Patents
Literature
180 results about "Human platelet" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Human platelet lysate is a growth factor-rich cell culture supplement derived from healthy donor human platelets at U.S. Food and Drug Administration (FDA)-licensed and Health Canada-licensed blood centers. Multiple donor units are pooled during manufacturing to minimize lot-to-lot variability.
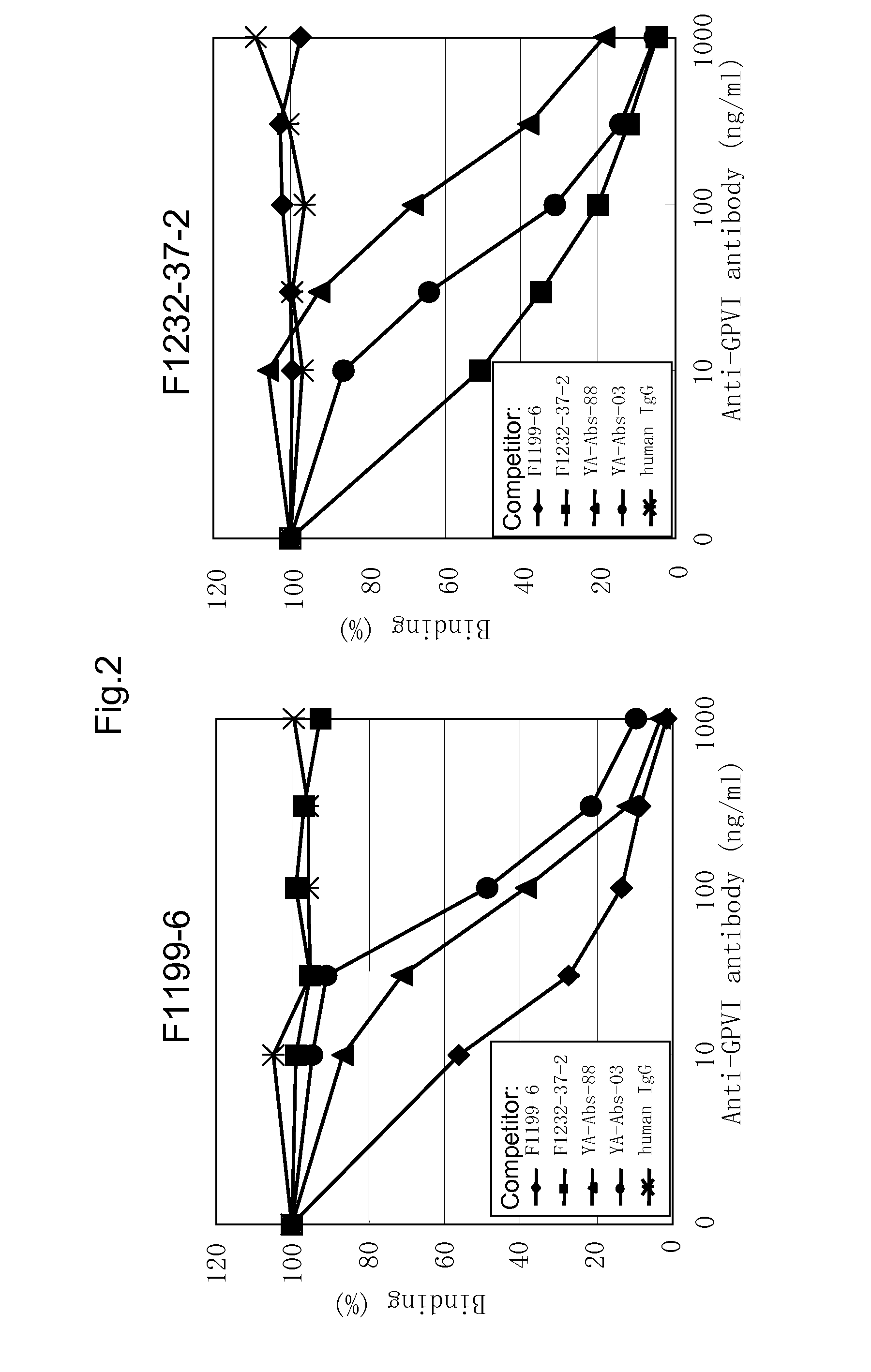
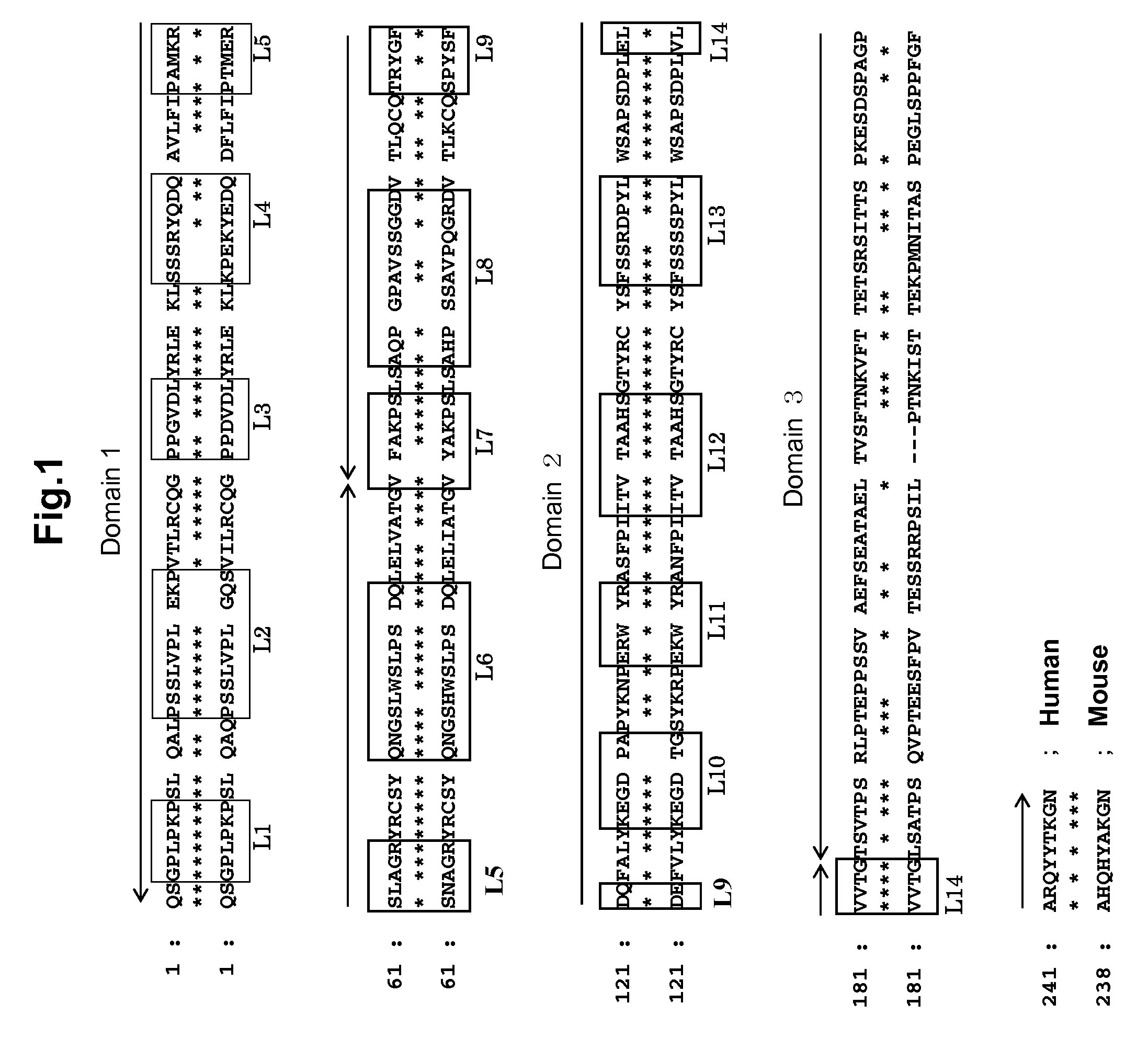
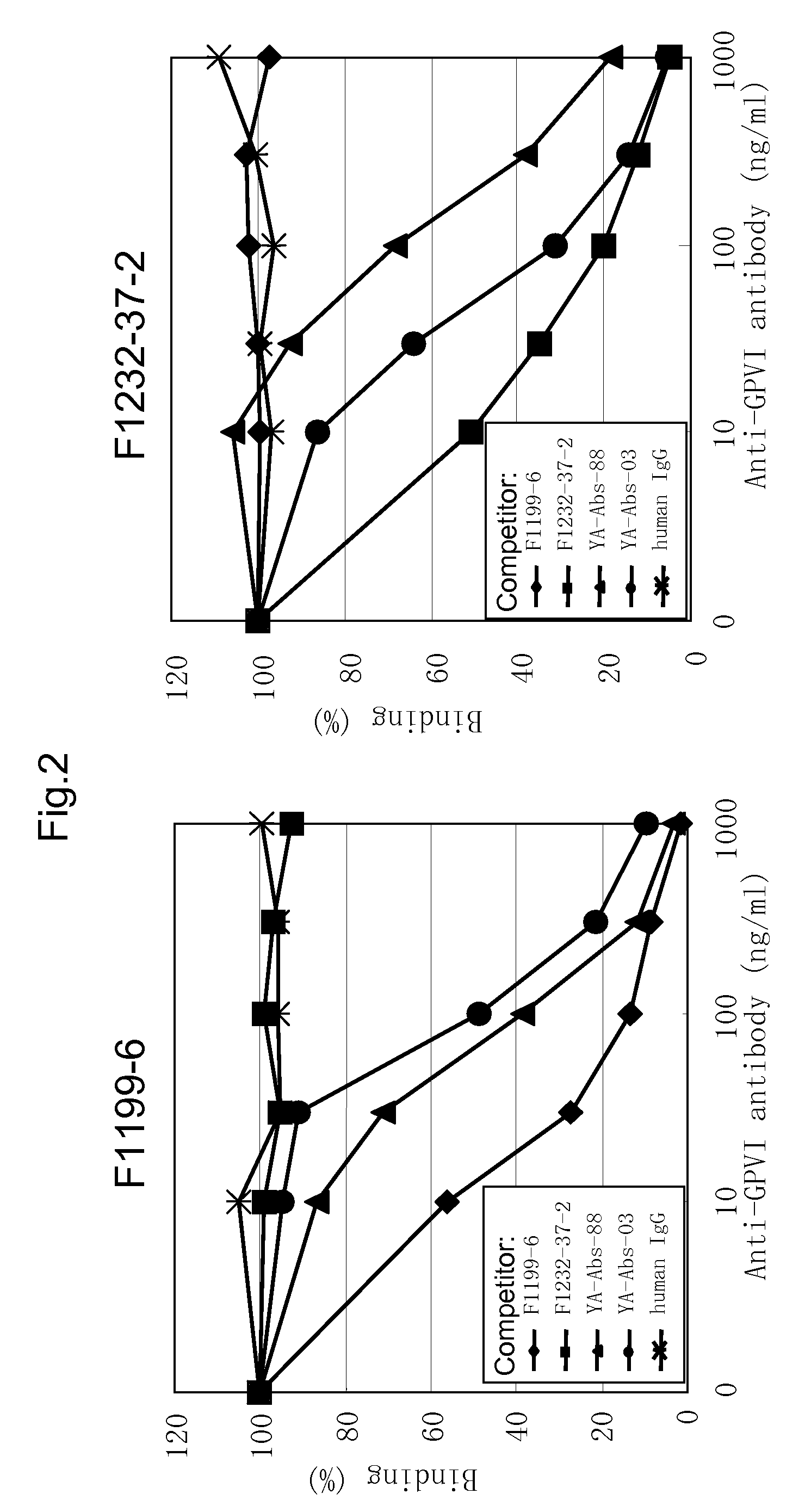
Anti-platelet membrane glycoprotein vi monoclonal antibody
InactiveUS20090041783A1Decrease platelet aggregabilitySuppresses collagen-induced human platelet aggregationImmunoglobulins against blood group antigensAntibody mimetics/scaffoldsHuman plateletNormal platelet morphology
The present invention provides an antibody which has the following features, its active fragment, or a derivative thereof:a) It specifically binds to human platelet membrane glycoprotein VI (GPVI);b) The function to activate a platelet and / or the function to induce a thrombocytopenia in vivo are low; andc) It at least partially depletes GPVI on the platelet membrane by contacting with a platelet.
Owner:MOCHIDA PHARM CO LTD
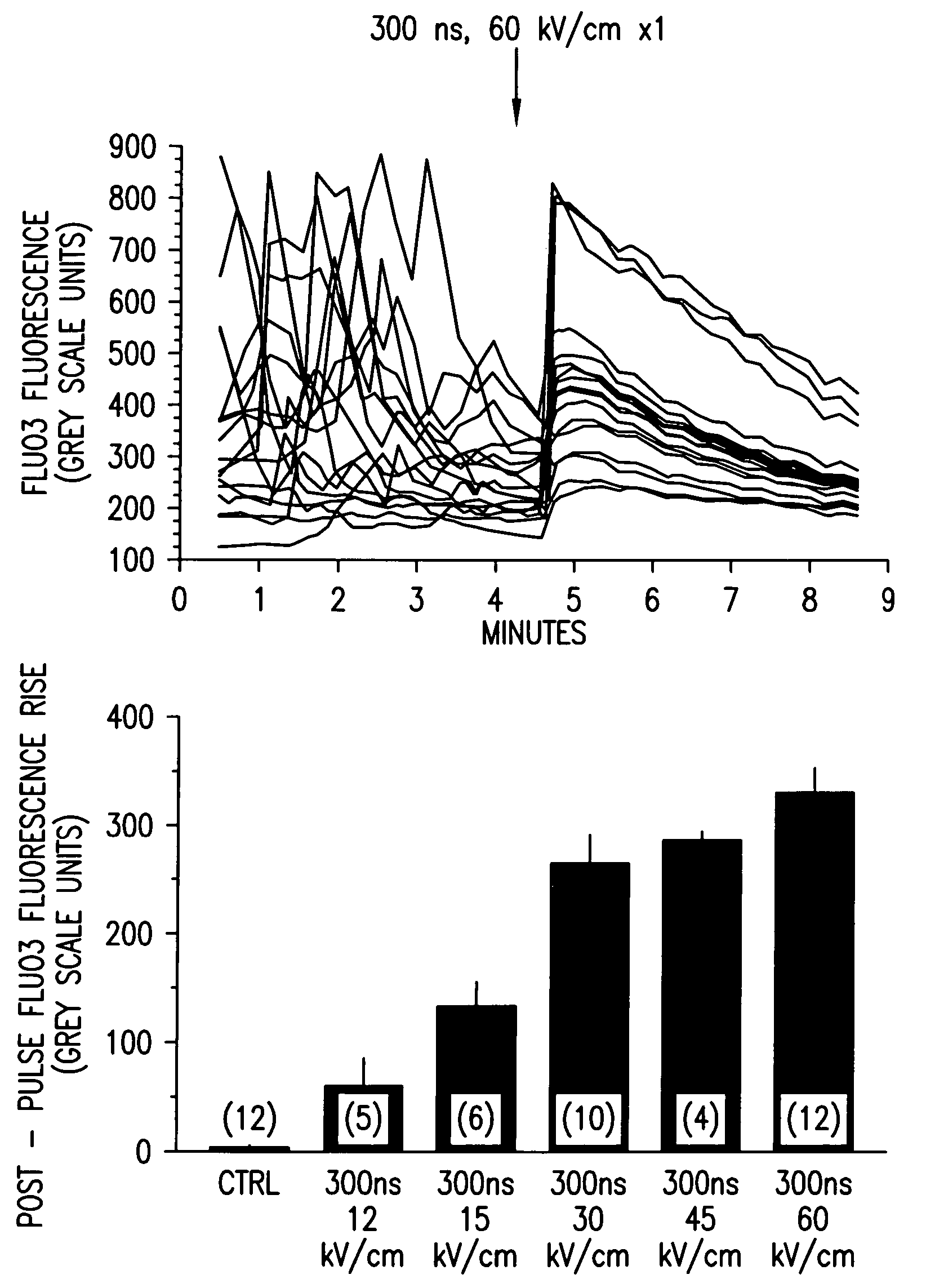
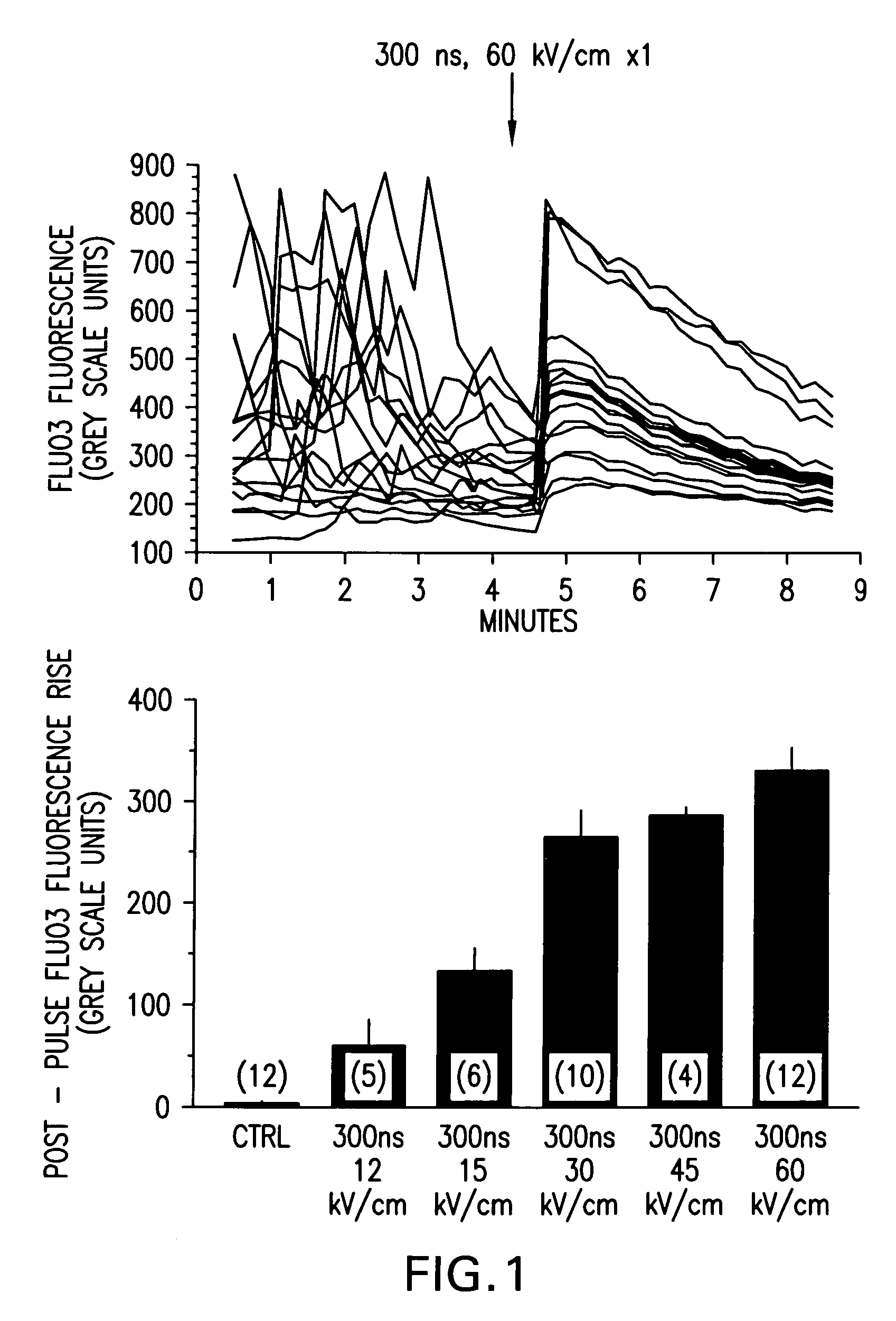
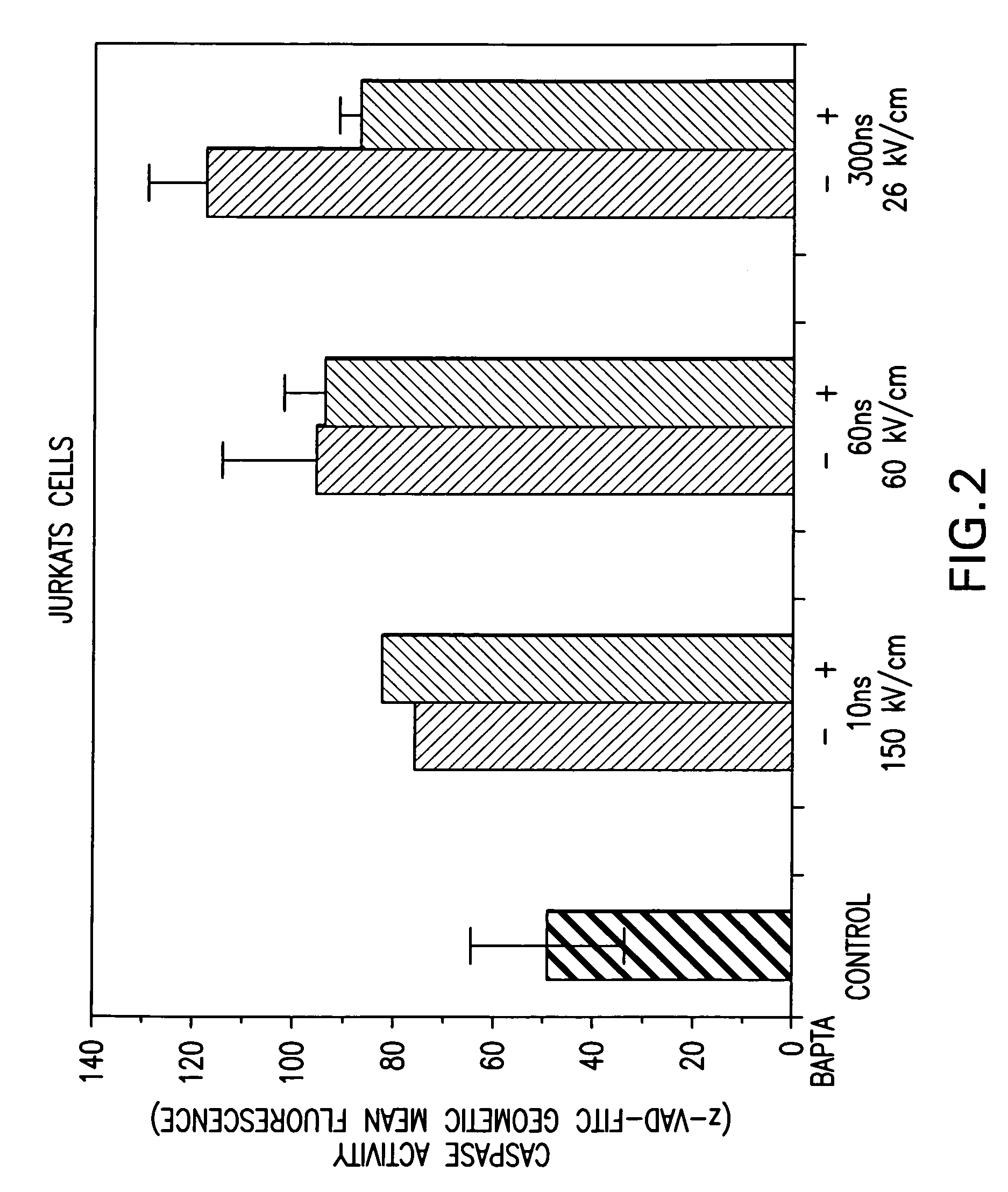
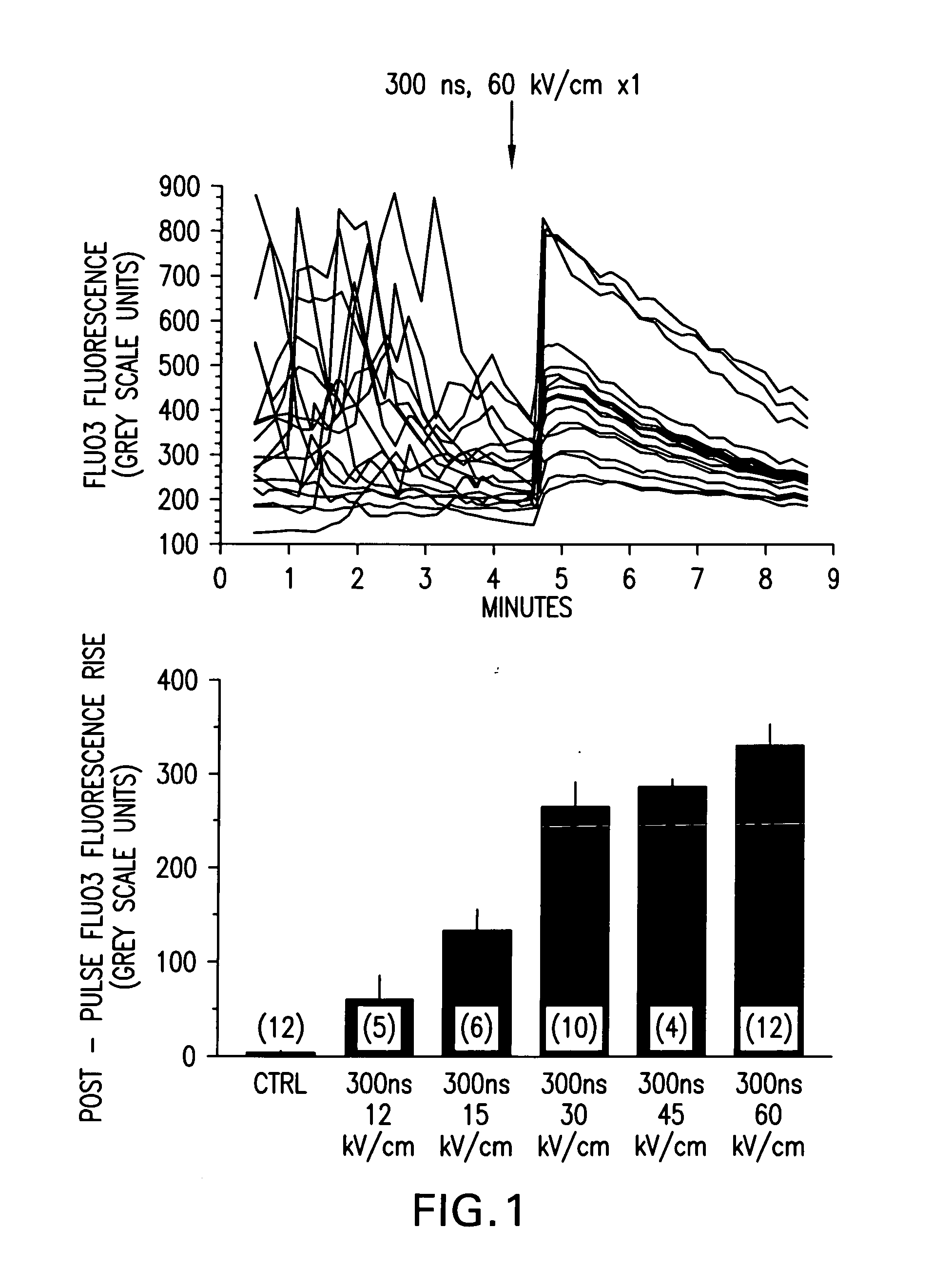
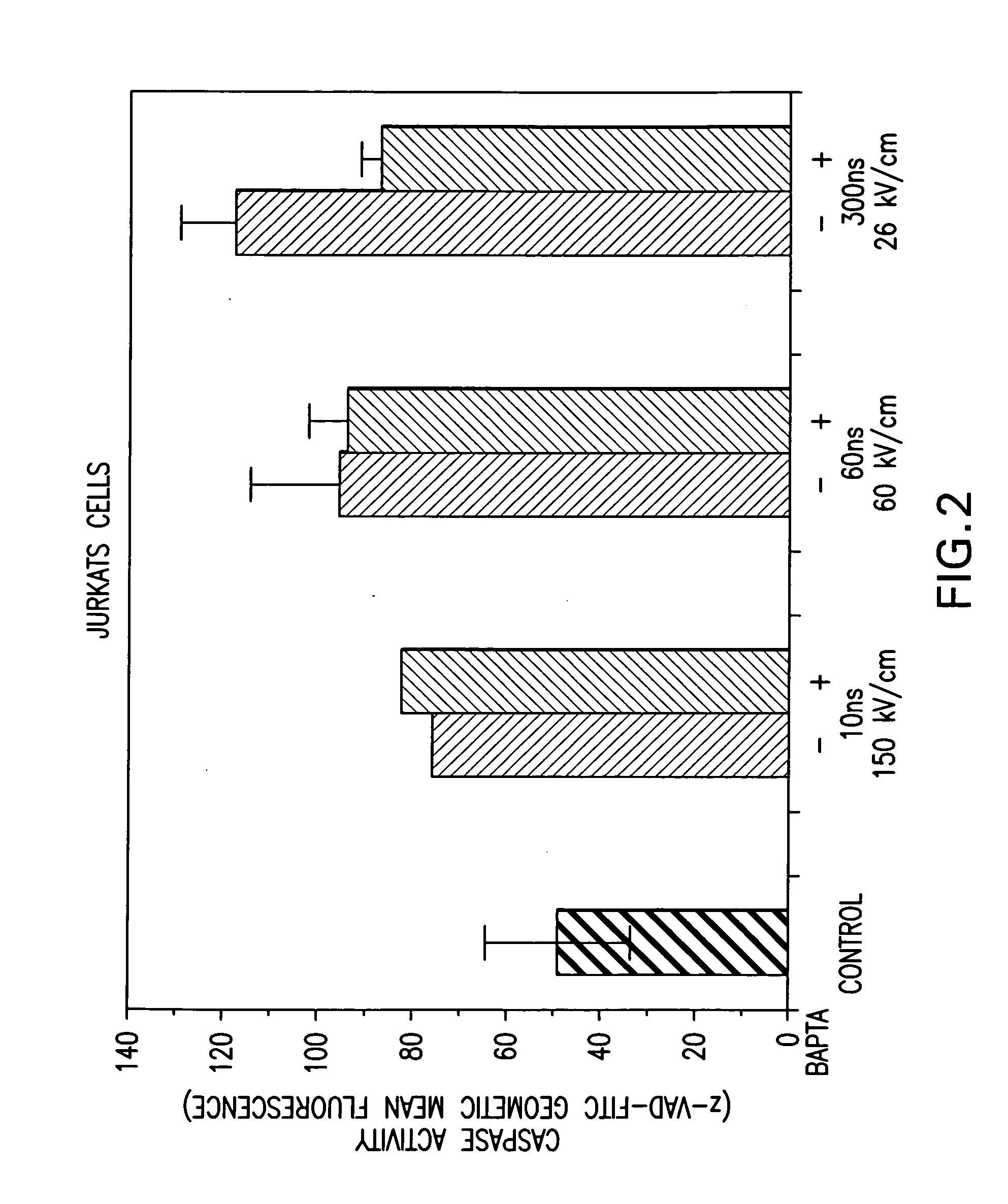
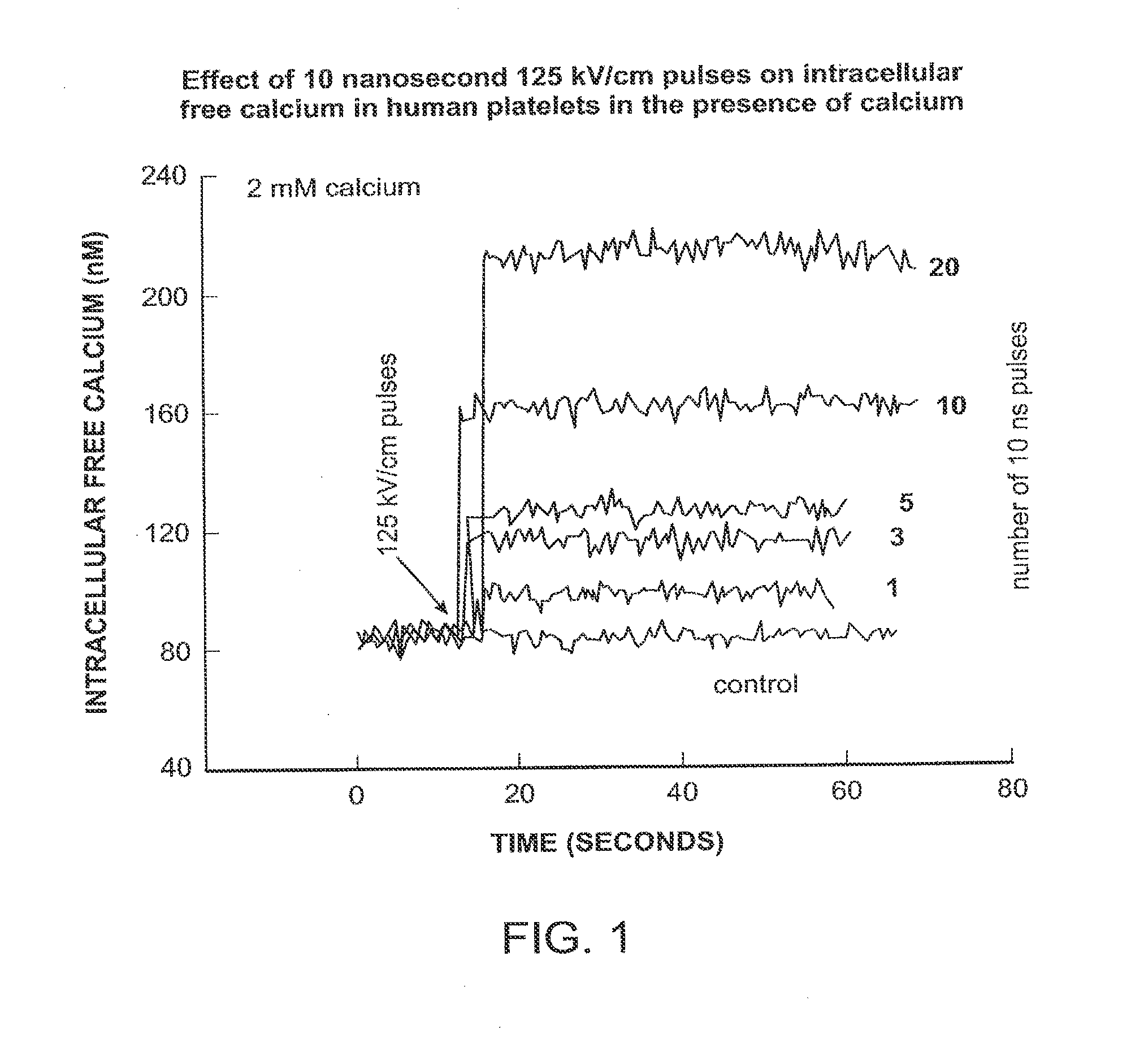
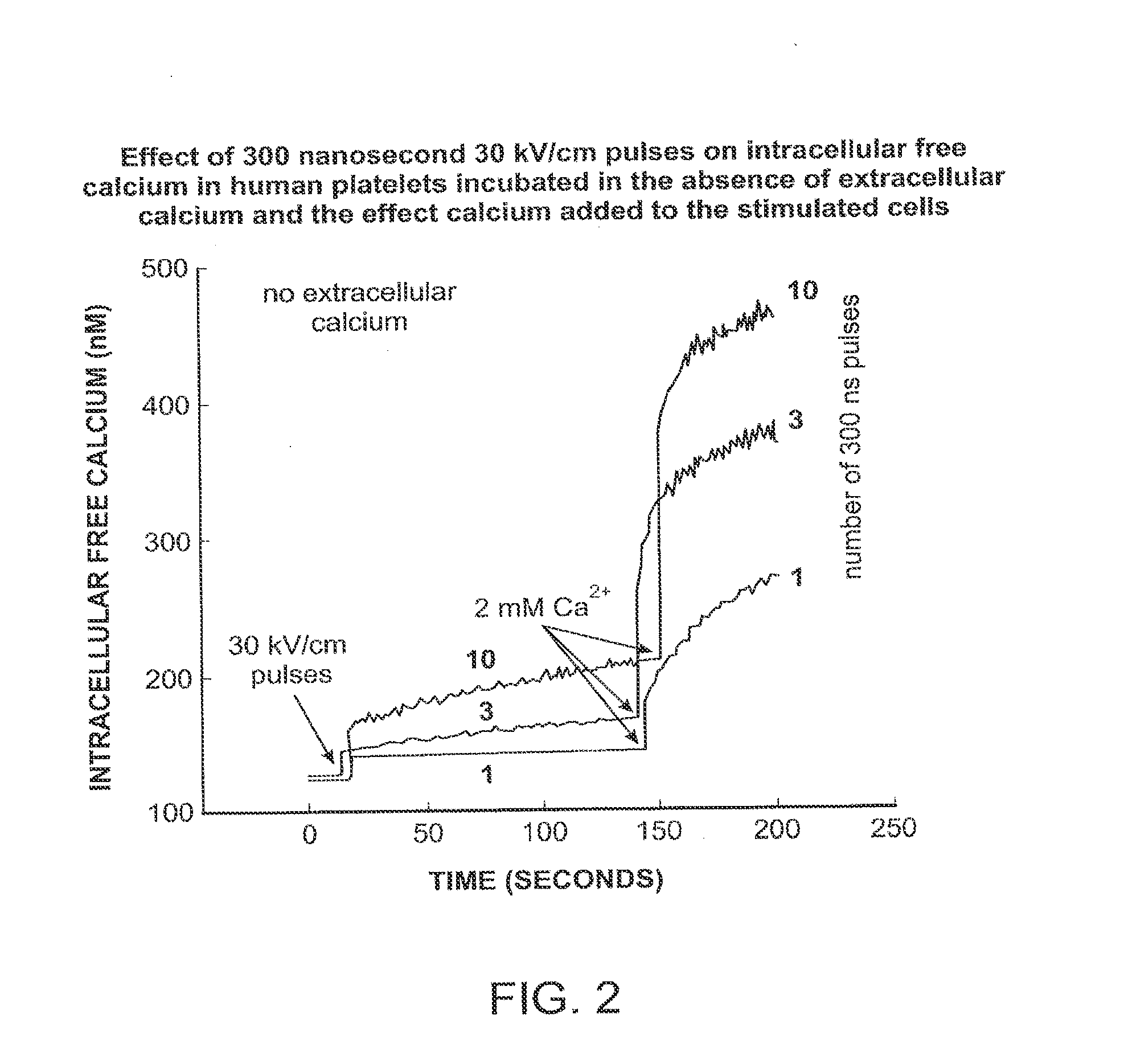
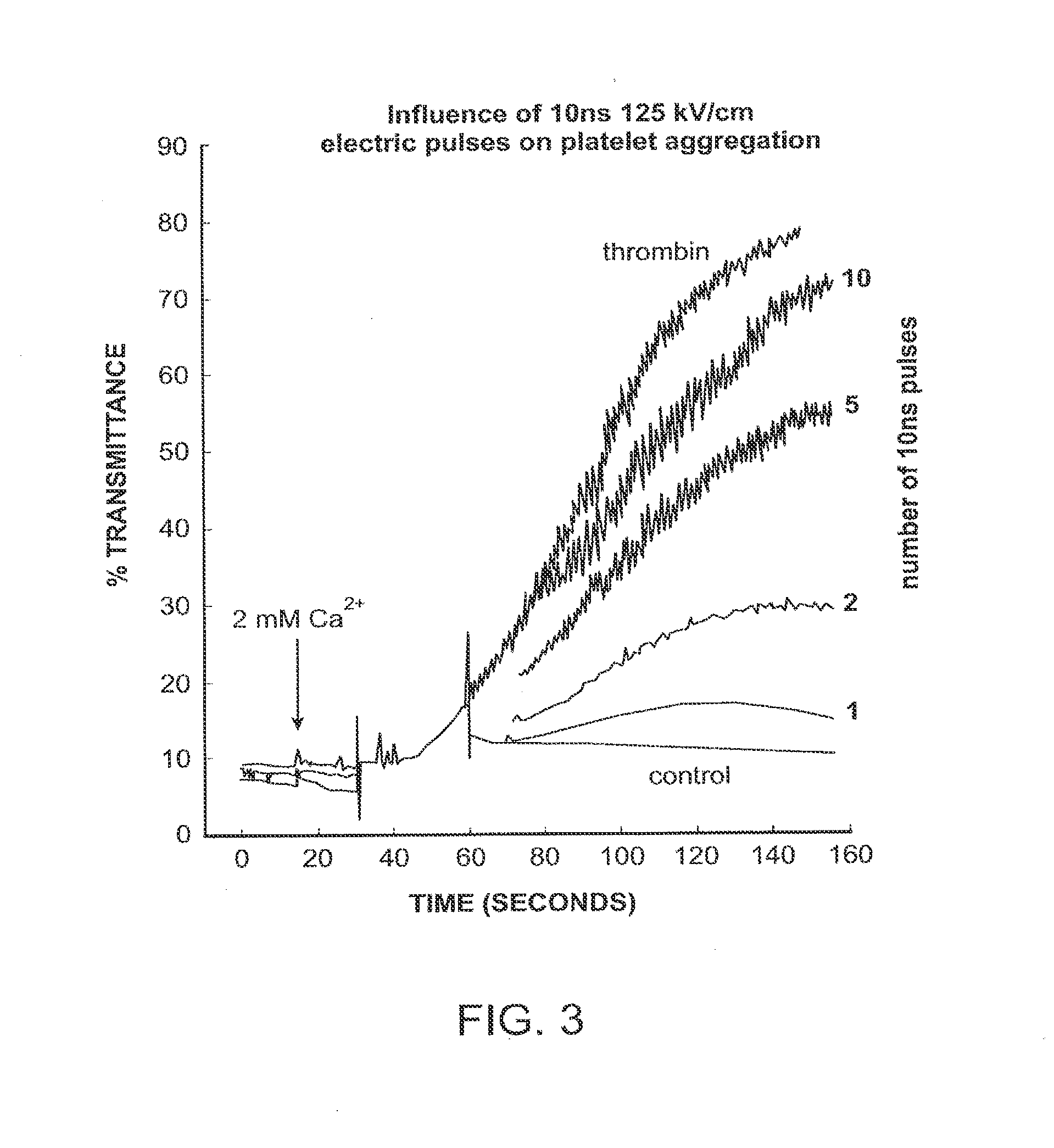
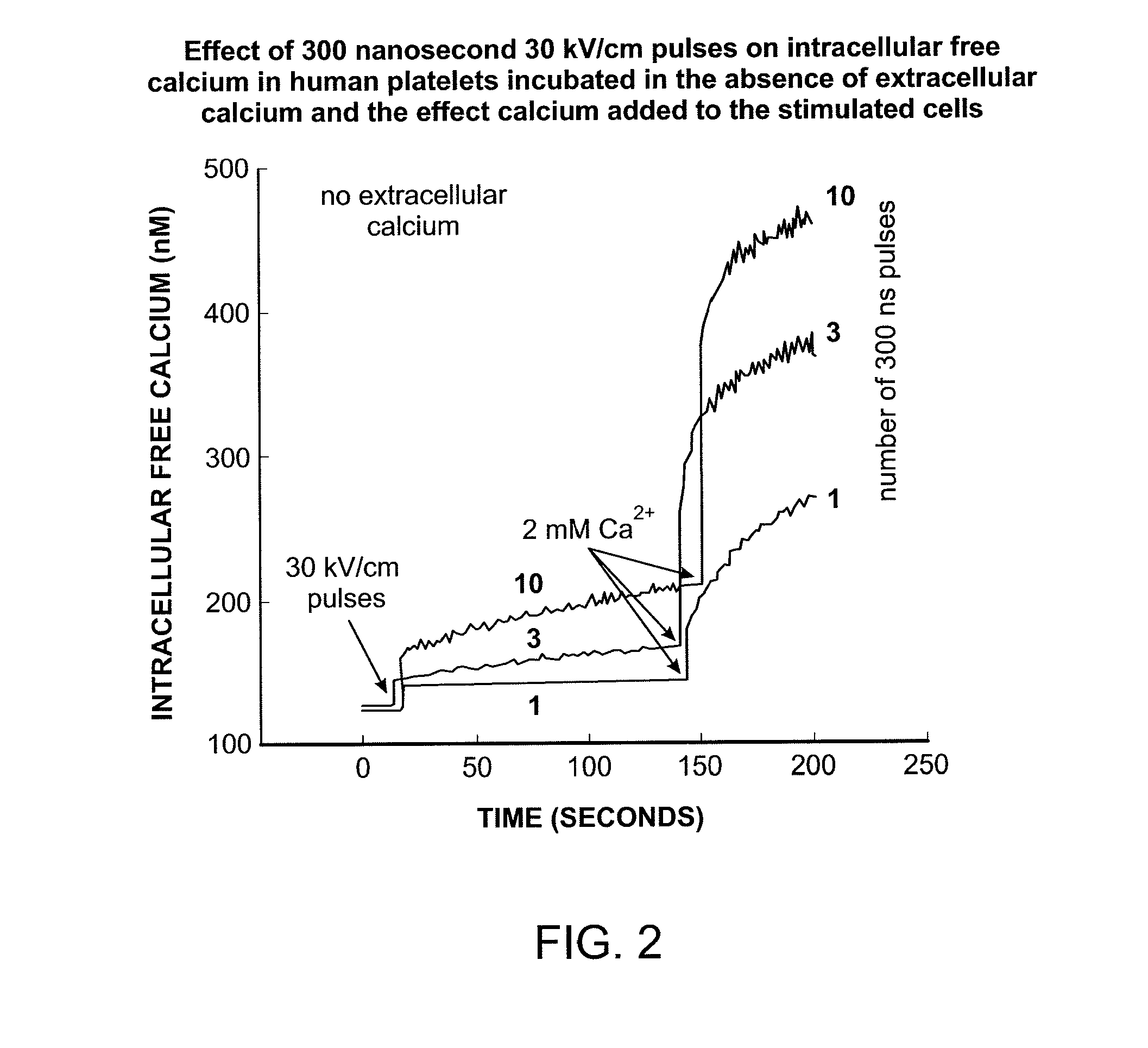
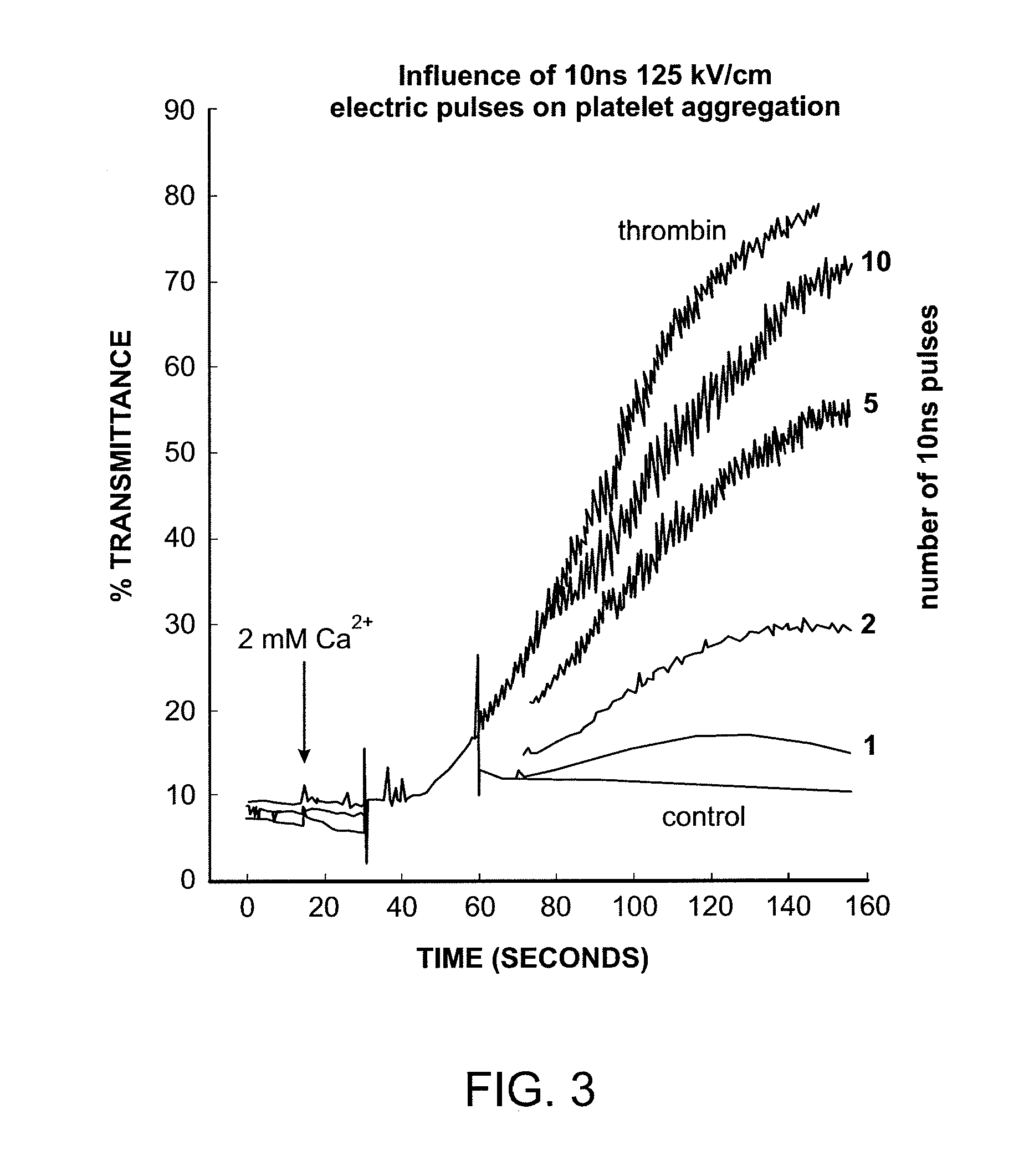
Activation of calcium-mediated cell functions in cells and tissues, including aggregation of human platelets. by nanosecond pulsed electric fields
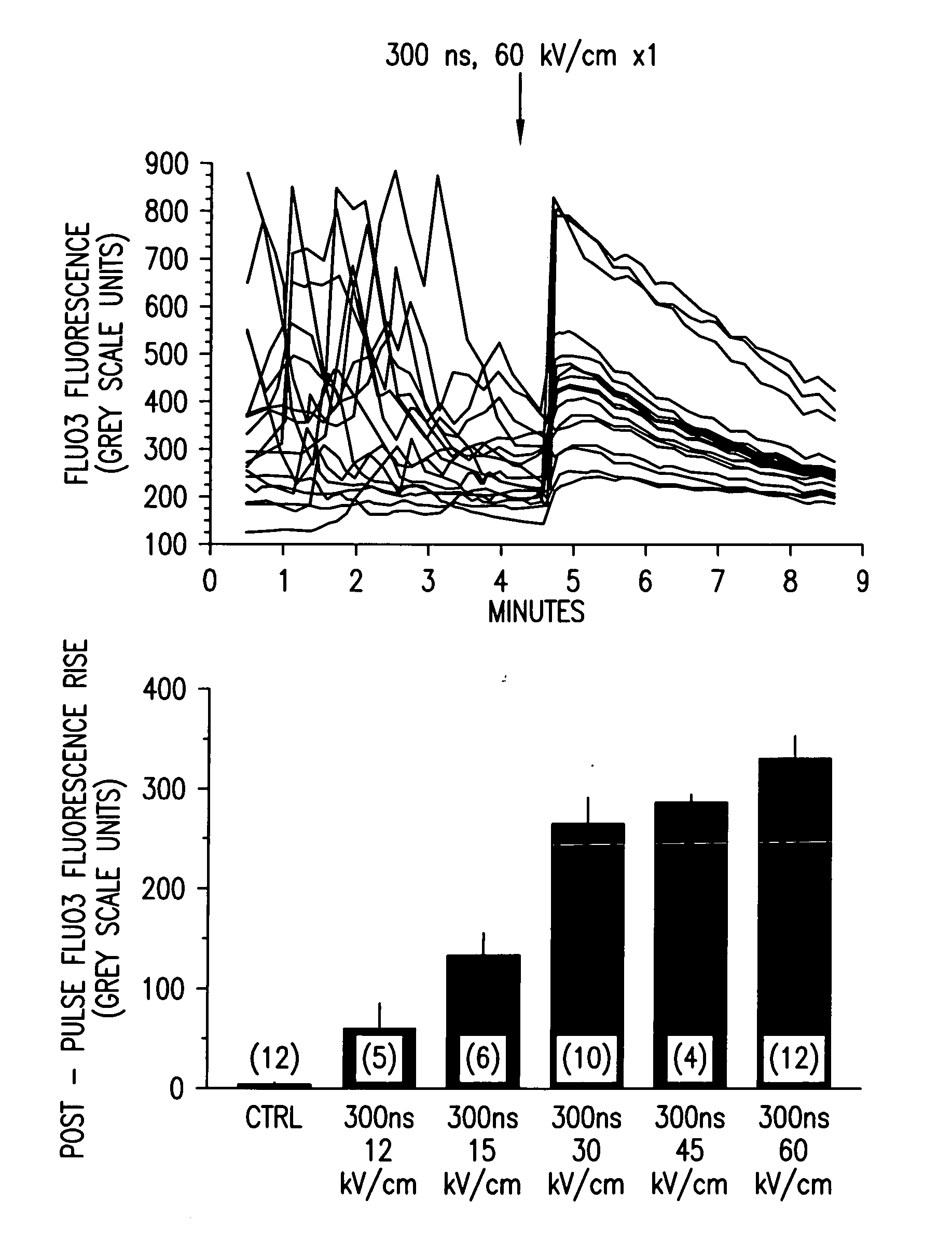
Methods for inducing calcium mobilization in cells through the application of nanosecond pulsed electric fields (“nsPEFs”) are provided. The invention also provides a method of increasing intracellular calcium in cells through the application of nsPEFs. In one embodiment of the invention, the cells are human platelets, whereby activation and aggregation of the platelets is induced. Methods for treating an injury, trauma, or loss of blood in a subject, through the application of nsPEFs are also provided.
Owner:EASTERN VIRGINIA MEDICAL SCHOOL +1
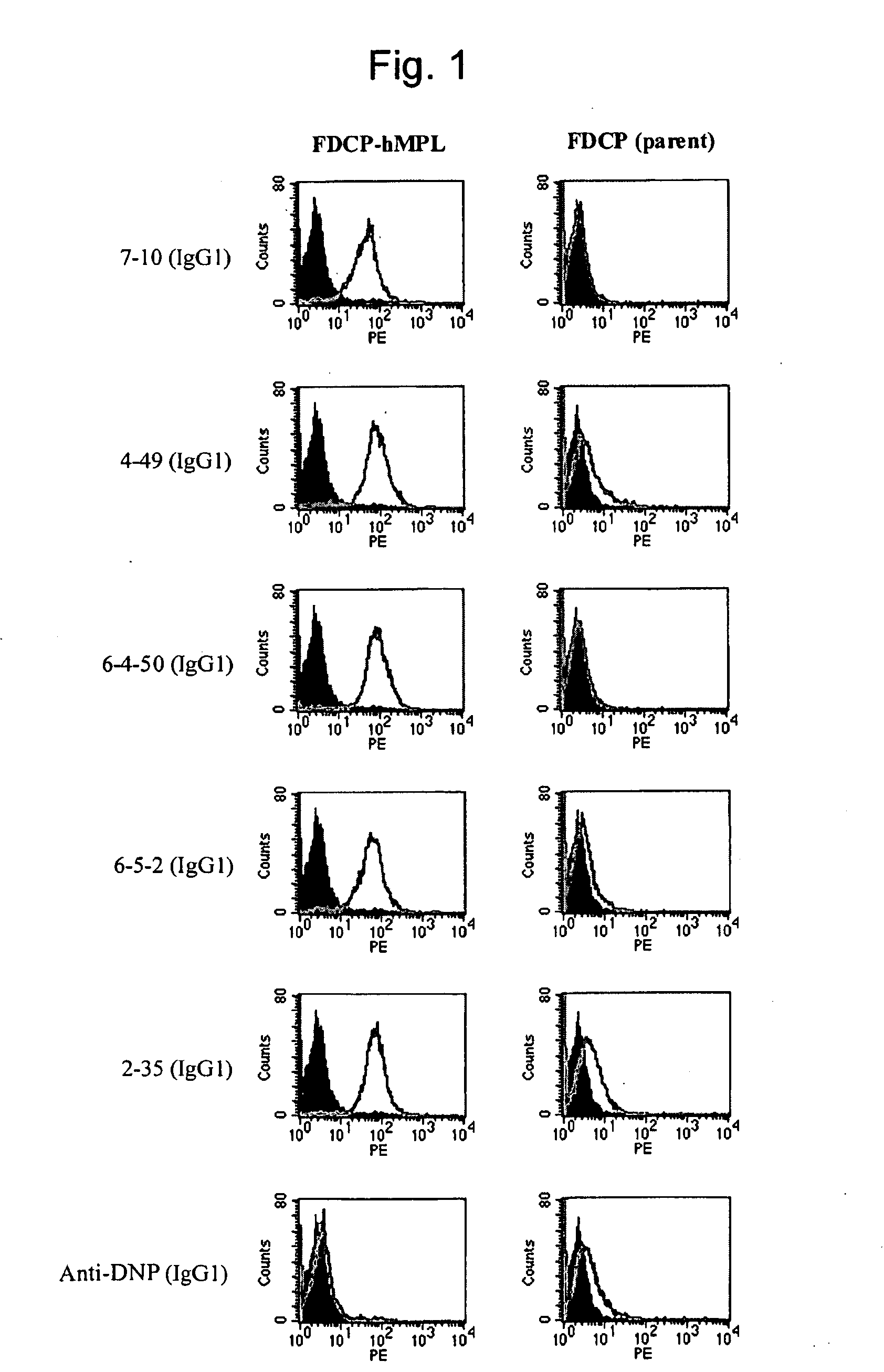
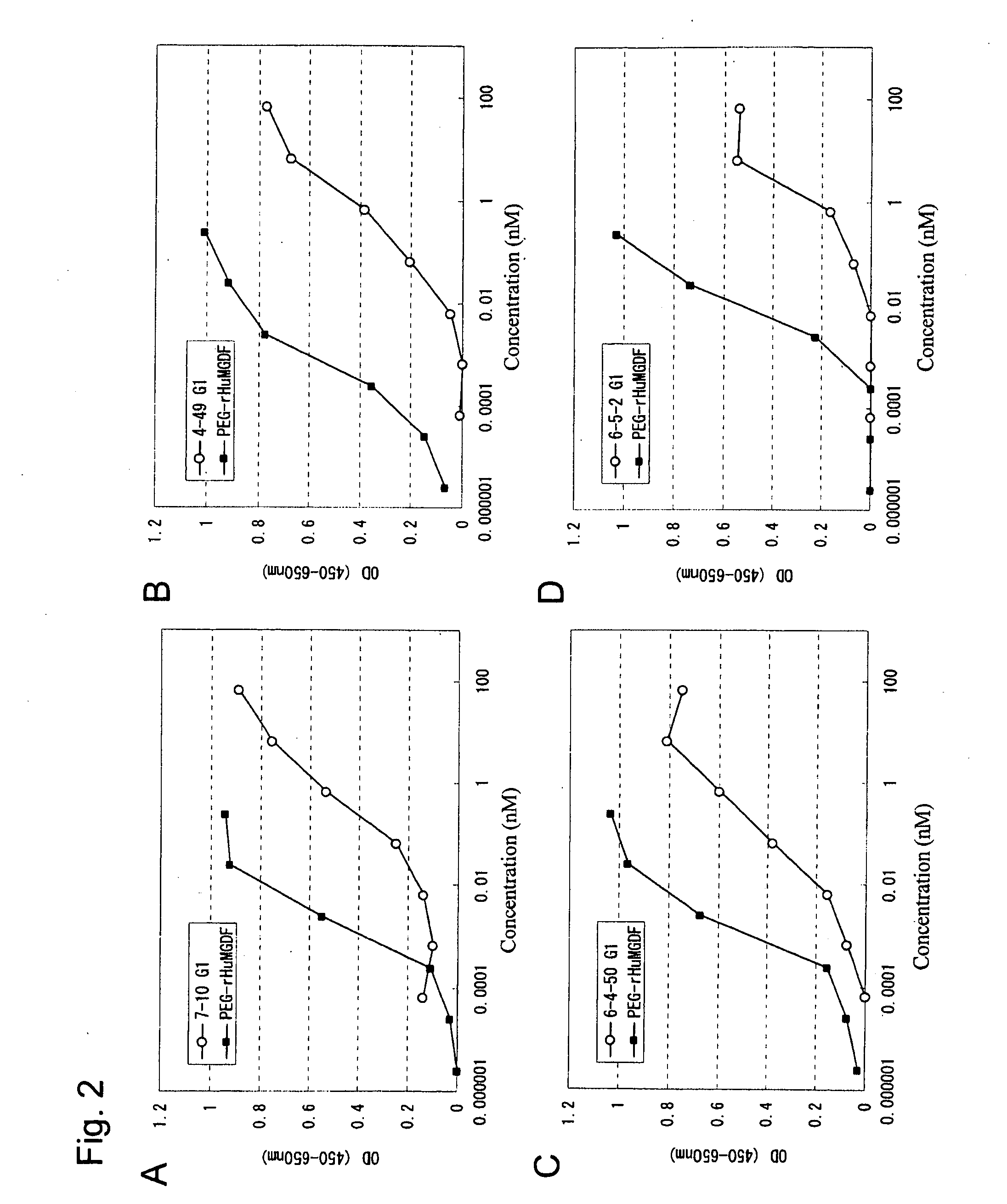
Agonist antibody to human thrombopoietin receptor
InactiveUS20100004429A1High activityLow antigenicityThrombopoietinHybrid immunoglobulinsHuman plateletUmbilical cord
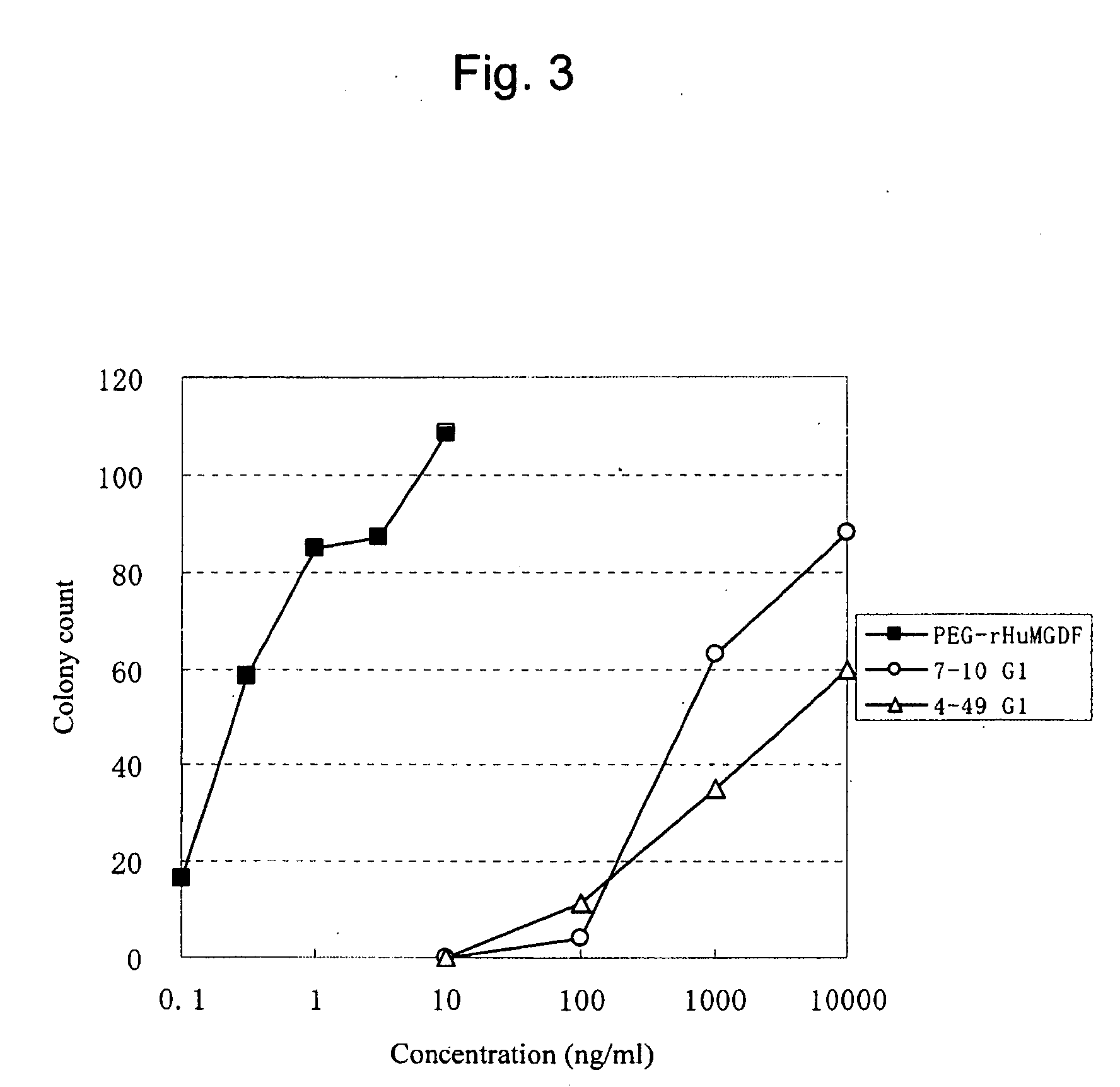
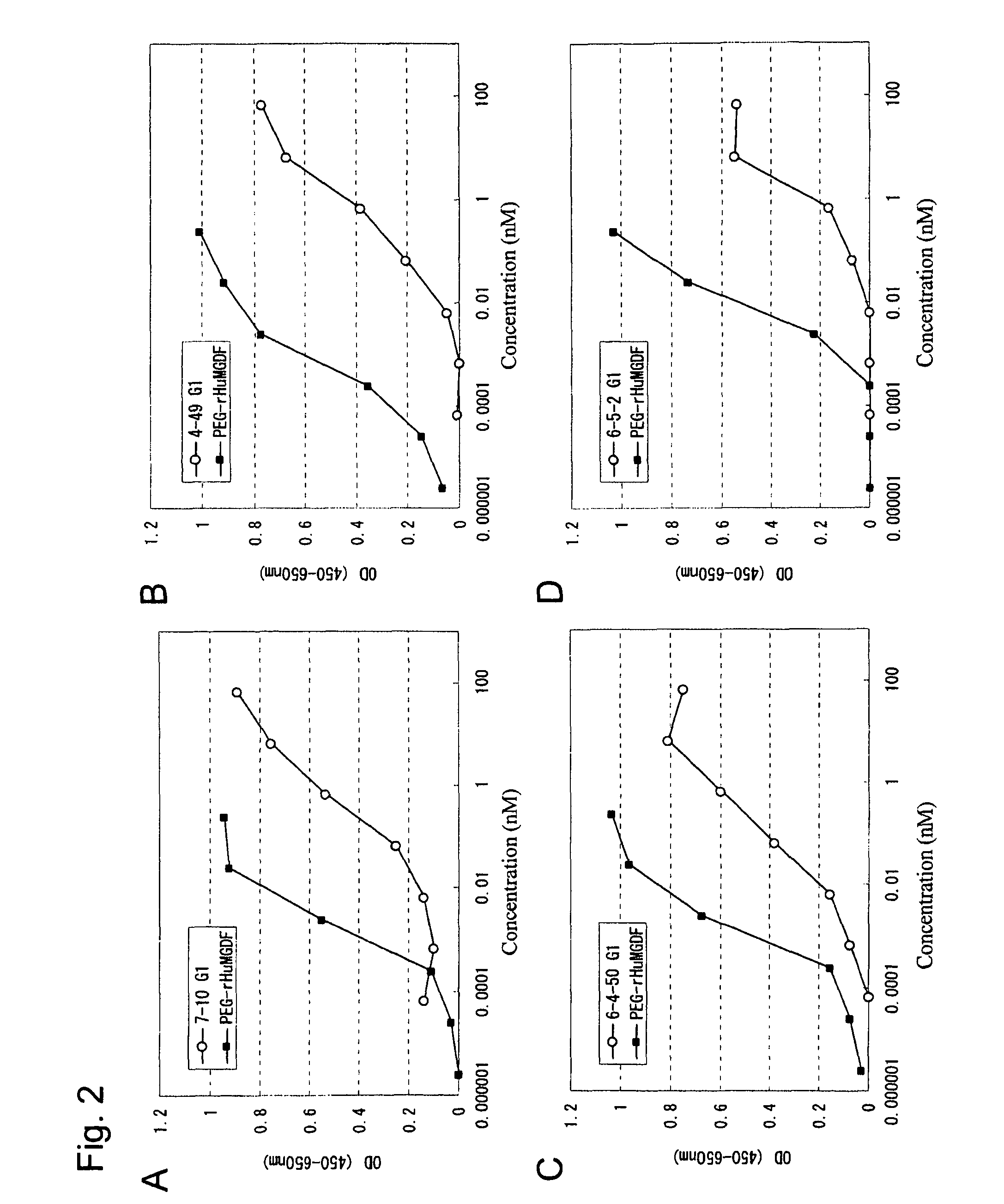
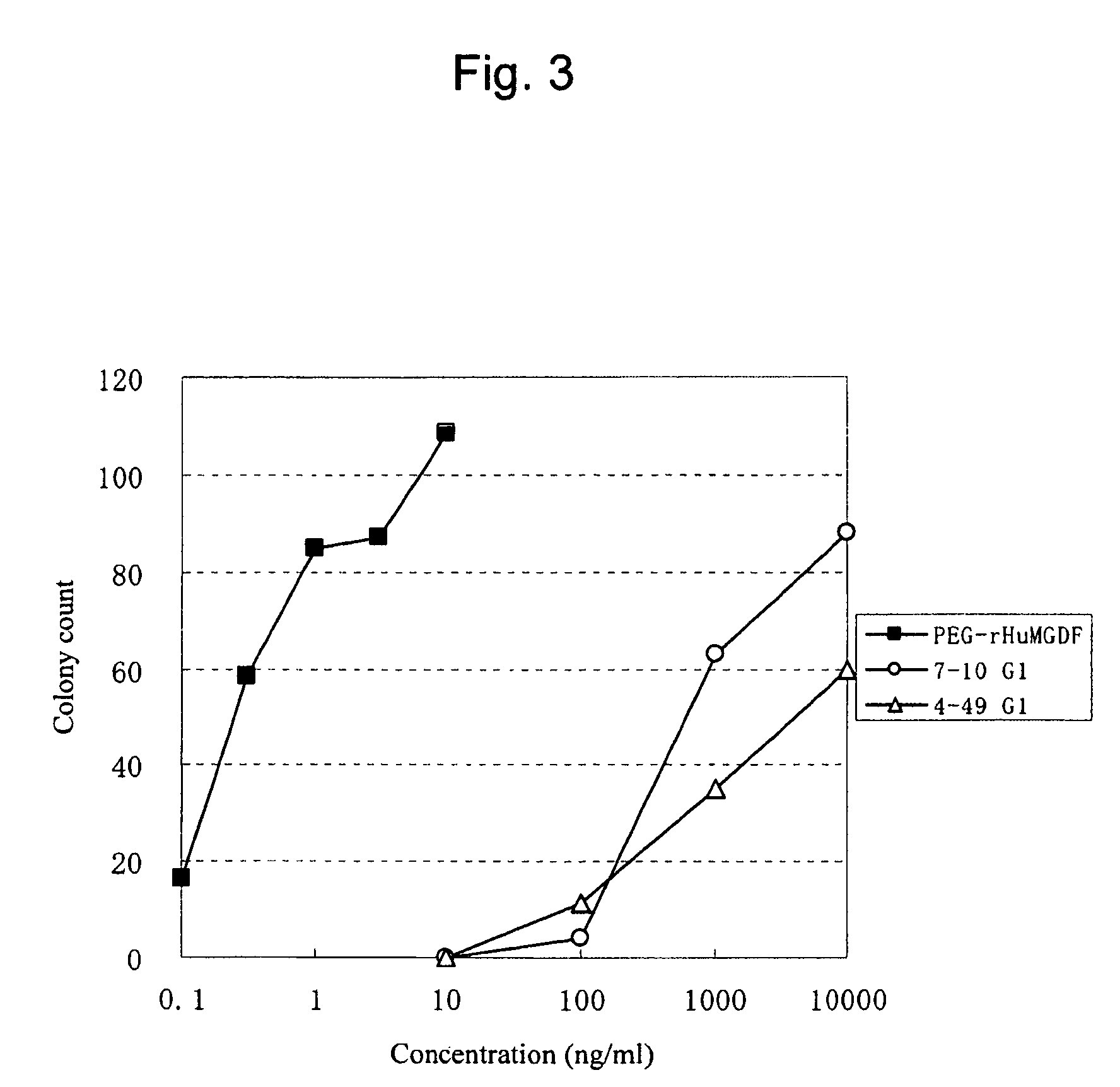
This invention provides an agonist antibody to a human thrombopoietin receptor (alias: human c-Mpl). More particularly, this invention provides an agonist antibody to a human thrombopoietin receptor, wherein the agonist antibody comprises: antibody constant regions comprising (1) amino acid sequences in a heavy chain constant region and a light chain constant region of a human antibody, (2) an amino acid sequence of a heavy chain constant region with a domain substituted between human antibody subclasses, and an amino acid sequence of a light chain constant region of a human antibody, or (3) amino acid sequences comprising a deletion(s), substitution(s), addition(s), or insertion(s) of one or several amino acid residues in the amino acid sequences of (1) or (2) above; and antibody variable regions capable of binding to and activating a human thrombopoietin receptor; and wherein the agonist antibody has the properties: (a) that the antibody induces colony formation at a concentration of 10,000 ng / ml or lower as determined by the CFU-MK colony formation assay using human umbilical-cord-blood-derived CD34+ cells; and (b) that the antibody has a maximal activity at least 50% higher than that of PEG-rHuMGDF and an 50% effective concentration (EC50) of 100 nM or less in the cell proliferation assay using UT7 / TPO cell. Also provided is a pharmaceutical composition for treating thrombocytopenia comprising said antibody.
Owner:KYOWA HAKKO KIRIN CO LTD
Monoclonal antibody against platelet membrane glycoprotein VI
InactiveUS20070025992A1Easy to manufactureSenses disorderAntibody mimetics/scaffoldsHuman plateletBULK ACTIVE INGREDIENT
The present invention provides a human antibody or an active-fragment thereof that specifically binds to human platelet membrane glycoprotein VI and does not induce a human platelet aggregation independently; a cell that produces the antibody or its active-fragment; a pharmaceutical composition that comprises the antibody or its active-fragment as an active ingredient, and so on. The above-mentioned cell can be obtained for example, as follows: a peripheral-blood-lymphocyte of the human that produces an autologous antibody to GPVI is activated by in vitro immunization under specific conditions; a hybridoma with mouse myeloma cell is prepared; and then the hybridoma that produces a monoclonal antibody, which has a binding capacity to GPVI and has an activity that suppresses collagen-mediated agglutinability of the human platelet is selected.
Owner:MOCHIDA PHARM CO LTD
Human CDR-grafted antibody and antibody fragment thereof
InactiveUS7504104B2Inhibiting cytokine-productionReduce or deplete CCR4-expressing cellsAntipyreticAnalgesicsExtracellularHuman platelet
A human CDR-grafted antibody or the antibody fragment thereof which specifically reacts with the extracellular region of human CC chemokine receptor 4 (CCR4) but does not react with a human blood platelet; a human CDR-grafted antibody or the antibody fragment thereof which specifically reacts with the extracellular region of CCR4 and has a cytotoxic activity against a CCR4-expressing cell; and a medicament, a therapeutic agent or a diagnostic agent comprising at least one of the antibodies and the antibody fragments thereof as an active ingredient.
Owner:KYOWA HAKKO KIRIN CO LTD
Biologically-safe quality control material for blood tester and preparation method thereof
ActiveCN103454410ASimple processGuaranteed stabilityBiological testingHuman plateletWhite blood cell
The invention discloses a biologically-safe quality control material for a blood tester and a preparation method thereof. The preparation method comprises that animal red blood cells having the sizes similar to sizes of human red blood cells are used for simulation of human red blood cells; animal red blood cells having the sizes similar to sizes of large cells in human white blood cells and animal red blood cells have the sizes similar to sizes of small cells in human white blood cells are used for simulation of human white blood cells; animal red blood cells have the sizes similar to sizes of human blood platelets are used for simulation of human blood platelets; the animal red blood cells for simulation of human white blood cells and human blood platelets are cured by formaldehyde having the content of 2-8%; the cured animal red blood cells are cleaned and then are added with bovine serum albumin so that the cure agent is removed; and according to requirements, through blending, the single-component or whole blood quality control material is obtained. The biologically-safe quality control material has good stability, a low cost and high safety, and is suitable for a blood cell analyzer, a urinary sediment analyzer and a hemoglobin analyzer.
Owner:URIT MEDICAL ELECTRONICS CO LTD
Method of prodcing megacaryocyle using umbilical blood CD 344+cell in vitro induction method
InactiveCN1556197AIncrease multipleImprove production efficiencyTissue cultureHuman plateletWhite blood cell
A process for generating megacaryocyte by in vitro induction to umbilical blood cell CD34+ includes in vitro amplifying the CD34+ in the culture medium containing fetal calf serum, human SCF, human TPO and / or ligand flt-3 and inducing the generation of megacaryocytes in the non-serum culture medium containing human TPO, human interleukin 3(IL-3) and / or human GM-CSF. Its advantage is high inducing efficiency.
Owner:上海伯瑞生物技术发展有限公司 +1
Activation of calcium-mediated cell functions in cells and tissues, including aggregation of human platelets, by nanosecond pulsed electric fields
Methods for inducing calcium mobilization in cells through the application of nanosecond pulsed electric fields (“nsPEFs”) are provided. The invention also provides a method of increasing intracellular calcium in cells through the application of nsPEFs. In one embodiment of the invention, the cells are human platelets, whereby activation and aggregation of the platelets is induced. Methods for treating an injury, trauma, or loss of blood in a subject, through the application of nsPEFs are also provided.
Owner:EASTERN VIRGINIA MEDICAL SCHOOL +1
Serum-free medium of stem cell
ActiveCN104805054APromote proliferationPromote differentiationSkeletal/connective tissue cellsSerum free mediaVitamin C
The invention discloses a serum-free medium of a stem cell, which comprises a basal medium and an additive, wherein the basal medium is DMEM / F12 (Dulbecco Modified Eagle Medium / F12); the additive comprises 2-15%(v / v) of serum replacement, 20-100ug / ml vitamin C, 0.5-10ng / ml stem cell growth factor, 5-20ng / ml human platelet-derived growth factor and 1-5mmol / ml L-glutamine. The serum-free medium of the stem cell for cell culture has the advantages of short cell cycle, strong multiplication capacity, good cell uniformity and high purity, effectively inhibits differentiation of the stem cell and adherence growth of an endothelial cell, ensures purity and dryness of the stem cell and is free from ingredients of animal origin such as fetal calf serum, and the stem cell obtained by the medium is suitable for clinical application.
Owner:钜威细胞(厦门)医学科技有限公司
Flow micro-sphere method for detecting plastocyte specificity immune body
InactiveCN101246173AIncreased sensitivityContribute to basic experimental researchMaterial analysisHuman plateletMicrosphere
The invention provides a method belonging to the field of cytometric bead array with high sensitivity for testing platelet specific antibody based on platelet basic experimental study. Platelet lysate is used to incubate the microsphere which is coated with platelet membrane glycoprotein monoclonal antibody, then goat-anti-human IgG polyclonal antibody labeled with phycoerythrin is added, which is analyzed in cytometric bead array. If autoantibodies exist on the surface of platelet, 'microsphere-platelet membrane glycoprotein monoclonal antibody-platelet membrane glycoprotein autoantibodies-goat-anti-human IgG polyclonal antibody labeled with phycoerythrin' complex structure is formed, the fluorescence intensity of the test microsphere is enhanced. The invention is easy to operate, and has mature technology, high sensitivity for testing platelet autoantibodies, which is good for basic experimental study of platelet antibody.
Owner:侯明 +2
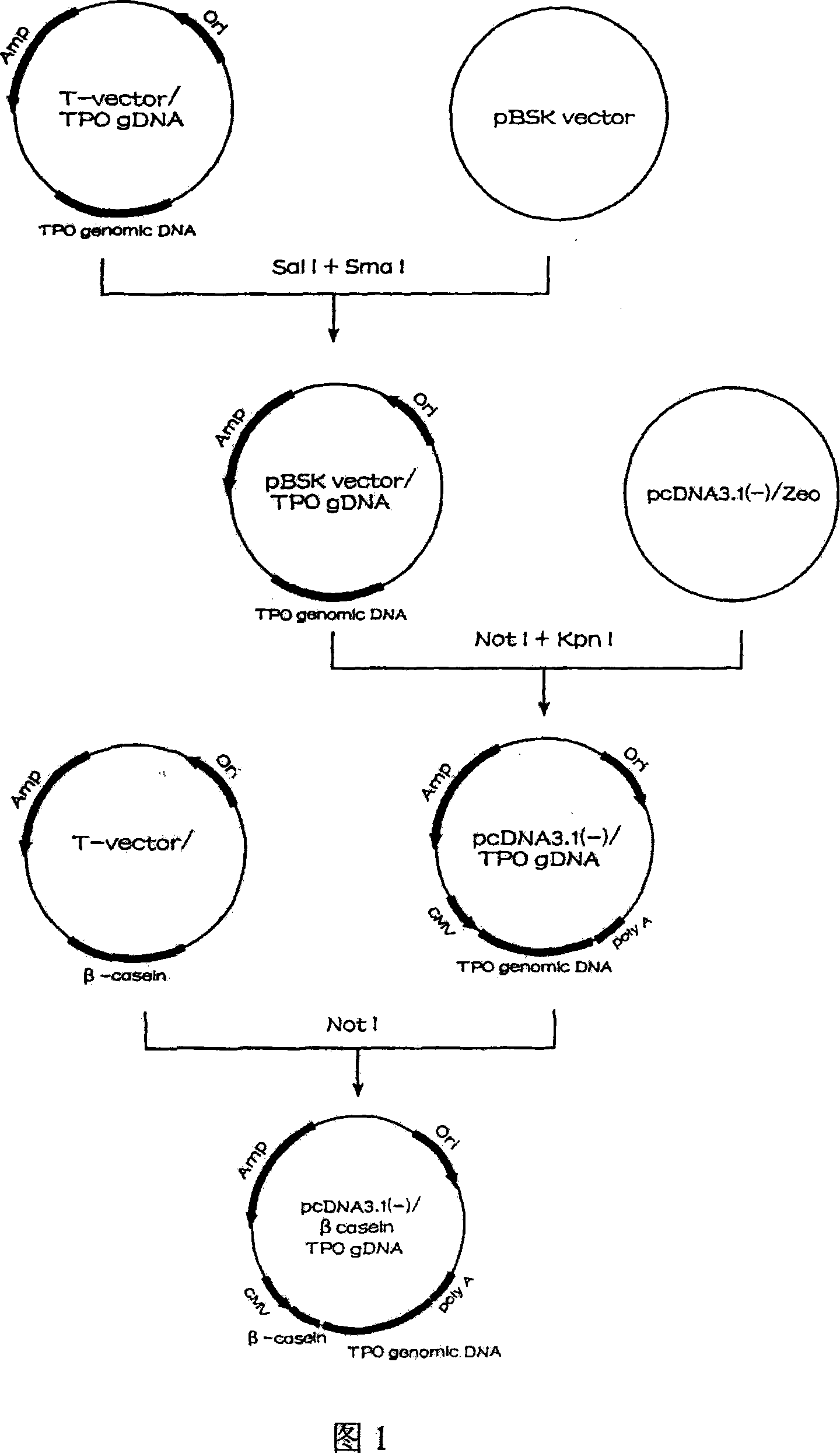
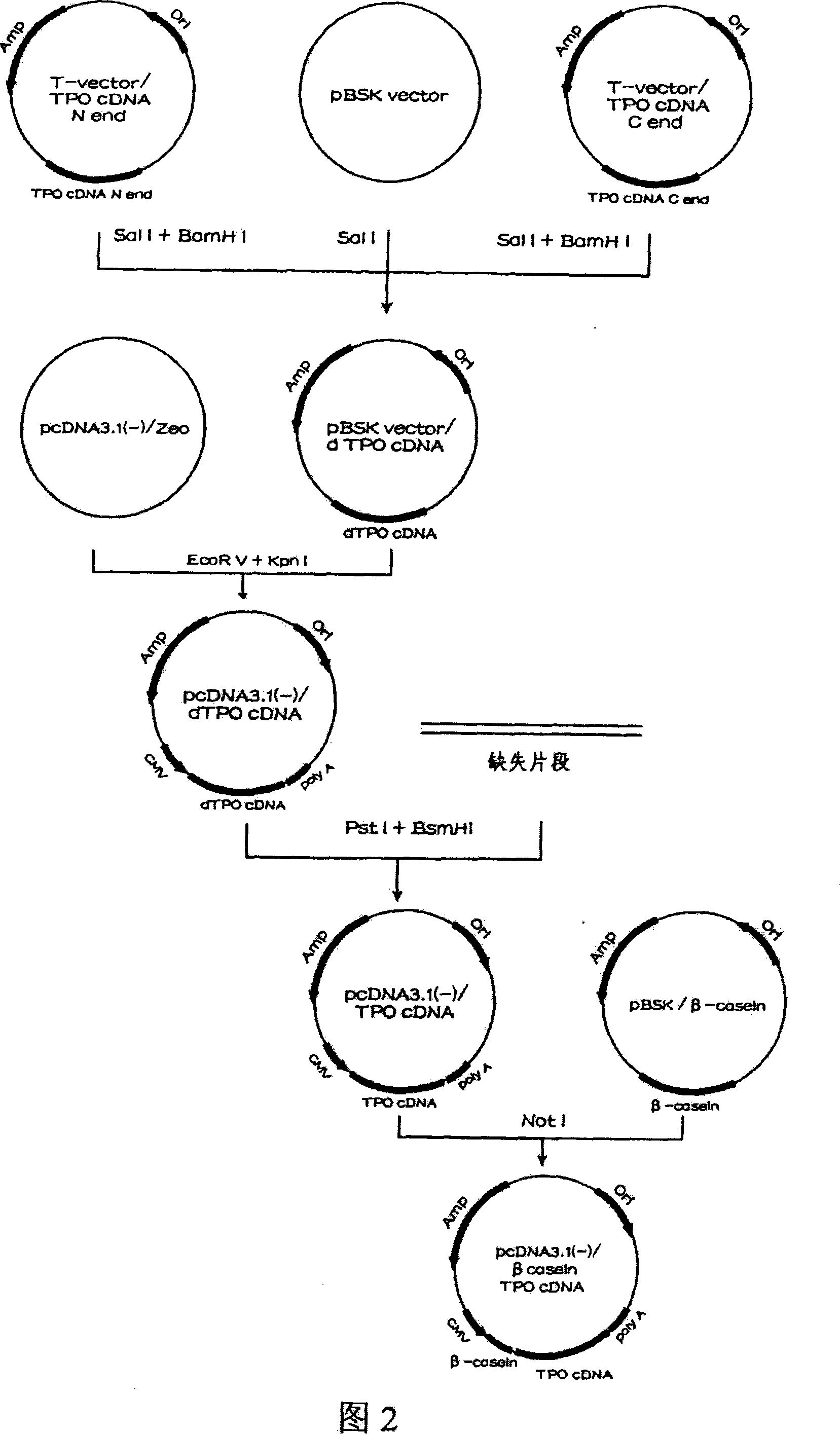
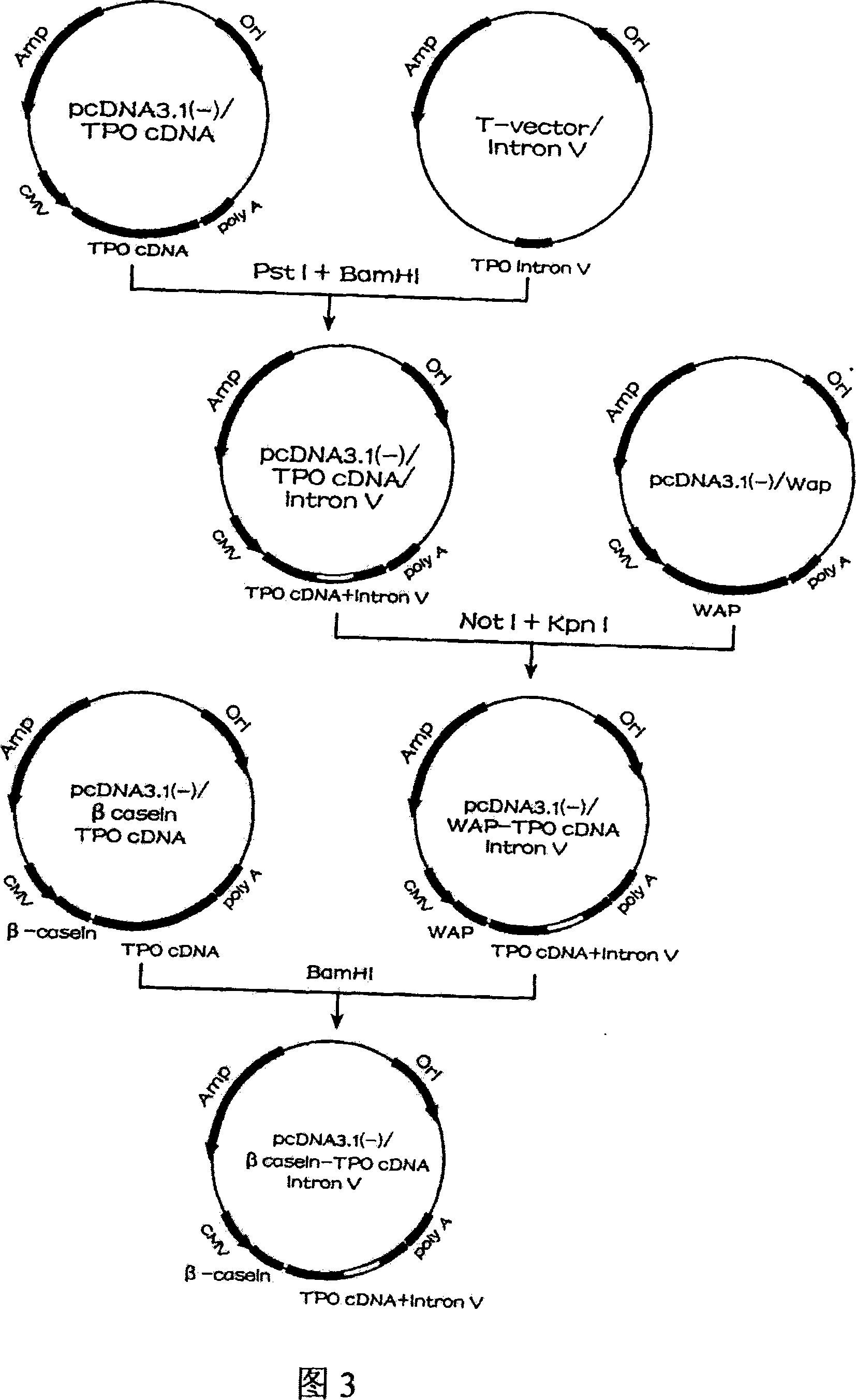
Human thrombopoietin expression vector and constructing method therefor
InactiveCN101250553AFermentationVector-based foreign material introductionHuman plateletThrombopoietin Gene
The invention belongs to the technical field of genetic engineering and transgenic engineering and in particular relates to a method for building mammary gland specific expression vectors of human thrombopoietin gene and goat beta-casein gene promoter. The vectors which are built are transferred to COS-7 and HC-11 cells and expressed on eukaryotic cell level verification hTPO. The built mammary gland specific expression vectors of the human thrombopoietin gene and the goat beta-casein gene promoter are applied to prepare a mammary gland bioreactor of transgenic animals and are expressed with high level in the milk of transgenic mammalian, and the vectors provide useful drug protein-hTPO for clinic. The mammary gland specific expression vectors of human thrombopoietin gene and goat beta-casein gene promoter which are provided by the invention provide a foundation for further research and development.
Owner:上海医学遗传研究所 +2
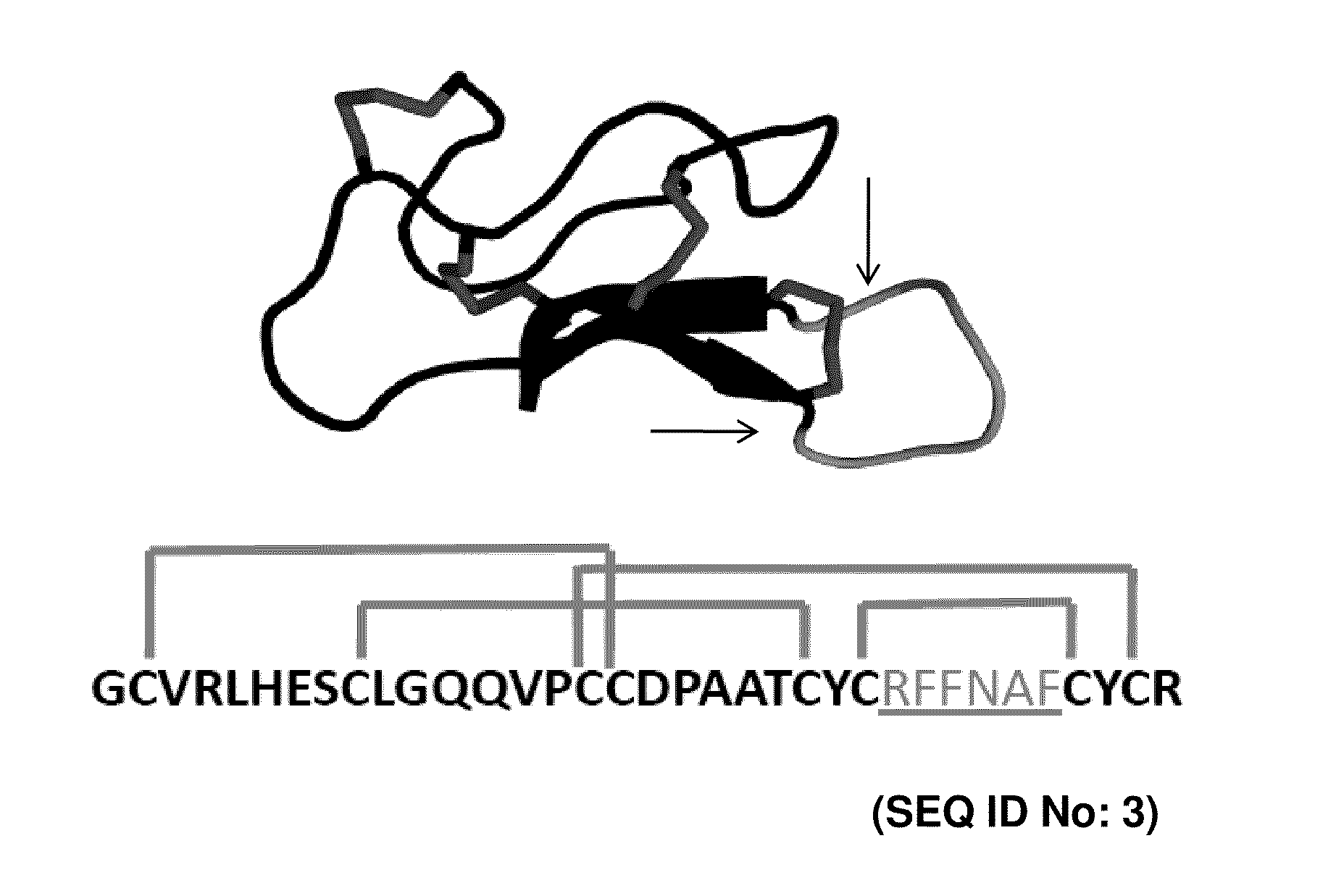
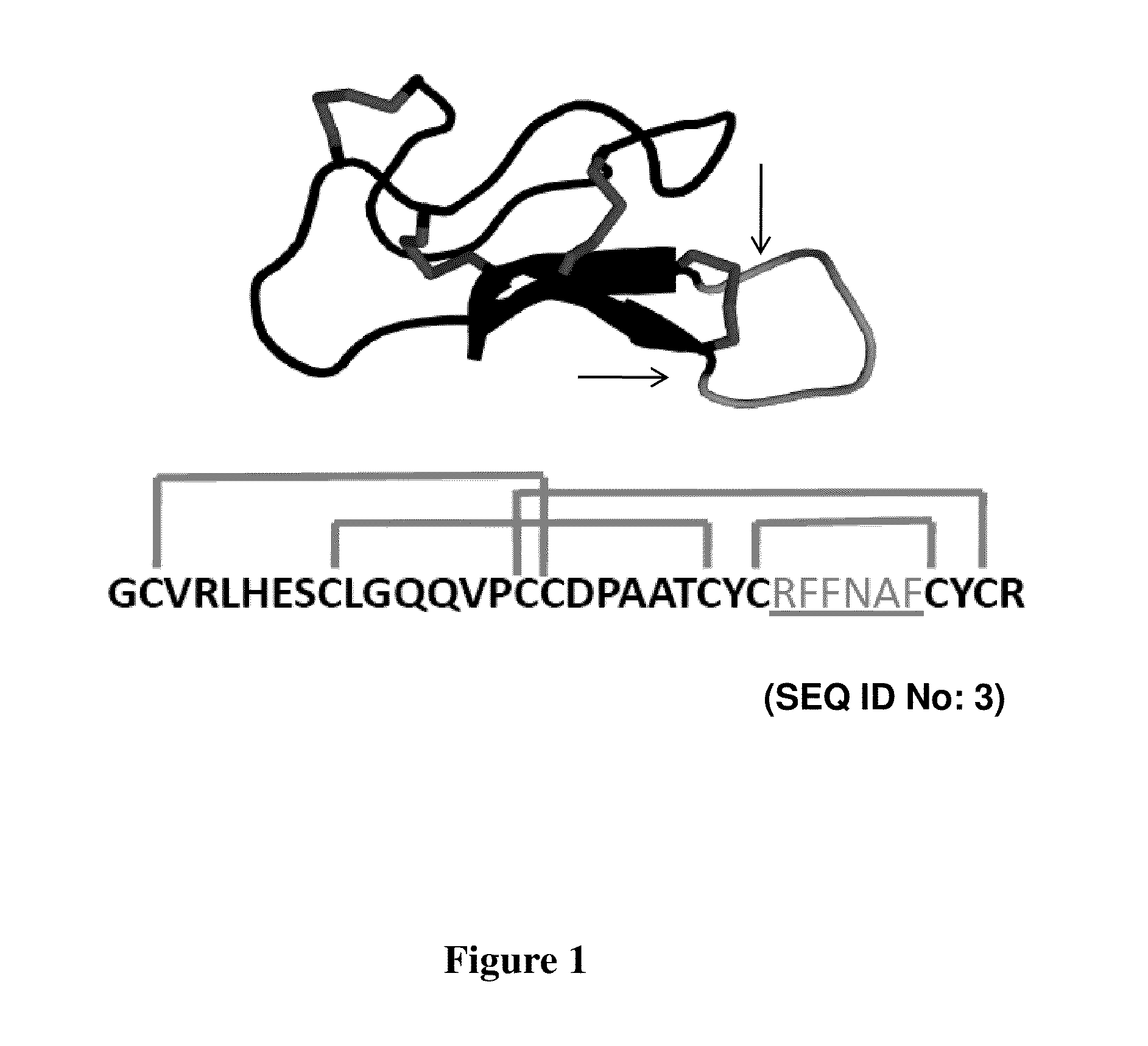
CYSTINE KNOT PEPTIDES BINDING TO ALPHA IIb BETA 3 INTEGRINS AND METHODS OF USE
InactiveUS20110136740A1Inhibits platelet aggregationImprove bindingDepsipeptidesPeptide preparation methodsCystine knotAlpha-IIb/Beta-3
Disclosed are peptides having a cystine knot structural motif and comprising a sequence engineered for specificity against αIIbβ3 integrin, found on platelets, and a method of using the same in anti-thrombotic therapies. The present peptides utilize a cystine knot scaffold derived from modified agouti-related protein or agatoxin, An alternate library screening strategy was used to isolate variants of peptides that selectively bound to αIIbβ3 integrin or to both αIIbβ3 and αVβ3 integrins. Unique consensus sequences were identified within the identified peptides suggesting alternative molecular recognition events that dictate different integrin binding specificities. In addition, the engineered peptides prevented human platelet aggregation in a plasma-based assay and showed high binding affinity for αIIbβ3 integrin.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Agonist antibody to human thrombopoietin receptor
This invention provides an agonist antibody to a human thrombopoietin receptor (alias: human c-Mpl). More particularly, this invention provides an agonist antibody to a human thrombopoietin receptor, wherein the agonist antibody comprises: antibody constant regions comprising (1) amino acid sequences in a heavy chain constant region and a light chain constant region of a human antibody, (2) an amino acid sequence of a heavy chain constant region with a domain substituted between human antibody subclasses, and an amino acid sequence of a light chain constant region of a human antibody, or (3) amino acid sequences comprising a deletion(s), substitution(s), addition(s), or insertion(s) of one or several amino acid residues in the amino acid sequences of (1) or (2) above; and antibody variable regions capable of binding to and activating a human thrombopoietin receptor; and wherein the agonist antibody has the properties: (a) that the antibody induces colony formation at a concentration of 10,000 ng / ml or lower as determined by the CFU-MK colony formation assay using human umbilical-cord-blood-derived CD34+ cells; and (b) that the antibody has a maximal activity at least 50% higher than that of PEG-rHuMGDF and an 50% effective concentration (EC50) of 100 nM or less in the cell proliferation assay using UT7 / TPO cell. Also provided is a pharmaceutical composition for treating thrombocytopenia comprising said antibody.
Owner:KYOWA HAKKO KIRIN CO LTD
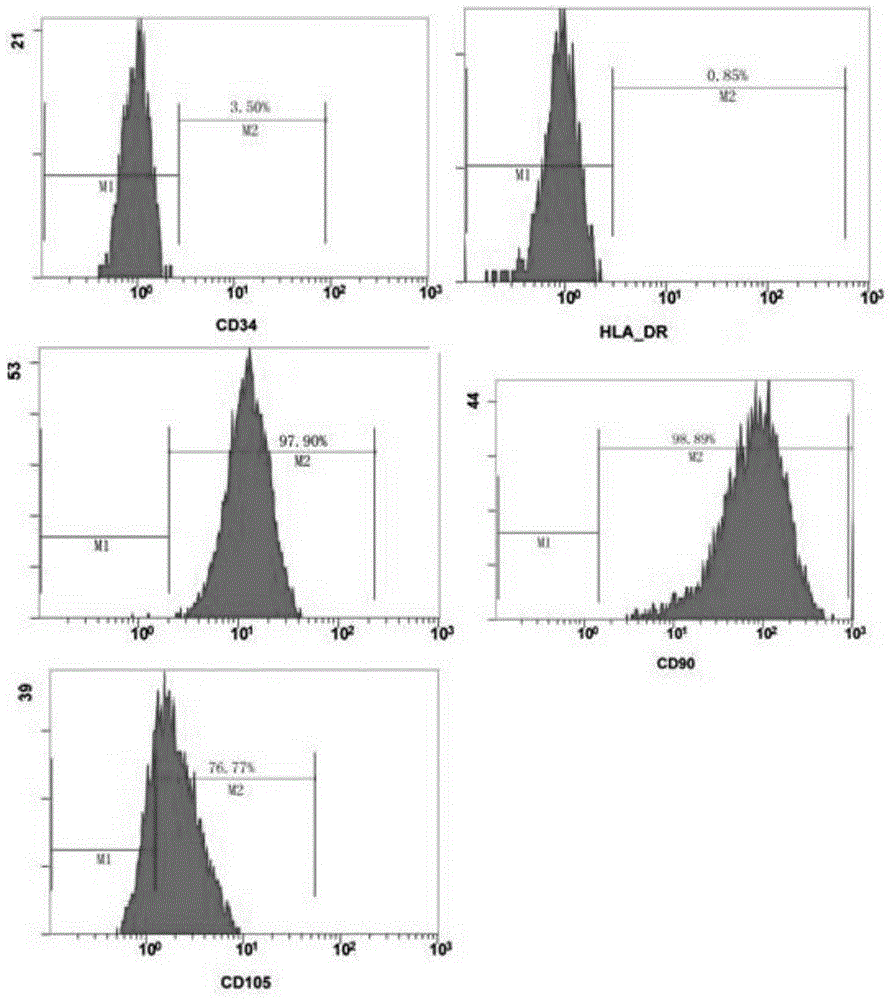
Preparation of human platelet-rich plasma and application of same in isolation and culture of human mesenchymal stem cells
ActiveCN102367435ARich sourcesTake advantage ofSkeletal/connective tissue cellsBiotechnologyHuman platelet
The invention provides preparation of human platelet-rich plasma and application of the same in isolation and culture of human mesenchymal stem cells. According to the invention, erythrocytes and a mixture of leucocytes and platelets are separated from human whole blood through centrifugation, then centrifugation with a centrifugal force greater than a centrifugal force used in above-mentioned centrifugation is carried out on residual blood plasma, obtained sediment is collected and is subjected to freezing-thawing treatment respectively at a temperature no more than -20 DEG C and a temperature no less than normal temperature, and the sediment is removed through separation so as to obtain human platelet-rich plasma. The invention has the following beneficial effects: raw materials used inthe method are widely available; the blood resource is fully utilized; the obtained product of platelets is purer, contains more abundant nutritional components including growth factors and has a more ideal effect when used for isolation and culture of human mesenchymal stem cells.
Owner:SICHUAN NEO LIFE STEM CELL BIOTECH
Mesenchymal stem cell serum-free medium and cell isolation and cultivation methods
PendingCN105950550ASimple ingredientsEliminate distractionsCulture processSkeletal/connective tissue cellsCells isolationSerum free media
The invention provides a mesenchymal stem cell serum-free medium. The mesenchymal stem cell serum-free medium is prepared from, by volume, 94-97 parts of DMEM / F12, 2-5 parts of human blood platelet lysate and 1 part of non-essential amino acid. The invention further provides application of the medium and isolation and cultivation methods of the mesenchymal stem cell serum-free medium. The serum-free medium can be used for effectively separating and cultivating mesenchymal stem cells derived from placentae and umbilical cords, the effects are superior to commercially available media, the cost is low, the safety is high, and the application prospect is good.
Owner:SICHUAN NEO LIFE STEM CELL BIOTECH
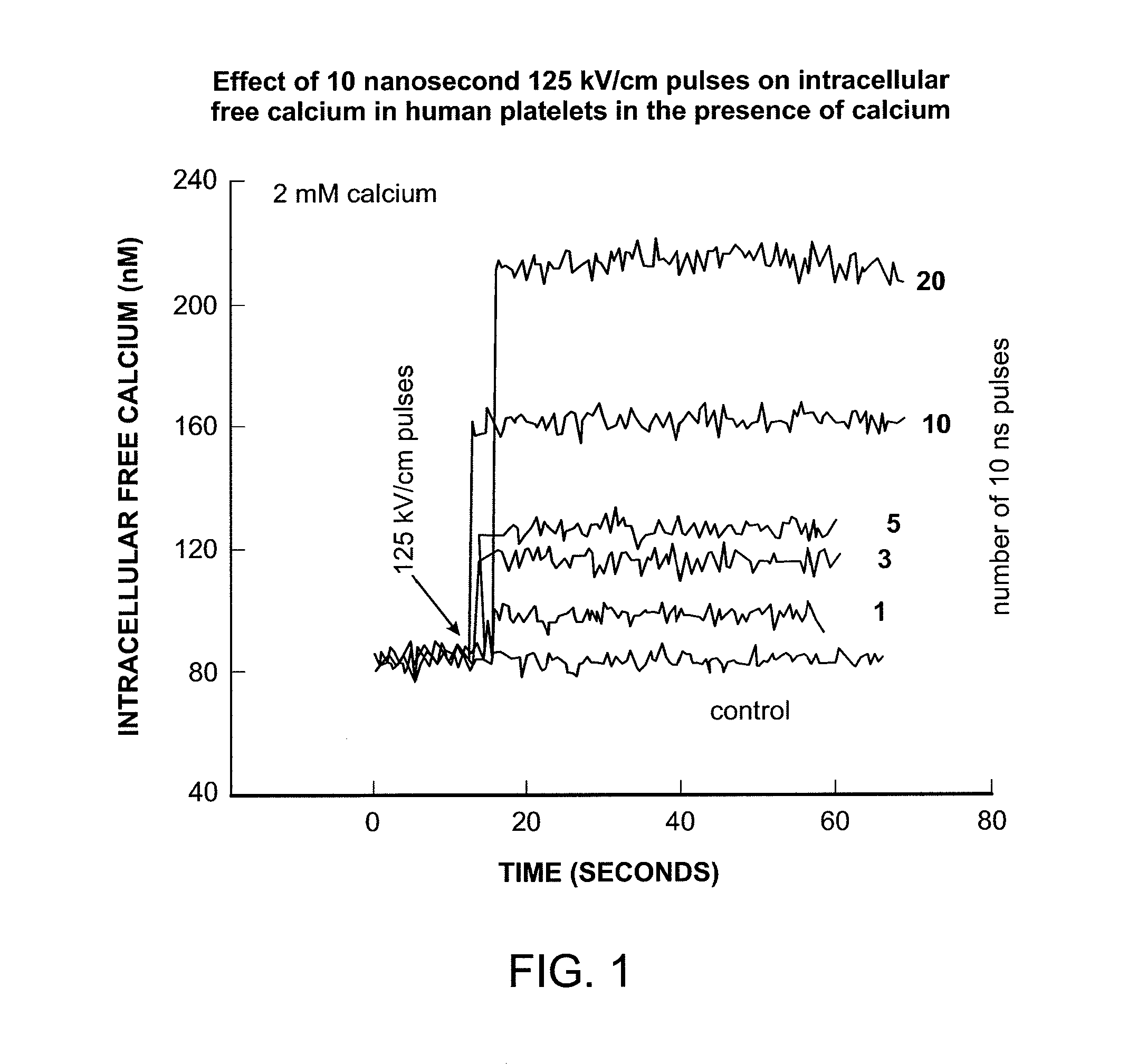
Activation and aggregation of human platelets and formation of platelet gels by nanosecond pulsed electric fields
InactiveUS20140106430A1ElectrotherapyMammal material medical ingredientsHuman plateletElectrical field strength
Owner:EASTERN VIRGINIA MEDICAL SCHOOL +1
Stem cell culture medium
InactiveCN105200008ADoes not affect potentialHigh speedNervous system cellsSkeletal/connective tissue cellsSodium bicarbonateHuman platelet
The invention discloses a stem cell culture medium. The stem cell culture medium comprises an improved DMEM (Dulbecco's Modified Eagle Medium) / F12 basal culture medium and an additive, wherein the additive comprises sodium bicarbonate, selenium amino acid chelate, recombinant human insulin growth factors, recombinant human basic fibroblast growth factors, recombinant human lactoferrin, ascorbic acid, recombinant human platelet-derived growth factors, recombinant human vascular endothelial cell growth factors, octacosanol, polyvinyl alcohol, polyvinylpyrrolidone and recombinant human epidermal growth factors. According to the stem cell culture medium, the potential of stem cells is not influenced while the stem cells can be proliferated rapidly, the proliferation speed of the stem cells is increased by 3-5 times compared with a common culture medium, and further, the stem cell culture medium can be used for culturing the stem cells of various kinds of tissue and has excellent applicability; the cultured stem cells have high differentiation capability, can be differentiated into multiple functional cells and have very high scientific research and medical application values, culture medium components are exact, the quality is stable, and accordingly, the cultured stem cells are not likely to generate human body rejection reaction after transplanting.
Owner:XINXIANG MEDICAL UNIV
ACTIVATION and AGGREGATION OF HUMAN PLATELETS AND FORMATION OF PLATELET GELS BY NANOSECOND PULSED ELECTRIC FIELDS
Methods for forming activated platelet gels using nsPEF's and applying the activated gels to wounds, such as heart tissue after myocardial infarction. The platelets are activated by applying at least one nsPEF with a duration between about 10 picoseconds to 1 microsecond and electrical field strengths between about 10 kV / cm and 350 kV / cm.
Owner:EASTERN VIRGINIA MEDICAL SCHOOL
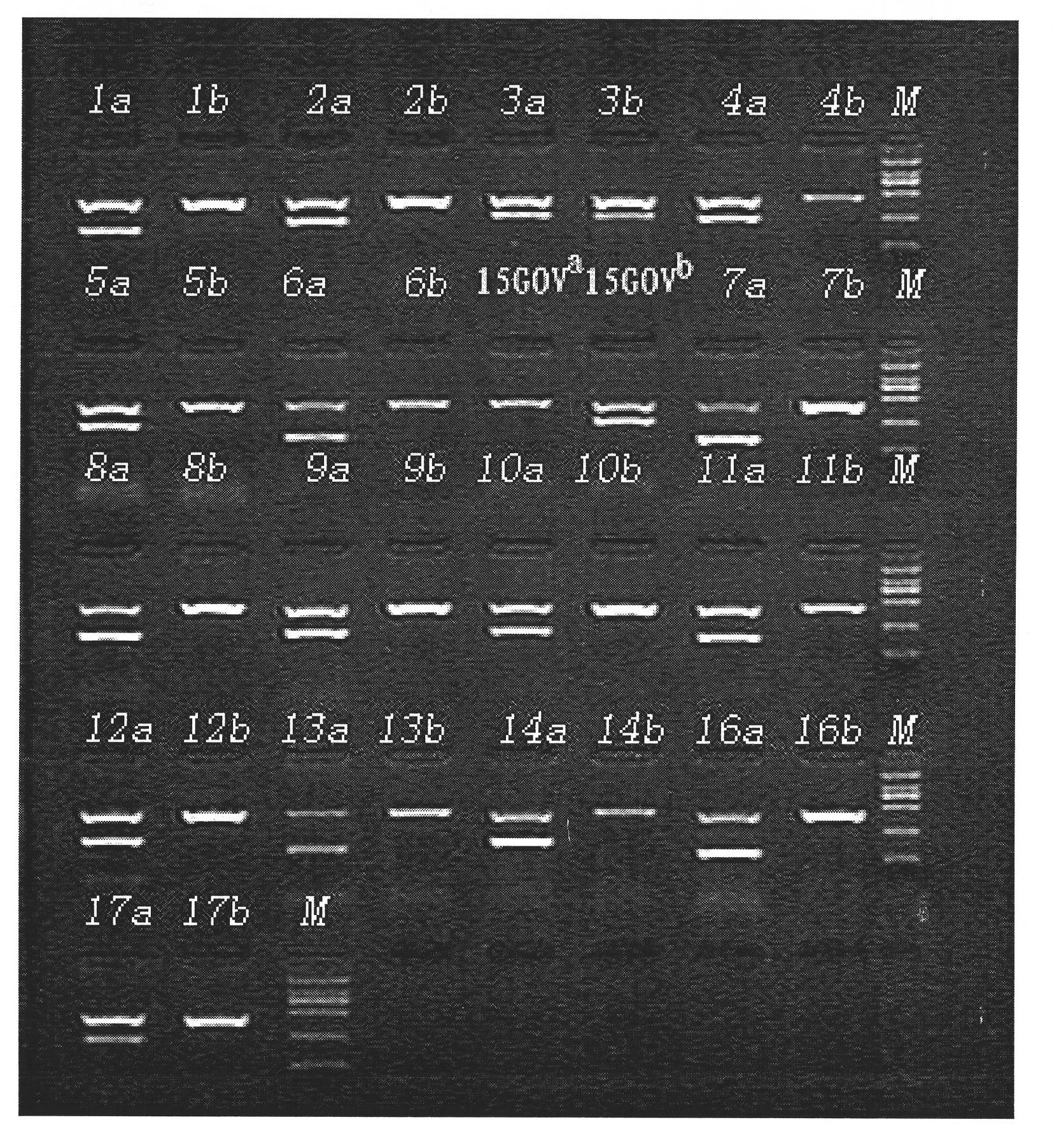
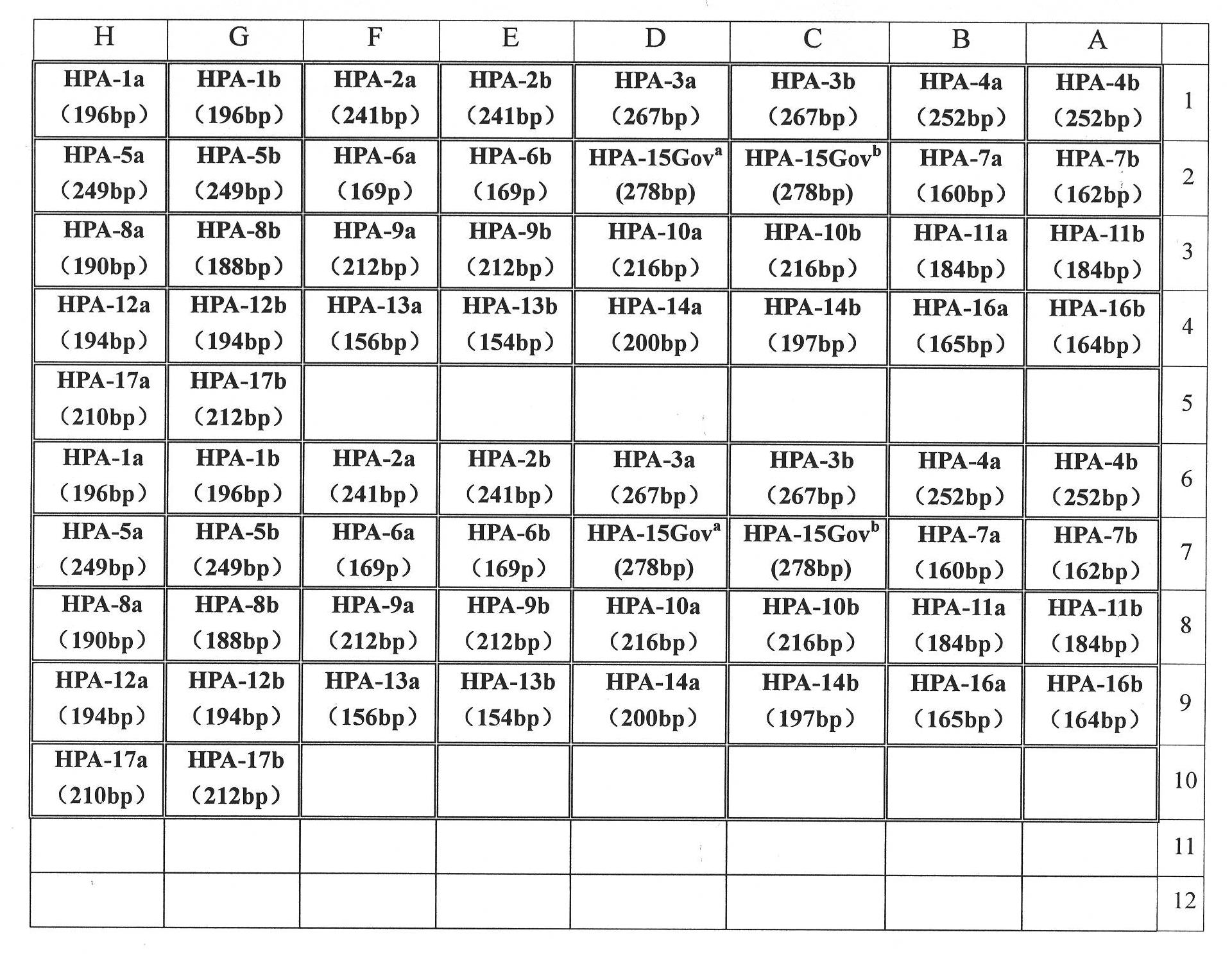
Primer group and kit for detecting human platelet alloantigen gene
ActiveCN101892314ARealize typingAvoid specific immunityMicrobiological testing/measurementDNA/RNA fragmentationHuman plateletWhole blood product
The invention discloses a primer group and a kit for detecting a human platelet alloantigen (HPA) gene. The primer group for detecting the human platelet alloantigen (HPA) gene comprises an HPA-6a primer pair, an HPA-6b primer pair, an HPA-15Gova primer pair, an HPA-15Govb primer pair and an internal reference primer pair. The kit of the invention realizes platelet genotyping and has great significance for effectively avoiding platelet specific immunity and selecting to transfuse blood products matched with HPA genotypes in clinical and blood transfusion.
Owner:天津市秀鹏生物技术开发有限公司
Methods for the Preservation of Platelet Efficacy During Storage
Invented is a method for the preservation of human platelet lifespan and / or efficacy during storage which comprises the addition of an effective amount of a non-peptide TPO receptor agonists to a storage solution containing human platelets.
Owner:SMITHKLINE BECKMAN CORP
Anti-platelet membrane glycoprotein vi monoclonal antibody
InactiveUS20090092612A1Efficiently obtainedImmunoglobulins against blood group antigensAntibody mimetics/scaffoldsHuman plateletMonoclonal antibody
The present invention provides an antibody which has the following features, its active fragment, or a derivative thereof:a) It specifically binds to human platelet membrane glycoprotein VI (GPVI);b) The function to activate a platelet and / or the function to induce a thrombocytopenia in vivo are low; andc) It at least partially depletes GPVI on the platelet membrane by contacting with a platelet.
Owner:MOCHIDA PHARM CO LTD
Preparation of platelet analogs
Erythrocytes from a vertebrate animal other than a human that have been size reduced and treated with a fixing agent by conventional methods to resemble human platelets are further treated to achieve a size distribution that is detected in a consistent manner by different methodologies of blood cell counting. The further treatment includes heating to a denaturing temperature followed by a second treatment with a fixing agent. The resulting cells are useful as controls for calibrating and checking the accuracy of automated blood cell counting equipment.
Owner:BIO RAD LAB INC
Method of preparing a growth factor concentrate derived from human platelets
The invention relates to a method of preparing an intra-dermally, intra-articularly, sub-dermally or topically administrable growth factor concentrate derived from human platelets. The method comprises the steps of suspending human platelets in multiple electrolyte isotonic solution; snap-freezing the suspension; thawing the frozen suspension; and sterile-filtering the suspension. In particular, in this method, a fixed number of platelets is suspended in a fixed volume of multiple electrolyte isotonic solution to obtain the required concentration of growth factors in the growth factor concentrate, snap-freezing of the suspension is carried out at a temperature of −120° C. to −200° C., thawing of the frozen suspension is carried out at 25° C. to 37° C., and cellular debris are separated from the thawed suspension and the resultant suspension of growth factors is diluted with an isotonic medium before sterile-filtering.
Owner:KASIAK RES PVT
Cell lines, ligands and antibody fragments for use in pharmaceutical compositions for preventing and treating haemostasis disorders
InactiveUS7332162B1Inhibit bindingPrevented platelet-dependent arterial thrombosisMicrobiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsMicroorganismHuman platelet
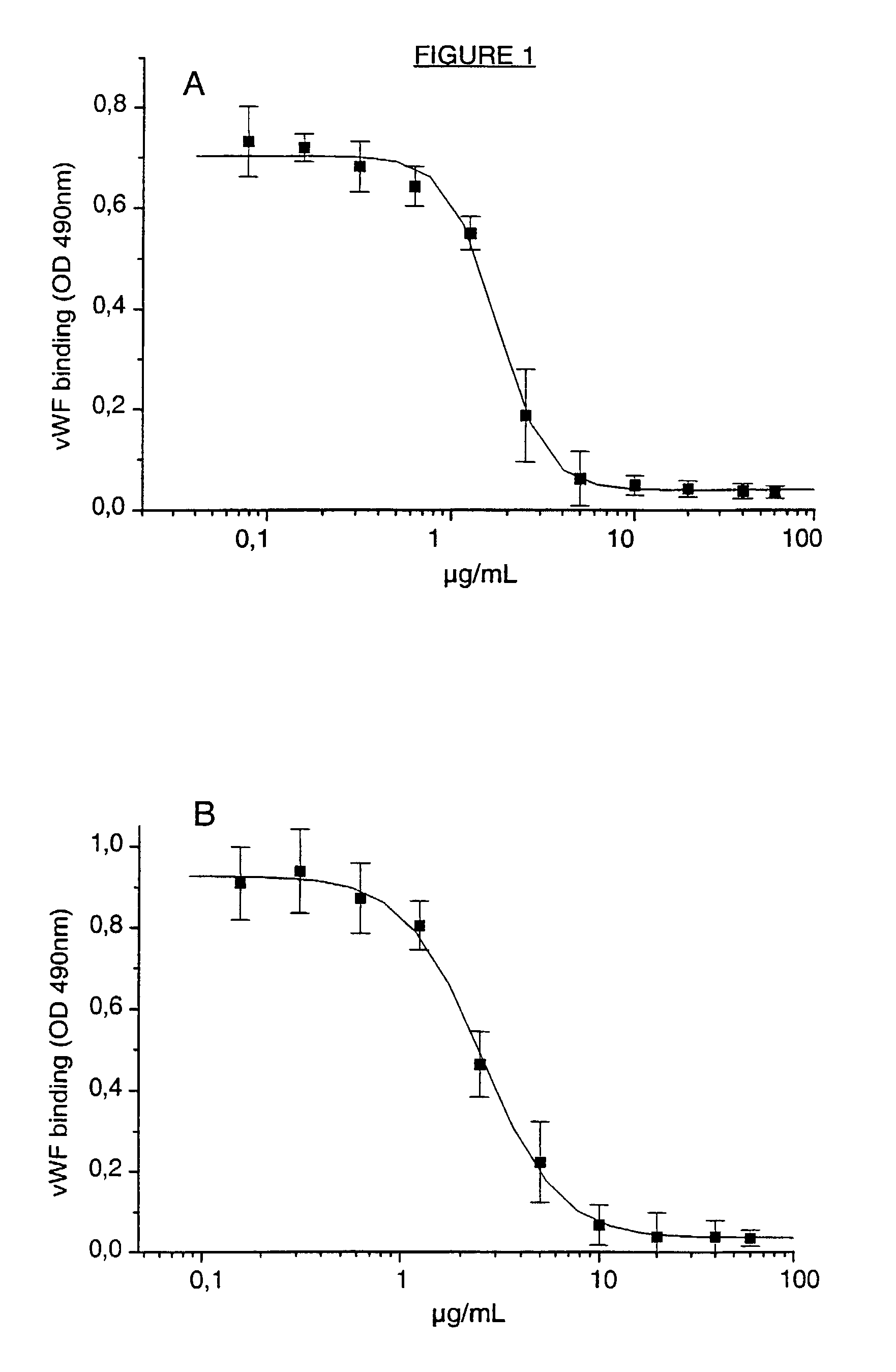
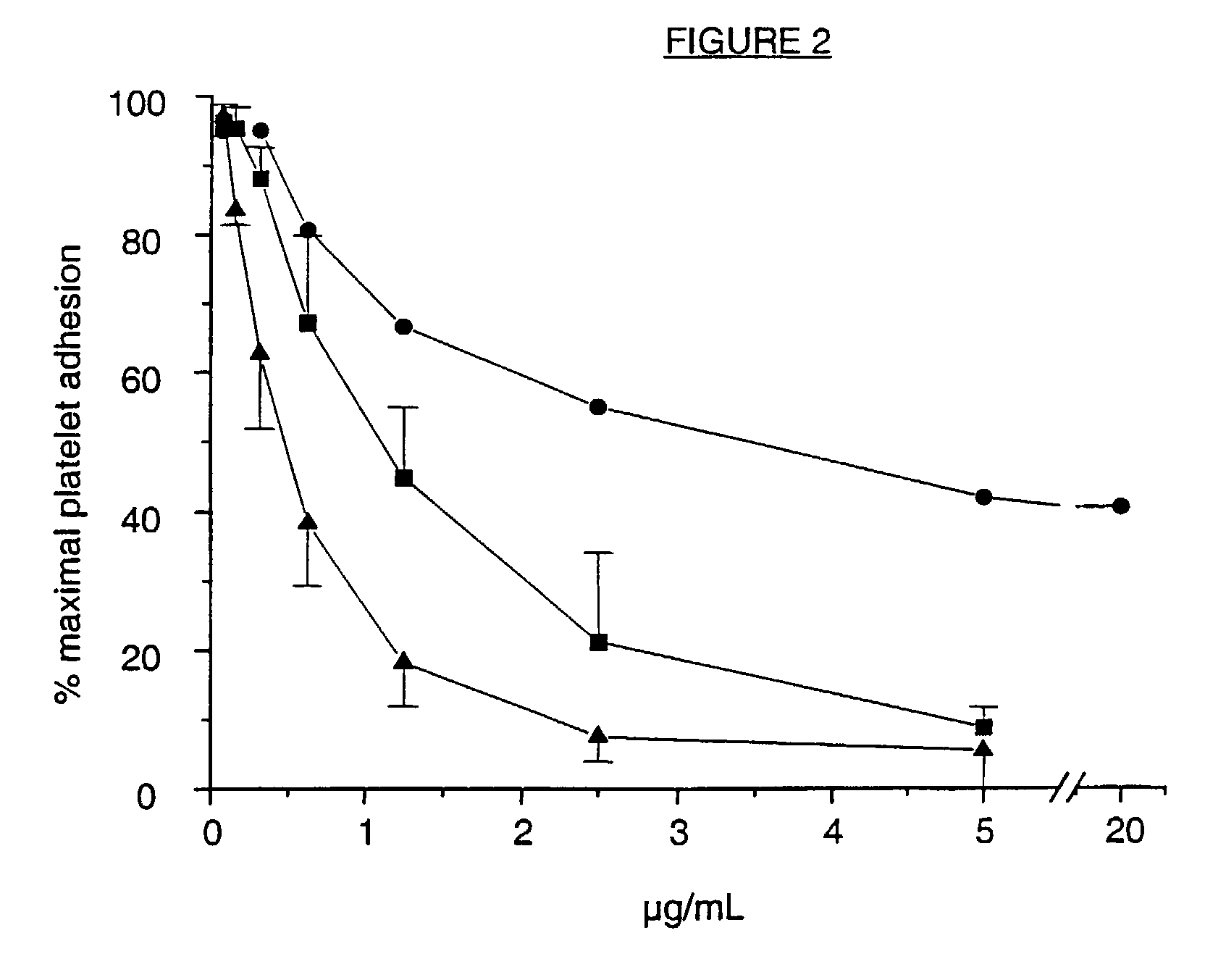
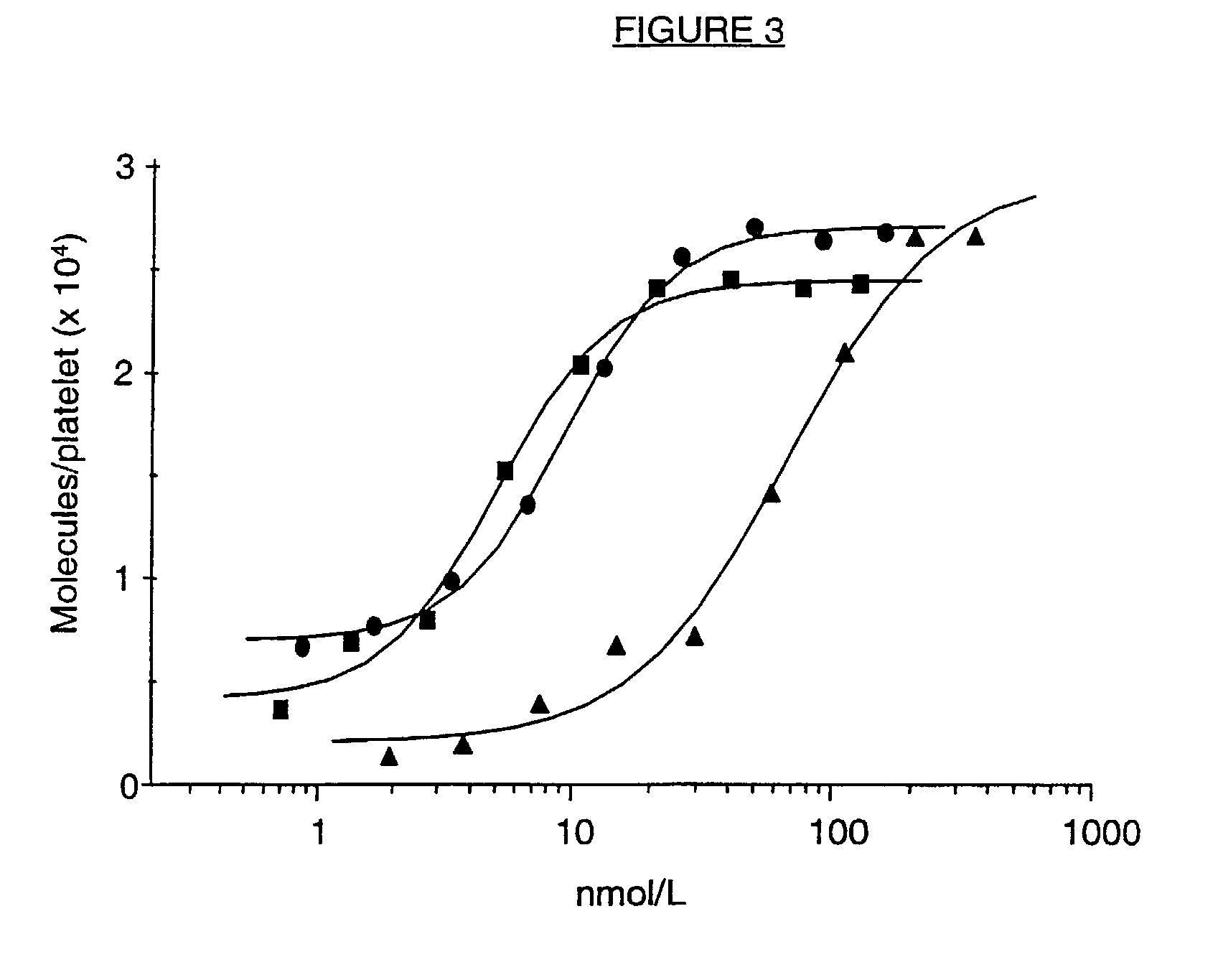
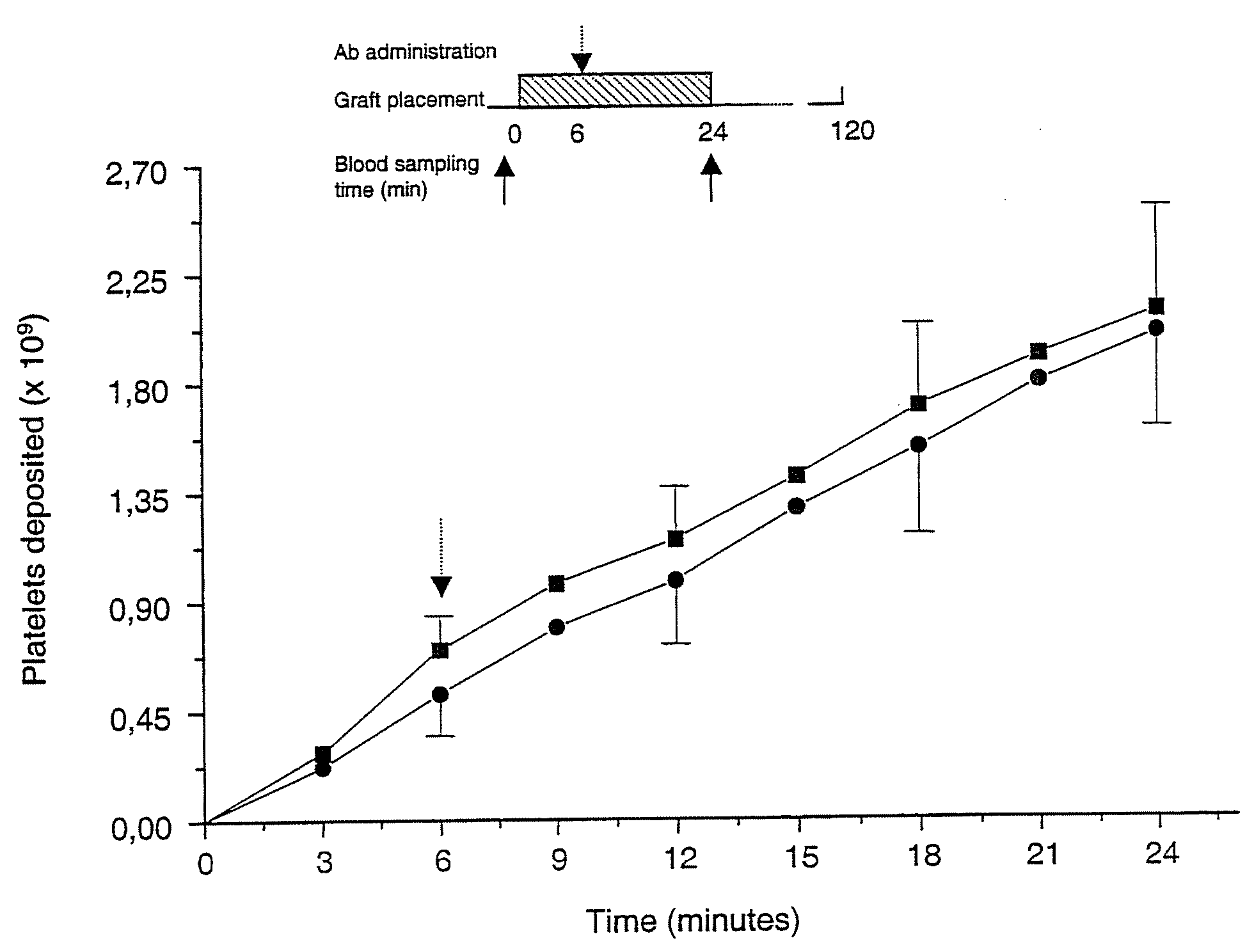
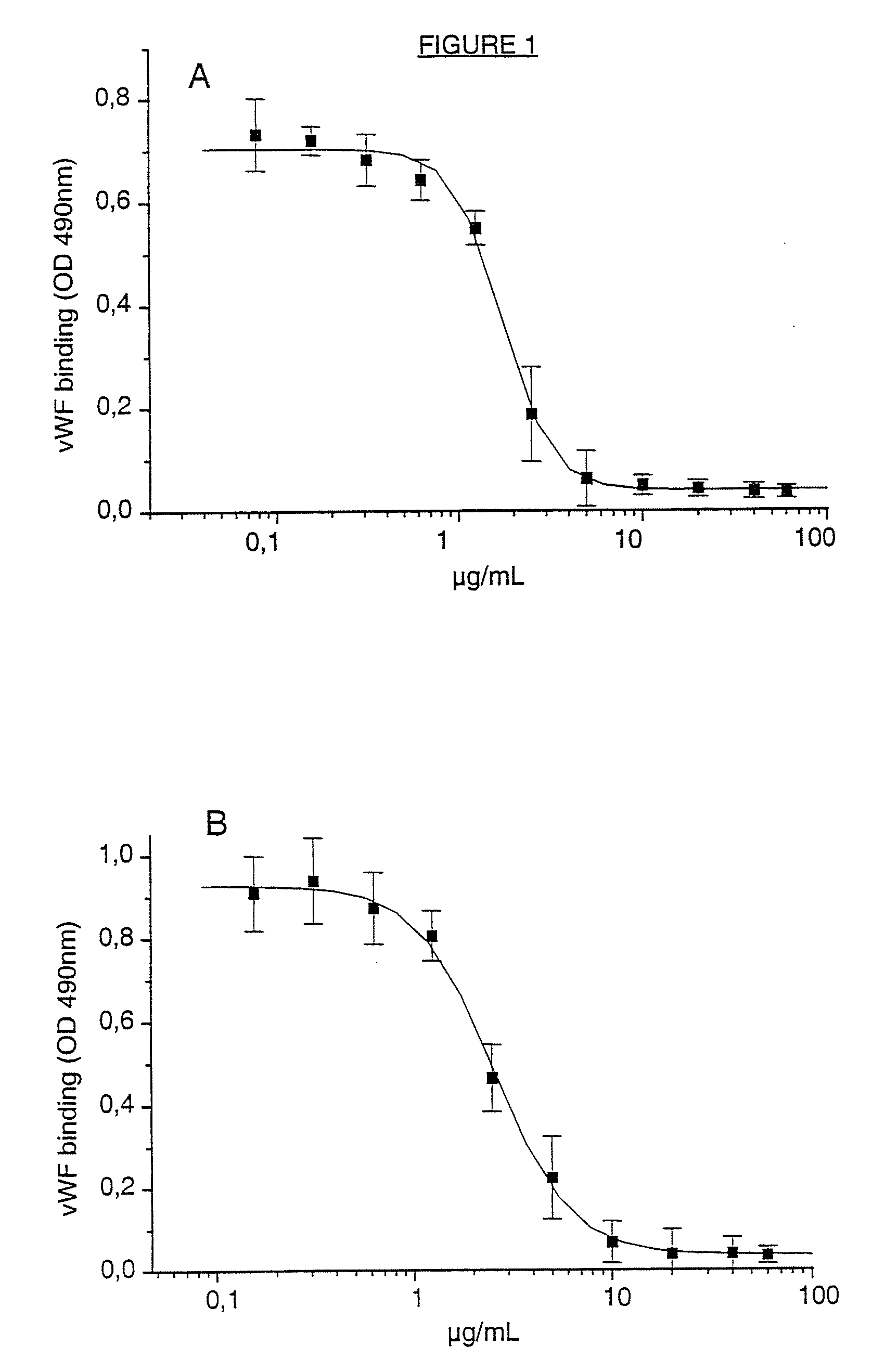
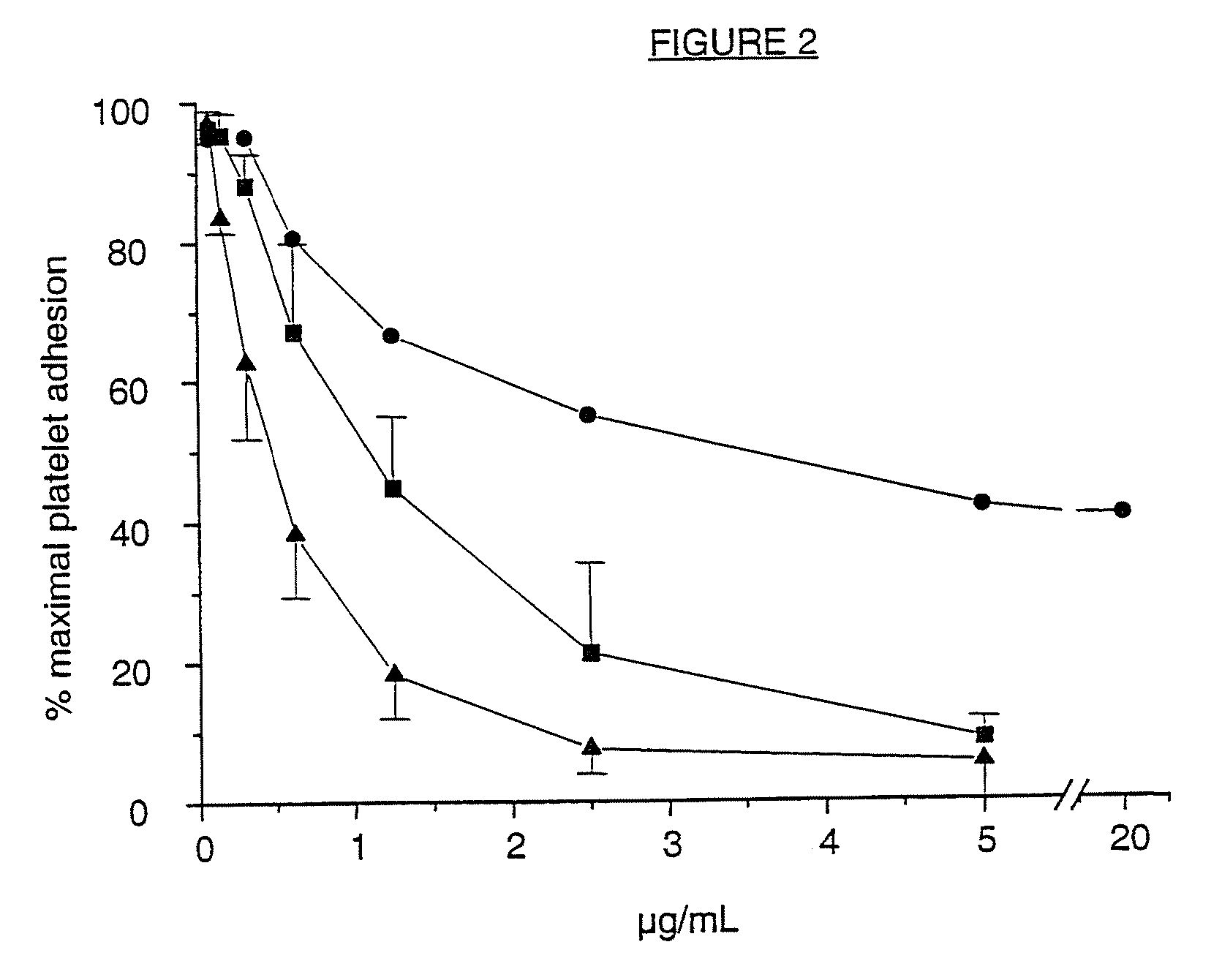
A ligand derived from, e.g. a Fab fragment of, a monoclonal antibody obtainable from the cell line deposited with the Belgian Coordinated Collections of Micro-organisms under accession number LMBP 5108CB binds to the human platelet glycoprotein GPib and prevents the binding of von Willebrand factor to said GPIb without inducing thrombocytopenia. The said ligand is useful, in admixture with a pharmaceutically acceptable carrier, in a pharmaceutical composition, optionally further comprising a thrombolytic agent, for preventing and / or treating haemostasis disorders.
Owner:K U LEUVEN RES & DEV
Embryo cattle serum substitute without serum for animal cell culture
InactiveCN1800368ALow costTo achieve the purpose of replacing serumAnimal cellsL929 cellHuman platelet
The invention relates to a non-serum animal cell culture used fetus cattle serum displace agent, which is formed by recombination human insulin growth factor-1:0.1g-0.5g / l, recombination human platelet sourced growth factor-B:0.1g-0.6g / l, recombination human alkali desmocyte growth factor: 0.05-0.4g / l, cattle transferring: 1g-5g / l, cattle serum protein (V component): 2g-15g / l, and mercaptoethanol 0.3-0.5mol / l.
Owner:上海尚优生物科技有限公司
Cell lines, ligands and antibody fragments for use in pharmaceutical compositions for preventing and treating haemostasis disorders
InactiveUS20090010934A1Prevention of platelet dependent thrombus formationWithout incurring thrombocytopeniaMicrobiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsFactor VIII vWFHuman platelet
A ligand derived from, e.g., a Fab fragment of a monoclonal antibody obtainable from the cell line deposited with the Belgian Coordinated Collections of Micro-Organisms under accession number LMBP 5108CB binds to the human platelet glycoprotein GPIb and prevents the binding of von Willebrand factor to said GPIb without inducing thrombocytopenia. The said ligand is useful, in admixture with a pharmaceutically acceptable carrier, in a pharmaceutical composition, optionally further comprising a thrombolytic agent, for preventing and / or treating haemostasis disorders.
Owner:K U LEUVEN RES & DEV
Serum-free and heterologous component-free mesenchymal stem cell culture medium and application thereof
ActiveCN111454893AEnhance in vitro proliferative abilitySimple recipeCulture processSkeletal/connective tissue cellsHuman plateletSerum free
The present invention discloses a serum-free and heterologous component-free mesenchymal stem cell culture medium and an application thereof, and firstly discloses the mesenchymal stem cell culture medium. The mesenchymal stem cell culture medium comprises a basal culture medium and a supplement, the basal culture medium is a DMEM / F12 culture medium, and the supplement is a human platelet lysate and / or human lipocalin 2. The present invention further discloses the application of the mesenchymal stem cell culture medium in culturing mesenchymal stem cells. The provided mesenchymal stem cell culture medium is free of serum and heterologous components, ensures safety of clinical application, is simple in components, good in batch-to-batch stability and strong in cell expansion ability in vitro, meets industrial transformation requirements, can well maintain immune regulation ability and multi-directional differentiation ability of the mesenchymal stem cells, and ensures effective functions in clinical treatment.
Owner:BEIJING TRANSGEN BIOTECH CO LTD
Efficient mesenchymal stem cell culture solution without serum component
InactiveCN109402050APromote growthGuaranteed feasibilitySkeletal/connective tissue cellsCell culture active agentsIfn alphaHuman platelet
The invention relates to the technical field of biology, in particular to a mesenchymal stem cell culture solution. The mesenchymal stem cell culture solution is prepared from the following components: human platelet lysate, mycillin (Pen Strep), long-acting glutamine, a chemotactic factor XCL1, a chemotactic factor CCL3, a heat shock protein (HSP70), a telomerase inhibitor IFN-alpha 2b and a basal culture medium DMEM. The components of the stem cell culture solution are wide in source, cultured mesenchymal stem cells are high in purity, quick in proliferation and good in dryness, and the mesenchymal stem cell culture solution is suitable for extracorporeal large-scale culture to conduct preclinical study and related clinical study.
Owner:沈阳中心血站
Polypeptide used for prevention and treatment of acute coronary syndrome and anticoagulation antithrombotic therapy and application thereof
ActiveCN102241735ATetrapeptide ingredientsTripeptide ingredientsHuman plateletFemoral artery thrombosis
Owner:SHAANXI MICOT TECH LTD
Modified thrombopoietin with reduced immunogenicity
InactiveUS20040071688A1Improve accuracyIncrease the number ofPeptide/protein ingredientsHydrolasesHuman plateletThrombopoietin
The present invention relates to polypeptides to be administered especially to humans and in particular for therapeutic use. The polypeptides are modified polypeptides whereby the modification results in a reduced propensity for the polypeptide to elicit an immune response upon administration to the human subject. The invention in particular relates to the modification of human thrombopoietin (TPO) to result in TPO proteins that are substantially non-immunogenic or less immunogenic than any non-modified counterpart when used in vivo.
Owner:MERCK PATENT GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com