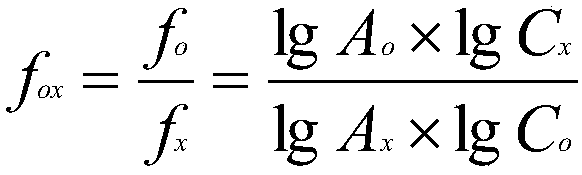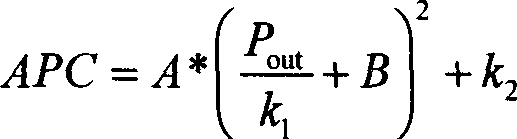Patents
Literature
83 results about "Relative correction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
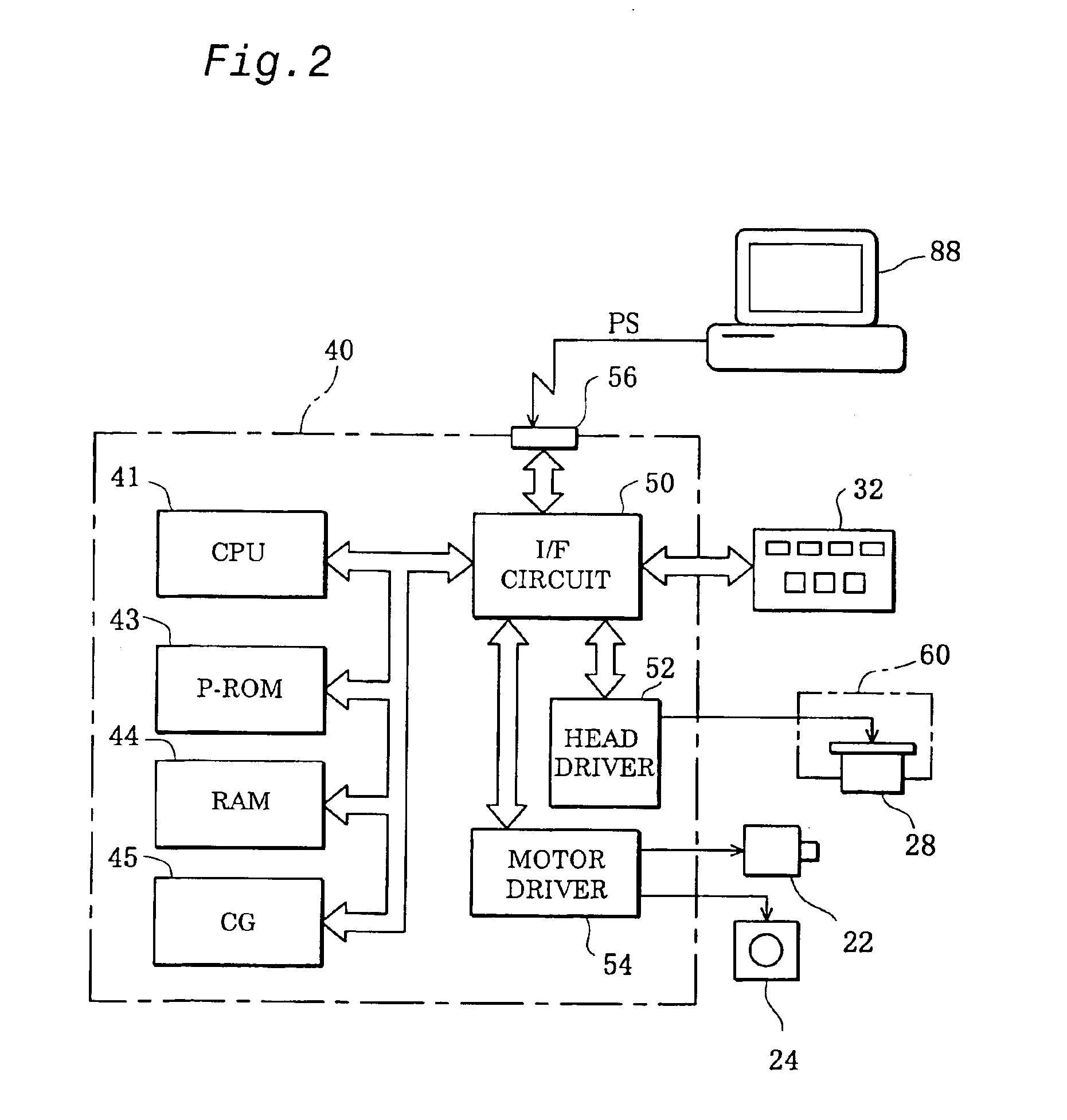
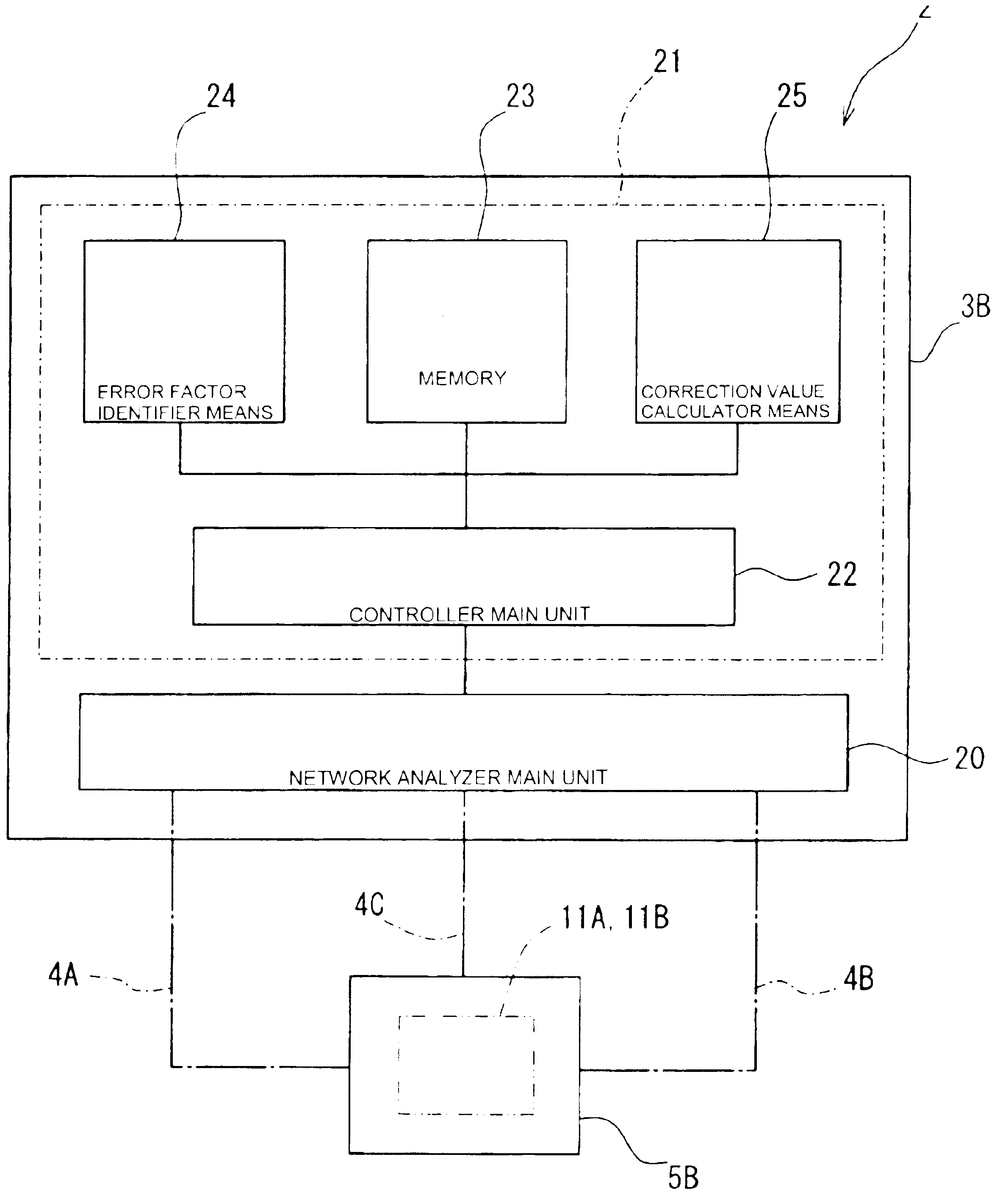
Method of correcting measurement error and electronic component characteristic measurement apparatus
ActiveUS6838885B2Easily and automatically expressedSuppress mutationResistance/reactance/impedenceElectrical testingObservational errorMeasurement device
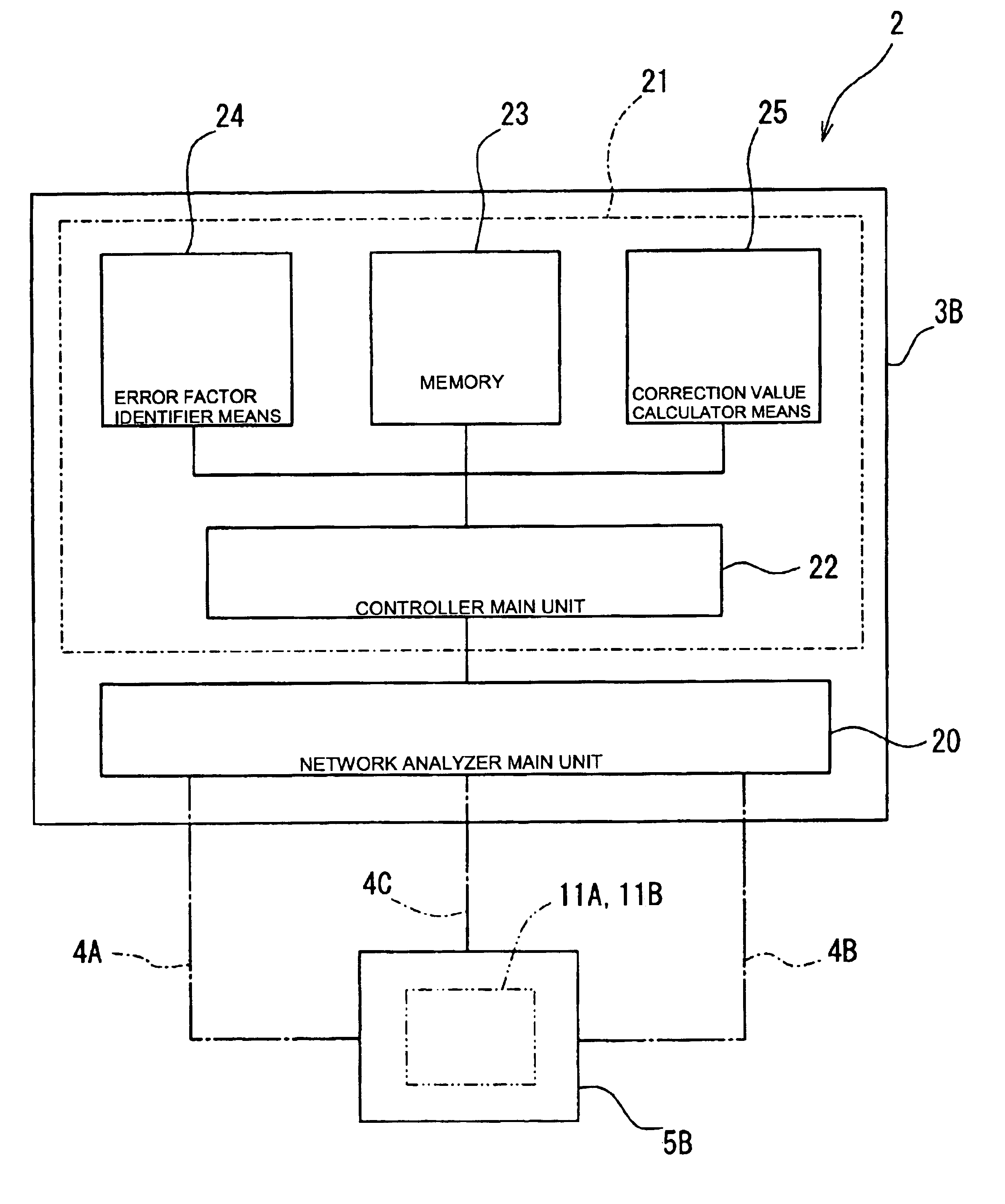
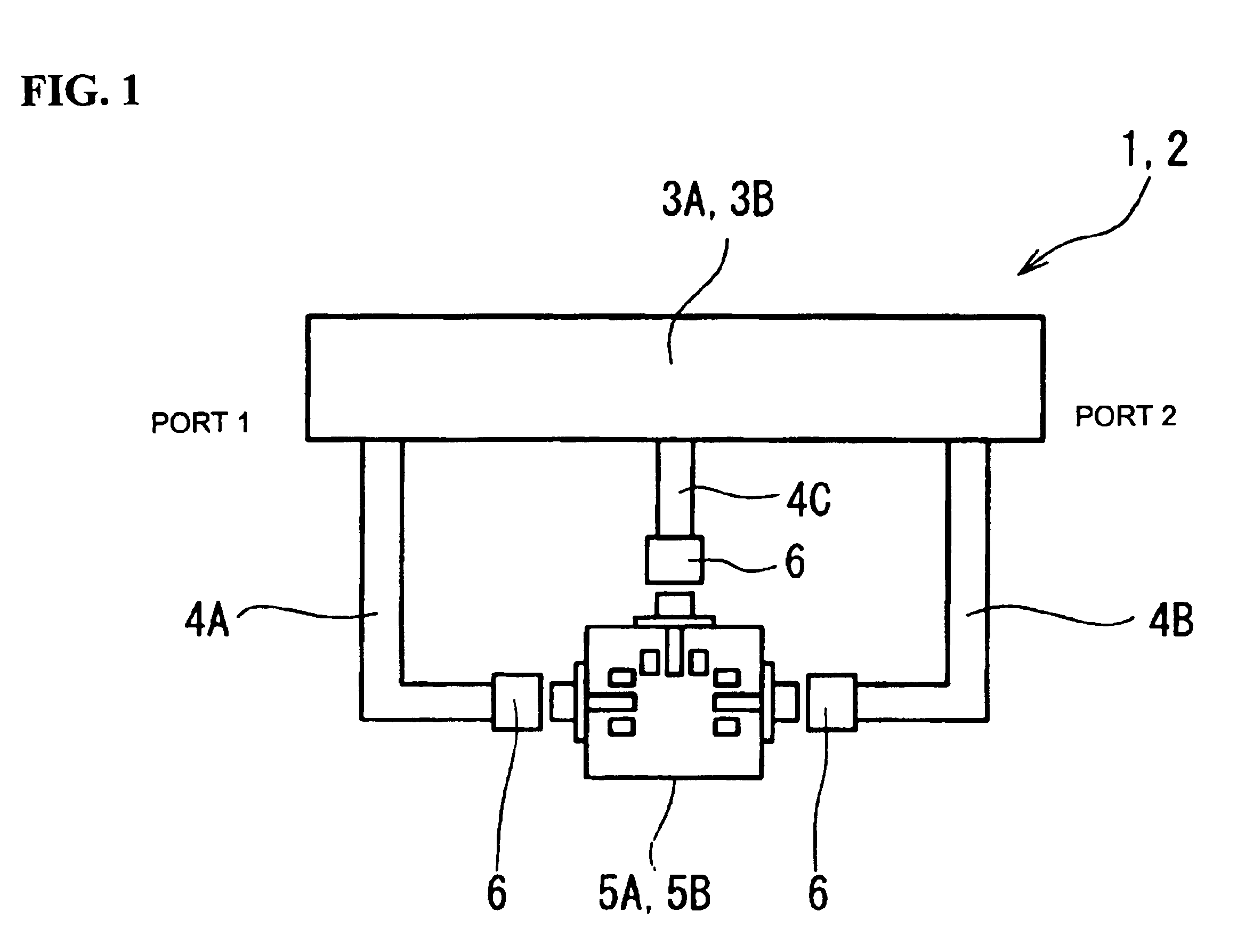
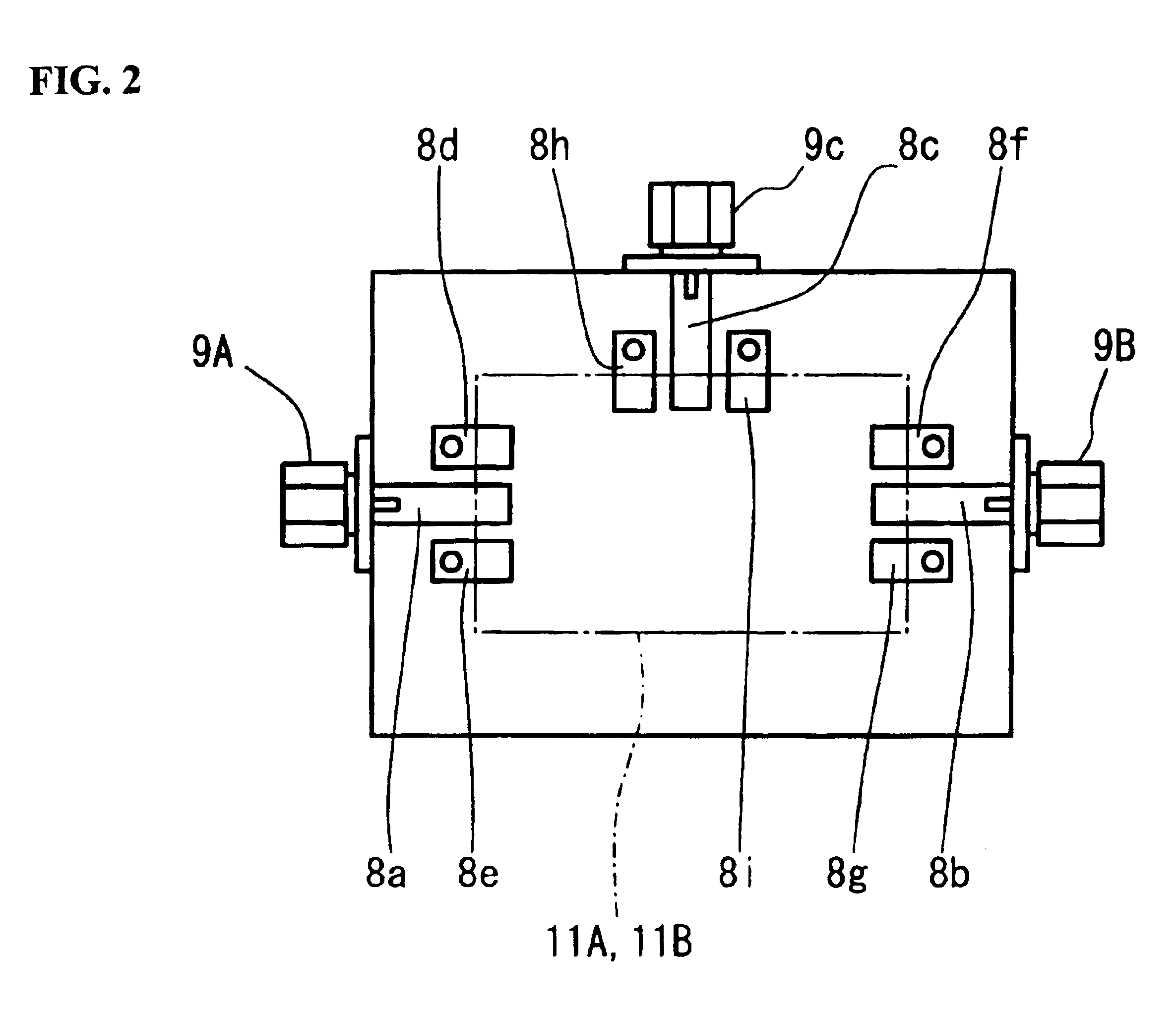
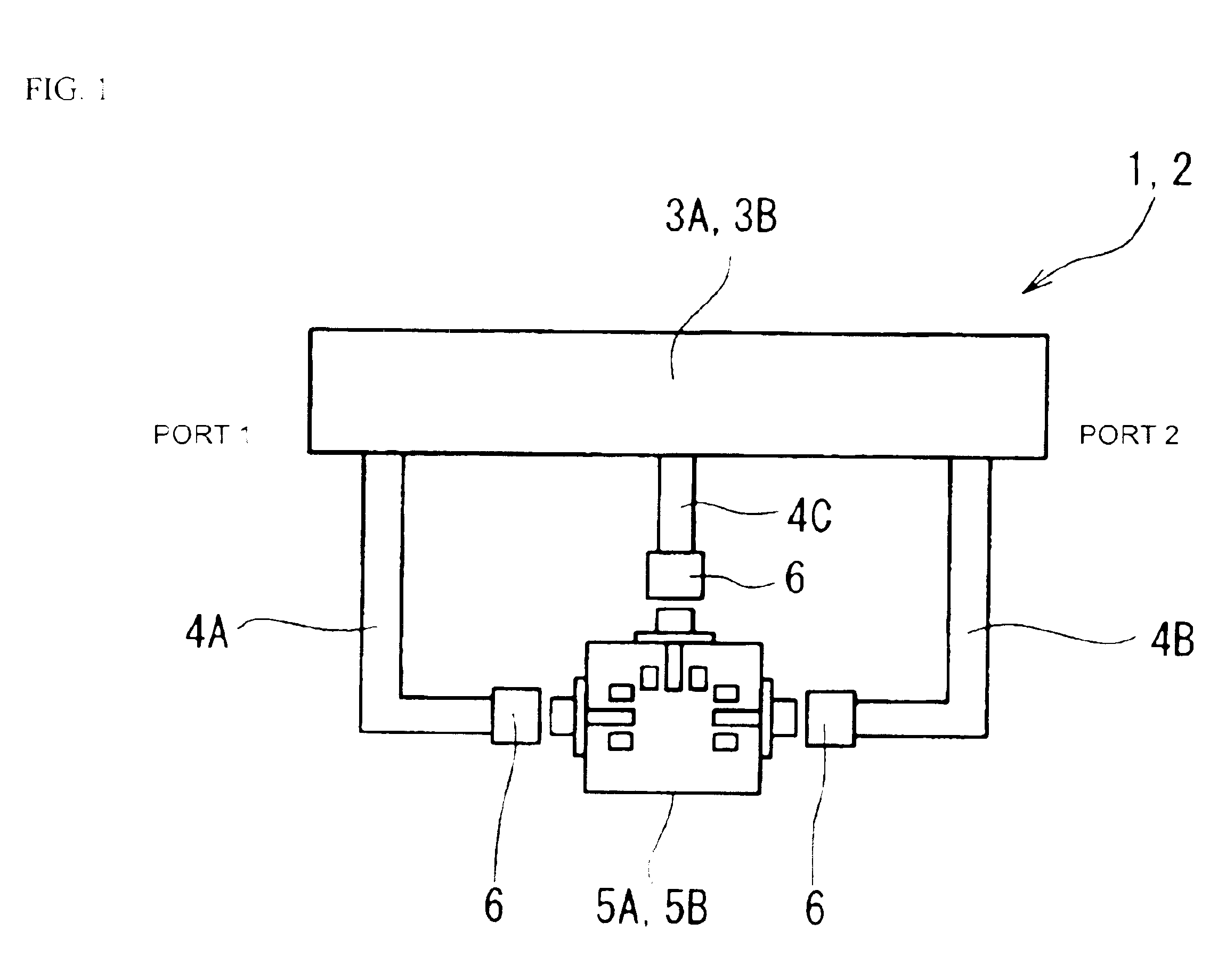
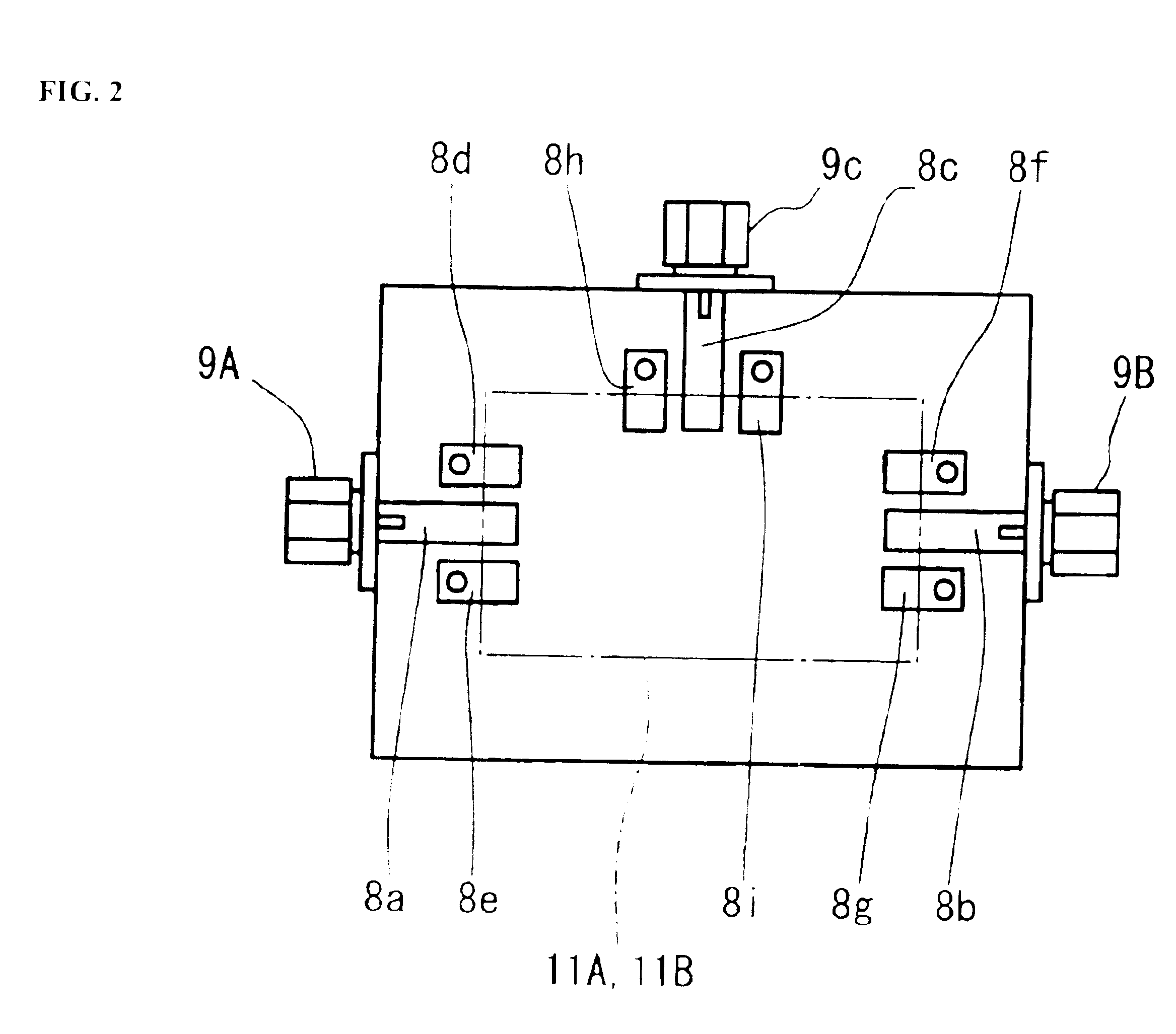
A high-precision, multi-port compatible, relative correction method and apparatus for correcting measurement errors covering an increase in the number of ports of a non-coaxial electronic component, in which a relative correction adapter 31 is provided that is formed of a two-port network connected to each port of a production test fixture 5B adjacent to a measurement apparatus. The relative correction adapter has a characteristic that modifies the electrical characteristics generated by the production test fixture 5B having an electronic component under test mounted thereon into electrical characteristics generated by a standard test fixture 5A having the electronic component under test mounted thereon. An error factor of the relative correction adapter 31 is identified from a standard test fixture measurement value and a production test fixture measurement value of a correction data acquisition specimen 11B. A production test fixture measurement value of the electronic component under test 11A is corrected with the error factor of the relative correction adapter 31 to thereby obtain the standard test fixture measurement value of the electronic component under test 11A which is assumed to be obtained when the electronic component under test 11A.
Owner:MURATA MFG CO LTD
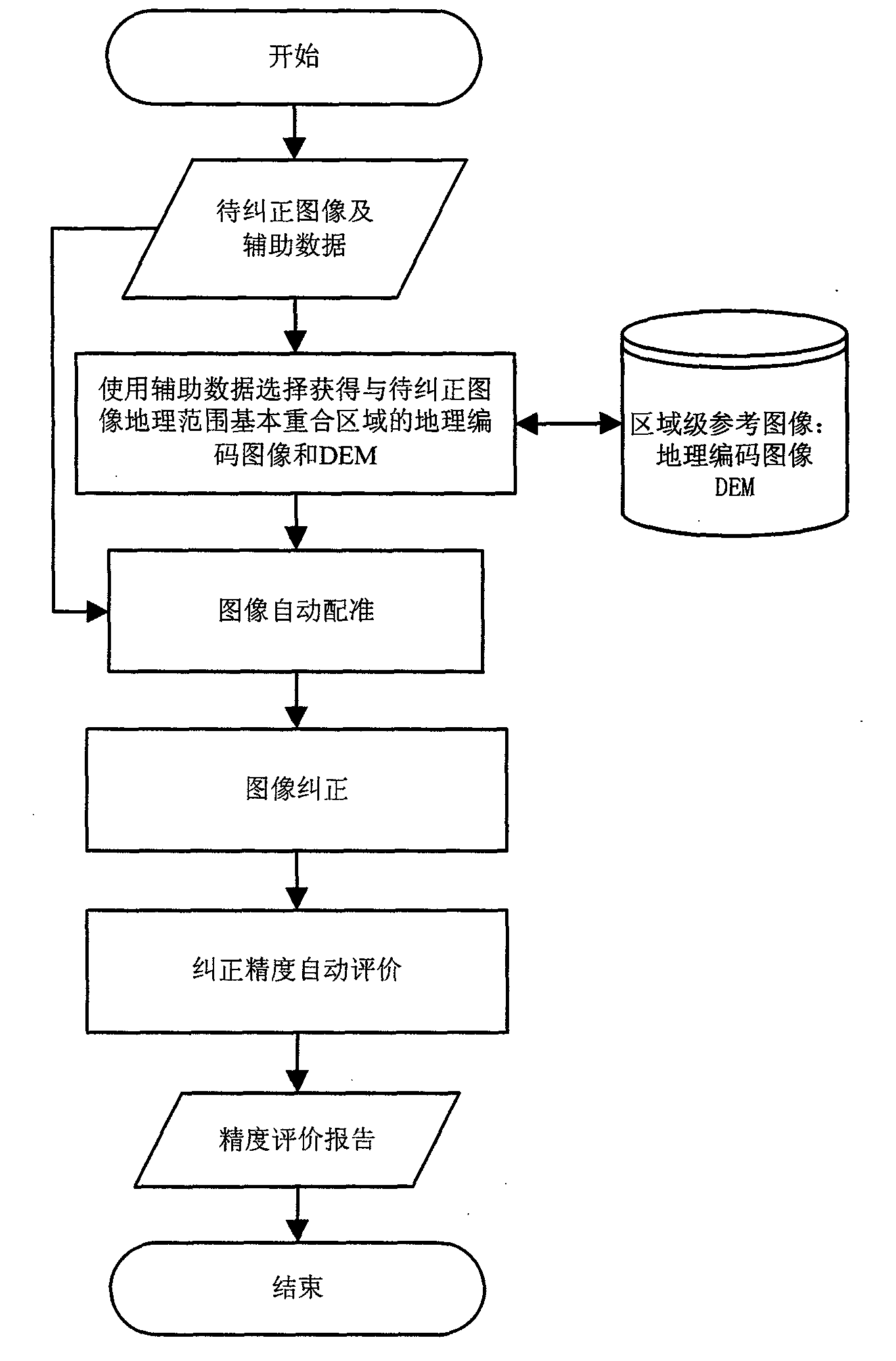
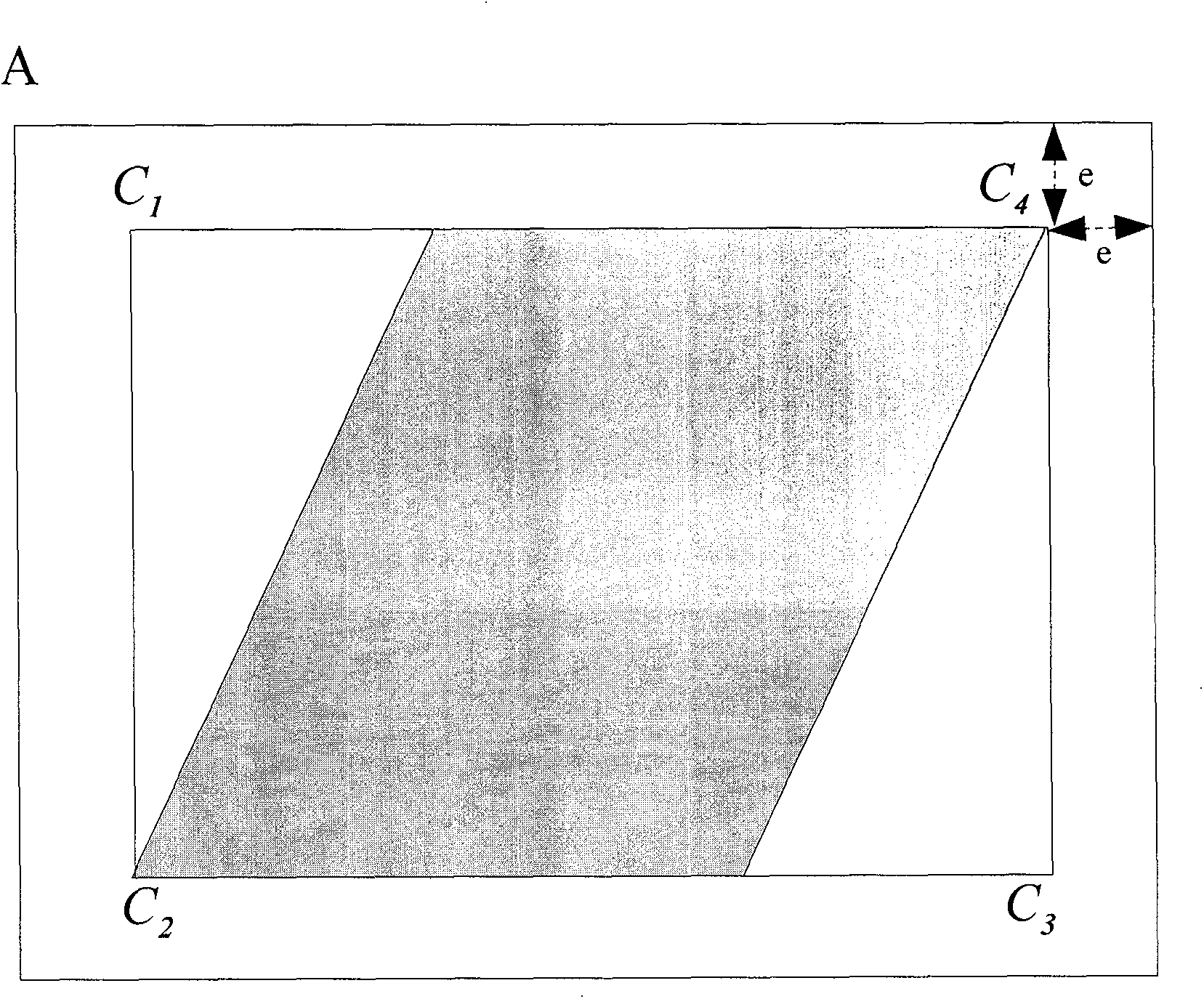
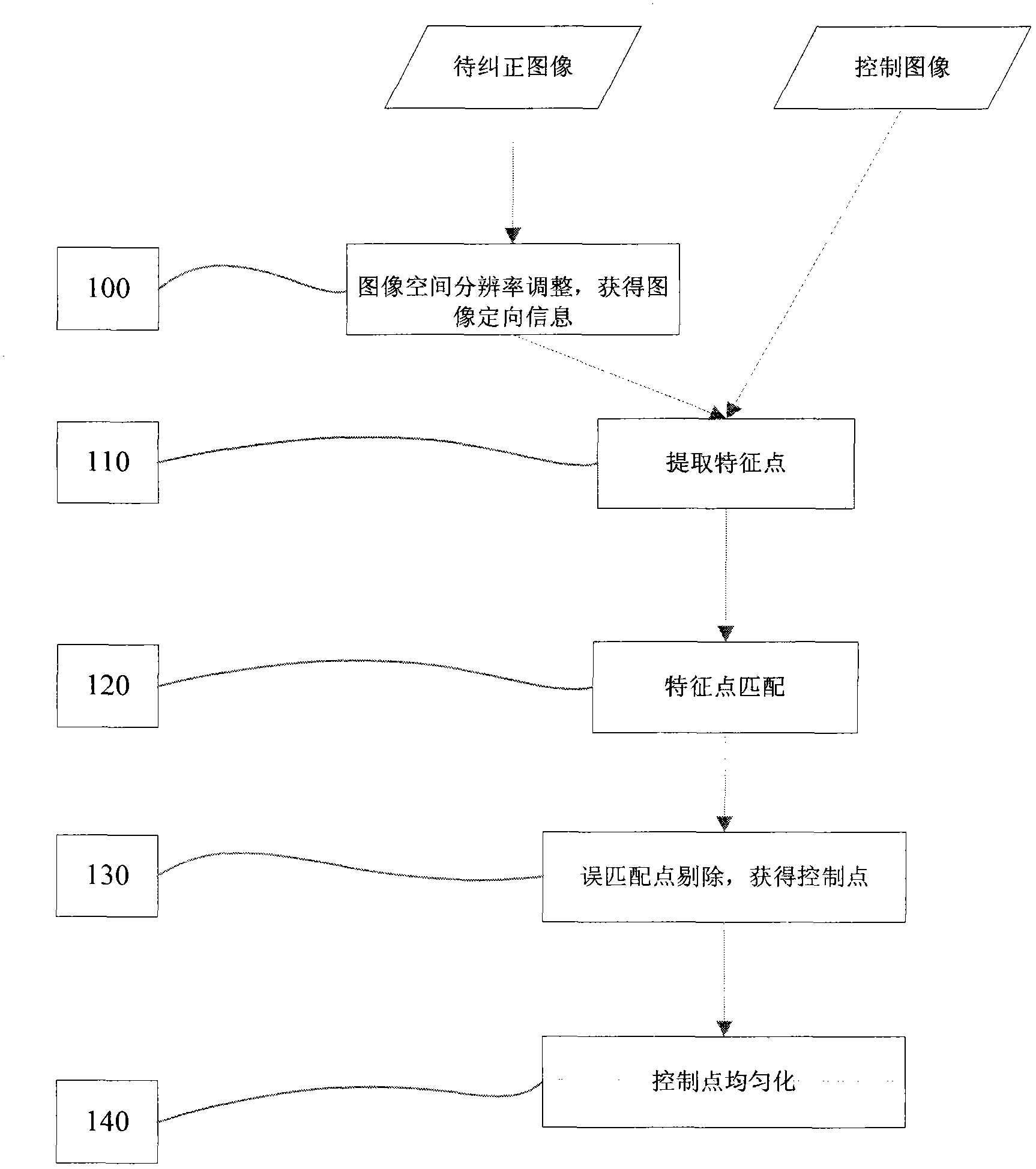
Automatic ortho-rectification frame and method for dynamically extracting remote sensing satellite image of image control points
Owner:INST OF REMOTE SENSING & DIGITAL EARTH CHINESE ACADEMY OF SCI +1
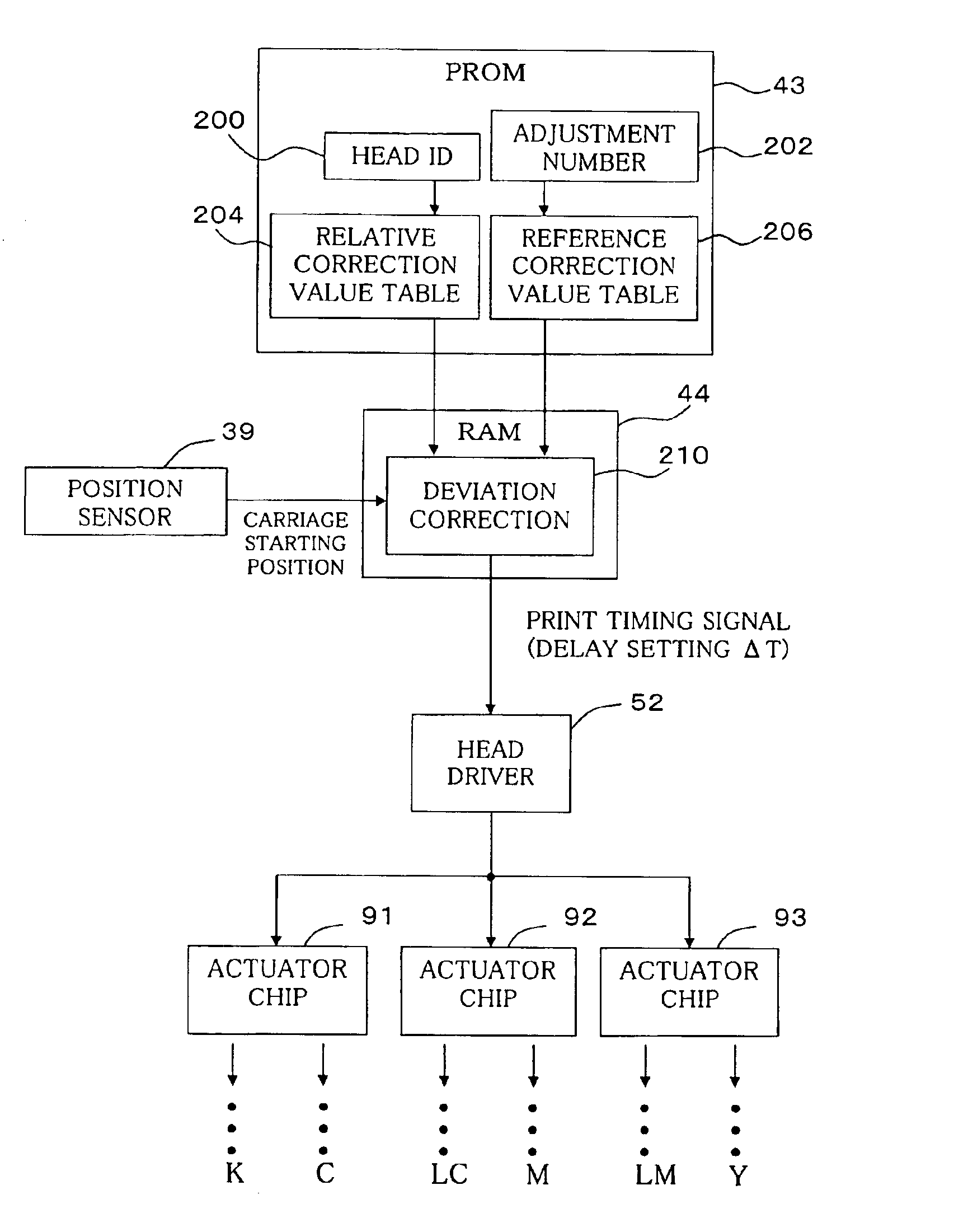
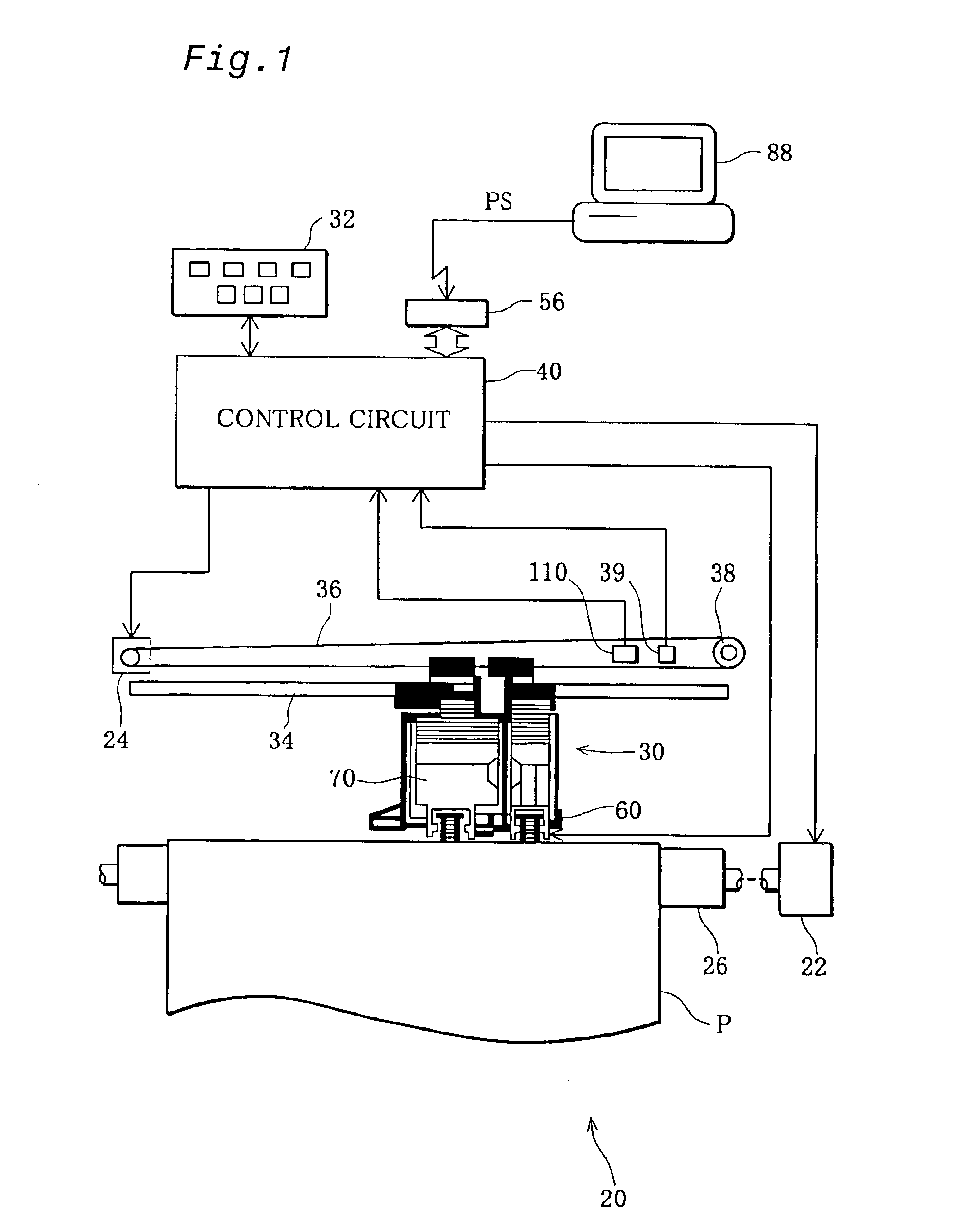
Positional deviation correction using reference and relative correction values in bi-directional printing
InactiveUS6908173B2Improve image qualityPrinting positional deviationInking apparatusSpacing mechanismsEngineeringControl theory
In the bi-directional printing, a reference correction value is set for correcting printing positional deviation arising between forward and reverse main scanning passes with respect to specific reference dots. An adjustment value is determined, using at least the reference correction value, to reduce printing positional deviation arising between forward and reverse main scanning passes. The printing positional deviation between forward and reverse main scanning passes is adjusted using the adjustment value. In a first adjustment mode, the adjustment value is determined by correcting the reference correction value with a relative correction value prepared beforehand for correcting the reference correction value.
Owner:SEIKO EPSON CORP
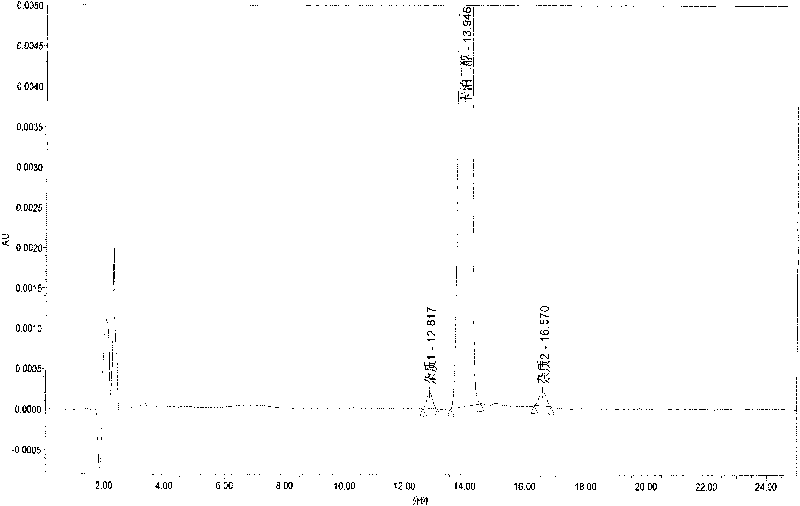
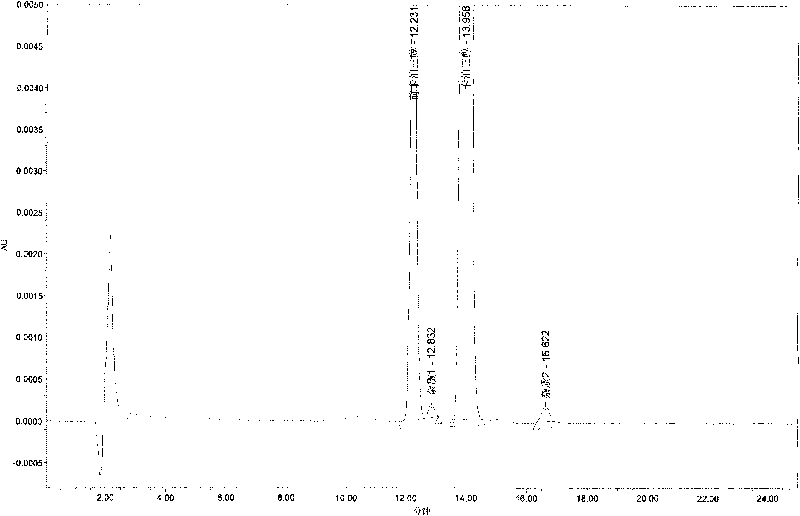
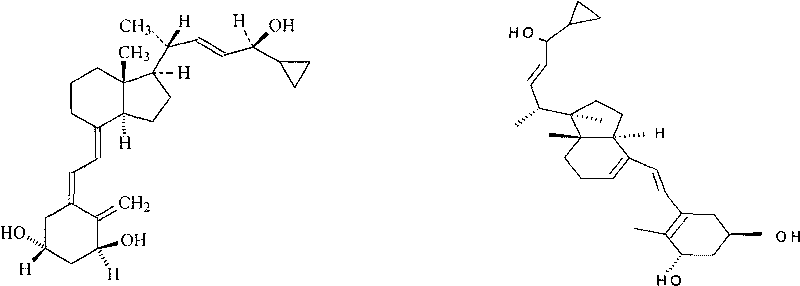
Method for determining content of compound by utilizing relative correction factor
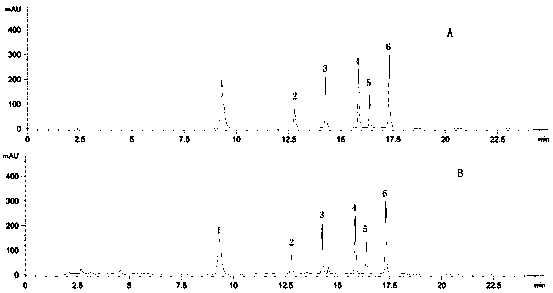
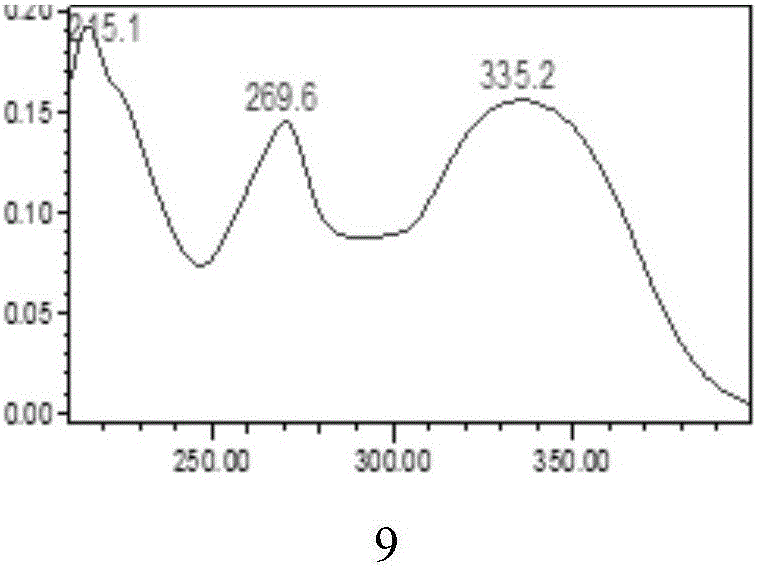
The invention relates to a method for determining the content of a compound easy to generate configuration conversion in a solution by utilizing high-efficiency liquid chromatography, comprising the following steps of: measuring the peak areas of a converted isomer and an unconverted isomer of a sample solution of the compound to be measured, multiplying the peak area of the converted isomer by arelative correction factor C and then adding the peak area of the unconverted isomer so that the marked content of the compound to be measured is calculated according to the result. When used for measuring the marked content of calcipotriol in a calcipotriol solvent, the method obtains a satisfactory result. The method has the advantages of simplicity, practicality and accurate result.
Owner:CHONGQING HUAPONT PHARMA
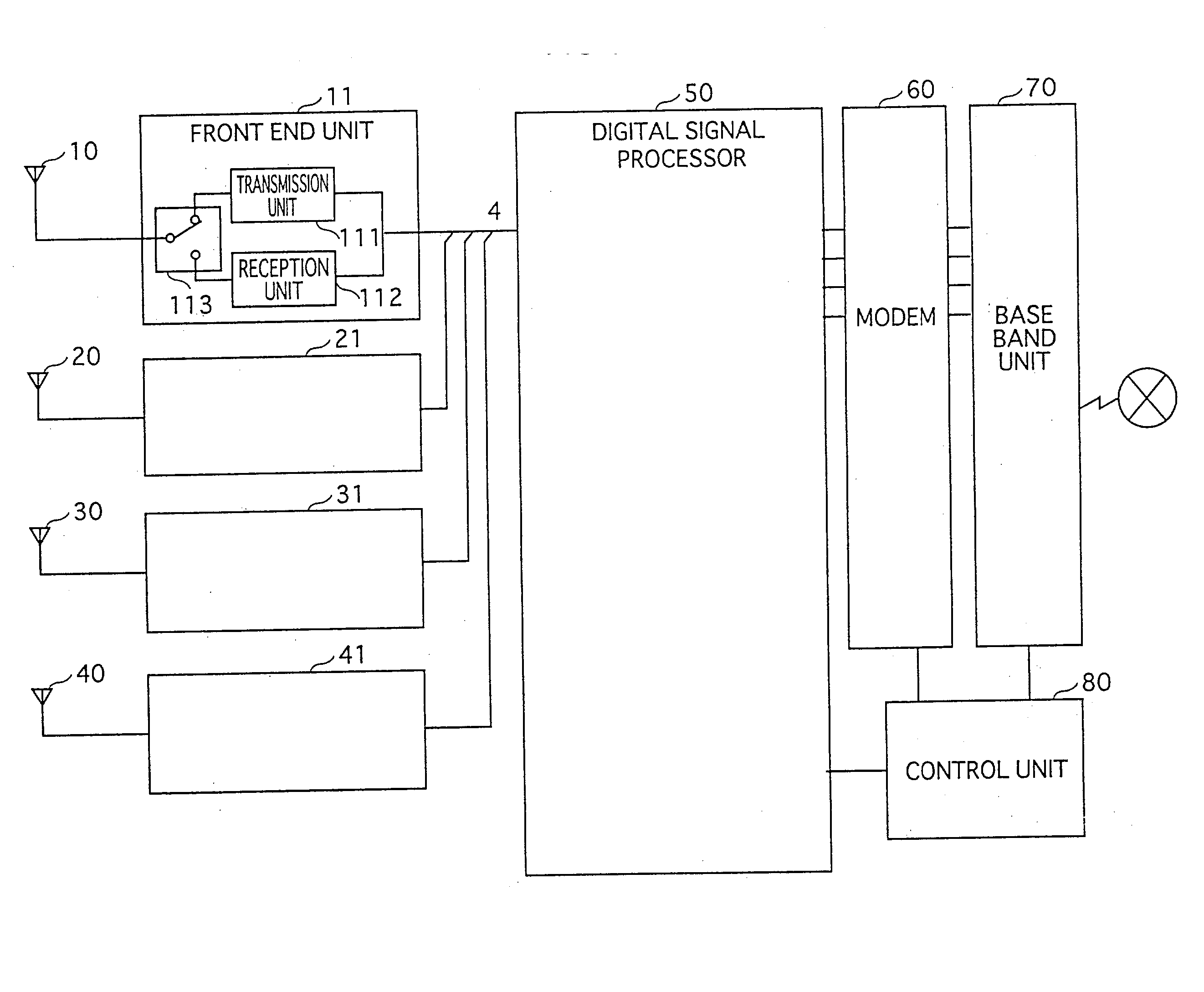
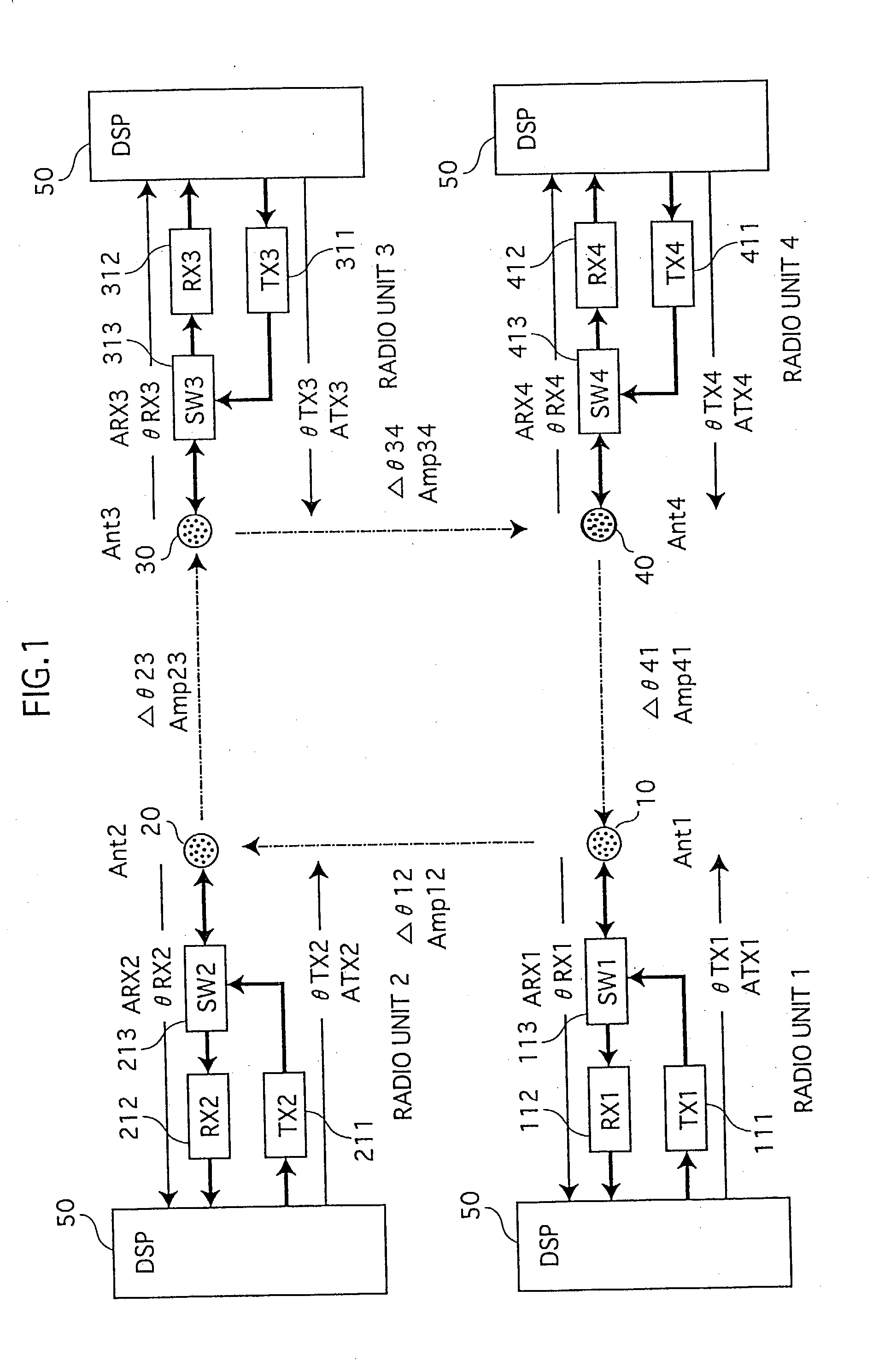
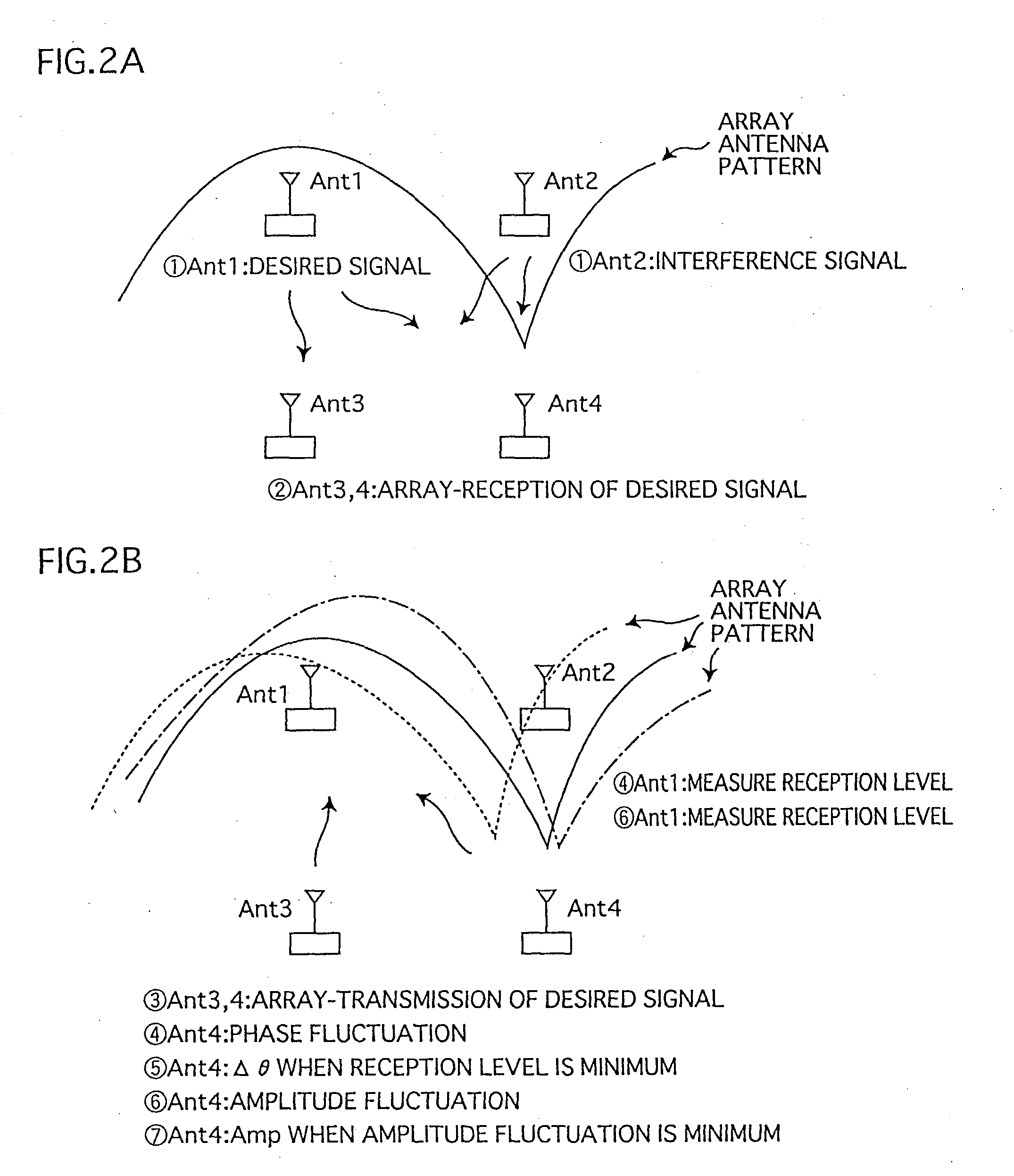
Adaptive array device, wireless base station and cellular telephone
InactiveUS20030096638A1Reduce circuit sizeTransmitters monitoringReceivers monitoringAudio power amplifierEngineering
Memory (237) stores relative correction values that indicate the differences of transfer characteristics between a) a radio unit made up of transmission circuit (211) and reception circuit (212), and b) a radio unit made up of transmission circuit (221) and reception circuit (222). Correction control unit (239), by means of phase shifter (240) and amplifier (241), uses the relative correction values to correct transmission signals. In similar fashion, the adaptive array apparatus and the radio base station perform corrections in order that identical array antenna patterns are formed at times of reception and transmission.
Owner:HERA WIRELESS
Method for correcting measurement error and electronic component characteristic measurement apparatus
InactiveUS6960920B2Easily and automatically expressedSuppress mutationResistance/reactance/impedenceElectrical testingObservational errorMeasurement device
A high-precision, multi-port compatible, relative correction method and apparatus for correcting measurement errors covering an increase in the number of ports of a non-coaxial electronic component, in which a relative correction adapter 31 is provided that is formed of a two-port network connected to each port of a production test fixture 5B adjacent to a measurement apparatus. The relative correction adapter has a characteristic that modifies the electrical characteristics generated by the production test fixture 5B having an electronic component under test mounted thereon into electrical characteristics generated by a standard test fixture 5A having the electronic component under test mounted thereon. An error factor of the relative correction adapter 31 is identified from a standard test fixture measurement value and a production test fixture measurement value of a correction data acquisition specimen 11B. A production test fixture measurement value of the electronic component under test 11A is corrected with the error factor of the relative correction adapter 31 to thereby obtain the standard test fixture measurement value of the electronic component under test 11A which is assumed to be obtained when the electronic component under test 11A.
Owner:MURATA MFG CO LTD
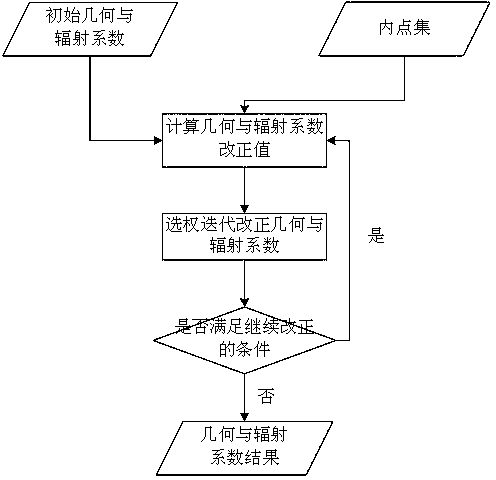
Remote-sensing image relative correction method and system integrating geometry and radiation
ActiveCN104050643ARealize integrationImprove calibration accuracyImage enhancementMaterial analysis using microwave meansImage calibrationTotal least squares
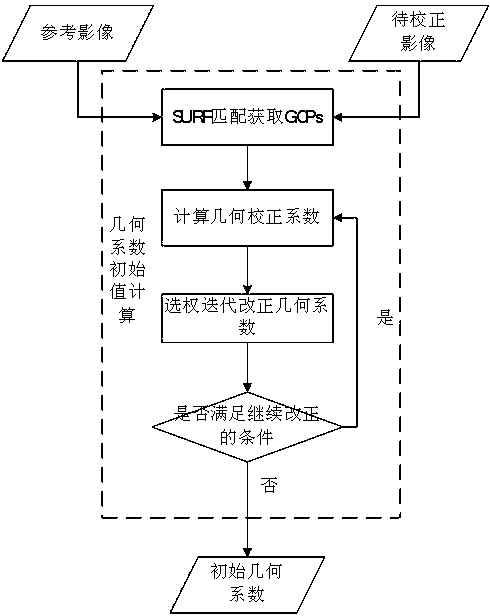
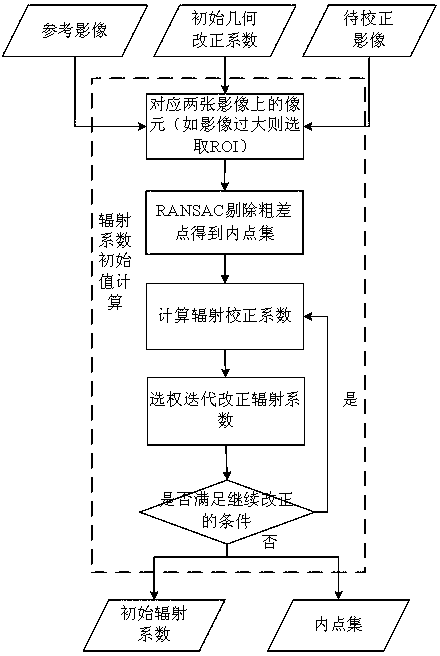
The invention discloses a remote-sensing image relative correction method and system integrating geometry and radiation. The method includes the steps of generating a control point on an image by means of combination of automatic matching and gross error detection, calculating an initial value of a geometry correction coefficient, detecting radiation invariant of the image to be corrected, calculating the initial value of a radiation correction coefficient, putting a geometric correction model into a linear radiation correction equation, building an error equation integrating geometry and radiation coefficients, calculating a correction coefficient by means of a weighing total least square method, solving the error equation to obtain a correction coefficient modification value and iterating and optimizing the correction coefficient modification value. According to the method, the geometry coefficient and the radiation coefficient in image correction can be calculated simultaneously, and the method lives up to the objective law, is scientific and can further improve correction precision.
Owner:HUAZHONG NORMAL UNIV
Methods for separation and content determination of chlorogenic acid type components in gynura procumbens
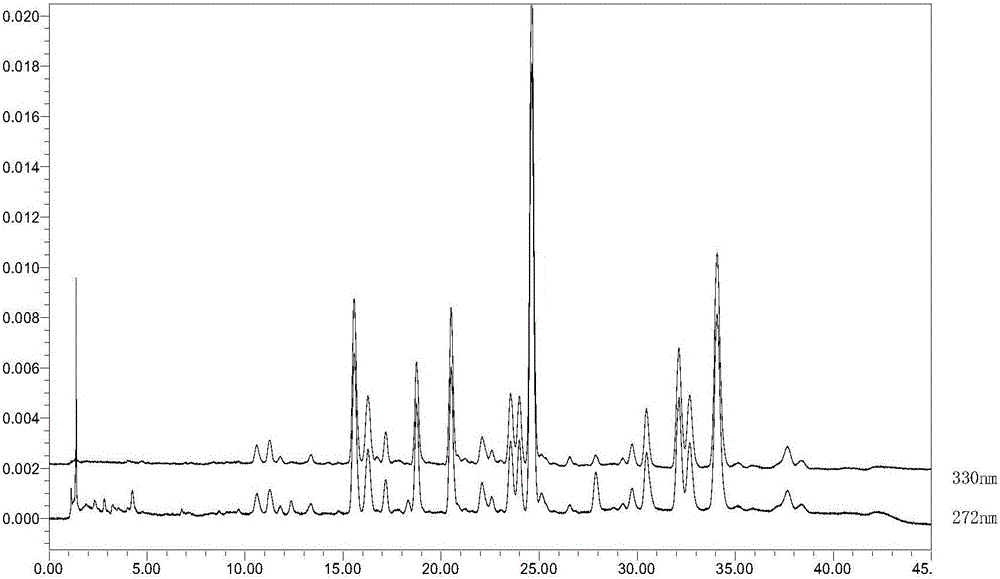
The invention discloses two methods for quantitative determination of chlorogenic acid, neochlorogenic acid and three isochlorogenic acids in gynura procumbens extract. The two methods comprise the steps that 1, the best chromatogram conditions are determined; 2, reference substances of the neochlorogenic acid, the chlorogenic acid and the three isochlorogenic acids are obtained, methyl alcohol is added to the reference substances, and then a reference solution is prepared; 3, the gynura procumbens extract is obtained, methyl alcohol is added to the gynura procumbens extract, and after filtering, a sample is obtained; 4, the reference solution and the sample solution are precisely absorbed, chromatography sample introduction is conducted, and the contents of the chlorogenic acid, the neochlorogenic acid and the three isochlorogenic acids are measured; 5, or, only a chlorogenic acid reference solution is prepared in the step 2, the chlorogenic acid is used as a reference substance, the relative correction factors of the isochlorogenic acid C, the isochlorogenic acid A, the isochlorogenic acid B and the neochlorogenic acid are 1.21, 1.12, 1.07 and 0.92, and the contents of the five substances are measured. According to the two methods, the chlorogenic acid is used as reference, fk / s between the chlorogenic acid and other components is established, the content of each component is calculated, the external standard method and the method for quantitative analysis of multi-components by a single marker are similar in accuracy and reliability, and therefore a brand-new mode is provided for evaluating the quality of gynura procumbens more authentically.
Owner:谭玉莲
Quality control method of rhizoma gastrodiae, decoction piece thereof and rhizoma gastrodiae extract
The invention relates to a quality control method of rhizoma gastrodiae, a decoction piece thereof and rhizoma gastrodiae extract. Based on high-performance liquid chromatography, by the aid of a one-standard multi-measurement analysis method, the contents of gastrodin components of gastrodin, p-hydroxybenzyl alcohol, parishin A, parishin B, parishin C and parishin E in the rhizoma gastrodiae arecalculated by relative correction factors, the low-cost gastrodin with stable property serves as an internal standard substance, and six effective components in the rhizoma gastrodiae are simultaneously measured. The multi-index quality control method is high in detection sensitivity, rapid in analysis and good in stability, can objectively, comprehensively and accurately evaluate the quality of the rhizoma gastrodiae, the decoction piece thereof and the rhizoma gastrodiae extract, is of great significance for ensuring the curative effect of the rhizoma gastrodiae and can solve the problems that parishin component reference substances are unstable, high in price and the like, and the quality of the rhizoma gastrodiae, the decoction piece thereof and the rhizoma gastrodiae extract cannot becomprehensively controlled.
Owner:王信
An adaptive correction method for channel mismatch of GNSS antenna array
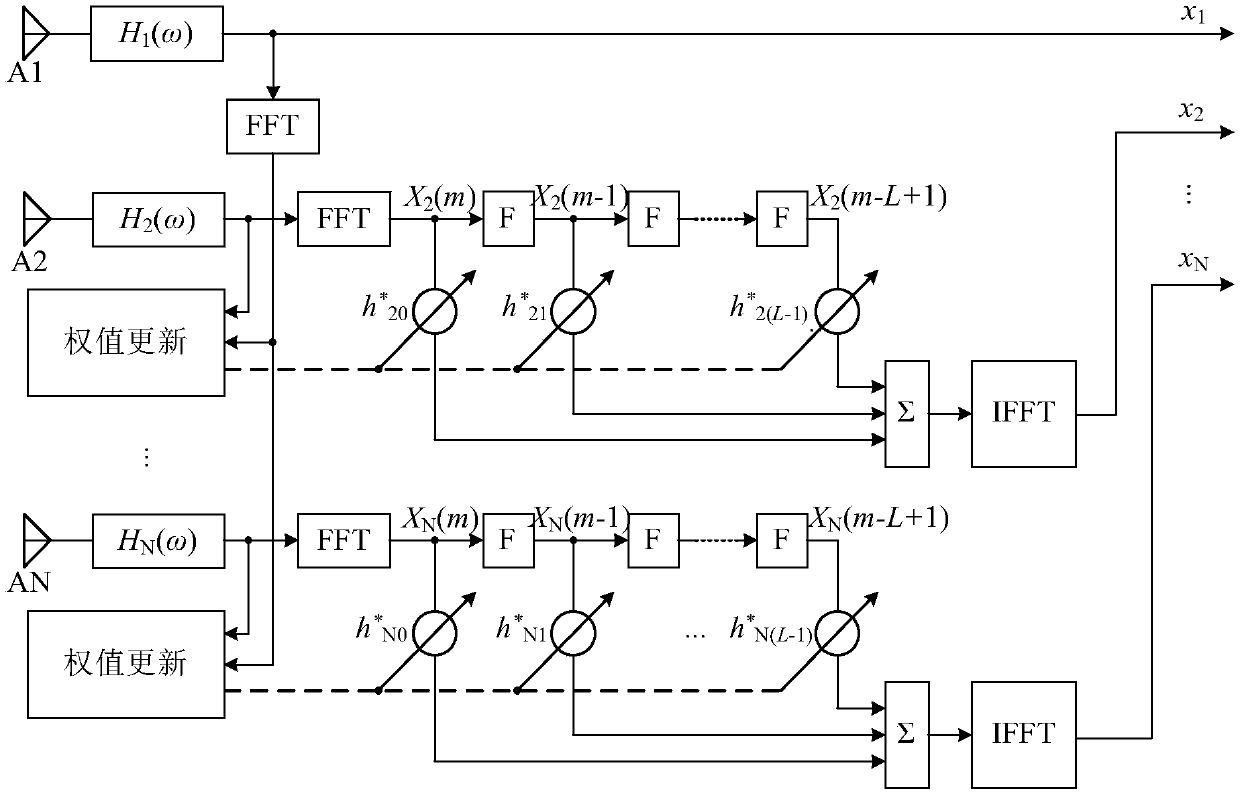
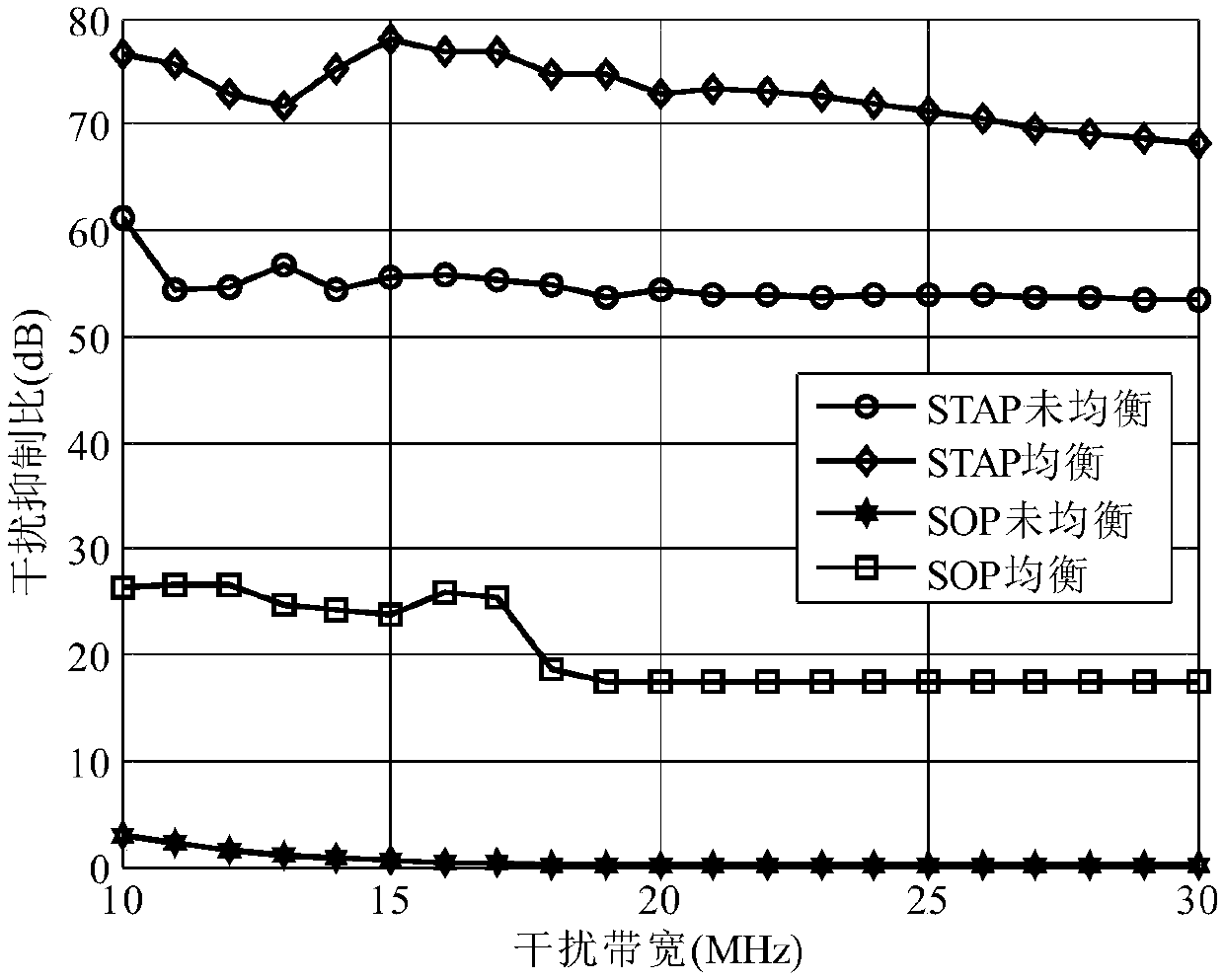
InactiveCN109104389AImprove consistencyAchieve relative correctionReceivers monitoringSatellite radio beaconingEngineeringArray element
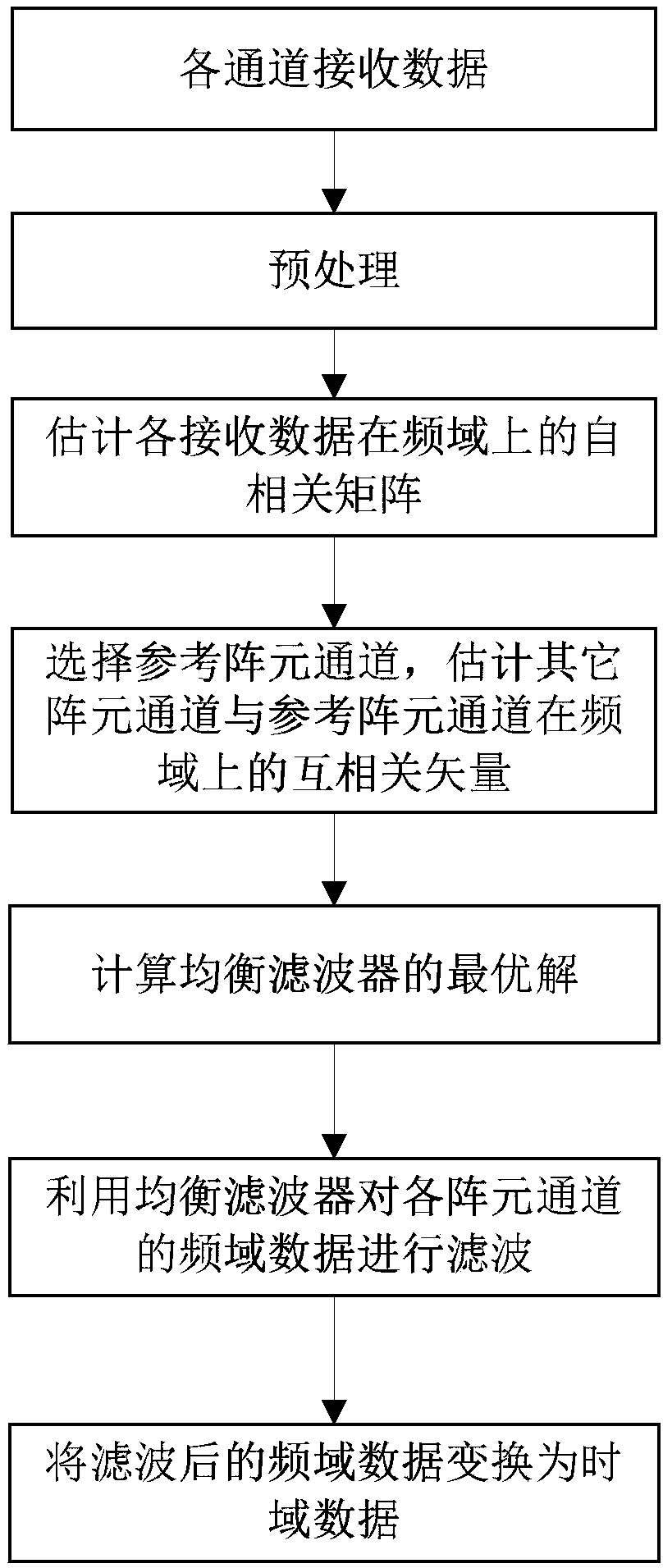
The invention relates to the technical field of array signal processing, in particular to an adaptive correction method for channel mismatch of a GNSS antenna array. The method comprises the followingsteps: (S1) converting the received data of each array element channel of the GNSS antenna array into frequency domain; (S2) estimating an autocorrelation matrix of the received data in the frequencydomain; 3) selecting a reference array element channel and estimating that cross-correlation vector of other array element channel and the reference array element channel in the frequency domain; (4)calculating the optimal solution h (n) of the equalization filter according to the LMS algorithm; (5) filtering the frequency domain data of each array element channel by using an equalization filter; (S6) converting the frequency domain data filtered by each array element channel in the step (S5) to the time domain by inverse Fourier transform. The invention takes the data of the reference arrayelement channel as a reference signal, corrects the received data of other array element channels, and realizes the purpose of improving the consistency of the channel data, that is, realizes the relative correction of the channel.
Owner:NAT UNIV OF DEFENSE TECH
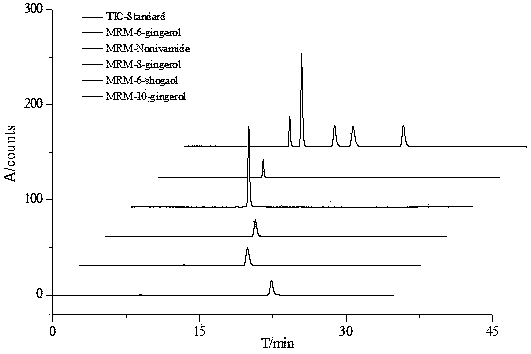
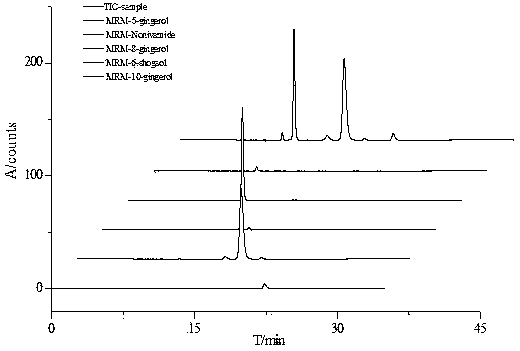
Method for measuring content of gingerol in ginger medicinal materials and preparations thereof
ActiveCN108414665AAccurate evaluationHigh sensitivityComponent separationMass spectrometry detectorHplc mass spectrometry
The invention discloses a method for measuring the content of gingerol in ginger medicinal materials and preparations thereof. By virtue of the separation and analysis technology of ultra-high performance liquid chromatography in series with a triple quadrupole mass spectrometer detector and an ultraviolet detector as well as a one-measurement multi-evaluation method, a pure substance N-vanillyl nonane amide reference substance which does not exist in a sample, has stable properties and is easy to obtain is adopted as an internal reference substance so as to establish a relative correction factor between the component and four gingerol components in the sample, thereby realizing measurement of the contents of the four gingerol components in the ginger medicinal materials and the preparations thereof by virtue of calculation of the correction factor. The method disclosed by the invention is simple in operation, high in sensitivity, accurate and efficient and low in cost, can be used forobjectively and accurately evaluating the quality of the ginger medicinal materials and the preparations thereof, can be used for quality control, can solve the problem that the quality of the medicinal materials and the preparations thereof can not be objectively and reasonably controlled due to the lack of reference substances, and has important significance for controlling quality and ensuringcurative effects.
Owner:河南省纳普生物技术有限公司
Detection method of content of polysaccharide in cordyceps sinensis mycelium powder and preparation thereof
PendingCN108490091AObjective evaluationComprehensive evaluationComponent separationPeak areaPrecipitation
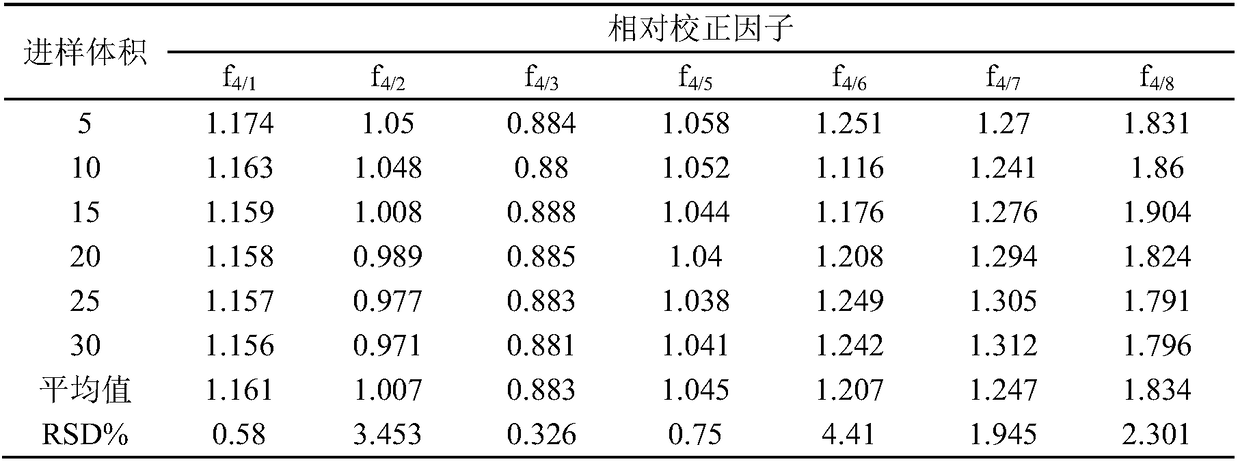
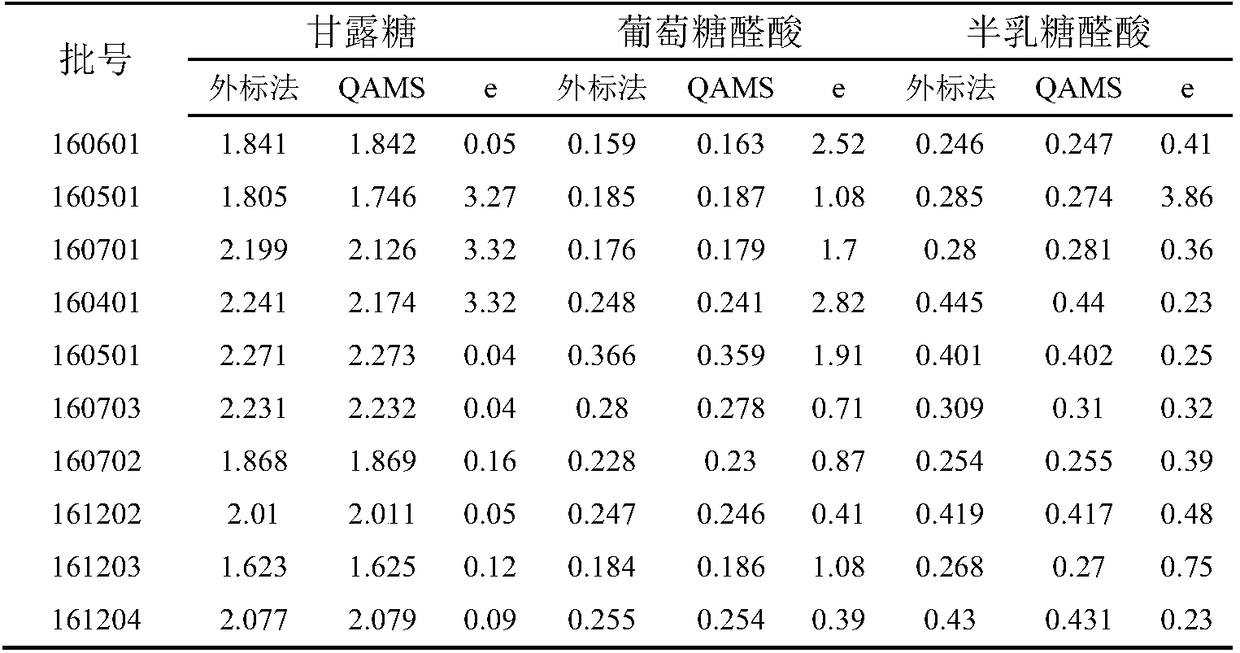
The invention relates to a detection method of the content of polysaccharide in cordyceps sinensis mycelium powder and a preparation thereof. The detection method has the characteristics of high detection sensitivity and good repeatability. The method basically comprises the following steps: (1), preparation of a reference liquor: accurately weighing an appropriate amount of monosaccharide, addingultrapure water to dissolve the monosaccharide, and performing derivation, so as to obtain a mixed reference solution; (2), preparation of a test liquor: weighing an appropriate amount of cordyceps sinensis mycelium powder, obtaining crude polysaccharide through a water-extraction and alcohol-precipitation method, and performing acid hydrolysis and PMP derivation, so as to obtain a test solution;(3), calculation of relative correction factors: weighing the mixed relative correction factor obtained in the step (1), and calculating the relative correction factors of glucose to mannose, glucuronic acid, galacturonic, galactose, xylose, arabinose and fucose; (4), determination of the content of the test liquor: taking the test solution in the step (2), detecting the peak area of each monosaccharide through a high performance liquid chromatography, and calculating the contents of effective components through the relative correction factors.
Owner:JIANGXI GUOYAO PHARMA LLC +1
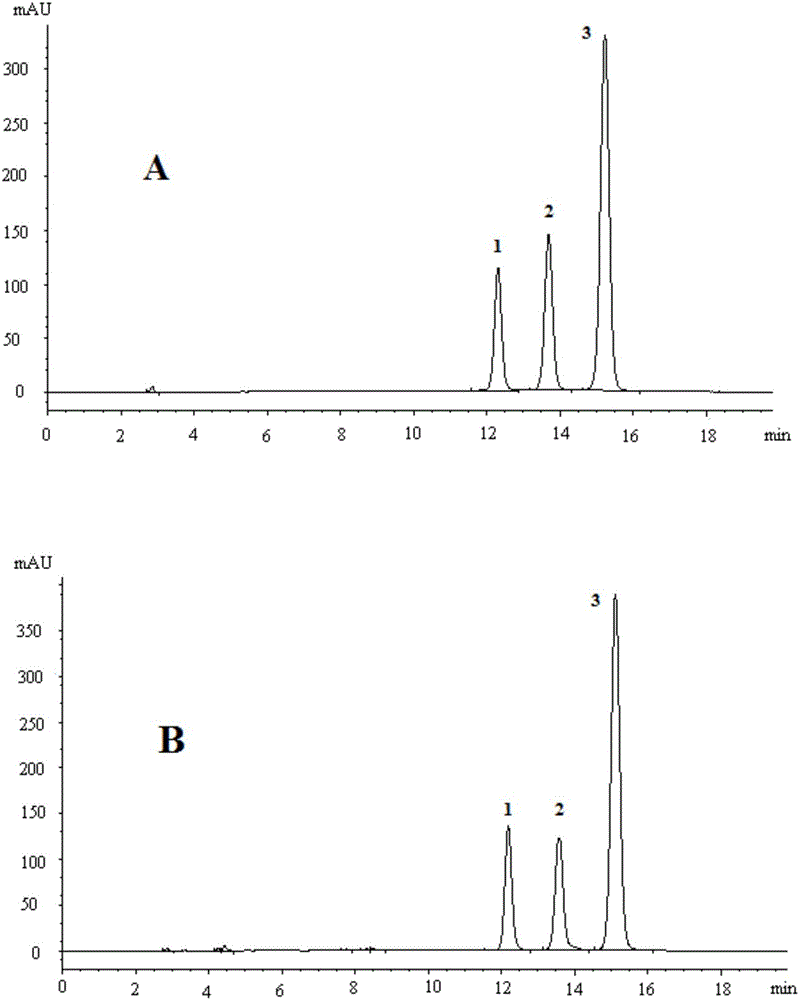
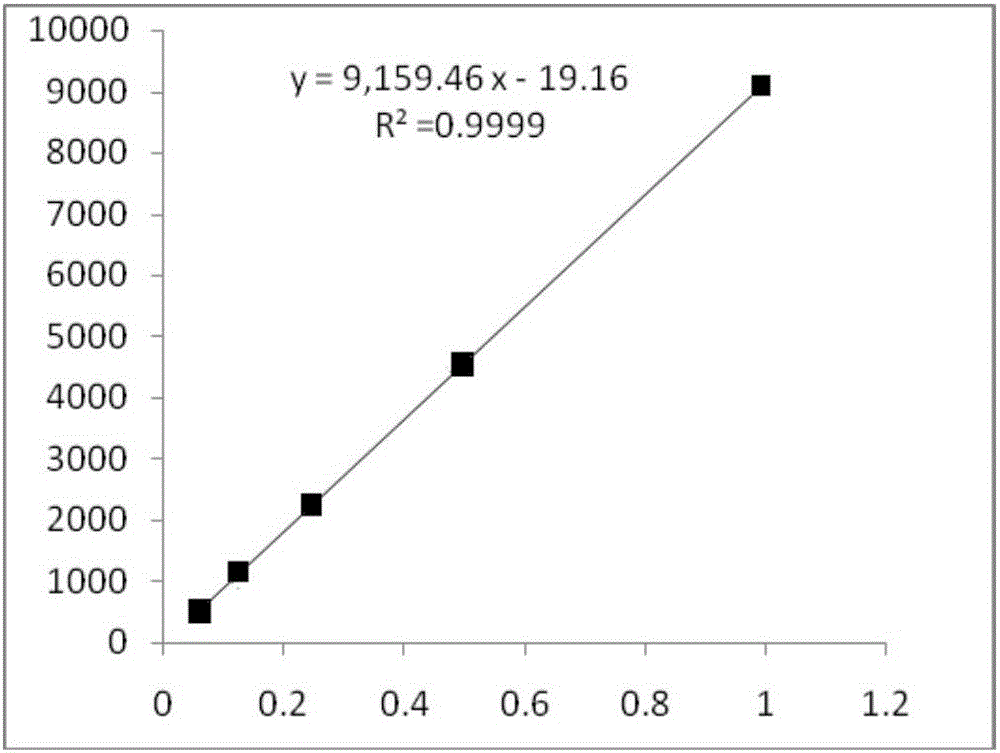
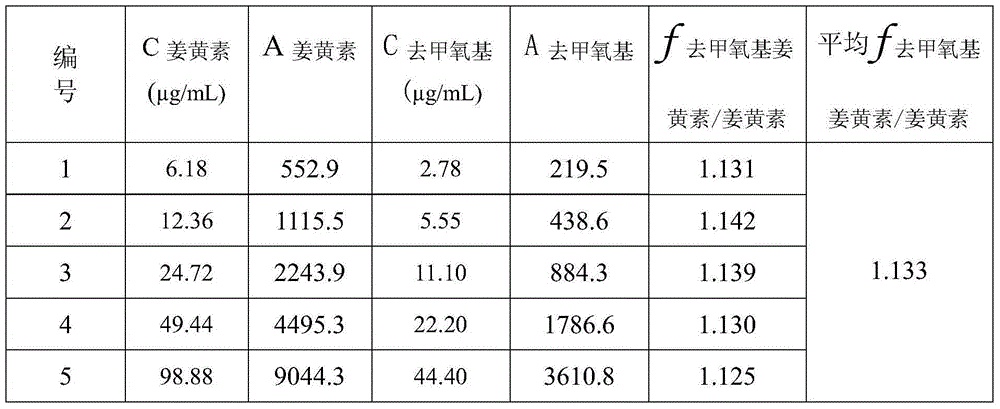
Method for calculating contents of three effective components in curcuma longa product through relative correction factor
InactiveCN105116061AReduce the number of weighings with the balanceReduce wasteComponent separationMedicineBisdemethoxycurcumin
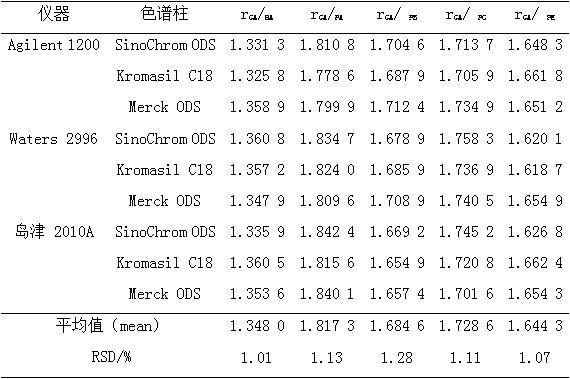
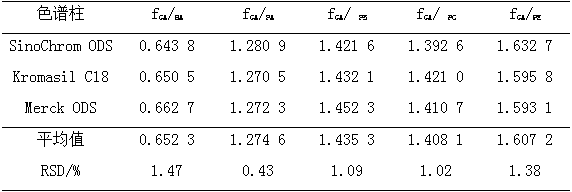
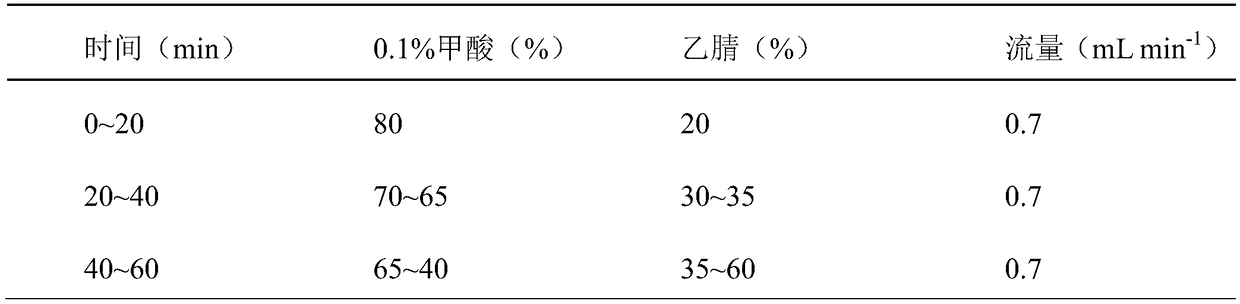
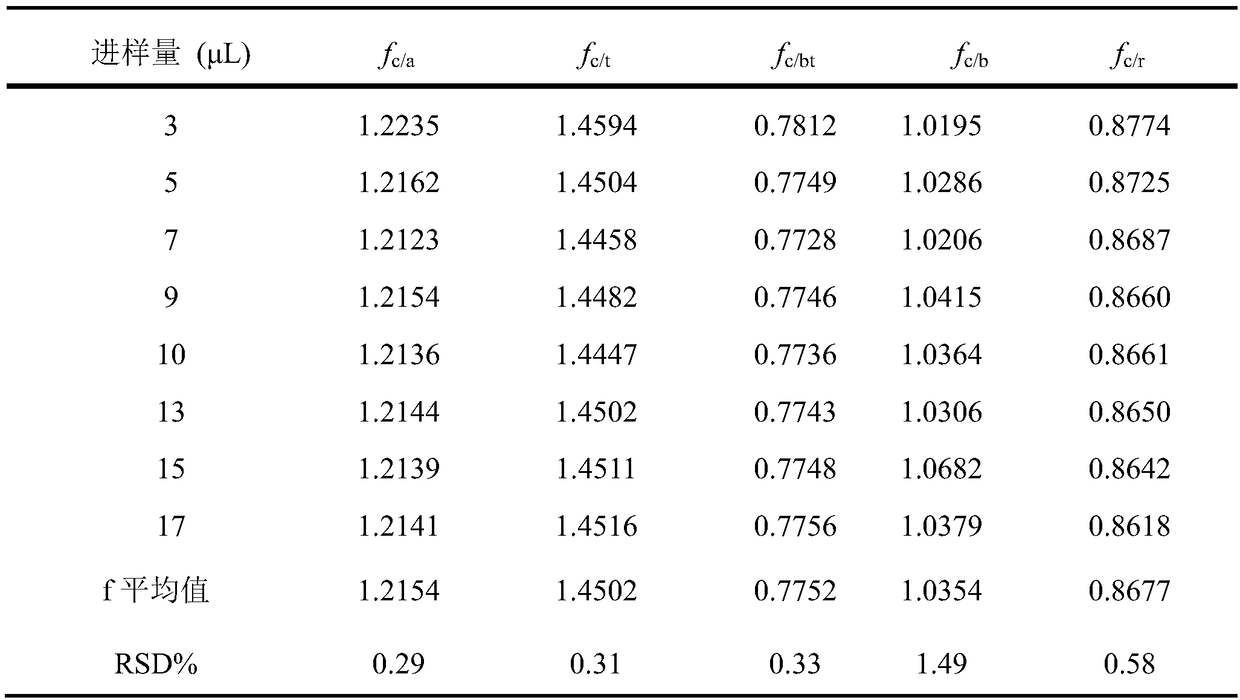
The invention relates to a method for calculating the contents of three effective components in a curcuma longa product through a relative correction factor, and belongs to the technical field of drug quality determination. The method comprises: (1) preparing a reference substance stock solution; (2) preparing a reference substance solution; (3) preparing a sample solution; (4) determining through high performance liquid chromatography; (5) determining the relative correction factor value; (6) calculating the curcumin content; and (7) calculating the demethoxycurcumin content and the bisdemethoxycurcumin content. According to the present invention, the relative correction factor is used to detect the contents of the three effective components in the curcuma longa product, such that the demethoxycurcumin reference substance preparation and the bisdemethoxycurcumin reference substance preparation in each experiment are eliminated, the detection cost is reduced, and the detection efficiency is improved; the relative correction factor f is verified under different detection equipment, different chromatographic columns, different detection wavelengths, different column temperatures, different flow rates and different flow relative ratios; and the method has characteristics of rapidness, efficiency, high precision, low cost and the like, and is the feasible and effective detection method.
Owner:SICHUAN NEO GREEN PHARMA TECH DEV
Method for extracting total flavonoid extract from desmodium styracifolium and method for measuring content of effective components in desmodium styracifolium
ActiveCN105079084AOvercoming the shortageContent monitoringComponent separationPlant ingredientsSchaftosideInternal standard
The invention provides a method for measuring the content of vicenin-1, vicenin-3 and schaftoside in desmodium styracifolium medicinal materials, a total flavonoid extract from desmodium styracifolium and a preparation thereof at the same time by using a single reference substance, namely the schaftoside. The method comprises the steps that the schaftoside in total flavonoid extract from desmodium styracifolium or the preparation is taken as an internal standard substance, wherein the content of effective components of the schaftoside is high and easy to be obtained; the relative correction factor of the schaftoside and the vicenin-1 and the vicenin-3 is calculated, and the relative correction factor is taken as a constant, the content of the vicenin-1, the vicenin-3 and the schaftoside in the total flavonoid extract from desmodium styracifolium can be measured at the same time, the quality of the total flavonoid extract from desmodium styracifolium or the preparation in industrial manufacture can be scientifically, sensitively and quickly monitored, meanwhile the problem of the shortage of reference substances is overcome.
Owner:WUHAN OPTICS VALLEY HUMANWELL BIO PHARMA
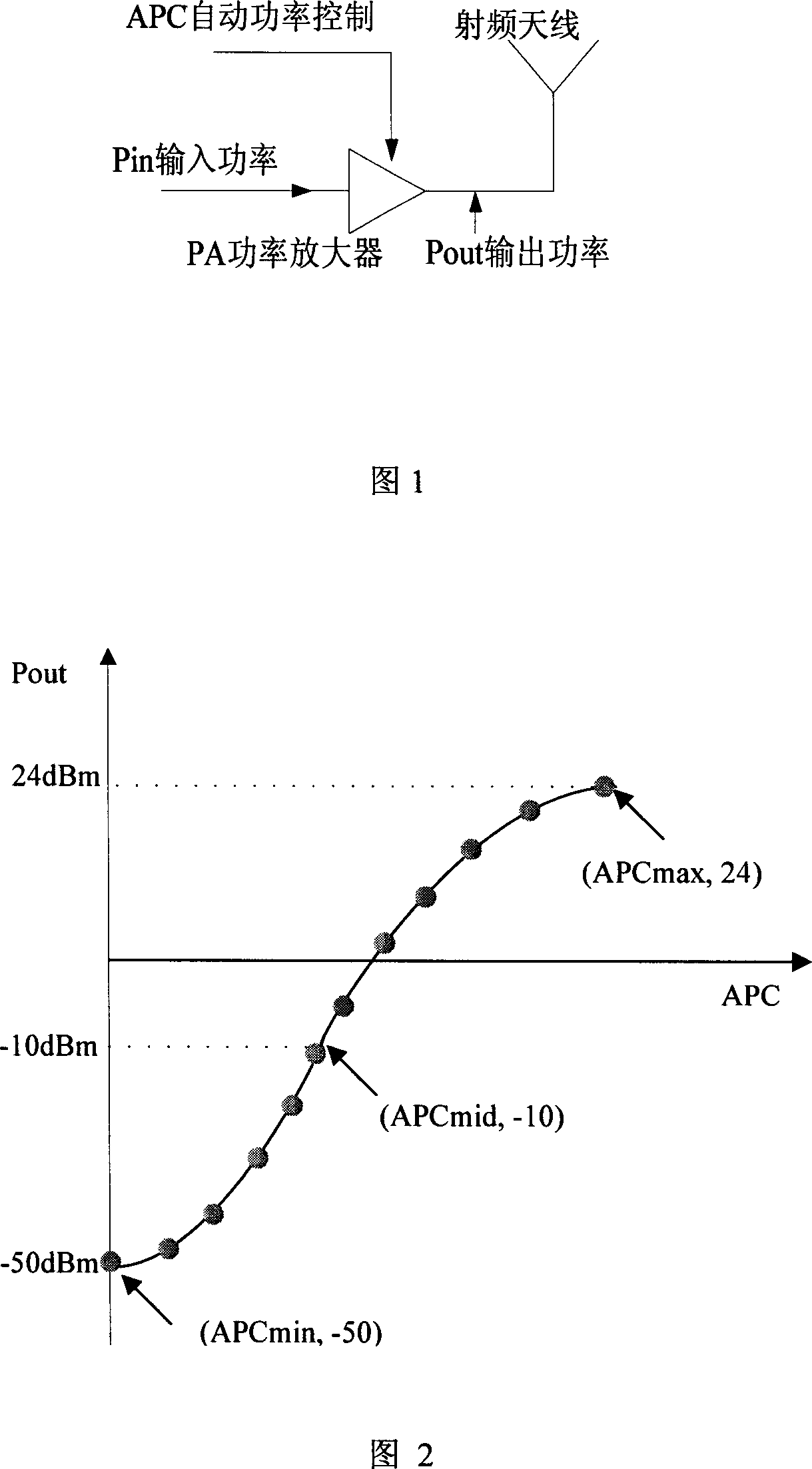
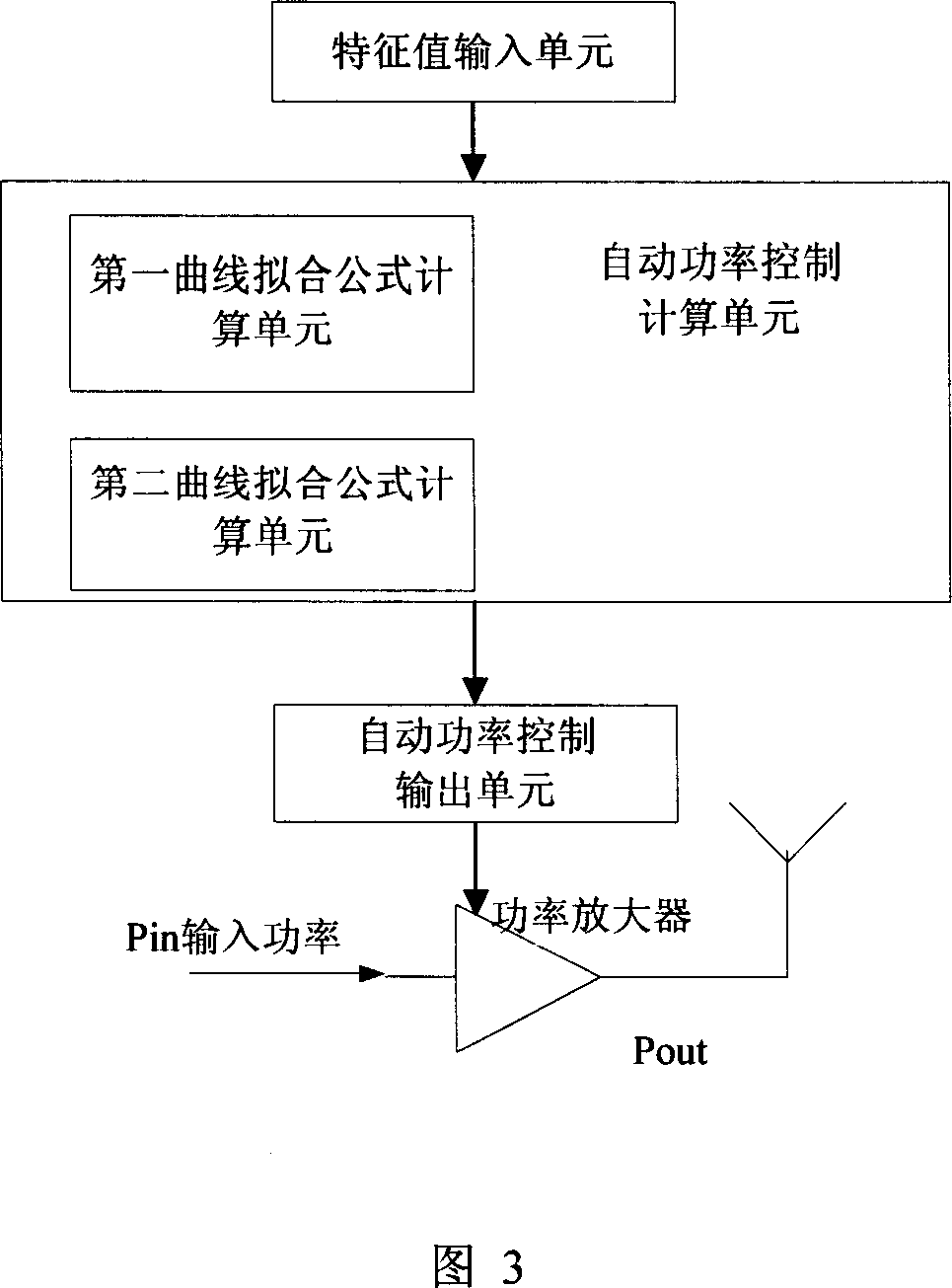
Production calibration method and apparatus for TD-SCDMA radio frequency power amplifier
InactiveCN1972149AImprove calibration accuracyMeet the precision requirementsTransmission control/equalisingTransmission monitoringAudio power amplifierTD-SCDMA
This invention discloses one TD-SCDMA radio frequency amplifier production and correction method and device, wherein, it selects upper limit characteristics value in the radio power amplifier output power correction range and down value and the third characteristics value in between and corrects their relative correction point to get the above values relative automatic power control values.
Owner:VIMICRO CORP
Fructus psoraleae medicinal material detection method based on quantitative analysis of multi-components by single marker
InactiveCN107389838AComprehensive evaluationAccurate evaluationComponent separationClinical efficacyRetention time
The invention provides a fructus psoraleae medicinal material detection method based on quantitative analysis of multi-components by single marker. The fructus psoraleae medicinal material detection method comprises the following steps: firstly, establishing an HPLC (High Performance Liquid Chromatography) content determination method for 10 main medicinal components in fructus psoraleae; taking psoralen as an internal-standard reference object, calculating relative correction factors of the other 9 active components and considering system applicability and method repeatability of the relative correction factors; carrying out chromatographic peak positioning according to relative retention time; calculating the content of each component to be determined by combining using the relative correction factors and proving that results have no remarkable difference through mutual verification of the quantitative analysis of multi-components by single marker and an external-standard method. By adopting the method provided by the invention, the difficulties that a reference substance has low the defect of high cost and is not easy to obtain and the like are overcome; the content of index components is calculated through the relative correction factors and the chromatographic peak positioning and synchronous determination of various medicinal substances in the fructus psoraleae is realized; the cost can be saved and the operation can be simplified; the efficiency is improved, the detection sensitivity is high, the stability is good and a determination result is accurate and reliable; the fructus psoraleae medicinal material detection method provided by the invention has great significance of in controlling the quality of the fructus psoraleae and ensuring the clinical effect.
Owner:ZHEJIANG UNIV
Method for detecting compounds in toad venom
InactiveCN108982719ASolve expensive and rare problemsQuality improvementComponent separationToad VenomReference product
The invention discloses a method for detecting compounds in a toad venom. The method for detecting the compounds in the toad venom comprises the steps of the preparation of a reference solution, the preparation of a test solution, determination of chromatographic conditions, determination of relative correction factor, determination of component content of the test solution, and the like. Cinobufagin is selected as an internal standard substance. The method for detecting the compounds in the toad venom not only solves a problem that a reference product is expensive and rare, but also complieswith a principle of environmentally friendly and green Chinese medicine, and the cinobufagin with high content, stable peak shape and low price is selected as the internal standard substance. At the same time, the content of six compounds in the toad venom is measured, the detection efficiency is greatly improved and the quality of the toad venom is comprehensively controlled. According to a detection object to determine a reasonable mobile phase, a chromatographic column, an elution procedure, a detection wavelength, a column temperature and other chromatographic conditions, under the selected chromatographic conditions, the content of the compounds in the toad venom can be quickly determined, precision is high, reproducibility is good, stability is good, recovery rate is high and measurement results are accurate .
Owner:山东宏济堂制药集团股份有限公司
Rapid qualitative and quantitative detection method for seven phenylethanoid glycoside components in medicinal material Herba Cistanche
ActiveCN107727763ASimple methodImprove accuracyComponent separationPreparing sample for investigationRetention timeBiology
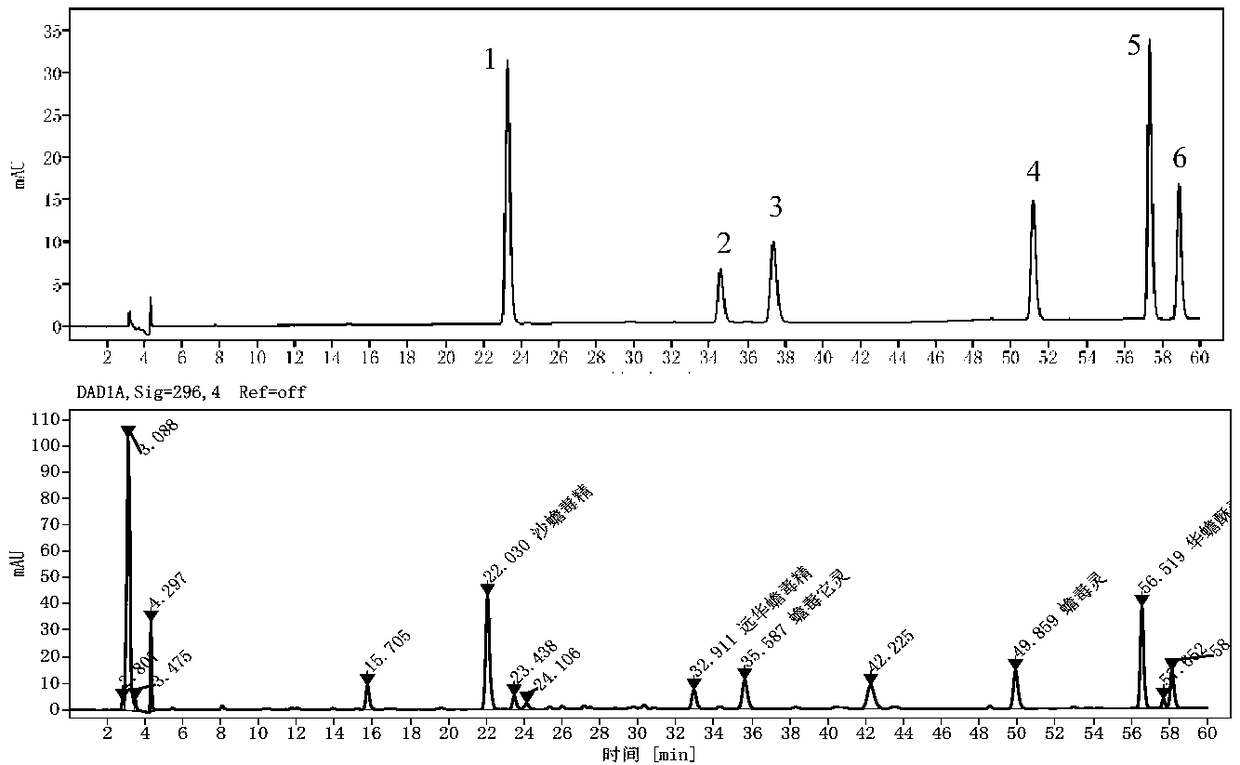
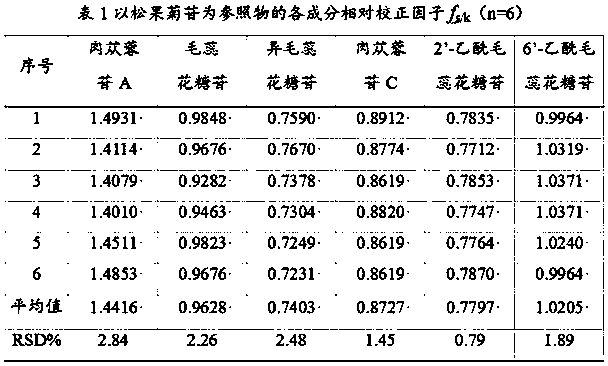
The invention discloses a rapid qualitative and quantitative detection method for seven phenylethanoid glycoside components in the medicinal material Herba Cistanche. The method can be used for determining the contents of active components in the medicinal material Herba Cistanche. The method comprises the following steps: with echinacoside as a reference substance, establishing relative calibration factors for echinacoside and verbascoside / isoacteoside / cistanoside C / cistanoside A / 2'-acetylverbascoside / 6'-acetylverbascoside by using a percent absorption coefficient; and then carrying out detection by using liquid chromatography, qualitatively determining the components according to relative retention time of each chromatographic peak and the peak of the reference substance, and quantitatively determining the components according to the peak areas of the components and the relative correction factors. Therefore, the method can simultaneously determine the contents of seven phenylethanoid glycoside components including echinacoside in the medicinal material Herba Cistanche by using only one reference substance, i.e., echinacoside.
Owner:NINGXIA MEDICAL UNIV
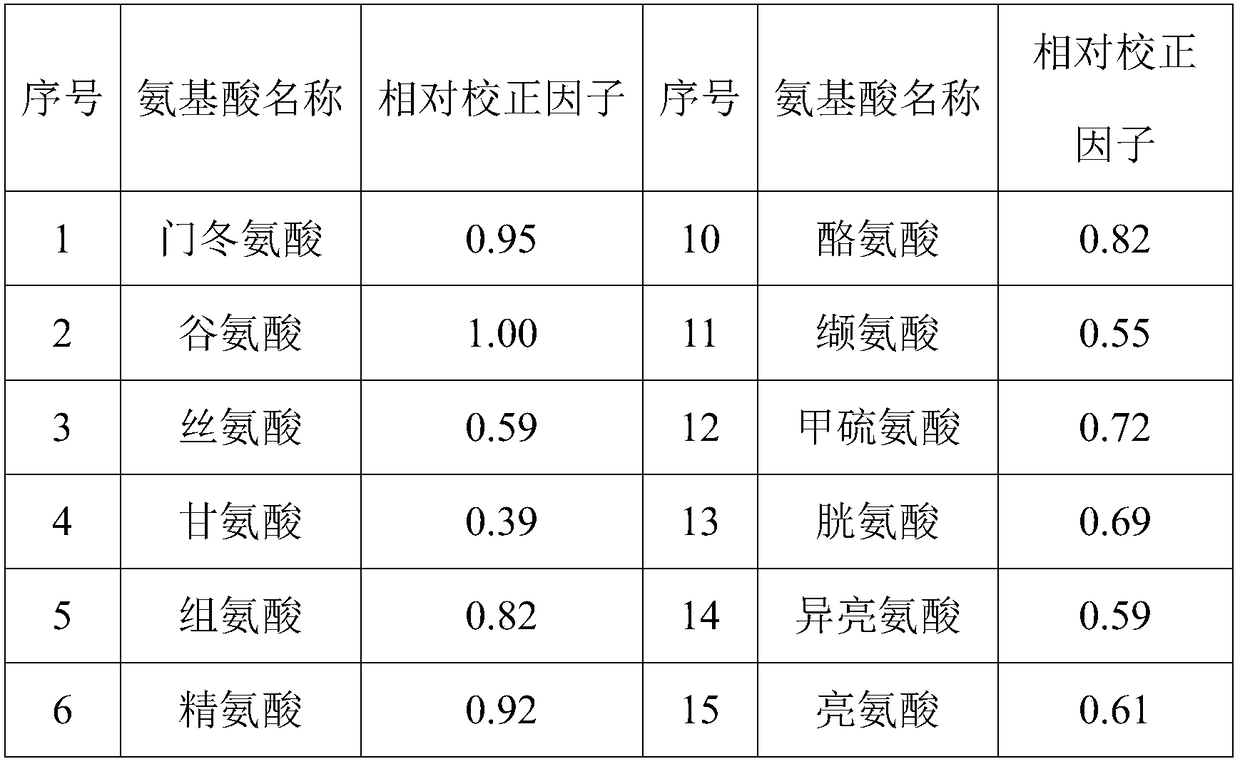
Method for quantificationally detecting total amino acids of transfer factor capsule
ActiveCN108828085ASimultaneous determination of amino acid contentReduce overlap ratioComponent separationRetention timeTotal amino acids
The invention discloses a method for detecting total amino acid content of a transfer factor capsule. The method solves the technical problem that the prior art only detects free amino acids and cannot detect bonded amino acids of the polypeptide substance and a transfer factor is a multi-component biochemical drug in the amino acid detection process so that the separation degree of amino acid peaks and unknown component peaks is poor. Through a one-measurement multi-evaluation method, based on a glutamic acid reference substance, 18 common amino acids are quantitatively detected so that the technical problems of more reference materials, a high detection cost and complicated operation are solved. The method utilizes glutamic acid as an internal reference substance. Through the systematicverification research, relative correction factors and relative retention time of other 17 kinds of amino acids are determined and quantitative detection of 18 kinds of amino acids is realized. The method is accurate and reliable, has good separation effects and strong specificity and can effectively detect and monitor total amino acids in the transfer factor capsule.
Owner:XIAN DAQING PHARMA FACTORY JINHUA ENTERPRISE GROUP CORP
Method for measuring content of ligustilide in ligusticum wallichii medical material through one-measurement multi-evaluation method
The invention relates to a method for measuring the content of the ligustilide in a ligusticum wallichii medical material through a one-measurement multi-evaluation method. Pure substance psoralen reference substance which does not exist in a testing sample and is stable in performance and easy to obtain is adopted as an internal standard, the relative correction factor of the component and the ligustilide in the medical material is established, the content of ligustilide is calculated through the correction factor, and the liquid chromatography is adopted for carrying out measurement. The specific method comprises the following steps that firstly, chromatographic conditions and a system suitability test are made; secondly, mother liquor of the psoralen reference substance is prepared; thirdly, a solution of a test article is prepared; fourthly, measurement is carried out. Psoralen is taken as the mother liquor of the reference substance and is stable in performance and easy to obtain, the internal function relation and the proportion relation of the active ingredients of the traditional Chinese medicine are used, the ingredient content of the ligustilide can be measured directly, the measurement efficiency is improved, and operation is convenient.
Owner:上海海虹实业(集团)巢湖今辰药业有限公司
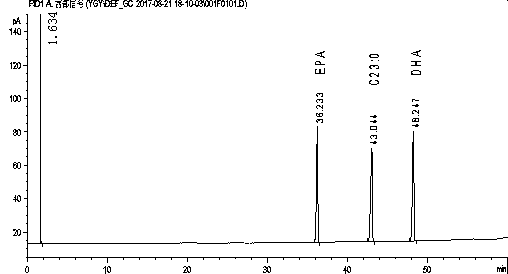
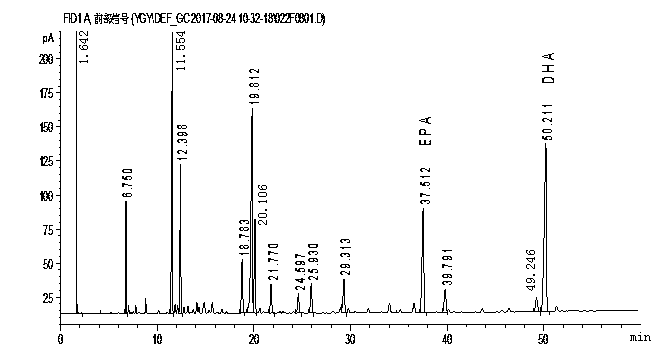
Method for rapidly determining contents of EPA and DHA in cod liver oil
InactiveCN108051506AAvoid harmOperational securityComponent separationCod liver oilInternal standard
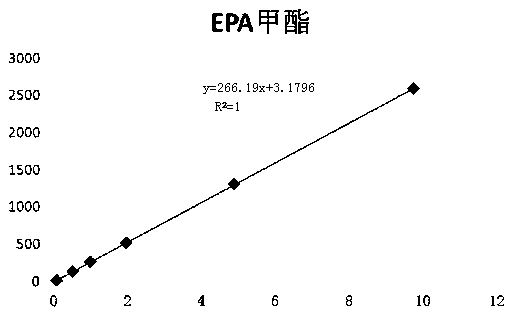
The invention relates to a method for rapidly determining the contents of omega-3 unsaturated fatty acids including EPA and DHA in cod liver oil. According to the method, EPA and DHA in cod liver oilis subjected to pre-column derivatization at first, the EPA and the DHA are determined by using gas chromatography and the contents of the EPA and the DHA in the cod liver oil are calculated by usinga correction factor-added internal standard method. The method comprises the following steps: 1, a pre-column derivatization reaction of the cod liver oil for preparation of a fatty acid methyl estersolution; 2, determination of relative correction factors of EPA methyl ester and DHA methyl ester; 3, gas chromatographic determination of the contents of the EPA and the DHA; and 4, calculation of the contents of the EPA and the DHA by using the correction factor-added internal standard method.
Owner:ZHOUSHAN INST FOR FOOD & DRUG CONTROL
Quality control method for 10 index components of traditional Chinese medicine conquering prescription for treating lung cancer
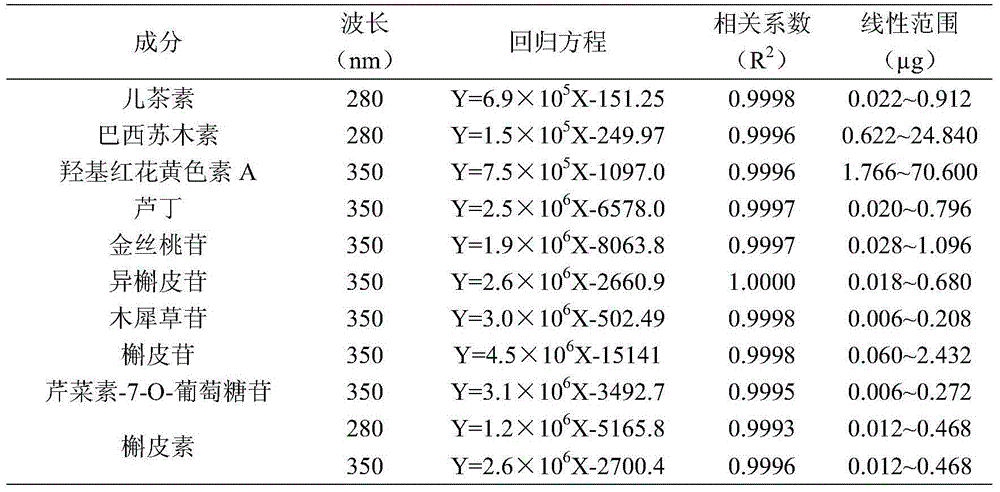
The invention discloses a quality control method for 10 index components of a traditional Chinese medicine conquering prescription for treating lung cancer. The method comprises the following steps: (1) extracting a test solution for preparing medicinal materials of the conquering prescription or preparation; (2) detecting hybrid reference substances catechinic acid, hyperin, rutin, isoquercitrin, galuteolin, quercitrin, apigenin-7-O-glucoside and quercetin chromatogram by adopting a liquid chromatography, and respectively calculating relative correction factors fkm of other components relatively to the quercetin by employing the quercetin as an internal standard compound; and (3) detecting the chromatogram of the test solution of the conquering prescription by the liquid chromatography, calculating the content according to the regression equation by employing the quercetin as an internal standard, and calculating the contents of other 9 components according to the peak area and the relative correction factors. Compared with the internal standard method, the method is accurate and reliable in data, high in detection sensitivity, and simple and convenient in method, and has relatively good adaptability on different model numbers of high performance liquid chromatographs and chromatographic columns; the problem that the medicinal material of the conquering prescription and the preparation quality of the medicinal material cannot be objectively and reasonably controlled due to shortage of reference substances can be solved; and the quality control method has important significance on control of the medicinal materials of the conquering prescription and the preparation quality and guarantee of the curative effect.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Quality evaluation method and application of wild chrysanthemum medicinal material
ActiveCN110579548AClear ingredientsFast contentComponent separationChrysanthemum FlowerQuality control
The invention relates to a quality evaluation method and application of a wild chrysanthemum flower medicinal material. According to the method, a fingerprint spectrum of the wild chrysanthemum flowermedicinal material is constructed, the fingerprint spectrum is analyzed through a mode recognition method, and the qualitative and quantitative information of multiple components in the wild chrysanthemum flower medicinal material in a once-detection-multi-evaluation method is reflected comprehensively. The method comprises the following steps: preparing a mixed reference solution and a test solution, then respectively detecting the mixed reference substance solution and the test solution by adopting high performance liquid chromatography, recording fingerprint spectrums of the mixed reference substance solution and the test solution, recording the peak areas of the 12 components by adopting the once-detection-multi-evaluation method, calculating a relative correction factor, and calculating the contents of the 12 components by using the correction factor. According to the method, the contents of the 12 components in the wild chrysanthemum flowers are simultaneously determined by highperformance liquid chromatography, operation is simple and convenient, the reproducibility is high, multiple index components in the wild chrysanthemum flowers are quantified rapidly and accurately at the same time, the separation degree of the 12 components is high, and a theoretical basis is laid for quality control of the wild chrysanthemum flowers.
Owner:河南省纳普生物技术有限公司
Detection method of phenolic acid compounds in compound radix salviae miltiorrhizae tablet
InactiveCN103776908AFully reflect the qualitySimple methodComponent separationSalvianolic acid BResource saving
The invention provides a detection method of phenolic acid compounds in a compound radix salviae miltiorrhizae tablet, and the detection method comprises the following steps: preparing a standard solution, a reference solution and a to-be-tested product solution, passing through a high performance liquid chromatography for detection; then calculating a relative correction factor of the phenolic acid compounds relative to salvianic acid A sodium; and using a formula IV shown in the specification too obtain the phenolic acid compound content in the compound radix salviae miltiorrhizae tablet. According to the detection method, by detection of one compound (salvianic acid A sodium), simultaneous determination of multiple compounds (salvianic acid A, protocatechuic aldehyde, rosmarinic acid, lithospermic acid and salvianolic acid B) can be realized, and the detection method is simple, rapid, accurate, good in universality, labor, and material and financial resource saving, and can more fully reflect the quality of the compound radix salviae miltiorrhizae tablet.
Owner:GUANGZHOU BAIYUSN HUTCHISON WHAMPOA CHINESE MEDICINE
Method for testing acetic acid distillation tower top composition by using gas chromatographic method
InactiveCN104655744AShorten the timeImprove accuracyComponent separationGas liquid chromatographicDistillation
The invention belongs to the technical field of chemical engineering analysis, and in particular provides a method for testing acetic acid distillation tower top composition by using a gas chromatographic method. The method comprises the following steps: (1) preparing a detection instrument and a reagent; (2) configuring standard solutions; (3) testing relative correction factors; and (4) testing samples. According to the method, reasonable gas phase chromatography configuration is chosen, and a proper chromatography condition is set, and three analysis projects of the samples are analyzed at a time according to the difference of water, acetic acid and sec-butyl acetate in the samples; moreover, according to the method, time-saving and labor-saving effects are achieved and the accuracy is high.
Owner:SHAANXI YANCHANG PETROLEUM GRP
Method for evaluating quality of gnaphalium affine by quantitative-analysis-of-multi-components-by-single-marker (QAMS) method
ActiveCN109991328ASimplified Quantitative Assay MethodReduce testing costsComponent separationChlorogenic acidClinical efficacy
The invention discloses a method for evaluating the quality of gnaphalium affine by a quantitative-analysis-of-multi-components-by-single-marker (QAMS) method. The method comprises the steps as follows: firstly, an HPLC content determination method for four main medicinal components in the gnaphalium affine is established; chlorogenic acid is used as an internal reference substance; relative correction factors of other three active components are calculated; the system applicability and the method reproducibility of the relative correction factors are inspected; chromatographic peak positioning is performed according to relative retention time; the content of each component to be determined is calculated in combination with the relative correction factors; and content determination resultsare proved to have no obvious difference by virtue of mutual verification of the QAMS method and an external standard method. The method disclosed by the invention solves the problems that the reference substance is high in cost and difficult to obtain; by virtue of the relative correction factors and the chromatographic peak positioning, the content of index components is calculated; synchronousdetermination of various medicinal substances in the gnaphalium affine is realized, so that the cost can be reduced, the operation is simplified, and the efficiency is improved; the method is high indetection sensitivity and high in stability; the determination results are accurate and reliable; and the method has a great significance on the quality control of the gnaphalium affine and the guarantee of the clinical effects.
Owner:XIAN MEDICAL UNIV
Triethylamine content determination method
ActiveCN107941926AEliminate distractionsImprove accuracyComponent separationLinear correlationInternal standard
The invention relates to the field of wastewater treatment, and discloses a triethylamine content determination method, which comprises: (1) preparing more than two different standard samples containing an internal standard substance and triethylamine and having different components, wherein the standard samples are used for determining a relative correction factor f1 and a calibration curve; (2)adding the internal standard substance to a triethylamine solution to be determined to obtain a sample to be determined; and (3) determining the internal standard substance peak areas and the triethylamine peak areas in the standard samples, the sample to be determined, and the triethylamine solution to be determined through chromatography, wherein f1, r, and the triethylamine content are calculated according to the following formulas defined in the specification. According to the present invention, the method has the simple operation, is especially suitable for the determination of the triethylamine content in the wastewater with large difference in the triethylamine content, can accurately calculate the relative correction factor f1 and the linear correlation coefficient r, can eliminatethe interference on the determination result due to the internal standard substance possibly existing in the wastewater, and can effectively improve the accuracy of the determination result.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for evaluating quality of Herba Cirsii Setosi through quantitative analysis of multicomponents by single marker
InactiveCN109991327ASimplified Quantitative Assay MethodReduce testing costsComponent separationClinical efficacyActive component
The invention provides a method for evaluating quality of Herba Cirsii Setosi through quantitative analysis of multicomponents by a single marker. According to the method for evaluating the quality ofthe Herba Cirsii Setosi through the quantitative analysis of multicomponents by the single marker, firstly, a HPLC content determination method for four main medicinal components in the Herba CirsiiSetosi is established; rutin is used as an internal reference substance, relative correction factors of another three active components are calculated, and the system suitability and the method reproducibility of the relative correction factors are investigated; chromatographic peak positioning is carried out according to relative retention time, the relative correction factors are combined for calculating the content of all to-be-detected components, through the mutual verification of the quantitative analysis of multicomponents by the single marker and an external standard method, it is proved that no significant difference exists in determination results; the problems that reference substances are high in costs and are not easy to obtain are solved, the content of index components is calculated through the relative correction factors and the chromatographic peak positioning, and the simultaneous determination of multiple medicinal substances in the Herba Cirsii Setosi is realized; and the costs can be saved, the operation can be simplified, the efficiency is improved, the detection sensitivity is high, the stability is good, the determination results are exact and reliable, andthe method has great significance for ensuring the quality control and the clinical effects of the Herba Cirsii Setosi.
Owner:XIAN MEDICAL UNIV
HPLC measurement method for content of multi-index components in rhizoma corydalis pain relieving drop pills
InactiveCN105467039ARealize multi-index quality evaluationQuality improvementComponent separationPharmacy medicineTetrahydropalmatine
The invention discloses an HPLC measurement method for content of multi-index components in rhizoma corydalis pain relieving drop pills. A high performance liquid chromatography (HPLC) method is adopted; a mixed contrast solution is used as a contrast, and tetrahydropalmatine is used as a reference; meanwhile, 7 main components, including protopine, palmatine, dehydro-corydaline, tetrahydropalmatine, corydaline, imperatorin and isoimperatorin, in the rhizoma corydalis pain relieving drop pills are measured simultaneously. According to the method, QAMS is realized; measurement of multiple components is realized by adopting relative correction factors among the components; the quality of the multiple components of a traditional Chinese medicine is controlled, thus realizing evaluation on quality of multiple indexes of the rhizoma corydalis pain relieving drop pills. The method is easy and convenient to operate, and quick; the detection cost and time are greatly reduced; the working efficiency and the practicability of the method are improved; the quality of traditional Chinese medicinal products can be controlled more effectively, comprehensively and accurately; stability and uniformity of the product quality are guaranteed, so that the aim of safe and effective taking of the product is fulfilled.
Owner:GANSU LONGSHENRONGFA PHARMACEUTICAL INDUSTRY CO LTD
Method for measuring contents of alkaloids, lignans and nucleosides in isatis root or preparation thereof by using quantitative analysis single-maker method
The invention discloses a method for measuring the contents of alkaloids, lignans and nucleosides in the isatis root or preparation thereof by using a quantitative analysis single-maker method. With low-cost stable-structure avaible alkaloids compound (R,S)-epigoitrin as a reference, relative correction factors with lignans compound clemastanin B and nucleoside compounds cytidine, uridine, vernine, and adenosine are established. Therefore, the contents of another five kinds of lignan and nucleoside contents with high content rates are calculated by using the correction factors only by measuring the content of the(R,S)-epigoitrin of the isatis root or preparation, thereby carrying out multi-indicator quality evaluation on the isatis root or preparation simply, conveniently, comprehensivelyand accurately. The quality controllability and curative effect stability of the product can be guaranteed; and the detection cost and time can be saved.
Owner:GUANGZHOU BAIYUSN HUTCHISON WHAMPOA CHINESE MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com