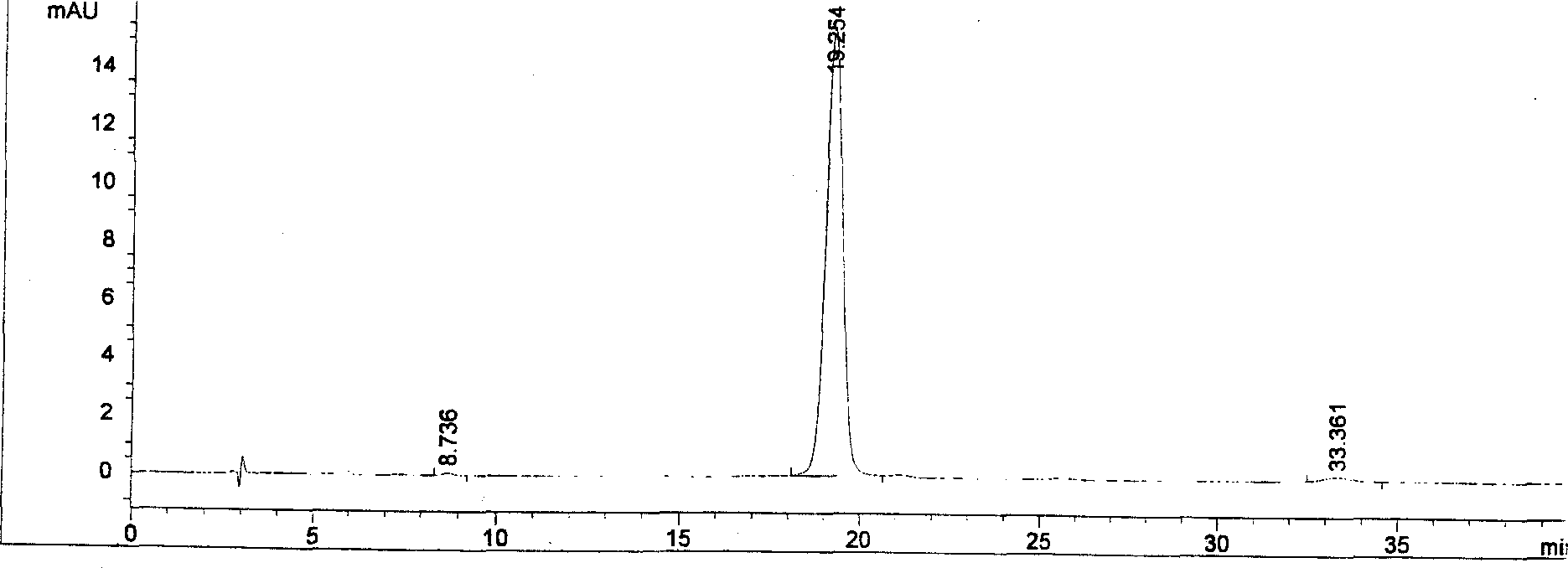
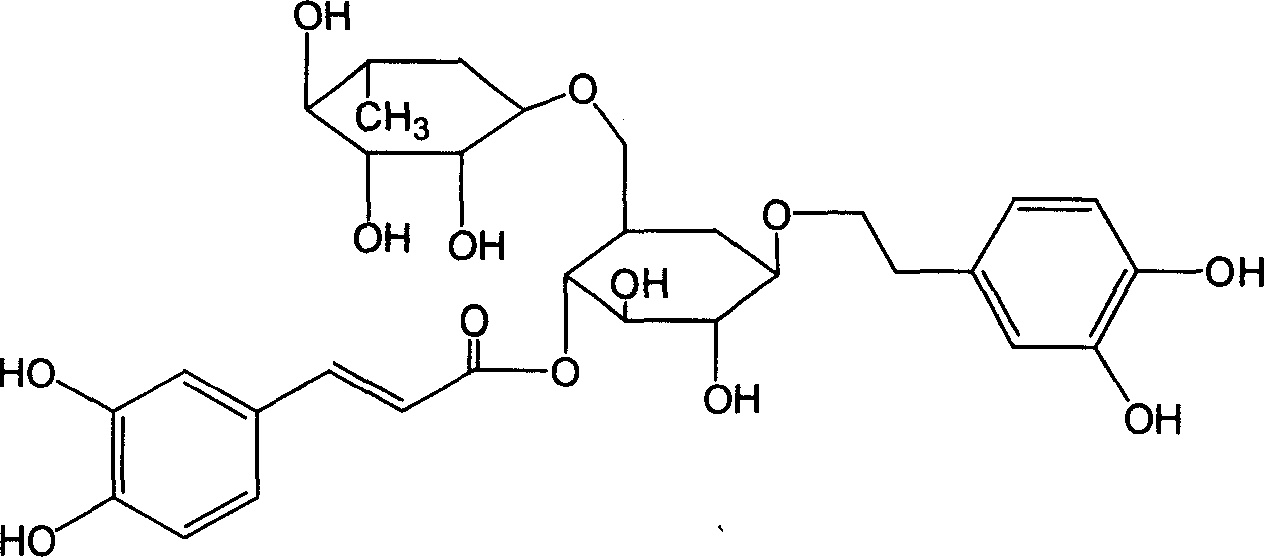
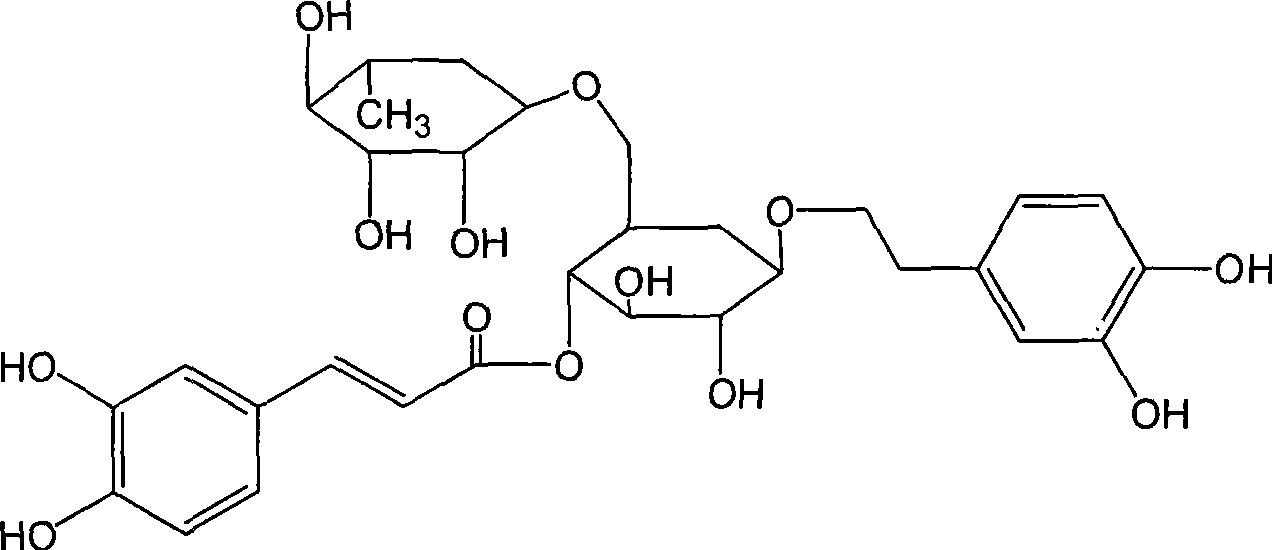
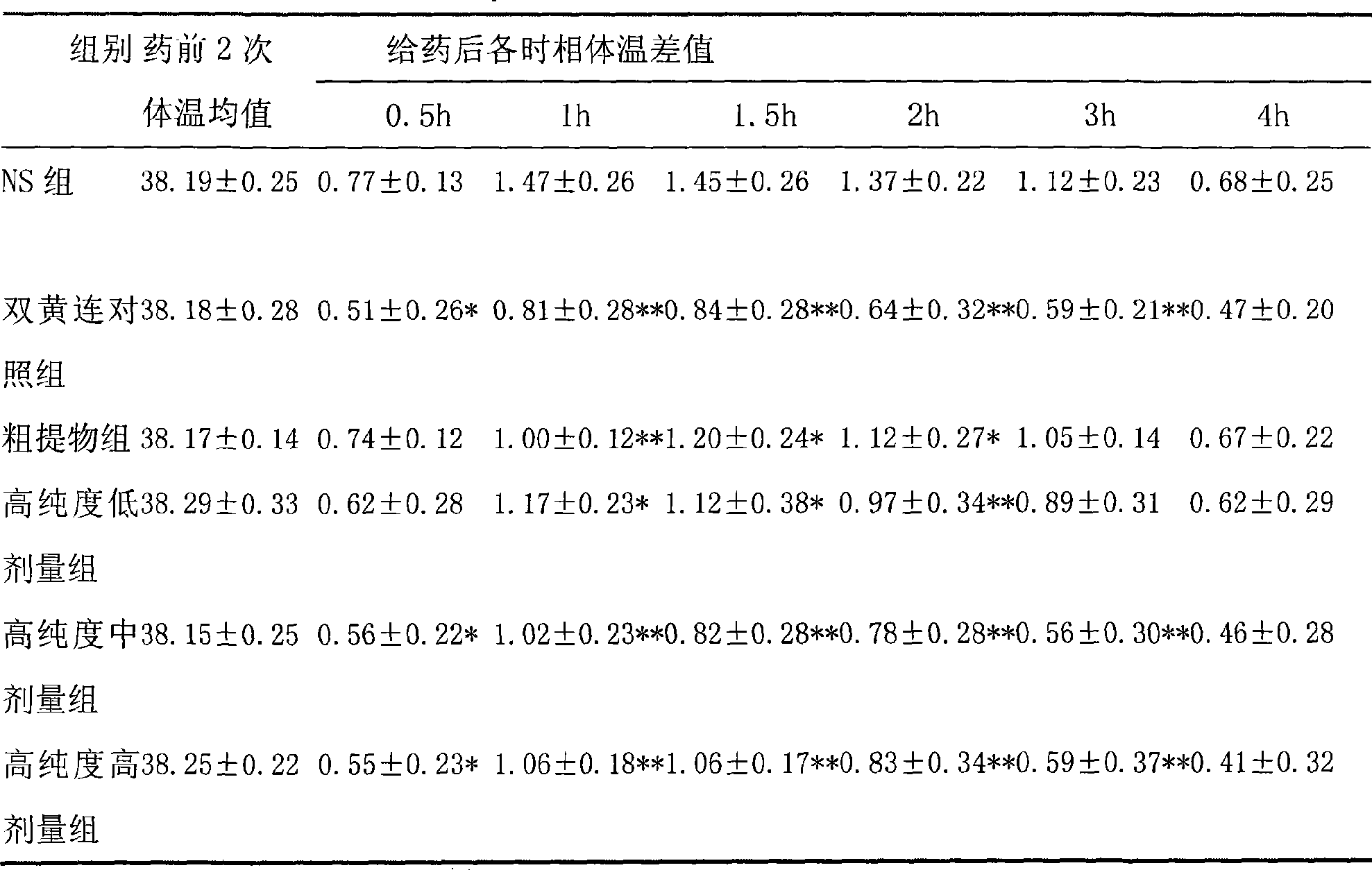
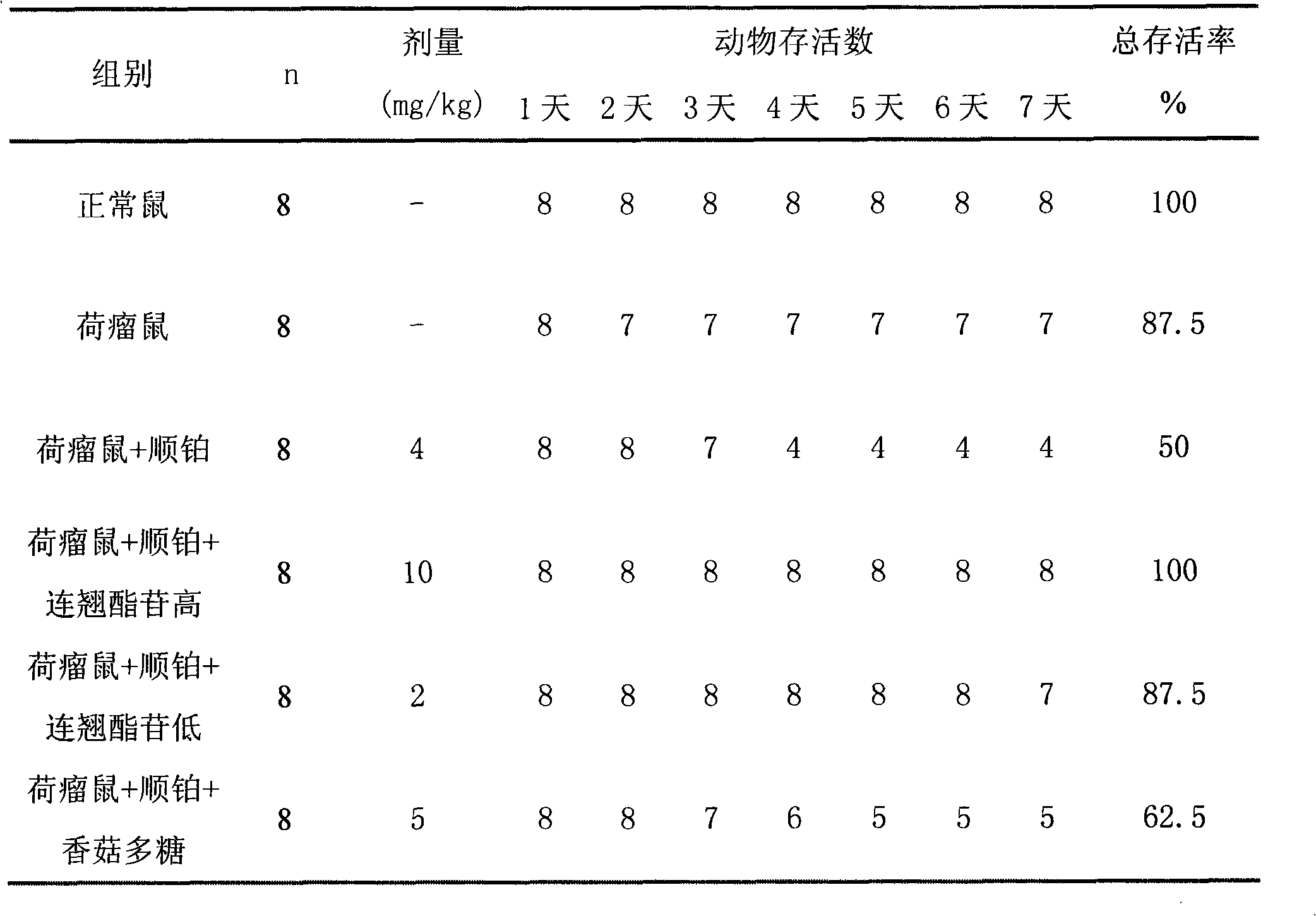
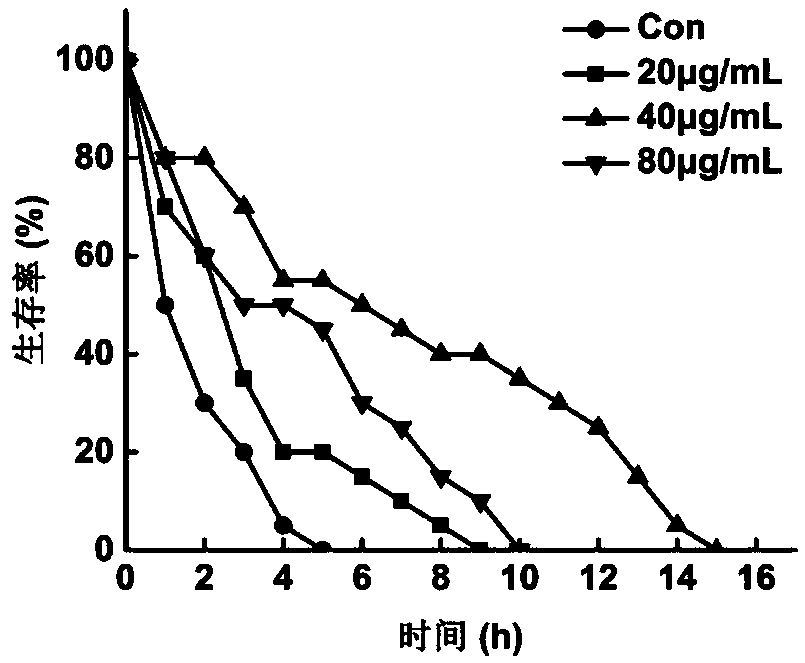
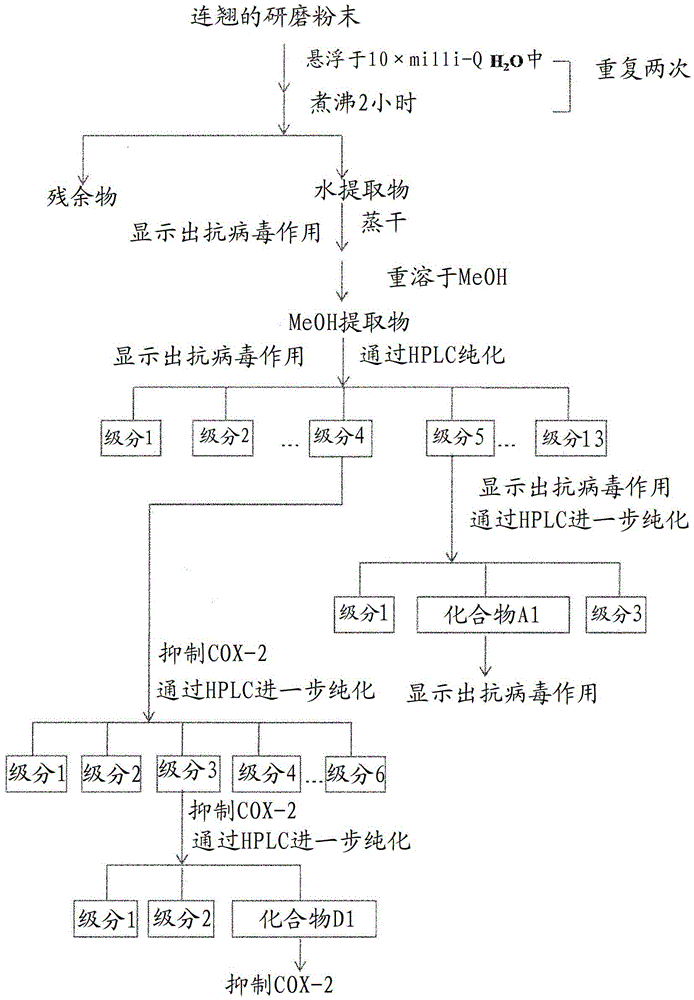
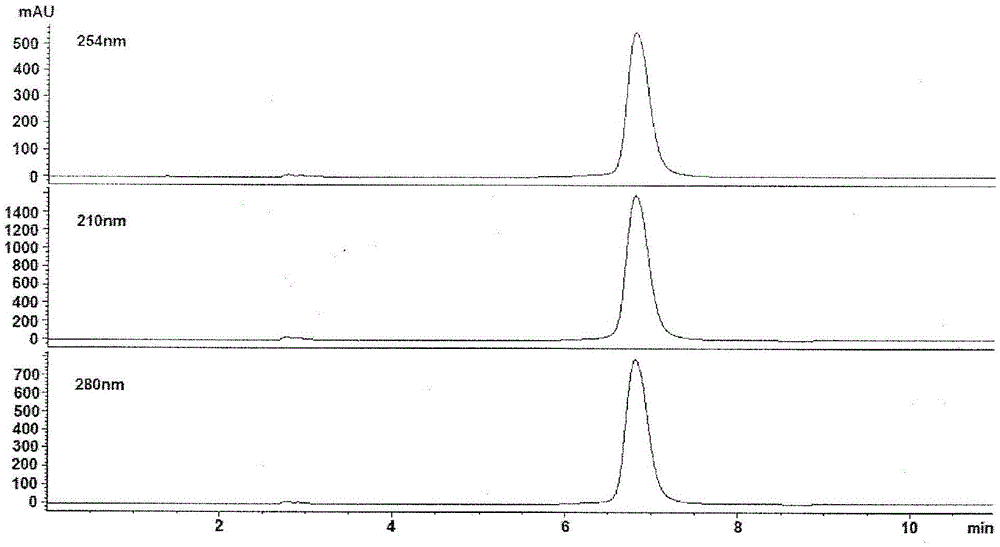
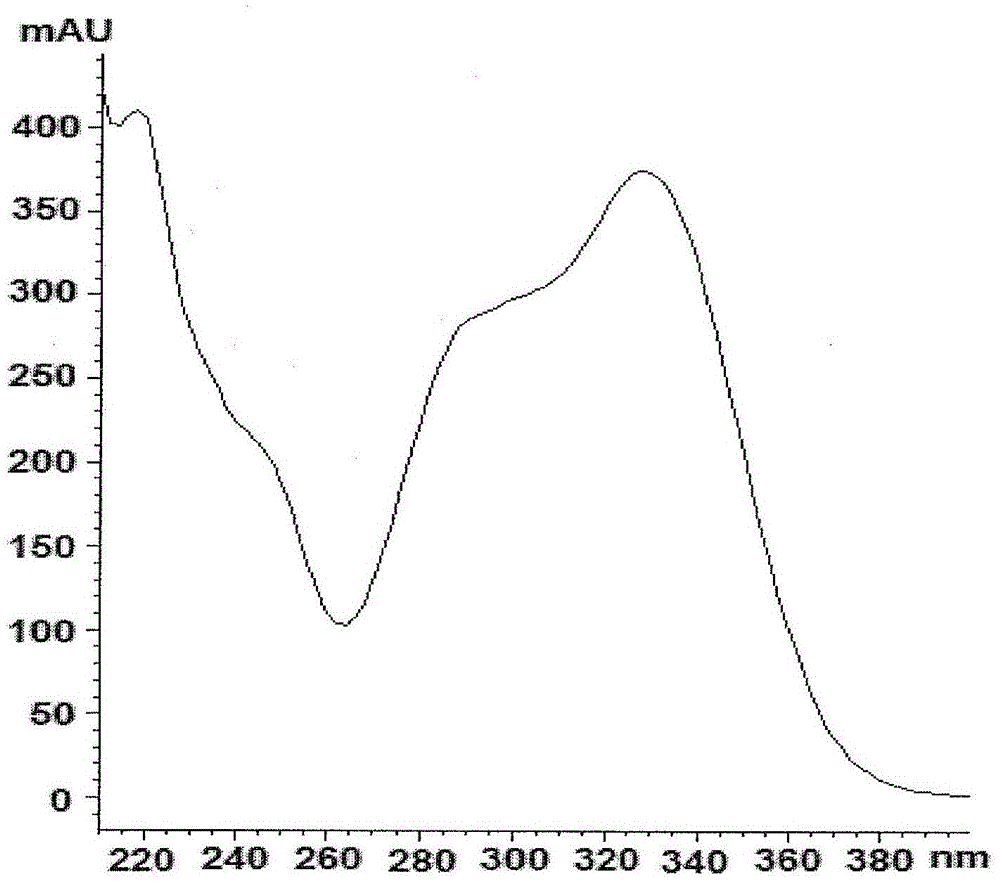
Patents
Literature
71 results about "Forsythoside A" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for purifying forsythiaside A from forsythia extractive
ActiveCN101081855AHigh purityReduce utilizationSugar derivativesSugar derivatives preparationForsythiaPhenol
The present invention relates to process of purifying forsythia extract to obtain forsythoside A. Specifically, forsythia phenols are further purified to obtain high purity forsythoside A in high extracting efficiency.
Owner:SHANDONG NEWTIME PHARMA
Use of high-purity forsythin in preparing bacteriostasis, antivirus and other medicine
ActiveCN101390869AIncrease contentEasy to removeAntibacterial agentsOrganic active ingredientsAnti virusBeta-hemolytic streptococcus
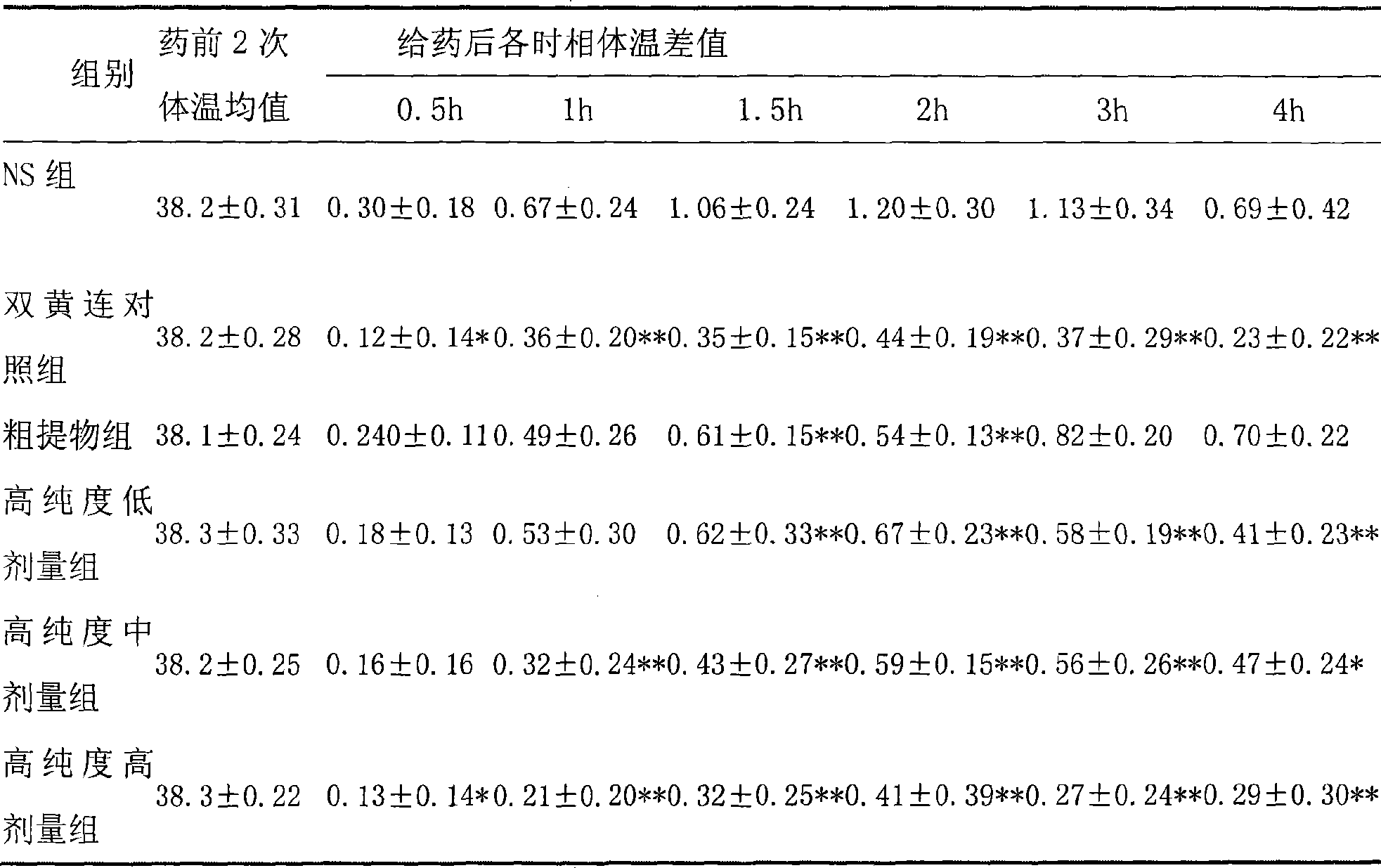
The invention belongs to the medical technical field and discloses the application of high-purity in the preparation of antibacterial medicine, antiviral medicine and other medicines. The invention proves the antipyretic, anti-inflammation, immunity enhancement, antibacterial and anti-virus effects of high-purity forsythiaside through the following experiments: (1) the experiment that high-purity forsythiaside has antipyretic effect on fever rats; (2) the experiment that high-purity forsythiaside inhibits xylene-induced mice ear edema, xylene-reduced capillary permeability of mice; (3) the experiment that high-purity forsythiaside increases carbon clearance ability of mice and increases the lymphocyte transformation rate; (4) the experiment that high-purity forsythiaside has vitro anti-influenza A type virus effect and in-vivo anti-respiratory syncytial virus effect; (5) the experiment that high-purity forsythiaside has vitro inhibition effects on beta-hemolytic streptococcus, staphylococcus aureus, streptococcus viridans and staphylococcus epidermidis.
Owner:SHANDONG NEWTIME PHARMA
Method for purifying forsythiaside A from forsythia extractive
ActiveCN101081857AHigh purityReduce utilizationSugar derivativesSugar derivatives preparationForsythiaPhenol
The present invention relates to process of purifying forsythia extract to obtain forsythoside A. Specifically, forsythia phenols are further purified to obtain high purity forsythoside A in high extracting efficiency.
Owner:SHANDONG NEWTIME PHARMA
Phenethyl alcohol glycoside-containing callicarpa kochiana extractive and preparation method thereof
InactiveCN101797307AHigh extraction rateSignificant efficacy in the treatment of Alzheimer's diseaseOrganic active ingredientsNervous disorderAlcoholCallicarpa kochiana
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
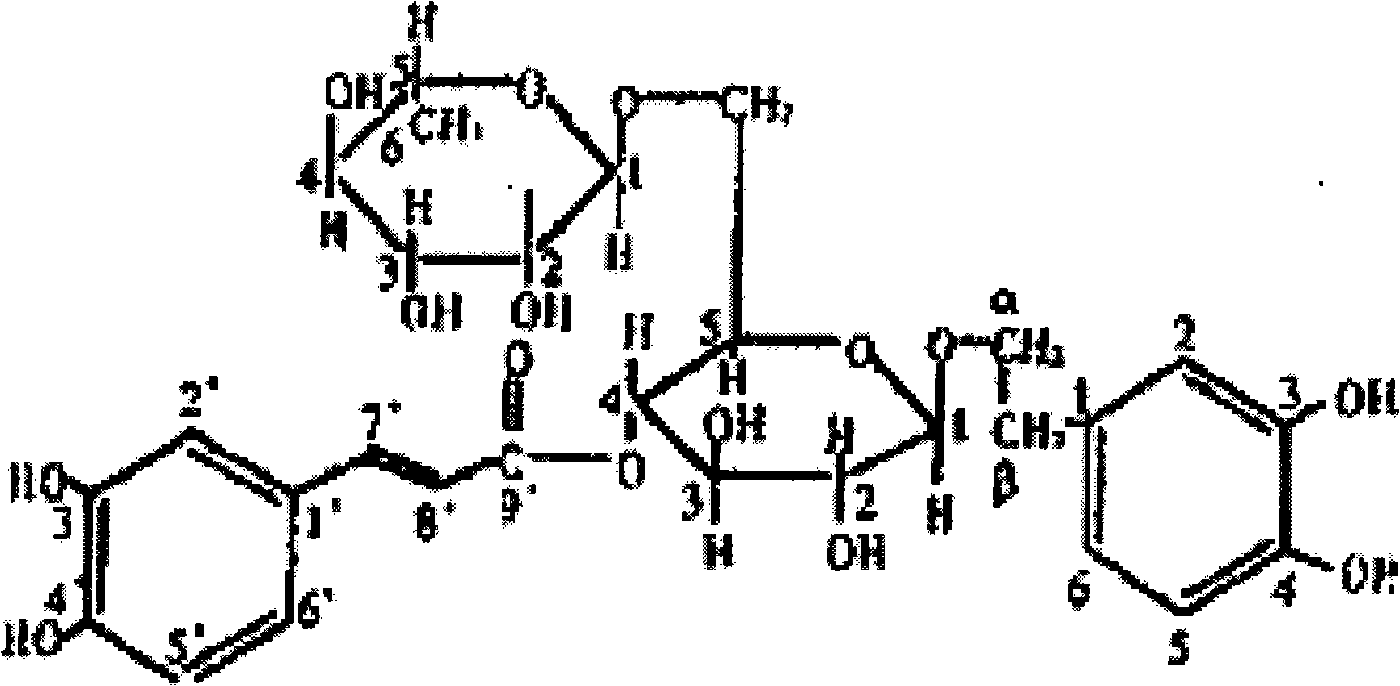
Application of Forsythoside A in preparing antineoplastic chemotherapy sensitivity-enhancing toxicity-reducing medicament or anti-AIDS sensitivity-enhancing toxicity-reducing medicament
InactiveCN101342185AGood curative effectLittle side effectsOrganic active ingredientsAntiviralsSide effectGlycoside formation
The present invention relates to the applications of forsythia ester glycoside in preparing a sensitivity-enhancing and toxicity-reducing medicine in resisting tumor chemotherapy or AIDS. The present invention is a medicinal compound prepared by forsythia ester glycoside and pharmaceutical carrier and / or excipient. The prepared medicinal compound is used for enhancing sensitivity and reducing toxicity in resisting tumor chemotherapy or AIDS, which has the advantages of good curing effect, less side effects and increasing the living quality of patients.
Owner:WUHAN UNIV
Method for testing quality of antiviral oral liquid for treating hand-foot-and-mouth disease
ActiveCN101843884ASimple methodComponent separationPharmaceutical delivery mechanismHand-foot-and-mouth diseaseMedicine
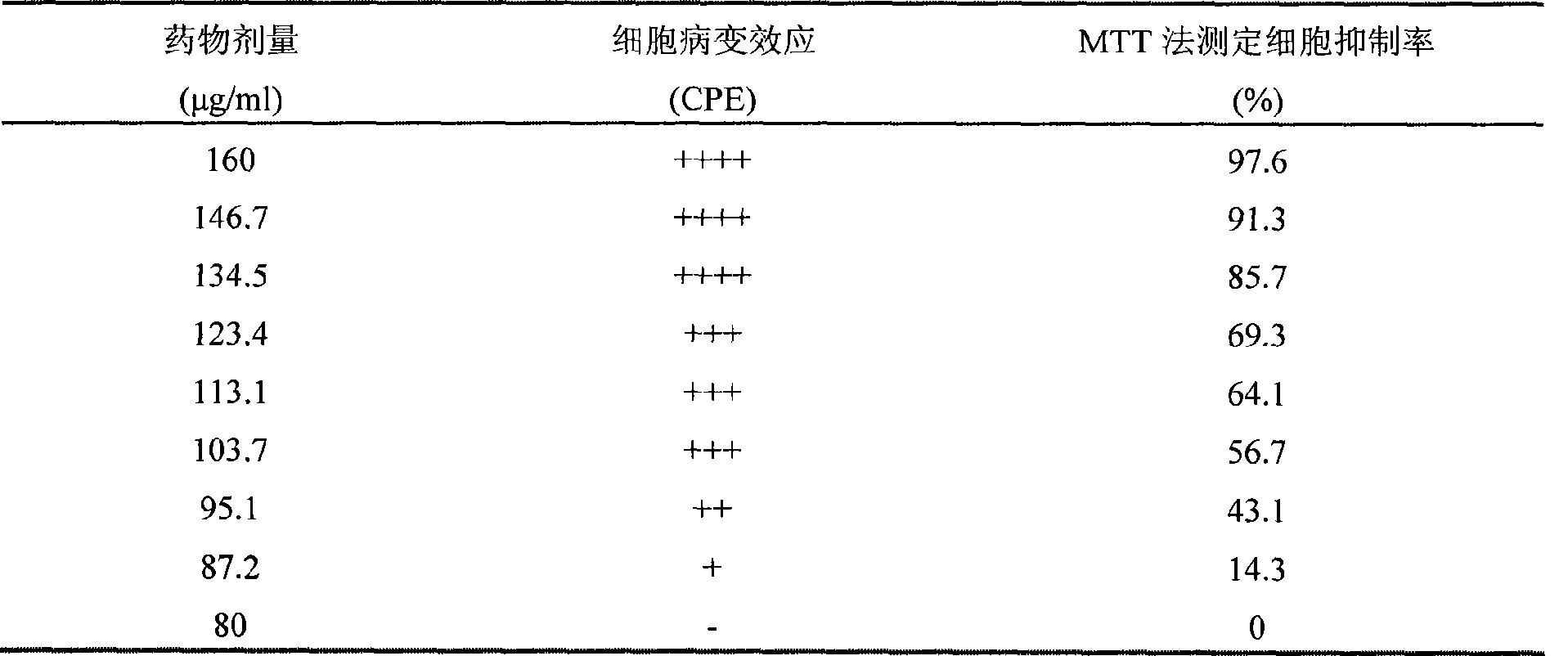
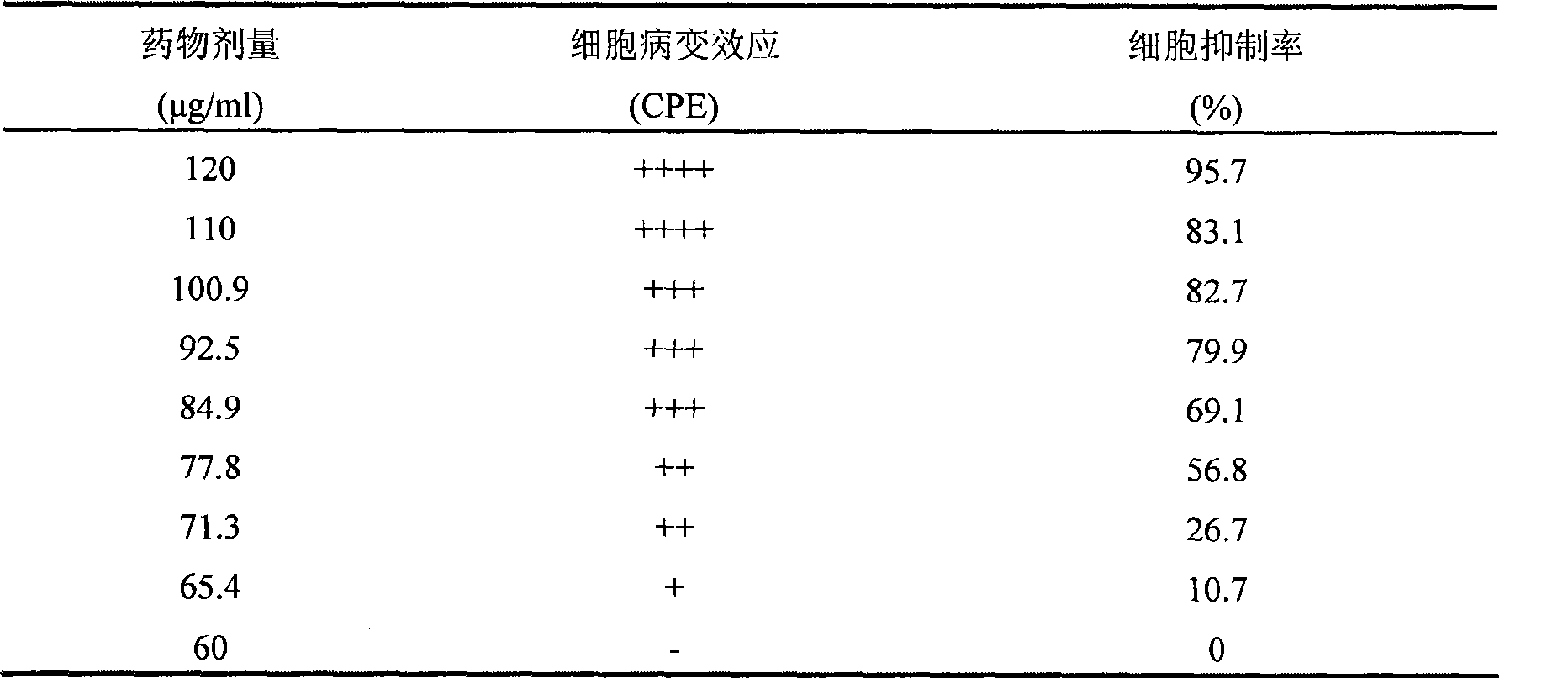
The invention discloses a method for testing the quality of antiviral oral liquid for treating the hand-foot-and-mouth disease and relates to the technical field of medicaments. In the method for testing the quality, the quality is tested by using a high performance liquid chromatogram single-test multi-evaluation method. 10 ml of antiviral oral liquid contains no less than 60 mu g of (R,S)-goitrin, no less than 1 mg of forsythoside A, no less than 0.25 mg of forsythin and no less than 0.12 mg of forsythingenin. The method has the advantages that: the method is simple and convenient; the content of four active ingredients of the antiviral oral liquid can be tested at the same time; the quality of the antiviral oral liquid and the curative effect of the antiviral oral liquid on the hand-foot-and-mouth disease can be comprehensively evaluated by establishing a quantitative analysis result of the active ingredients of the antiviral oral liquid, so the antiviral oral liquid has a truly-controllable standard; and simultaneously, the medicinal material basis of a Chinese medicinal preparation, namely, the antiviral oral liquid in the aspect of curing the hand-foot-and-mouth disease is put forward in China for the first time.
Owner:GUANGZHOU XIANGXUE PHARMA CO LTD
Olive total phenylethanoid glycoside composition and preparation and application thereof
InactiveCN102670631AGood against liver fibrosisGood liver protectionOrganic active ingredientsDigestive systemLiver fibrosisBULK ACTIVE INGREDIENT
The invention discloses an olive total phenylethanoid glycoside composition. The olive total phenylethanoid glycoside composition comprises 6 types of particular phenylethanoid glycoside compositions which include cistanoside E, calcelarioside B, verbascoside, isoverbascoside, forsythoside B and alyssonoside and are varied from a general formula A. Pathological tests show that the olive total phenylethanoid glycoside composition in the general formula A has good anti-liver fibrosis and liver protection effects, can be used as an active ingredient of medicines for resisting liver diseases, canbe independently used or combined with proper excipients and the like, and is prepared into oral or non-oral dosage forms by conventional methods to be used for preparing medicines for treating liverdiseases.
Owner:HENAN UNIV OF SCI & TECH
Research approach of IFN-alpha anti PCV2 and PRRSV induced by forsythiaside
InactiveCN101380330AEasy to get materialsExperimental data is reliableOrganic active ingredientsAntiviralsIfn alphaAnti virus
The invention relates to an application of forsythiaside in anti-PCV2 and anti-PRRSV. The measured prevention effect of the forsythiaside in anti-PCV2 and anti-PRRSV is greater than the treatment effect and further greater than the direct killing effect. The IFN-Alpha secretion after being acted in cells is gradually increased along with the increase of the concentration of the forsythiaside within the range of the safe concentration of the forsythiaside, the virus proliferation is gradually decreased, while the virus proliferation and the amount of the IFN-Alpha show negative correlation. The forsythiaside can induce the IFN-Alpha to express and play the anti-virus role. The invention provides a new method for researching anti-virus traditional Chinese medicine, provides a drug for treating PCV2 and PRSSV and lays the foundation for the action mechanism of anti-PCV2 and anti-PRRSV in vitro of the forsythiaside. The application has the advantages that: materials are easy to obtain, cells are a cell system which can be passaged, the application has no individual difference of animals, and the obtained experimental data is comparatively reliable.
Owner:BEIJING UNIV OF AGRI
Traditional Chinese medicine composition with effects of clearing away heat and toxic materials as well as preparation method and drug preparation thereof
InactiveCN107126465AGood treatment effectAvoid lostOrganic active ingredientsAntipyreticChlorogenic acidCurative effect
The invention relates to a traditional Chinese medicine composition with effects of clearing away heat and toxic materials as well as preparation method and drug preparation thereof, and belongs to the field of traditional Chinese medicines. The traditional Chinese medicine composition with effects of clearing away heat and toxic materials comprises baicalin, chlorogenic acid, galuteolin, forsythiaside a, (R, S)-epigoitrin and total volatile oil, wherein a weight ratio of the baicalin to chlorogenic acid to galuteolin to forsythiaside a to (R, S)-epigoitrin to total volatile oil is (150-600):(10-60):(1-6):(10-50):(2-9):(50-200); and the total volatile oil is extracted from lonicera japonica, fructus forsythia and isatis root. The traditional Chinese medicine composition disclosed by the invention is high in content of active ingredients and excellent in curative effect of the product.
Owner:HEILONGJIANG ZBD PHARMA +1
Preparation method of forsythoside A
InactiveCN102060884AProcess safetyNo pollution in the processSugar derivativesSugar derivatives preparationAlcoholControllability
The invention discloses a preparation method of forsythoside A, comprising the following steps of: (1) extraction; (2) alcohol precipitation; (3) separation; (4) purification; and (5) enrichment. The preparation method has the advantages of simple and safe process, no pollution, more convenience for production and operation, capability of realizing mass production, conformity to industrial requirement, lower cost, reliable and stable process data, good reproducibility, strong controllability for process, and conformity to GMP (Good Manufacturing Practice) production requirement.
Owner:TIANJIN TASLY PHARMA CO LTD
Quality control reference substance for antelope's horn tablets for common cold and application of quality control reference substance
InactiveCN104374841AAdaptableGreat application potentialComponent separationChlorogenic acidChinese patent
Owner:SHANDONG INST FOR FOOD & DRUG CONTROL
Application of forsythiaside I and preparation method thereof
ActiveCN105287610AGood effectInhibition of syncytial virusOrganic active ingredientsSugar derivativesBiotechnologyPharmaceutical drug
The invention discloses application of forsythiaside I in preparation of drugs for inhibiting respiratory syncytial viruses, and belongs to the field of application of plant extracts. An experiment proves that the forsythiaside I can effectively inhibit the respiratory syncytial viruses.
Owner:SHIJIAZHUANG YILING PHARMA
Antioxidant honey tea powder
The invention discloses antioxidant honey tea powder. The antioxidant honey tea powder comprises, by weight, 280-320 parts of green tea water extract liquid, 28-32 parts of honey, 14-16 parts of beta-cyclodextrine, 2-3 parts of lecithin, 1-2 parts of sodium carboxymethyl celluloses, 0.9-1.1 parts of gelatin, 6-7 parts of soluble corn starch and 2-3 parts of antioxidant compositions. The antioxidant compositions comprise, by weight, 1-2 parts of radix et rhizoma rhodiolae extract, 1-3 parts of flavone from radix puerariae, 1-2 parts of puerarin, 3-5 parts of total flavone from radix glycyrrhizae, 2-4 parts of polysaccharides from fructus lycii, 2-3 parts of pectin from fructus crataegi, 1-3 parts of forsythiaside, 3-4 parts of total flavone from herba epimedii and 1-2 parts of rhizoma polygoni cuspidate extract. The antioxidant honey tea powder has the advantages of high antioxidant capacity, good solubility, dispersibility, stability and taste and abundant nutrition.
Owner:FANCHANG COUNTY BEISI ENTREPRENEUR SERVICE
Forsythoside A drug composite
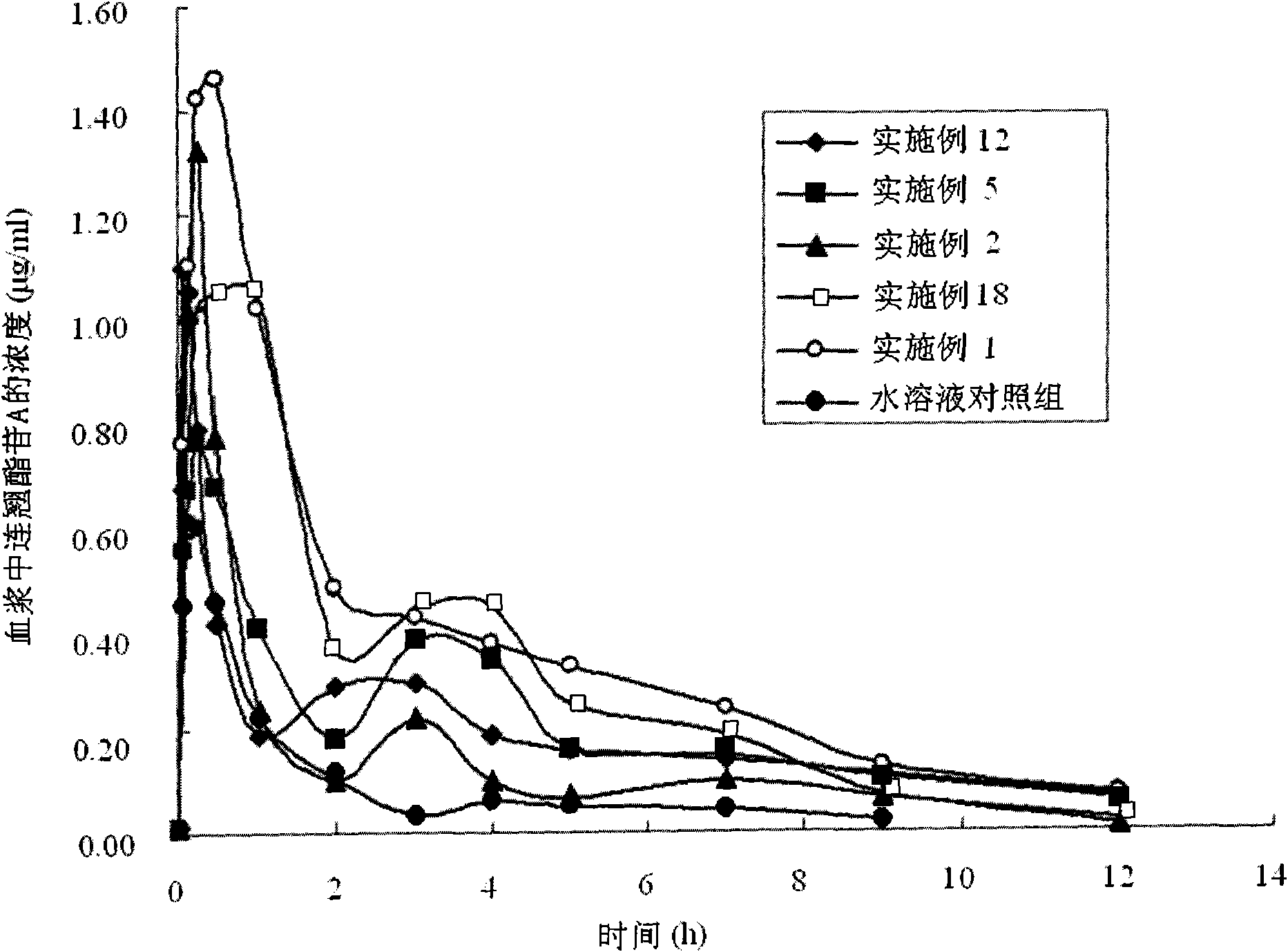
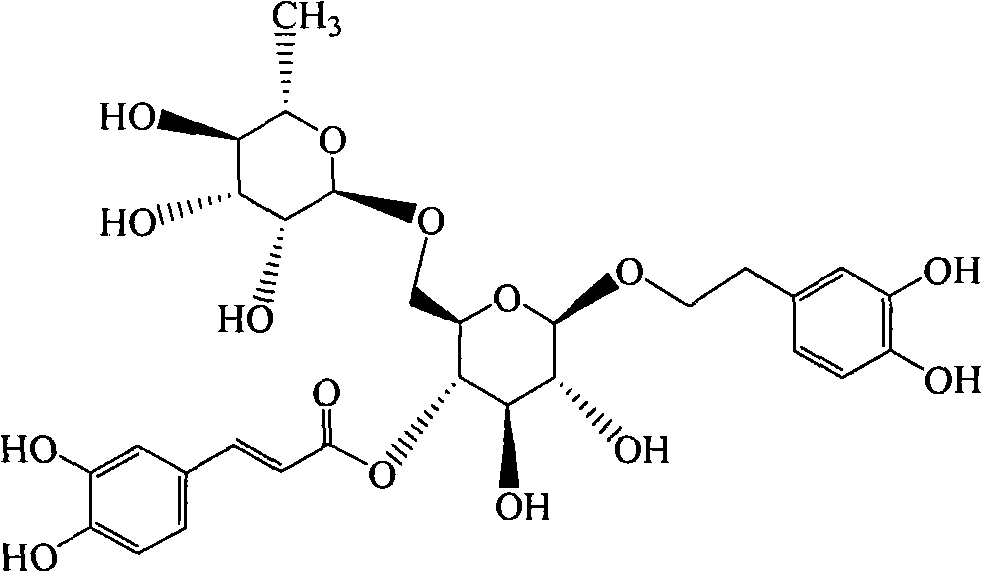
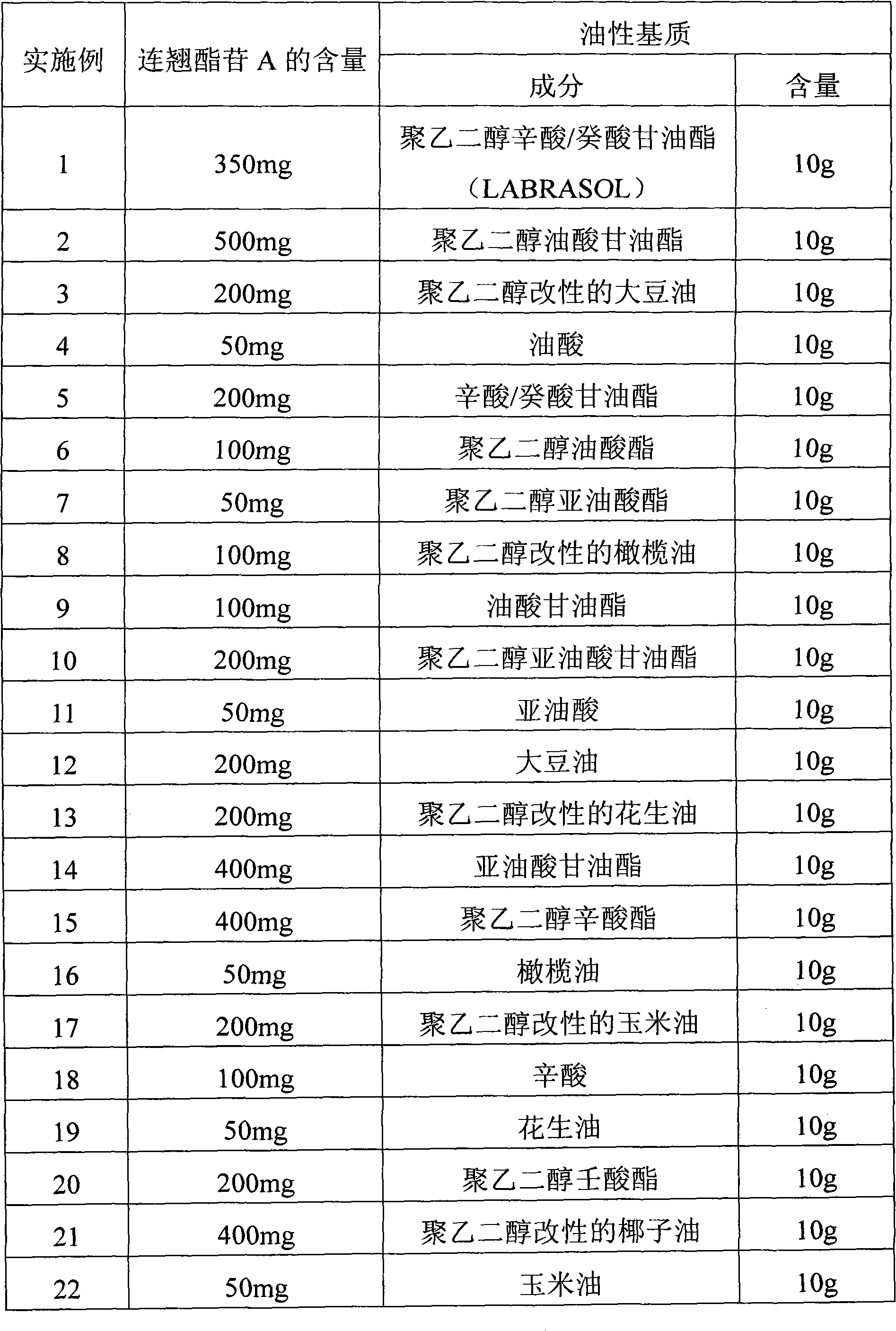
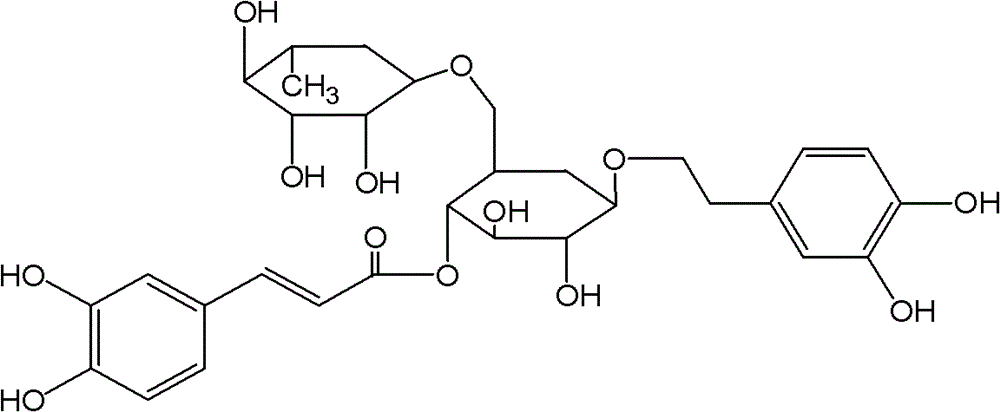
InactiveCN101919869APromote absorptionIncrease blood concentrationOrganic active ingredientsDigestive systemBlood concentrationBioavailability
The invention discloses a forsythoside A drug composite, which contains forsythoside A and oleaginous base plant extract, wherein, the mass ratio of forsythoside A to oleaginous base and / or oleaginous base plant extract is 0.05:10-0.5:10. The forsythoside A drug composite can be well absorbed in gastrointestinal tracts, can acquire higher blood concentration, and has high oral bioavailability.
Owner:SHANGHAI INST OF PHARMA IND
Preparation method for forsythoside A
InactiveCN102060885AReduce manufacturing costSimple processSugar derivativesSugar derivatives preparationAlcoholPolyamide
The invention discloses a preparation method for forsythoside A. The preparation method for the forsythoside A comprises the following steps of: (1) immersing raw material medicament in acid, and extracting; (2) separating by using resin; (3) separating by using polyamide; (4) separating by using resin; (5) purifying by using reverse phase silica gel; and (6) purifying by using dextrangel. According to the method, an alcohol-water system is used for extraction and separation, and resin materials used in the process are recycled, so the production cost is reduced. By the extraction method of the preparation method for the forsythoside A, the content of the final product forsythoside A is up to 90 to 95 percent; therefore, the process is simple and efficient, and is suitable for industrial production.
Owner:TIANJIN TASLY PHARMA CO LTD
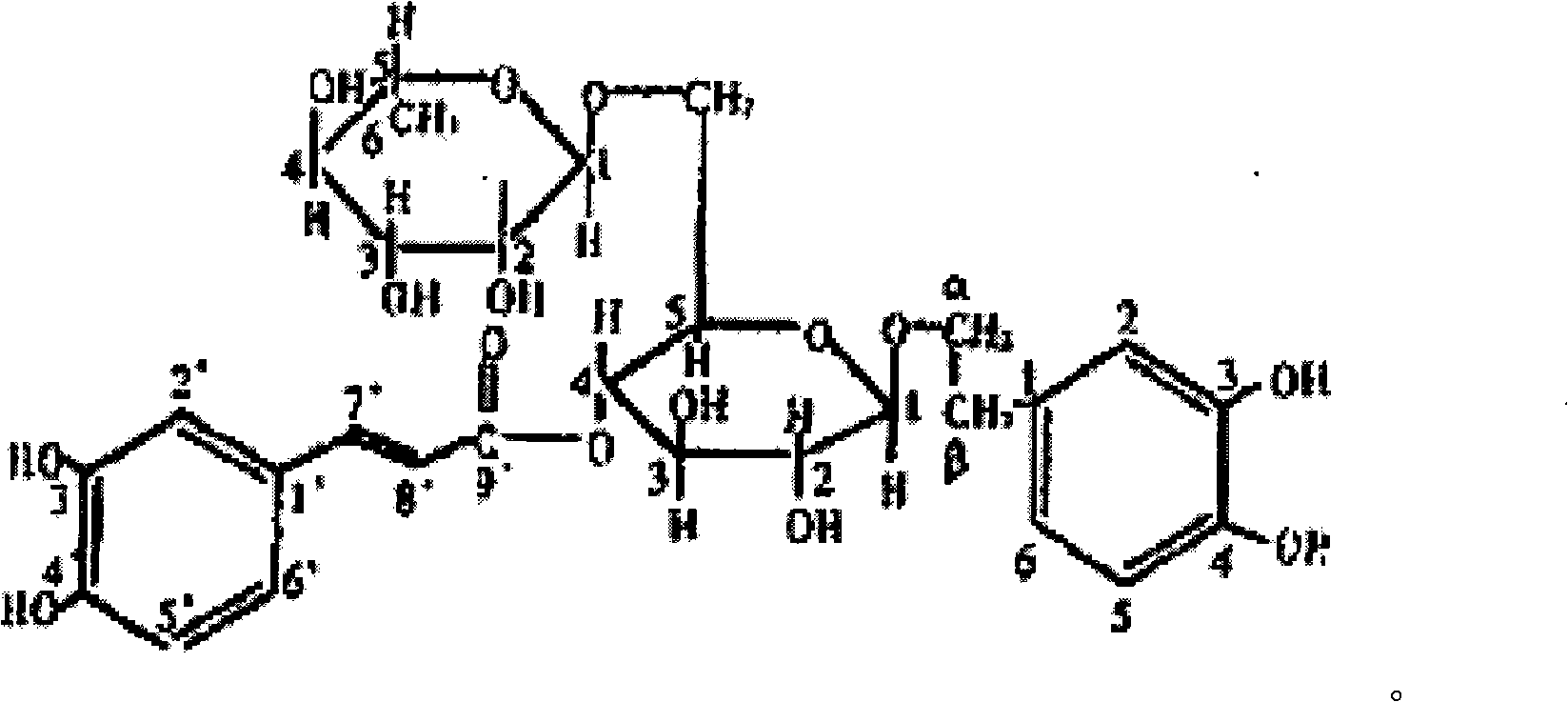
Forsythoside A and preparation method thereof
The invention provides a forsythoside A and a preparation method thereof, and belongs to the field of medicines. In the preparation method, fruits of a traditional Chinese medicine of fructus forsythia are taken as raw materials, and are extracted, separated, crystallized, recrystallized and the like, and the high-content forsythoside A with good effect of clearing heat and releasing toxin and capacity of preventing acute or chronic hepatic injury is prepared; and a high performance liquid chromatography is adopted for measuring, and the content range is 60-99.8 percent. The modern separation and purification technology for traditional Chinese medicines is adopted, so the preparation method is novel in process, simple and convenient to operate and suitable for industrial production, and has high practicability.
Owner:JIANGSU TIANSHENG PHARMA
Application of Forsythoside A in preparation of anti-influenza A H1N1 virus medicine and preparation thereof
Belonging to the field of traditional Chinese medicines, the invention discloses application of Forsythoside A in preparation of an anti-influenza A H1N1 virus medicine and a preparation thereof. In vivo pharmacodynamic experimental study on the Forsythoside A provided in the invention in treatment of respiratory virus infection and in vitro anti-respiratory virus pharmacodynamic experimental study on the Forsythoside A show that the Forsythoside A has an obvious inhibiting effect on influenza A H1N1 viruses.
Owner:LUNAN PHARMA GROUP CORPORATION
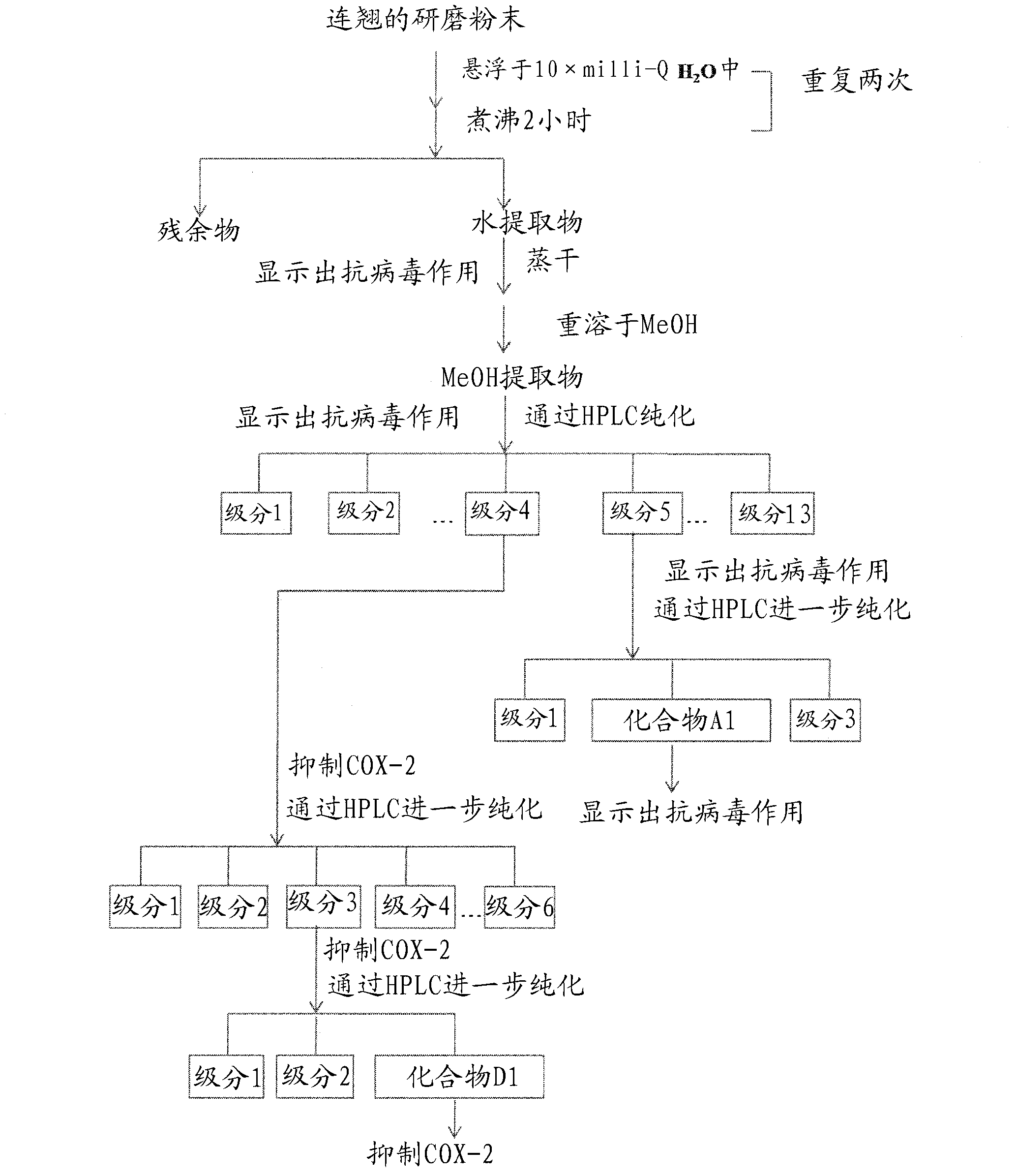
Materials and Methods for Prevention and Treatment of Viral Infections
The subject invention provides a novel and advantageous method for preventing and treating viral infection. Specifically exemplified herein are therapeutic uses of forsythoside A and jacaranone, compounds isolated from traditional Chinese medicinal material such as Fructus forsythiae (Lian Qiao). Also provided is use of a Yin Qiao San composition for preventing and treating viral infection.
Owner:BAGI RES +1
Materials and methods for prevention and treatment of viral infections
The subject invention provides a novel and advantageous method for preventing and treating viral infection. Specifically exemplified herein are therapeutic uses of forsythoside A and jacaranone, compounds isolated from traditional Chinese medicinal material such as Fructus forsythiae (Lian Qiao). Also provided is use of a Yin Qiao San composition for preventing and treating viral infection.
Owner:BAGI RES +1
Preparation method of forsythoside-beta-cyclodextrin inclusion compound
InactiveCN101474412AReduce manufacturing costSimple processOrganic active ingredientsAntimycoticsBeta-CyclodextrinsColloid mill
The invention provides a method for preparing a forsythoside-beta-cyclodextrin inclusion compound. Forsythoside extract with the purity of 50%-60% is used as raw material, and the forsythoside-beta-cyclodextrin inclusion compound is prepared by a colloid mill inclusion method. The content of the forsythoside in the forsythoside-beta-cyclodextrin inclusion compound is 10%-20%, the inclusion ratio of the forsythoside is more than 85 percent, and the inclusion compound yield is more than 70 percent.
Owner:SHANXI UNIV
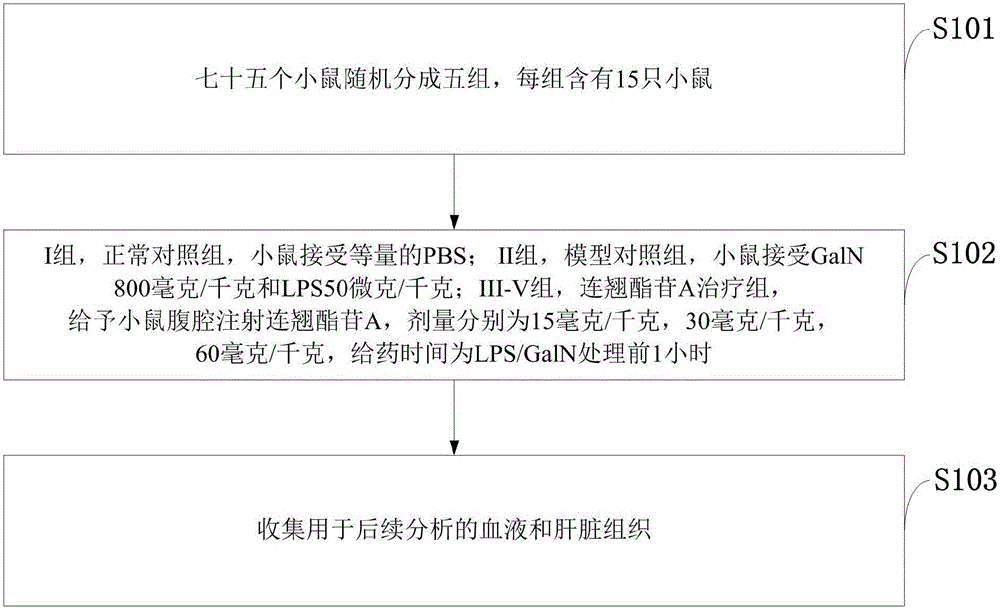
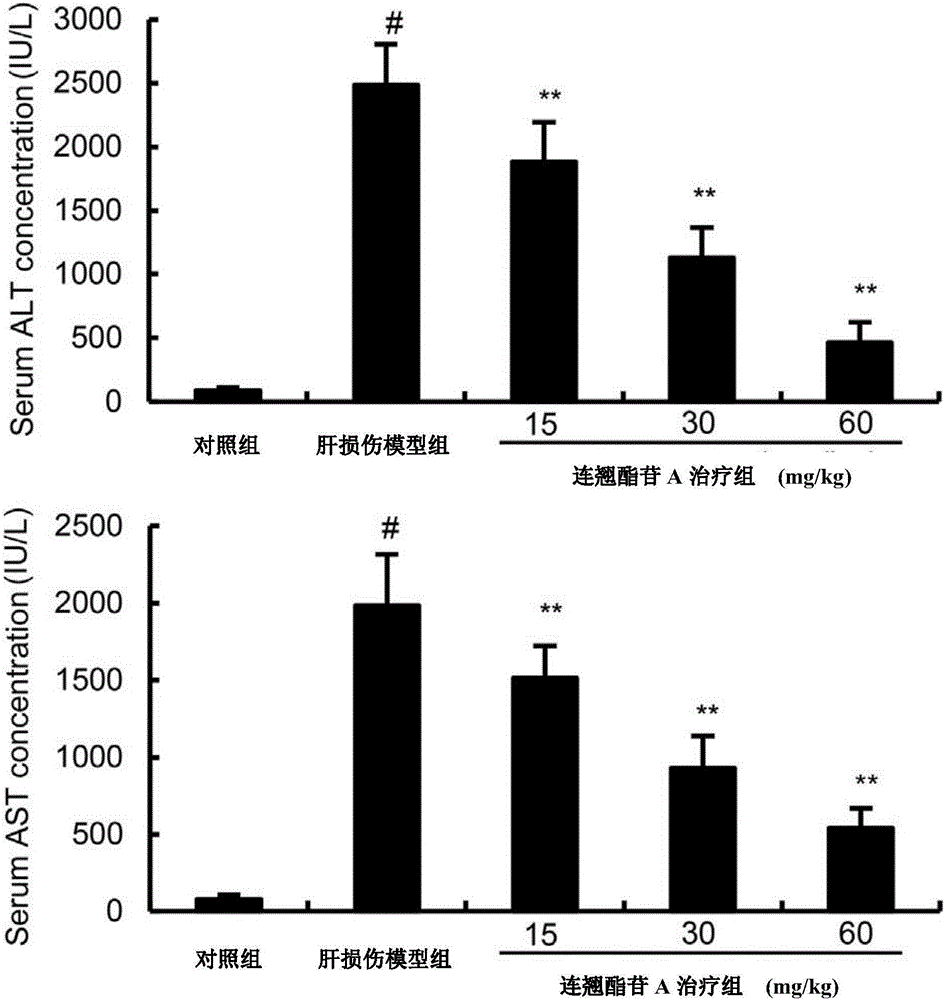
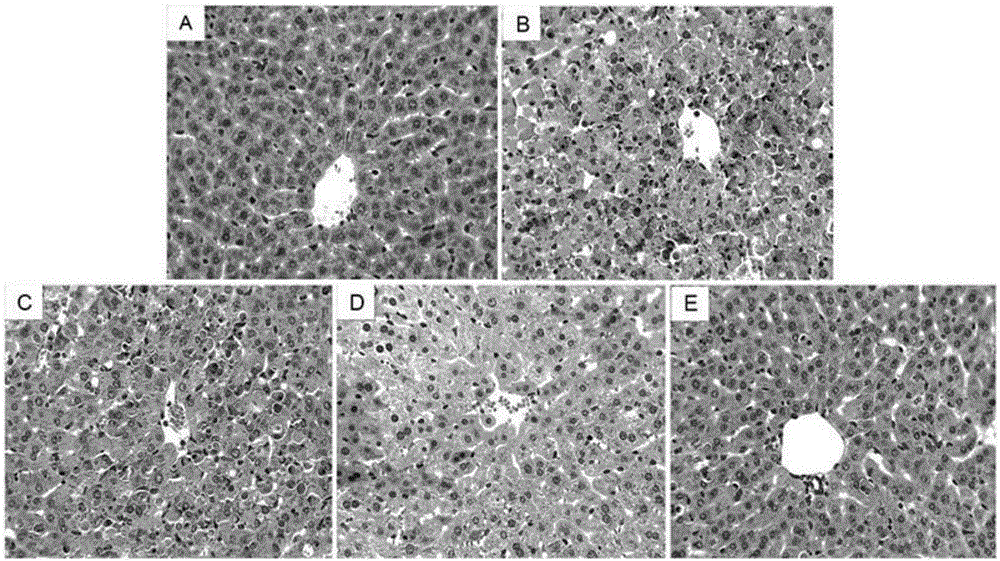
Medicine for treating lipopolysaccharide/D-aminogalactose induced liver injury
InactiveCN106822159AReduce pathological damageReduce malondialdehyde (MDA) contentCompounds screening/testingOrganic active ingredientsHeme oxygenaseAlanine aminotransferase
The invention discloses a medicine for treating lipopolysaccharide / D-aminogalactose (LPS / GalN) induced liver injury. The medicine for treating lipopolysaccharide / D-aminogalactose (LPS / GalN) induced liver injury is forsythiaside A. The forsythiaside A is capable of alleviating liver pathological injury induced by lipopolysaccharide / D-aminogalactose (LPS / GalN), reducing content of malonaldehyde (MDA) and serum ALT (Alanine Aminotransferase) and AST (Aspartate Amino Transferase) levels. In addition, the forsythiaside A is capable of inhibiting NF-chiB activation, serum TNF-alpha and liver TNF-alpha levels caused by LPS / GalN. In addition, the forsythiaside A is capable of increasing expression of Nrf2 and haem oxygenase 1. Results show that the protection function of the forsythiaside A on LPS / GalN induced liver injury is achieved by activating Nrf2 and inhibiting NF-chiB activation.
Owner:THE SECOND HOSPITAL AFFILIATED TO WENZHOU MEDICAL COLLEGE
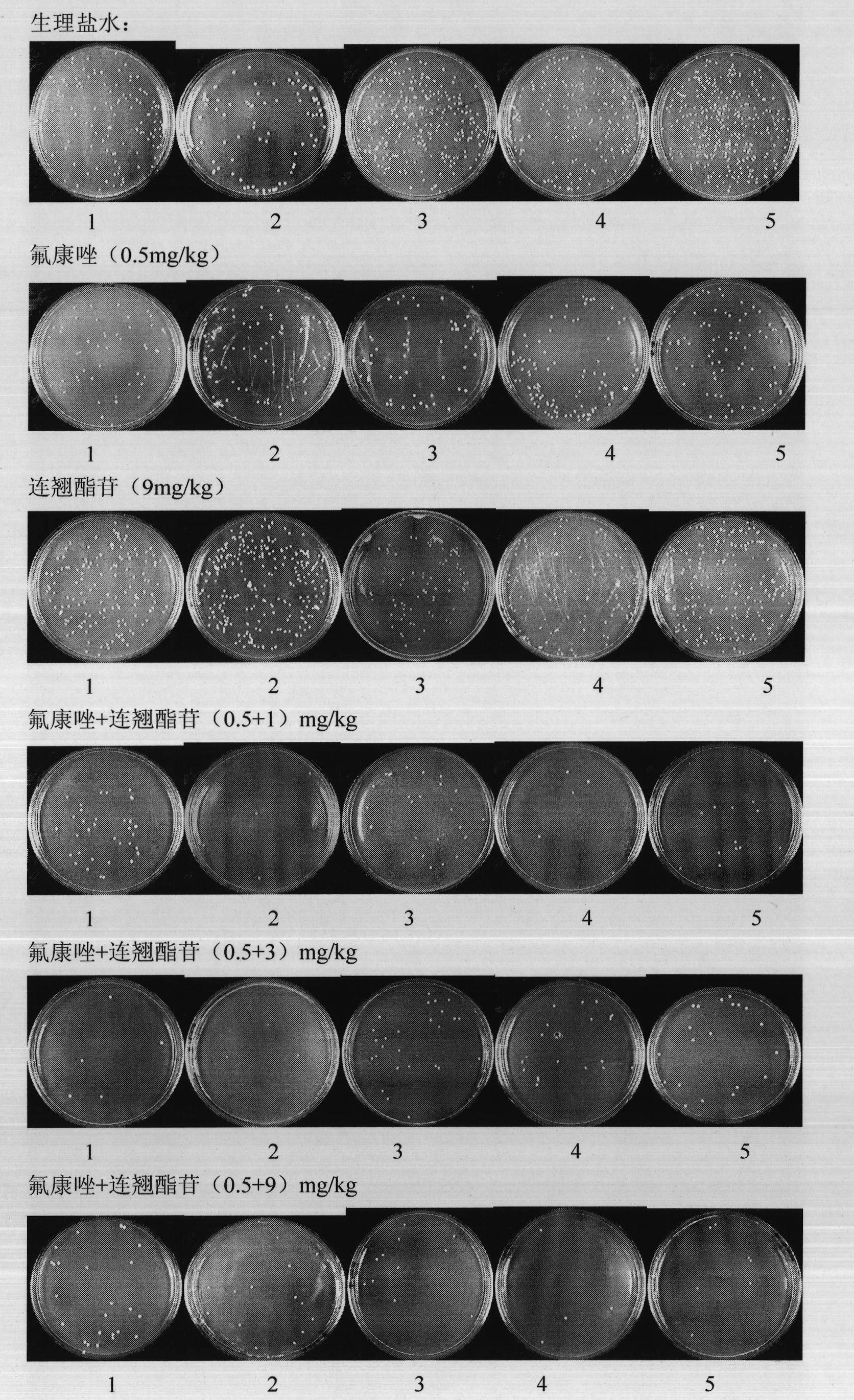
Use of forsythiaside as synergist of antifungal agents
InactiveCN101816666AReduce doseEnhanced inhibitory effectOrganic active ingredientsAntimycoticsSide effectFluconazole
The invention relates to the technical field of medicines and discloses a new use of forsythiaside as a synergist of antifungal agents. In vivo and in vitro experiments show that the forsythiaside can be combined with the antifungal agents, such as fluconazole, itraconazole, miconazole, ketoconazole and the like, thereby improving the treatment effect of superficial and deep fungal infection with different degrees, leading the antifungal agents to recover the role to fungi with drug resistance and further using the forsythiaside as the synergist of the antifungal agents. The invention opens up the new use of the forsythiaside, and the use of the forsythiaside as the synergist of the antifungal agents can not only improve the antifungal role of drugs, but also lead the antifungal agents to recover the role to the fungi with drug resistance under the situations that the clinical drug resistance of the fungi is increasingly common and the drug resistance degree is increasingly serious, and further reduce the dosage of the antifungal agents, thereby saving medical expenses for patients and reducing toxicity and side effects of the drugs.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Chinese patent medicine preparation for pneumonia and preparation method thereof
InactiveCN113082071AAvoid adverse effects such as drug resistanceGood effectOrganic active ingredientsRespiratory disorderAntibiotic drugChinese patent medicine
The invention discloses a Chinese patent medicine preparation for pneumonia and a preparation method thereof. The Chinese patent medicine preparation for pneumonia is prepared from the following raw materials in parts by weight: 40-50 parts of dry honeysuckle extract powder, 8-10 parts of forsythoside A, 5-10 parts of arctigenin, 1-5 parts of platycodin D, 2-5 parts of saikoside, 2-6 parts of amygdalin and 1-10 parts of licoflavone. According to the Chinese patent medicine preparation for pneumonia, pure traditional Chinese medicinal materials are used as raw materials, and effective components directed at pneumonia are extracted; and compared with direct taking of traditional Chinese medicines, the effect of the the Chinese patent medicine preparation is faster, taking of the the Chinese patent medicine preparation is convenient, adverse effects such as body weight losing and drug resistance generation caused by large-amount taking of western antibiotic medicines are avoided, and pneumonia can be fundamentally treated and is not prone to relapse.
Owner:南京康天生物科技有限公司
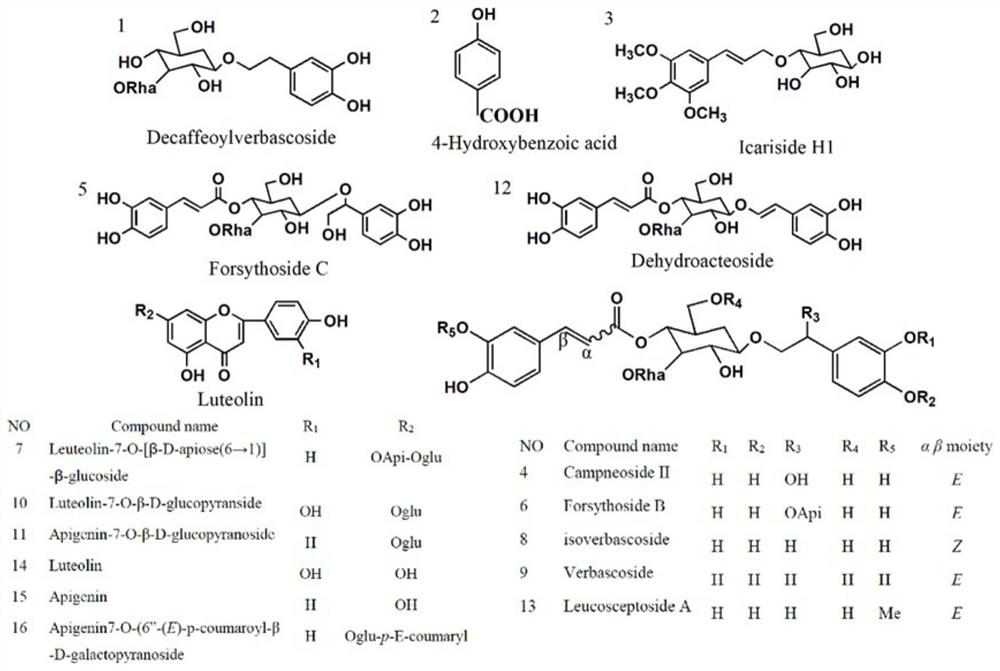
Extraction method of lamiophlomis rotata phenolic glycoside and application in medicine or health care product for preventing and treating hepatic fibrosis
The invention belongs to the field of medicines and health-care products, and particularly relates to application of a phenolic glycoside mixture in preparation of medicines or health-care products for preventing and treating hepatic fibrosis, in particular to a phenolic glycoside mixture of a total phenolic glycoside extract of lamiophlomis rotata, and an extraction method of the total phenolic glycoside extract of lamiophlomis rotata. The total phenolic glycoside extract of the lamiophlomis rotata comprises eight symbolic components, namely verbascoside, forsythiaside B, 4-hydroxybenzoic acid, icariin H1, Decaffeoylverbascoside, cosmosiin, luteolin and apigenin, has an anti-hepatic fibrosis effect, and can be used for preparing a medicine for preventing and treating hepatic fibrosis or a health-care product for assisting in treating CCl4 liver injury. According to the extraction method of the lamiophlomis rotata total phenolic glycoside extract, the polyacrylamide gel resin is adopted, so that separation and purification of the lamiophlomis rotata total phenolic glycoside extract are smoother, and the yield is higher.
Owner:CHONGQING MEDICAL UNIVERSITY
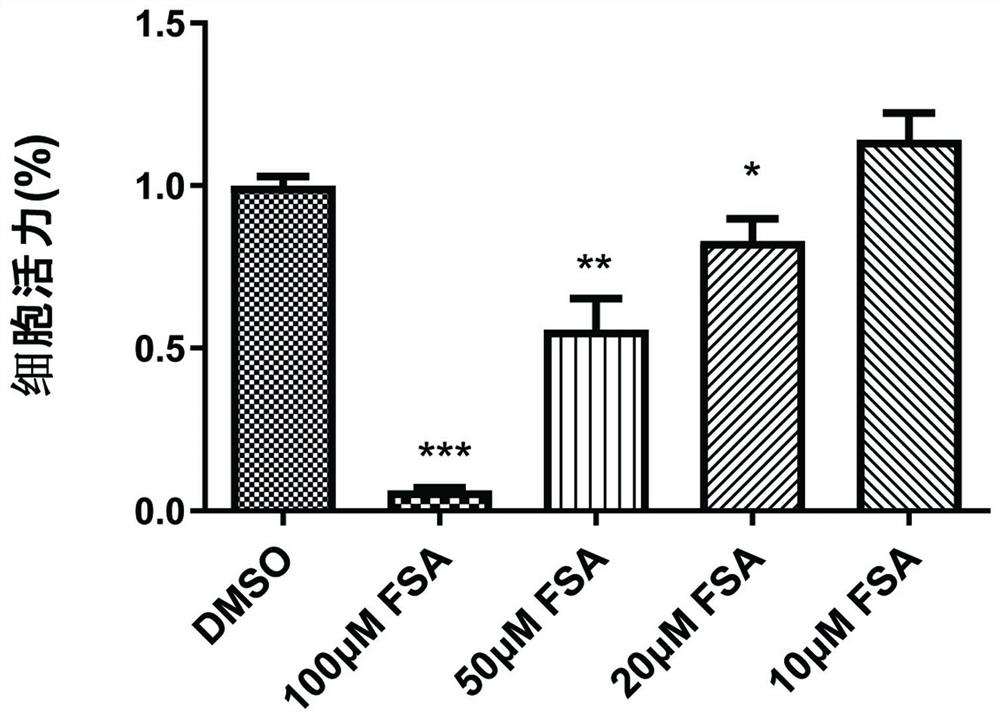
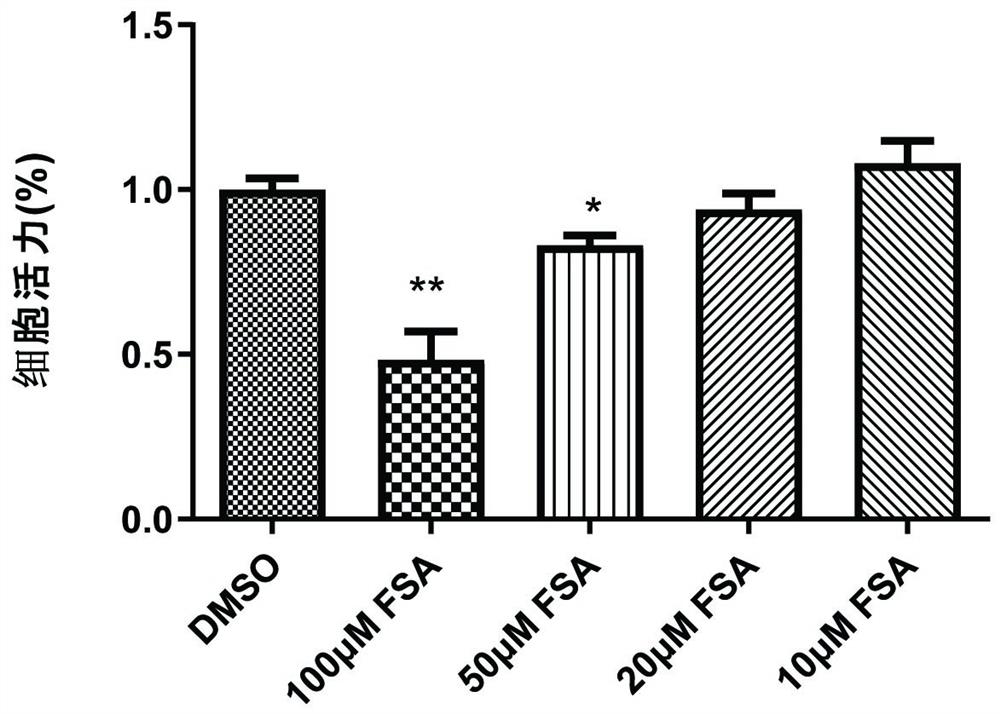
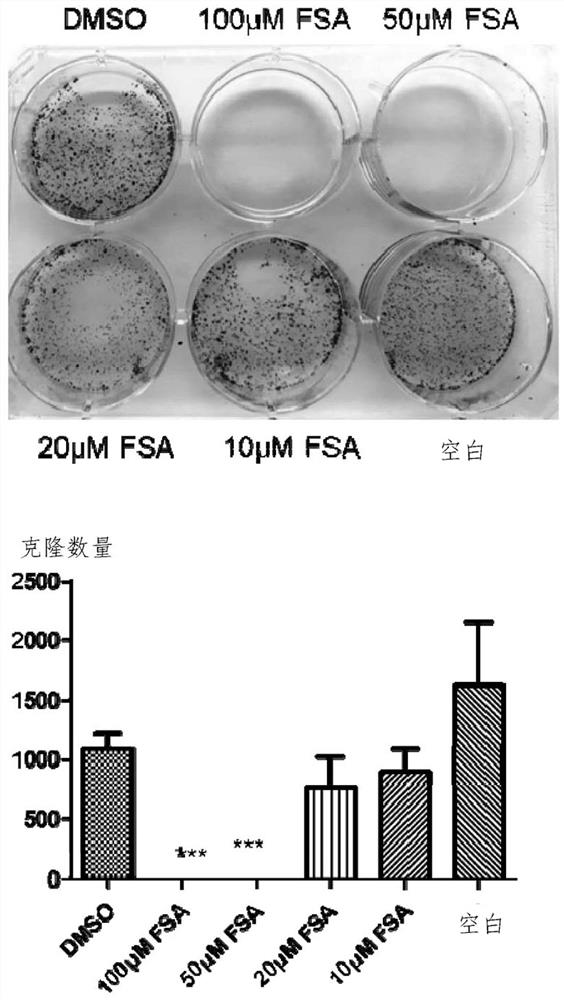
Application of forsythiaside A to preparation of anti-esophageal cancer medicine
ActiveCN113209113APromote apoptosisTo achieve the purpose of resisting esophageal squamous cell carcinomaOrganic active ingredientsAntineoplastic agentsMalignant phenotypeOncology
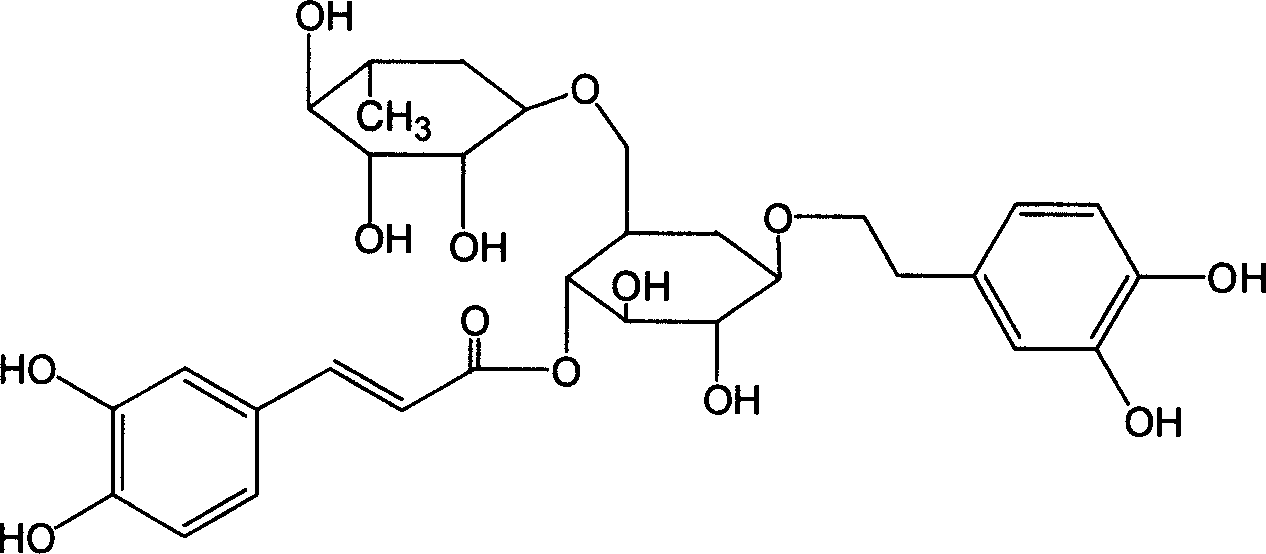
The invention provides an application of forsythiaside A to preparation of an anti-esophageal squamous cell carcinoma medicine. The forsythiaside A is used as an active component in the anti-esophageal squamous cell carcinoma medicine, and the molecular formula of the forsythiaside A is C29H36O15. The forsythoside A mainly inhibits proliferation, clone formation and cell migration of esophageal squamous carcinoma cells, or promotes apoptosis of the esophageal squamous carcinoma cells by retarding the cycle of the esophageal squamous carcinoma cells so as to achieve the purpose of resisting the esophageal squamous carcinoma. Therefore, the forsythiaside A has an inhibiting effect on the malignant phenotype of the esophageal squamous carcinoma.
Owner:ZHENGZHOU NORMAL UNIV
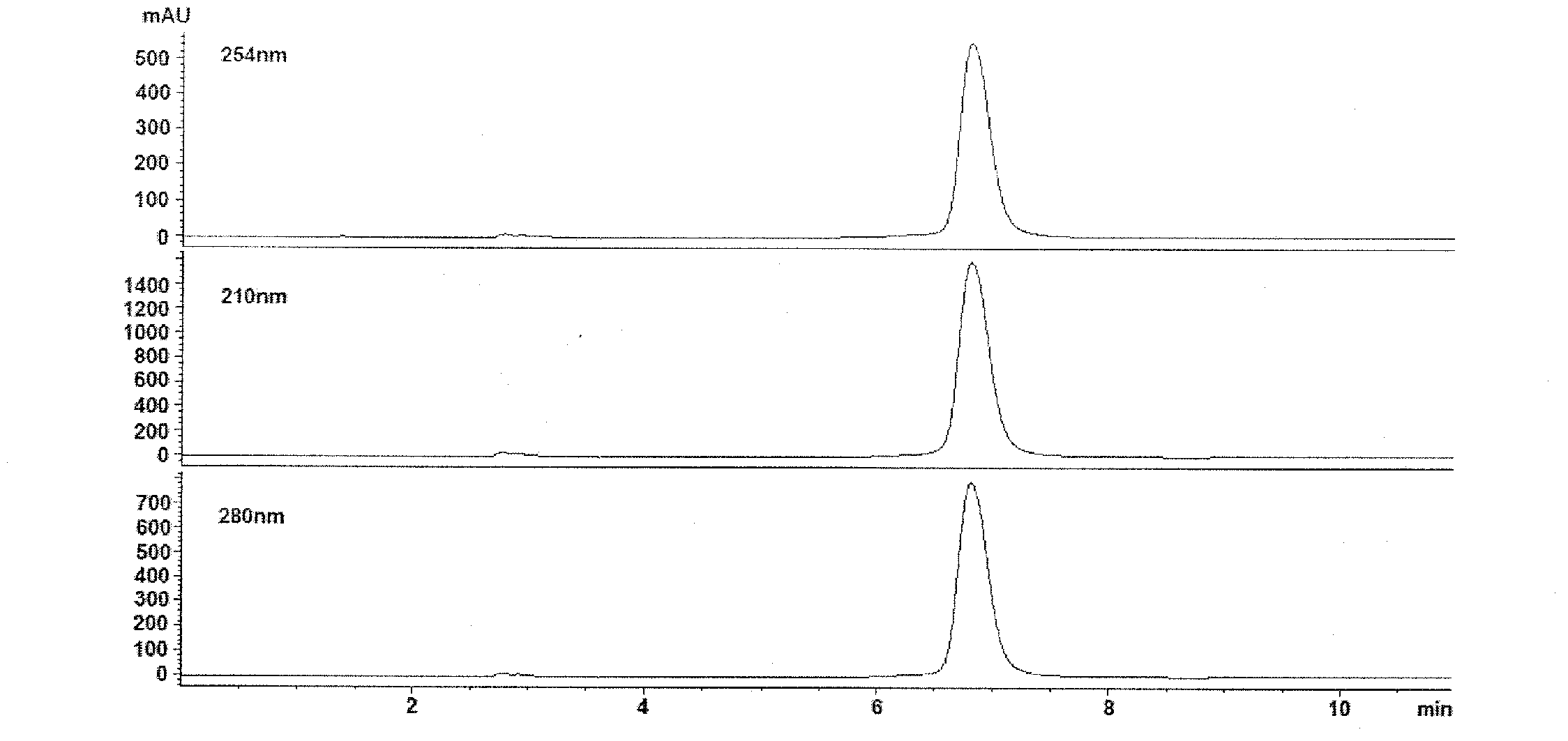
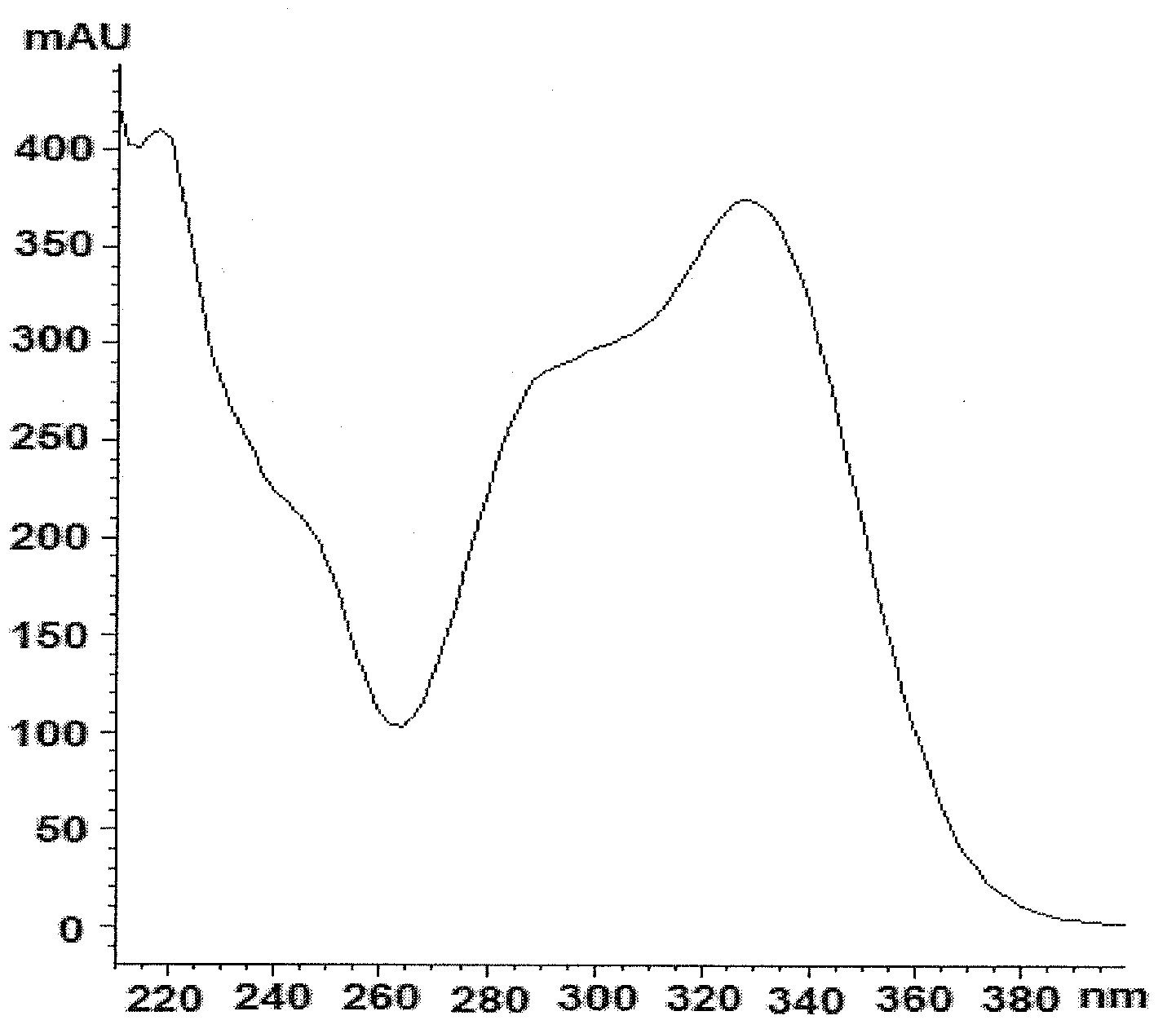
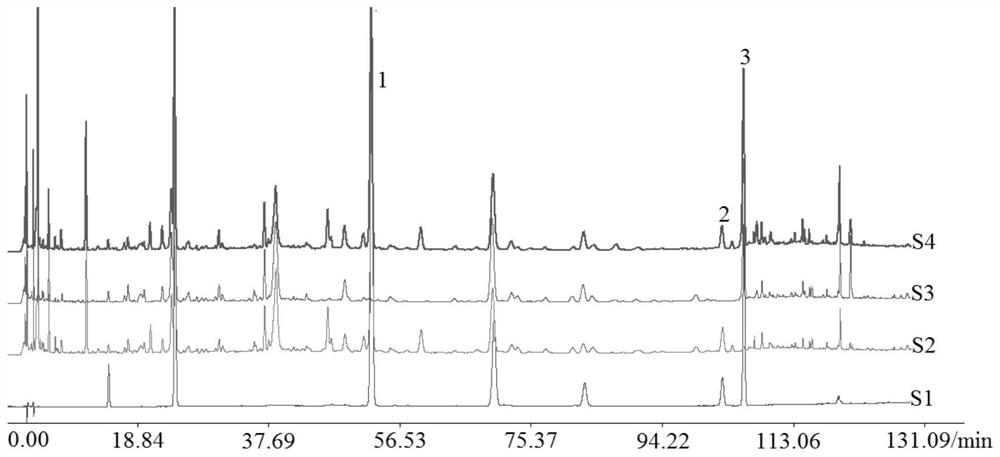
Method for determining contents of seven components in lonicera and forsythia powder by adopting dual-wavelength quantitative analysis of multi-components by single marker
PendingCN113075314AImprove detection efficiencyReduce testing costsComponent separationBiotechnologyIsochlorogenic acid
The invention discloses a method for determining the contents of seven components in lonicera and forsythia powder by adopting dual-wavelength quantitative analysis of multi-components by a single marker, which comprises the following steps of: respectively taking neochlorogenic acid, chlorogenic acid, forsythiaside A, isochlorogenic acid A, isochlorogenic acid C, forsythin and arctiin as reference substances and forsythiaside A as an internal reference substance, calculating the relative correction factors of arctiin and forsythin at 237 nm, calculating the relative correction factors of neochlorogenic acid, chlorogenic acid, isochlorogenic acid A and isochlorogenic acid C at 327 nm, taking a forsythiaside A reference substance solution and a test solution, injecting the solutions into a high performance liquid chromatograph, and calculating the contents of seven components including forsythiaside A, forsythin, arctiin, neochlorogenic acid, chlorogenic acid, isochlorogenic acid A and isochlorogenic acid C in the lonicera and forsythia powder according to the relative correction factors. According to the invention, the same test solution is adopted to determine the contents of seven components under two absorption wavelengths, so that the detection efficiency is improved, and the detection cost is reduced.
Owner:SHANDONG ACAD OF CHINESE MEDICINE
Livestock and poultry feed additives and application thereof
PendingCN110613051AImprove reproductive performanceGain weight fastAnimal feeding stuffAccessory food factorsGestationAnimal science
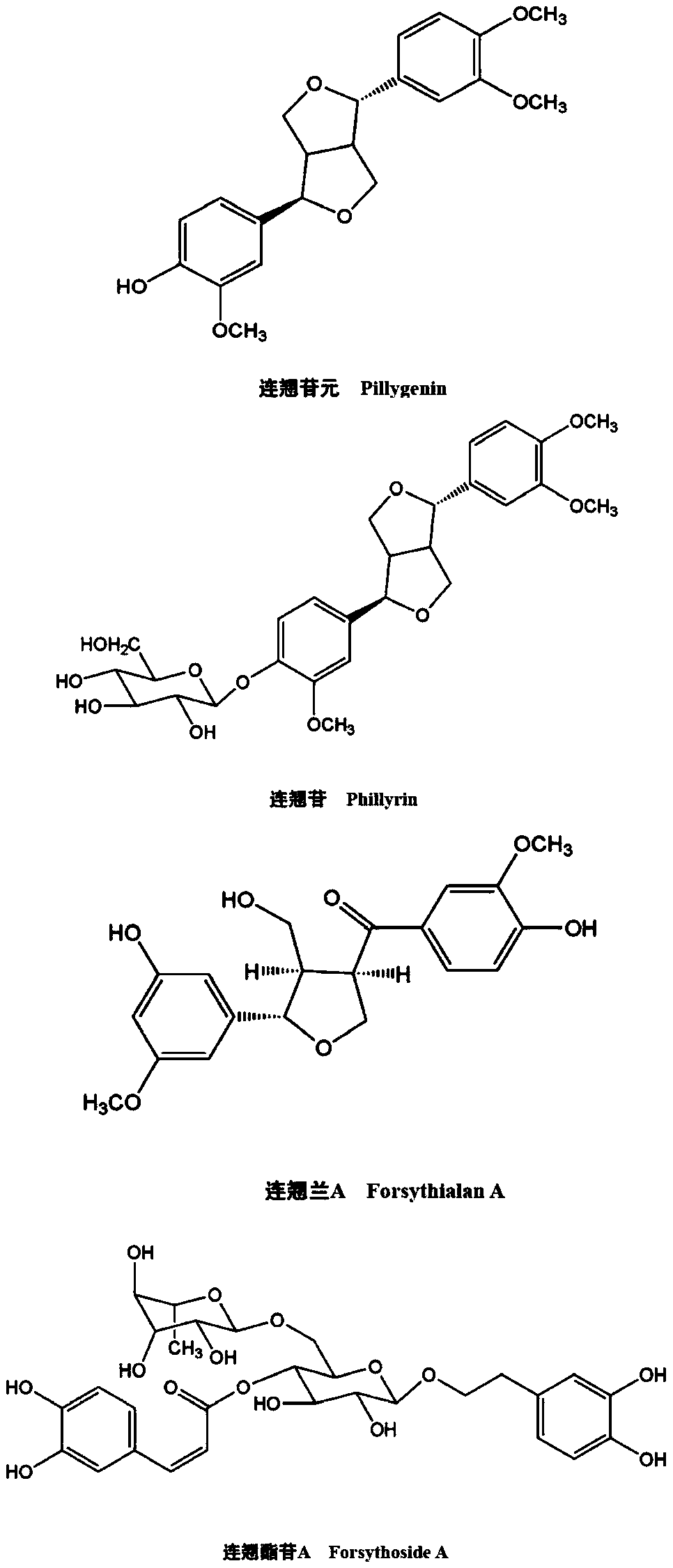
The invention discloses livestock and poultry feed additives and application thereof. As for one of the feed additives, dried fructus forsythia fruits are taken as raw materials, a fructus forsythia extract is obtained through alcohol extraction, main active components in the fructus forsythia extract comprise phillygenin, phillyrin, forsythialan A and forsythoside A, and the other feed additive is 25-hydroxyl vitamin D3. According to two methods for improving reproductive performance of multiparous sows and improving breast milk components through the additives at the latter half of gestation, by using the fructus forsythia extract, the newborn litter weight of lactating sows can be increased significantly, the mortality rate of newborn piglets is decreased, and the content of milk fat and milk proteins in colostrums is increased; and by using the 25-hydroxyl vitamin D3, the average newborn piglet weight of the lactating sows can be increased significantly, and the content of the milkfat and the milk proteins in the colostrums is increased. Effective means are provided for improving the immunity function of the sows and effectively resisting stress caused by metabolic enhancementduring gestation, parturition and lactation, and thus the development of sow breeding industry is promoted.
Owner:CHINA AGRI UNIV
Establishhment method for fructus forsythiae medicine fingerprint
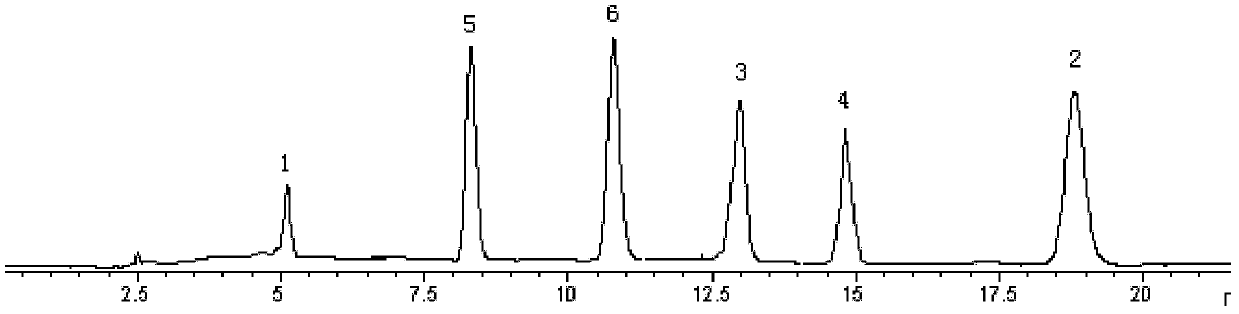
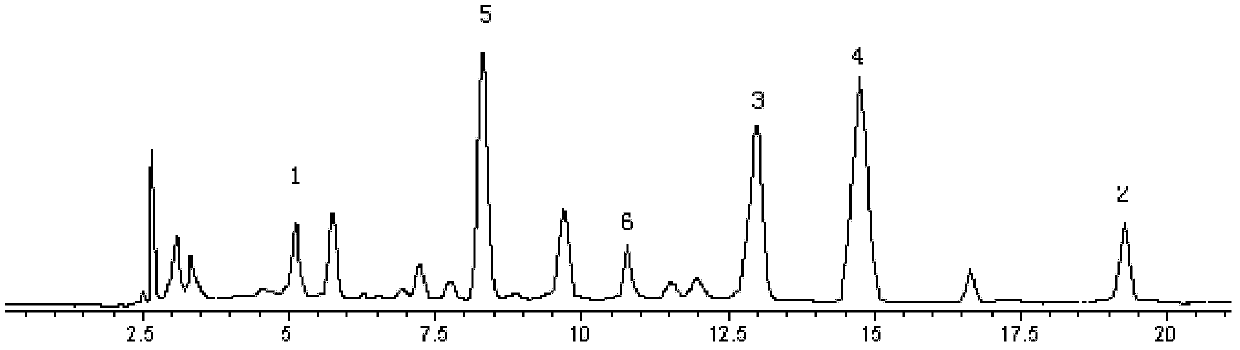
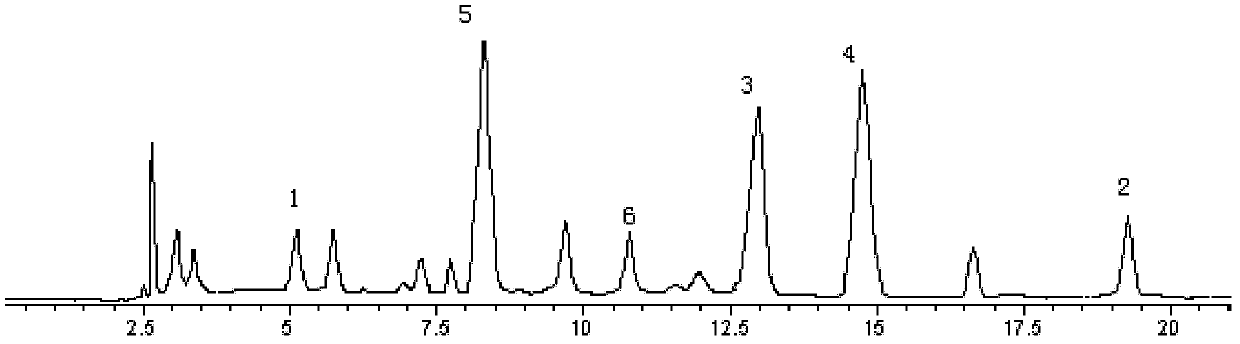
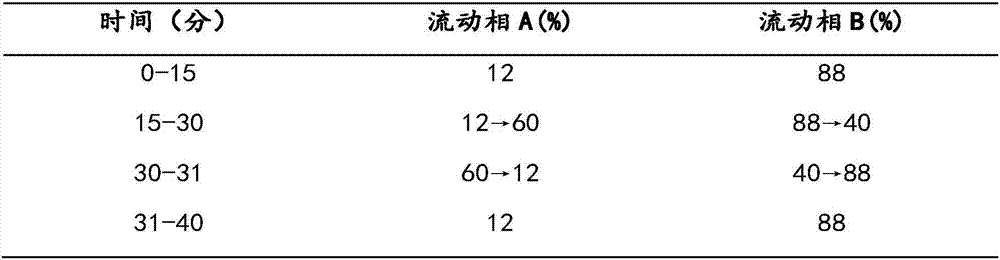
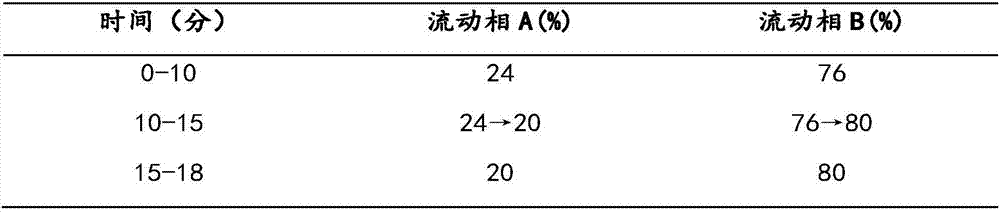
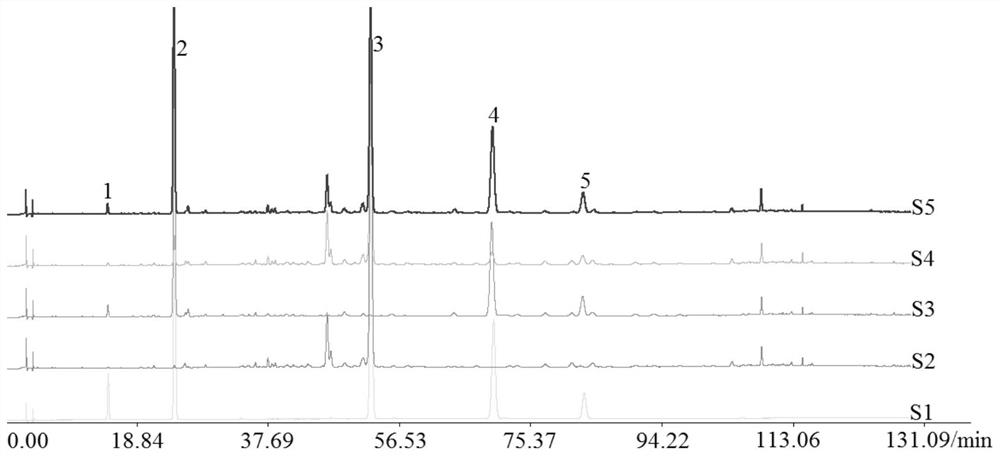
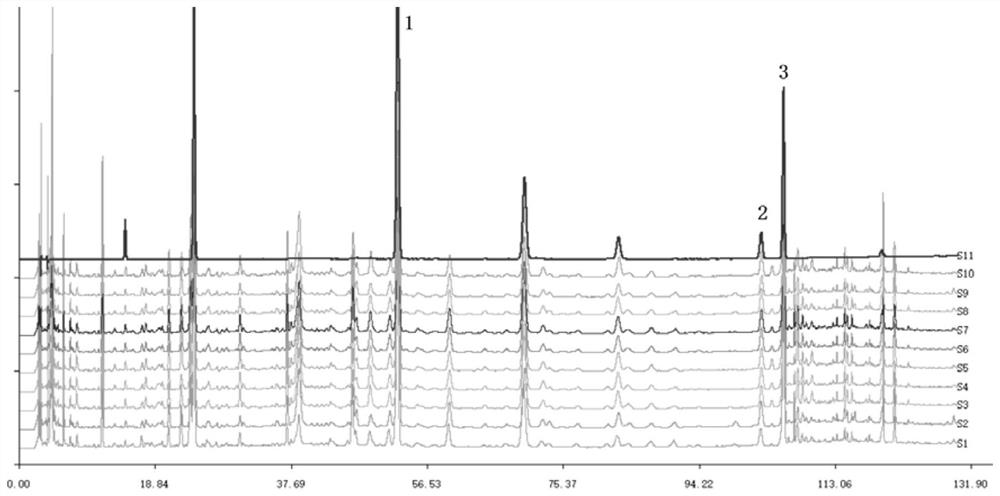
The invention belongs to the technical field of medicine analysis and specifically relates to an establishment method for a fructus forsythiae medicine fingerprint. The method specifically is realizedby the following steps of preparing a reference substance solution, taking appropriate amount of forsythin, forsythoside A, isoforsythiaside, rutin and forsythingenin reference substances, and preparing the reference substances into the reference substance solution; preparing a test sample solution, weighing fructus forsythiae medicine powder, carrying out heating reflux extraction, taking subsequent filtrate, carrying out filtering through utilization of filter membrane, taking the subsequent filtrate as a sample solution, measuring the sample solution and a puerarin water solution, and carrying out mixing uniformly to obtain the test sample solution; and carrying out detection through adoption of a high performance liquid chromatography and establishing the fingerprint. According to themethod, high performance liquid chromatography detection is carried out on the fructus forsythiae medicine through adoption of different gradient elution, a separation effect is good; through external addition of an internal standard, the established fingerprint has a semiquantitative function, so change of components among samples can be effectively expressed, and quality of the samples can be judged relatively precisely; and quality monitoring can be carried out on the raw material fructus forsythiae medicine relatively comprehensively.
Owner:SHANDONG ACAD OF CHINESE MEDICINE
Application of forsythia flower yellow pigment in preparing anti-oxidative stress injury drug
PendingCN109602787AExtend your lifeImprove antioxidant enzyme activityAntinoxious agentsNatural extract food ingredientsWater bathsFiltration
The invention provides an application of forsythia flower yellow pigment in preparing an anti-oxidative stress injury drug. The preparation step of the yellow pigment is as follows: adding dried forsythia flower powder into 95% ethanol according to a solid-liquid ratio of 1:100, performing water bath at 50 DEG C, and performing extraction by a hot reflux method; and performing suction filtration separation, repeating extraction twice, collecting the extractive liquid, performing rotary evaporation concentration, carrying out freeze drying, and collecting dry powder. The yellow pigment contains: rutin, forsythoside A, rosin, forsythin and the like. The yellow pigment can effectively eliminate ROS free radicals in caenorhabditis elegans body by up-regulating the expression of DAF-16 and SKN-1 target genes, increase the activity of antioxidant enzymes in elegans body, reduce the free radical content in the elegans body and weaken the oxidative stress damage in the elegans body by juglone,and can significantly prolong the lifespan of wild-type elegans N2 and daf-16 mutant elegans under oxidative stress, thereby increasing the resistance of elegans to oxidative stress. The yellow pigment can also be used in the preparation of functional foods resistant to oxidative stress damage.
Owner:SHANXI UNIV
Methods and substances for the prevention and treatment of viral infections
The present invention provides an advantageous new method for the prevention and treatment of viral infections. Specifically, the present invention exemplifies the therapeutic use of forsythiaside A and jacarandaone, which are compounds isolated from Chinese medicinal materials such as Fructus forsythiae (Lian Qiao). The present invention also provides the use of Yin Qiao San (Yin Qiao San) composition for preventing and treating virus infection.
Owner:BAGI RES +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com