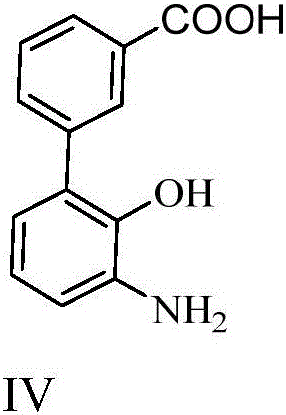
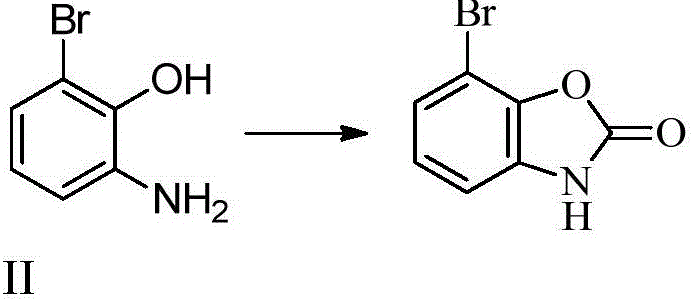
Synthesizing method for 3'-amino-2'-hydroxy biphenyl-3-carboxylic acid
A technology of hydroxybiphenyl and synthesis method, which is applied in the synthesis of 3'-amino-2'-hydroxybiphenyl-3-carboxylic acid, the key intermediate field of synthesizing Eltrombopag, and can solve the problem of shortening the reaction route and dangerous and other problems, to achieve the effect of novel method, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
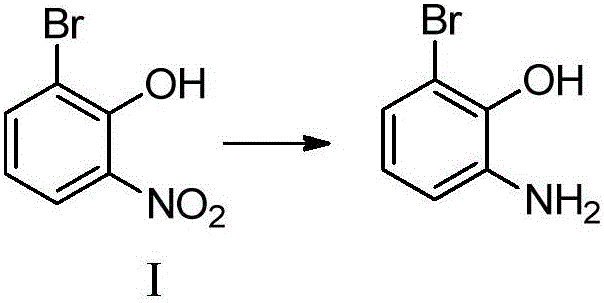
[0056] Embodiment 1: the preparation of 2-bromo-6-aminophenol (compound I)
[0057] 15g (68.8mmol) of 2-bromo-6-nitrophenol, 300mL of methanol, stirred and dissolved, added 1.84g (34.4mmol) of ammonium chloride, 19.27g (344mmol) of iron powder were added in batches, after the addition was completed, the temperature was raised to reflux Reacted for 2 hours, TLC detected that the reaction was complete, added saturated sodium bicarbonate to adjust pH = 7-8, filtered, the filtrate was spin-dried, added ethyl acetate and water for extraction, and the organic phase was spin-dried to give 2-bromo-6-aminophenol (compound I ) 11.2g, yield 86.5%.
Embodiment 2
[0058]Embodiment 2: the preparation of 2-bromo-6-aminophenol (compound I)
[0059] 15g (68.8mmol) of 2-bromo-6-nitrophenol, 400mL of methanol, stirred and dissolved, 22.36g (344mmol) of zinc powder was added in batches, after the addition was completed, the temperature was raised to reflux for 3 hours, TLC detected that the reaction was complete, and saturated carbonic acid was added Sodium hydrogen was used to adjust the pH to 7-8, filtered, the filtrate was spin-dried, ethyl acetate and water were added for extraction, and the organic phase was spin-dried to obtain 11.38 g of 2-bromo-6-aminophenol (compound I), with a yield of 88%.
Embodiment 3
[0060] Embodiment 3: the preparation of 2-bromo-6-aminophenol (compound I)
[0061] 15g (68.8mmol) of 2-bromo-6-nitrophenol, 300mL of methanol, and 150mL of glacial acetic acid were stirred and dissolved, and 19.27g (344mmol) of iron powder was added in batches. After the addition was completed, the reaction was carried out at room temperature for 3 hours. The reaction was detected by TLC and filtered. , washed with water, the filtrate was spin-dried, washed with saturated sodium bicarbonate, then washed with saturated brine, and the organic phase was spin-dried to obtain 11.38 g of 2-bromo-6-aminophenol (compound I), with a yield of 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com