Method for testing quality of antiviral oral liquid for treating hand-foot-and-mouth disease
An antiviral oral liquid and quality detection method technology, applied in the field of medicine, can solve the problem of not establishing content determination indicators and the like, and achieve the effect of a simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation of antiviral oral liquid
[0032] (A) Sample:
[0033] Prescription: Banlangen 129g, gypsum 57g, reed root 61g, rehmannia glutinosa 32g, turmeric 25g, Anemarrhena 25g, Shichangpu 25g, patchouli 29g, forsythia 46g
[0034] Preparation method: add water to extract the above nine medicinal materials twice, add 8 times the amount of water for the first time, decoct for 3 hours, collect volatile oil and volatile oil emulsion at the same time, add hydroxypropyl-β-cyclodextrin for inclusion; Add 6 times the amount of water for the second time, decoct for 1 hour and 20 minutes, combine the decoction liquid, concentrate to a relative density of 1.08 (60°C), add more than 85% ethanol to make the alcohol content reach 70%, let stand for 24 hours, filter Collect the filtrate, recover ethanol from the filtrate under reduced pressure, concentrate to a relative density of 1.10 (60°C), filter, add volatile oil clathrate, honey, sucrose, etc., add purified...
Embodiment 2
[0040] Example 2 Determination of the contents of the four components of (R, S)-Gaoyichun, Forsythin, Forsythiaside, and Forsythialin in the sample (A) of Example 1
[0041] 1. Instruments and reagents
[0042] Instruments and reagents Agilent 1200 high performance liquid chromatography, antiviral oral liquid provided by the applicant, acetonitrile is chromatographically pure, and water is ultrapure water. (R, S)-Gaoyichun, forsythin, forsythiaside A, forsythialin and other control drugs were provided by China National Institute for the Control of Pharmaceutical and Biological Products.
[0043] 2. High performance liquid chromatography
[0044] Chromatographic conditions: use octadecylsilane bonded silica gel as filler; use acetonitrile as mobile phase A, use water (adjust pH value to 2.85 with phosphoric acid) as mobile phase B, carry out gradient elution according to the table below, and the detection wavelength is 236nm. The number of theoretical boards is calculated ac...
Embodiment 3
[0050] Example 3: Determination of the contents of the four components (R, S)-Cuyichun, Forsythin, Forsythiaside and Forsythialin in the sample (B) of Example 1.
[0051] Method steps are with embodiment 2
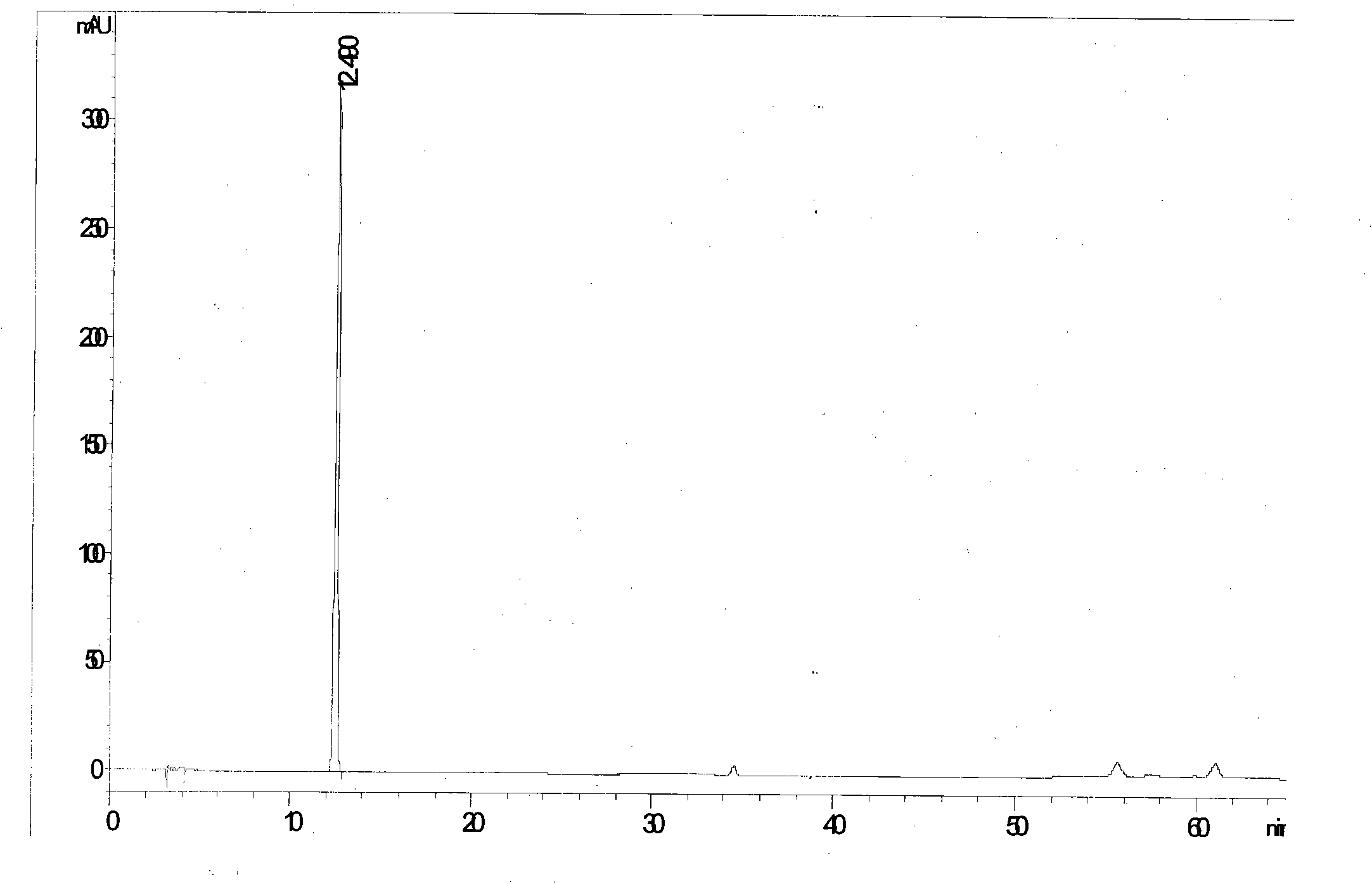
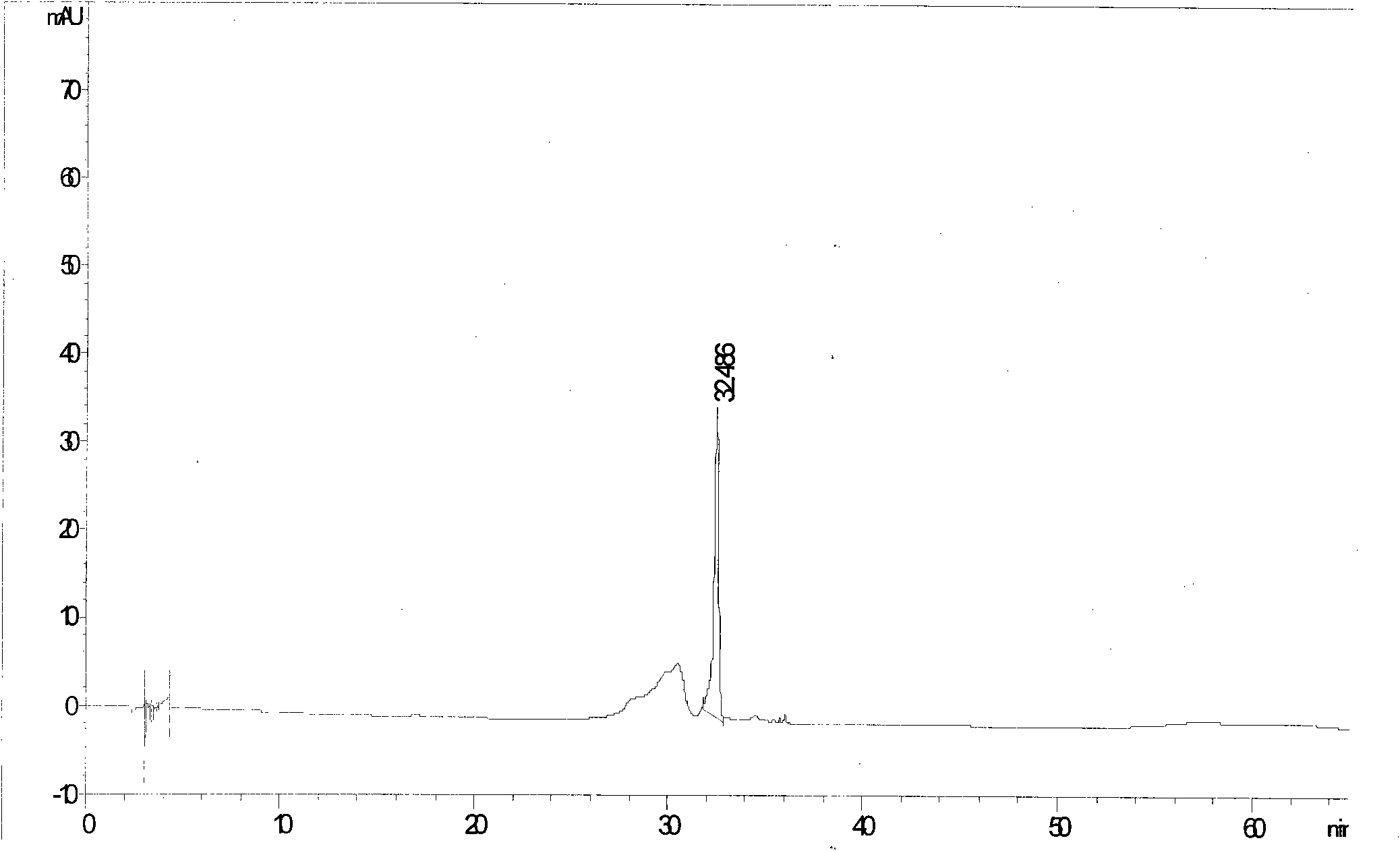
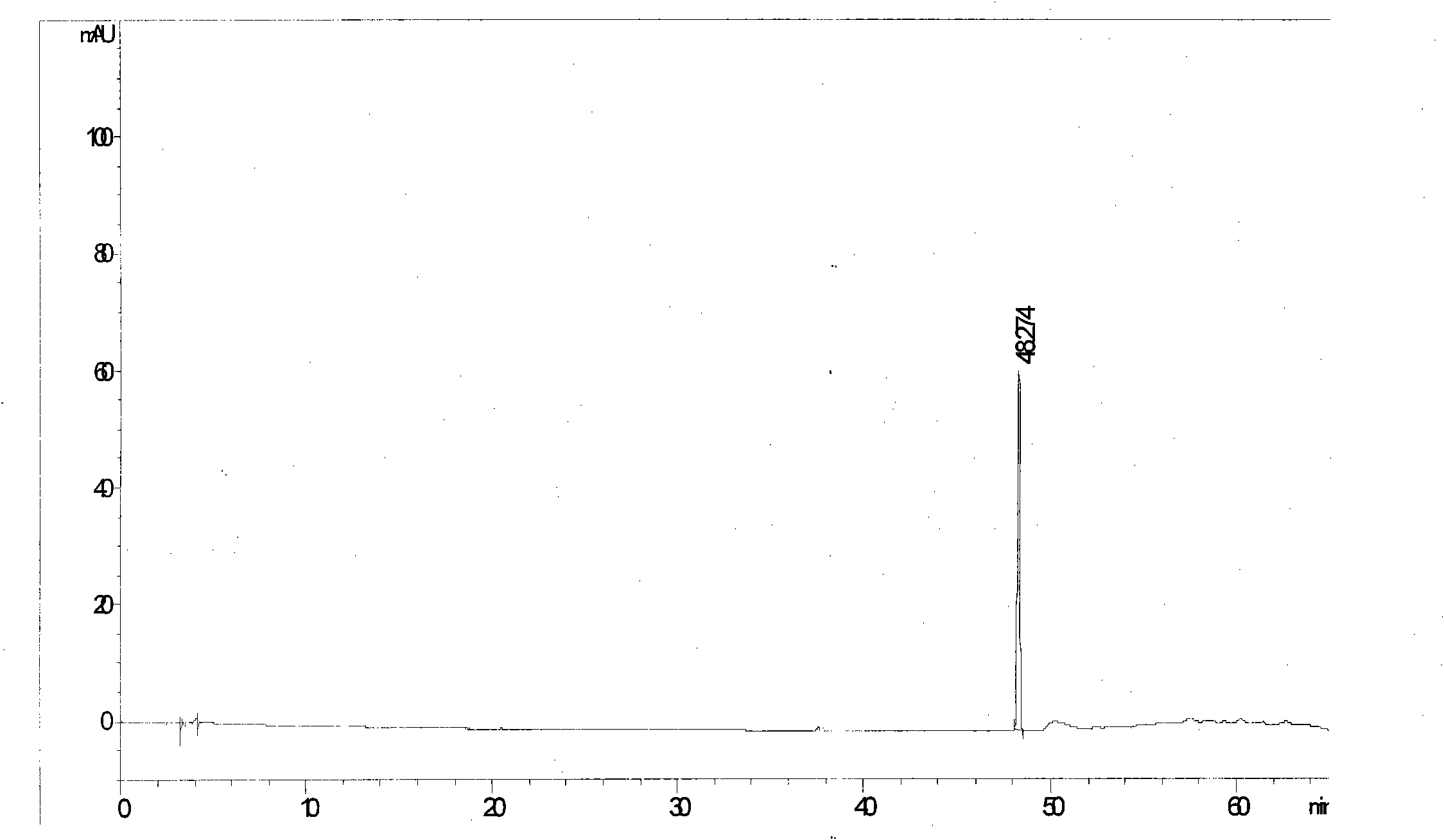
[0052] See attached figure 2
[0053] Calculated: each antiviral oral solution contains (R, S)-Gaoyichun 68.9μg / bottle, forsythiaside A 1.47mg / bottle, forsythin 0.27mg / bottle, forsythiaside 0.14mg / bottle branch.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com