Method for content determination of multiple components in traditional Chinese medicinal preparation Shuanghuanglian for injection
The technology of a traditional Chinese medicine preparation and a determination method is applied in the field of content determination of various components in Shuanghuanglian, which can solve the problems of occupying a large number of personnel, equipment, high detection cost, and inability to fully reflect the integrity of fingerprints, and achieve the effect of shortening the inspection period.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
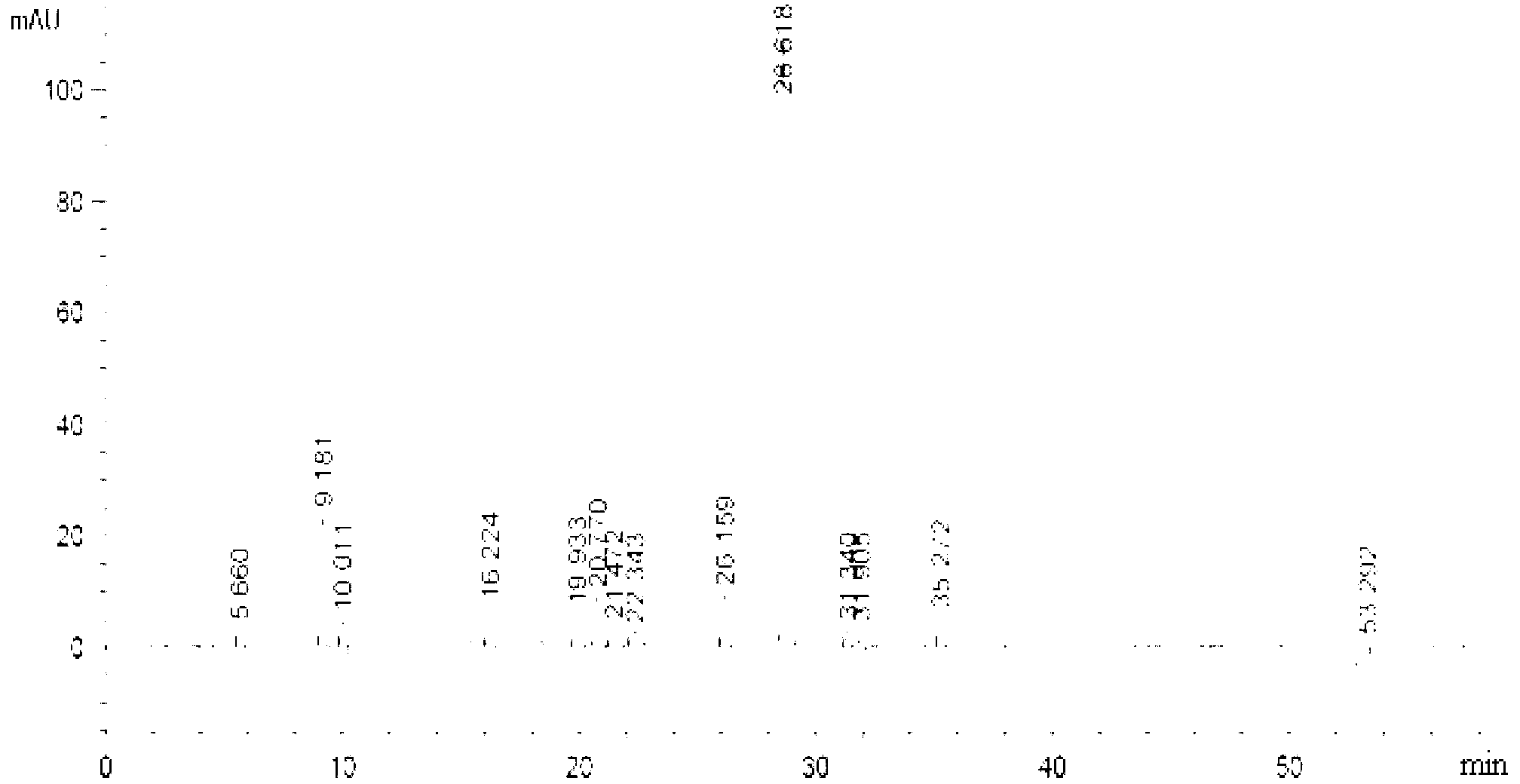
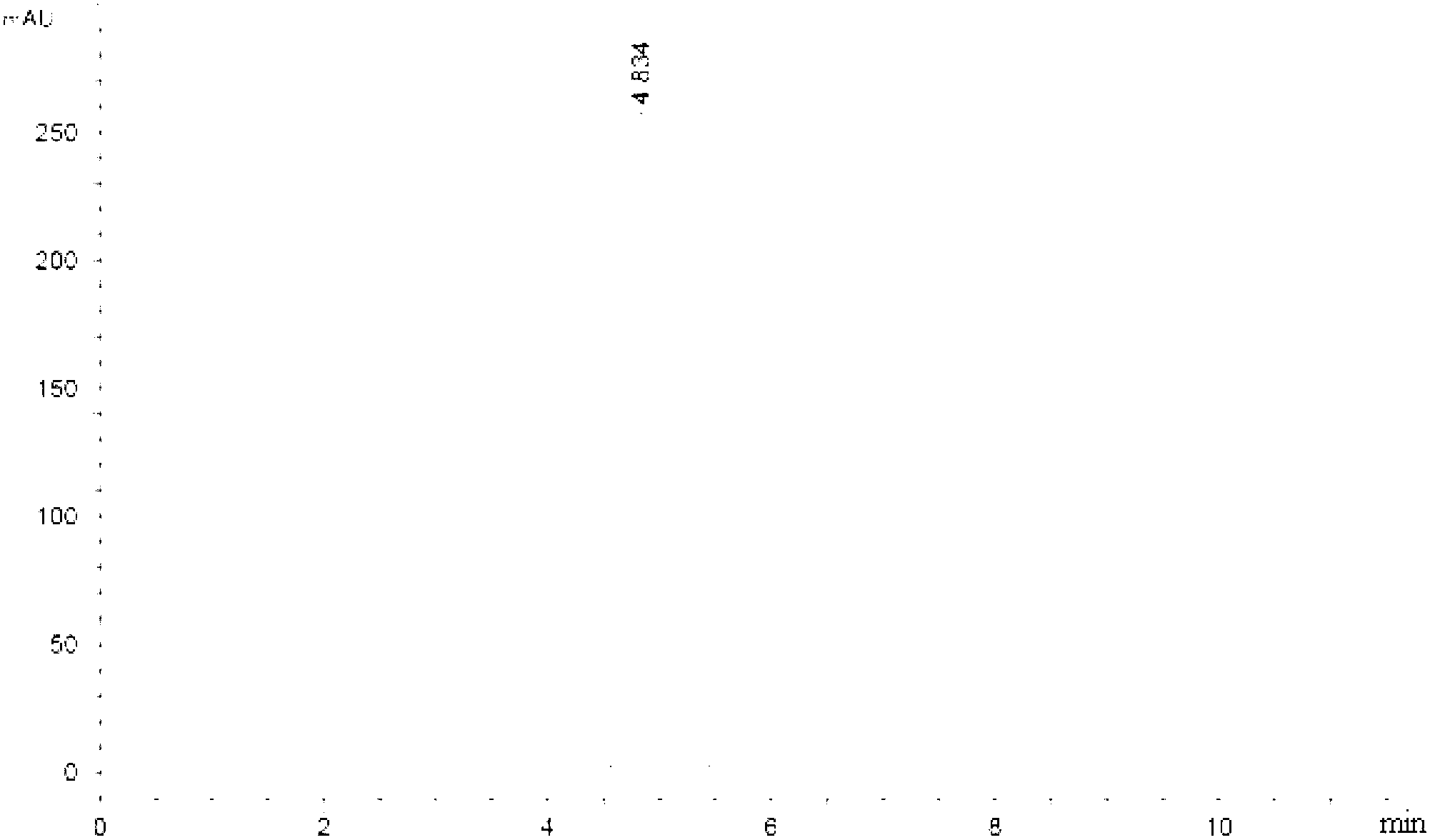
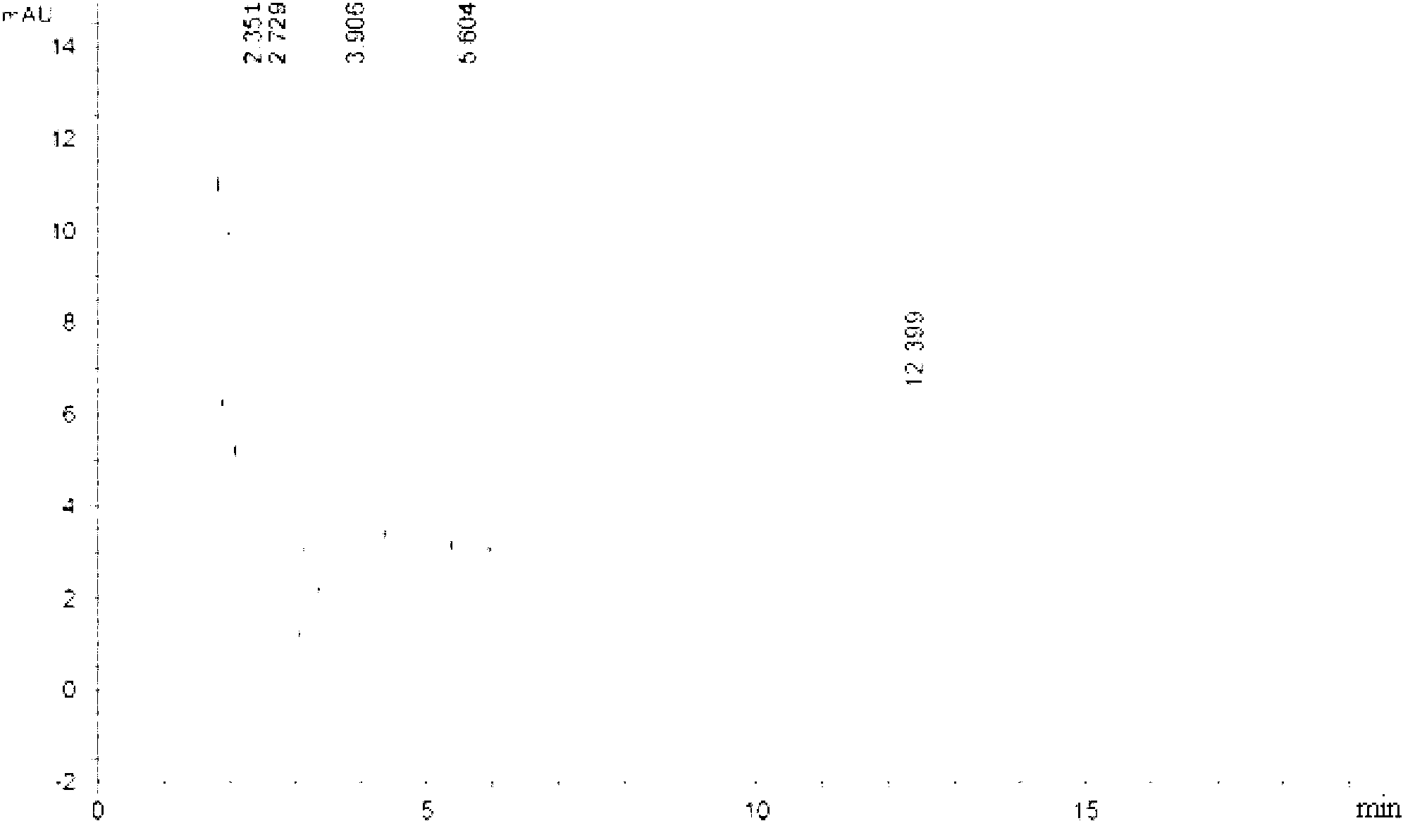
[0028] Embodiment 1: A method for determining the content of various components in Shuanghuanglian for injection of a traditional Chinese medicine preparation according to the present embodiment is carried out according to the following steps:
[0029] 1. Preparation of the baicalin reference substance: take the baicalin reference substance, add buffered saline solution to make a solution with a concentration of 0.5 mg / mL, and use it as the baicalin reference substance;
[0030] 2. Preparation of mixed reference substance solution: Weigh the reference substance, add buffered saline solution to make each 1mL containing 10μg neochlorogenic acid, 40μg chlorogenic acid, 8.0μg cryptochlorogenic acid, 4.0μg isochlorogenic acid A, 10.0 μg of isochlorogenic acid C, 8.0 μg of forsythin, 20 μg of scutellarin-7-O-glucuronide, 3.0 μg of wogonin, 2.0 μg of baicalein and 0.6 μg The solution of wogonin is used as a mixed reference substance;
[0031] 3. Preparation of the test solution: tak...
specific Embodiment approach 2
[0034] Specific embodiment 2: The difference between this embodiment and specific embodiment 1 is that the multi-wavelength simultaneous monitoring or wavelength monitoring over time refers to the neochlorogenic acid and chlorogenic acid in the test sample of Shuanghuanglian for injection of Chinese medicine preparations Acid, Cryptochlorogenic Acid, Caffeic Acid, Forsythiaside A, Isochlorogenic Acid A, Isochlorogenic Acid C, Melaleucain-7-O Glucuronide, Wogonin, Baicalein and Chinese Scutellariae luteolin and rutin were detected at 350nm, forsythin was detected at 208nm or 230nm; baicalin was detected at 240nm or 350nm. Others are the same as in the first embodiment.
specific Embodiment approach 3
[0035] Specific embodiment three: the difference between this embodiment and specific embodiment one or two is that the buffered saline solution is phosphate buffer with pH=5.0-6.0, acetic acid-sodium acetate buffer with pH=4.5, phosphate Buffer mixed solution or acetic acid-sodium acetate buffer mixed solution; wherein, the phosphate buffer mixed solution is added to methanol or acetonitrile by adding phosphate buffer solution with pH=5.0 to 6.0 to make the volume percentage 5% to 50% of the mixed solution (the volume percentage refers to the volume of the phosphate buffer solution in the mixed solution); the acetic acid-sodium acetate buffer solution is composed of acetic acid-sodium acetate with pH=4.5 The buffer solution is added to methanol or acetonitrile to prepare a mixed solution with a volume percentage of 5-50% (the volume percentage refers to the volume of the acetic acid-sodium acetate buffer solution in the mixed solution). Others are the same as in the first or ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com