Analysis method for evaluating in-vitro activity of thrombopoietin receptor stimulant
A thrombopoietin and receptor agonist technology, applied in the field of protein medicine, bioengineering and the field, can solve the problems of low safety and inaccurate test results, and achieve the effect of improving stability and reliable data results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1 In vitro activity evaluation of recombinant human thrombopoietin mimetic peptide-Fc fusion protein (TMP-Fc) (5-fold dilution gradient)
[0070] (1) Cell recovery and passage
[0071] Take the cell line out of the liquid nitrogen tank, quickly put it into a 37°C water bath, and shake it gently to make it melt quickly. Transfer it to a centrifuge tube in an ultra-clean bench, add 10% FBS+1640 medium to resuspend the cells. After centrifugation at 800rpm for 5min, discard the supernatant, add cell culture medium and growth factor recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF) at a final concentration of 10ng / ml, mix well by pipetting, and transfer to a cell culture flask. The cell name, operation date, and cell generation are marked on the bottle wall.
[0072] The cells in the logarithmic growth phase were pipetted and mixed, and 100 μl of cell suspension was taken to determine the concentration of viable cells, cell survival rat...
Embodiment 2
[0087] Example 2 In vitro activity evaluation of recombinant human thrombopoietin mimetic peptide-Fc fusion protein (TMP-Fc) (10-fold dilution gradient)
[0088] Cell recovery and subculture, cell viability evaluation, sample detection, color development method, and data processing detailed steps are shown in Case 1. The sample preparation is 10-fold serial dilution, and 9 concentrations of sample solutions are prepared.
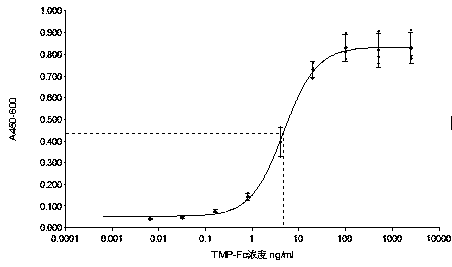
[0089] The result is as image 3 As shown, in the range of TMP-Fc concentration from 0.0005ng / ml to 5000ng / ml, MO7e cell proliferation and the logarithm of TMP-Fc concentration with base 10 showed a good linear relationship, R 2 =0.999. The half-effective concentration of TMP-Fc was 3.0 ng / ml.
Embodiment 3
[0090] Embodiment three romigrastim in vitro activity evaluation
[0091] See Case 1 for detailed steps of cell recovery and passage, cell viability evaluation, sample detection, color development method, and data processing.
[0092] Sample preparation: take a romigrastim sample, dilute the protein concentration to 0.05 mg / ml with 10% FBS+1640 medium, and use 10-fold serial dilution to prepare sample solutions with 9 concentrations.
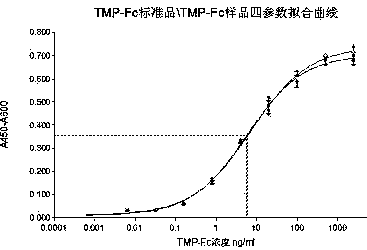
[0093] The result is as Figure 4 As shown, in the range of romigrastim concentration of 0.0005ng / ml to 5000ng / ml, the MO7e cell proliferation and the logarithm of the romigrastim concentration present a good linear relationship, R 2 =0.995. The half-effective concentration of romigrastim is 3.0 ng / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com