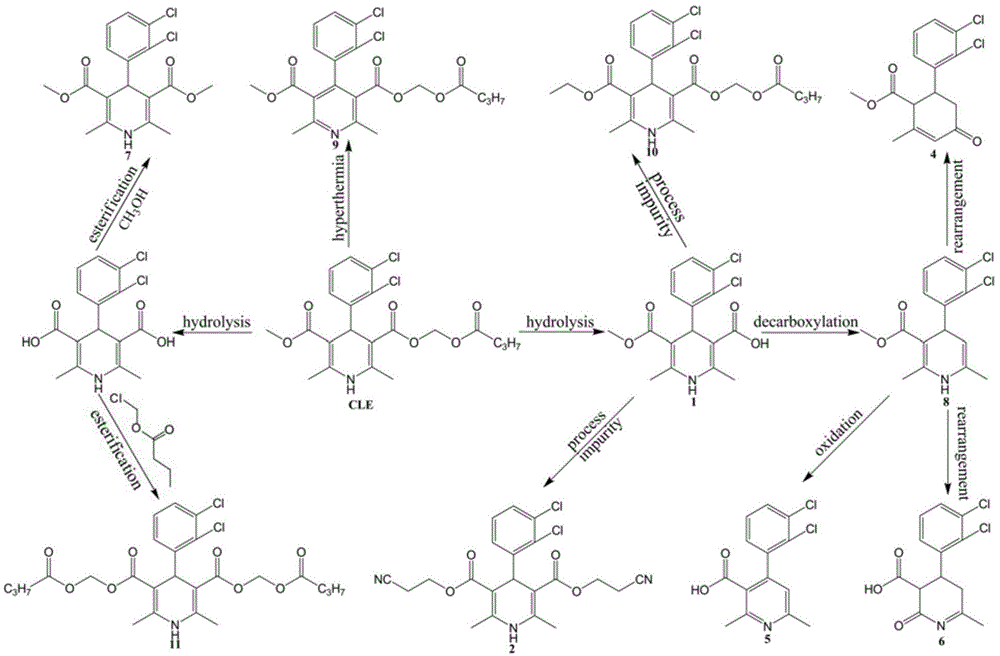
Method for simultaneously detecting clevidipine butyrate and related substances
A technology for clevidipine butyrate and related substances, which is applied in the field of raw drug detection of clevidipine butyrate, can solve the problems of not giving three kinds of impurity names, analysis of other related substances, etc., and achieves good reproducibility and precision high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The optimization of embodiment one high performance liquid chromatography conditions
[0065] In this embodiment, by analyzing the mixed reference substance solution, the optimal high performance liquid chromatography conditions are determined, and the optimal chromatographic conditions are explored from the aspects of detection wavelength, chromatographic column, mobile phase, flow rate and column temperature.
[0066] In this example, the standard for achieving effective separation of clevidipine butyrate and its 11 impurities is: separation between each chromatographic peak≥resolution.
[0067] (1) Investigation of ultraviolet wavelength
[0068] It can be known from the ultraviolet absorption characteristics of each substance that impurities ⑤, ⑧, and ⑨ only absorb at the end. Considering the stability of the baseline, 220nm is selected as the detection wavelength. The main components and other impurities also have good absorption at this detection wavelength.
...
Embodiment 2
[0083] Embodiment two, mixed reference substance solution, clevidipine butyrate contrast solution and need testing solution test
[0084] The present embodiment adopts the chromatographic conditions in embodiment one to carry out the test of mixed reference substance solution, clevidipine butyrate contrast solution and need testing solution.
[0085] (1), detection of mixed reference substance solution and clevidipine butyrate reference solution
[0086] According to the method of solvent configuration in 3, configure mixed reference substance solution and clevidipine butyrate contrast solution respectively, accurately draw 20 μ L of each of the two solutions, inject into the high performance liquid chromatograph supplemented by photodiode array detector, and then follow the embodiment One of the preferred chromatographic conditions for detection.
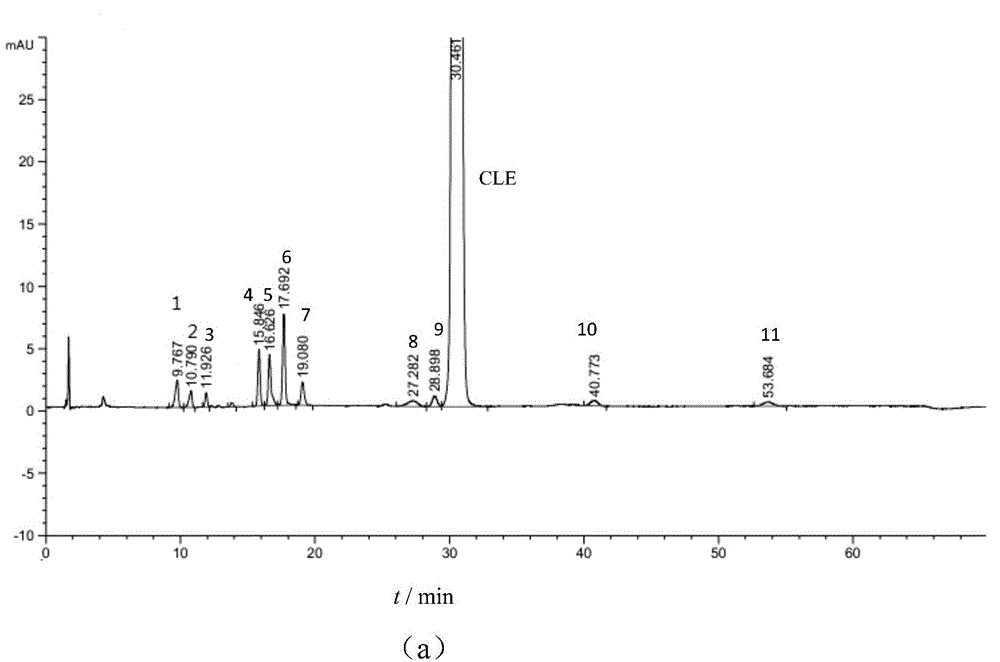
[0087] figure 2 (a) is the chromatogram of the mixed reference substance in the present embodiment.
[0088] figure 2 (b) i...
Embodiment 3
[0103] Embodiment three method verification
[0104] This embodiment carries out methodological verification to the preferred chromatographic conditions in embodiment one, mainly in the following five aspects:
[0105] (1), linear range, detection limit and quantification limit
[0106] Weigh the appropriate amount of 11 kinds of impurities and clevidipine butyrate reference substance respectively, and prepare a series of concentration solutions corresponding to 50%, 80%, 100%, 120% and 150% under the conditions of content determination, and measure and investigate the linearity of each component. relationship and relative correction factor. Take the injection concentration (mg / L) as the abscissa, and the peak area as the ordinate, perform regression curve analysis, and calculate the quantitative limit of the method with 10 times the signal-to-noise ratio (S / N=10), and use 3 times the signal-to-noise ratio (S / N=3) the detection limit of calculation method, the results are ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com