water so as to remove a large amount of inorganic impurities; and step C, the crystals purified by acetonitrile are dissolved in an ethanol solution with a concentration of 50 to 90% so as to remove a small amount of inorganic impurities. The products are dissolved completely by mixing the poor solvent with the good solvent so as to realize complete purification; the purity of clevidipine butyrate reaches 99.5%; and arsenic, lead and chloride indexes are all much lower than that of standards.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Pharmaceutical compositions and methods for producing low impurity concentrations of the same
InactiveUS20120322835A1Lower Level RequirementsHigh bloodBiocideOrganic chemistryMedicineClevidipine
A composition having clevidipine as an active ingredient is described. The composition includes clevidipine as an active ingredient and an amount of the impurity H168 / 79 that is no greater than about 1.5%, or where the ratio between clevidipine and H168 / 79 is equal or above 60 to 1.
Owner:MOTHERAM RAJESHWAR +4
Clevidipine emulsion formulations containing antimicrobial agents
ActiveUS8658676B2Stable against formationReduction tendencyBiocideAnimal repellantsClevidipinePharmaceutical formulation
Pharmaceutical formulations comprising clevidipine in an oil-in-water formulation that is resistant to microbial growth and stable against the formation of impurities.
Owner:CHIESI FARM SPA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com