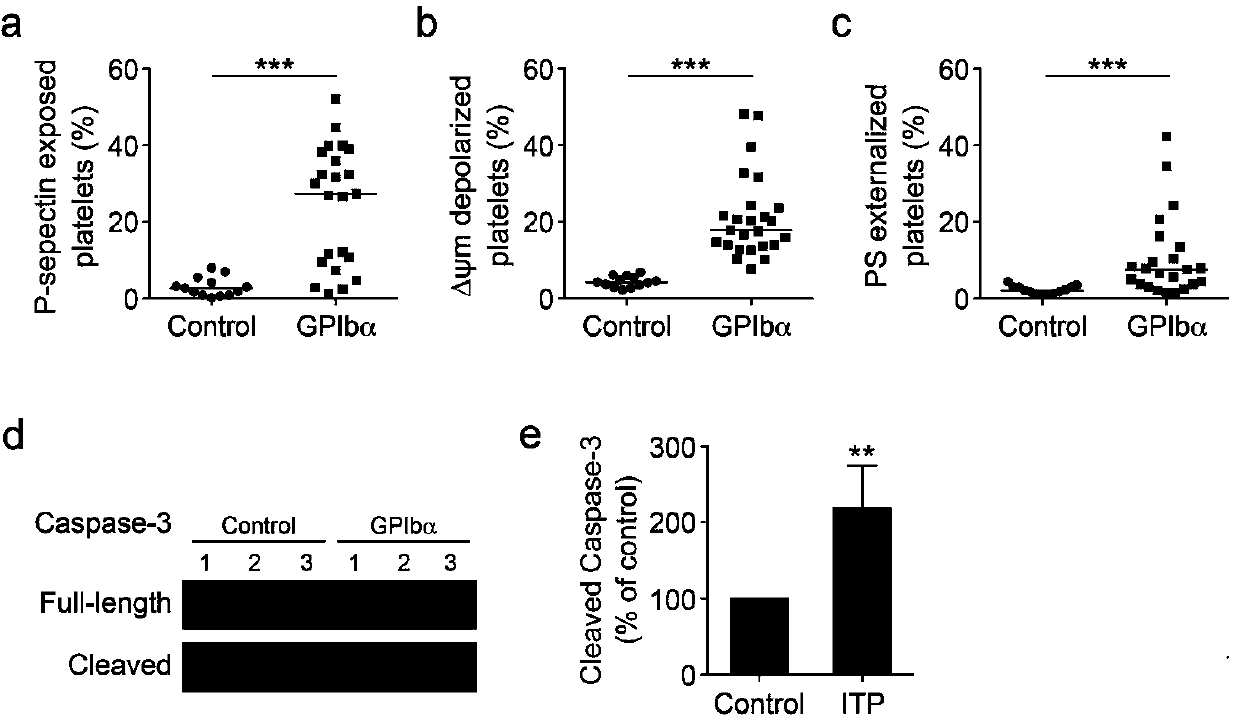
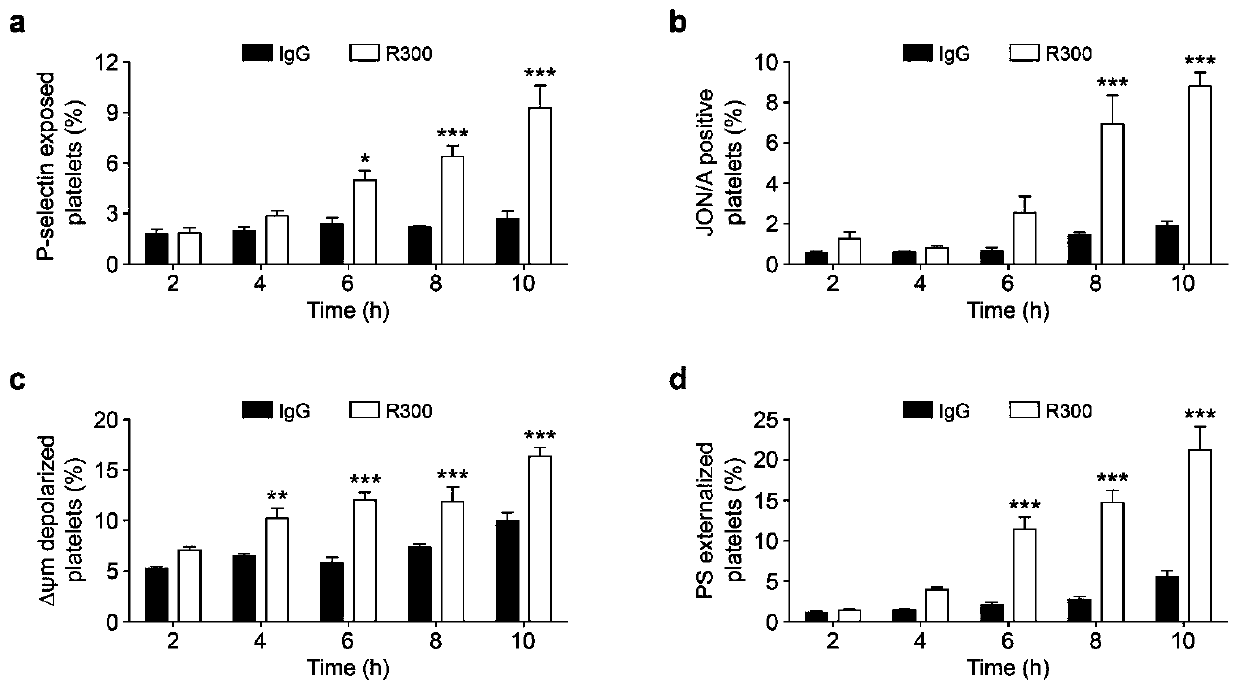
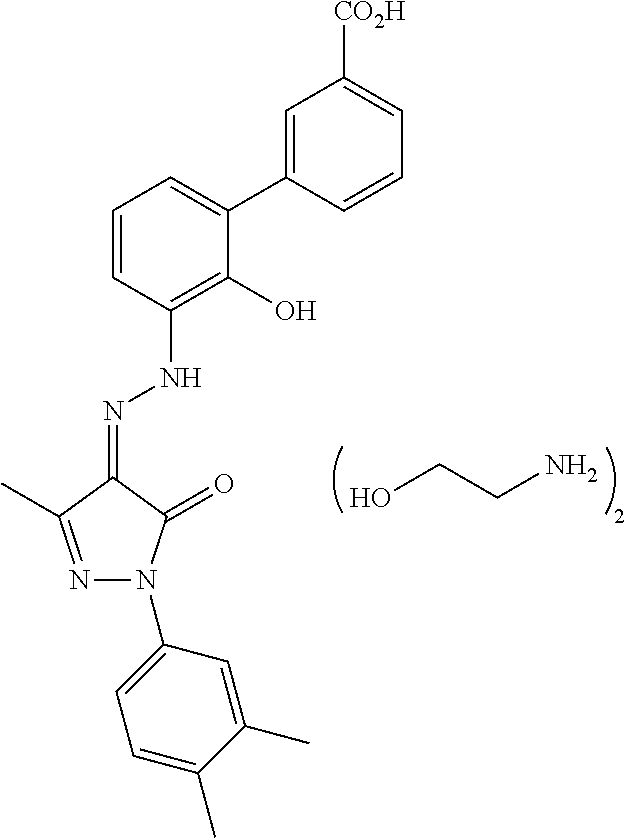
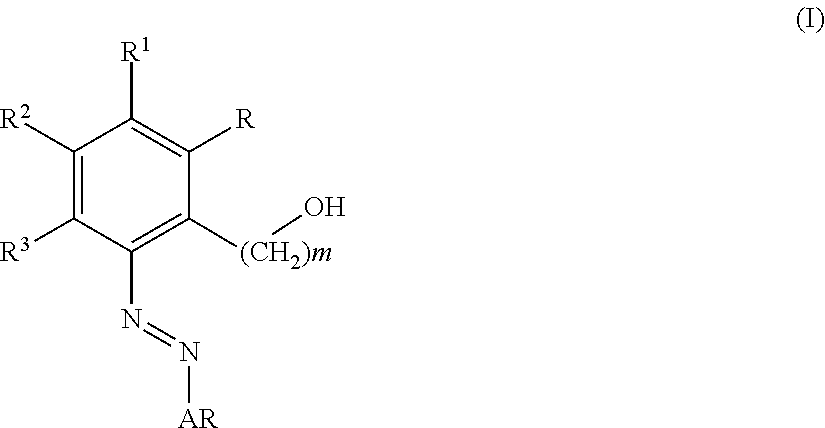
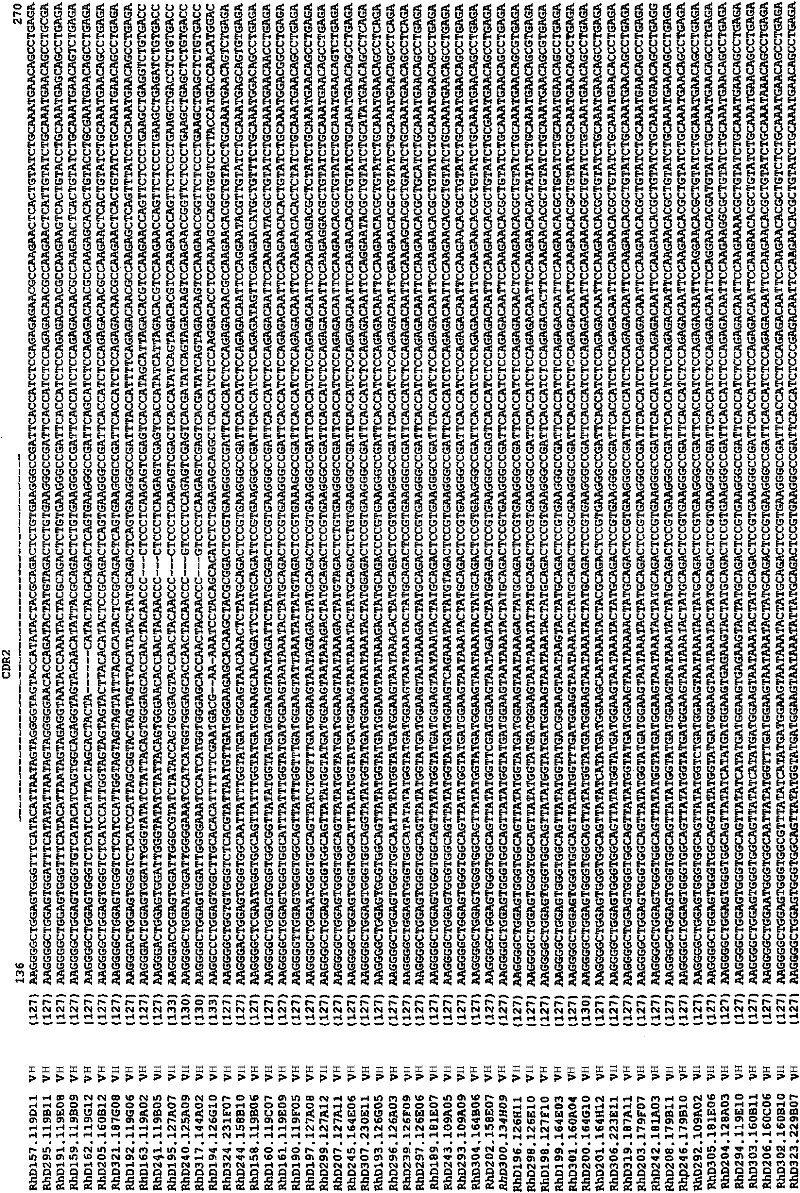Patents
Literature
63 results about "Thrombasthenia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
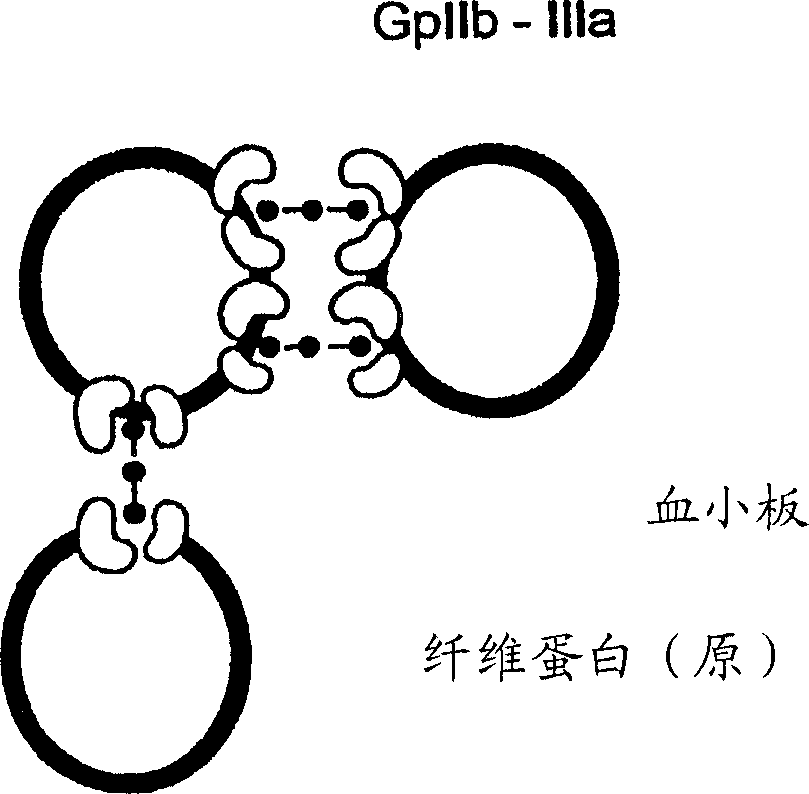
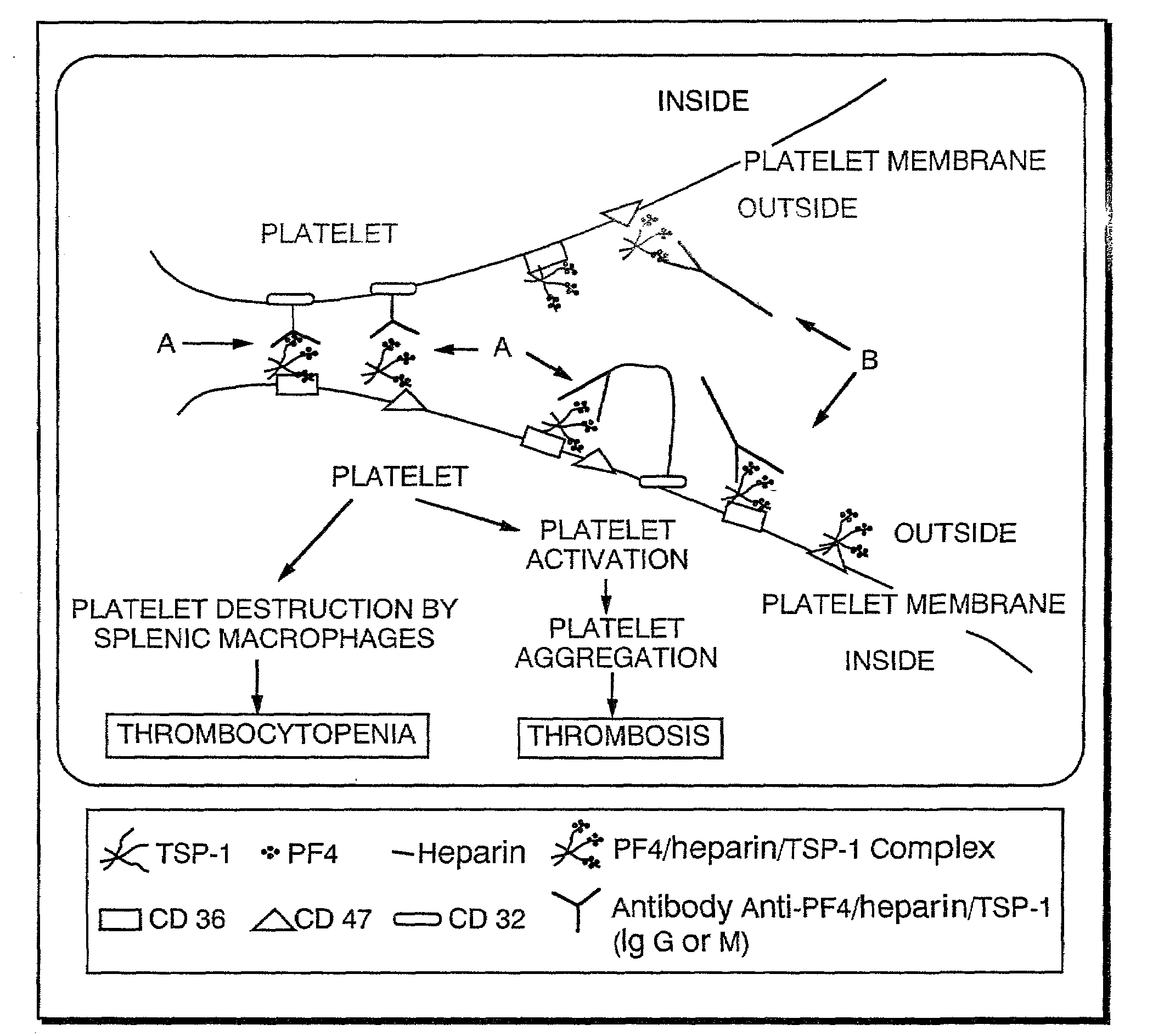
A congenital bleeding disorder with prolonged bleeding time, absence of aggregation of platelets in response to most agents, especially ADP, and impaired or absent clot retraction. Platelet membranes are deficient in or have a defect in the glycoprotein IIb-IIIa complex (PLATELET GLYCOPROTEIN GPIIB-IIIA COMPLEX).
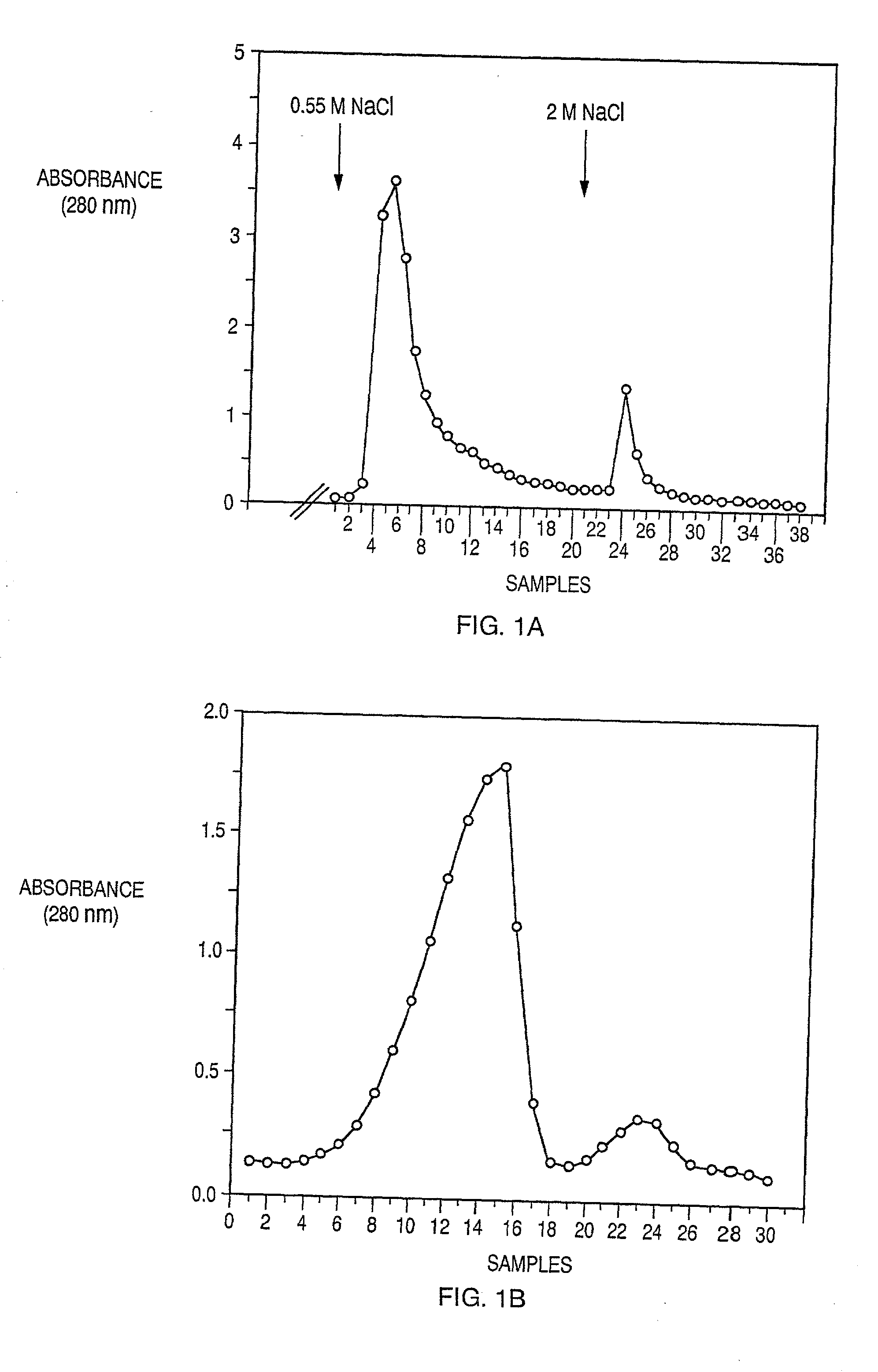
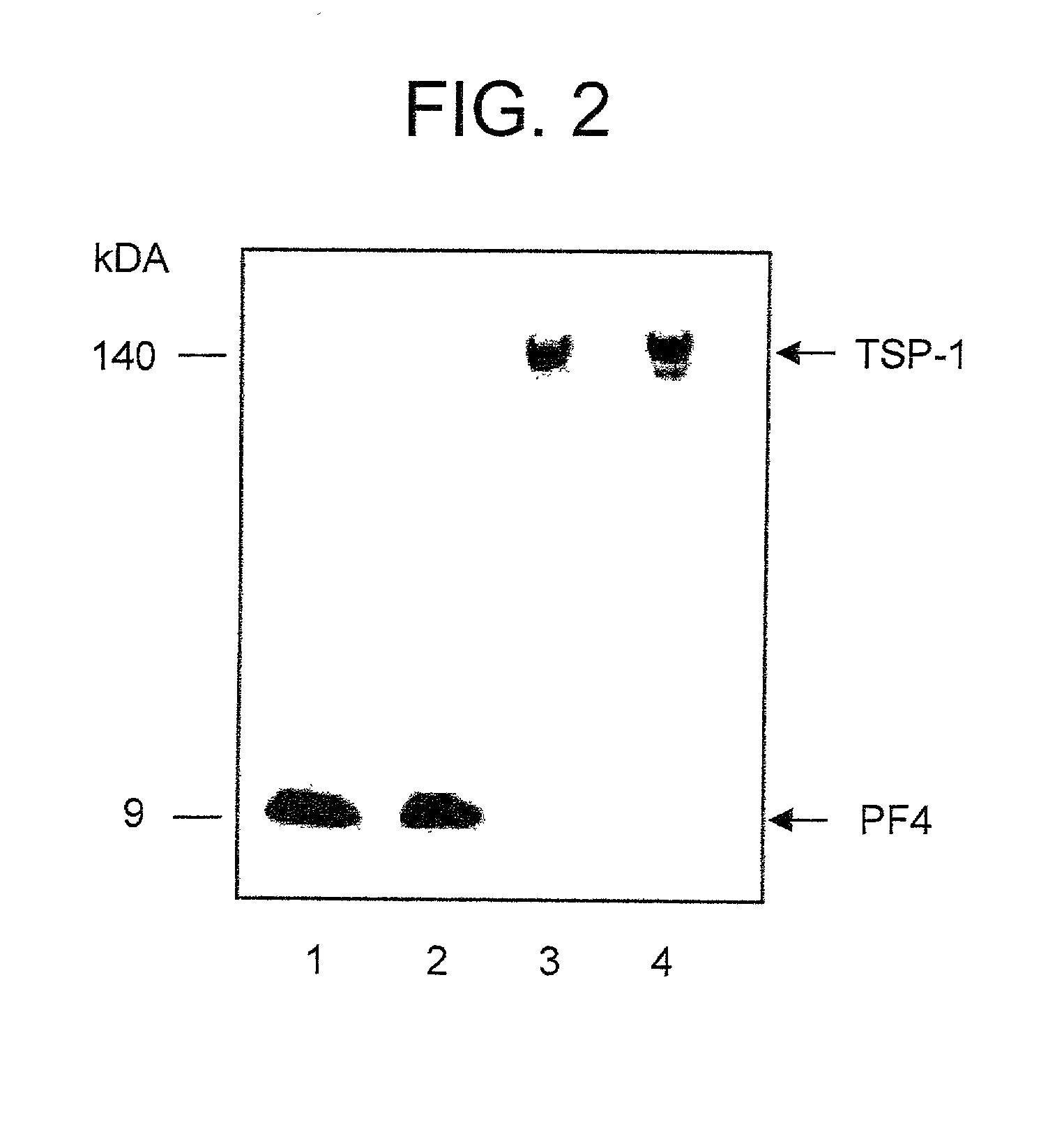
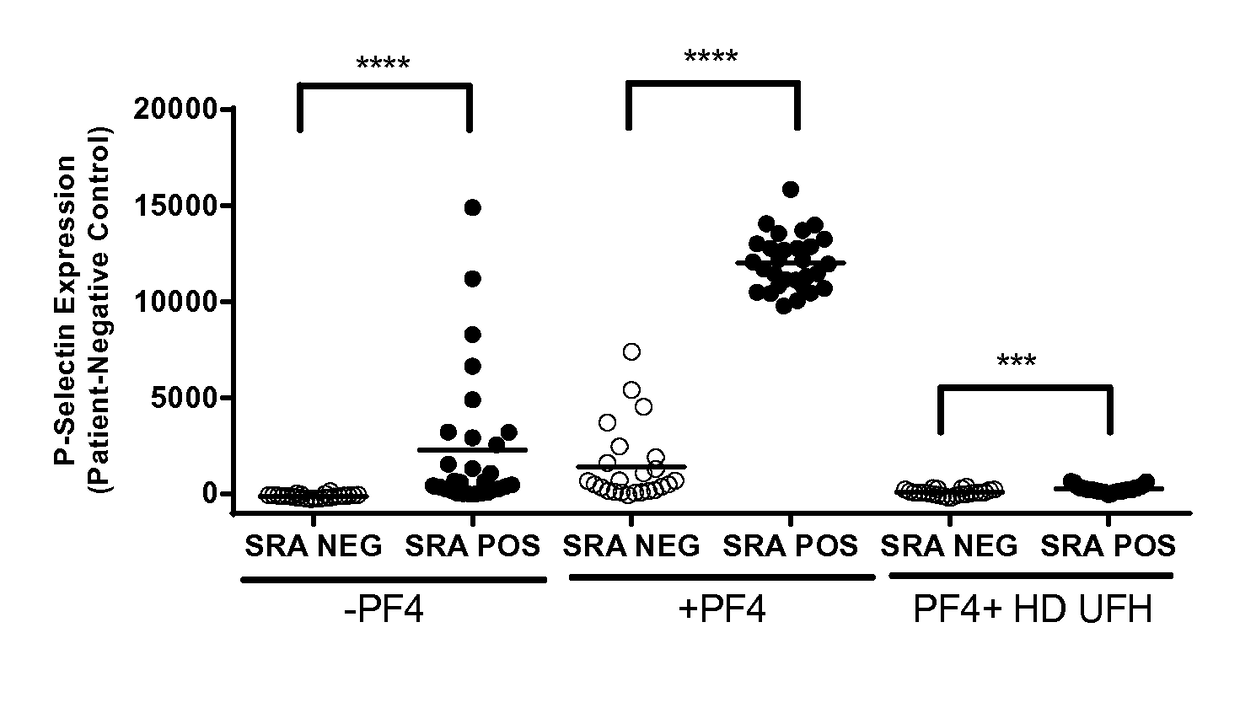
Compositions and methods useful for the diagnosis and treatment of heparin induced thrombocytopenia/thrombosis
InactiveUS6964854B1Animal cellsImmunoglobulins against cytokines/lymphokines/interferonsHeparin antibodyMammal
The invention includes compositions, kits and methods comprising a monoclonal antibody which shares key functional properties with the polyclonal antibodies which participate in the pathogenesis of heparin induced thrombocytopenia / thrombosis (HIT / HITT) in a mammal. The monoclonal antibody of the invention preferentially binds with a PF4 / heparin complex relative to the binding of the antibody with PF4 or heparin alone. The monoclonal antibody of the invention also binds specifically with PF4 in a complex with other glycosaminoglycans besides heparin, and also activates platelets. The monoclonal antibody of the invention is useful in methods for diagnosing and treating HIT / HITT in a mammal. A humanized version of the monoclonal antibody of the invention is also included, along with a process for humanizing the monoclonal antibody of the invention.
Owner:STC UNM
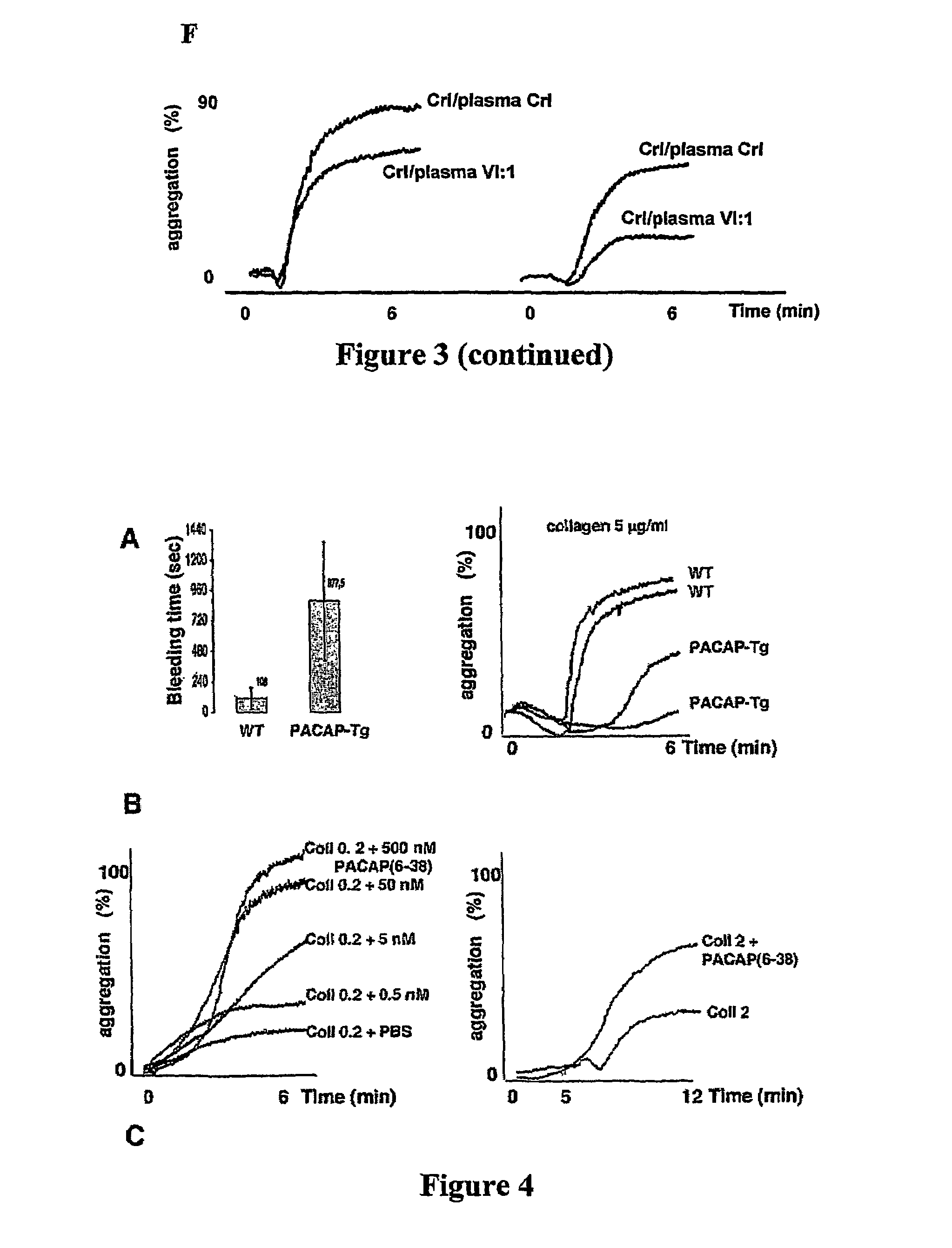
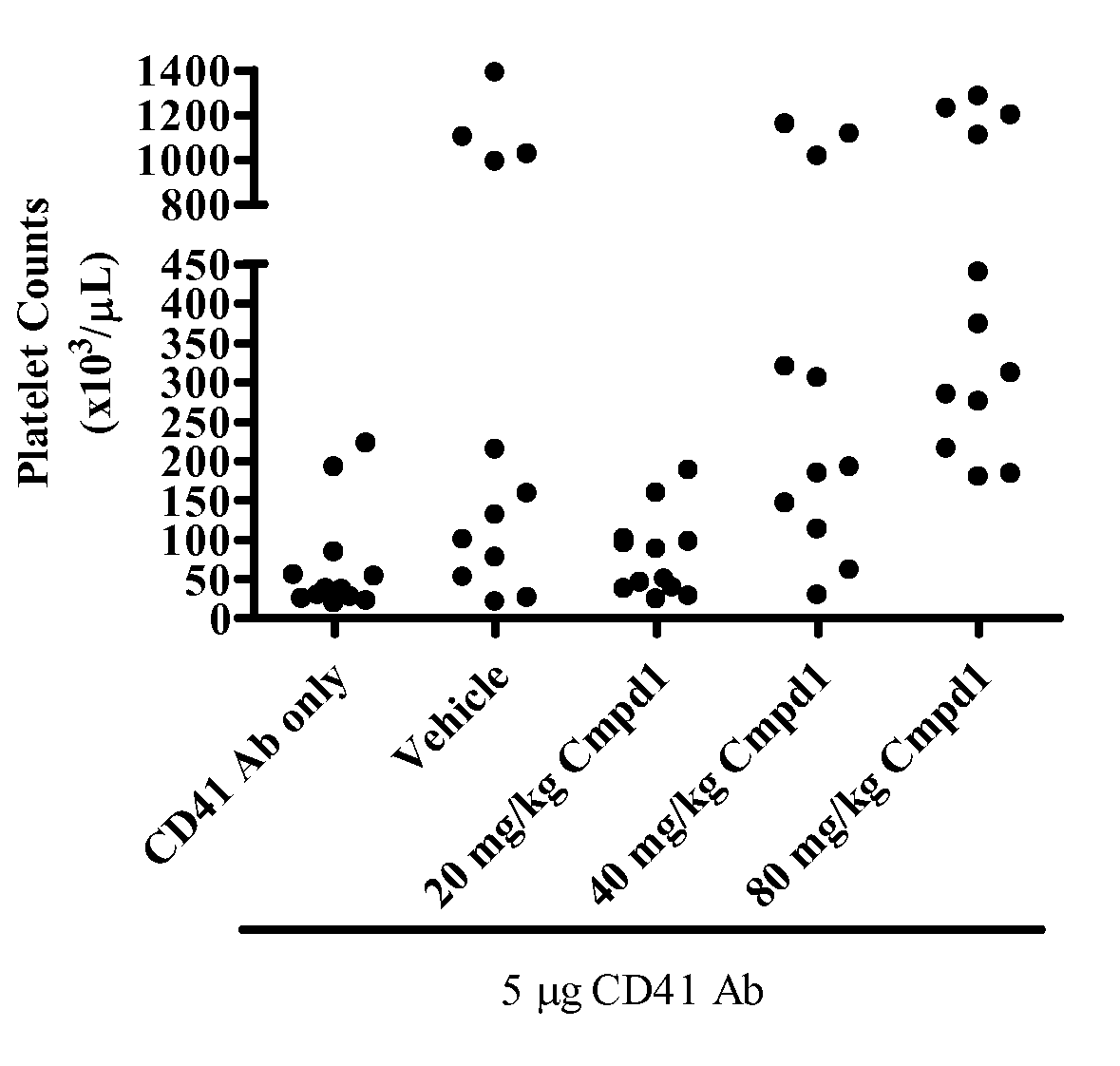
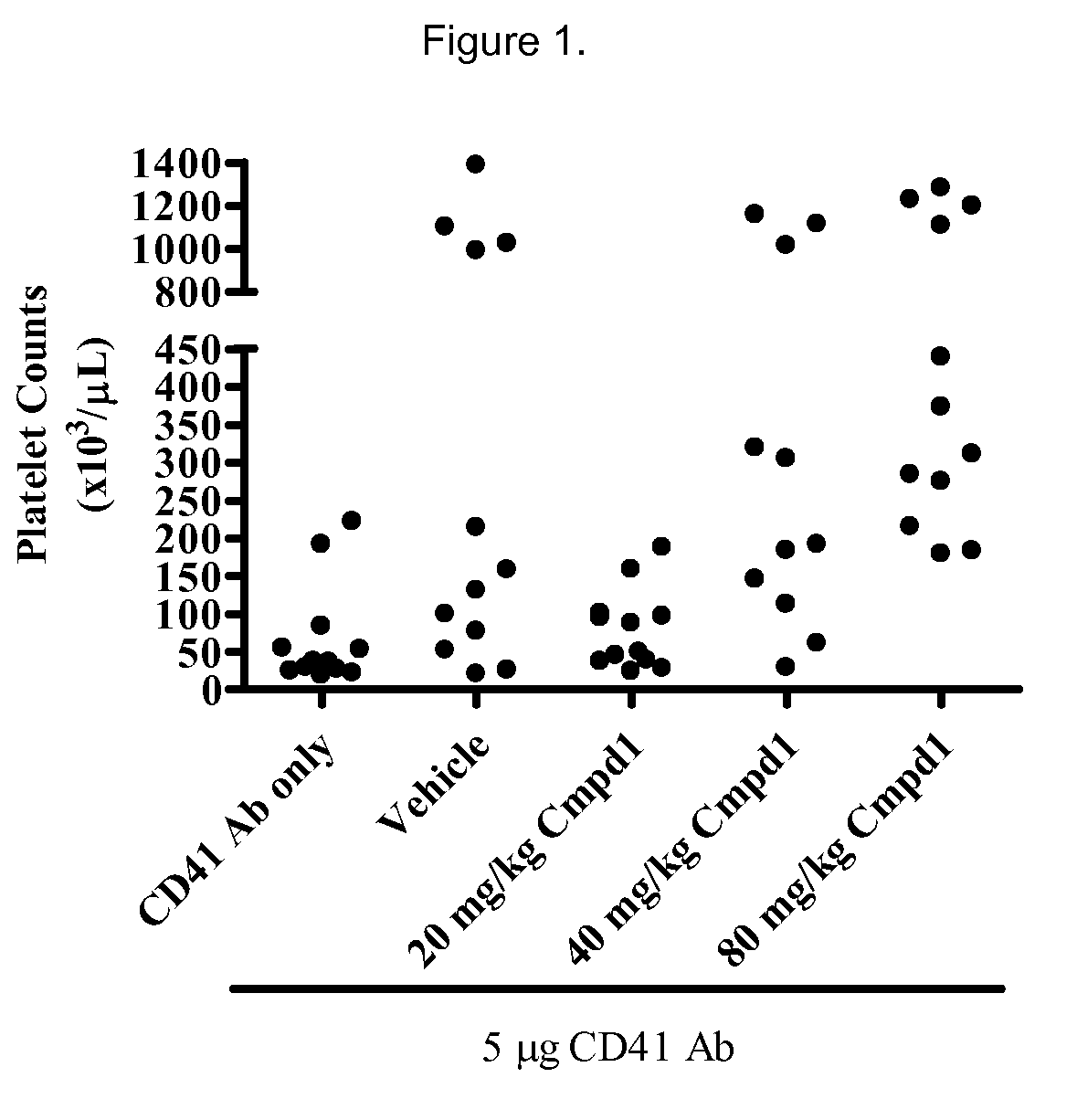
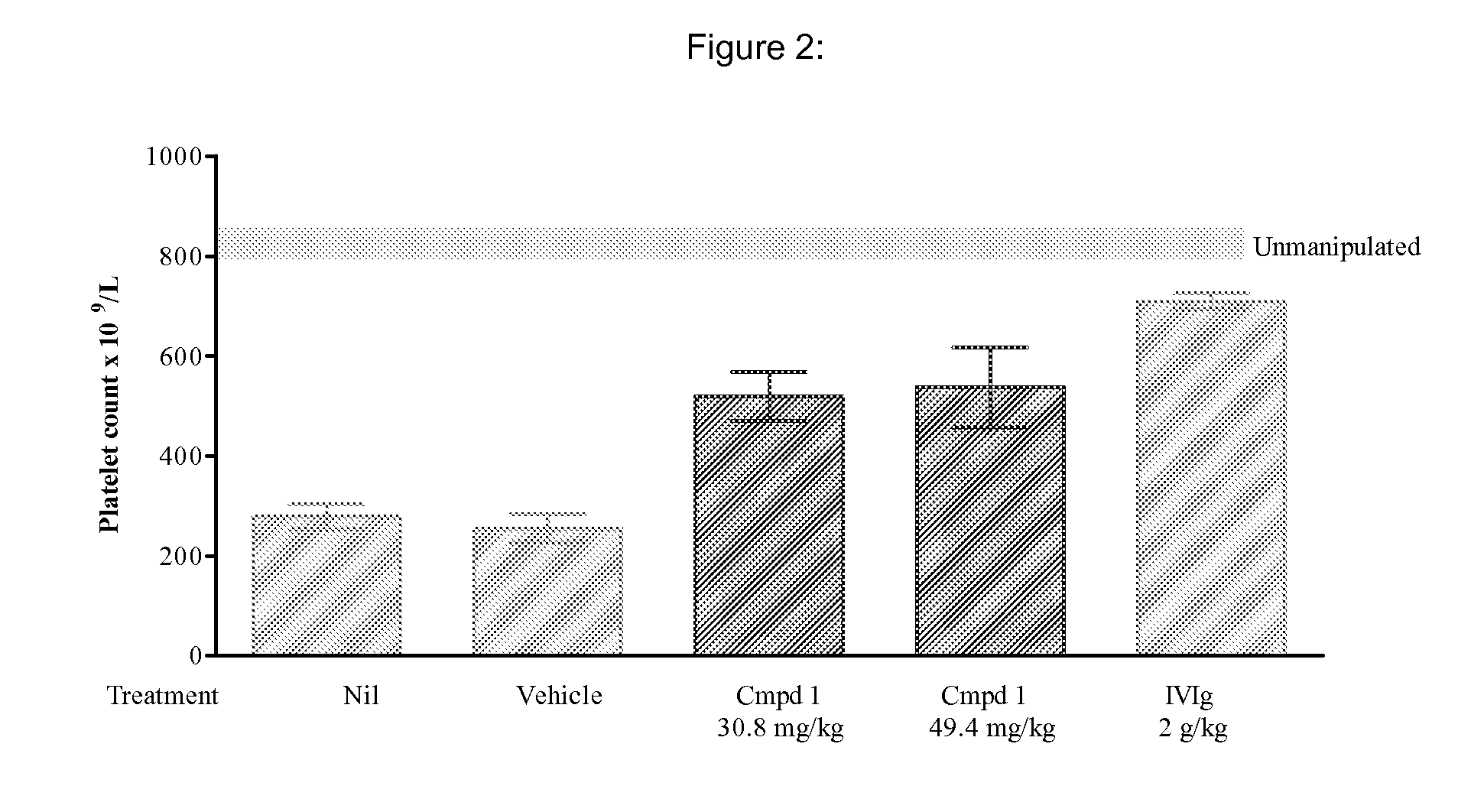
Inhibition of pacap signalling for the prevention and treatment of thrombocytopenia
InactiveUS20100129372A1Organic active ingredientsPeptide/protein ingredientsAntagonistThrombasthenia
The present invention discloses the use of inhibitors and / or antagonists of PACAP signalling for the manufacture of a medicament for the prevention or treatment of decreased blood platelet numbers (thrombocytopenia).
Owner:LIFE SCI RES PARTNERS VZW
Therapeutic uses of allogeneic myeloid progenitor cells
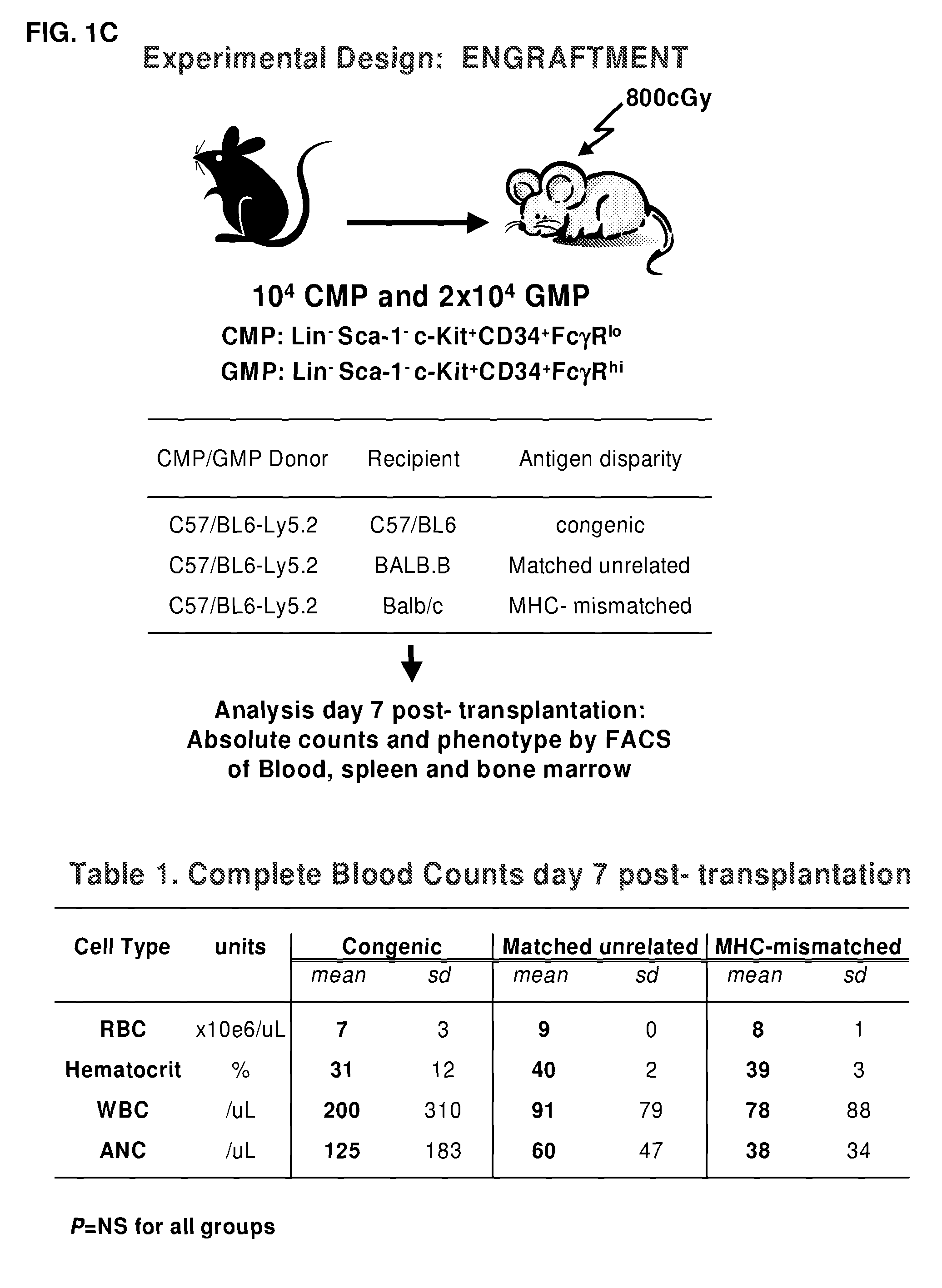
Myeloid function is enhanced by transplantation or infusion of allogeneic myeloid progenitor cells, including CMP, GMP, MEP and MKP cell subsets. Myeloid progenitors ameliorate sequelae of anemia and thrombocytopenia, and can prevent or treat gastrointestinal mucositis associated with chemotherapy, radiotherapy, and the like. The transplantation or infusion may be performed in the absence of HLA typing, and the cells may be mismatched at one or more Class I HLA loci. The transplantation may provide for treatment of ongoing disease, or prevention of disease in high risk patients.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Interleukin-11 compositions and methods of use
InactiveUS20070160577A1Prevents thrombocytopeniaReduces thrombocytopeniaPeptide/protein ingredientsAntipyreticWhite blood cellInterleukin 11
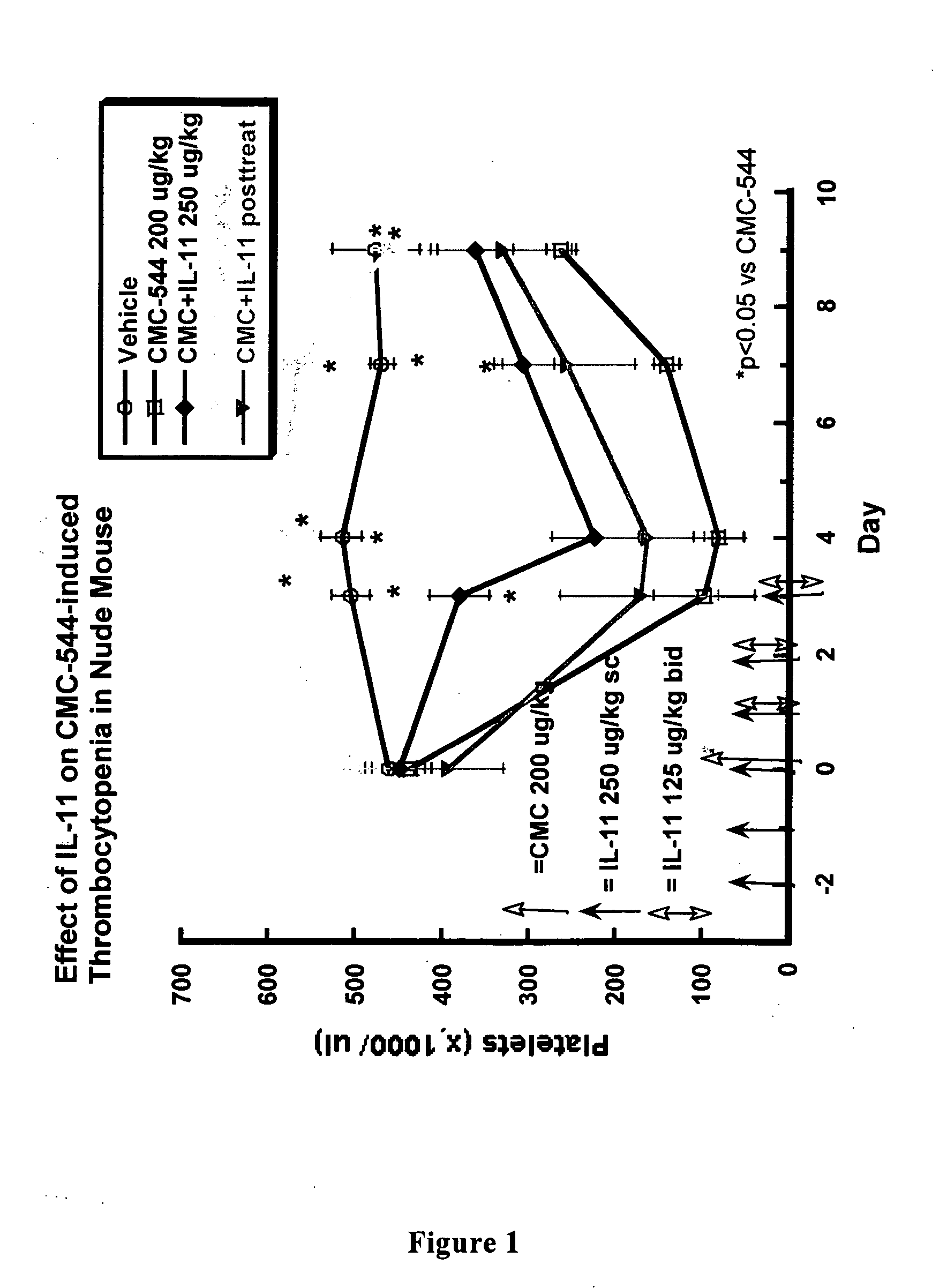
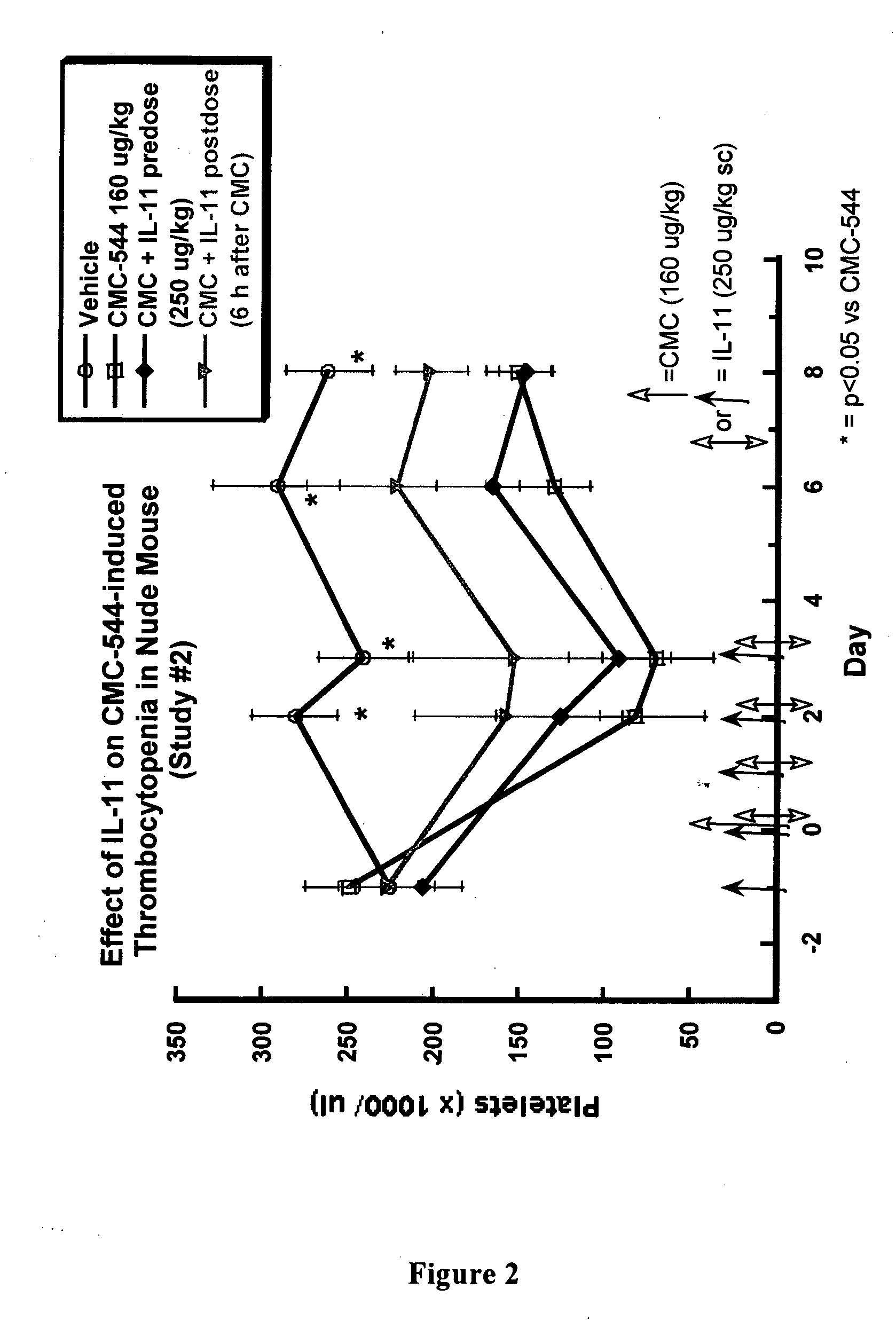
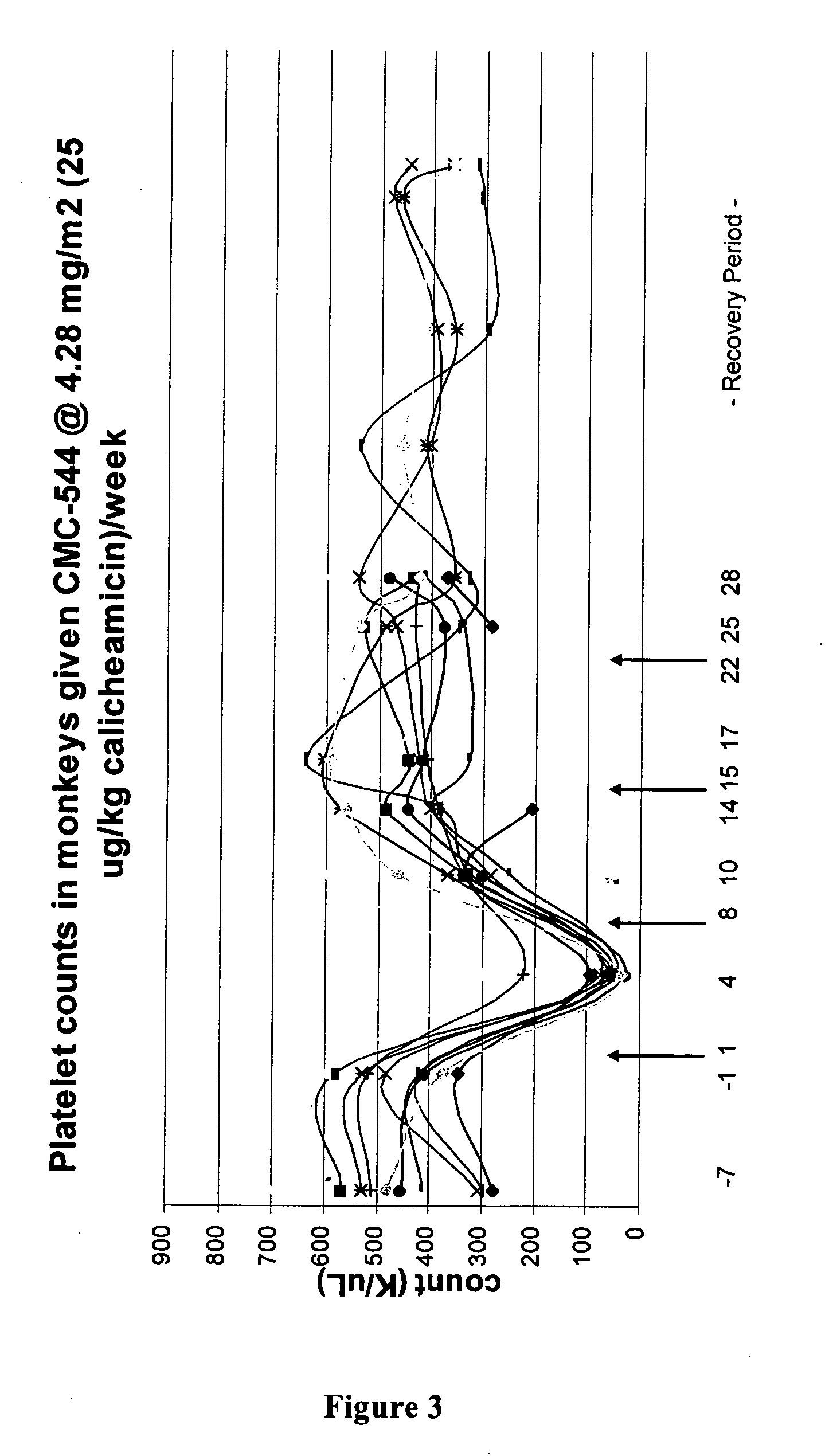
The present invention provides methods for the treatment and / or prevention of thrombocytopenia including thrombocytopenia associated with drug-induced liver damage and thrombocytopenia associated with drug-induced bone marrow destruction. The methods of treatment of the invention include administration of interleukin-11 to a subject suffering from or susceptible to thrombocytopenia and / or receiving or about to receive a treatment involving a conjugate therapeutic agent whose administration results in thrombocytopenia. Also provided are pharmaceutical compositions and kits useful for carrying out such methods of treatment.
Owner:WYETH
Thrombopoietin mimetics
Invented are non-peptide TPO mimetics. Also invented is a method of treating thrombocytopenia, in a mammal, including a human, in need thereof which comprises administering to such mammal an effective amount of a selected hydroxy-1-azobenzene derivative.
Owner:SMITHKLINE BECKMAN CORP
Inhibition of PACAP signalling for the prevention and treatment of thrombocytopenia
The present invention discloses the use of inhibitors and / or antagonists of PACAP signalling for the manufacture of a medicament for the prevention or treatment of decreased blood platelet numbers (thrombocytopenia).
Owner:LIFE SCI RES PARTNERS VZW
Combination therapy with syk kinase inhibitor
The invention encompasses methods of increasing platelet levels in a patient having or at risk for immune thrombocytopenia comprising co-administering a Syk kinase inhibitor and a thrombopoietin receptor agonist, and methods of treating thrombocytopenia comprising co-administering a Syk kinase inhibitor and a thrombopoietin receptor agonist to a patient in need thereof, as well as pharmaceutical compositions for use in these methods.
Owner:RIGEL PHARMA
Methods of Modulating Thrombocytopenia and Modified Transgenic Pigs
InactiveUS20130024961A1Reduced platelet uptakeInhibit expressionOrganic active ingredientsSugar derivativesThrombastheniaNormal platelet morphology
The application provides methods of modulating platelet uptake by liver sinusoidal endothelial cells and of modulating thrombocytopenia. Transgenic pigs modified to bind fewer platelets are provided.
Owner:BURLAK CHRISTOPHER +1
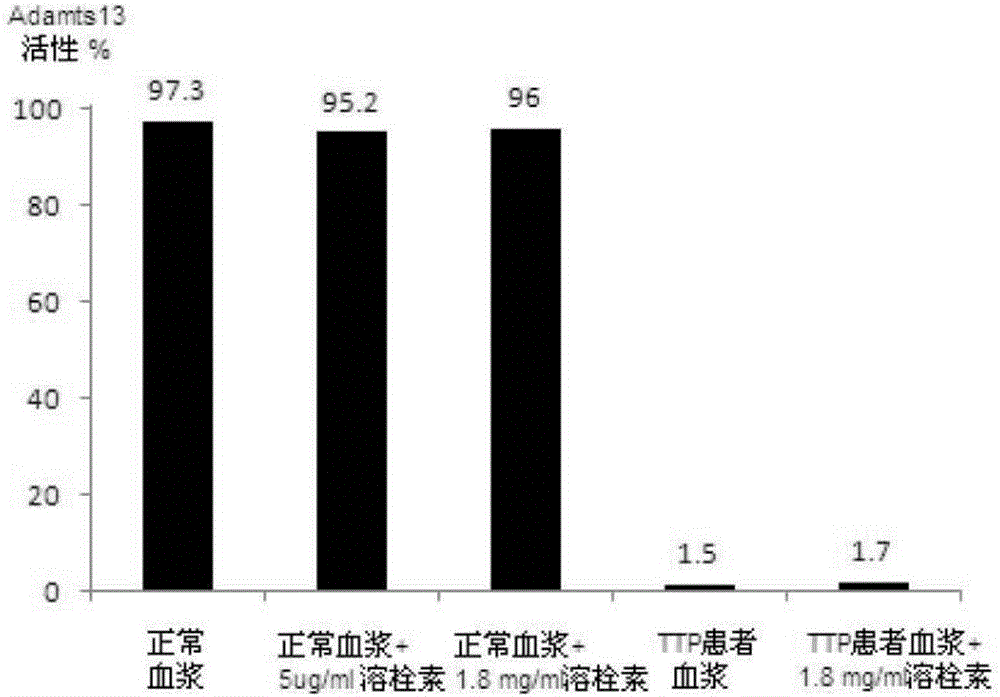
Application of antiplatelet thrombolysin in preparation of medicine for treating thrombotic thrombocytopenic purpura (TTP)
PendingCN105833255ARecovery levelLower levelPeptide/protein ingredientsBlood disorderAnti plateletThrombasthenia
The invention relates to the field of medicines and especially relates to an application of antiplatelet thrombolysin in preparation of a medicine for treating thrombotic thrombocytopenic purpura (TTP). The antiplatelet thrombolysin limits adhesion and aggregation of platelets, recovers levels of the platelets, erythrocytes and hemoglobin, reduces level of lactic dehydrogenase and effectively inhibits thrombocytopenia and cleaved-cell hemolyticanemia, thereby treating the TTP. The antiplatelet thrombolysin has great clinical application prospect.
Owner:ZHAOKE PHARMA HEFEI
Methods of modulating thrombocytopenia and modified transgenic pigs
ActiveUS20150135344A1Reduced uptakeSymptoms improvedDead animal preservationMammal material medical ingredientsThrombastheniaPlatelet
The application provides methods of modulating platelet uptake by liver sinusoidal endothelial cells and of modulating thrombocytopenia. Transgenic pigs modified to bind fewer platelets are provided.
Owner:INDIANA UNIV RES & TECH CORP
Anti-platelet membrane glycoprotein vi monoclonal antibody
InactiveUS20090092612A1Efficiently obtainedImmunoglobulins against blood group antigensAntibody mimetics/scaffoldsHuman plateletMonoclonal antibody
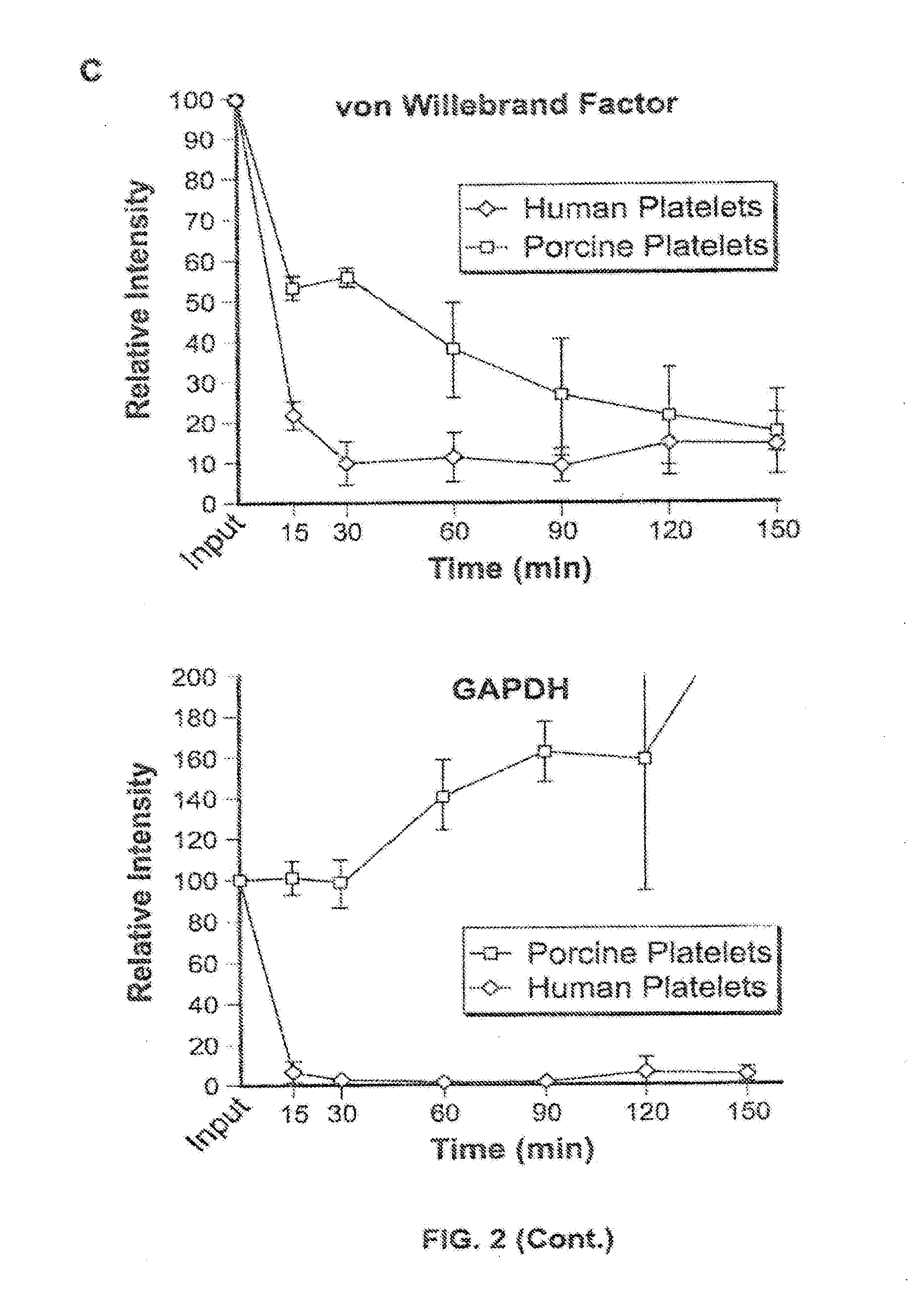
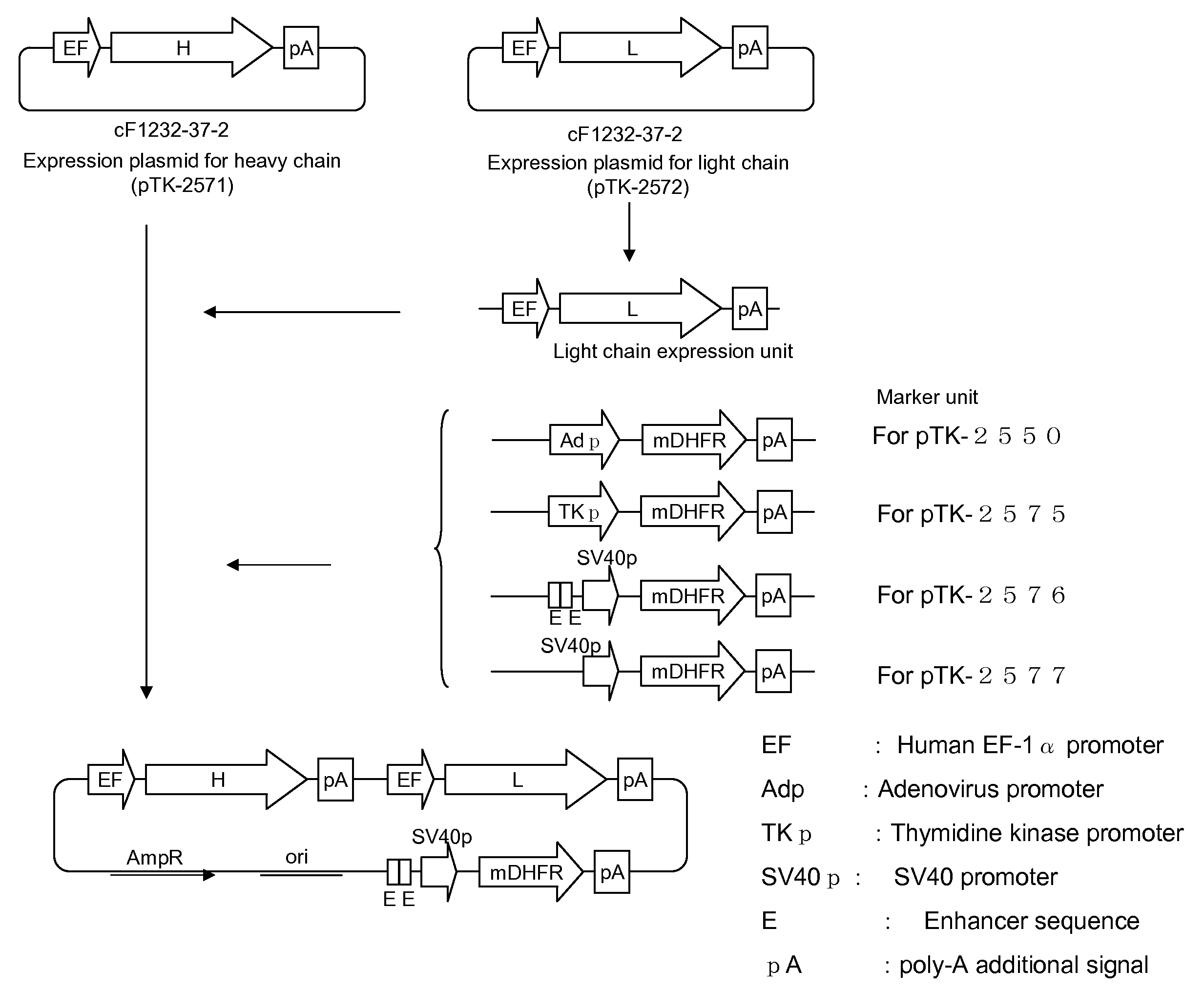
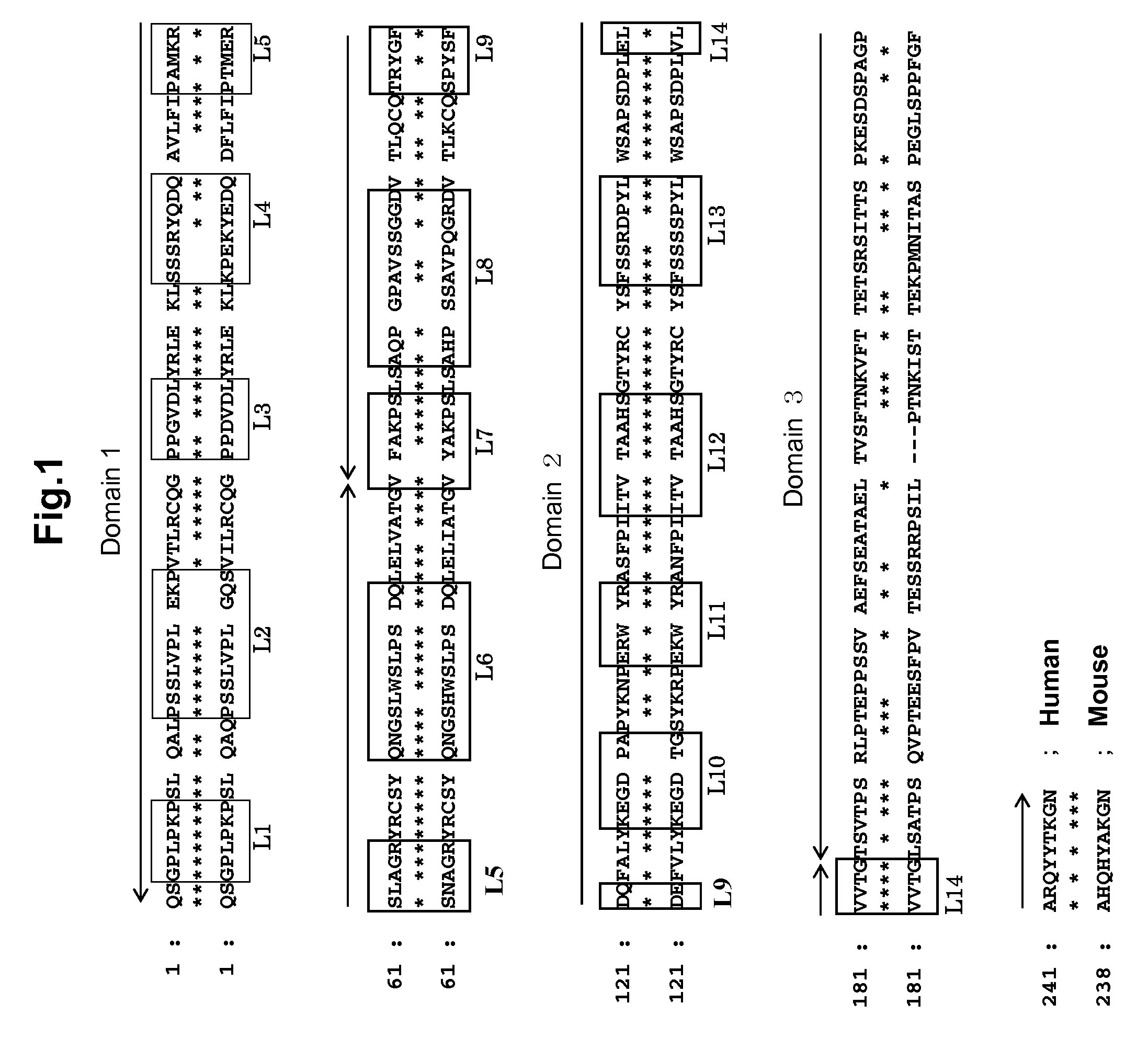
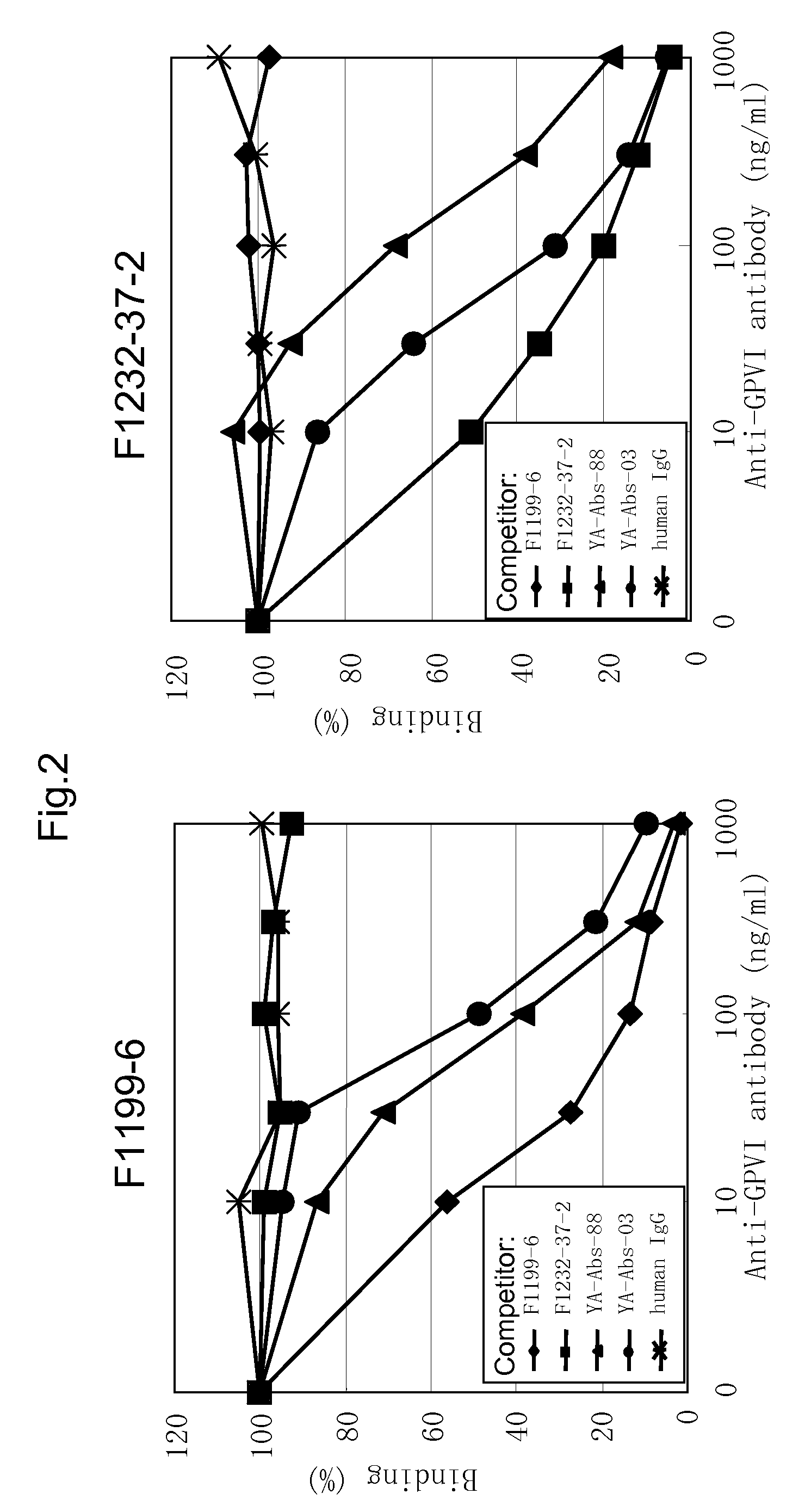
The present invention provides an antibody which has the following features, its active fragment, or a derivative thereof:a) It specifically binds to human platelet membrane glycoprotein VI (GPVI);b) The function to activate a platelet and / or the function to induce a thrombocytopenia in vivo are low; andc) It at least partially depletes GPVI on the platelet membrane by contacting with a platelet.
Owner:MOCHIDA PHARM CO LTD
Thrombopoietin Mimetics
The invention relates to compounds and their use in the treatment of thrombocytopenia resulting from diseases or conditions such as immune thrombocytopenic purpura, cancer chemotherapy, surgery, bone marrow or stem cell transplantation, radiation injury or treatment, chronic viral infection, and pancytopenia. The invention further relates to pharmaceutical compositions containing the compounds and compositions of the invention as well as methods for treating such diseases or conditions in a mammal, including a human, by administering to such mammal an effective amount of a selected thrombopoietin receptor agonist.
Owner:STATEGICS
Recombinant human fibrinogen for treatment of bleeding in trauma and platelet disorders
InactiveUS20100279939A1Save livesFast and efficient arrestSaccharide peptide ingredientsBlood disorderPlatelet disorderDisease
The present invention provides methods of using recombinant human fibrinogen to prevent or treat excessive bleeding in pre-hospital and hospital settings. In particular, the present invention relates to methods for treating bleeding using recombinant human fibrinogen in individuals suffering from traumatic hemorrhages in pre-hospital settings and in individuals having thrombocytopenia or qualitative platelet disorders.
Owner:FRIES DIETMAR RUDOLF +2
Liquid, aqueous pharmaceutical composition of Factor VII polypeptides
InactiveUS20060166882A1Improve stabilityHeavy metal active ingredientsBiocideClotting factor deficiencyOxidation state
Owner:NOVO NORDISK HEALTH CARE AG
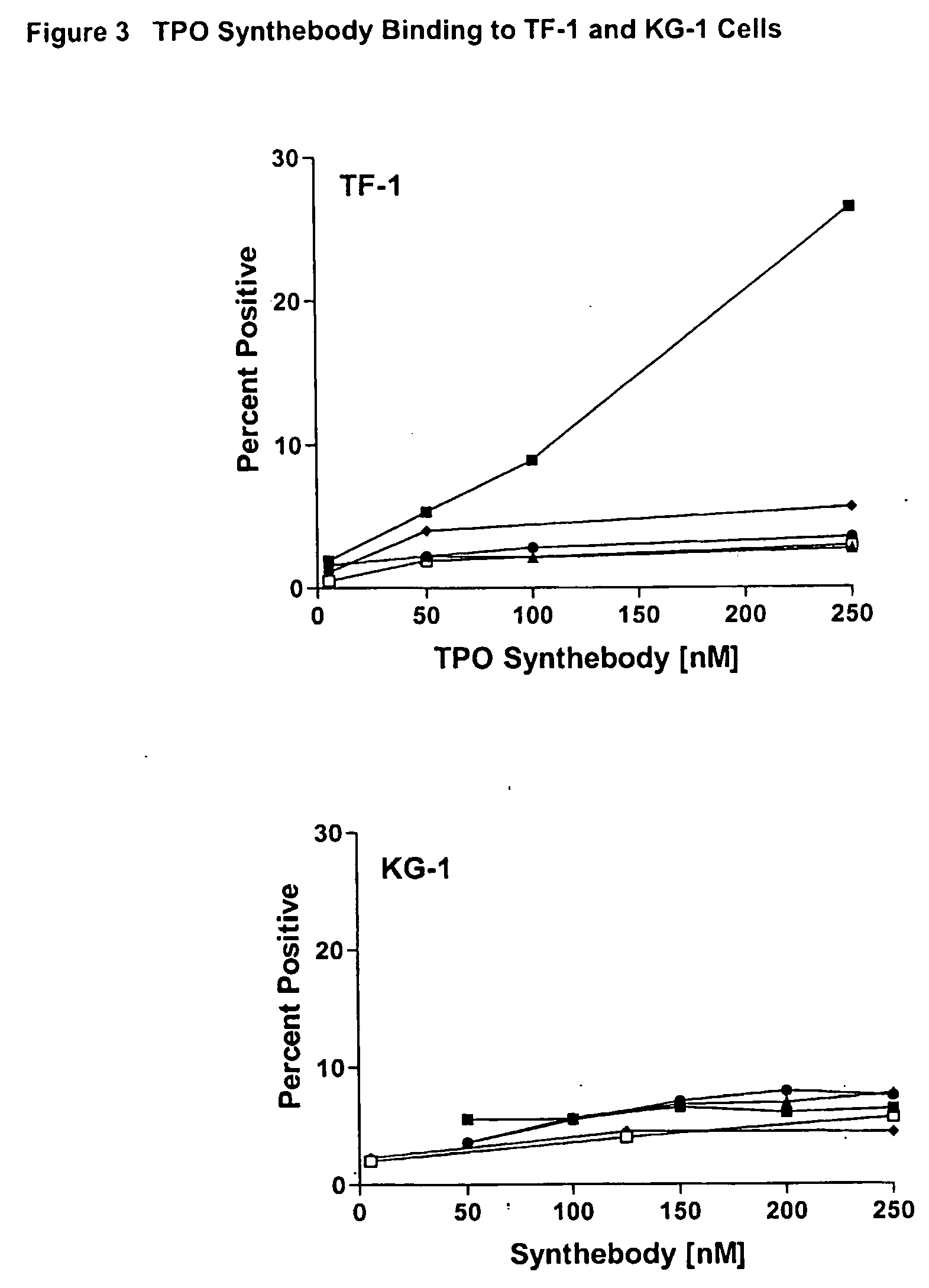
Thrombopoietin(tpo) synthebody for stimulation of platelet production
InactiveUS20040136980A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsProgenitorHematopoietic cell
The present invention relates to a synthetic variable region of an immunoglobin construct which contains in at least one of its CDRs a sequence of thrombopoietin, e.g., IEGPTLRQWLAARA or its derivatives. This construct can efficiently bind and activate a thrombopoientin receptor (MPL) leading to stimulation of proliferation, growth or differentiation or modulation of apoptosis of hematopoietic cells, especially platelet progenitor cells. The invention further relates to the use of the synthebody to treat hematopoietic or immune disorders, and particularly thrombocytopenia resulting from chemotherapy, radiation therapy, or bone marrow transfusions.
Owner:EURO-CELTIQUE SA
Liquid, Aqueous Pharmaceutical Composition of Factor VII Polypeptides
InactiveUS20100166730A1Improve stabilityHeavy metal active ingredientsPeptide/protein ingredientsClotting factor deficiencyOxidation state
The present invention is directed to liquid, aqueous pharmaceutical compositions containing Factor VII polypeptides, and methods for preparing and using such compositions, as well as vials containing such compositions, and the use of such compositions in the treatment of a Factor VII-responsive syndrome, e.g., bleeding disorders, including those caused by clotting Factor deficiencies (e.g. haemophilia A, haemophilia B, coagulation Factor VII deficiency); by thrombocytopenia or von Willebrand's disease, or by clotting Factor inhibitors, and intra cerebral haemorrhage, or excessive bleeding from any cause. The preparations may also be administered to patients in association with surgery or other trauma or to patients receiving anticoagulant therapy. More particularly, the invention relates to liquid compositions stabilised against chemical and / or physical degradation. The main embodiment is represented by a liquid, aqueous pharmaceutical composition comprising a Factor VII polypeptide (i); a buffering agent (ii) suitable for keeping pH in the range of from about 4.0 to about 9.0; at least one metal-containing agent (iii), wherein said metal is selected from the group consisting of first transition series metals of oxidation state +II, except zinc, such as chromium, manganese, iron, cobalt, nickel, and copper; and a non-ionic surfactant (iv).
Owner:NOVO NORDISK HEALTH CARE AG
Agonistic antibody directed against human thrombopoietin receptor
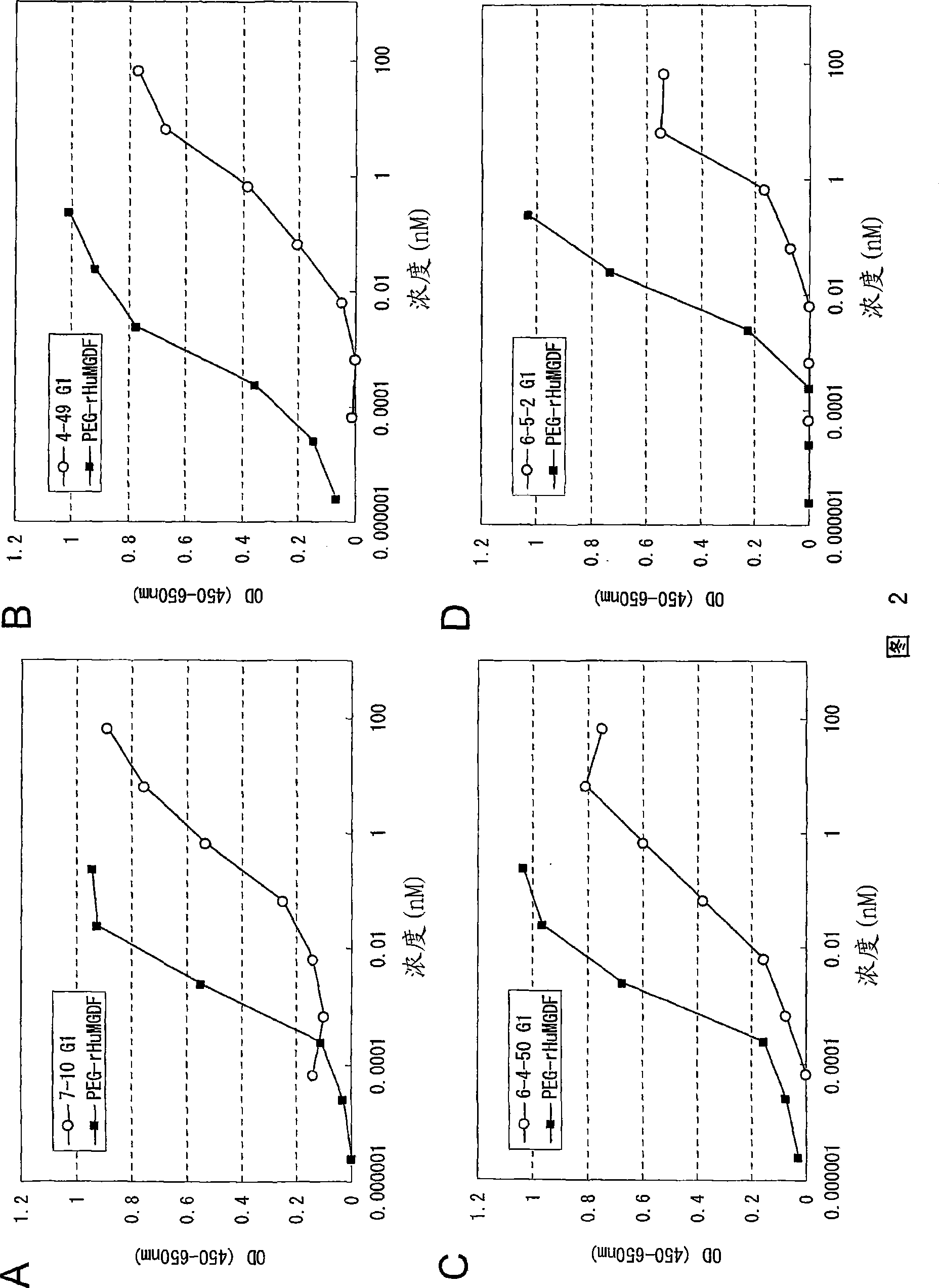
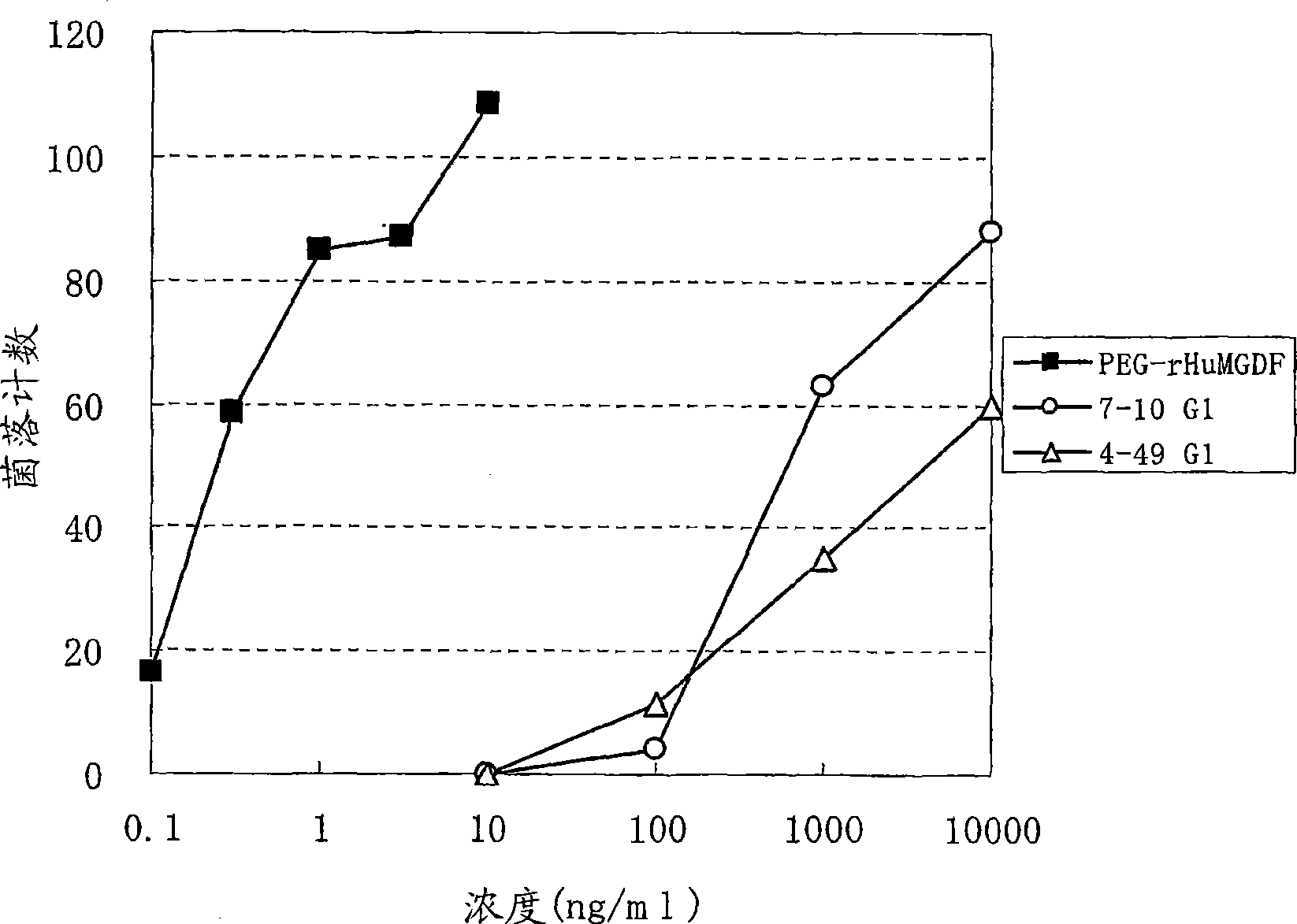
The present invention discloses an agonistic antibody directed against human thrombopoietin receptor (also referred to as 'human c-Mpl). Specifically, the antibody has a constant region having a set of amino acid sequences selected from the following items (1) to (3): (1) amino acid sequences for a heavy-chain constant region and a light-chain constant region of a human antibody; (2) an amino acid sequence for a human antibody heavy-chain constant region in which the domain is replaced by one of other human antibody subclass and an amino acid sequence for a human antibody light-chain constant region; and (3) a set of amino acid sequences having the deletion, substitution, addition or insertion of one or several amino acid residues in each of the amino acid sequences shown in (1) and (2), and the antibody has a variable region capable of binding to a human thrombopoietin receptor to activate the receptor. The antibody also has the following properties: (a) the antibody can induce the formation of a colony at a concentration of 10,000 ng / mL or less in the CFU-MK colony formation assay using a human umbilical cord blood CD34+ cell; and (b) the antibody has the maximum activity higher than that of PEG-rHuMGDF by 50% or more and a 50% effective concentration (EC50) of 100 nM or less in the cell growth assay using an UT7 / TPO cell. Also disclosed is a pharmaceutical composition for the treatment of thrombocytopenia, which comprises the antibody.
Owner:KIRIN PHARMA
Methods for treating leukopenia and thrombocytopenia
ActiveUS20160151323A1Reduce neutrophil migrationInhibit transferBiocideHeavy metal active ingredientsLeukopeniaThrombasthenia
The disclosure provides methods for treating, controlling or mitigating leukopenia (e.g. neutropenia) and / or thrombocytopenia, for example in the context of cancer chemotherapy, comprising administration of a monoacetyl-diacyl-glycerol compound, as well as compositions useful therefor.
Owner:ENZYCHEM LIFESCI CORP
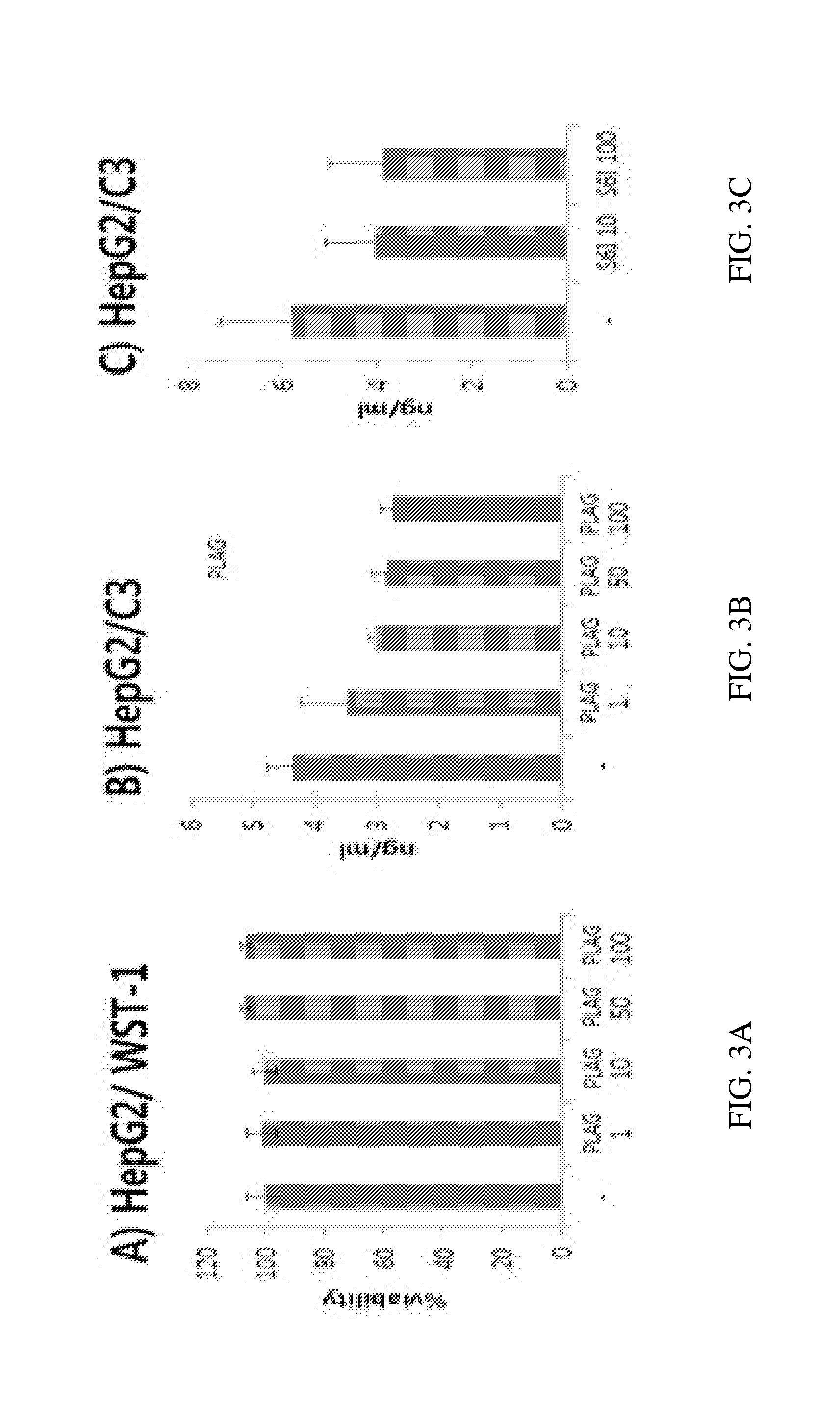
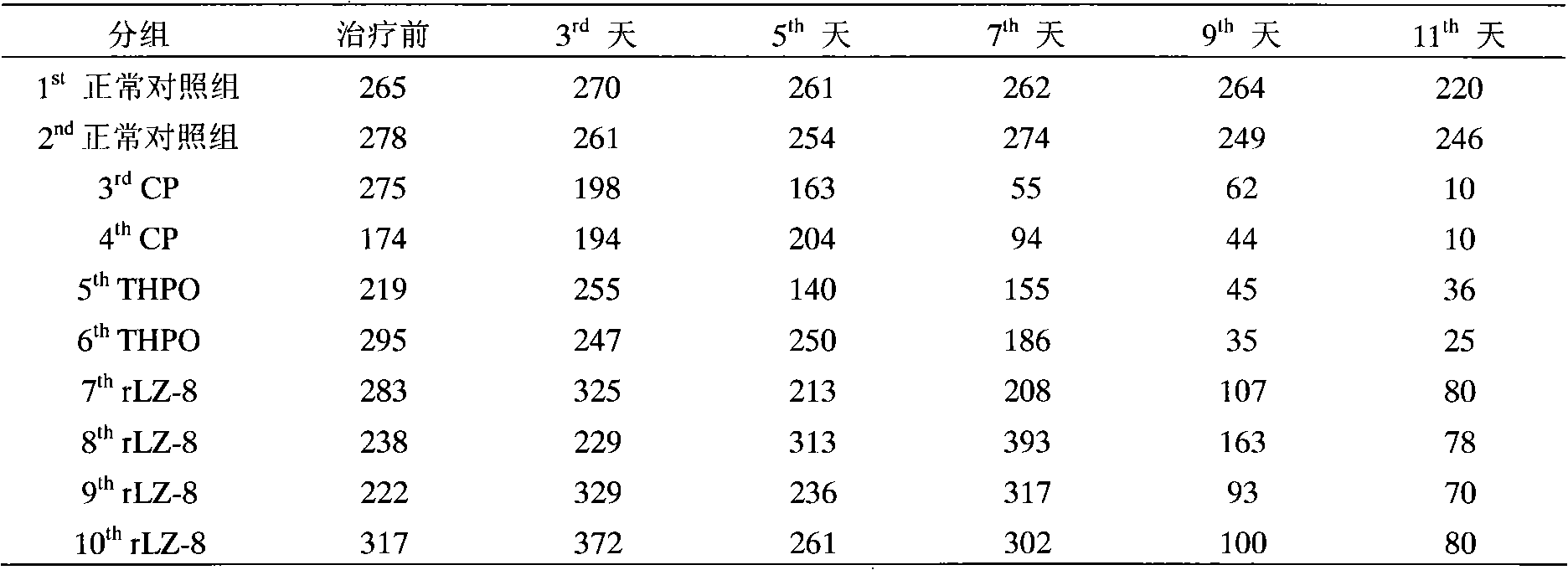
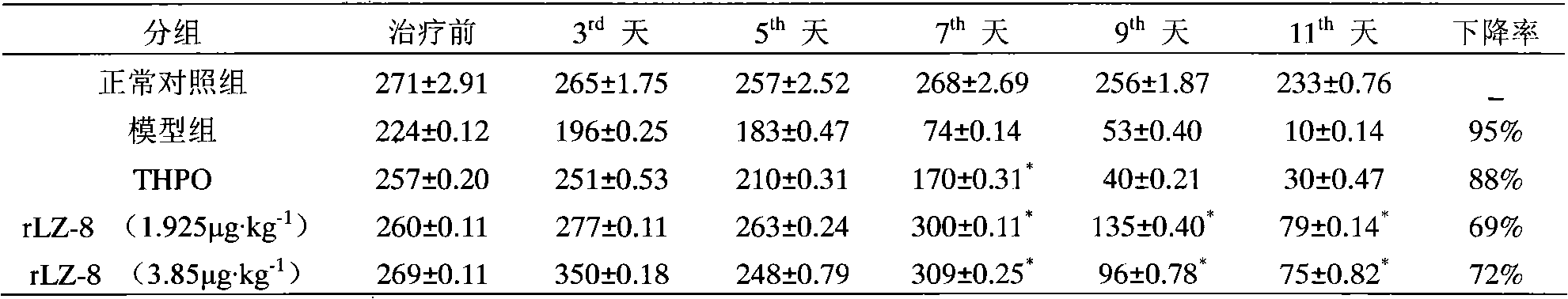
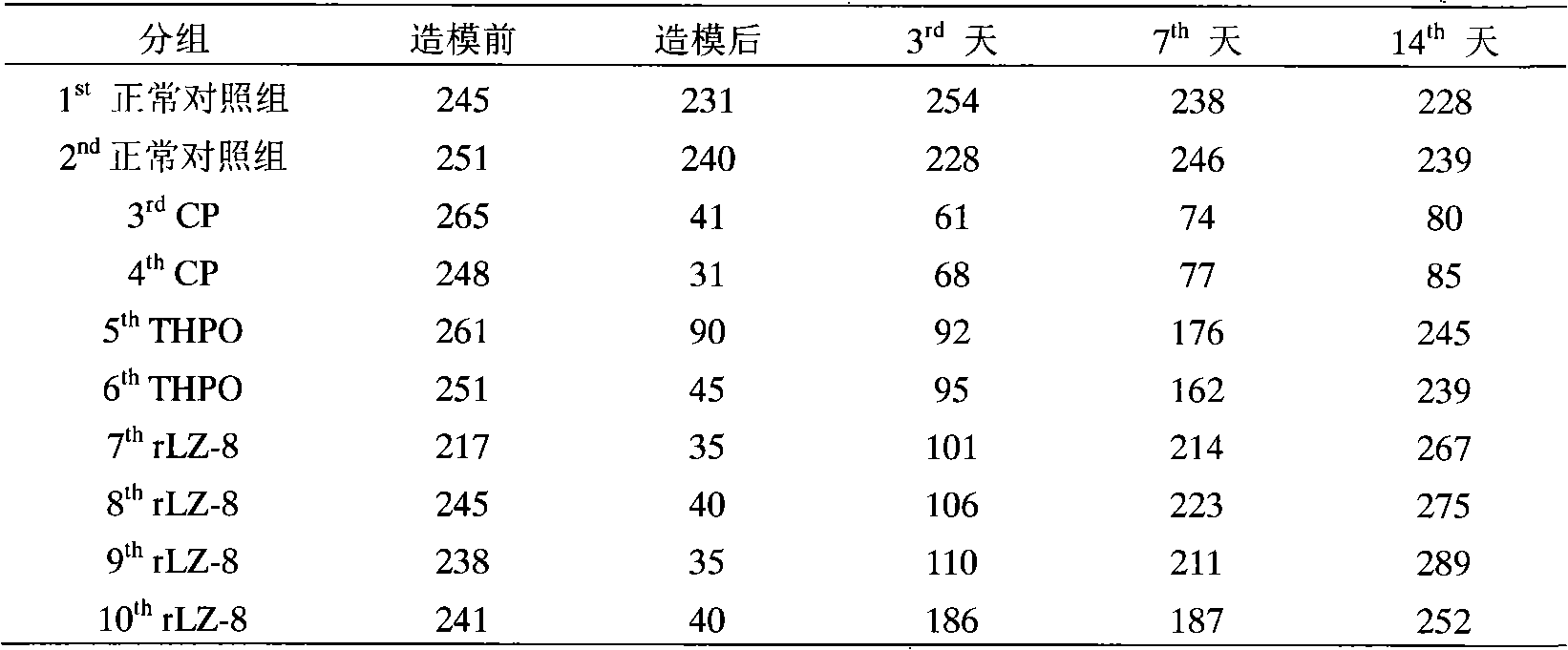
Application of rLZ-8 in treating thrombopenia and preparation thereof
The invention provides a novel application of a recombinant ganoderma lucidum immunological regulation protein (rLZ-8) in treating thrombopenia. In the experiments of preventing and treating the thrombopenia of rats and dogs caused by cyclophosphamide, the rLZ-8 prepared from physiological saline carries out administration on an experimental animal model with lower thrombocytes, THPO and IL-6 are used as positive medicines, continuous administration is carried out, the number of the thrombocytes in blood is detected, the changes of the number of the thrombocytes before and after therapy are contrasted, and the medicine effect is analyzed. Compared with a model group, the rLZ-8 medicine group already obviously stimulates the thrombocyte proliferation of rats, Guinea pigs and dogs in the initial period (the 3rd day to the 5th day) of the administration, the difference is very obvious, the thrombocytes are recovered to a normal level in the middle period (the 7th day to the 10th day) of the administration, and the invention also obtains a very good effect in treating the thrombopenia of a Guinea pig experimental animal model caused by injecting anti-thrombocyte serum.
Owner:张喜田
Compositions and methods for treating thrombocytopenia
InactiveUS20100041668A1Reduce morbidityOrganic active ingredientsBlood disorderBinding siteThrombasthenia
Owner:ASTELLAS PHARMA INC
Application of phosphatidylserine (PS) blocking agent to preparation of medicines for treating platelet number reduction related diseases
The invention discloses application of phosphatidylserine (PS) blocking agent to preparation of medicines for treating platelet number reduction related diseases. The invention proves that PS exposurecaused by apoptotic and activated platelets promotes that the platelets are eliminated in liver through experiments for the first time. Research proves that the platelets in the body of a patient suffering from thrombocytopenia are subjected to both activation and apoptosis, and apoptosis and activation cause the PS of the platelets to be exposed, so that the platelets are swallowed by phagocyticcells in the liver. Closing of the PS or blockage of PS extroversion can inhibit the platelets from being eliminated, which indicates that the PS blocking agent can take part in the treatment processof the platelet number change related diseases and can inhibit platelet number reduction in peripheral circulation blood. Therefore, the invention discloses a mechanism for eliminating the plateletsthrough the PS extroversion; furthermore, the blocking agent for inhibiting from eliminating PS exposure dependency platelets has the potential to be developed into novel platelets protective medicines and medicines for treating thrombocytopenia, and has great scientific research and economic value.
Owner:SUZHOU UNIV
Blood coagulation factor XIII for treating platelet disorders
Use of factor XIII for treating the symptoms of thrombocytopenia. A patient having thrombocytopenia, either chemically- or metabolically induced, is treated by administering factor XIII.
Owner:ZYMOGENETICS INC
Method of treating thrombocytopenia
Invented is a method of treating thrombocytopenia in a human, in need thereof which comprises the in vivo administration of a therapeutically effective amount of a peptide or a non-peptide TPO receptor agonist and an anti-clotting agent or agents, and optional further active ingredients, to such human.
Owner:NOVARTIS AG
Thrombopoietin mimetics
Owner:STATEGICS
(Trifluoromethoxy) pyrrolidine benzamide compound and application of compound in treating thrombocytopenia
The invention discloses a (trifluoromethoxy) pyrrolidine benzamide compound. A structural formula of the compound is as shown in the specification. A pharmacological experiment confirms that the compound has a better proliferation effect on 32D-MPL (myeloproliferative leukemia) cells containing a TPO (thrombopoietin) receptor; in-vivo activity and in-vitro activity are higher than those of a positive drug, eltrombopag; and the compound can be concluded to have an effect of a TPO receptor agonist. After the compound is administrated to a blood loss mouse, data analysis finds that data of the compound 6 in a test group on the seventh day and on the fourteenth day have significance as compared with a model group; a platelet concentration of tail blood on the fourteenth day is higher than thatof orbit blood; and the deeper research can be conducted in the aspect of thrombocytopenia related diseases.
Owner:窦玉玲
Method of detection of platelet-activating antibodies that cause heparin-induced thrombocytopenia/thrombosis
Owner:VERSITI BLOOD RES INST FOUND INC
Treatment of thrombocytopenia
InactiveCN102186882AImmunoglobulins against blood group antigensAntibody ingredientsBULK ACTIVE INGREDIENTActive ingredient
The present invention relates to treatment of thrombocytopenia with apharmaceutical composition comprisingrecombinant polyclonal anti-RhesusD antibody productas the active ingredient.
Owner:SYMPHOGEN AS
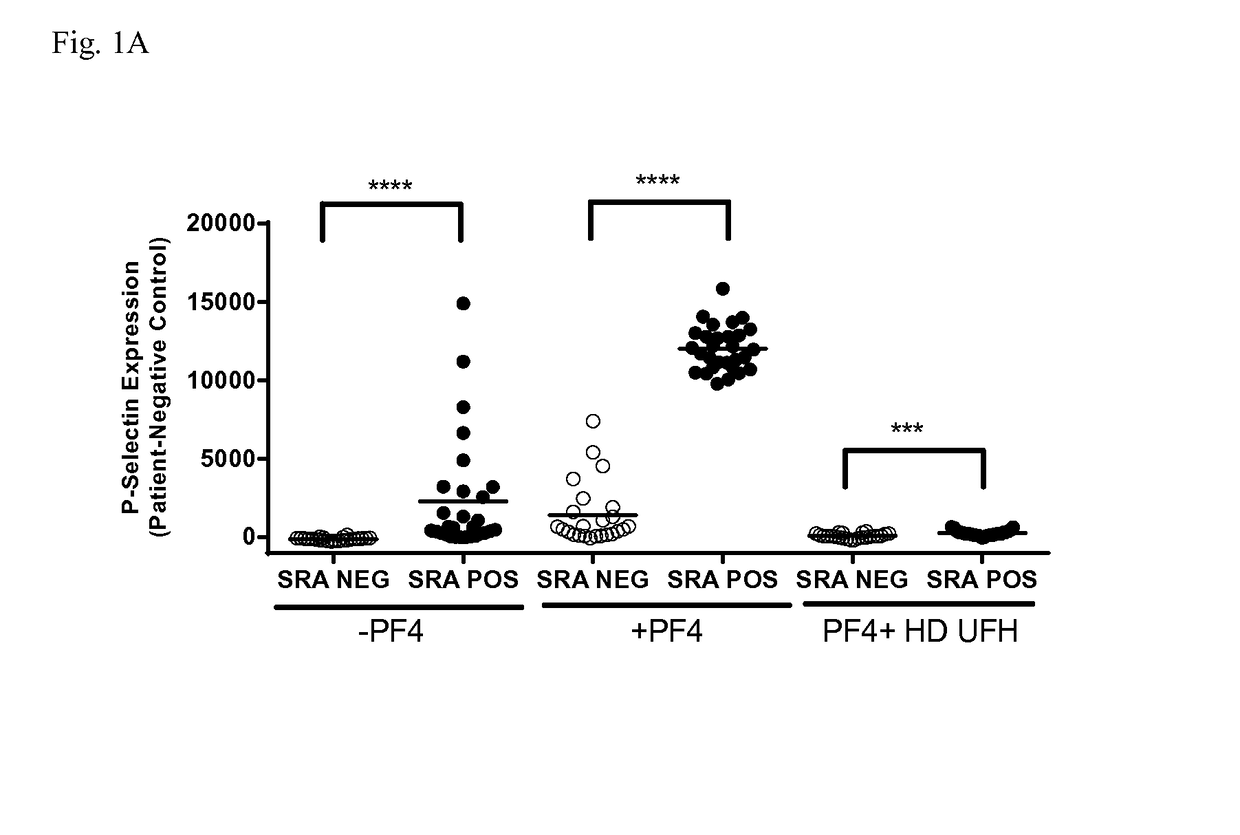
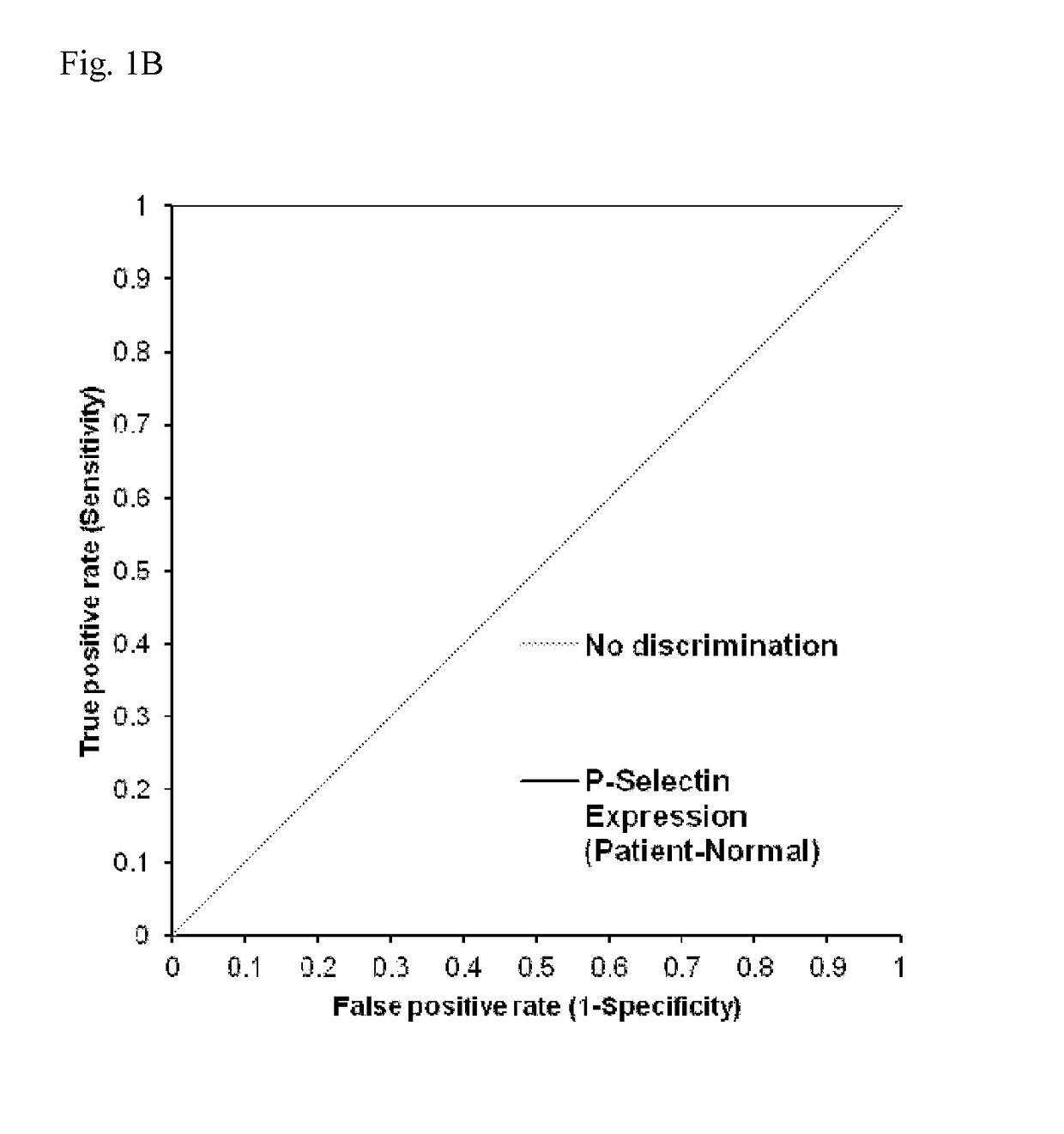
Atomic description of immune complex that causes heparin-induced thrombocytopenia
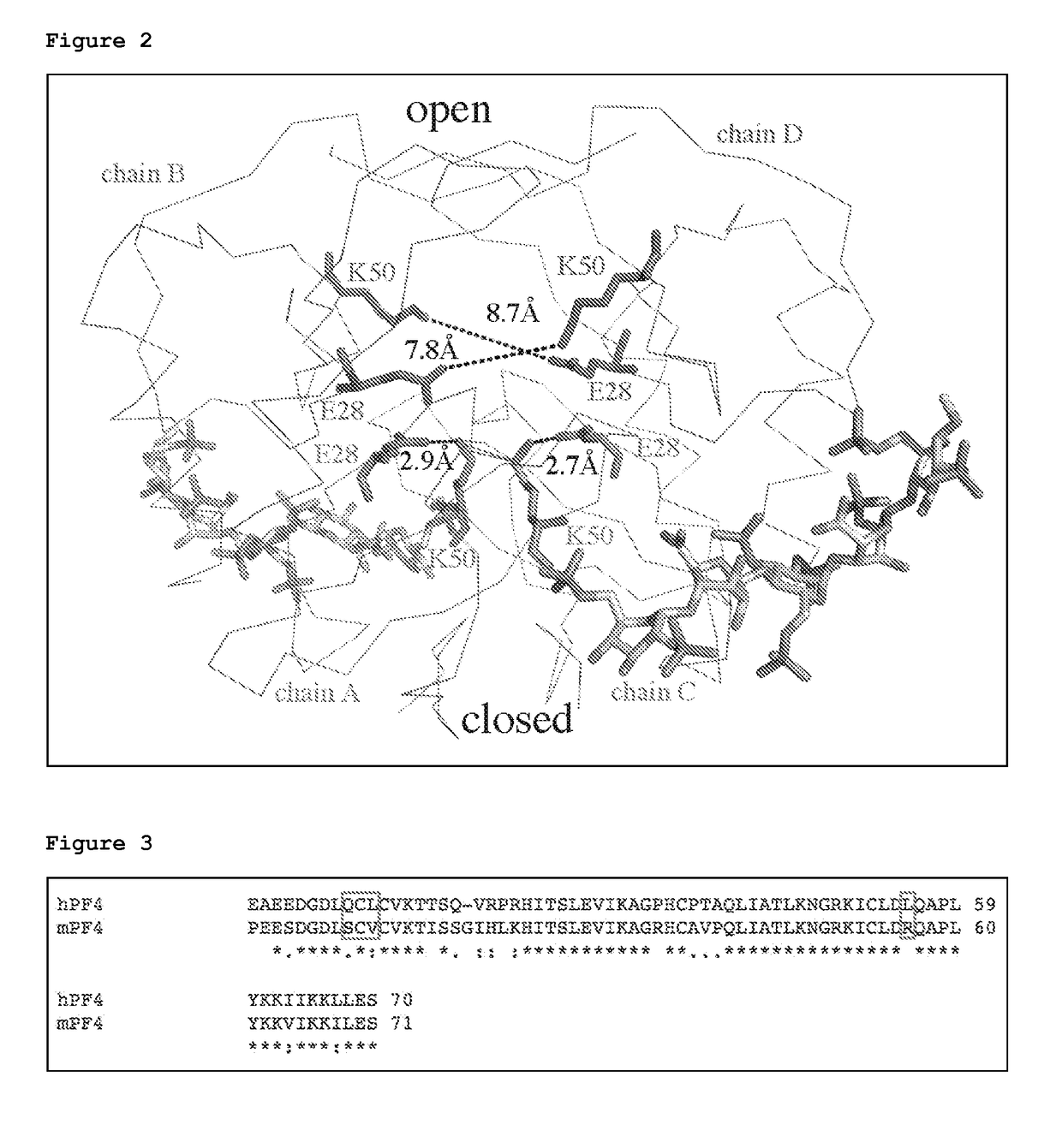
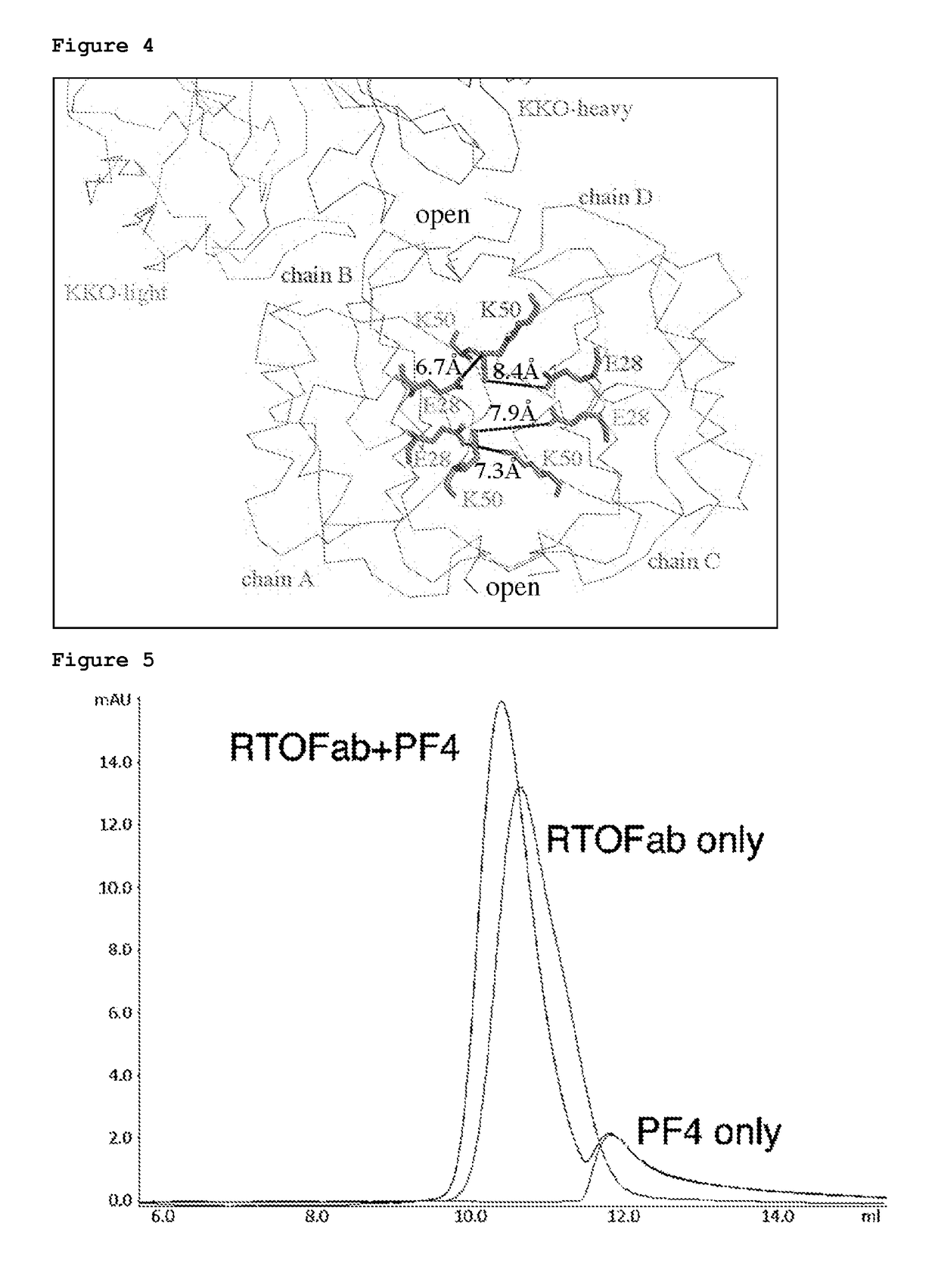
ActiveUS20180024140A1Reduce platelet factor (PF4) oligomerizationReduce oligomerizationChemokinesPeptide/protein ingredientsComplementarity determining regionHeparin antibody
The present invention provides a humanized antibody or antibody fragment comprising (a) a humanized light chain comprising 1) Complementarity Determining Region (CDR)-L1, the sequence of which is identical to the sequence of SEQ ID NO: 3; 2) CDR-L2, the sequence of which is identical to the sequence of SEQ ID NO: 4; and 3) CDR-L3, the sequence of which is identical to the sequence of SEQ ID NO: 5, and (b) a humanized heavy chain comprising 1) CDR-H1, the sequence of which is identical to the sequence of SEQ ID NO: 6; 2) CDR-H2, the sequence of which is identical to the sequence of SEQ ID NO: 7; and 3) CDR-H3, the sequence of which is identical to the sequence of SEQ ID NO: 8, as well as methods for treating, diagnosing, and monitoring the progression of HIT. The present invention also provides methods for assessing the antigenicity and ability to cause HIT of anionic anticoagulants. The present invention also provides a mutant protein which has the same amino acid sequence of a wild type PF4 monomer except that (i) at least one amino acid of the wild type PF4 monomer has been deleted, (ii) at least one amino acid of the wild type PF4 monomer has been replaced by another amino acid, or (iii) a combination of such changes has been made. The present invention also provides methods of treating or reducing the likelihood of HIT, treating angiogenesis, treating abnormal cell growth, or affecting coagulation pathologies that lead to thrombus formation, by administering such mutant proteins to a patient.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com