Use of forsythiaside as synergist of antifungal agents
An antifungal drug, the technology of forsythiaside, which is applied in the field of medicine, can solve the problems of no reports of antifungal effects and no fungal diseases, and achieve the effects of saving medical expenses, improving antifungal effects, and reducing drug doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0009] Example 1. The effect of the combination of forsythiaside and fluconazole on different clinical fungal strains
[0010] Materials and methods
[0011] 1. Test drug:
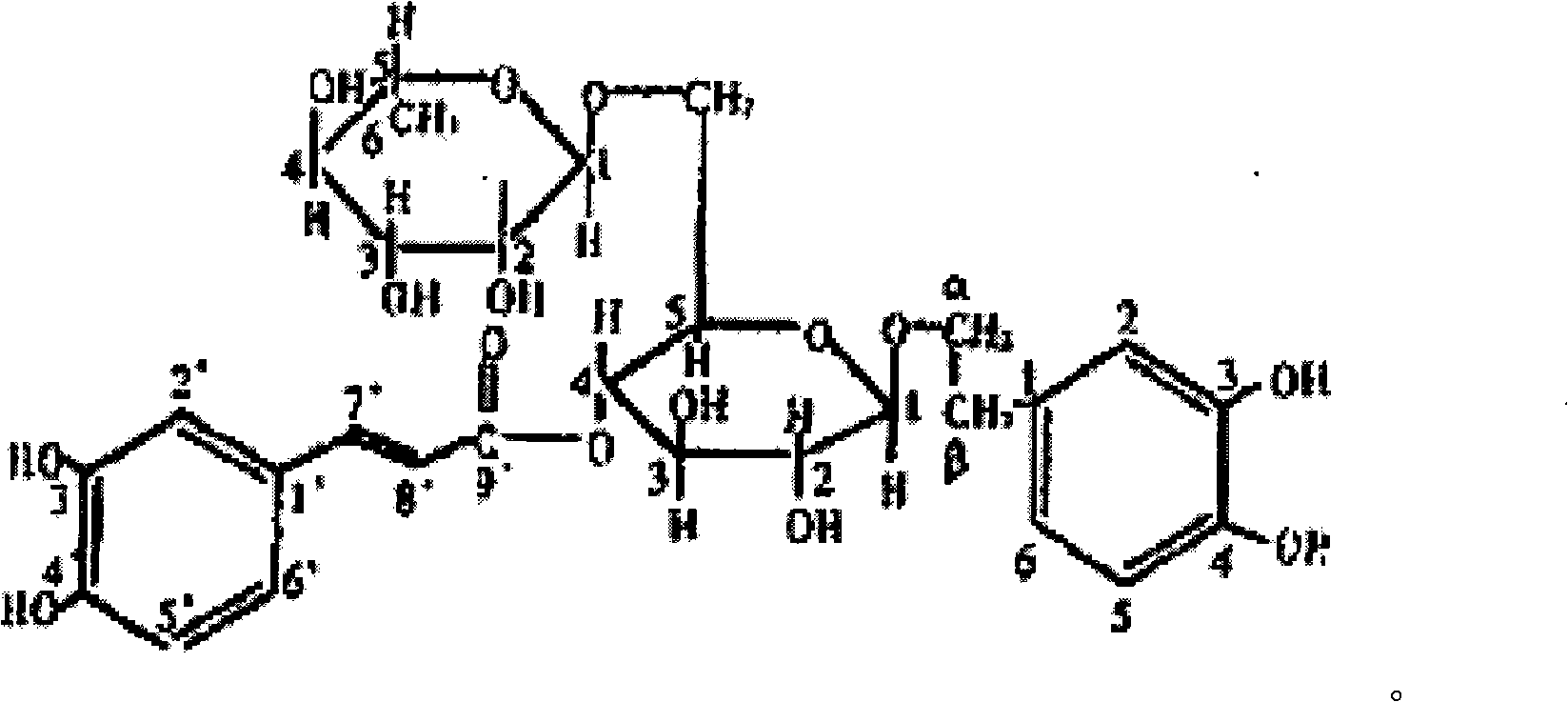
[0012] Forsythiaside: Shanghai Ronghe Pharmaceutical Technology Co., Ltd., batch number 090923.
[0013] Fluconazole injection: Pfizer Pharmaceutical Co., Ltd., batch number 9167802.
[0014] Dimethyl sulfoxide: Shanghai Shiyi Biotechnology Co., Ltd.
[0015] Forsythiaside was formulated with dimethyl sulfoxide at a concentration of 6.4 mg / ml.
[0016] All reagents were stored at -20°C. Before the experiment, the drug was taken out and placed in a 35°C incubator to melt, mixed well, and pharmacodynamic tests were carried out respectively.
[0017] 2. Strains:
[0018] Clinical strains: 1) Drug-resistant Candida albicans, including five strains 103, 904, 18, 38, and 0710448; 2) Candida tropicalis, including five strains 409, 936, 422, 0710453, and 0710087; 3) Cryptococcus neoformans, including 34880 ...
Embodiment 2
[0049] Example 2: Combination of forsythiaside and other azole antifungal drugs
[0050] Materials and methods
[0051] 1. Drugs:
[0052] Itraconazole, ketoconazole and miconazole were all provided by the School of Pharmacy, Second Military Medical University.
[0053] Itraconazole, ketoconazole and miconazole were prepared with dimethyl sulfoxide to prepare 6.4 mg / ml, and forsythiaside was prepared with dimethyl sulfoxide to prepare 6.4 mg / ml, and each reagent was stored at -20°C. Before the experiment, the drug was taken out and placed in a 35°C incubator to melt, mixed well, and pharmacodynamic tests were carried out respectively.
[0054] Other experimental steps and methods are the same as in Example 1, and the experimental results are shown in Tables 4, 5, and 6.
[0055]Table 4 MIC of forsythiaside and itraconazole (ITR) in combination with 4 strains of clinical drug-resistant Candida albicans 80 value
[0056]
[0057] Table 5 The MIC of the combination of for...
Embodiment 3
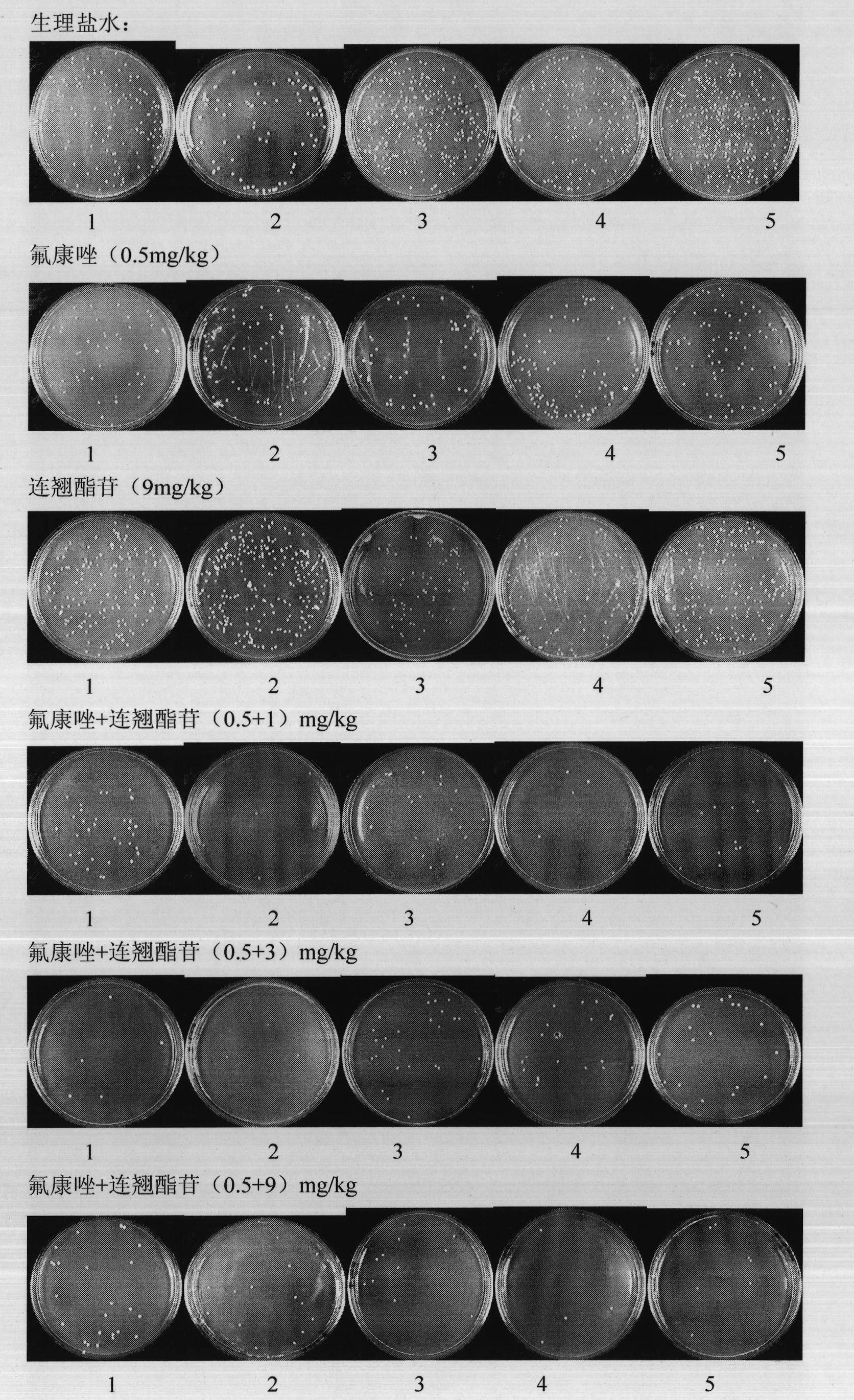
[0062] Example 3: The protective effect of forsythiaside combined with fluconazole on systemic fungal infection in mice
[0063] experimental method:
[0064] The mouse strain used in the experiment is a clean grade ICR female mouse (18-20g), certificate number: 2007000501825, provided by Shanghai Slack Experimental Animal Co., Ltd.
[0065] Drug-resistant Candida albicans (103 clinical drug-resistant strains), after the virulence recovery in mice, was reactivated on SDA medium, and grown in YEPD liquid medium to logarithmic growth phase. Adjust the bacterial concentration to 3.0×10 with normal saline 6 Each / ml, inject 0.1ml / 10g into the tail vein of mice according to body weight, and cause the model of deep fungal infection in mice. The mice in the normal control group were injected with 0.1ml / 10g normal saline through the tail vein.
[0066] 33 mice were randomly divided into 7 groups, except for 3 in the normal control group, and 5 in each group, which were fungal infect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com