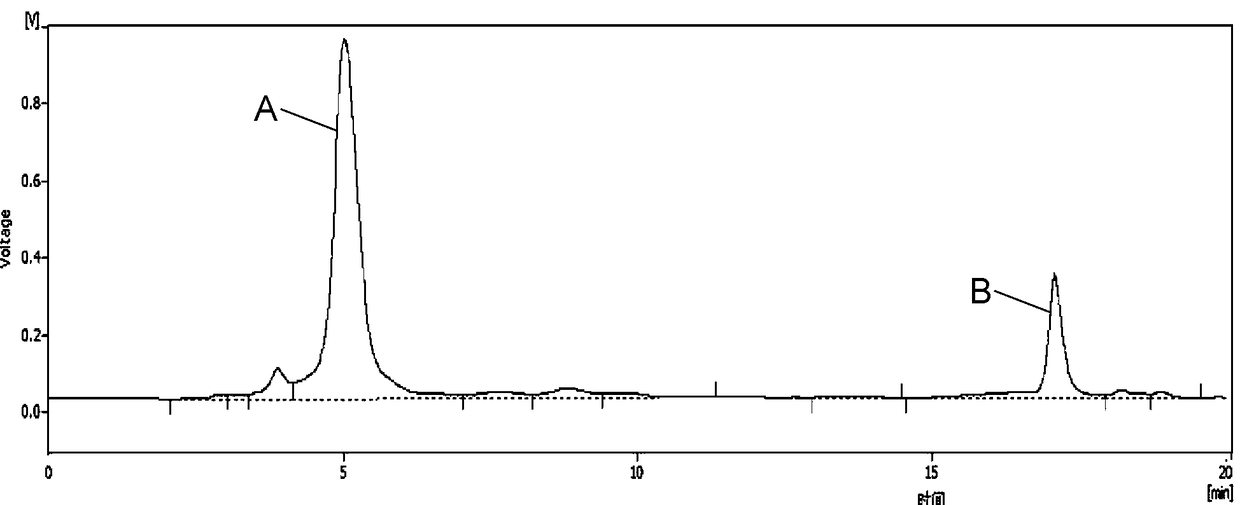
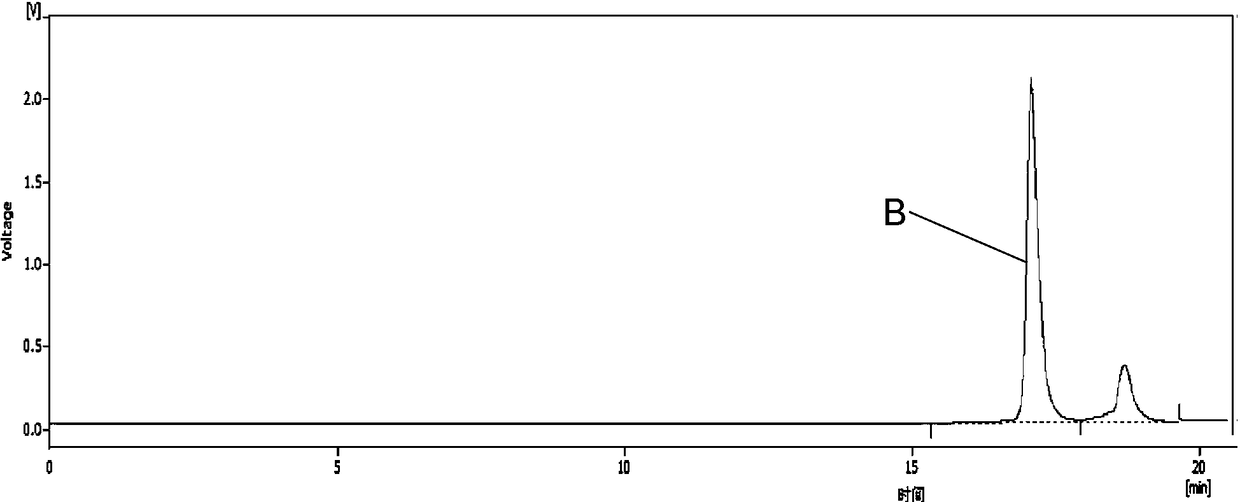
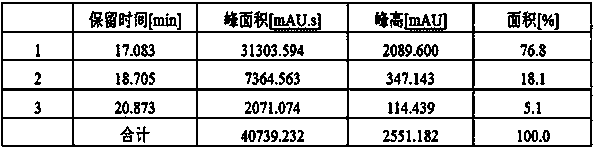
Method for extracting chlorogenic acid and isochlorogenic acid from gynura procumbens
A technology of flat-lying chrysanthemum notoginseng and isochlorogenic acid, which is applied in the field of extracting chlorogenic acid and isochlorogenic acid, can solve the problem of not being able to meet the market demand for high-purity chlorogenic acid, not suitable for large-scale industrial production, and restricting pharmacological drugs Effective in-depth research and other issues, to achieve good market prospects, low cost, high production safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Firstly, the modified silica microspheres are prepared for future use; the micron-sized silica microspheres for preparing the modified silica microspheres are commercially available known raw materials.
[0038] A preparation method of modified silicon dioxide microspheres, which comprises the following specific steps:
[0039] (a) 5 g of micron-sized silica microspheres with a particle size of 1 μm were vacuum-dried at 130°C for 3 hours, and then treated with 0.5 g of 30% sodium hydroxide to activate the surface active group silanol, Then rinse with pure water until neutral;
[0040] (b), add 3ml of ethyl chloroacetate to the solution washed to neutral in step a, mix and stir in the 70% volume concentration ethanol solution for 2h, continue to add 3ml of ethylenediamine, react at room temperature for 5h, and put The solution was centrifuged to collect the product, and then washed with dry dichloromethane at a dosage of 5ml each time for 3 times to remove the remaining...
Embodiment 2
[0051] First prepare modified silica microspheres for subsequent use; prepare micron-sized silica microspheres of modified silica microspheres to be synthesized using the improved Stöber method, which includes the following specific steps:
[0052] Step a1. Dissolve 0.03g KCl in 10 g deionized water, transfer the KCl solution to a 250 mL three-neck flask, add 75 g absolute ethanol, 4 mL NH 3 •H 2 O and 2g tetraethyl orthosilicate, mix and stir until the solution is milky white;
[0053] Step a2. Disperse 4g of tetraethyl orthosilicate in 25g of absolute ethanol. The mixed solution obtained after ultrasonic dispersion is added dropwise at room temperature at a rate of 0.1 mL / min to the milky white solution in the three-necked flask in step a1. 3 After the addition of h is completed, the reaction is continued for 1 h, and after the reaction is completed, the product is collected;
[0054] In step a3, the obtained product is washed with water and ethanol several times until the...
Embodiment 3
[0068] First prepare modified silica microspheres for subsequent use; prepare micron-sized silica microspheres of modified silica microspheres to be synthesized using the improved Stöber method, which includes the following specific steps:
[0069] Step a1. Dissolve 0.06g KCl in 15 g deionized water, transfer the KCl solution to a 250 mL three-neck flask, add 80 g absolute ethanol, 7 mL NH 3 •H 2 O and 5g ethyl orthosilicate, mix and stir until the solution is milky white;
[0070] In step a2, 8 g of tetraethyl orthosilicate is dispersed in 33 g of absolute ethanol, and the mixed solution obtained after ultrasonic dispersion is added dropwise at room temperature at a speed of 0.3 mL / min to the milky white solution in the three-necked flask in step a1, 3 After the addition of h is completed, the reaction is continued for 1 h, and after the reaction is completed, the product is collected;
[0071] In step a3, the obtained product is washed with water and ethanol several times ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com