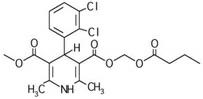
Clevidipine butyrate emulsion, preparation method thereof and purpose thereof
A technology of clevidipine butyrate and dipine emulsion, which is applied in the direction of pharmaceutical formula, emulsion delivery, medical preparations of non-active ingredients, etc., and can solve problems such as endangering immune function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] In a preferred embodiment of the present invention, the preparation method further includes an optional final filtration and / or autoclaving step.
[0034] In another aspect, the present invention provides a pharmaceutical composition comprising the above-mentioned clevidipine butyrate. The pharmaceutical composition uses the above-mentioned clevidipine butyrate as an active ingredient, and optionally includes one or more pharmaceutically acceptable excipients and / or carriers. The pharmaceutically acceptable excipients and / or carriers include emulsifiers such as lecithin, polyoxyethylene castor oil; pH regulators such as phosphate buffer, borate buffer, etc.; bacteriostatic agents such as paraben Class (parabens), benzyl alcohol, sodium benzoate, etc.; co-solvents such as Tween-80, propylene glycol, etc.
[0035] In yet another aspect, the present invention also provides ophthalmic preparations comprising the clevidipine butyrate, preferred dosage forms include eye drop...
Embodiment 1
[0043] Example 1 : Clevidipine Butyrate Emulsion
[0044] 0.01-20% clevidipine butyrate
[0045] 10-25% lipid phase
[0046] Emulsifier accounting for 0.1-1.5 times the weight of lipid phase
[0047] 40-99% water or buffer.
Embodiment 2
[0048] Embodiment 2: Clevidipine butyrate emulsion
[0049] Clevidipine Butyrate 0.7g
[0050] Medium Chain Triglycerides 180g
[0052] Glycerin 25g
[0053] Appropriate amount of sodium hydroxide
[0054] Add water for injection to 1000ml
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com