Method for evaluating quality of gnaphalium affine by quantitative-analysis-of-multi-components-by-single-marker (QAMS) method
A technology for quality evaluation and sagebrush, which is applied in measuring devices, instruments, scientific instruments, etc., can solve the problems of many varieties of standard products and complex content determination methods, so as to simplify the method, reduce the testing cost and testing time, and improve the efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
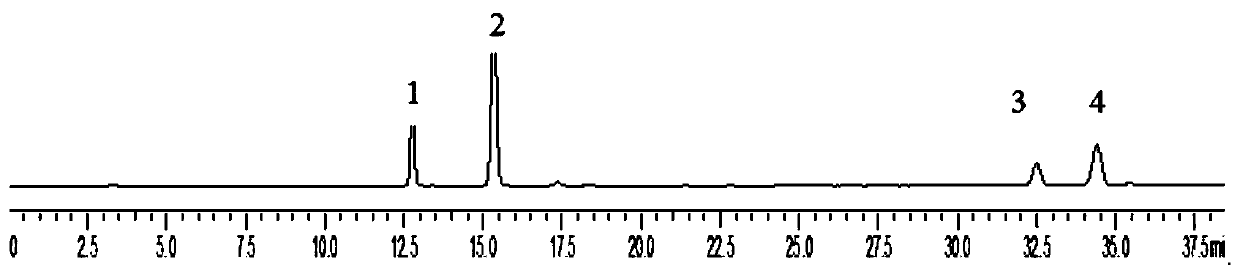
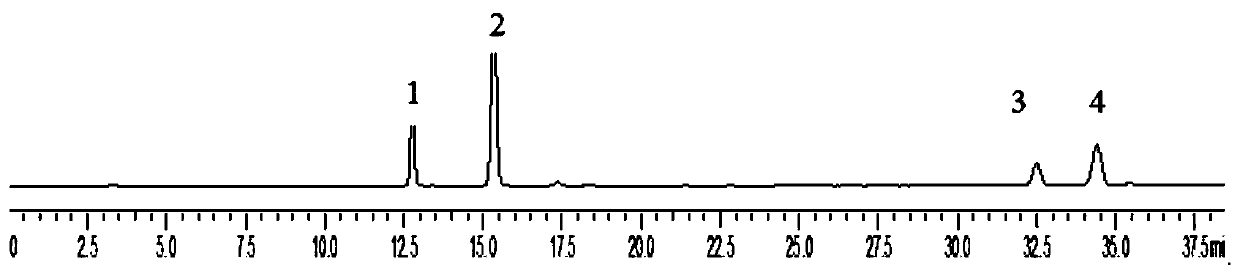
[0041] Preparation of the test solution: crush the sage grass, sieve through a 40-mesh sieve, accurately weigh the sieved powder of the sage grass, add methanol solution to constant volume, cold soak for 1h-10h, and filter through 0.22-0.45 μm microporous membrane, spare;
[0042]Take the sample solution prepared in step 2, accurately draw 10uL of each test solution, detect and analyze according to the HPLC chromatographic conditions in step 3, obtain a liquid chromatogram, perform peak positioning according to the relative retention time, and calculate each target compound in combination with the relative correction factor content. The results were compared with those calculated by the external standard method, and the content of each target compound was calculated. The present invention uses chlorogenic acid as an internal reference, calculates the relative correction factors of caffeic acid, isobracoside, and eupholzanthin, and investigates the reappearance of the relative...
Embodiment 1
[0046] Step 1, preparation of standard solution: take 5 mg of chlorogenic acid, isobracoside and eupholzanthin reference substance, and 10 mg of caffeic acid reference substance, weigh them accurately, place them in 10mL volumetric flasks, add methanol to dissolve, The volume was adjusted to the scale, and the mass concentration was prepared to be 0.52 mg / ml of chlorogenic acid, 1.06 mg / ml of caffeic acid, 0.53 mg / ml of isoverbacoside and 0.50 mg / ml of eupholzanthin. Accurately measure 1ml of the above-mentioned reference substance solution and place it in a 10ml volumetric flask, add methanol to the volume, and prepare the mass concentrations of chlorogenic acid 0.052mg / ml, caffeic acid 0.106mg / ml, isomacoside 0.053mg / ml and A mixed reference substance solution of 0.050mg / ml isoeuplavin;
[0047] Step 2, preparation of the test solution: Accurately weigh 0.1 g of the Herba skeletalum herb powder, put it in a 25 mL volumetric flask, add methanol to the volume, filter after col...
Embodiment 2
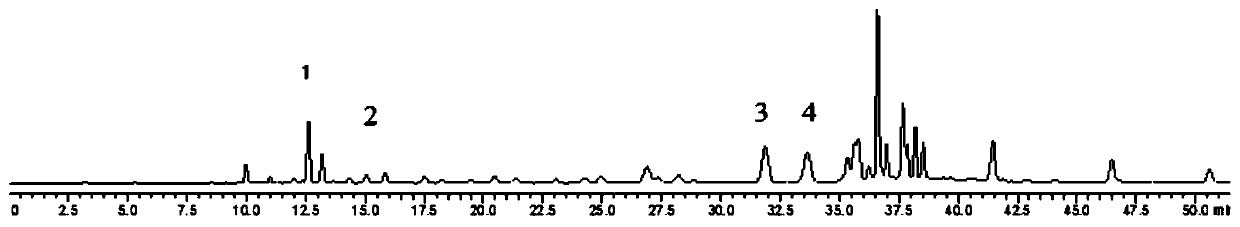
[0071] Step 1, preparation of standard solution: take 5 mg of chlorogenic acid, isobracoside and eupholzanthin reference substance, and 10 mg of caffeic acid reference substance, weigh them accurately, place them in 10mL volumetric flasks, add methanol to dissolve, The volume was adjusted to the scale, and the mass concentration was prepared to be 0.52 mg / ml of chlorogenic acid, 1.06 mg / ml of caffeic acid, 0.53 mg / ml of isoverbacoside and 0.50 mg / ml of eupholzanthin. Accurately measure 1ml of the above-mentioned reference substance solution and place it in a 10ml volumetric flask, add methanol to the volume, and prepare the mass concentrations of chlorogenic acid 0.052mg / ml, caffeic acid 0.106mg / ml, isomacoside 0.053mg / ml and A mixed reference solution of eupholzanthin 0.050mg / ml.
[0072] Step 2, preparation of the test solution: Accurately weigh 0.1g of the Herba sageria medicinal material powder, add 50mL of methanol, reflux for extraction for 1h, filter while hot and evapo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com