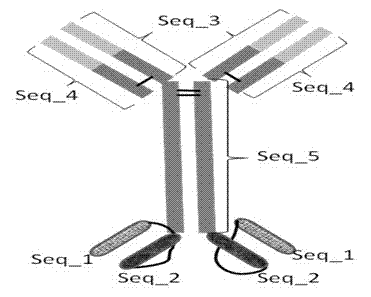
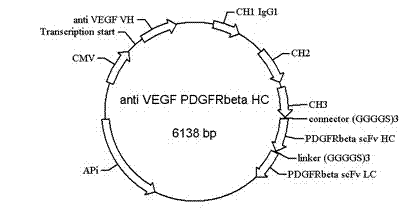
Bispecific antibody to VEGF/PDGFR beta and application thereof
A bispecific antibody, antibody technology, applied in the direction of antibodies, anti-tumor drugs, hybrid immunoglobulins, etc., can solve the problems of toxicity and high toxicity, achieve good tumor activity, inhibit growth, and avoid the increase of drug dosage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Preparation method of anti-VEGF-PDGFRbeta bispecific antibody
[0071] 1. Nucleotide sequence design and synthesis of anti-VEGF-PDGFRbeta bispecific antibody (bsAb):
[0072] According to the bsAb heavy chain VH VEGF -CH1 VEGF -CH2 VEGF -CH3 VEGF -(GGGGS) 3 -VH PDGFRbeta -(GGGGS) 3 -VL PDGFRbetaThe heavy chain nucleotide sequence was designed according to the amino acid sequence and connection form, and a Sac I restriction site, KOZAK sequence and leader sequence were added at the 5' end of the sequence, and an Xho I restriction site was added at its 3' end. Design three oligonucleotide primers containing EcoRI restriction site at the 3' end to synthesize the heavy chain VH respectively VEGF -CH1 VEGF , CH2 VEGF -CH3 VEGF -(GGGGS) 3 and VH PDGFRbeta -(GGGGS) 3 -VL PDGFRbeta Fragment, the full-length 2118bp bsAb heavy chain gene was synthesized by PCR direct ligation method (SDL PCR).
[0073] According to the amino acid sequence of human anti-VEGF-A IgG1...
Embodiment 2
[0080] Antigen-Antibody Binding Experiment
[0081] Antigen-antibody binding experiments were performed using Biacore T 100 system (GE Healthcare) with high-density VEGF-A (1000RU) immobilized on a CM4 chip through covalent binding, and bsAb was diluted to 100nM and injected into the system so that it flowed at a flow rate of 10uL / min Over-immobilize the VEGF-A surface on the chip for a total of 5 minutes. PDGFRβ (500nM) was then infused at a flow rate of 30uL / min for 10 minutes. The binding curves in the experimental results were automatically generated by Biacore Evaluation Software vl.1.1. The curve obtained from the co-binding experiment is basically consistent with the curve generated by the binding of the bispecific antibody to VEGF-A or PDGFRβ respectively, indicating that the antibody can specifically bind to VEGF-A and PDGFRβ at the same time. See Figure 4 . Anti-VEGF-PDGFRβ bispecific antibody (bsAb) simultaneously binding hVEGF-A and PDGFRβ assay (Biacore). In ...
Embodiment 3
[0083] Comparison of Antibody Inhibition Blood Vessel Experiments
[0084] HUVECs cells were inserted into 96-well plates with a density of about 1000 cells per well, cultured in EGM-2MV medium at 37°C for 48 hours, and then cells were placed in serum-free medium containing 1 times insulin-transferrin-sodium selenium Incubate at 37°C. After 24 hours, the original medium was removed, replaced with anti-VEGF-A full antibody (bevazimumab), anti-VEGF-A Fab or bsAb containing 2.6nM hVEGF-A and different concentrations (0.0005nM-500nM), and cultured at 37°C for 24 hours. After that, 1uCi / well of 3H-thymidine was added to the cells and cultured at 37°C for 24 hours.
[0085] HBVPs cells were inserted into 96-well plates with a density of about 500 cells per well. The cells were cultured at 37°C for 48 hours in a medium containing serum and PGS, and then cultured at 37°C for 24 hours in a serum-free medium. Add 0.4nM PDGFR β to stimulate cell growth, and at the same time add differ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com