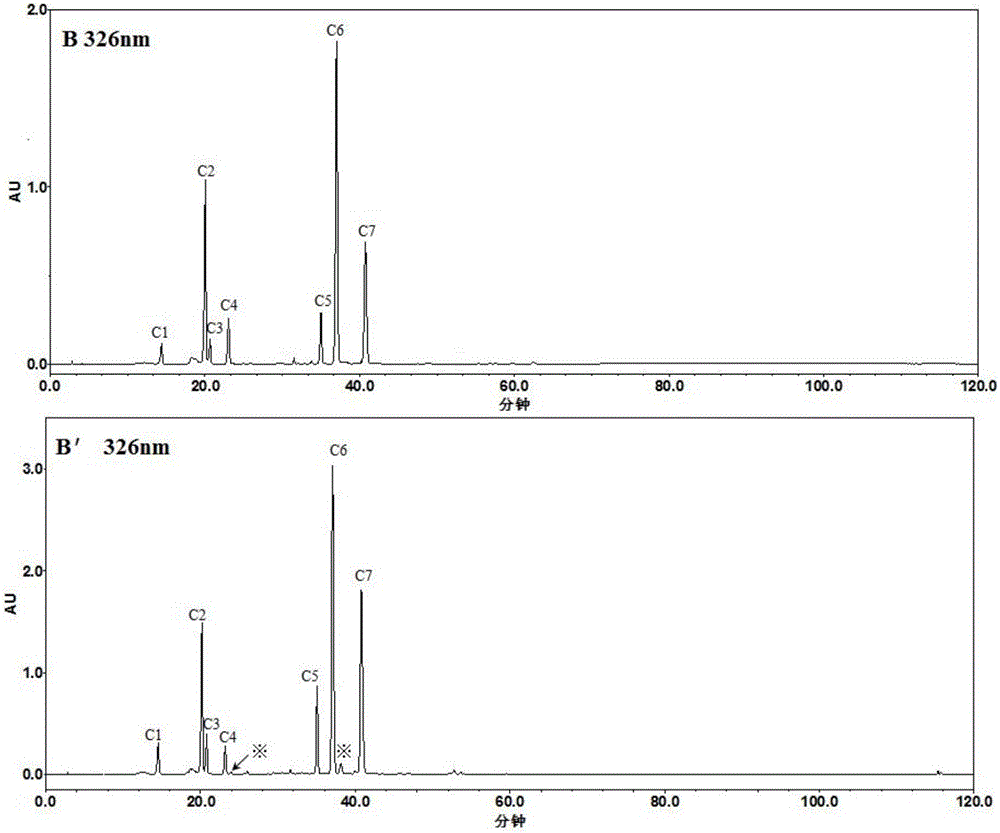
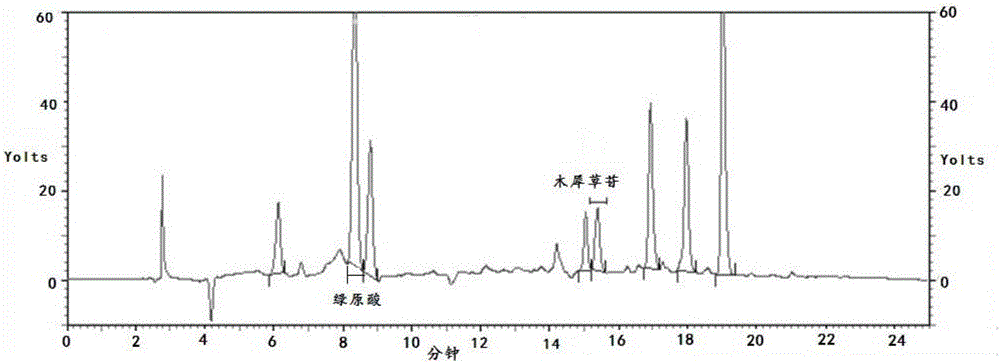
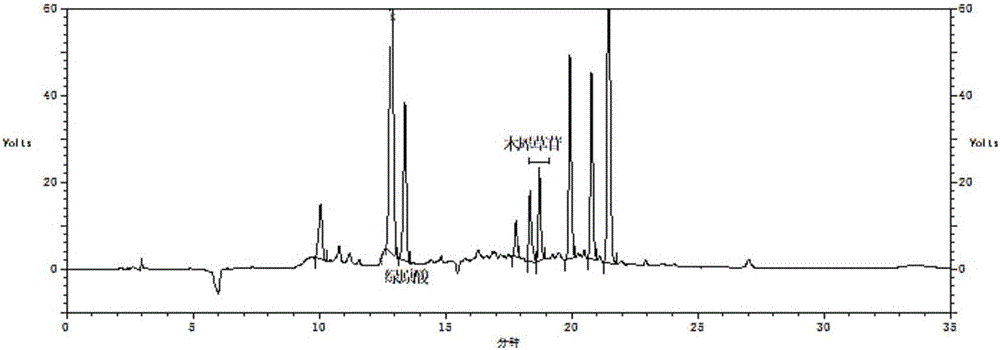
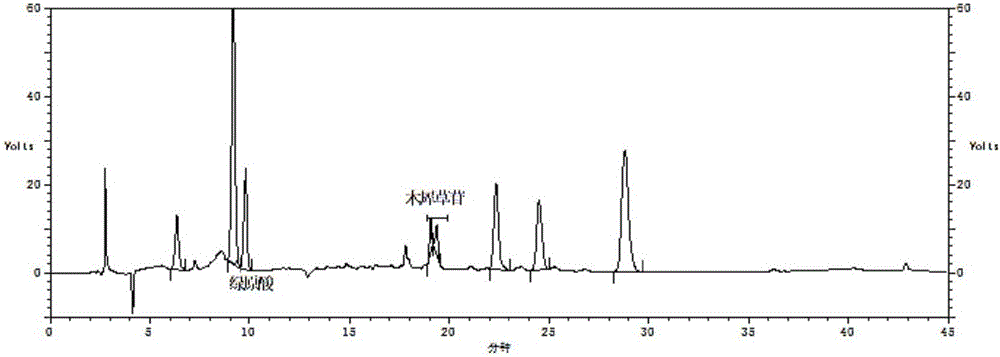
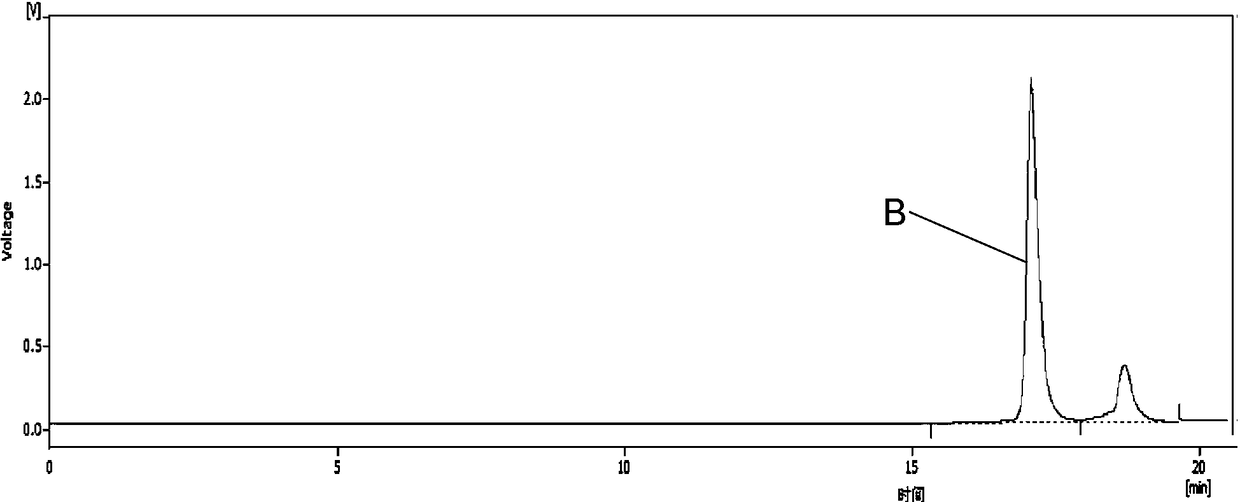
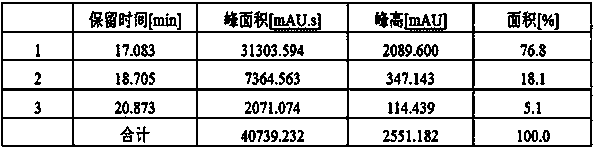
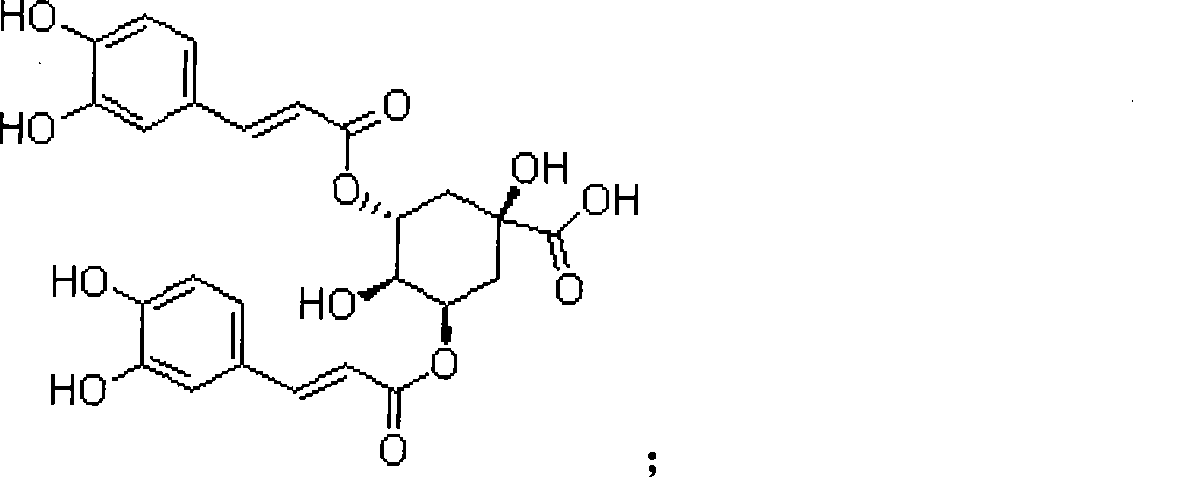
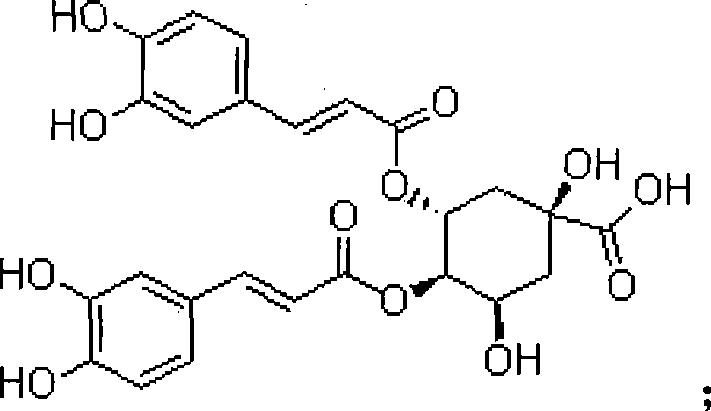
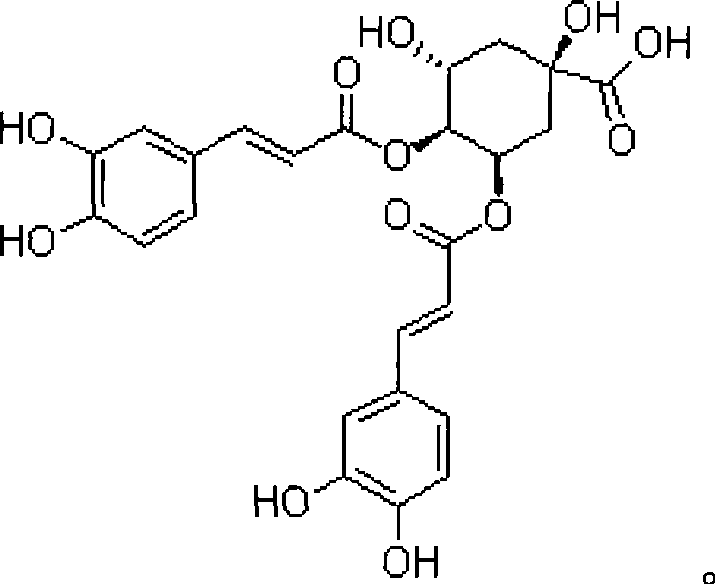
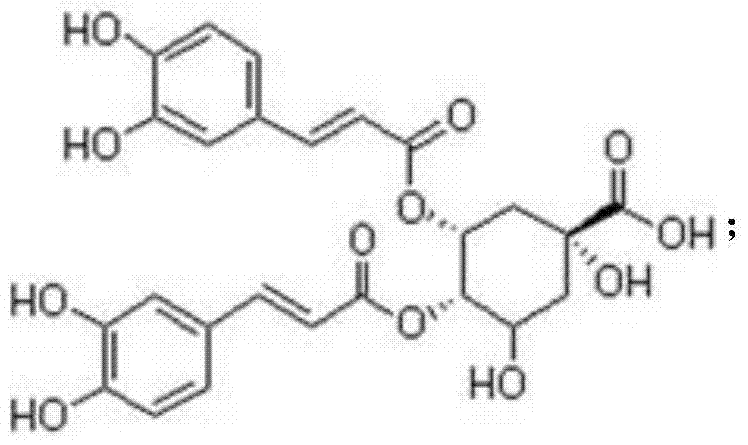
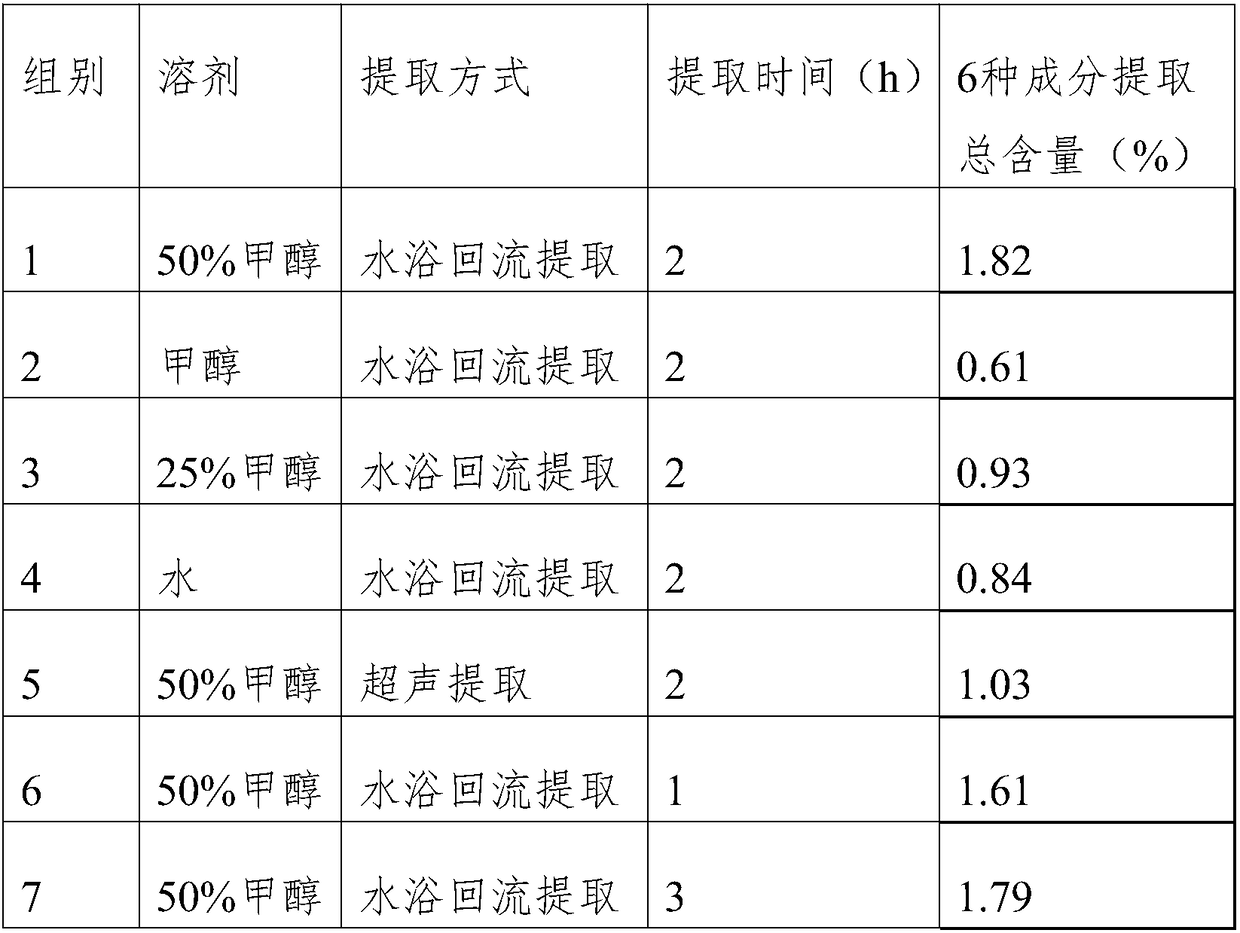
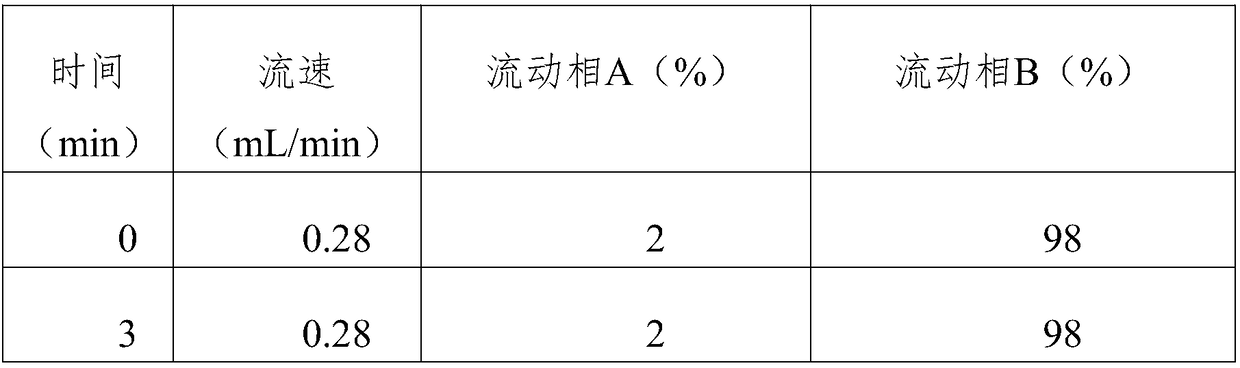
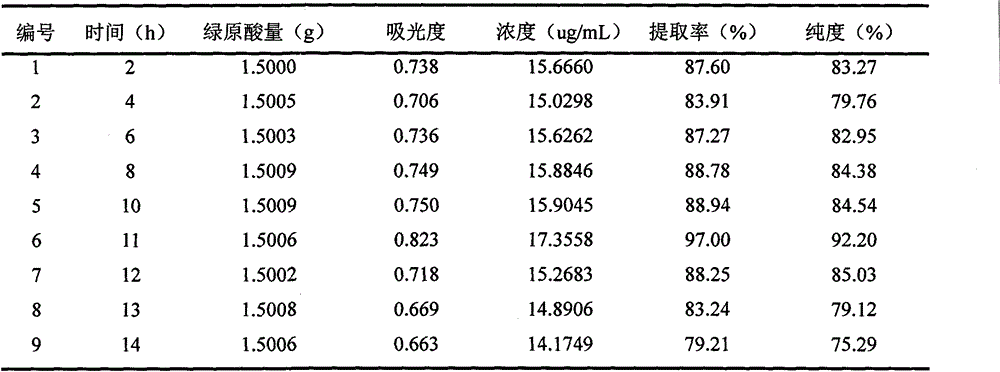
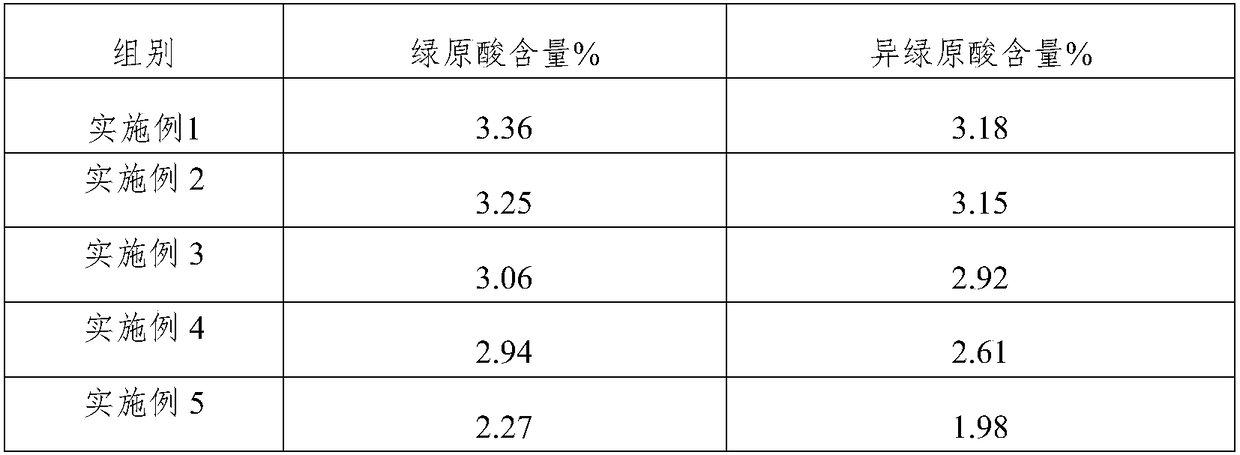
Patents
Literature
86 results about "Isochlorogenic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Content measurement method for Chinese patent medicine prepared from sweet wormwood, honeysuckle and gardenia jasminoides fruit
ActiveCN102233021AGood reproducibilityImprove quality controlComponent separationAntipyreticPhosphoric acidIsochlorogenic acid
The invention belongs to the field of Chinese medicine analysis and particularly relates to a content measurement method for Chinese patent medicine prepared from sweet wormwood, honeysuckle and gardenia jasminoides fruit. The method measures the content of nine ingredients including neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, caffeic acid, geniposide, secoxyloganin, isochlorogenic acid B, isochlorogenic acid A and isochlorogenic acid C in the Chinese patent medicine by reversed-phase high performance liquid chromatography, wherein the chromatographic conditions include: a chromatographic column is C18 column; methanol or acetonitrile is used as a flowing phase A; 0.05-to-1.0 percent phosphoric acid or 0.05-to-1.0 percent acetic acid is used a flowing phase B; the totalpercentage of flowing phases A and B is 100 percent; and gradient elute is performed. The content measurement method has high repeatability; and the method completely reflects the main ingredients and the change of the main ingredient content of the medicine from a qualitative prospect respectively and thus improves the level of the control over the quality of the medicine.
Owner:JIANGSU KANION PHARMA CO LTD
Method for content determination of multiple components in traditional Chinese medicinal preparation Shuanghuanglian for injection
ActiveCN103308615AAvoid driftingSolve the precipitation problemComponent separationIsochlorogenic acidContent determination
The invention relates to a method for content determination of multiple components in traditional Chinese medicinal preparation Shuanghuanglian for injection, aiming at solving the problems of an existing method for content determination of Shuanghuanglian for injection which is complex in sample processing, time, labor and detection equipment-consuming to detect Shuanghuanglian, and long in detection period. The detection method provided by the invention can be used for synchronously determining the contents of more than 10 components such as forsythin, forsythiaside A, caffeic acid, neochlorogenic acid, chlorogenic acid, cryptochlorogenin acid, isochlorogenic acid A, isochlorogenic acid C, baicalin, scutellarin and oroxylin-7-O-glucuronic acid in the Shuanghuanglian for injection, and meanwhile monitoring a fingerprint spectrum. The method can be used for completing previous detection in one time just by one detection system, and is more accurate, stable, comprehensive and rapid in control over the quality of the Shuanghuanglian for injection.
Owner:哈药集团中药有限公司
Honeysuckle contrasting extract and preparation method thereof
ActiveCN103823034AHigh content of target ingredientsAccurate Qualitative and Quantitative DeterminationTesting medicinal preparationsBiotechnologyIsochlorogenic acid
The invention relates to the technical field of traditional Chinese medicine extracts and traditional Chinese medicine quality control, in particular to a honeysuckle contrasting extract. The honeysuckle contrasting extract is prepared from the following components of neochlorogenic acid, chlorogenic acid, cryptochlorogenin acid, isochlorogenic acid A, isochlorogenic acid B and isochlorogenic acid C at the mass ratio: (0.20-0.40) : 1.00 : (0.30-0.50) : (0.15-0.45) : (0.10-0.40) : (0.15-0.45), and the total mass fraction exceeds 70%. The preparation method comprises the following steps: performing water extracting and alcohol precipitating to obtain crude extracts; dissolving the crude extracts, regulating pH to be acidic, and centrifuging; separating the supernatant by macroporous adsorption resin, silica gel column and gel column to reach the target content. The honeysuckle contrasting extract has high content of target components, can be applied as a mixed reference substance, is used for quality control of traditional Chinese medicine that contains honeysuckle in the prescription and for accurately qualitative and quantitative measurement, and is efficient in detection, low in cost, accurate in results and lower in preparation cost.
Owner:SHANDONG INST FOR FOOD & DRUG CONTROL
Anti-hepatitis B virus composition taken from fresh dandelion and application of anti-hepatitis B virus composition in preparation of anti-hepatitis B virus drug
InactiveCN103099838AHas anti-HBV activityHigh development valueOrganic active ingredientsDigestive systemCaffeic acidIsochlorogenic acid
The invention relates to an anti-hepatitis B virus composition taken from fresh dandelion and an application of the anti-hepatitis B virus composition in preparation of an anti-hepatitis B virus drug and belongs to the field of traditional Chinese medicines. The anti-hepatitis B virus composition provided by the invention comprises a fresh dandelion polysaccharide component, a phenolic acid component and a flavone component and is characterized in that the weight ratio of the fresh dandelion polysaccharide component to the phenolic acid component to the flavone component is (2-40): (3-70): (2-50). According to an optimized scheme, the phenolic acid component takes caffeic acid, chlorogenic acid and isochlorogenic acid A as typical ingredients; the flavone component takes galuteolin, quercetin and luteolin as typical ingredients; and the weight ratio of the caffeic acid to the chlorogenic acid to the isochlorogenic acid A to the galuteolin to the quercetin to the luteolin is (0.25-10): (2-40): (0.25-20): (0.5-20): (0-10): (1.5-20). According to the invention, the fresh dandelion is taken as raw material, effective ingredients are separated, purified and enriched by macroporous resin, the effective ingredients are mixed to obtain the fresh dandelion anti-hepatitis B virus composition, a process is simple and convenient, related diseases caused by hepatitis B viruses can be effectively treated, cost is low, and mass production can be achieved.
Owner:苏州艾费堂医药科技有限公司
Quantitative determination method of chlorogenic acid and three kinds of isochlorogenic acid in lonicera flower medicinal material and preparation thereof
ActiveCN102507769ASimple methodFast wayComponent separationIsocratic elutionQuantitative determination
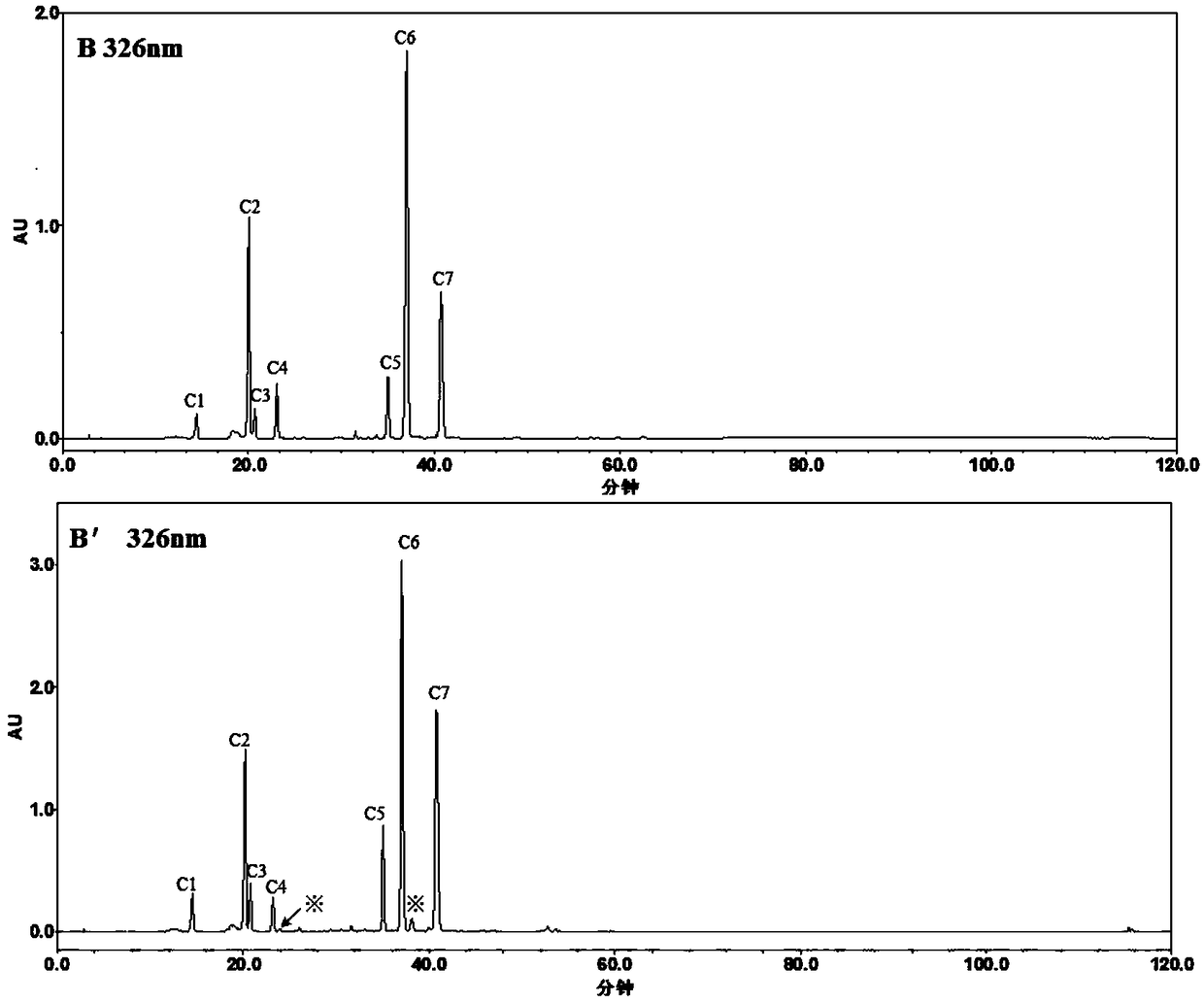
The invention relates to a quantitative determination method of chlorogenic acid and three kinds of isochlorogenic acid in lonicera flower medicinal material and preparation thereof, which is characterized in that: ordinary isocratic elution is adopted at the first time, acetonitrile-methanol-0.1% phosphoric acid with the volume ratio of (15 to 14): (6.5 to 6): (78.5 to 80) is adopted as a flow phase, and the content of the chlorogenic acid and three kinds of isochlorogenic acid in the lonicera flower medicinal material and preparation thereof is simultaneously determined at 326 minus or plus2nm. The method is simple, convenient and rapid to operate and is easy to popularize and master; separation of each peak of a quantitative chromatogram map of each component on three different chromatographic columns is good, the basic line is stable, and the peak appearance is completed within 20 to 30 minutes. Compared with the gradient elution, the cost of instruments is greatly reduced, the popularization rate of the method is improved, and the determination time is reduced. Due to the adoption of the quantitative determination method, a determination method and reference data can be supplied for evaluating the quality of the lonicera flower medicinal material and the preparation thereof through multiple indexes.
Owner:承德燕峰药业有限责任公司
Finger-print spectrum construction method and quality detection method of chrysanthemum cell-disruption decoction pieces
ActiveCN104833749AQuality improvementControl and identify counterfeit goodsComponent separationHplc fingerprintActive component
The invention relates to a finger-print spectrum mutual mode construction method and a quality detection method of chrysanthemum cell-disruption decoction pieces. In the method, with isochlorogenic acid A as a contrast peak, mutual mode standard spectrums and determination indexes are established through HPLC analysis to not less than 10 batches of the samples under following chromatographic conditions: column temperature: 35 DEG C; wavelength: 348 nm; mobile phase: an acetonitrile-0.5% phosphoric acid solution; elution gradient: 0-8 min-24 min-50 min-75 min; and acetonitrile change: 14%-18%-18%-25%-45%. The spectrums of the samples to be detected under the same chromatographic condition are compared with the mutual mode standard spectrums to detect the quality of the samples to be detected. The invention firstly discloses the HPLC finger-print spectrum and the quality detection method aiming to the chrysanthemum cell-disruption decoction pieces. The spectrums comprehensively contain the spectrum information of main active components of the chrysanthemum cell-disruption decoction pieces. The method is strong in specificity, is quick and accurate in detection, and can effectively control the total quality of medicines, cell-disruption powders and cell-disruption decoction pieces.
Owner:ZHONGSHAN ZHONGZHI PHARMA GRP
Quality control method for simultaneous realization of content analysis and similarity evaluation of 18 components in Ilex kudingcha
ActiveCN106198782ARealize simultaneous content determinationComprehensive evaluationComponent separationIlex kudingchaHydroxytyrosol
The invention discloses a quality control method for simultaneous realization of content analysis and similarity evaluation of 18 components in Ilex kudingcha. Rutin, isochlorogenic acid A and kudinoside A are used as internal references; correction factors of rutin for 6-hydroxy-7,7a-dihydro-2(6H)-benzofuran and hydroxytyrosol glucoside, correction factors of isochlorogenic acid A for protocatechuic acid, kudinoside E, kudinoside D, neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, caffeic acid, isochlorogenic acid B, and isochlorogenic acid C, and correction factors of kudinoside A for latifoloside G, kudinoside G, ilex kudingcha ilexoside T and latifoloside H are calculated, and the factors are used as constants for determining content. Only three common reference substances are needed for simultaneous determination of contents of 18 kinds of components in ilex kudingcha, quality of the ilex kudingcha can be rapidly, economically and scientifically controlled, and further cluster analysis, main component analysis and similarity calculation of medicinal materials can be carried out by using contents of the 18 kinds of components, in order to comprehensively control quality of ilex kudingcha.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Method for establishing fingerprint of flos lonicerae medicinal preparation
The invention relates to a method for establishing a fingerprint of a flos lonicerae medicinal preparation. The method comprises the following steps of by taking flos lonicerae formula granules as detection objects, establishing a method for a fingerprint of a medicinal preparation, and obtaining more comprehensive fingerprint information; determining common characteristic peaks: first peak neochlorogenic acid, second peak chlorogenic acid, third peak cryptochlorogenic acid, fourth peak rutin, fifth peak galuteolin, sixth peak, seventh peak isochlorogenic acid A and eighth peak isochlorogenic acid C; selecting the second peak chlorogenic acid as an inner reference peak, and determining relative retention time of the common characteristic peaks of the flos lonicerae formula granules. In addition, by combining information of multiple chromatographic peaks in the fingerprint, the quality of the flos lonicerae formula granules can be detected comprehensively and quickly, and comprehensive quality detection and integral quality control of the flos lonicerae formula granules are facilitated, thereby benefiting the improvement on the safety and the stability of medicine use. Meanwhile, the method has the advantages of high stability, high precision, good repeatability and the like.
Owner:华润三九现代中药制药有限公司
Methods for separation and content determination of chlorogenic acid type components in gynura procumbens
The invention discloses two methods for quantitative determination of chlorogenic acid, neochlorogenic acid and three isochlorogenic acids in gynura procumbens extract. The two methods comprise the steps that 1, the best chromatogram conditions are determined; 2, reference substances of the neochlorogenic acid, the chlorogenic acid and the three isochlorogenic acids are obtained, methyl alcohol is added to the reference substances, and then a reference solution is prepared; 3, the gynura procumbens extract is obtained, methyl alcohol is added to the gynura procumbens extract, and after filtering, a sample is obtained; 4, the reference solution and the sample solution are precisely absorbed, chromatography sample introduction is conducted, and the contents of the chlorogenic acid, the neochlorogenic acid and the three isochlorogenic acids are measured; 5, or, only a chlorogenic acid reference solution is prepared in the step 2, the chlorogenic acid is used as a reference substance, the relative correction factors of the isochlorogenic acid C, the isochlorogenic acid A, the isochlorogenic acid B and the neochlorogenic acid are 1.21, 1.12, 1.07 and 0.92, and the contents of the five substances are measured. According to the two methods, the chlorogenic acid is used as reference, fk / s between the chlorogenic acid and other components is established, the content of each component is calculated, the external standard method and the method for quantitative analysis of multi-components by a single marker are similar in accuracy and reliability, and therefore a brand-new mode is provided for evaluating the quality of gynura procumbens more authentically.
Owner:谭玉莲
Method for extracting chlorogenic acid and isochlorogenic acid from gynura procumbens
ActiveCN108689852AEfficient removal of impuritiesHigh purityOther chemical processesOrganic compound preparationIsochlorogenic acidSolvent
The invention discloses a method for extracting chlorogenic acid and isochlorogenic acid from gynura procumbens. The method comprises the following steps of drying and smashing fresh gynura procumbensleaves, and obtaining powder; mixing the powder and an ethanol solution, adopting ultrasonic extraction to obtain an extracting solution, and then centrifuging, filtering and concentrating to obtaina turbid dark green concentrated solution; adding a clarifying agent into the concentrated solution for clarifying; adding modified silicon dioxide pellets into the clarified concentrated solution, de-coloring, and enabling the color of the concentrated solution to change into light gray from dark green; adding absolute ethyl alcohol into the light-gray concentrated solution, and eliminating carbohydrates; adsorbing the carbohydrates-eliminated through a middle0high-pressure chromatographic column; after completely adsorbing, desorbing in a classification way, concentrating, and drying to obtain a chlorogenic acid finished product dry powder and isochlorogenic acid mixture. The method provided by the invention is simple in process, convenient to operate, low in cost, free of harmful solvent, high in product purity, better in purification and impurity cleaning effects, energy saving, environmental-friendly, high in production safety, and suitable for industrial production, and has a favorable market prospect.
Owner:江西省蔓三七健康科技有限公司 +2
Use of isochlorogenic acid compound and different combinations in hepatitis treatment
InactiveCN101380318AAbundant resourcesThe extraction process is simpleOrganic active ingredientsDigestive systemHepatitisIsochlorogenic acid
The invention relates to the application of a compound to treating hepatitis and aims at providing an isochlorogenic acid compound which has stronger effects of anti-HBV, anti-inflammation and liver protection, and has wide and rich sources, and simple and convenient extraction process. The technical proposal is as follows: the isochlorogenic acid compound is made from one or more than one of isochlorogenic acid A, isochlorogenic acid B and isochlorogenic acid C according to any mixture ratio; in addition, the technical proposal further comprises the application of the compound to preparing drugs for resisting hepatitis B virus, and resisting inflammation and protecting liver.
Owner:伍义行
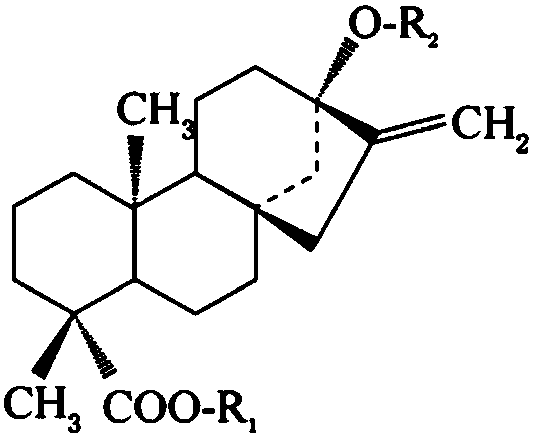
Method for preparing stevia rebaudiana polyphenols through composite chitosan flocculation method
ActiveCN109432153ARealize comprehensive utilizationReduce manufacturing costPlant ingredientsHigh concentrationIsochlorogenic acid
The invention discloses a method for preparing stevia rebaudiana polyphenols through a composite chitosan flocculation method and belongs to the technical field of plant extraction. The method comprises flocculating stevia rebaudiana water extract at room temperature with composite chitosan for impurity removal, and then absorbing supernatant through microporous resin to obtain effluent for further extraction of stevioside; then eluting the microporous resin with alcohol aqueous solutions of different concentrations, combining eluent eluted by low-concentration alcohol aqueous solutions with the undiluted effluent to obtain a combined solution with a content of total phenols lower than 0.5% on a dry basis for extracting stevioside; drying the eluent eluted by high-concentration alcohol aqueous solutions to obtain stevia rebaudiana polyphenols mainly containing isochlorogenic acid. The method for preparing the stevia rebaudiana polyphenols through the composite chitosan flocculation method achieves production of the stevia rebaudiana polyphenols with high content of isochlorogenic acid on the basis of an original stevioside production process, thereby achieving comprehensive utilization of stevia rebaudiana and increasing the economic benefits for enterprises.
Owner:DONGTAI HIRYE BIOTECH CO LTD +1
Beverage
ActiveUS20050147728A1Easy to drinkMilk preparationTea flavoringAdditive ingredientIsochlorogenic acid
The present invention provides a beverage comprising: (a) 0.05 to 10% by weight (wt %) of a chlorogenic acids family mixture comprising isochlorogenic acids(s) family wherein the weight content of said isochlorogenic acid(s) ranges from 1 / 20 to 1 / 3 of the total ingredient (a); (b) hydroxycarboxylic acid(s) in a quantity ranging from 5 to 30 times the weight content of said ingredient (a) and from 0.25 to 10 wt % of the beverage, and / or vegetable-derived or fruit-derived flavor substance(s) in a quantity ranging from 0.1 to 30 times the weight content of said ingredient (a) and from 0.25 to 10 wt % of the beverage; and (c) 30 to 99.7 wt % of water. The present invention provides a flavorous beverage having a stable antihypertensive action and long-term storage stability.
Owner:KAO CORP
Industrialized utilization method of stevia rebaudiana and chlorogenic acid and stevioside
ActiveCN109265346AAvoid hydrolysisGuaranteed validitySugar derivativesOrganic compound preparationHigh concentrationAlcohol
The invention relates to an industrialized method for extracting chlorogenic acid and stevioside of stevia rebaudiana. The method has the main improvement points that firstly, stevia rebaudiana powderis extracted by a high-concentration alcohol solution; then, extraction liquid is adsorbed by polar adsorption resin subjected to hydroxyl modification; chlorogenic acid type substances are obtained;finally, glycoside resin is used for separation to obtain stevioside. The method provided by the invention has the advantages that the hydrolysis of isochlorogenic acid ingredients in the tevia rebaudiana can be prevented; the effective ingredient content and efficiencies of a stevia rebaudiana chlorogenic acid product can be ensured; the successful separation and extraction of the isochlorogenicacid are realized. The method can also realize the effective separation of the stevioside; the production efficiency is improved; the stevioside quality is improved.
Owner:CHENGUANG BIOTECH GRP CO LTD
Beverage
Owner:KAO CORP
Preparation containing isochlorogenic acid and application of preparation
InactiveCN104490761AGood inhibitory effectEnhanced inhibitory effectOrganic active ingredientsPharmaceutical delivery mechanismIsochlorogenic acidWilms' tumor
The invention discloses a preparation containing isochlorogenic acid. The preparation comprises 1-100 parts by weight of isochlorogenic acid and 1-100 parts by weight of additives. The preparation disclosed by the invention inhibits the tumor development by improving the organism immune response and is suitable for tumors with immunodeficiency, wherein the tumors with immunodeficiency comprise lung cancer, kidney cancer, liver cancer, uterine cancer, breast cancer, pancreatic cancer, leukemia and brain tumor.
Owner:SICHUAN JIUZHANG BIO TECH CO LTD
Industrial method for synchronously preparing stevia rebaudiana chlorogenic acid and stevioside
PendingCN109438241AAvoid hydrolysisGuaranteed validitySugar derivativesOrganic compound preparationWater insolubleOrganic layer
The invention relates to an industrial method for synchronously preparing stevia rebaudiana chlorogenic acid and stevioside. The industrial method includes carrying out alcohol extraction on stevia rebaudiana which is used as a raw material, and then adjusting feed liquid states to allow chlorogenic acid to be in free molecular states; carrying out extraction separation by water-insoluble moderate-polarity organic solvents; enriching the stevia rebaudiana chlorogenic acid on organic layers; enriching the stevioside on water layers. Compared with the traditional water extraction processes, theindustrial method has the advantages that chlorogenic acid components in the stevia rebaudiana can be prevented from being hydrolyzed, so that the contents and effects of effective components in stevia rebaudiana chlorogenic acid products can be guaranteed; effective separation can be carried out on the premise that the quality and the production efficiency of stevioside products are unaffected, the production efficiency can be greatly improved, and the proportion of isochlorogenic acid in the obtained products is close to the proportion of isochlorogenic acid in the raw material; production water consumption can be reduced to a great extent, discharge of sewage and flocculation residues can be decreased, accordingly, the industrial method is a green production process with high benefits,and the progress of the industry can be promoted to a great extent.
Owner:CHENGUANG BIOTECH GRP CO LTD
Ultra-high performance liquid chromatography detecting method for traditional Chinese medicine composition
The invention provides a method for detecting a traditional Chinese medicine composition. According to the method, seven components of chlorogenic acid, caffeic acid, rutin, isochlorogenic acid B, isochlorogenic acid C, forsythiaside A and phillyrin in the traditional Chinese medicine composition are detected through an ultra-high performance liquid chromatography, and the quality of the traditional Chinese medicine composition can be represented more comprehensively.
Owner:SHIJIAZHUANG YILING PHARMA
PVC film antiseptic
The invention relates to a PVC film antiseptic. The PVC film antiseptic comprises, by weight, 20 parts of angelica root essential oil, 8 parts of chlorogenic acid, 8 parts of isochlorogenic acid, 2 parts of luteolin and 1.5 parts of common salt. The PVC film antiseptic is a pure natural antiseptic, produces obvious antibacterial effects, has a low cost and can be widely used in fields of foods and medicines.
Owner:QINGDAO KECHUANG PLASTIC MACHINERY
Diabetes treating traditional Chinese medicine preparation identification method
ActiveCN104849397AHigh sensitivityGood repeatabilityComponent separationIsochlorogenic acidEthyl acetate
The present invention discloses a diabetes treating traditional Chinese medicine preparation identification method. According to the present invention, the traditional Chinese medicine preparation is prepared from Keyaoluoqu, plantago depressa willd, agrimonia pilosa ledeb, and lonicera confusa dc; the identification is carrying out thin-layer chromatography on Keyaoluoqu, plantago depressa willd, agrimonia pilosa ledeb, and lonicera confusa dc, wherein the Keyaoluoqu identification method is the thin-layer chromatography method adopting a luteoloside reference substance as a control and adopting a mixed solution comprising ethyl acetate, acetone, formic acid and water according to a ratio of 7:3:1:1.2 as a developing solvent, the plantago depressa willd identification method is the thin-layer chromatography method adopting a plantamajoside reference substance as a control and adopting a mixed solution comprising ethyl acetate, acetone, formic acid and water according to a ratio of 7:3:1:1.2 as a developing solvent, the lonicera confusa dc identification method is the thin-layer chromatography method adopting an isochlorogenic acid A reference substance as a control and adopting a mixed solution comprising ethyl acetate, acetone, formic acid and water according to a ratio of 7:3:1:1.2 as a developing solvent; and the identification method of the present invention has characteristics of high sensitivity, good reproducibility, convenience, feasibility, and no negative control interference.
Owner:GUIZHOU BAILING GRP PARMACEUTIAL CO LTD
Method for determining content of chemical components in chrysanthemum
ActiveCN111537653AHigh sensitivityFast analysisComponent separationMedicinal herbsIsochlorogenic acid
The embodiment of the invention provides a method for determining the content of chemical components in chrysanthemum, which adopts ultra-high performance liquid chromatography-mass spectrometry to determine the content of 13 chemical components in chrysanthemum at the same time. The 13 chemical components comprise isochlorogenic acid C, hesperidin, coreopsis tinctoria glycoside, quercetin, farnetin, hyperoside, chlorogenic acid, luteolin-7-O-glucuronide, unuronide, luteolin, apigenin, diosmetin, isoquercitrin and cryptochlorogenic acid. By adopting the method disclosed by the invention, the contents of 13 chemical components in the chrysanthemum can be simultaneously determined by reasonably selecting chromatographic conditions and mass spectrometric conditions. And the method has the advantages of simplicity, convenience, high sensitivity, high analysis speed, strong specificity and the like, so that the method can be used for controlling the quality of the chrysanthemum medicinal material.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Method for obtaining chlorogenic acid and isochlorogenic acid from stevia rebaudiana waste residues
InactiveCN113501759AFacilitate subsequent separation and purification workGood for separation and purificationOrganic compound preparationCarboxylic acid esters separation/purificationSteviolmonosideIsochlorogenic acid
The invention belongs to the technical field of plant extraction, and particularly relates to a method for extracting chlorogenic acid and isochlorogenic acid from stevia rebaudiana waste residues. The invention relates to a method for obtaining chlorogenic acid and isochlorogenic acid from stevia rebaudiana waste residues, which mainly comprises the following steps: by taking industrial production stevia rebaudiana flocculation waste residues as raw materials, dispersing the stevia rebaudiana waste residues with water, regulating the pH value to 3.0-4.0, and filtering and separating filtrate and filter residues; filtering the filtrate with a ceramic membrane and an organic membrane in sequence to remove impurities, and drying to obtain chlorogenic acid; and continuously dissolving and dispersing the filter residue with ethanol, filtering and collecting the filtrate, concentrating under reduced pressure and recovering the ethanol, extracting and refining the extract with ethyl acetate, and drying to obtain the isochlorogenic acid. According to the method, industrial waste residues in the stevia rebaudiana production process are fully utilized, production of stevioside is not affected, meanwhile, chlorogenic acid and isochlorogenic acid are extracted and separated through the waste residues, resin does not need to be used for separation and purification in the whole production process, operation is easy, and the method is suitable for industrial large-scale production.
Owner:GUILIN NATURAL INGREDIENTS CORP
A quality control method capable of simultaneous content analysis and similarity evaluation of 18 components in Kudingcha
ActiveCN106198782BRealize simultaneous content determinationComprehensive evaluationComponent separationIlex kudingchaHydroxytyrosol
The invention discloses a quality control method for simultaneous realization of content analysis and similarity evaluation of 18 components in Ilex kudingcha. Rutin, isochlorogenic acid A and kudinoside A are used as internal references; correction factors of rutin for 6-hydroxy-7,7a-dihydro-2(6H)-benzofuran and hydroxytyrosol glucoside, correction factors of isochlorogenic acid A for protocatechuic acid, kudinoside E, kudinoside D, neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, caffeic acid, isochlorogenic acid B, and isochlorogenic acid C, and correction factors of kudinoside A for latifoloside G, kudinoside G, ilex kudingcha ilexoside T and latifoloside H are calculated, and the factors are used as constants for determining content. Only three common reference substances are needed for simultaneous determination of contents of 18 kinds of components in ilex kudingcha, quality of the ilex kudingcha can be rapidly, economically and scientifically controlled, and further cluster analysis, main component analysis and similarity calculation of medicinal materials can be carried out by using contents of the 18 kinds of components, in order to comprehensively control quality of ilex kudingcha.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Method for inhibiting Aspergillus fumigatus biofilm from formation
InactiveCN103710418AEasy to killEnhanced inhibitory effectOrganic active ingredientsAntimycoticsBiotechnologySporeling
The invention relates to a method for inhibiting a Aspergillus fumigatus biofilm from formation, belonging to the technical field of biology. The method comprises the following steps: preparing a drug, collecting a strain, preparing a carrier, preparing a spore suspension, carrying out drug sensitivity test, inhibiting an Aspergillus fumigatus biofilm, and scanning with an electron microscope, wherein the Aspergillus fumigatus biofilm inhibiting process comprises the following steps: adding prepared isochlorogenic acid A with different concentrations and a tested strain spore suspension into a 24-pore plate filled with the carrier, standing in a 35-37 DEG C biochemical incubator for culture, and taking out the carrier at the 24th hour and 48th hour and scanning with the electron microscope to observe the shape of the biofilm. Only hypha can be seen in the drug composed of the isochlorogenic acid A and spore suspension, and little and even no extracellular matrix can be seen, which indicates that the isochlorogenic acid A can inhibit the Aspergillus fumigatus biofilm from formation.
Owner:GUANGXI MEDICAL UNIVERSITY
Feed additive for enhancing anti-stress capability of breeding ducks in egg producing period and preparation method of feed additive
PendingCN113057260AStrong in vitro antioxidantHigh antibacterial activityAnimal feeding stuffAccessory food factorsBiotechnologyBenzoic acid
The invention discloses a feed additive for enhancing the anti-stress ability of breeding ducks in the egg laying period and a preparation method of the feed additive. The additive comprises the following raw materials in percentage by weight: 3-5 parts of isochlorogenic acid, 1-3 parts of tributyrin, 2-4 parts of acetylglucosamine benzoate and 6-9 parts of puffed chickpea powder. The additive further comprises 1-3 parts of hyperforin perforatum and 1-3 parts of bile acid. The preparation method comprises the following steps: preparing a hyperforin perforatum extract: adding water twice for extraction, combining the two extracting solutions, concentrating to obtain paste, and drying to obtain the hyperforin perforatum extract; mixing and granulating to obtain granular products: wherein the variable coefficient of the mixing uniformity is less than or equal to 4%; sequentially spraying a tributyrin solution and an acetylglucosamine benzoate solution on the granular product; and drying. When the additive is added into the breeding duck feed, the physique, the resistance, the adaptive capacity to change of external factors such as feed changing and illumination increasing of the breeding ducks and the anti-stress capacity after egg laying can be effectively enhanced, and the overall economic benefit of duck breeding is improved.
Owner:江苏中煤长江生物科技有限公司
Method for detecting five chlorogenic acid substances in bilberry fruits through ultra-high performance liquid chromatography-tandem mass spectrometry
PendingCN113624863AQuick QualificationQuick checkComponent separationFluid phaseMass Spectrometry-Mass Spectrometry
The invention relates to the technical field of analysis and detection, in particular to a method for detecting five chlorogenic acid substances in bilberry fruits through ultra-high performance liquid chromatography-tandem mass spectrometry. The invention provides a method for detecting chlorogenic acid substances in bilberry fruits, which comprises the following steps: extracting the chlorogenic acid substances in the bilberry fruits, purifying, and detecting by adopting ultra-high performance liquid chromatography-tandem mass spectrometry, and the chlorogenic acid substances comprise chlorogenic acid, neochlorogenic acid, cryptochlorogenic acid, isochlorogenic acid A and isochlorogenic acid B. According to the method, an extraction process, a separation and purification process and detection and analysis conditions of the five chlorogenic acid substances in the bilberry fruits are systematically optimized, and an ultra-high performance liquid chromatography-tandem mass spectrometry method is established for determining the chlorogenic acid substances in the bilberry fruits. According to the method, rapid, accurate and efficient qualitative and quantitative detection of chlorogenic acid substances in cowberry can be realized.
Owner:FRUIT TREE INST OF CHINESE ACAD OF AGRI SCI
Method for detecting content of various kinds of ingredients in herba gerberae piloselloidis medicinal material
InactiveCN108490095AEasy to separateGood repeatabilityComponent separationUplc pdaAdditive ingredient
The invention relates to the technical field of traditional Chinese medicine detection, in particular to a method for detecting the content of various kinds of ingredients in herba gerberae piloselloidis medicinal material. The method adopts a UPLC-PDA method, takes Waters BET C18 (2.1*150mm, 1.7microns) as a chromatographic column, acetonitrile-0.1% formic acid water solution is subjected to gradient elution, the detection wavelength is 280nm and 328nm, the flowing velocity is 0.28L / min, and the column temperature is 40 DEG C. According to the method, chlorogenic acid, isochlorogenic acid A,isochlorogenic acid B, isochlorogenic acid C, galuteolin and arbutin in herba gerberae piloselloidis medicinal material can be well separated, the average recovery of the method is 97.20%-103.57%, andRSD (relative standard deviation) is less than or equal to 3.0%. The method is simple and convenient and precise, the repeatability is good, the method can be used for detecting the content of chlorogenic acid, isochlorogenic acid A, isochlorogenic acid B, isochlorogenic acid C, galuteolin and arbutin in herba gerberae piloselloidis medicinal material, and reference foundations are provided for quality control of herba gerberae piloselloidis.
Owner:GUIZHOU MEDICAL UNIV
A method for extracting chlorogenic acid by enzymatically activating honeysuckle leaves
InactiveCN103086889BHigh extraction rateImprove permeabilityCarboxylic acid esters separation/purificationResource utilizationIsochlorogenic acid
The invention discloses a method for extracting chlorogenic acid from Lonicera japonica leaves activated under catalysis of enzyme, belonging to the technical field of chlorogenic acid extraction. According to the method, the solid waste of Lonicera japonica leaves, used as raw material, is prepared into the target product by the simple processes of treatment on Lonicera japonica leaves, enzymatic hydrolysis, ultrasonic wave synergistic digestion and chlorogenic acid preparation. The raw material used in the method provided by the invention is cheap and easily available, and the method has the characteristics that the waste is fully used, the prepared chlorogenic acid product is high in extraction ratio and purity and complete in biological activity, energy consumption in production process is low, the production equipment is hardly corroded, the consumption of organic solvent is low, environment protection is facilitated, and so on. The method provided by the invention can be widely used for extracting natural products, such as chlorogenic acid, isochlorogenic acid and Ginnol, from the solid waste of Lonicera japonica leaves, and effectively realizes the resource utilization of the Lonicera japonica leaves.
Owner:王星敏
Method for processing flos lonicerae
The invention relates to the technical field of Chinese medicinal material processing, in particular to a method for processing flos lonicerae. The method for processing the flos lonicerae harvested at 7-9 o'clock in the mornings includes steps of cleaning, soaking, pre-freezing, drying and shaping, screening, vacuum microwave sterilization and the like. The method has the advantages that flos lonicerae finished products processed by the aid of the method are yellowish green and are free of browning or blackening phenomena, and the flos lonicerae has distinct petals without adhesion and has faint scent; chlorogenic acid and isochlorogenic acid in the flos lonicerae are high in content and efficacy, and heat clearing, toxicity removing, anti-inflammation, deficiency tonifying and wind treating effects of the flos lonicerae can be enhanced.
Owner:遵义源创生产力促进中心有限公司
Novel method for controlling quality of abdominal Kefuan tablet
ActiveCN112051352AImprove quality controlSimple compositionComponent separationAgainst vector-borne diseasesIlex rotundaMedicinal herbs
The invention belongs to the technical field of medicine quality control, and particularly relates to a novel method for controlling the quality of an abdominal Kefuan tablet. For improving the effectiveness of the quality control of the abdominal Kefuan tablet, based on an original quality standard, thin-layer chromatography identification is carried out on ovateleaf holly bark by adopting a thin-layer chromatography identification developing solvent and a sample preparation method which are different from those in the prior art; the contents of two components, namely isochlorogenic acid B and isochlorogenic acid C, which are not reported in the ilex rotunda thunb medicinal material of the ilex rotunda thunb tablet are determined, and the contents of the isochlorogenic acid B and the isochlorogenic acid C in the ilex rotunda thunb are used as the quality control standard of the ilex rotunda thunb tablet; the method provided by the invention has strong specificity and reproducibility,provides a new method for the quality standard of the fukean tablet, can replace or supplement the existing quality control method, and can simultaneously determine the contents of the two componentsin the fukean tablet, so that the result is more reliable, and the quality control of the fukean tablet is better facilitated.
Owner:GUANGDONG MEDIHEALTH PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com