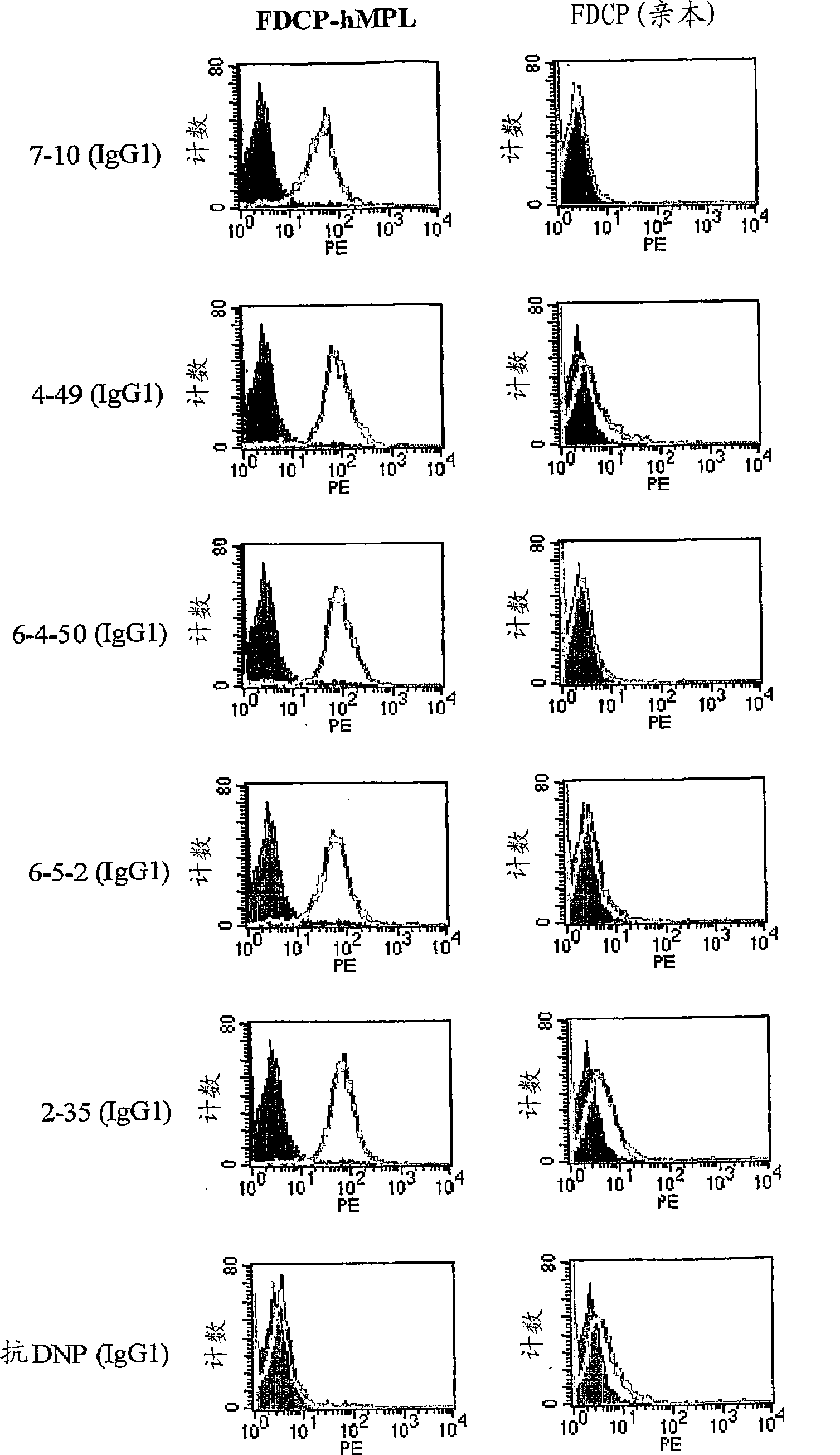
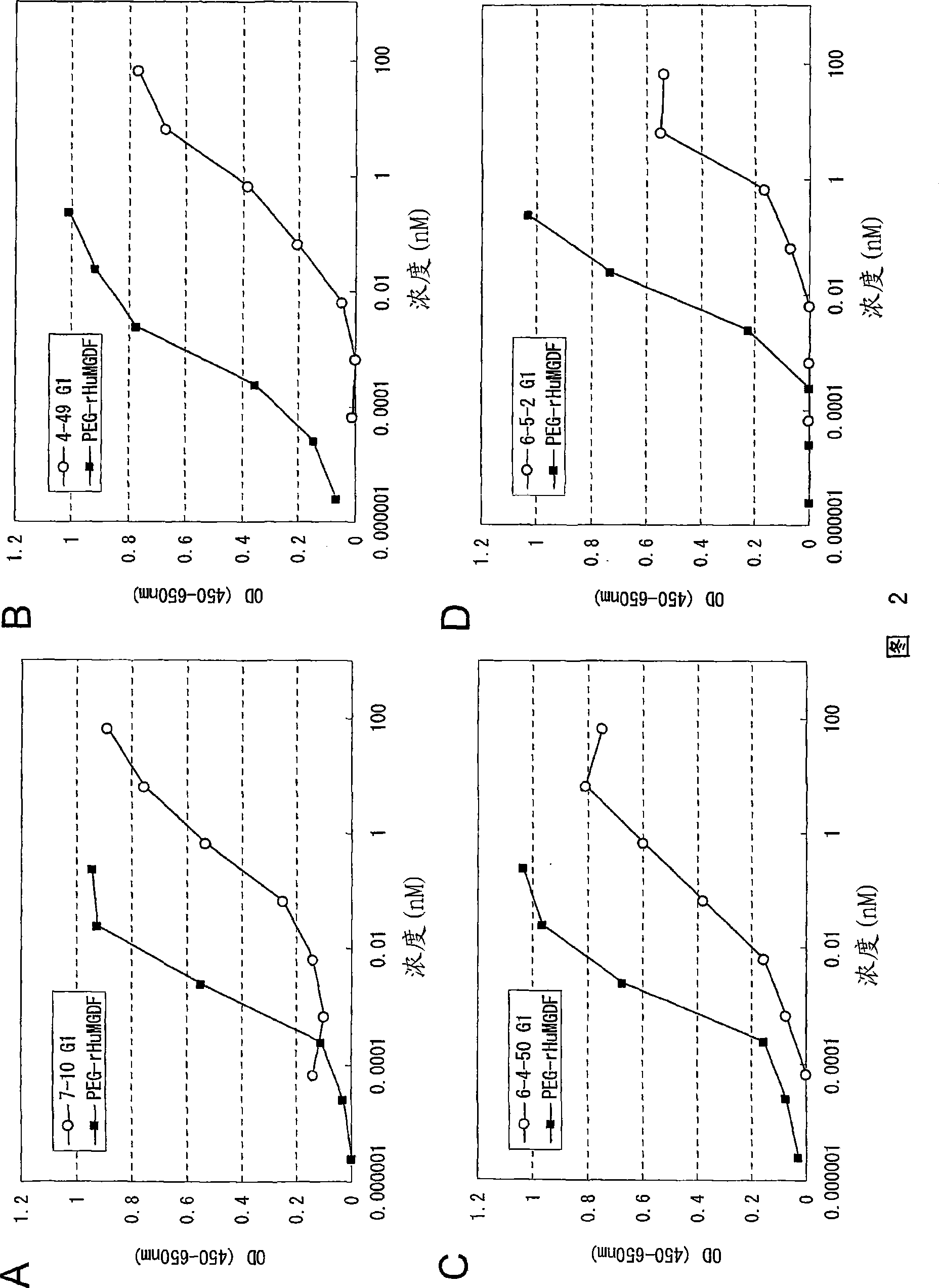
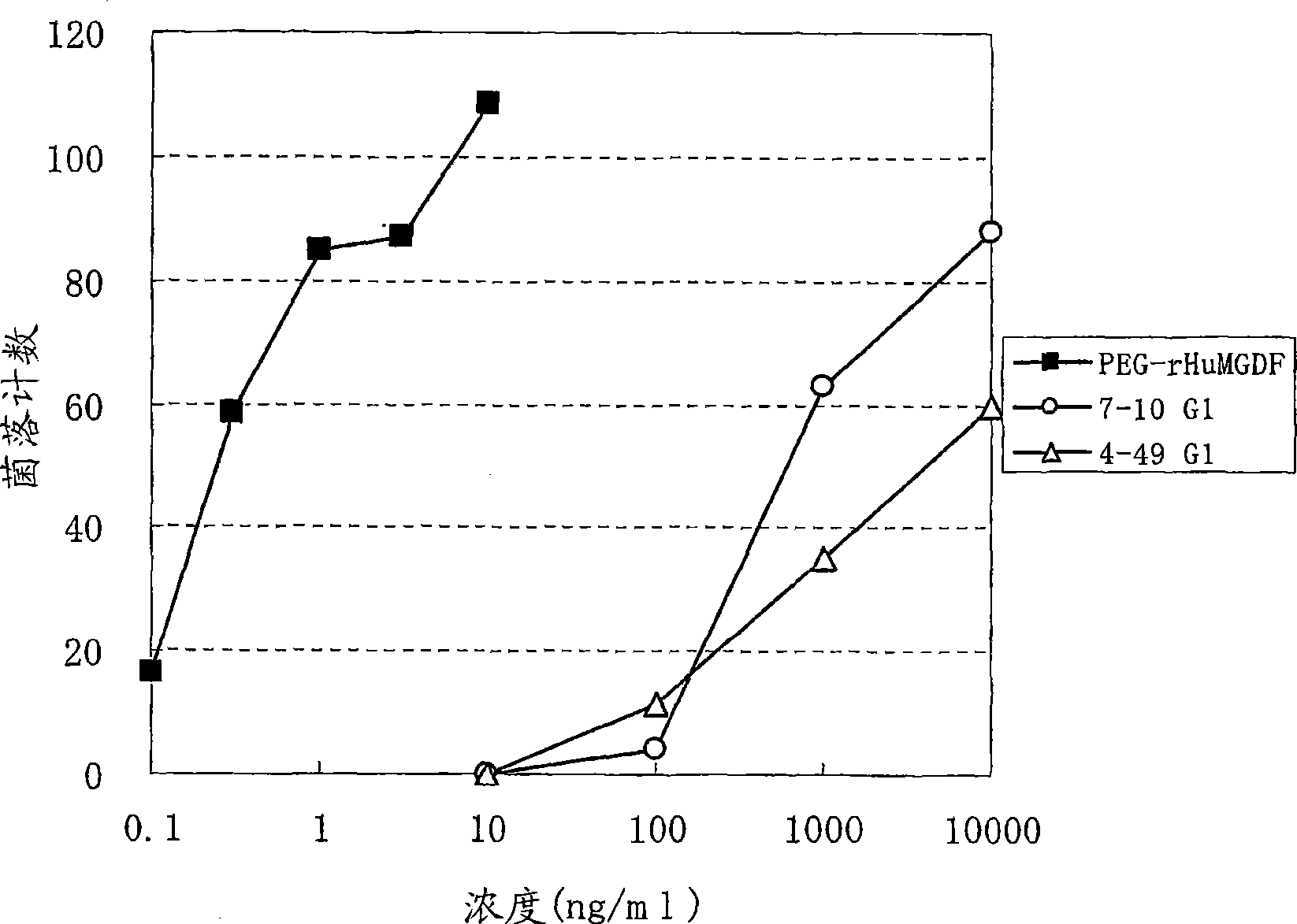
Agonistic antibody directed against human thrombopoietin receptor
A thrombopoietin and agonist technology, applied in the direction of antibodies, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, anti-animal/human immunoglobulin, etc. Antibodies, low antigenicity, long-lasting half-life, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0250] Antigen preparation
[0251] 1-1: Preparation of human c-Mpl-expressing cells
[0252] In general, when an antigenic protein expressing cell line is used for immunization, it is more advantageous to use a cell line showing a higher expression level for antibody production. Various types of human megakaryocyte cell lines or forced expression cell lines are known to be human c-Mpl-expressing cell lines; but the expression level of c-Mpl in such cell lines is only several thousand molecules per cell, so such Cell lines are not suitable for use as antigens. When immunized with the expressing cell line FDCP-hMpl (see FEBS Lett. Oct 21, 1996; 395(2-3): 228-234) comprising human c-Mpl introduced into FDCP2 (ie, a murine hematopoietic cell line), Human antibody mouse (such as KM mouse TM ), the increase in antibody titer was actually not sufficient, and the hMpl-specific human antibody could not be obtained. Antibodies reactive with other membrane molecules were also induce...
Embodiment 2
[0282] Preparation of monoclonal antibodies
[0283] Mice (KM mice) that produce human antibodies were immunized with the antigen TM ) to prepare monoclonal antibodies, thereby obtaining the antibodies of the present invention, and the above-mentioned mice can produce human antibodies through genetic modification. KM mice lack endogenous mouse immunoglobulin (Ig) heavy chains and mouse kappa light chains, while carrying a chromosome 14 fragment with a human Ig heavy chain gene (SC20) and a human Ig kappa chain transgene (KCo5). In particular, KM mice are capable of producing human antibodies and lack murine Ig heavy and kappa chains. The mice were prepared by crossing lineage A mice with the human Ig heavy chain locus and lineage B mice with the human Ig kappa chain transgene. Lineage A mice are homozygous for deficient endogenous Ig heavy chain and kappa light chain genes and carry a chromosome 14 segment (SC20) repertoire that is transmitted to offspring (see Tomizuka. et ...
Embodiment 3
[0297] Preparation of purified antibodies from hybridoma culture supernatants
[0298] Anti-human c-Mpl monoclonal antibody was purified from hybridoma culture supernatant in the following manner. Using rmp protein A (Amersham Pharmacia Biotech), 0.8 × 40cm column (Bio-Rad), PBS as adsorption buffer and 0.02M glycine buffer (pH 3) as elution buffer, the culture supernatant containing antibody Perform affinity purification. The pH of the eluted fractions was adjusted to around 7.2 by adding 1M Tris (pH 9.0). The prepared antibody solution was replaced with PBS using a dialysis membrane (10,000 cutoff, Spectrum Laboratories), and passed through a membrane filter MILLEX-GV (Millipore), pore size 0.22 μm, to obtain purified anti-human c-Mpl monoclonal antibody . The concentration of the purified antibody was determined by measuring the absorbance at 280 nm and calculating by specifying 1 mg / ml to be equal to 1.4 OD.
[0299] Culture supernatants containing anti-human c-Mpl mon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com