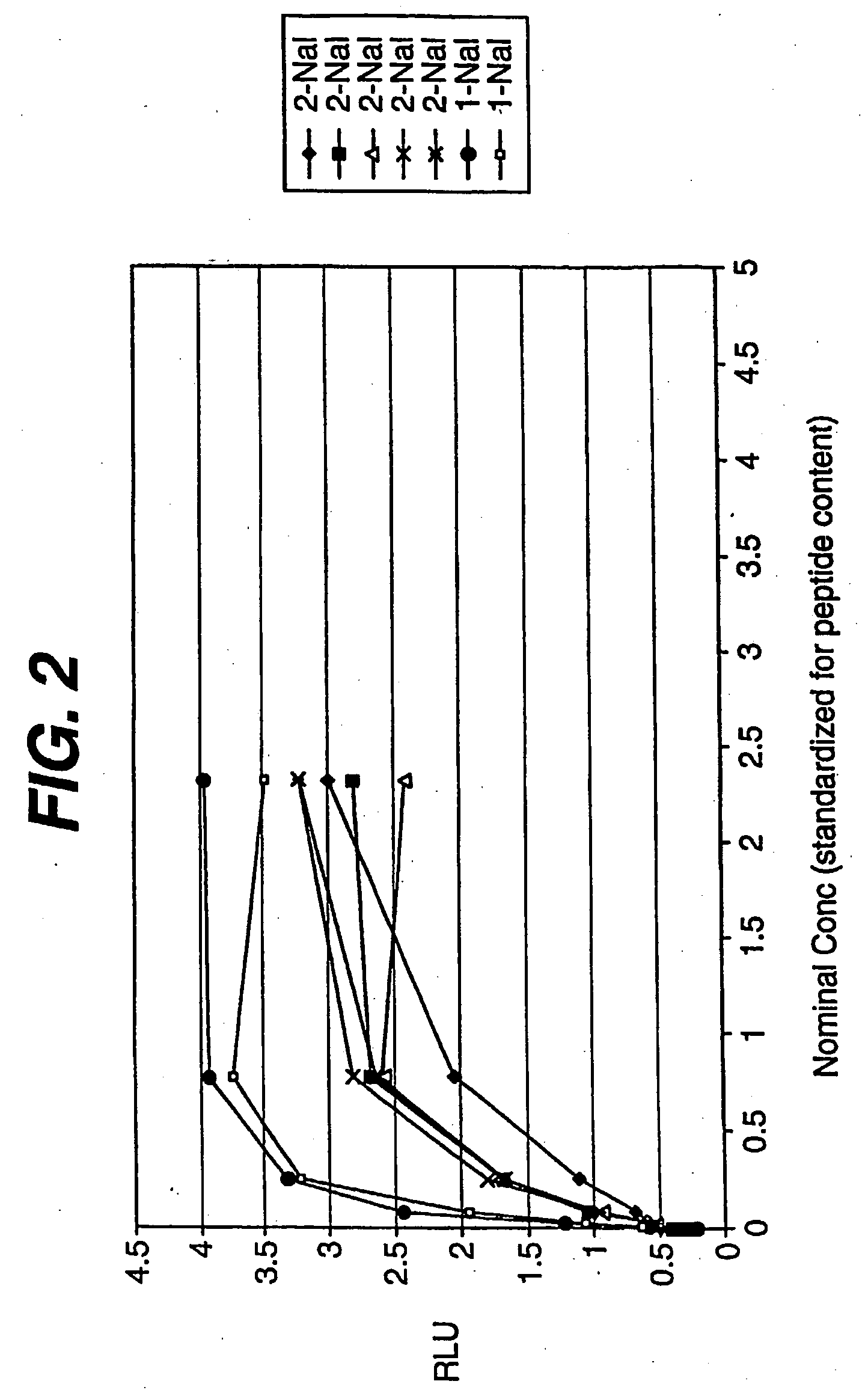
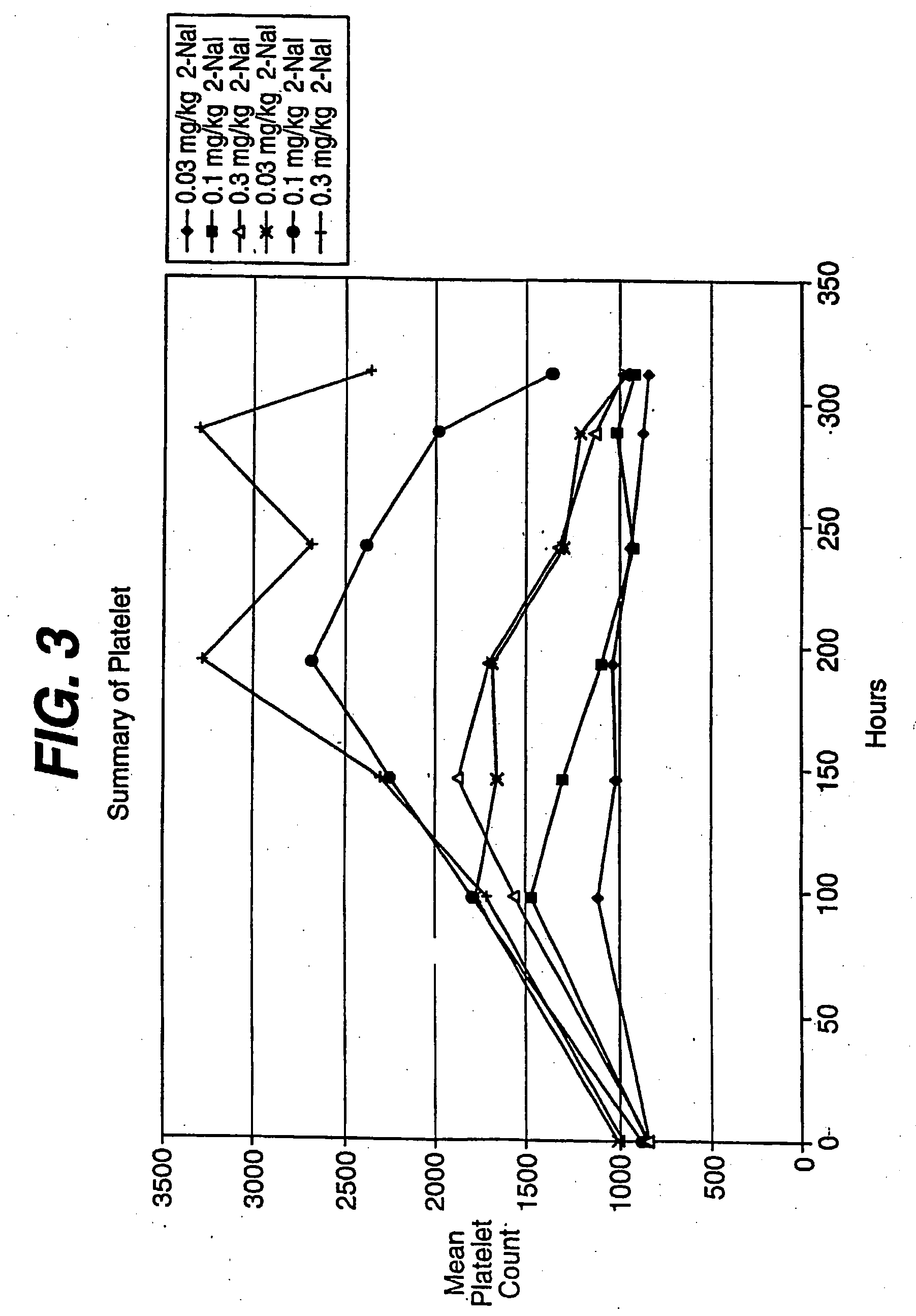
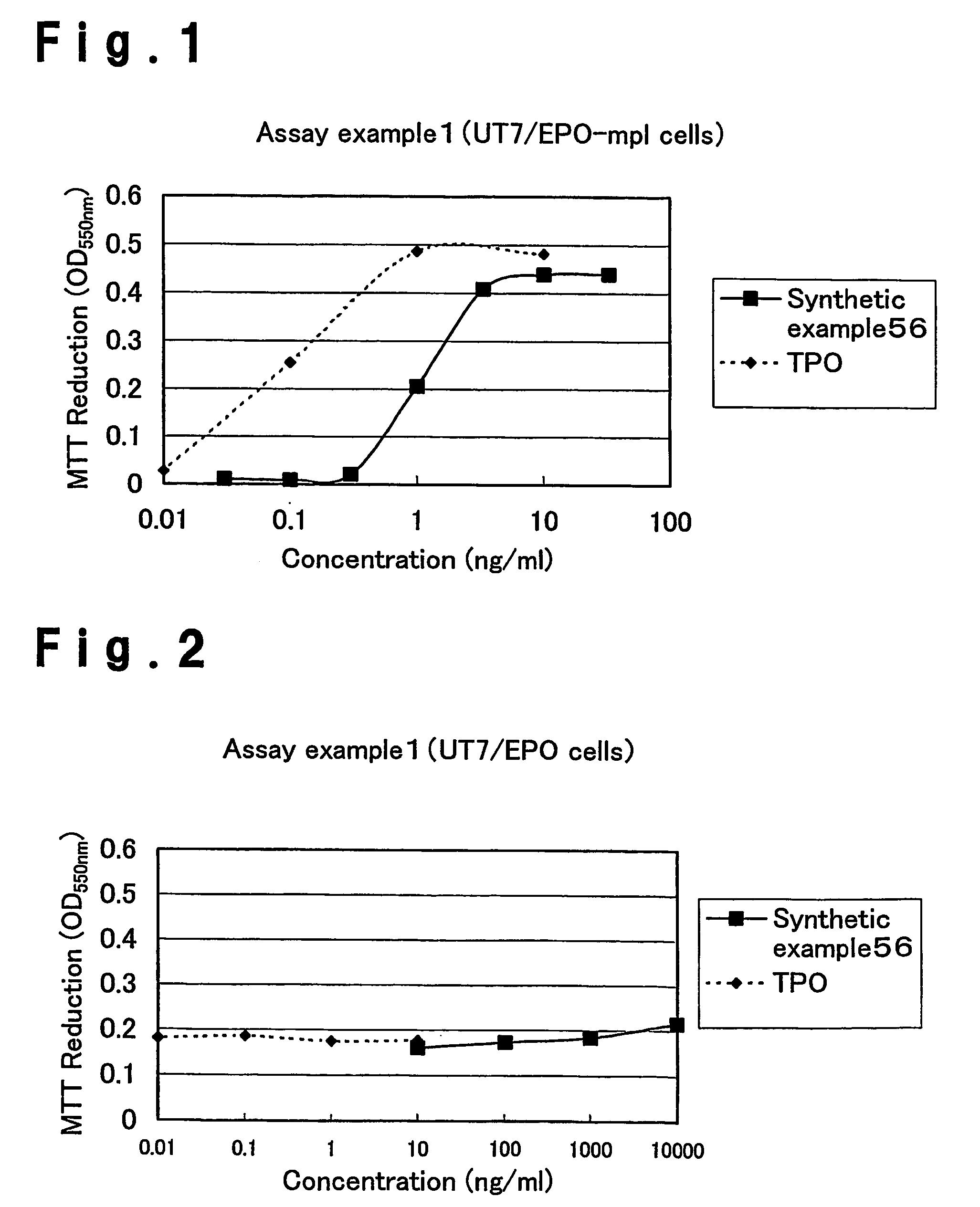
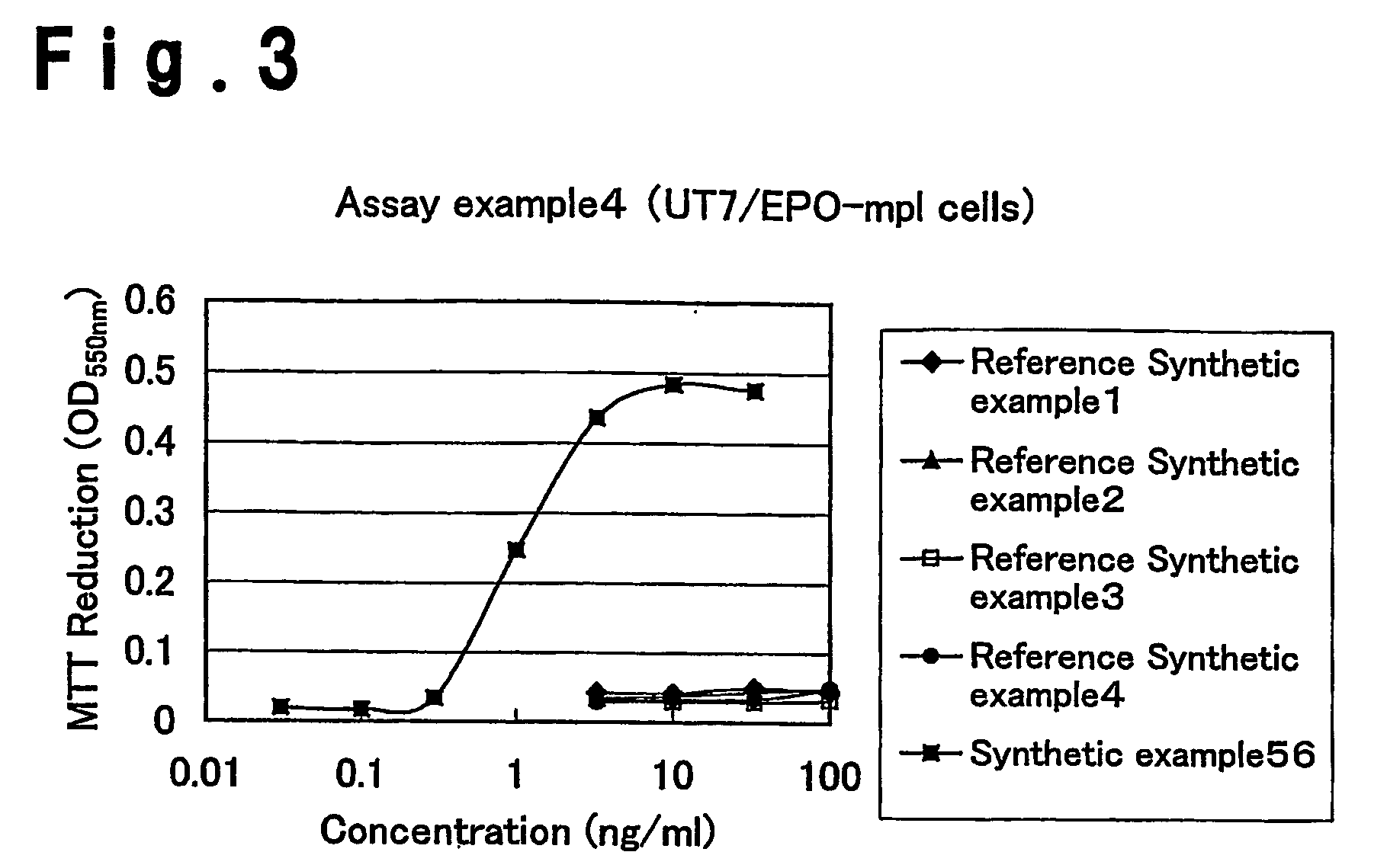
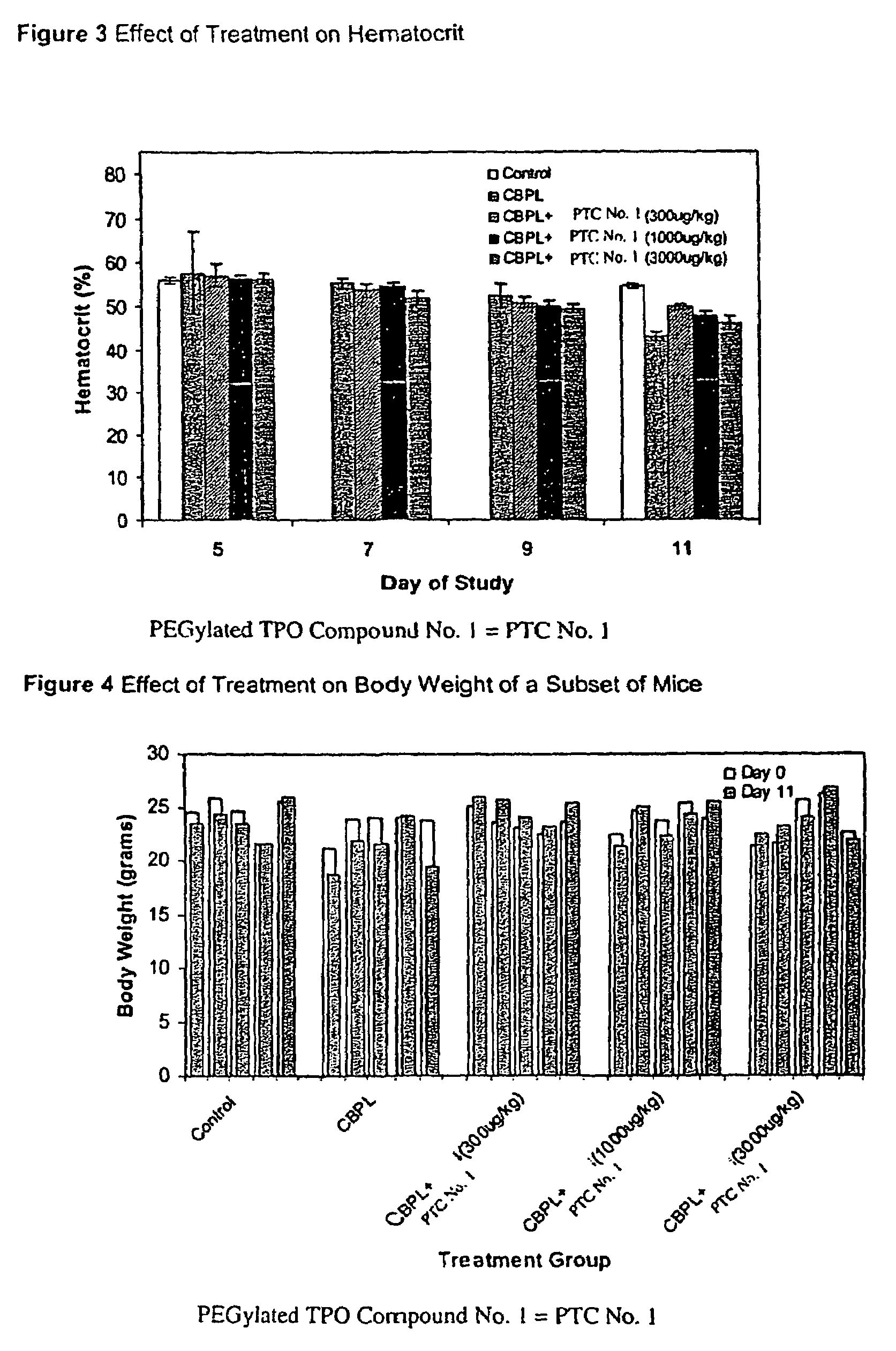
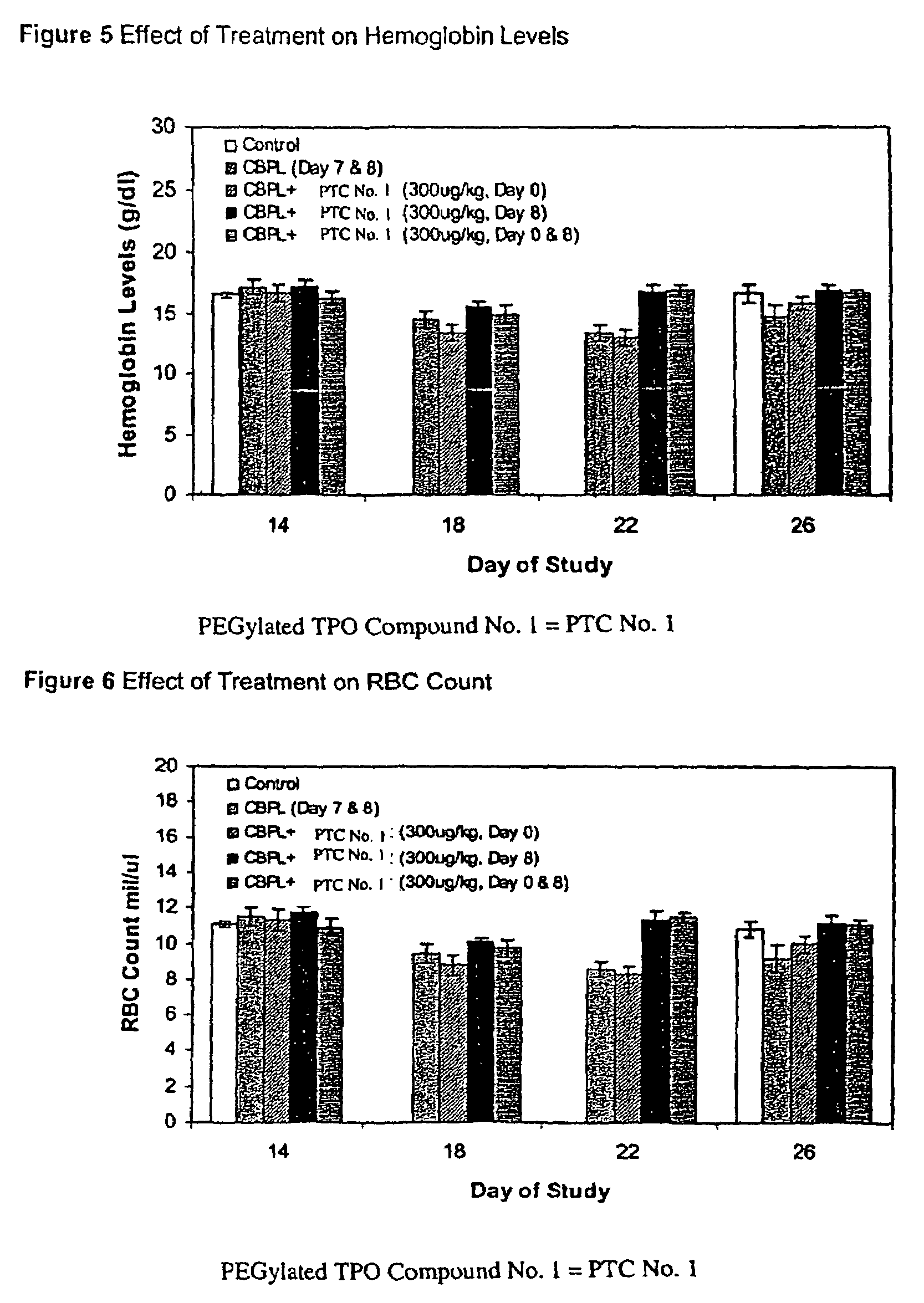
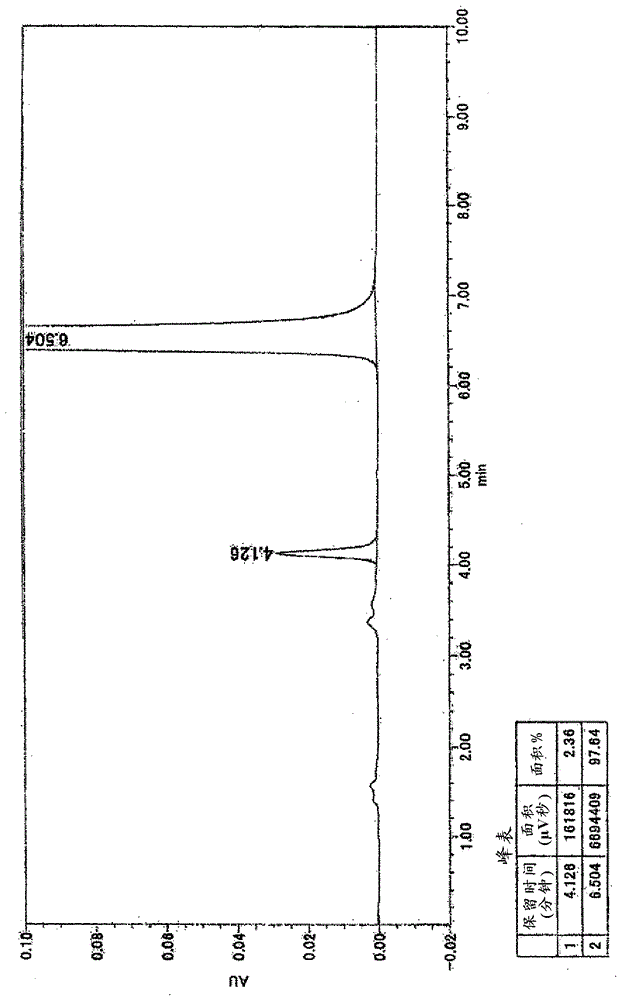
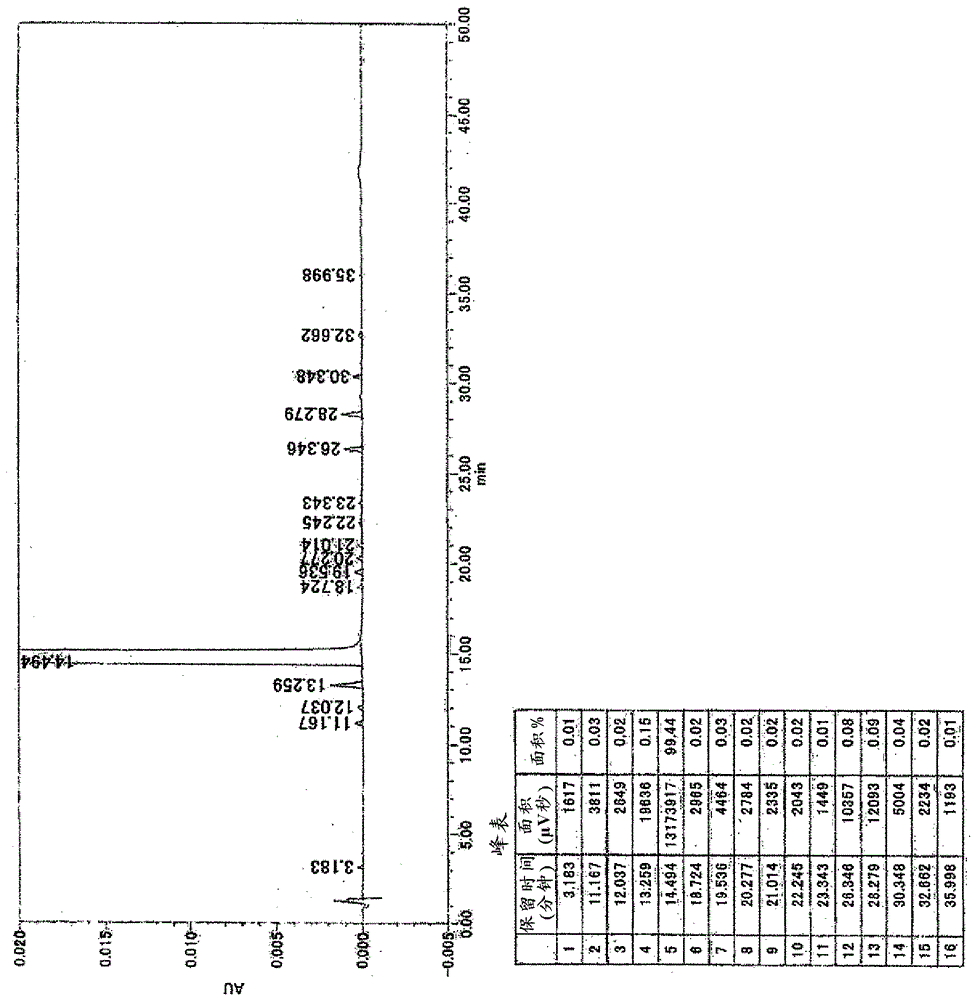
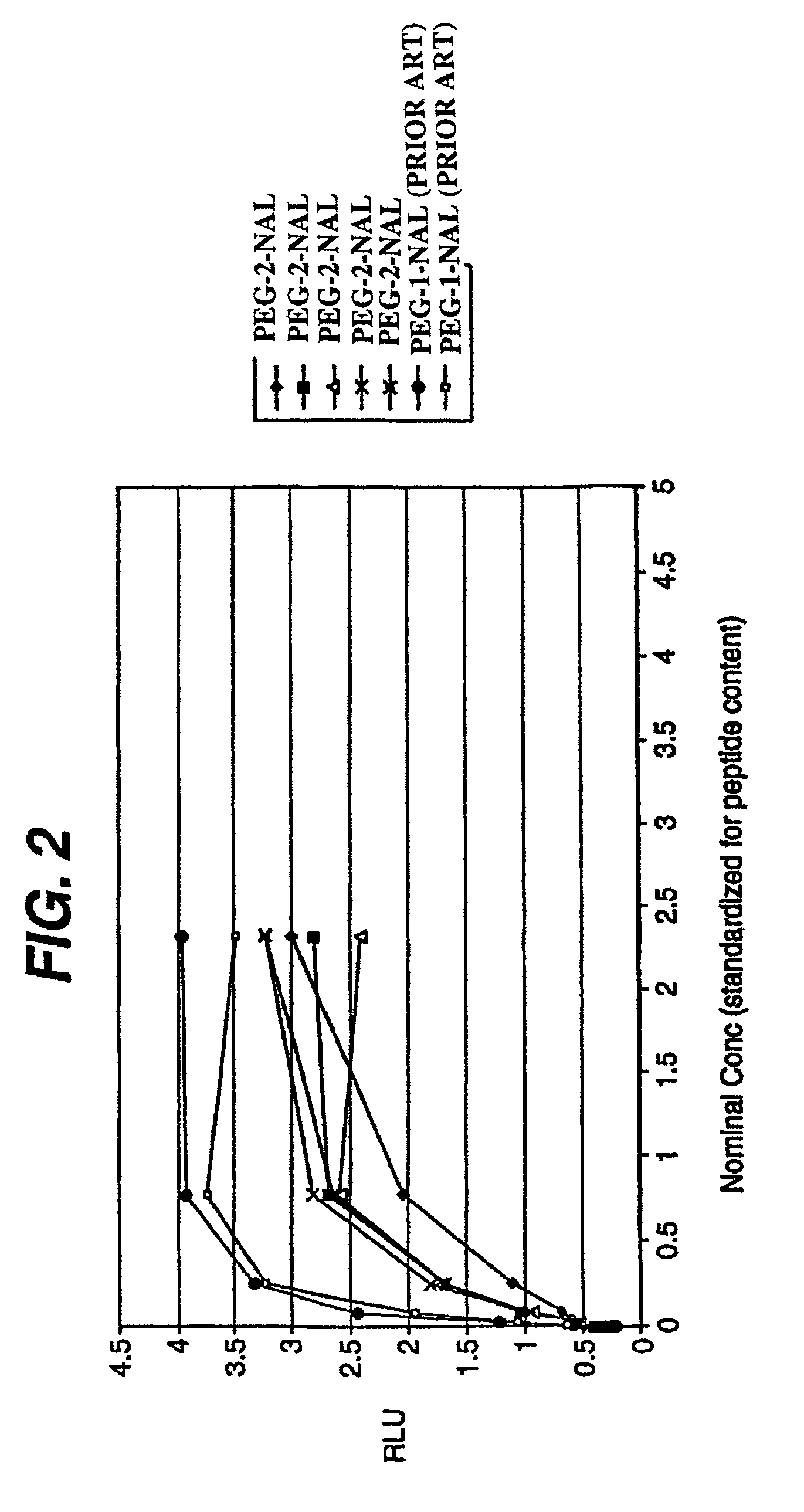
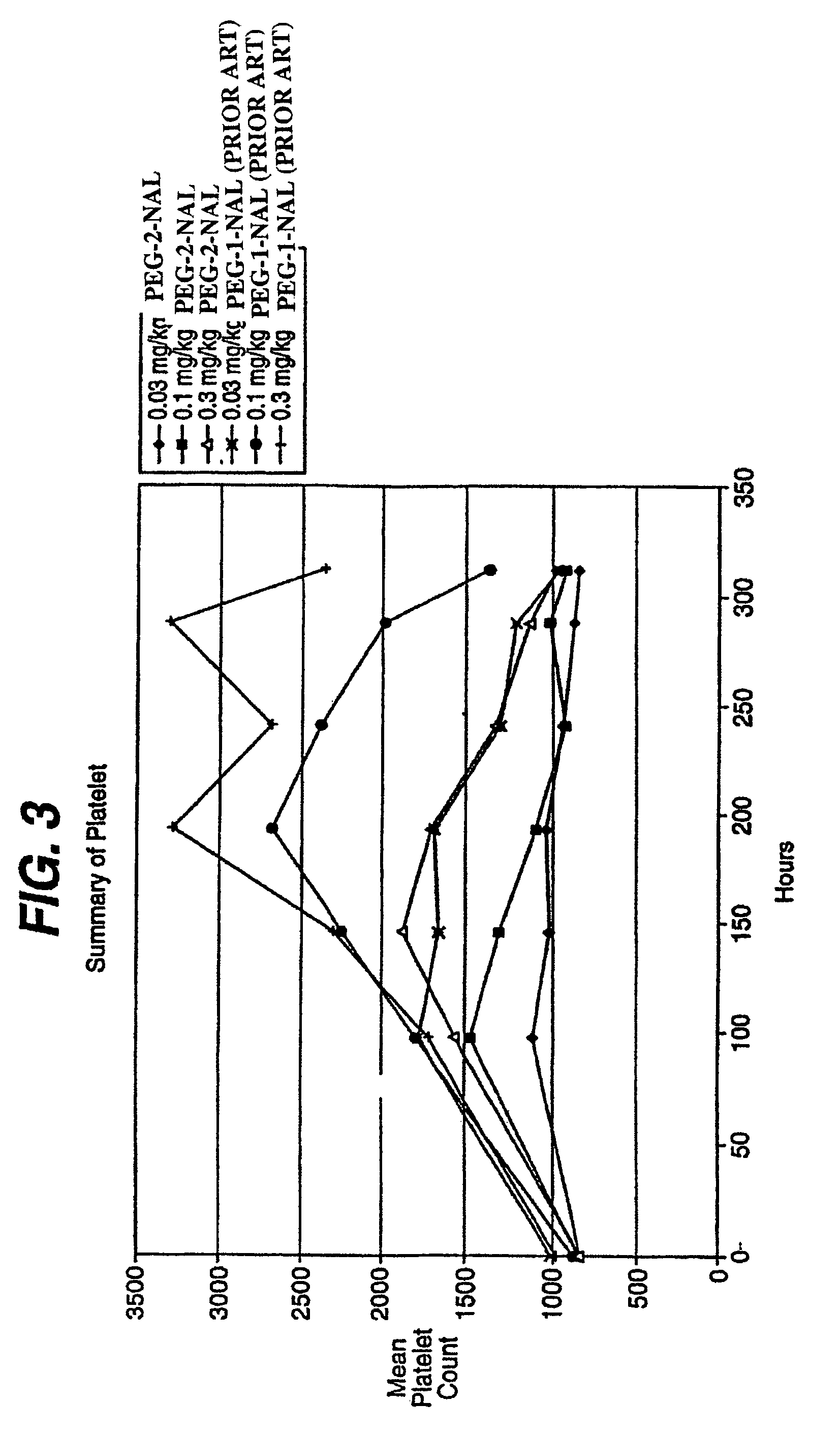
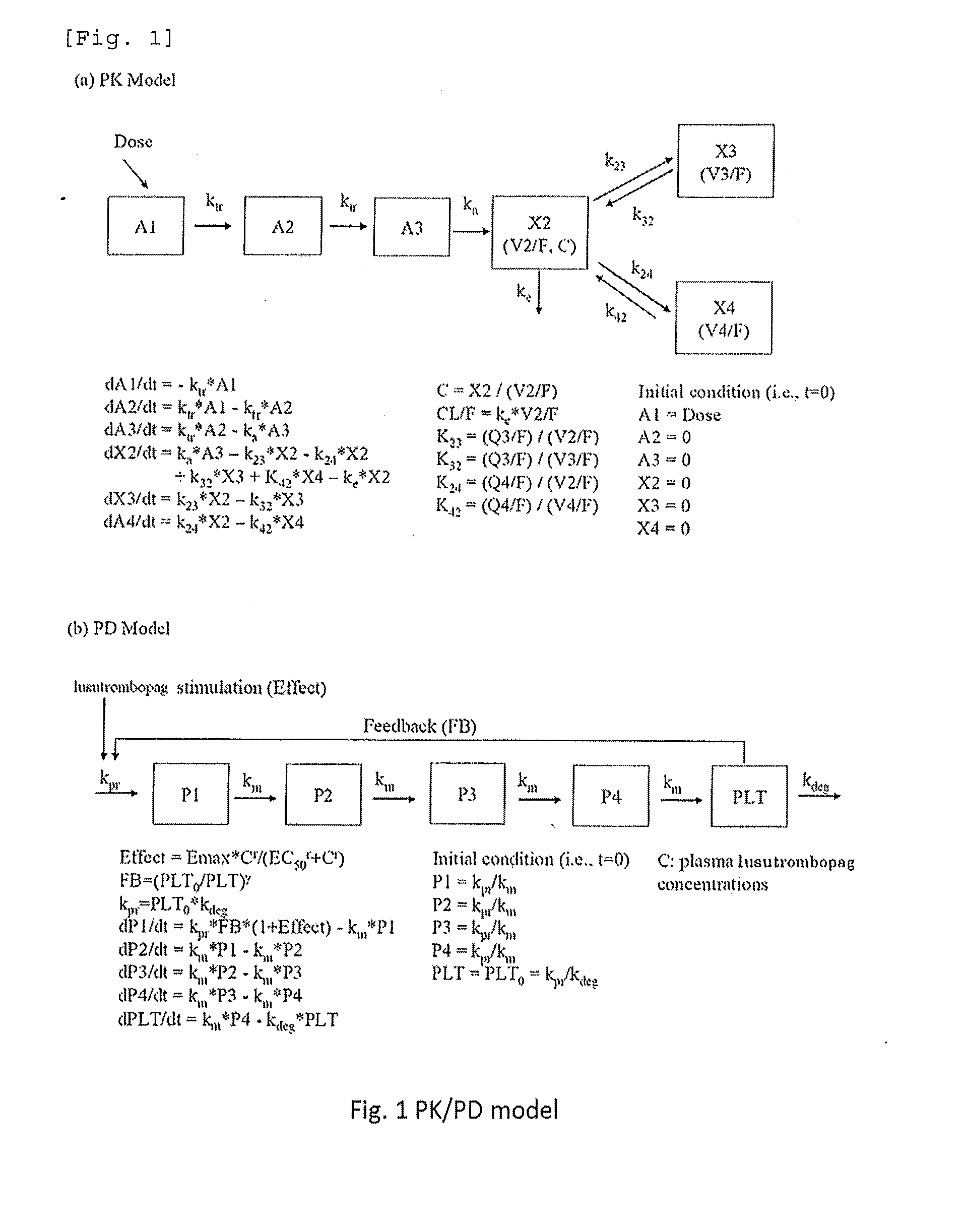
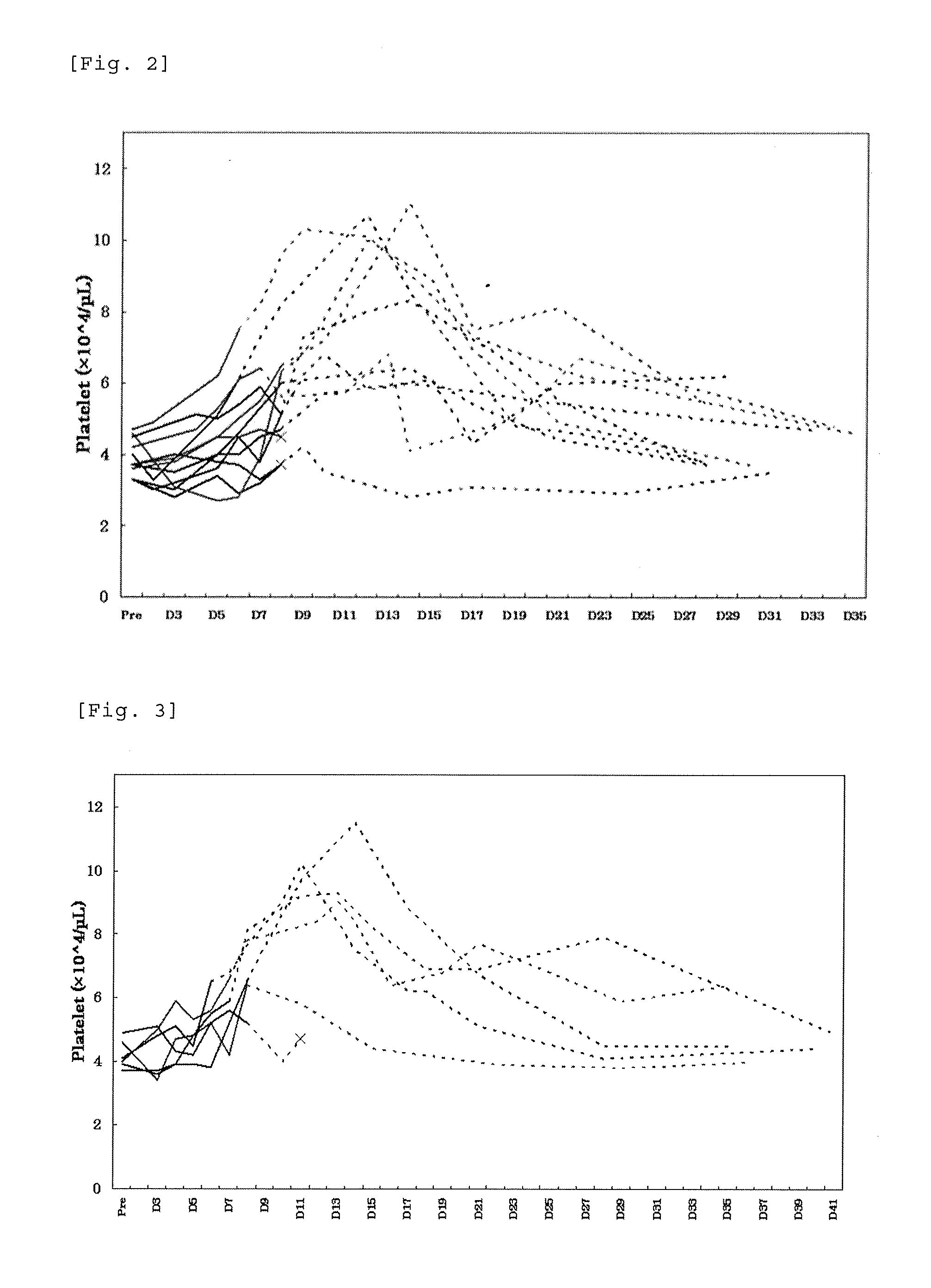
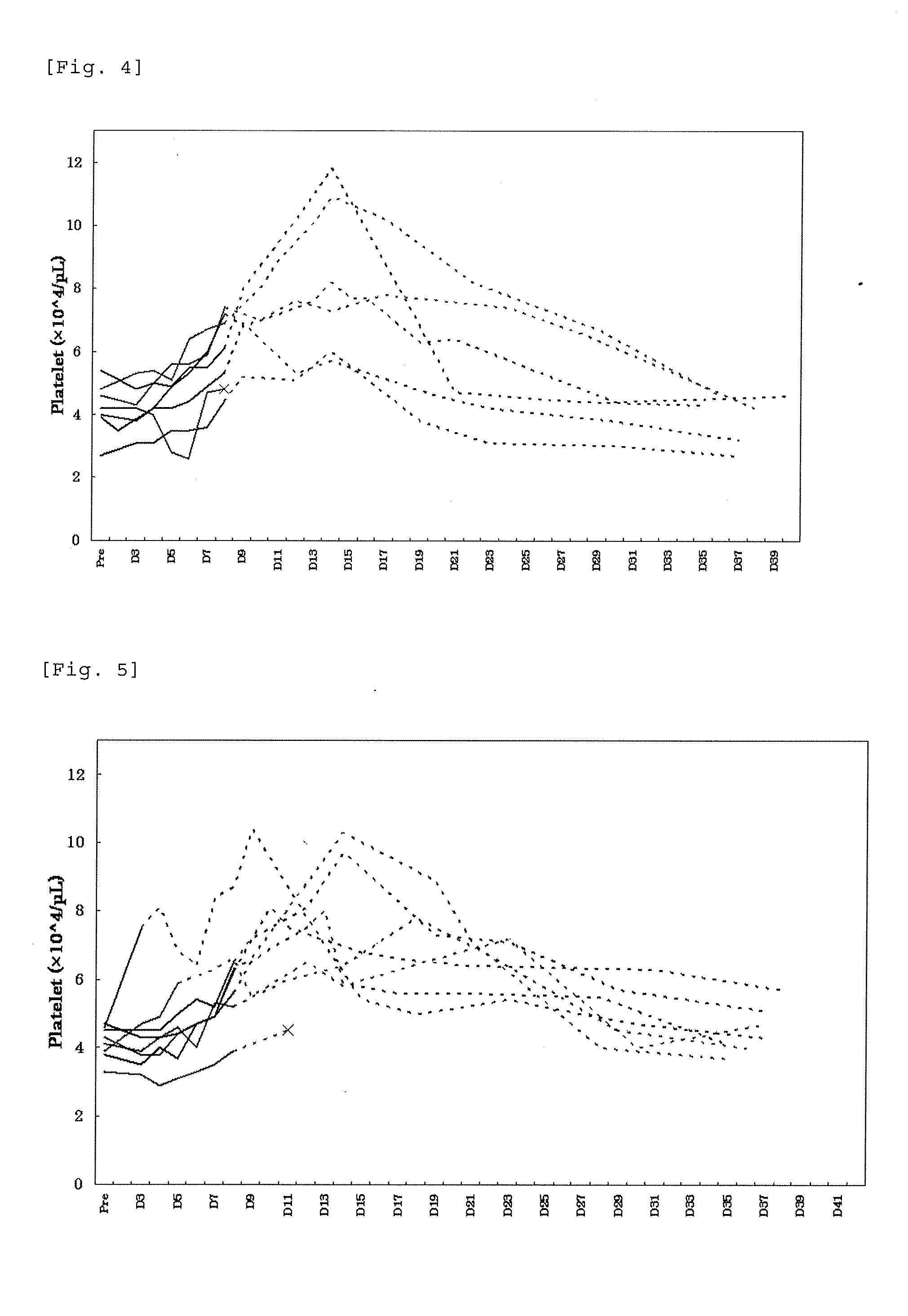
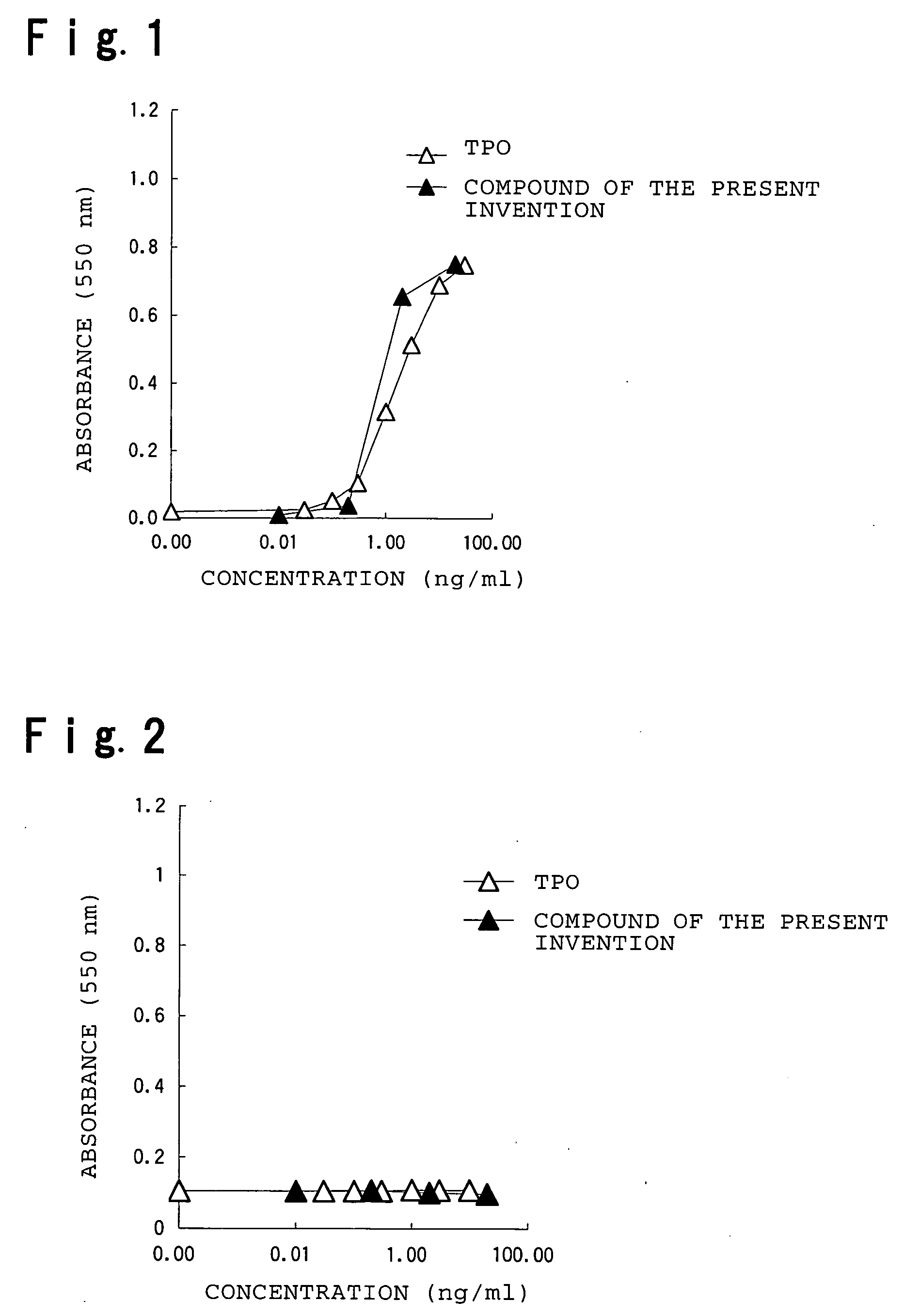
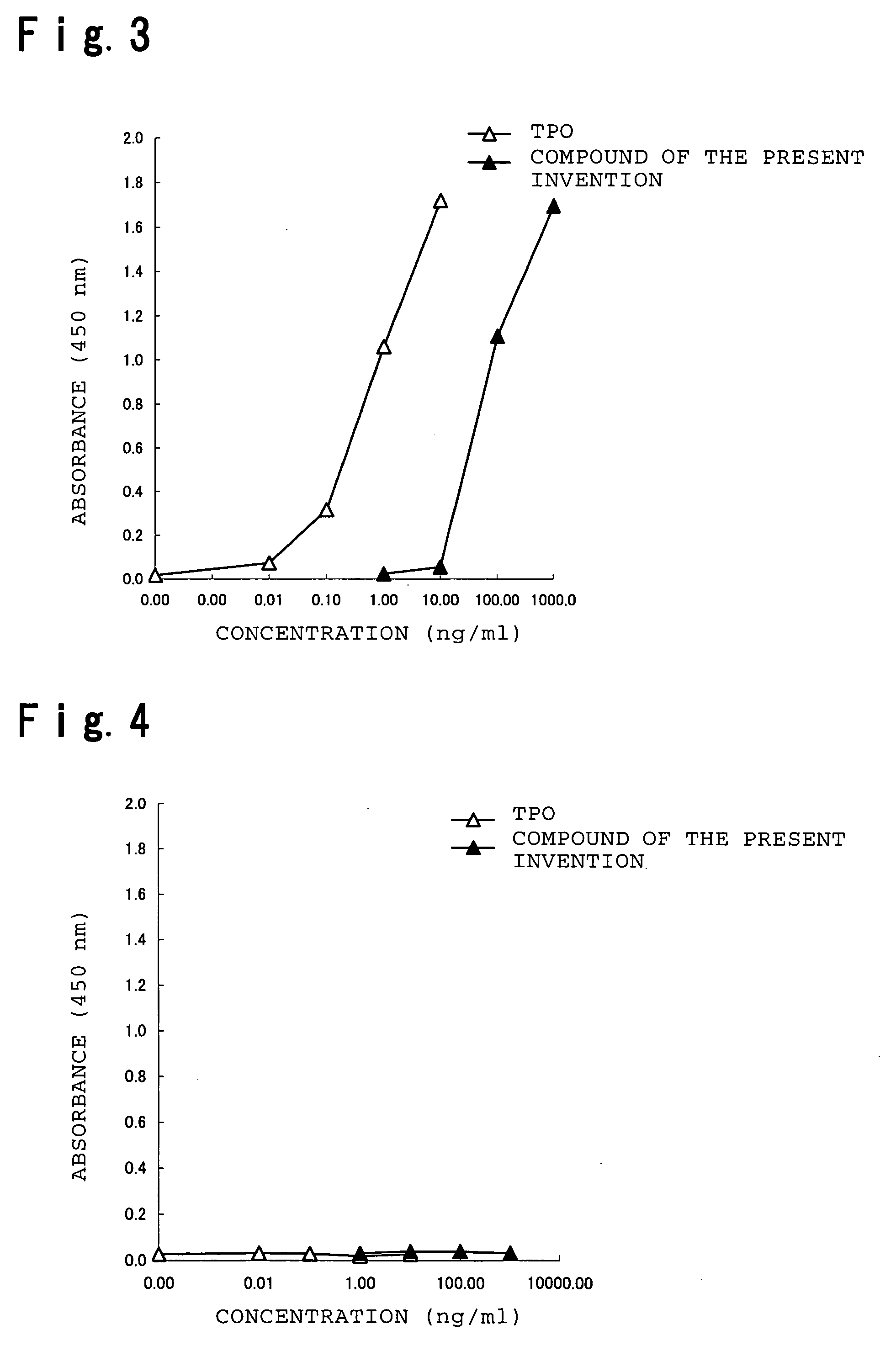
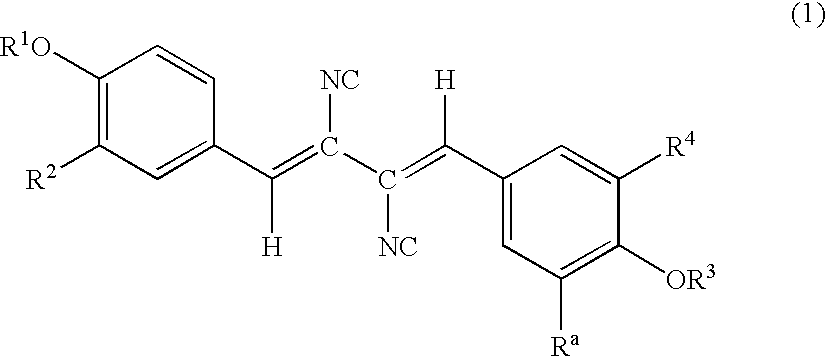
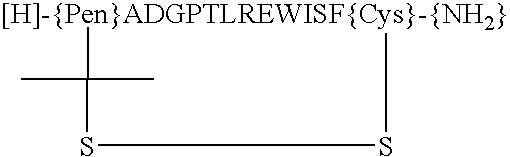Patents
Literature
51 results about "Thrombopoietin receptor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The thrombopoietin receptor also known as the myeloproliferative leukemia protein or CD110 (Cluster of Differentiation 110) is a protein that in humans is encoded by the MPL (myeloproliferative leukemia virus) oncogene.
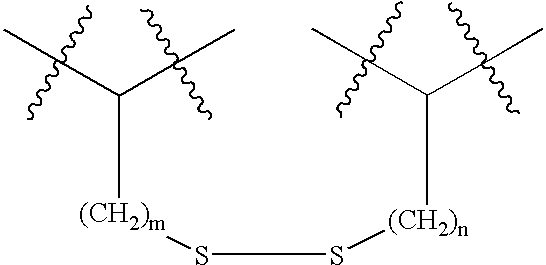
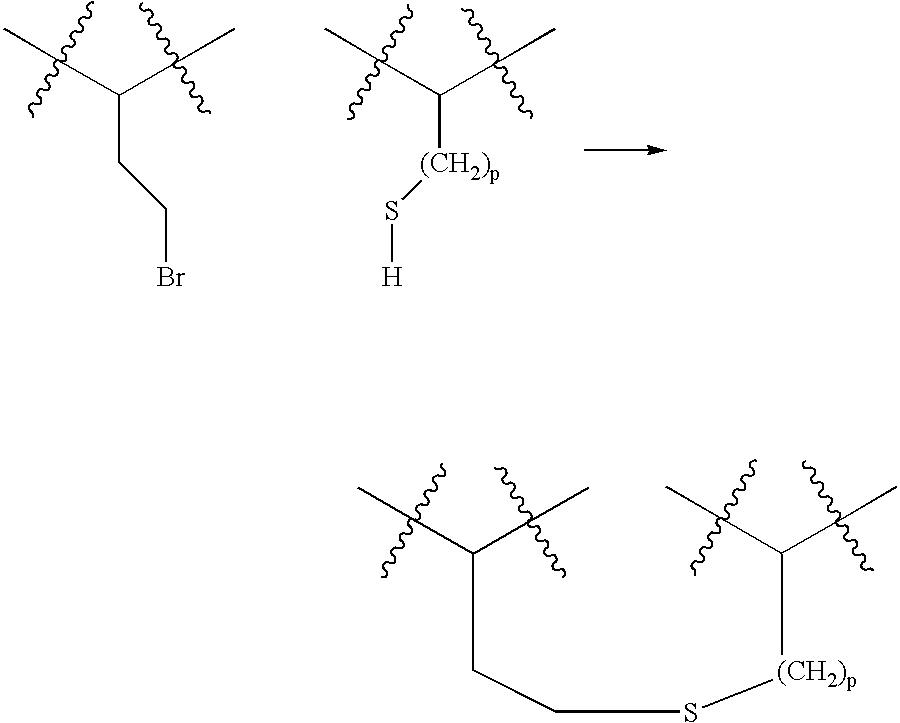
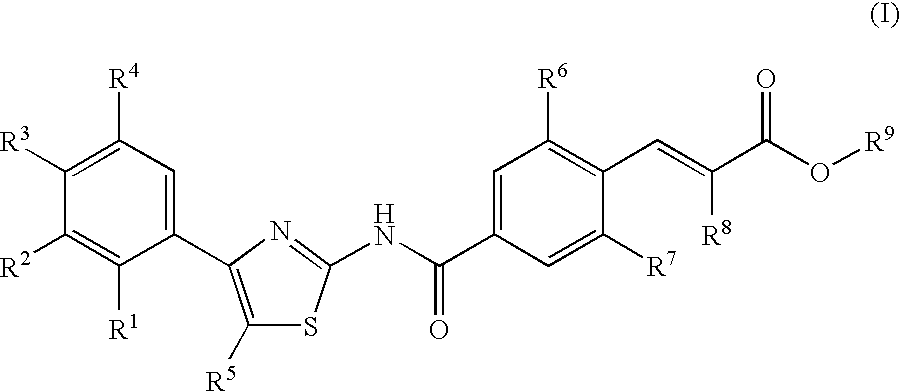
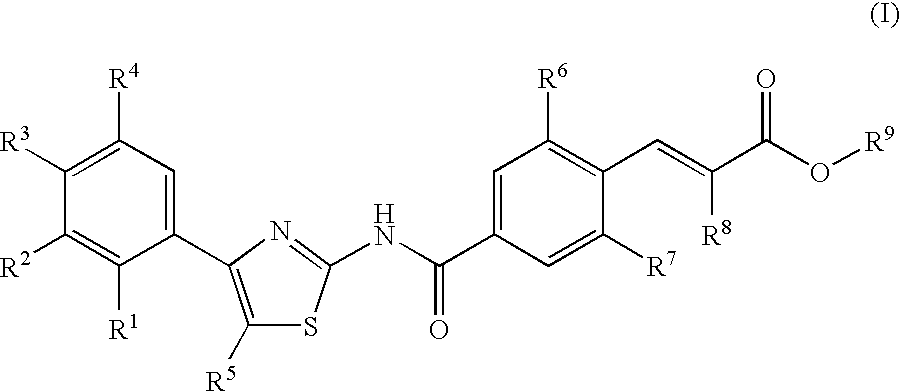
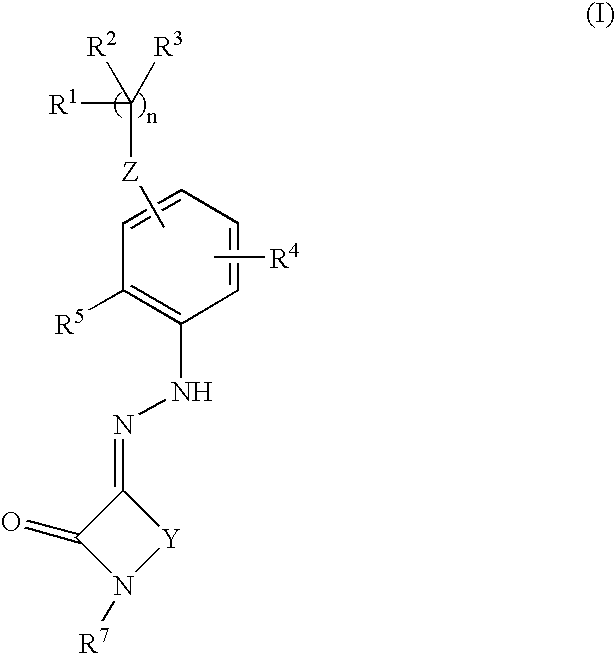
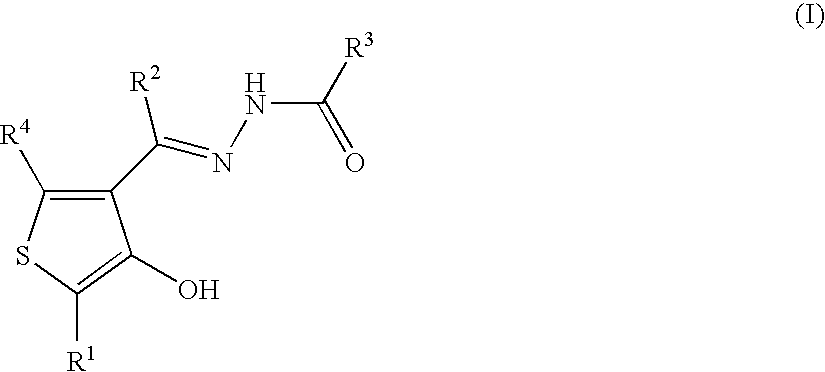
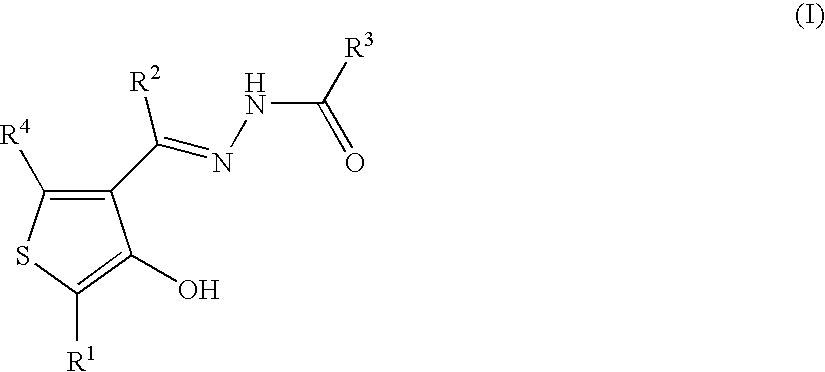
Cyclic compounds exhibiting thrombopoietin receptor agonism
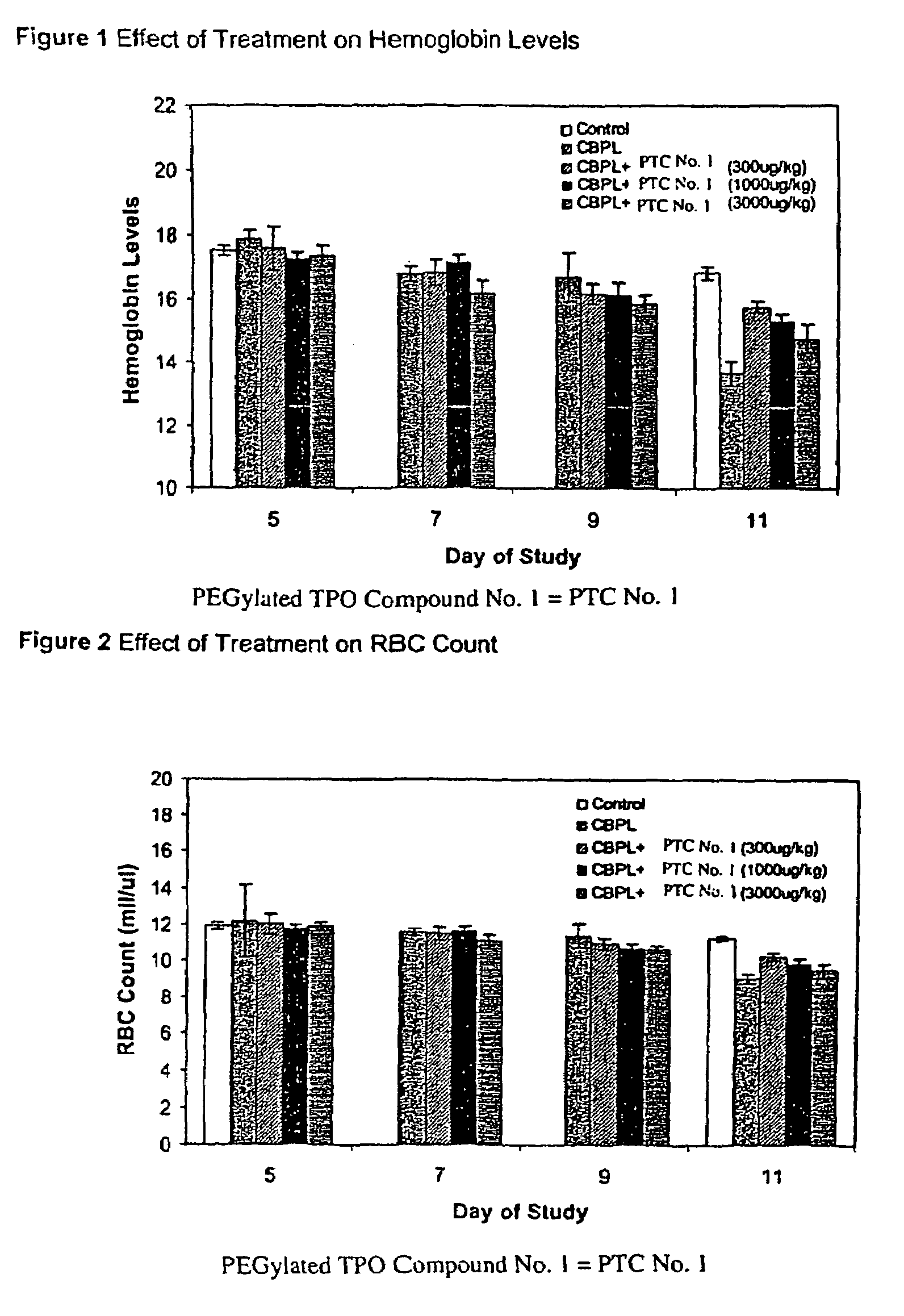
InactiveUS20040082626A1Increase the number ofMaintain normalBiocideOrganic chemistryArylHydrogen atom
Pharmaceutical compositions containing as an active ingredient compounds of the general formula (I), prodrugs of the same, pharmaceutically acceptable salts of both, or solvates of them and exhibiting thrombopoietin receptor agonism: X<1>-Y<1>-Z<1> (I) wherein X<1 >is optionally substituted aryl, optionally substituted heteroaryl or the like; Y<1 >is -NRCO-(CH2)0-2- or the like (wherein Ris hydrogen atom or the like); and Z<1 >is a cyclic group fused the same or different two ring selected from optionally substituted carbocyclic group and optionally substituted heterocyclic group.
Owner:SHIONOGI & CO LTD
Agonist antibody to human thrombopoietin receptor
InactiveUS20100004429A1High activityLow antigenicityThrombopoietinHybrid immunoglobulinsHuman plateletUmbilical cord
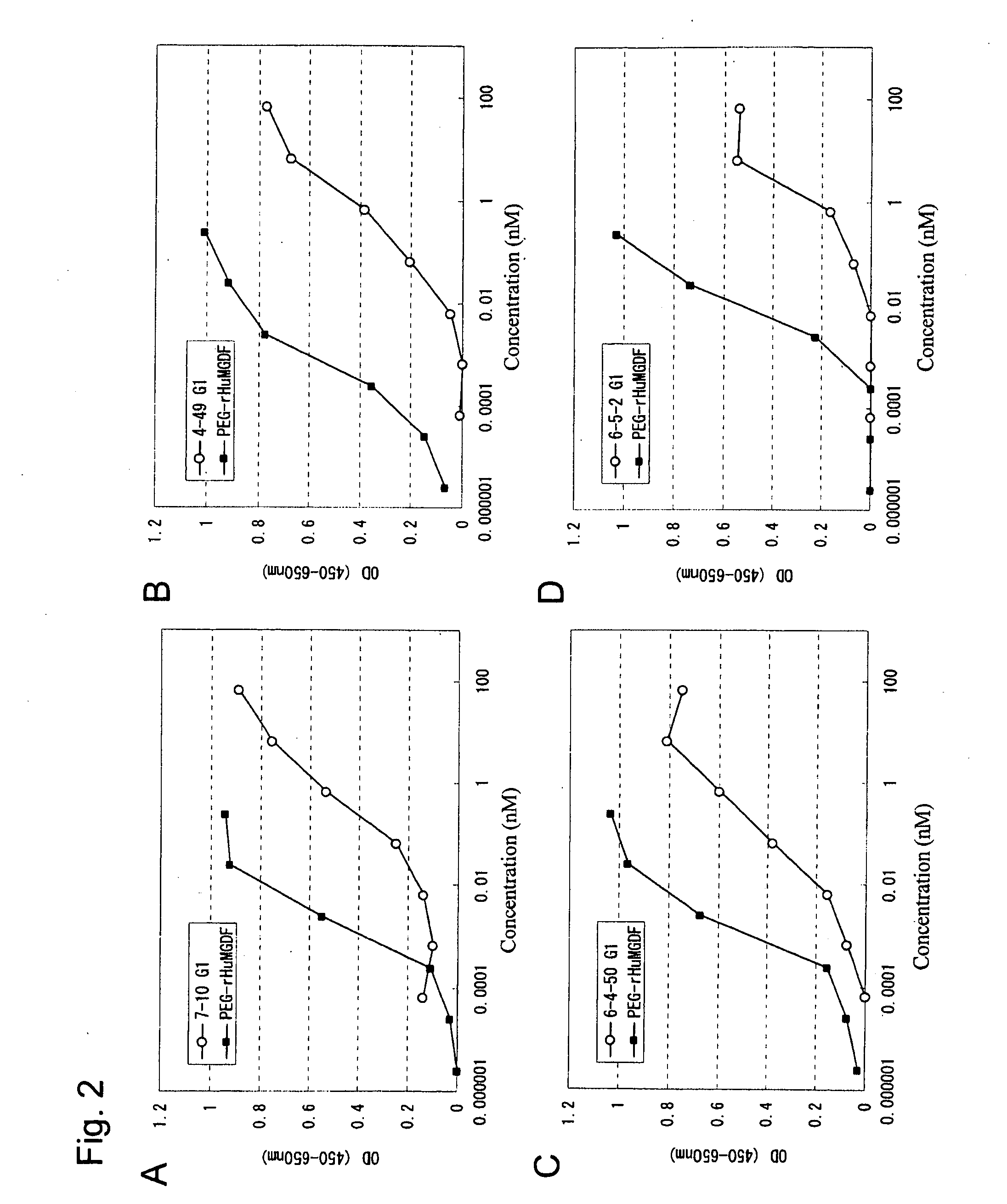
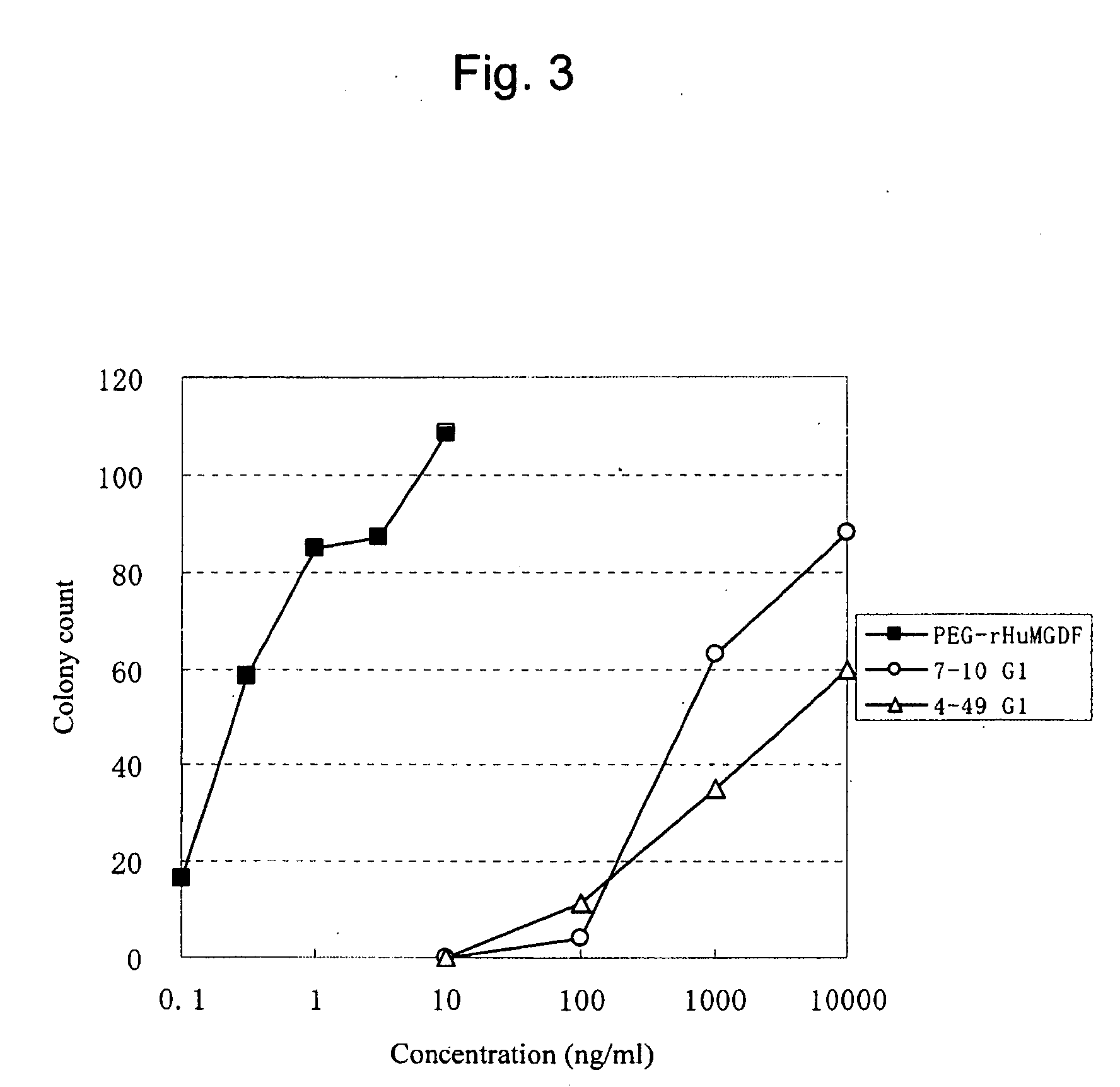
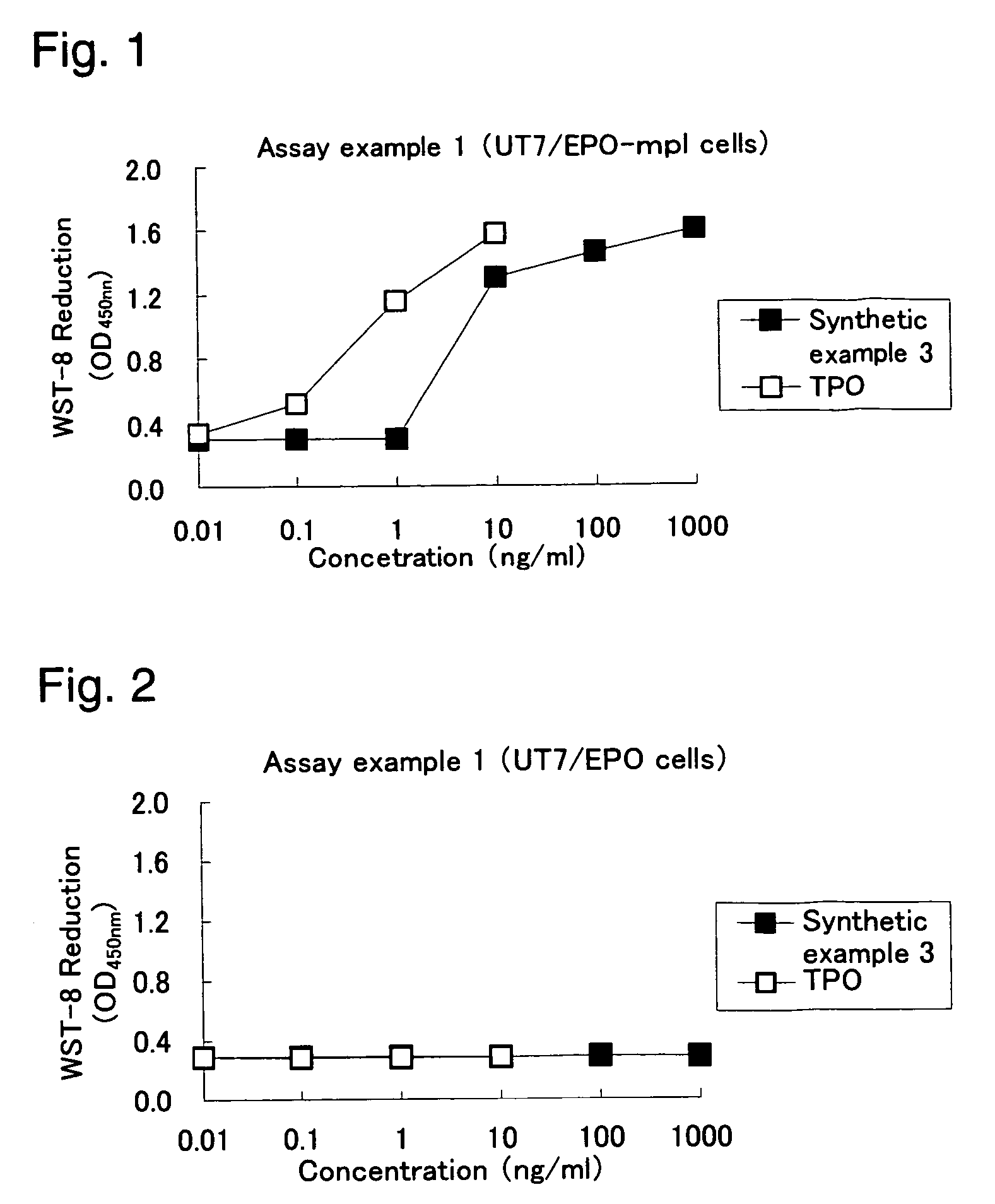
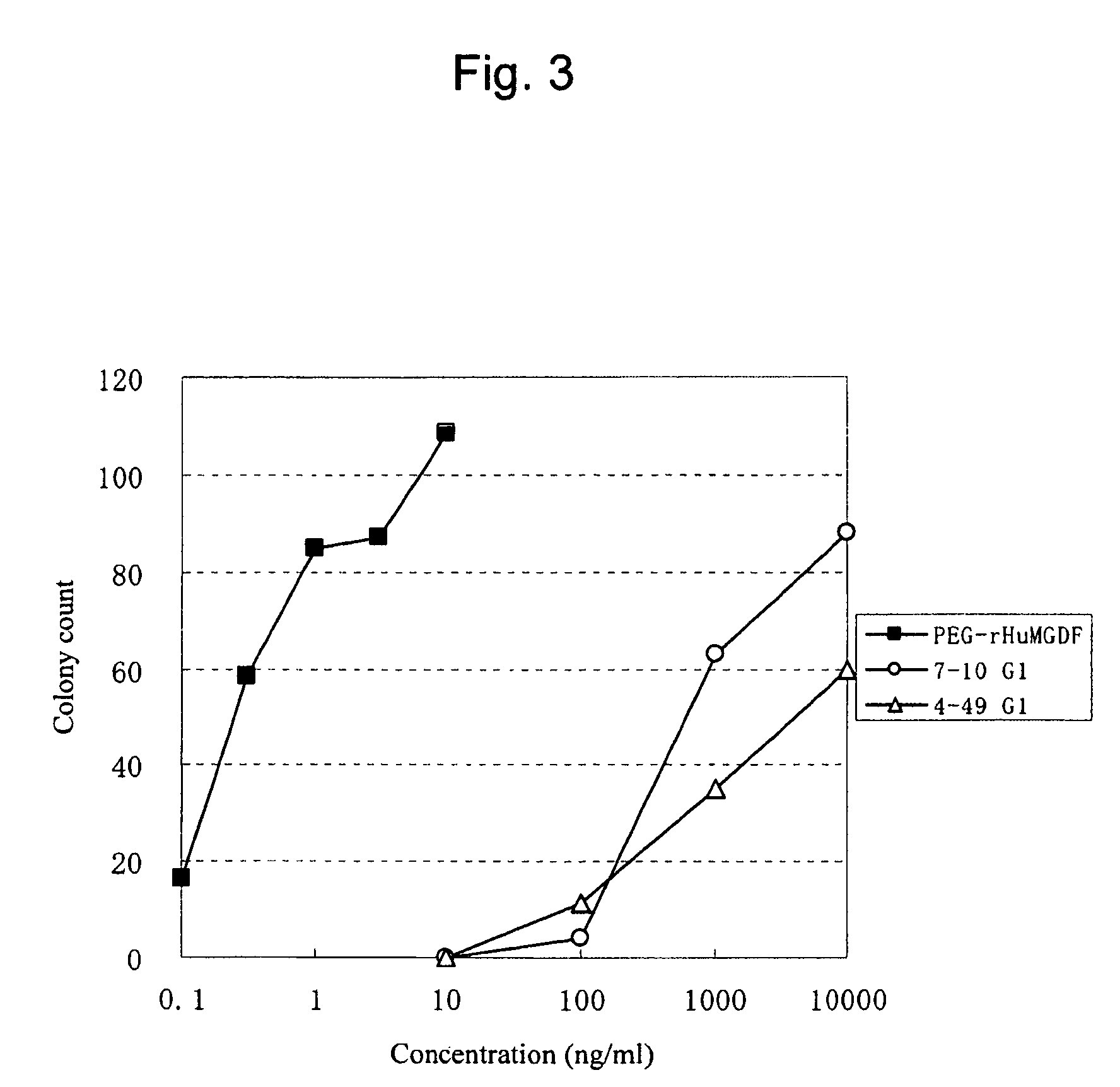
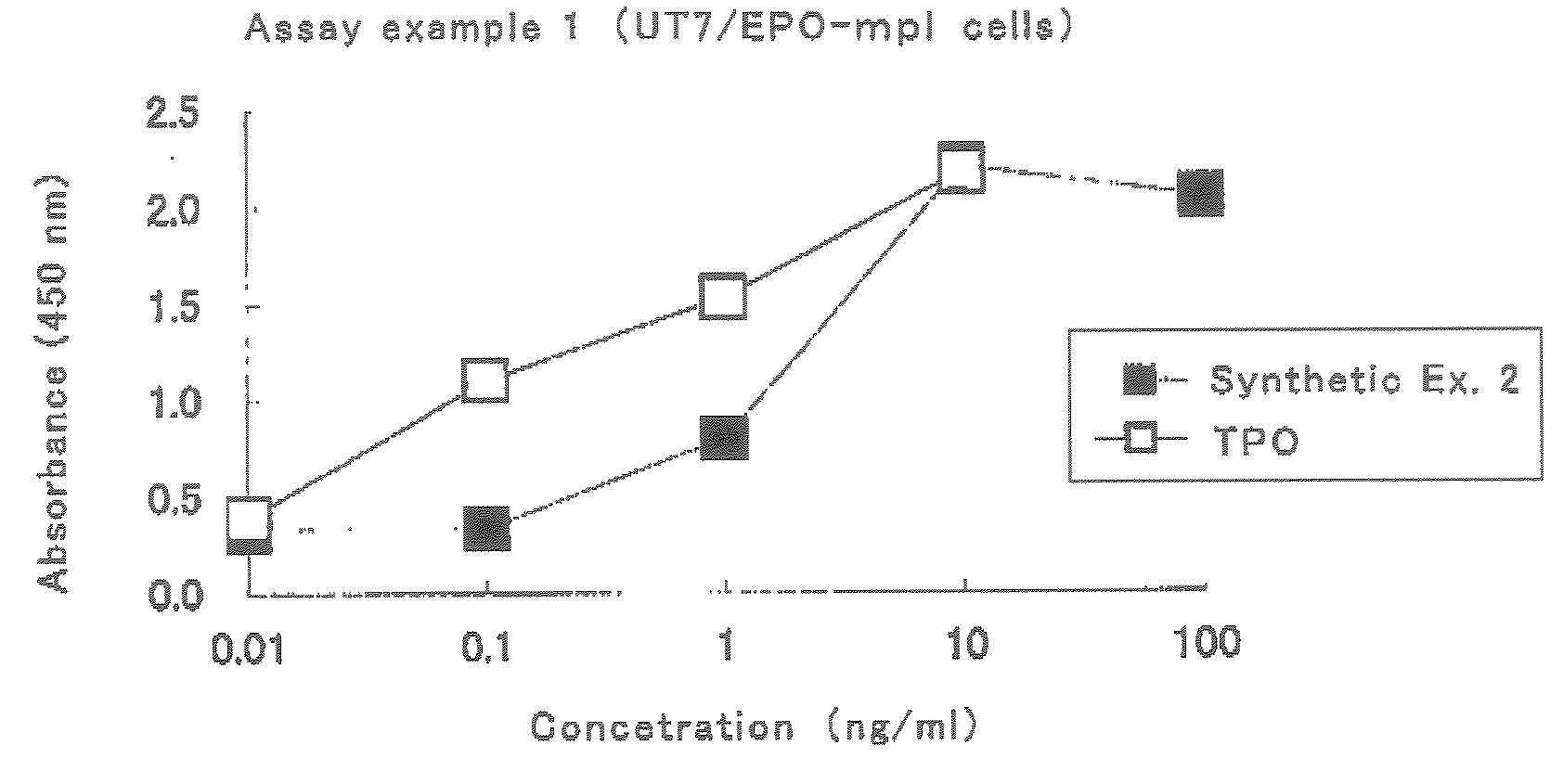
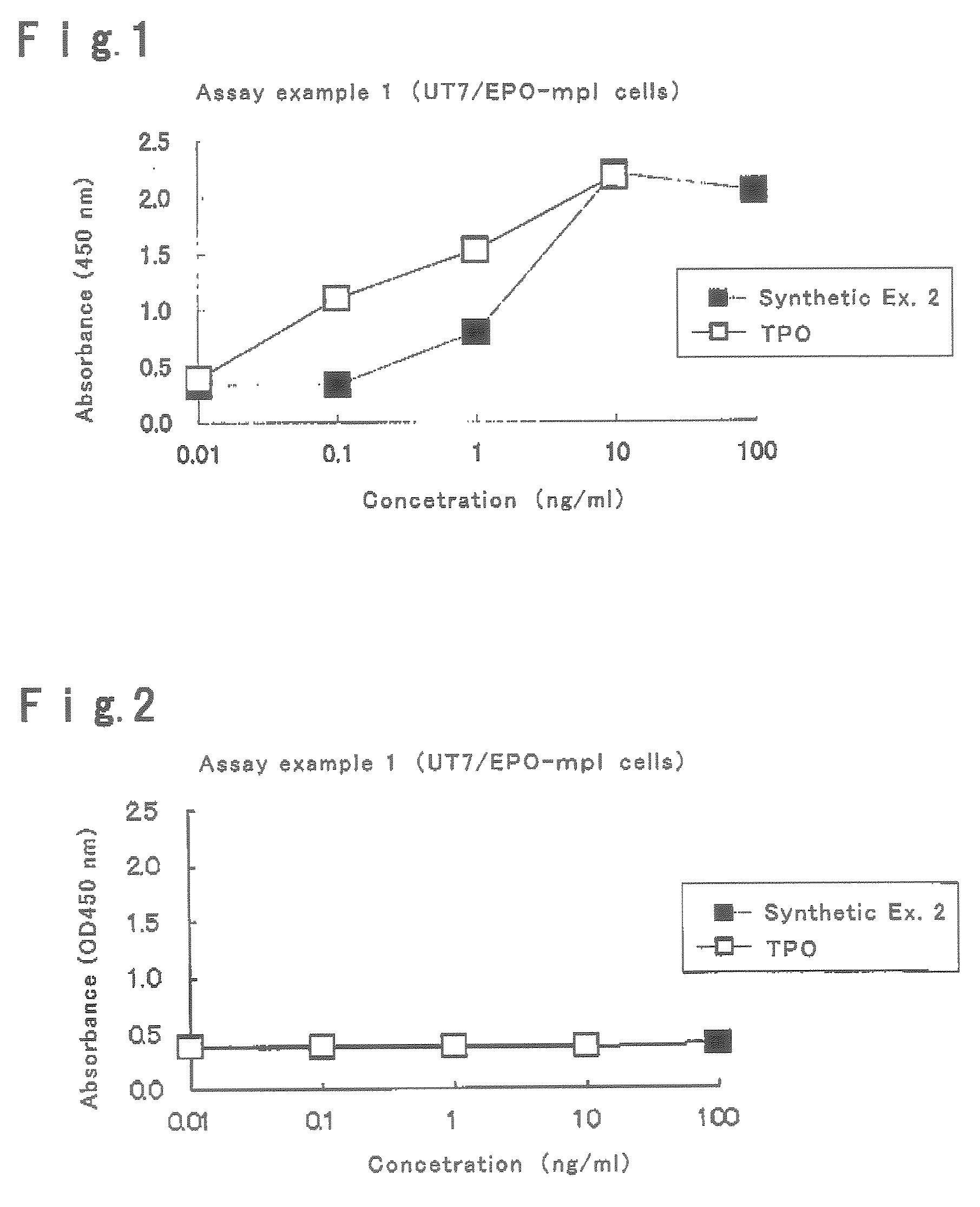
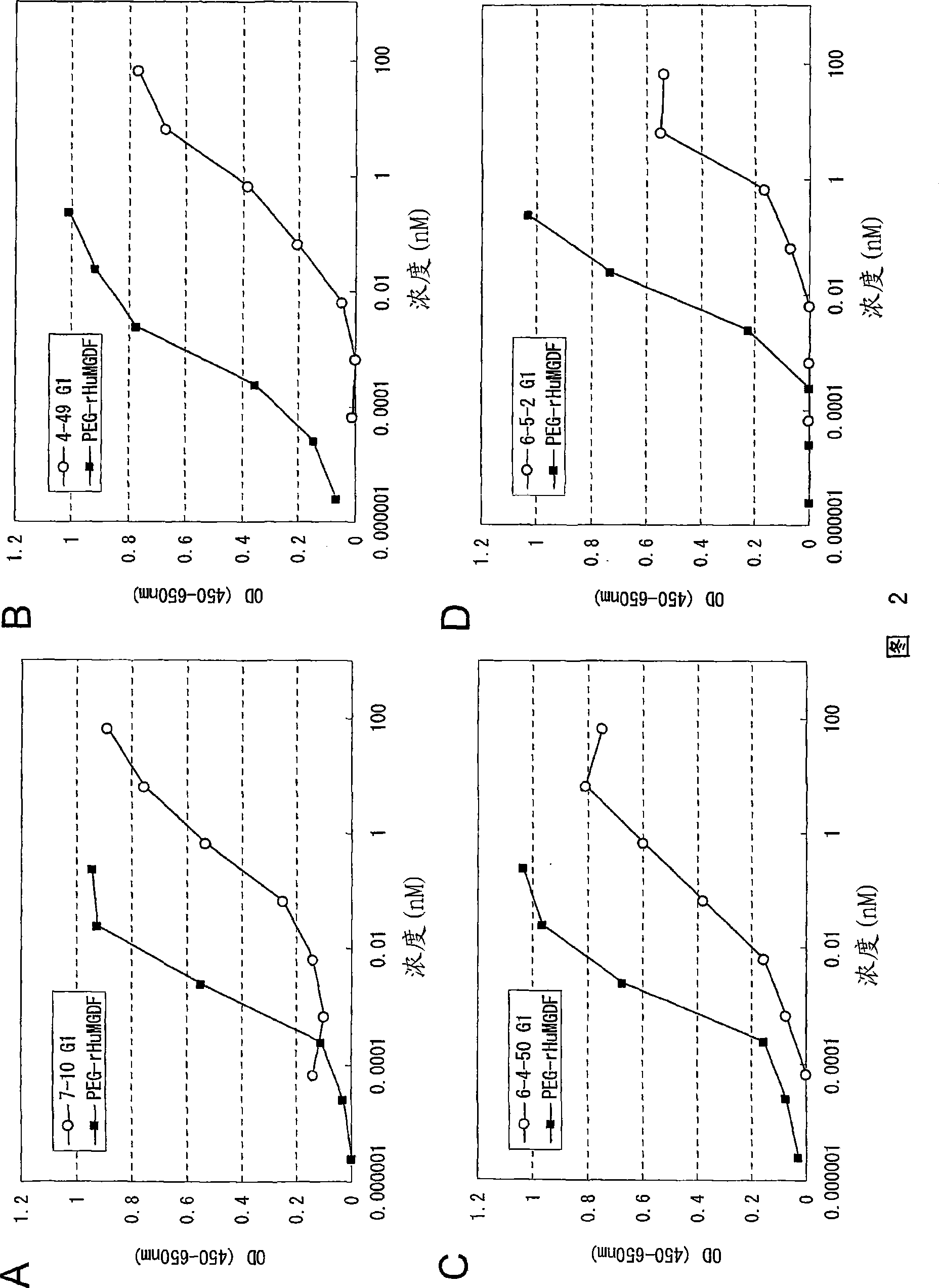
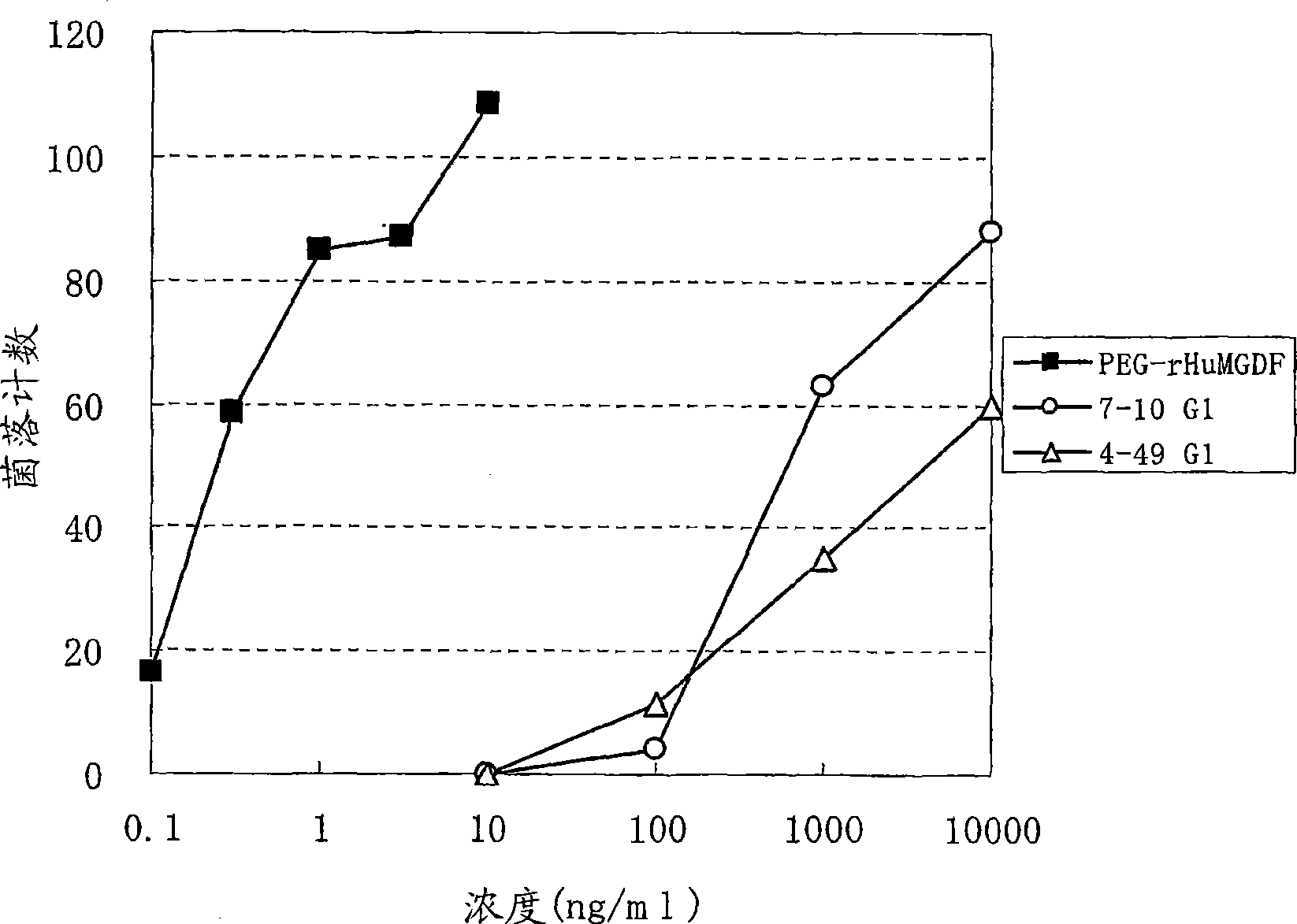
This invention provides an agonist antibody to a human thrombopoietin receptor (alias: human c-Mpl). More particularly, this invention provides an agonist antibody to a human thrombopoietin receptor, wherein the agonist antibody comprises: antibody constant regions comprising (1) amino acid sequences in a heavy chain constant region and a light chain constant region of a human antibody, (2) an amino acid sequence of a heavy chain constant region with a domain substituted between human antibody subclasses, and an amino acid sequence of a light chain constant region of a human antibody, or (3) amino acid sequences comprising a deletion(s), substitution(s), addition(s), or insertion(s) of one or several amino acid residues in the amino acid sequences of (1) or (2) above; and antibody variable regions capable of binding to and activating a human thrombopoietin receptor; and wherein the agonist antibody has the properties: (a) that the antibody induces colony formation at a concentration of 10,000 ng / ml or lower as determined by the CFU-MK colony formation assay using human umbilical-cord-blood-derived CD34+ cells; and (b) that the antibody has a maximal activity at least 50% higher than that of PEG-rHuMGDF and an 50% effective concentration (EC50) of 100 nM or less in the cell proliferation assay using UT7 / TPO cell. Also provided is a pharmaceutical composition for treating thrombocytopenia comprising said antibody.
Owner:KYOWA HAKKO KIRIN CO LTD
Heterocyclic compounds and thrombopoietin receptor activators
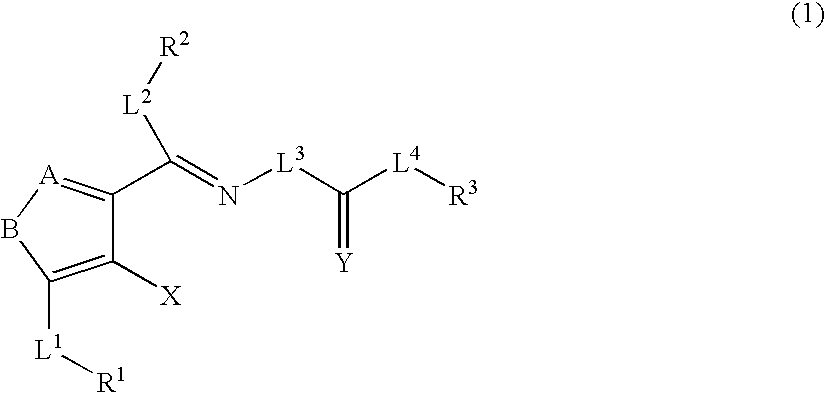
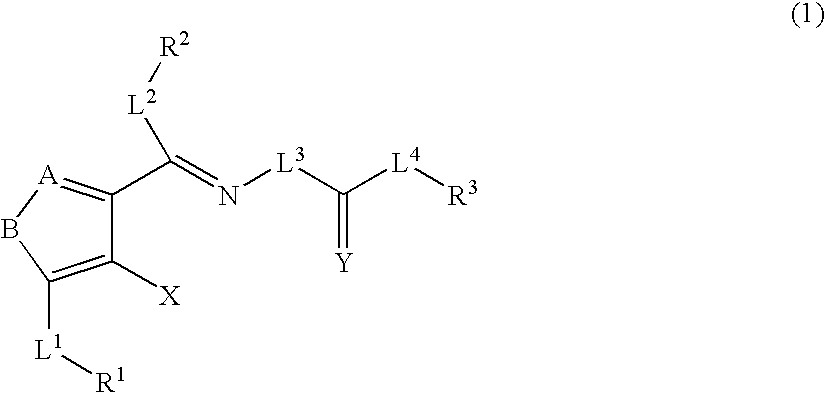
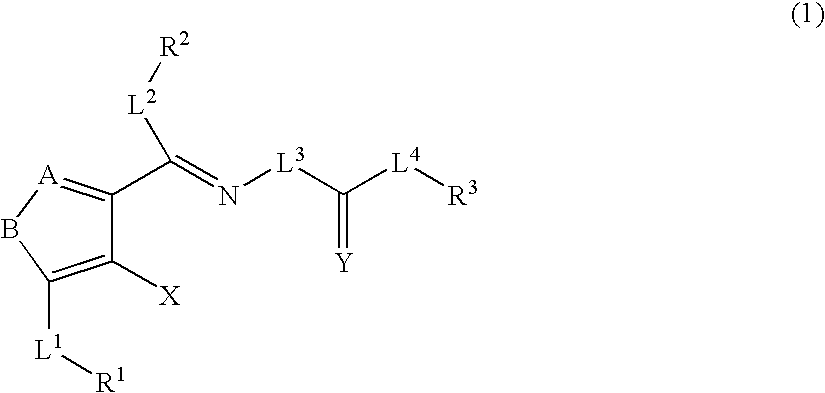
A compound represented by the formula (1): wherein A is a nitrogen atom or CR4, B is an oxygen atom, a sulfur atom or NR9 (provided that when A is a nitrogen atom, B is not NH), R1 is a C2-14 aryl group, L1 is a bond, CR10R11, an oxygen atom, a sulfur atom or NR12, X is OR13 SR13 or NR14NR15, R2 is a hydrogen atom, a formyl group, a C1-10 alkyl group or the like, L2 is a bond or the like, L3 is a bond, CR17R18, an oxygen atom, a sulfur atom or NR19, L4 is a bond, CR20R21, an oxygen atom, a sulfur atom or NR22, Y is an oxygen atom, a sulfur atom or NR23, and R3 is a C2-14 aryl group, a tautomer, prodrug or pharmaceutically acceptable salt of the compound or a solvate thereof.
Owner:NISSAN CHEM IND LTD
Heterocyclic compounds and thrombopoietin receptor activators
Owner:NISSAN CHEM CORP
Ligand having agonistic activity to mutated receptor
InactiveUS20060189794A1X-ray/infra-red processesImmunoglobulins against cell receptors/antigens/surface-determinantsThrombopoiesisThrombopoietin
The present inventors used antibody engineering techniques to prepare functional antibodies that correspond to individual mutations in causative genes of diseases, and discovered that such antibodies enable the treatment of the diseases. Specifically, the inventors succeeded in preparing ligands, particularly minibodies, which have agonistic activity to receptors that have almost completely lost responsiveness to their natural ligands because of gene mutations (for example, a thrombopoietin (TPO) receptor whose reactivity to TPO has been markedly impaired), and which can transduce signals by interacting with these mutant receptors at levels comparable to normal.
Owner:CHUGAI PHARMA CO LTD
Peptides and compounds that bind to a receptor
Peptides and compounds that bind to and activate the thrombopoietin receptor (c-mpl or TPO-R) or otherwise act as a TPO agonist are disclosed.
Owner:JANSSEN PHARMA NV +1
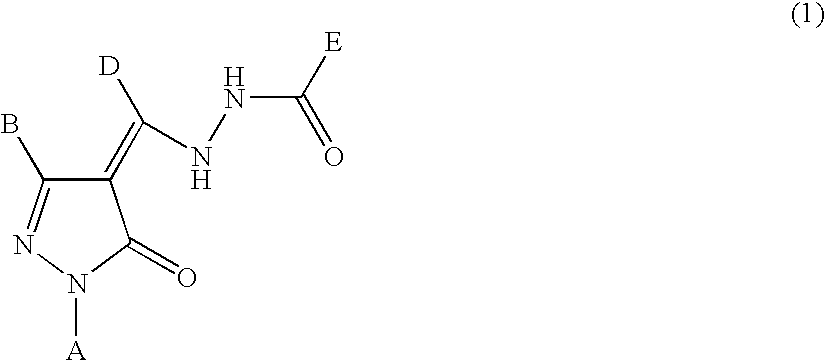
Pyrazolone compounds and thrombopoietin receptor activator
InactiveUS20060069140A1Potent platelet increasing actionHigh affinityBiocideOrganic chemistryBULK ACTIVE INGREDIENTBiological activation
A preventive, therapeutic or improving agent for diseases against which activation of the thrombopoietin receptor is effective or a platelet increasing agent, which contains a thrombopoietin receptor activator represented by the formula (1): wherein A is a C?2-14#191 aryl group, B is a hydrogen atom, a C?1-6#191 alkyl group, a C?1-3#191 alkyl group substituted with one or more fluorine atoms or a C?2-14#191 aryl group, D is a hydrogen atom, a C?1-6#191 alkyl group, a C?1-3#191 alkyl group substituted with one or more fluorine atoms or a C?2-14#191 aryl group, and E is a C?2-14#191 aryl group, a tautomer, prodrug or pharmaceutically acceptable salt of the activator or a solvate thereof, as an active ingredient.
Owner:NISSAN CHEM IND LTD
Use of TPO peptide compounds and pharmaceutical compostions in the treatment of anemia
ActiveUS20080119384A1Improve bindingEliminate side effectsThrombopoietinPeptide/protein ingredientsAgonistPeptide
Peptide compounds that bind to and activate the thrombopoietin receptor (c-mpl or TPO-R) or otherwise act as a TPO agonist are disclosed.
Owner:JANSSEN PHARMA NV
Cyclic compounds exhibiting thrombopoietin receptor agonism
Owner:SHIONOGI & CO LTD
Peptides and compounds that bind to a receptor
ActiveUS7576056B2Eliminate side effectsImprove bindingThrombopoietinPeptide/protein ingredientsAgonistEphA Receptors
Peptide compounds that bind to and activate the thrombopoietin receptor (c-mpl or TPO-R) or otherwise act as a TPO agonist are disclosed.
Owner:JANSSEN PHARMA NV +1
Agonist antibody to human thrombopoietin receptor
This invention provides an agonist antibody to a human thrombopoietin receptor (alias: human c-Mpl). More particularly, this invention provides an agonist antibody to a human thrombopoietin receptor, wherein the agonist antibody comprises: antibody constant regions comprising (1) amino acid sequences in a heavy chain constant region and a light chain constant region of a human antibody, (2) an amino acid sequence of a heavy chain constant region with a domain substituted between human antibody subclasses, and an amino acid sequence of a light chain constant region of a human antibody, or (3) amino acid sequences comprising a deletion(s), substitution(s), addition(s), or insertion(s) of one or several amino acid residues in the amino acid sequences of (1) or (2) above; and antibody variable regions capable of binding to and activating a human thrombopoietin receptor; and wherein the agonist antibody has the properties: (a) that the antibody induces colony formation at a concentration of 10,000 ng / ml or lower as determined by the CFU-MK colony formation assay using human umbilical-cord-blood-derived CD34+ cells; and (b) that the antibody has a maximal activity at least 50% higher than that of PEG-rHuMGDF and an 50% effective concentration (EC50) of 100 nM or less in the cell proliferation assay using UT7 / TPO cell. Also provided is a pharmaceutical composition for treating thrombocytopenia comprising said antibody.
Owner:KYOWA HAKKO KIRIN CO LTD
Peptides and compounds that bind to a receptor
Described are peptides and peptide mimetics that bind to and activate the thrombopoietin receptor. Such peptides and peptide mimetics are useful in methods for treating hematological disorders and particularly, thrombocytopenia resulting from chemotherapy, radiation therapy, or bone marrow transfusions as well as in diagnostic methods employing labeled peptides and peptide mimetics.
Owner:SMITHKLINE BECKMAN CORP
Compounds exhibiting thrombopoietin receptor agonism
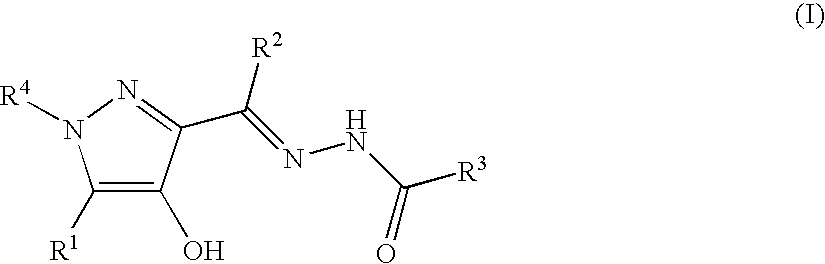
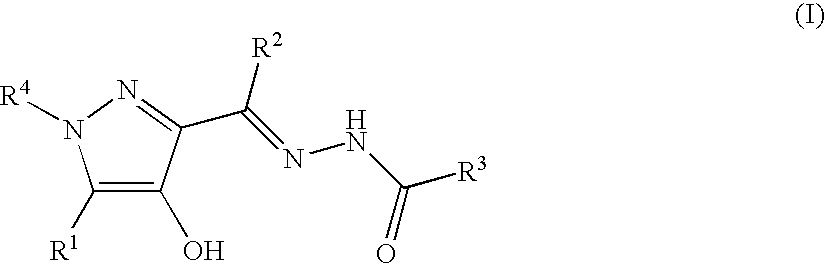
A compound represented by the general formula (I): wherein R1 is a hydrogen atom, a halogen atom, or the like; R2, R3, and R4 are each independently a hydrogen atom, a halogen atom, C1-C15 alkyl optionally substituted with one or more C1-C12 alkyloxy or the like, or the like; R5 is a hydrogen atom or the like; R6 and R7 are a hydrogen atom or the like; R8 is C1-C3 alkyl or the like; R9 is a hydrogen atom or the like), a prodrug, a pharmaceutically acceptable salt, or solvate thereof.
Owner:SHIONOGI & CO LTD
TPO peptide compounds for treatment of anemia
ActiveUS7615533B2Improve bindingEliminate side effectsThrombopoietinPeptide/protein ingredientsAgonistAnemia
Peptide compounds that bind to and activate the thrombopoietin receptor (c-mpl or TPO-R) or otherwise act as a TPO agonist are disclosed.
Owner:JANSSEN PHARMA NV
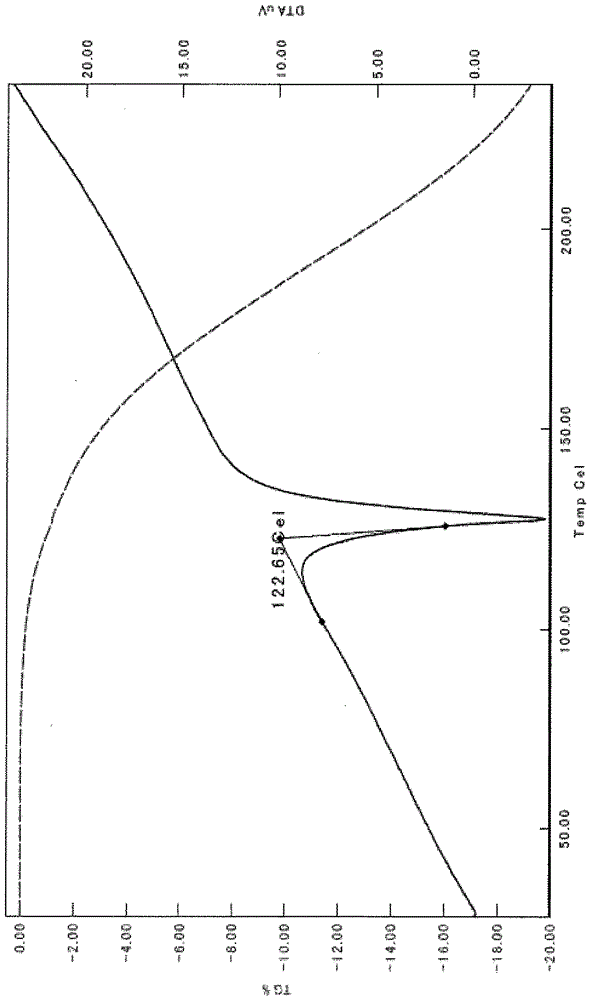
Methods respectively for producing optically active compound having agonistic activity on thrombopoietin receptors and intermediate of said compound
InactiveCN105992761AHigh chemical purityOrganic compound preparationCarboxylic acid esters preparationBiochemistryPerylene derivatives
The present invention relates to: a method for producing an intermediate having high chemical purity and / or high optical purity; a crystal of an intermediate having high chemical purity and / or optical purity; a method for producing an intermediate, in which two reactions can be carried out sequentially substantially in a single step; and a method for producing an optically active 1,3-thiazole derivative having an agonistic activity on thrombopoietin receptors.
Owner:SHIONOGI & CO LTD
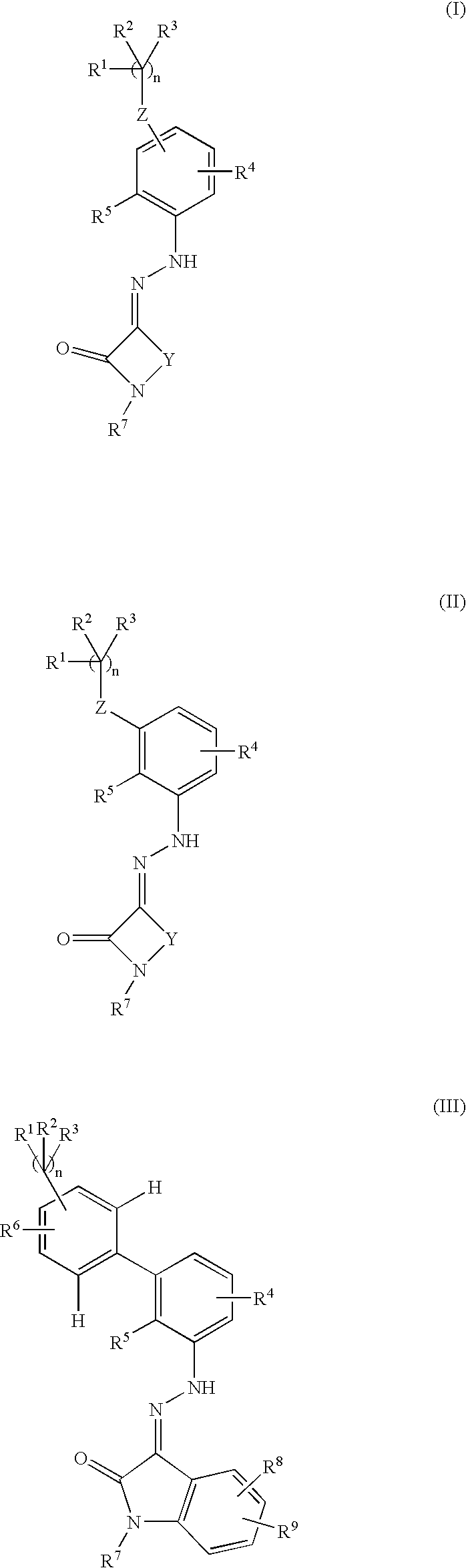
Thrombopoietin activity modulating compounds and methods
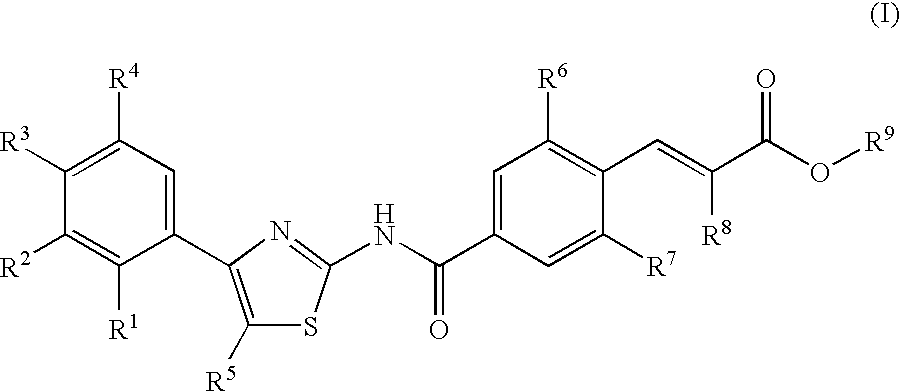
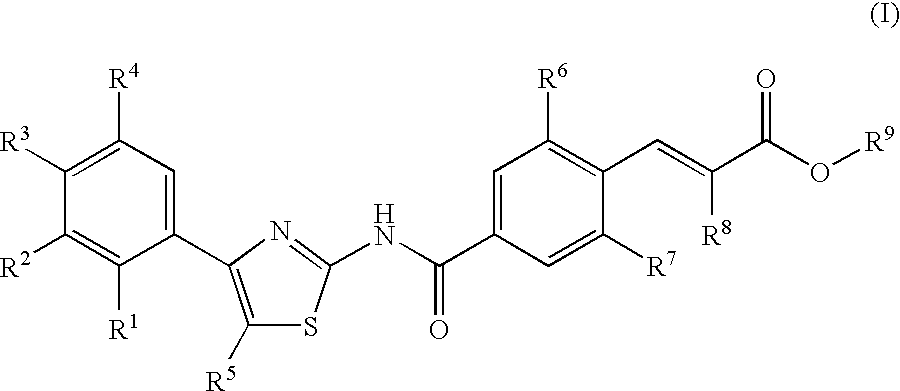
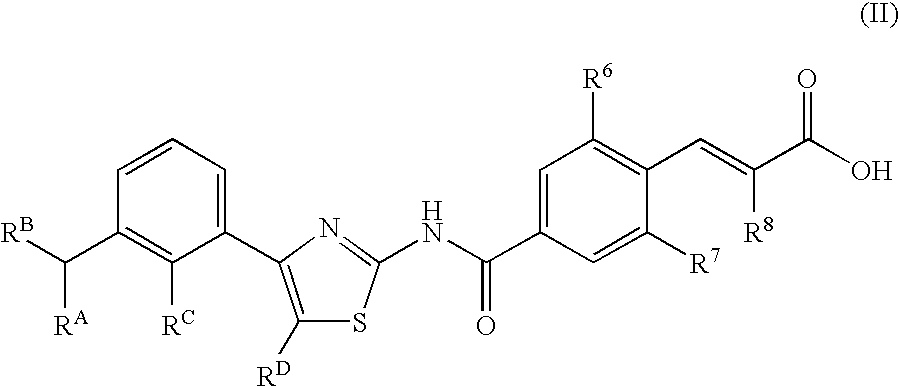
Disclosed herein are compounds of Formula (I), (II), and (III)pharmaceutical compositions comprising the same, methods of modulating the activity a thrombopoietin receptor using the same, methods of identifying compounds as thrombopoietin receptor modulators, and methods of treating disease by administering a compound of the invention to a patient in need thereof.
Owner:LIGAND PHARMA INC
Amide compound and thrombopoietin receptor activator
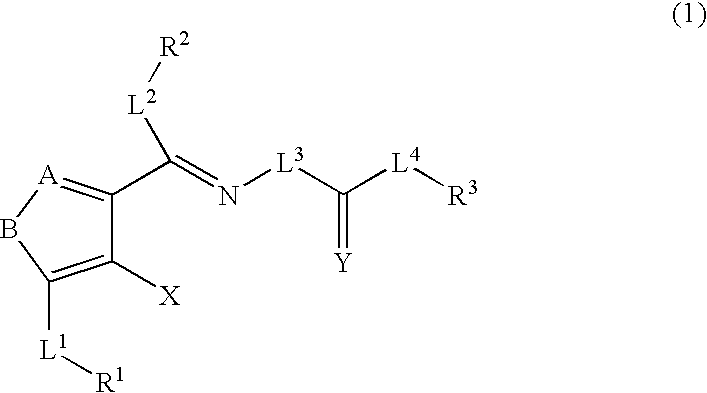
The present invention provides compounds useful for prevention, treatment or alleviation of diseases against which activation of the thrombopoietin receptor is effective.A compound represented by the formula (1):wherein A, B, R1, L1, R2, L2, L3, Y, L4, R3 and X are the same as defined in the description, a tautomer, prodrug or pharmaceutically acceptable salt of the compound or a solvate thereof.
Owner:NISSAN CHEM IND LTD
Peptides and compounds that bind to a receptor
ActiveUS7723295B2Eliminate side effectsImprove bindingAntibacterial agentsPeptide-nucleic acidsAgonistEphA Receptors
Peptide compounds that bind to and activate the thrombopoietin receptor (c-mpl or TPO-R) or otherwise act as a TPO agonist are disclosed.
Owner:JANSSEN PHARMA NV +1
Pharmaceutical composition containing a compound having a thrombopoietin receptor agonistic activity
InactiveUS20150148385A1Increase plateletReduce riskBiocideOrganic active ingredientsIncreased platelet countEndocrinology
The present inventor has found out that the following criteria enable to ensure an effect for increasing the platelet count while preventing an excessive increase in the platelet count;“when the platelet count has increased by a certain amount and reached to a sufficient level of the platelet count during administration of a pharmaceutical composition containing a compound having a thrombopoietin receptor agonistic activity, administration of the pharmaceutical composition is discontinued thereafter”.
Owner:SHIONOGI & CO LTD
Thrombopoetin receptor activator and process for producing the same
A preventive, therapeutic or improving agent for diseases against which activation of the thrombopoietin receptor is effective or a platelet increasing agent, which contains a thrombopoietin receptor activator represented by the formula (1): [wherein each of R1 and R3 is independently a hydrogen atom, SO3H, a C1-6 alkyl group, a C1-6 alkylcarbonyl group or a C6-18 arylcarbonyl group (the C1-6 alkyl group, the C1-6 alkylcarbonyl group and the C6-18 arylcarbonyl group may be optionally substituted with a halogen atom, a hydroxyl group, a C2-6 alkenyl group, a C1-6 alkoxy group, a C1-6 alkoxycarbonyl group, a C6-18 aryl group, a 2-pyridyl group, a 3-pyridyl group, a 4-pyridyl group, a 2-furanyl group, a 3-furanyl group, a 2-thienyl group, a 3-thienyl group or NR9R10) , and each of R2, R4 and Ra is independently a hydrogen atom, a hydroxyl group or a C1-6 alkoxy group].
Owner:NISSAN CHEM IND LTD
Thiophene compounds and thrombopoietin receptor activators
InactiveUS20090118500A1Potent platelet increasing actionPromote absorptionBiocideOrganic chemistryThrombopoietin receptorProdrug
Owner:NISSAN CHEM IND LTD
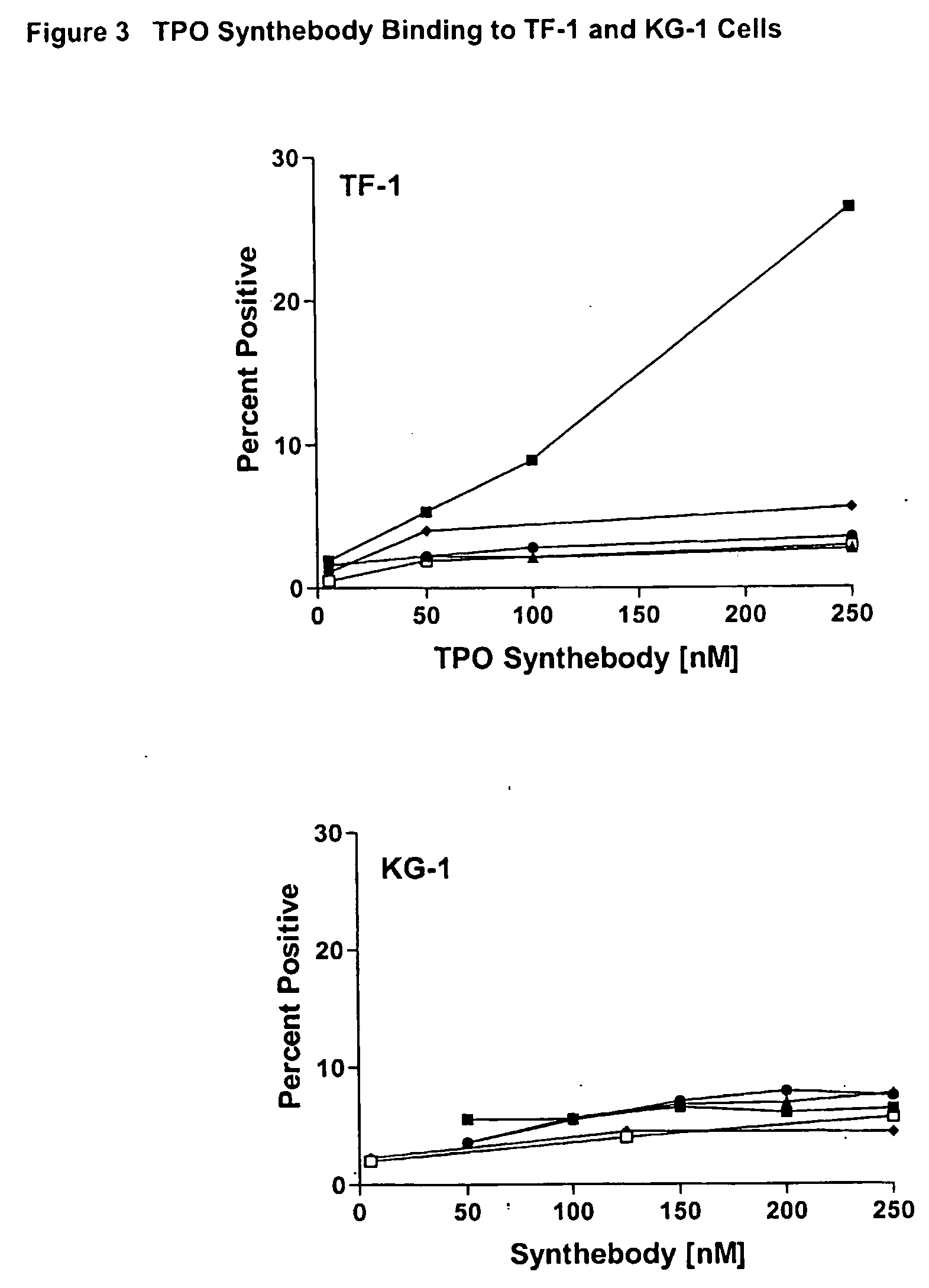
Thrombopoietin(tpo) synthebody for stimulation of platelet production
InactiveUS20040136980A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsProgenitorHematopoietic cell
The present invention relates to a synthetic variable region of an immunoglobin construct which contains in at least one of its CDRs a sequence of thrombopoietin, e.g., IEGPTLRQWLAARA or its derivatives. This construct can efficiently bind and activate a thrombopoientin receptor (MPL) leading to stimulation of proliferation, growth or differentiation or modulation of apoptosis of hematopoietic cells, especially platelet progenitor cells. The invention further relates to the use of the synthebody to treat hematopoietic or immune disorders, and particularly thrombocytopenia resulting from chemotherapy, radiation therapy, or bone marrow transfusions.
Owner:EURO-CELTIQUE SA
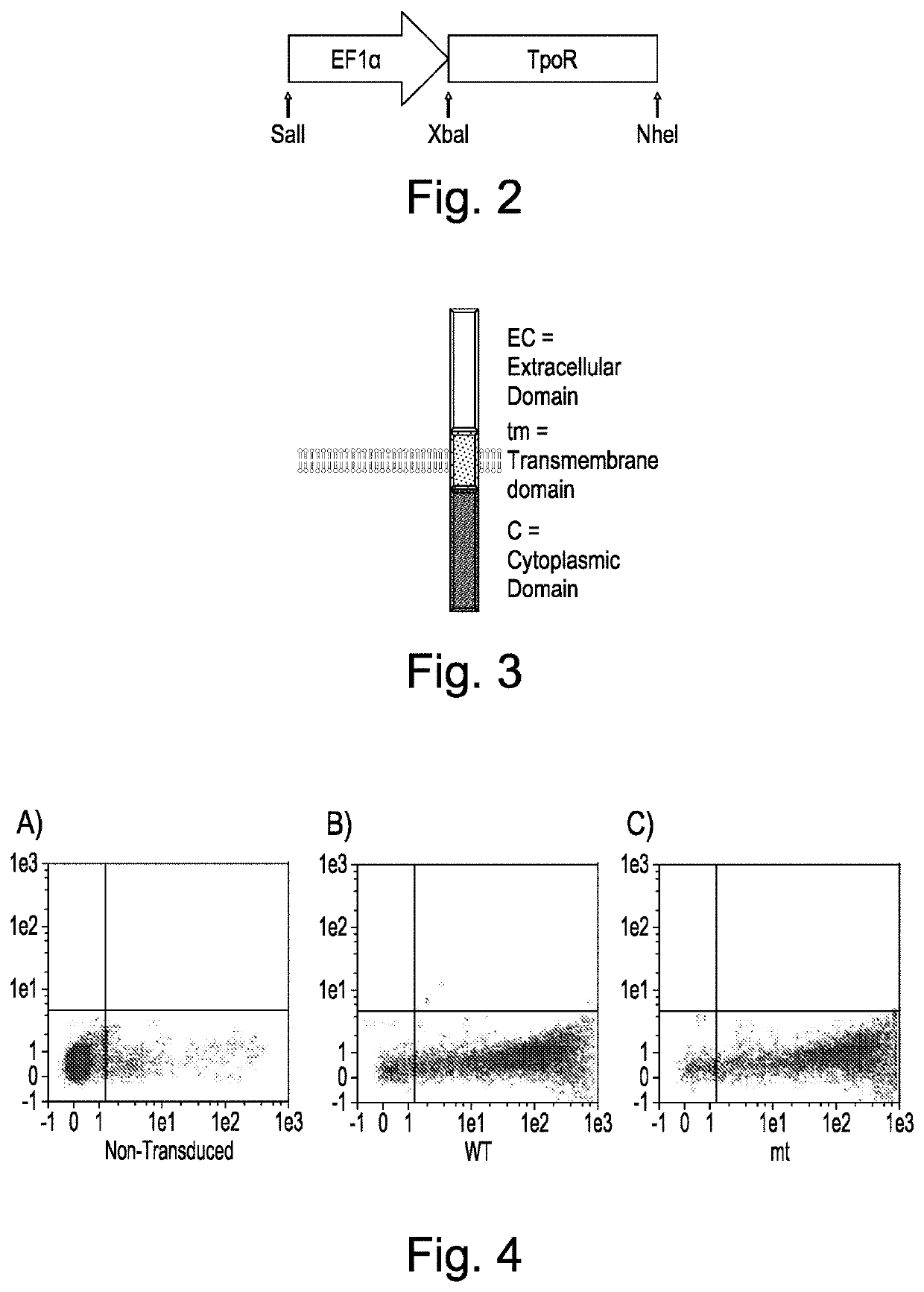
Cells expressing recombinant growth factor receptors
ActiveUS20200263130A1Augment lymphocyte expansionInduced proliferationPolypeptide with localisation/targeting motifOrganic active ingredientsAdoptive cellular therapyNatural Killer Cell Inhibitory Receptors
The present invention discloses cell lines and recombinant growth factor receptors useful in adoptive cell therapy (ACT), wherein the recombinant growth factor receptor can act as a molecular switch enabling cells expressing the rGFR protein to be expanded in-vitro or in- vivo. Thus the invention provides a T or NK cell, comprising a recombinant growth factor receptor (rGFR) comprising: (i) an extracellular (EC) domain; (ii) a thrombopoietin receptor transmembrane (TM) domain; and (iii) a growth factor receptor intracellular (IC) domain.
Owner:INSTIL BIO UK LTD
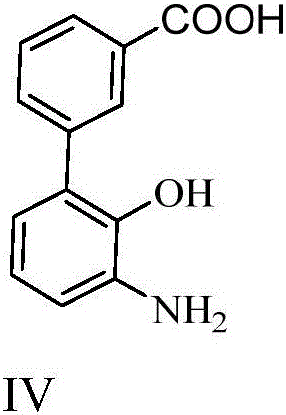
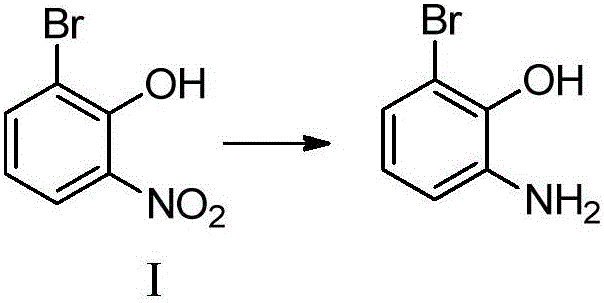
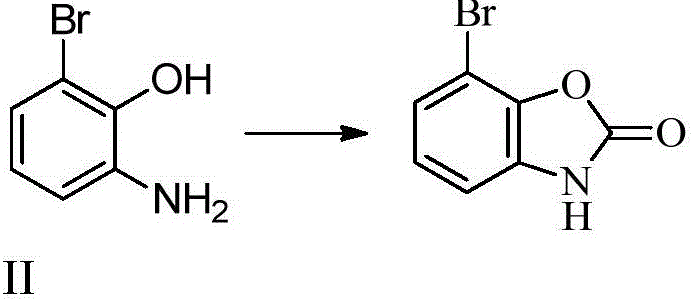
Synthesizing method for 3'-amino-2'-hydroxy biphenyl-3-carboxylic acid
ActiveCN105801444AMethod raw materials are readily availableRaw materials are easy to getOrganic compound preparationAmino-carboxyl compound preparationEltrombopag OlamineBromine
The invention discloses a synthesizing method for 3'-amino-2'-hydroxy biphenyl-3-carboxylic acid.2-bromine-6-nitrophenol serves as a staring material, 2-bromine-6-aminophenol is obtained through a reduction reaction, ring formation and Suzuki coupling are carried out, a hydrolysis reaction is carried out, and 3'-amino-2'-hydroxy biphenyl-3-carboxylic acid is obtained. According to the method, raw materials are easy to obtain, the path is short, and the method is novel, low in cost, high in yield and environmentally friendly. Tests show that the obtained product is reliable in quality, stable in performance and capable of being further used for preparing a thrombopoietin receptor stimulant, namely, eltrombopag olamine.
Owner:启东东岳药业有限公司 +1
Agonistic antibody directed against human thrombopoietin receptor
The present invention discloses an agonistic antibody directed against human thrombopoietin receptor (also referred to as 'human c-Mpl). Specifically, the antibody has a constant region having a set of amino acid sequences selected from the following items (1) to (3): (1) amino acid sequences for a heavy-chain constant region and a light-chain constant region of a human antibody; (2) an amino acid sequence for a human antibody heavy-chain constant region in which the domain is replaced by one of other human antibody subclass and an amino acid sequence for a human antibody light-chain constant region; and (3) a set of amino acid sequences having the deletion, substitution, addition or insertion of one or several amino acid residues in each of the amino acid sequences shown in (1) and (2), and the antibody has a variable region capable of binding to a human thrombopoietin receptor to activate the receptor. The antibody also has the following properties: (a) the antibody can induce the formation of a colony at a concentration of 10,000 ng / mL or less in the CFU-MK colony formation assay using a human umbilical cord blood CD34+ cell; and (b) the antibody has the maximum activity higher than that of PEG-rHuMGDF by 50% or more and a 50% effective concentration (EC50) of 100 nM or less in the cell growth assay using an UT7 / TPO cell. Also disclosed is a pharmaceutical composition for the treatment of thrombocytopenia, which comprises the antibody.
Owner:KIRIN PHARMA
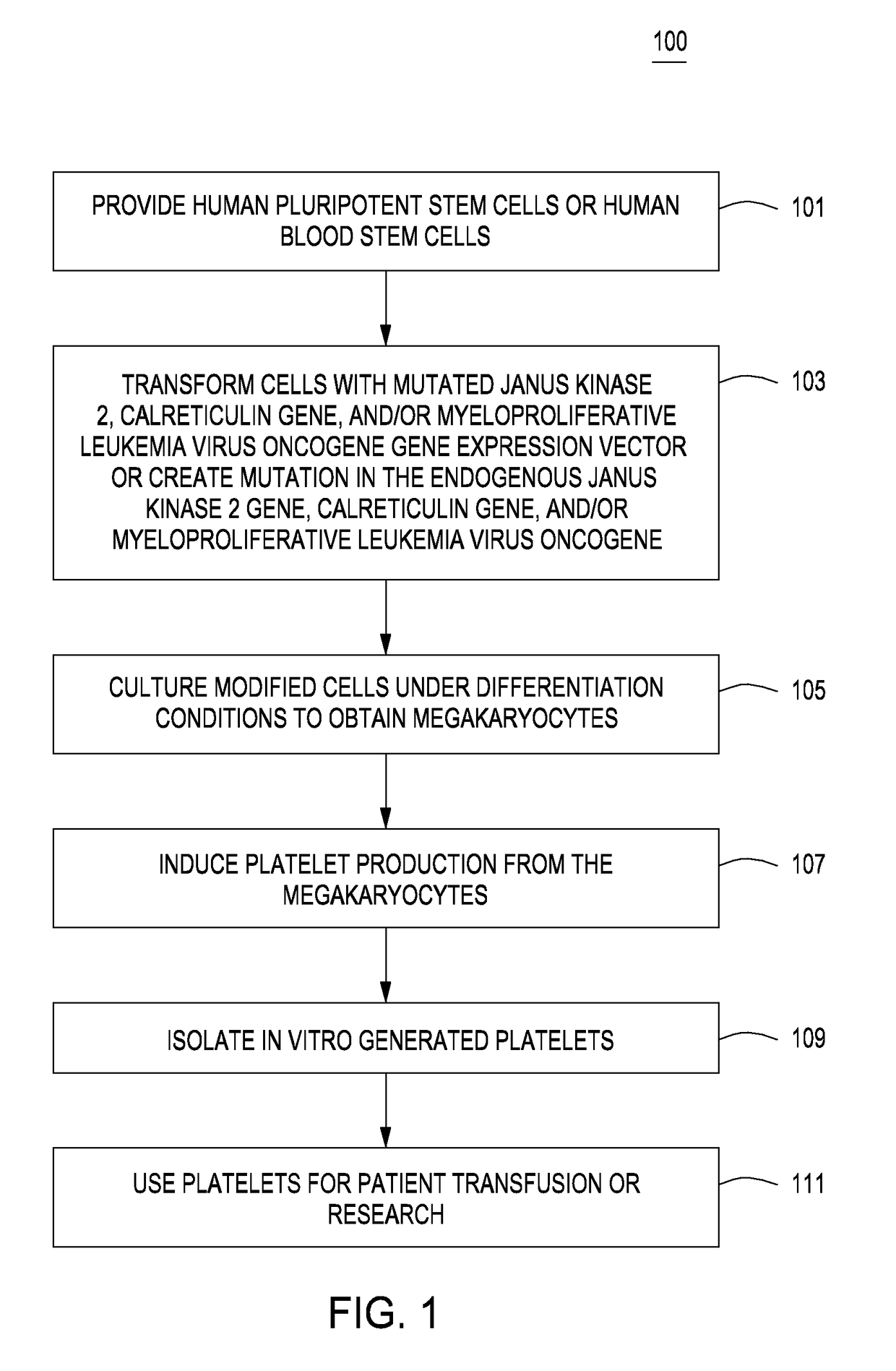
Methods for in vitro production of platelets and compositions and uses thereof
Owner:LOH JEFFREY THOMAS
Pyrazole compounds and thrombopoietin receptor activators
ActiveUS20090131676A1Potent platelet increasing actionPromote absorptionBiocideOrganic active ingredientsPyrazole CompoundThrombopoietin receptor
Owner:NISSAN CHEM IND LTD
Compositions and methods for treating immune thrombocytopenia
PendingUS20200024344A1Raise countImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAgonistImmune thrombocytopenia
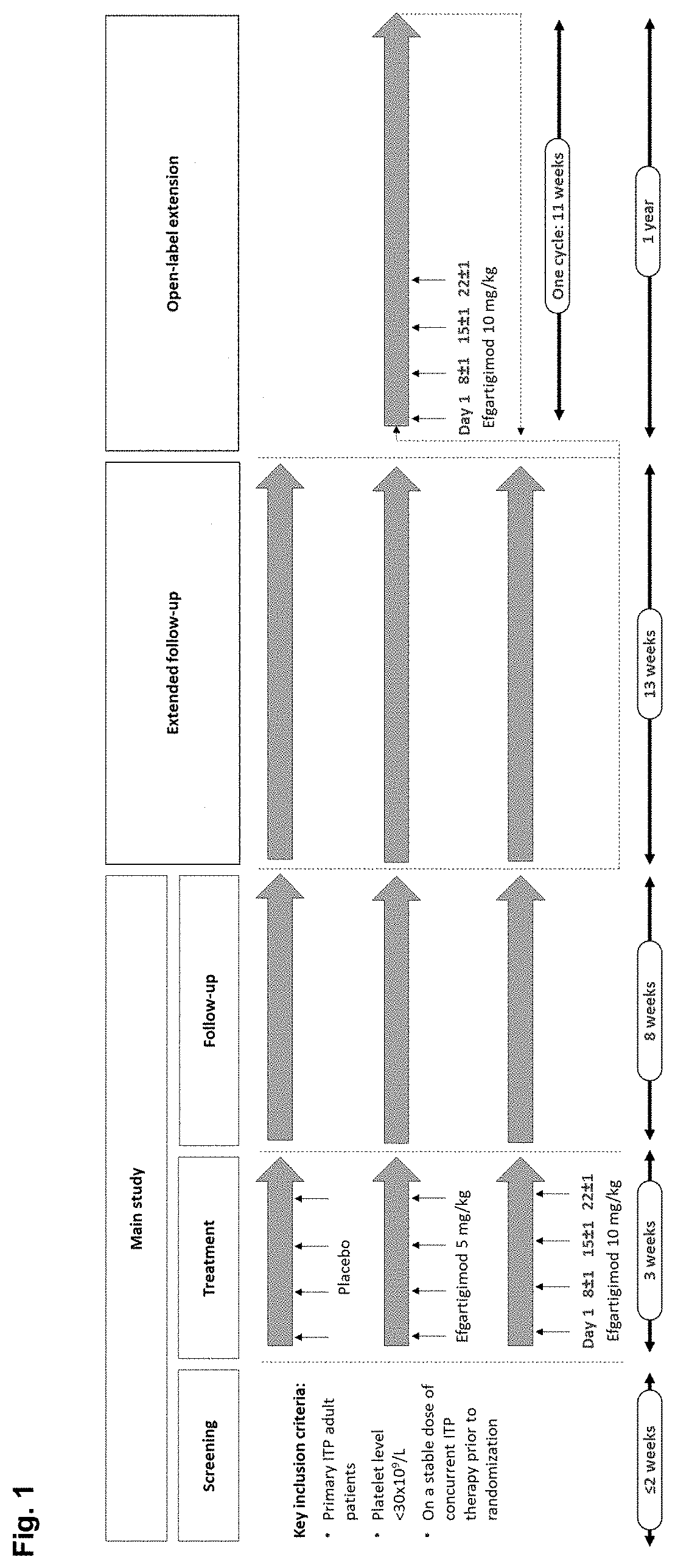
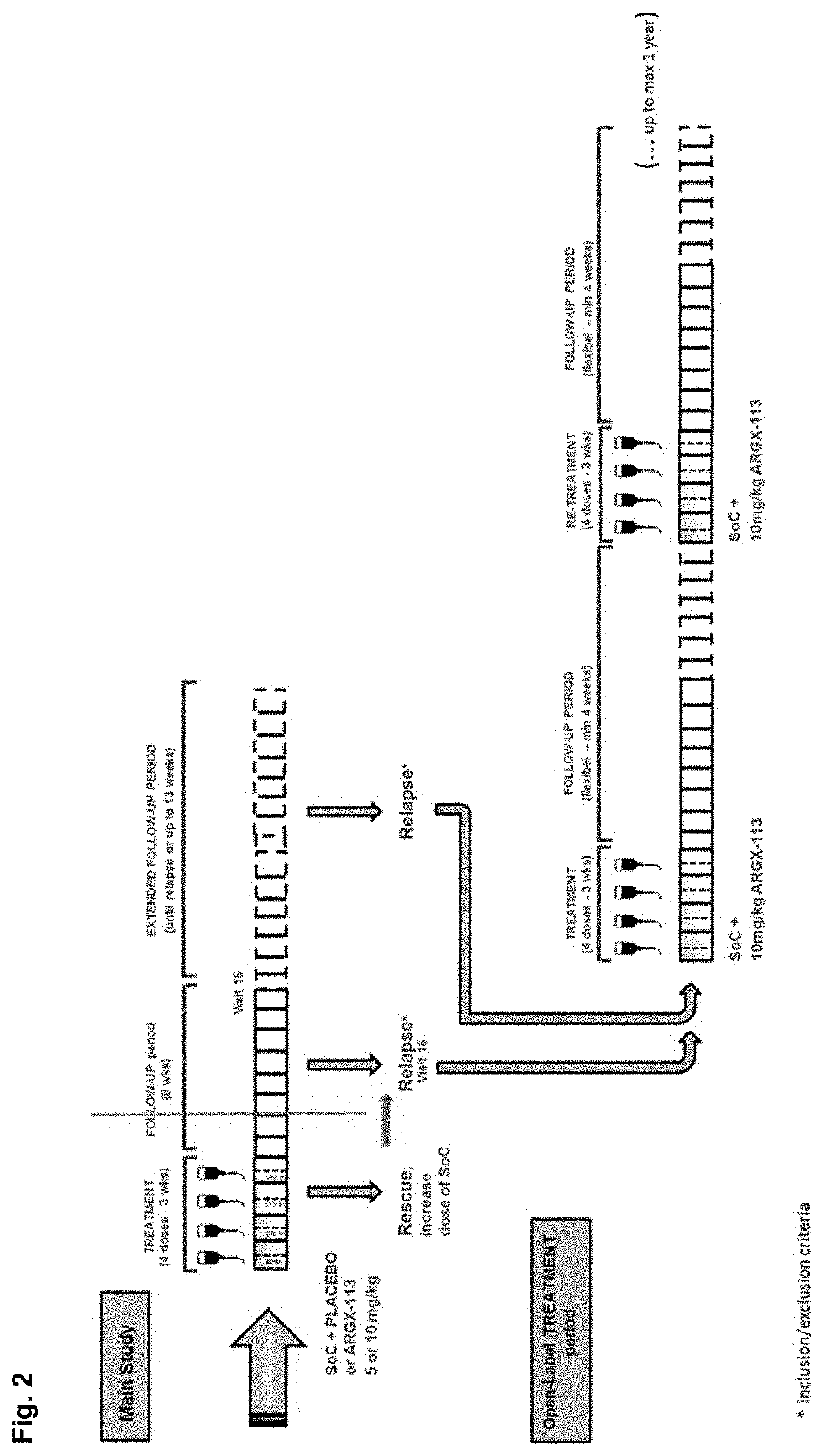
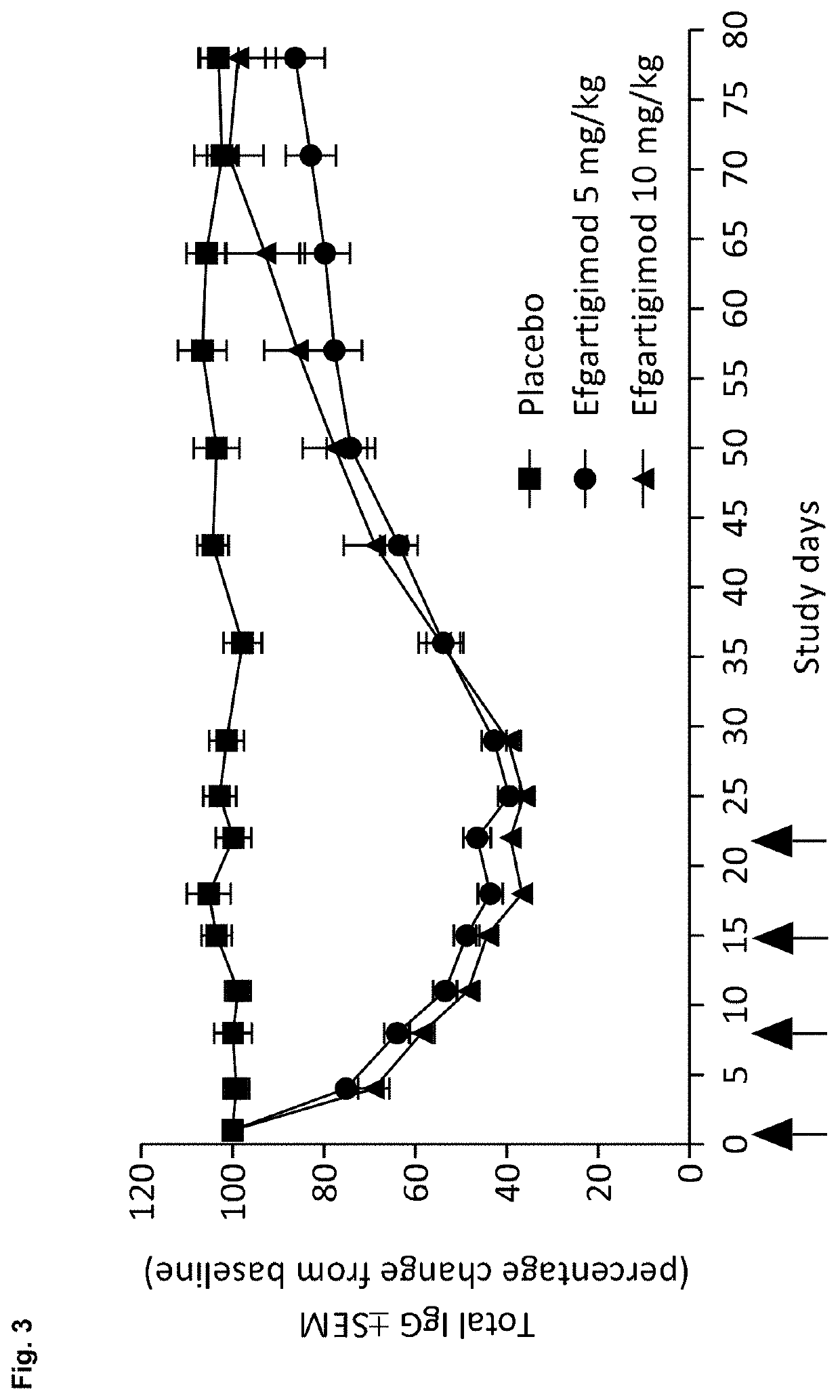
A method is disclosed for the treatment of human subjects diagnosed with immune thrombocytopenia (ITP). The method comprises administering to a human subject a human neonatal Fc receptor (hFcRn) antagonist, optionally in combination with standard-of-care ITP treatment. In certain embodiments, the hFcRn antagonist is efgartigimod (ARGX-113). Standard-of-care ITP treatment may comprise administration of corticosteroids, immunosuppressants, and / or thrombopoietin receptor (TPO-R) agonists.
Owner:ARGENX BV
Compounds exhibiting thrombopoietin receptor agonism
A compound represented by the general formula (I):wherein R1 is a hydrogen atom, a halogen atom, or the like; R2, R3, and R4 are each independently a hydrogen atom, a halogen atom, C1-C15 alkyl optionally substituted with one or more C1-C12 alkyloxy or the like, or the like; R5 is a hydrogen atom or the like; R6 and R7 are a hydrogen atom or the like; R8 is C1-C3 alkyl or the like; R9 is a hydrogen atom or the like), a prodrug, a pharmaceutically acceptable salt, or solvate thereof.
Owner:SHIONOGI & CO LTD
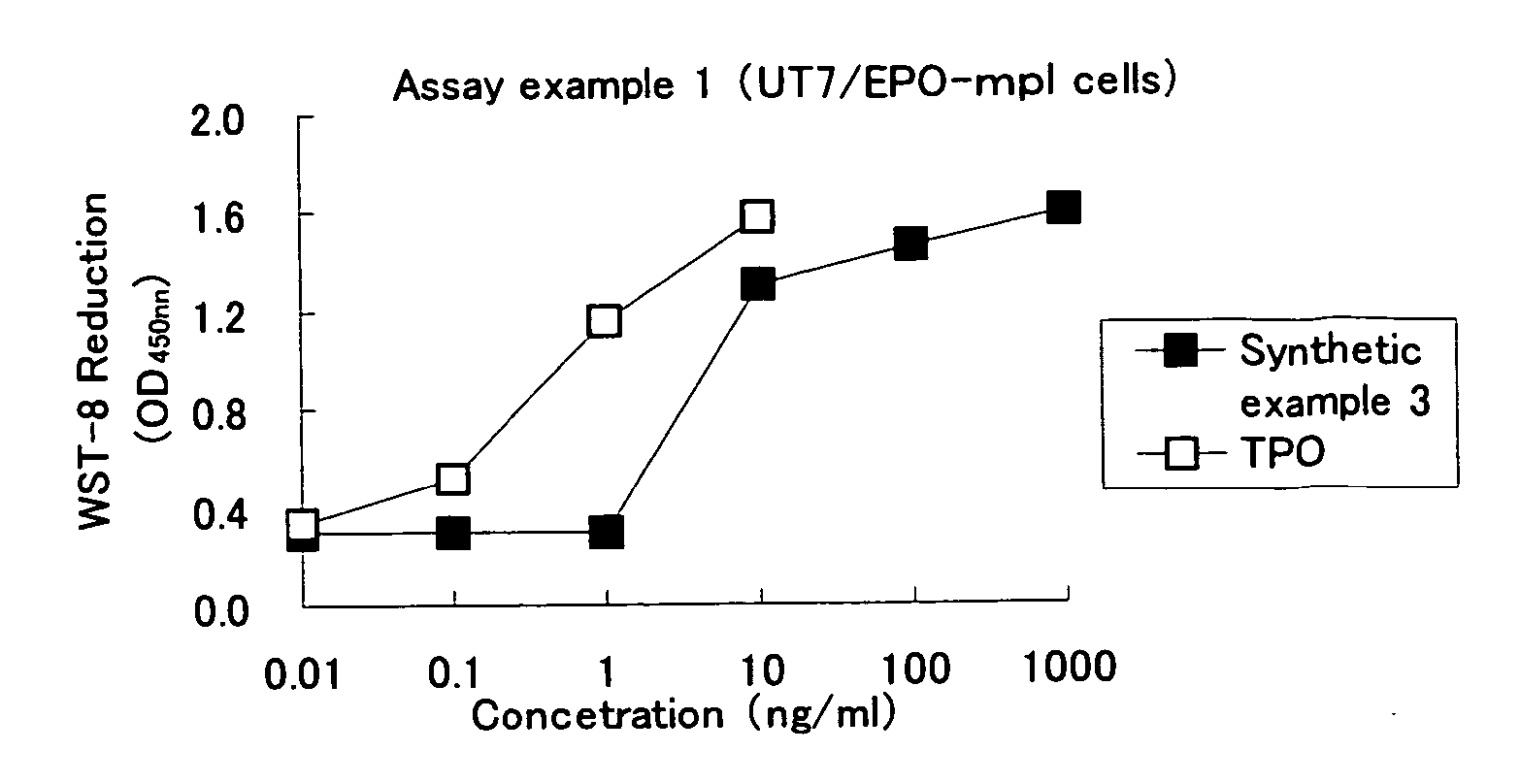
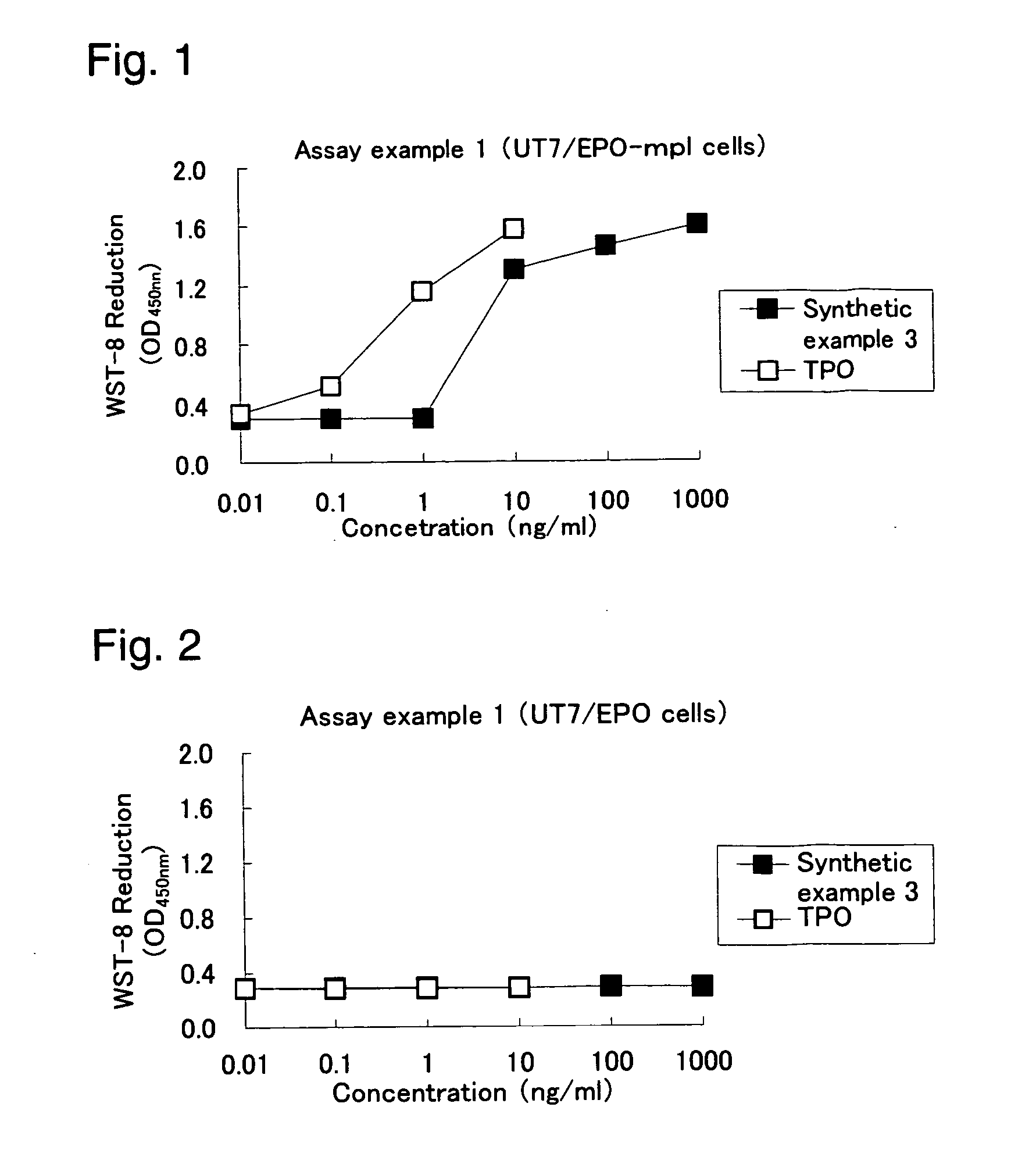
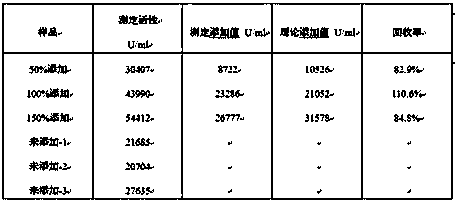
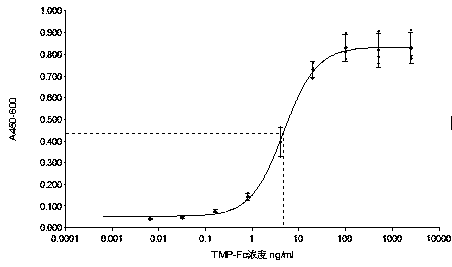
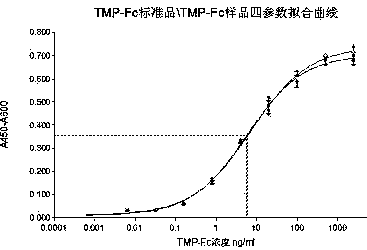
Analysis method for evaluating in-vitro activity of thrombopoietin receptor stimulant
The invention discloses an analysis method for evaluating in-vitro activity of a thrombopoietin (TPO) receptor stimulant. According to the method, the binding activity of a sample and a TPO receptor and the activity of the thrombopoietin are reflected by evaluating the proliferation-promoting development capacity of the sample on cells. By adopting the method, on one hand, the problem that BaF3-hMpl cell system in the prior art is great in establishing difficulty, great in time consumption and instable in detection result can be solved; on the other hand, the problems that by adopting a suspension cell MTT method, the color developing time is long, an insoluble formazan product is generated, the result reproductivity is poor and the accuracy is low can be solved; meanwhile, a four-parameter data processing method which is better than survival rate evaluation indexes is adopted, so that the activity value of the sample can be directly defined.
Owner:BEIJING TIDE PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com