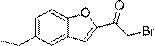(Trifluoromethoxy) pyrrolidine benzamide compound and application of compound in treating thrombocytopenia
A technology of trifluoromethoxy and benzamide, applied in the field of thrombocytopenia and pyrrolidine benzamide compounds, which can solve the problems of not emphasizing the adjustment of platelet count, easy recurrence of patients, and many adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Synthesis of 2-bromo-1-(5-ethylbenzofuran-2-yl)ethan-1-one:
[0024]
[0025] 1-(5-Ethylbenzofuran-2-yl)ethan-1-one (compound 1) (4.18g, 22.21mmol) was dissolved in ether (30mL), and bromine water ( 1.5 mL) was added to the above solution, and after the addition was complete, the reaction mixture was stirred at room temperature for 2 hours. After the reaction was completed, add water and stir, separate the layers, and separate the organic phase. The organic layer was washed with brine and dried over anhydrous sodium sulfate. Then the solvent was distilled under reduced pressure to obtain 2-bromo-1-(5-ethylbenzofuran-2-yl)ethan-1-one (compound 2), 5.05 g, yield 85.1%. 1 H-NMR (400 MHz, CDCl 3 ) δ: 1.18(t, 3H), 2.72(q, 2H), 4.59(s, 2H), 7.13(d, 1H), 7.29(s, 1H), 7.50(d, 1H), 7.53(s, 1H ). 13 C-NMR (125 MHz, CDCl 3 ) δ: 13.19, 28.44, 32.01, 112.58, 112.86, 122.31, 126.10, 127.46, 136.67, 154.56, 158.66, 187.05. LC-MS(ESI, pos, ion) m / z: 267[M+1].
Embodiment 2
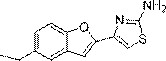
[0026] Example 2: Synthesis of 4-(5-ethylbenzofuran-2-yl)thiazol-2-amine:
[0027]
[0028] Compound 2 (5.05 g, 18.91 mmol) was dissolved in ethanol (30 mL), then thiourea (2.1 g, 27.59 mmol) was added at room temperature, and the reaction mixture was stirred at 80° C. overnight. After the reaction was completed, water was added, the precipitate was filtered, and the obtained solution was concentrated under reduced pressure, then chloroform was added, and the layers were separated. The organic layer was washed with potassium carbonate aqueous solution and brine in turn, and then dried over sodium sulfate. The obtained crude product was recrystallized from a solution of cyclohexane:EtOAc=1:1 to obtain 4.17 g of light yellow solid 4-(5-ethylbenzofuran-2-yl)thiazol-2-amine (compound 3), Yield 90.3%. 1 H-NMR (400 MHz, CDCl 3 ) δ: 1.18(t,3H), 2.72(q, 2H), 7.03(s, 1H), 7.11(s, 1H), 7.34(d, 1H), 7.50(d, 1H), 7.63(s,2H ), 7.67(s, 1H). 13 C-NMR (125 MHz, CDCl 3 ) δ: 13.19, 28.4...
Embodiment 3
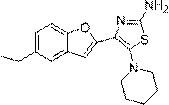
[0029] Example 3: Synthesis of 4-(5-ethylbenzofuran-2-yl)-5-(piperidin-1-yl)thiazol-2-amine:
[0030]
[0031] Compound 3 (4.17g, 17.07mmol) was dissolved in 50mL of DMF, then NBS (4.00g, 22.47mmol) was added under ice-water bath conditions, the mixture was stirred at 0°C for 1 hour, and then added to the reaction mixture Piperidine (2.91 g, 34.14 mmol) and triethylamine (6 mL) were added successively, and the mixture was stirred at 70° C. for 3 days. After the reaction was complete, the solvent was distilled off under reduced pressure, dissolved in chloroform, washed with potassium carbonate aqueous solution and brine successively, and dried over sodium sulfate. The obtained crude product was purified by silica gel column chromatography (eluent is cyclohexane:EtOAc=1:1) to give off-white 4-(5-ethylbenzofuran-2-yl)-5-(piperidine- 1-yl)thiazol-2-amine (compound 4) powder, 5.18 g, yield 92.6%. 1 H-NMR (400 MHz, CDCl 3 ) δ: 1.18(t, 3H), 1.52-1.67(m, 6H), 2.72(q, 2H), 3.36(m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Internal diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com