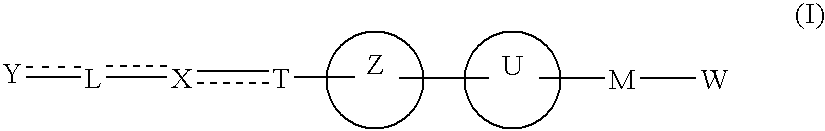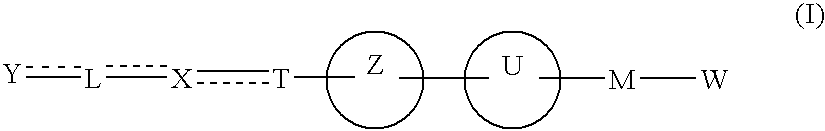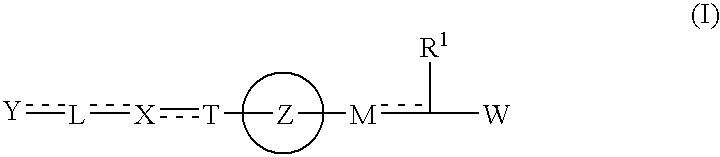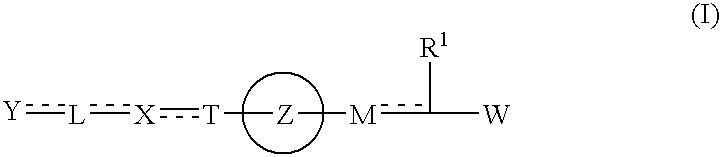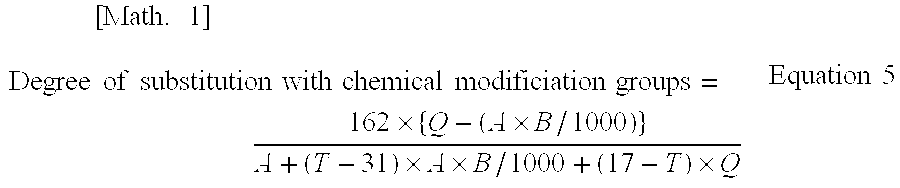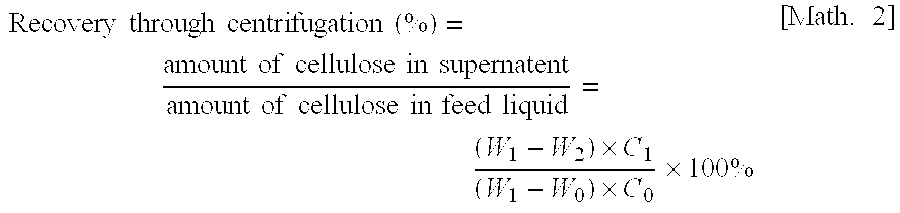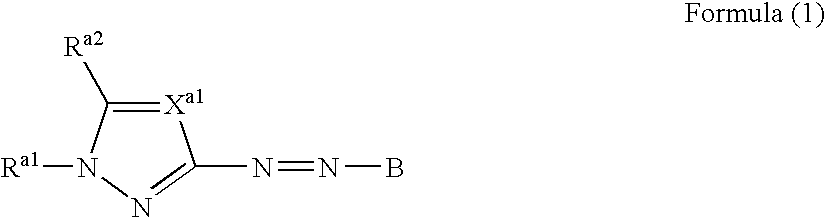Patents
Literature
712 results about "Formyl group" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
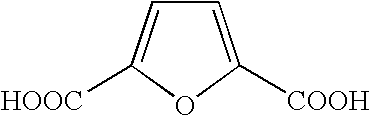
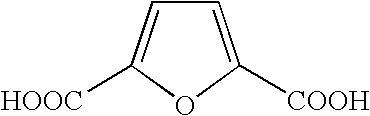
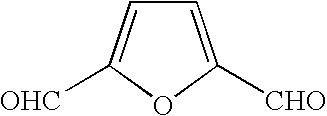
Formyl group. formyl group fôr´mĭl [key], in chemistry, functional group that consists of a carbonyl group joined by a single bond to a hydrogen atom. Aldehydes are compounds that contain a formyl group joined by a single bond to a hydrogen atom, an alkyl group , or an aryl group . The Columbia Electronic Encyclopedia, 6th ed.
Method for producing furan-2,5-dicarboxylic acid
ActiveUS20070232815A1Efficiently and quantitatively producingReduce energy consumptionOrganic chemistryFuranAlkaline earth metal
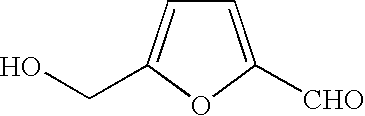
A method for producing furan-2,5-dicarboxylic acid (FDCA) is provided which can efficiently and quantitatively producing FDCA under mild conditions, without employing an expensive catalyst and with a reduced energy consumption. A furan ring compound having two functional groups selected from a hydroxymethyl group, a formyl group and a carboxyl group in the 2- and 5-positions of the furan ring, is oxidized with a metal permanganate in an alkaline environment to produce furan-2,5-dicarboxylic acid. Advantageously, the alkaline environment contains at least one of alkali metal hydroxides and alkali earth metal hydroxides, and the oxidation is performed at a temperature of from 1 to 50° C. by adding the permanganate metal salt to the alkaline aqueous solution containing the furan ring compound.
Owner:CANON KK
Method for producing furan-2,5-dicarboxylic acid
ActiveUS7411078B2Efficiently and quantitatively producingReduce energy consumptionOrganic chemistryFuranAlkaline earth metal
Owner:CANON KK
Carboxylic acid derivative and medicine comprising salt or ester of the same
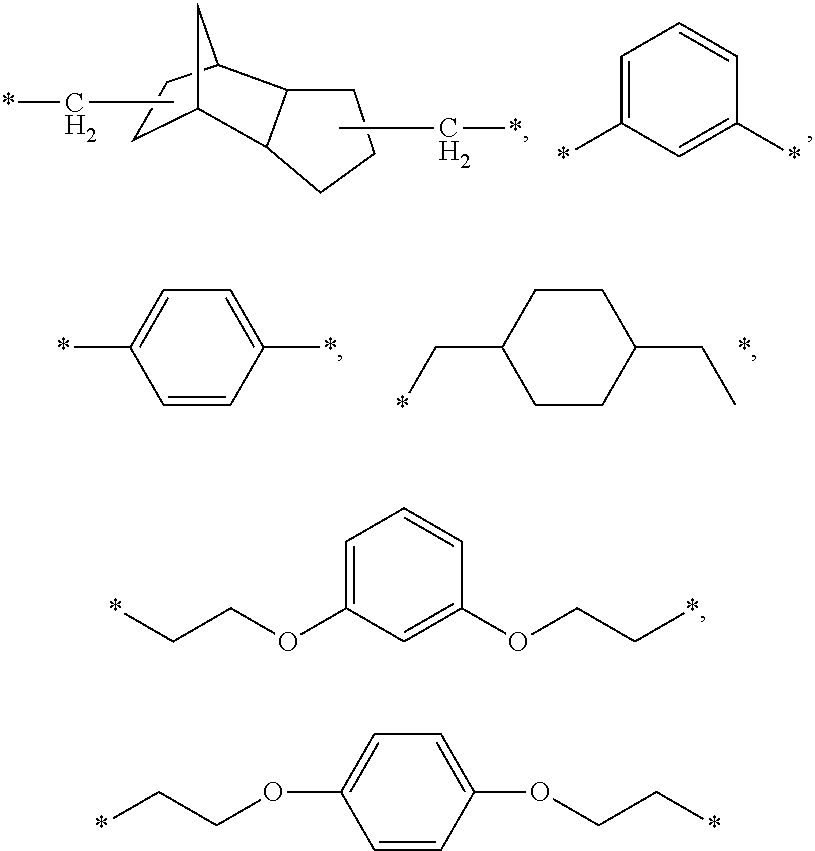
The present invention provides novel carboxylic acid derivatives useful as an insulin sensitizer, a salt thereof or a hydrate of them, and a medicament comprising the derivative as the active ingredient. Specifically, it provides a carboxylic acid derivative represented by the following formula: (wherein L represents a single bond, or a C1 to C6 alkylene group, a C2 to C6 alkenylene group or a C2 to C6 alkynylene group, each of which may have one or more substituent groups; M represents a single bond, or a C1 to C6 alkylene group, a C2 to C6 alkenylene group or a C2 to C6 alkynylene group, each of which may have one or more substituent groups; T represents a single bond, or a C1 to C3 alkylene group, a C2 to C3 alkenylene group or a C2 to C3 alkynylene group, each of which may have one or more substituent groups; W represents a carboxyl group; X represents a single bond, an oxygen atom, or a group represented by the various substituent groups including -NR<X1>CQ<1>O- (wherein Q<1 >represents an oxygen atom or a sulfur atom; R<X1 >represents a hydrogen atom, a cyano group, a formyl group, or various groups including a C1 to C6 alkyl group and a C1 to C6 hydroxyalkyl group, each of which may have one or more substituent groups), ONR<X1>CQ<1>-, -NR<X1>CQ<1>-, -CQ<1>NR<X1>-, -NR<X1a>CQ<1>NR<X1b>-, -Q<2>SO2- and -SO2Q<2>-; Y represents a 5 to 14-membered aromatic group which may have one or more substituent groups and one or more hetero atoms, or a C3 to C7 alicyclic hydrocarbon group; and the rings Z and U may be the same as or different from each other and each represents a 5 to 14-membered aromatic group which may have 1 to 4 substituent groups and one or more hetero atoms, and the ring may be partially saturated.), a salt thereof, an ester thereof or a hydrate of them.
Owner:EISIA R&D MANAGEMENT CO LTD
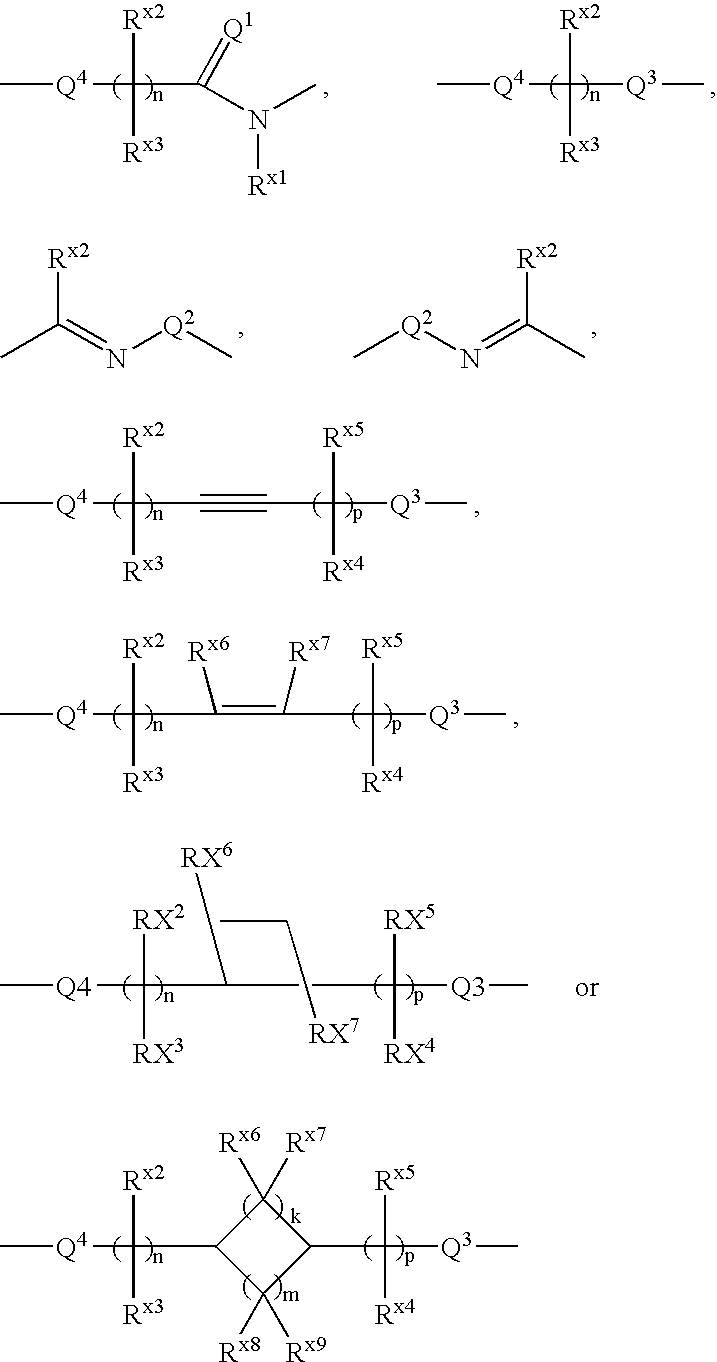
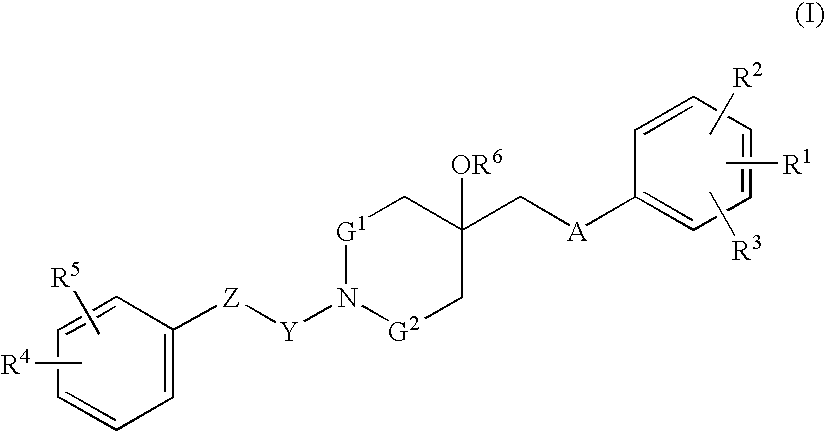
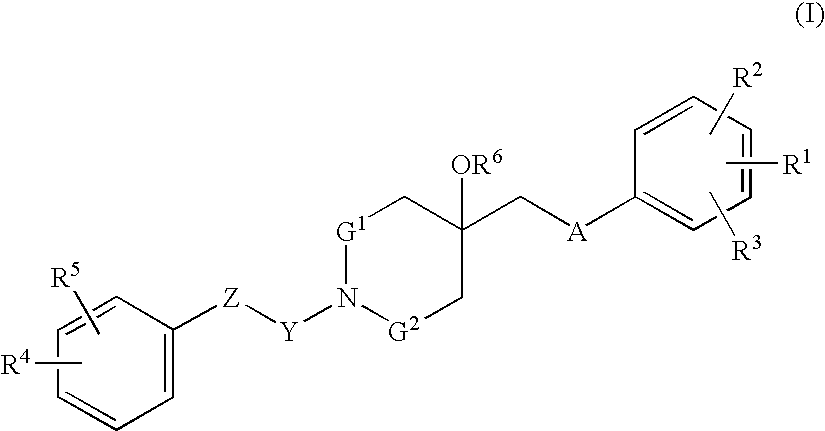
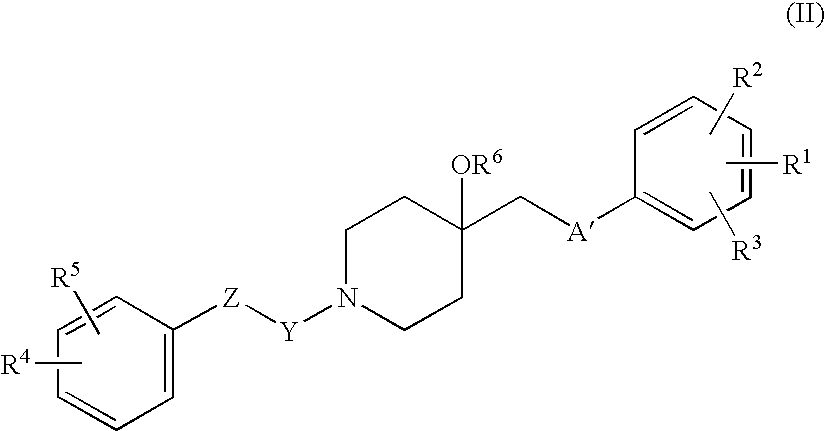
Agent for treating respiratory diseases containing 4-hydroxypiperidine derivative as active ingredient
InactiveUS7494987B2Potent antitussive actionImprove securityAntibacterial agentsBiocideDiseaseCarboxyl radical
An agent for preventing / treating respiratory diseases contains, as an active ingredient, a compound represented by following Formula (I):wherein A is a group represented by L-W [wherein L is a bond or CH2; and W is O, SOn (wherein n is 0 to 2), or —NR7— (wherein R7 is hydrogen or lower alkyl)]; each of G1 and G2 is (CH2)r (wherein r is 0 to 2), provided that when n is 1, G1 and G2 may be bridged by lower alkylene; Y is a lower alkylene or (substituted) benzylidene; Z is a bond or O, provided that when Z is a bond, Y may form a 5- or 6-membered ring with carbon on the benzene ring; R1 is, for example, NO2, a lower alkoxycarbonyl, (substituted) carbamoyl, (protected) hydroxyl group, (protected) carboxyl, or (protected) N-hydroxycarbamoyl; each of R2 and R3 is hydrogen, halogen, (halogenated) lower alkyl, (halogenated) lower alkoxy or NO2; each of R4 and R5 is, for example, hydrogen, halogen, (halogenated) lower alkyl, (halogenated) lower alkoxy, CN, or lower alkylsulfonyl; and R6 is hydrogen or lower alkyl, a salt thereof or a solvate of them. It has excellent antitussive activity when used as an agent for preventing / treating respiratory diseases such as lung cancer, common cold syndrome, pulmonary tuberculosis, pneumonia, acute bronchitis or chronic bronchitis.
Owner:MOCHIDA PHARM CO LTD
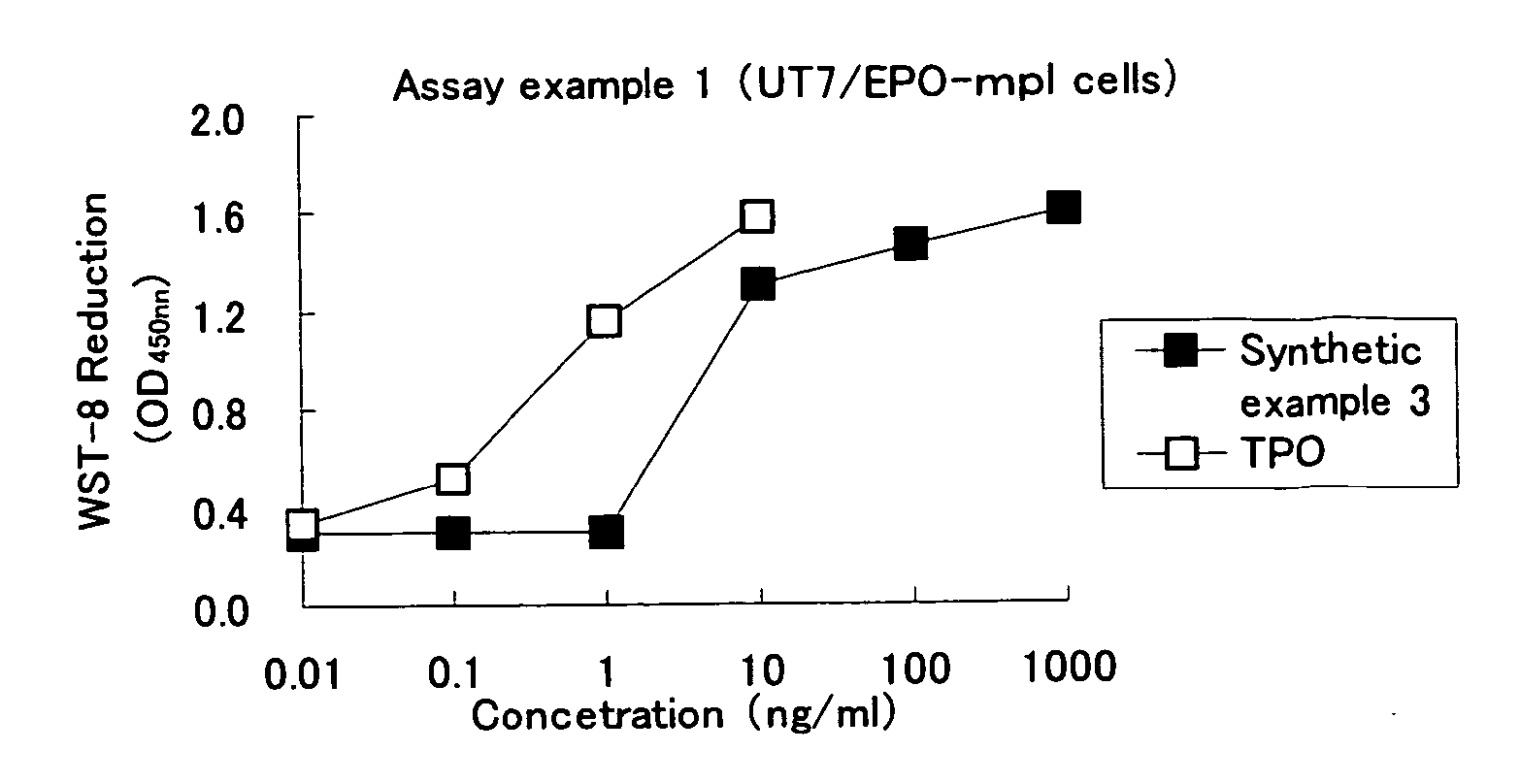
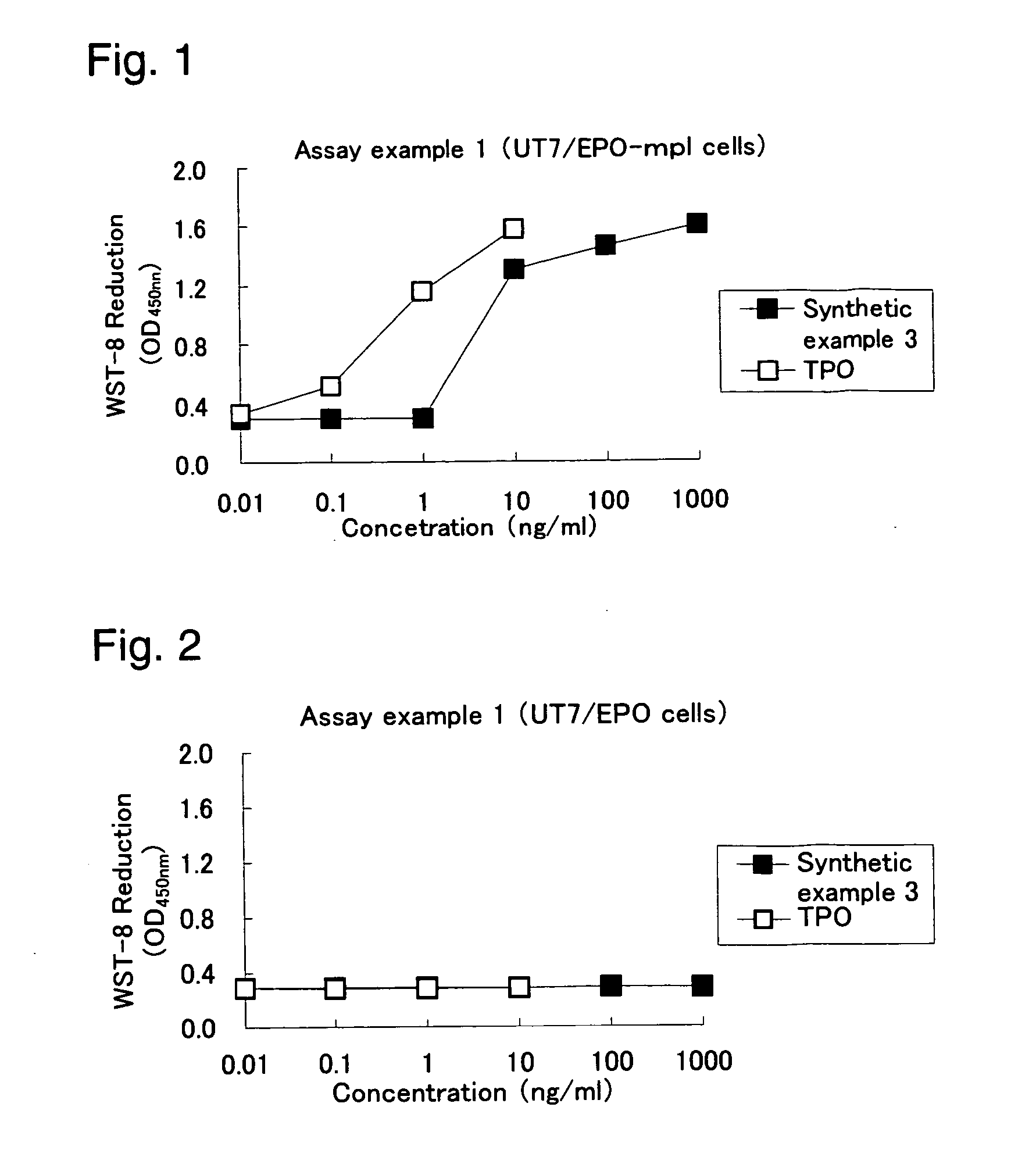
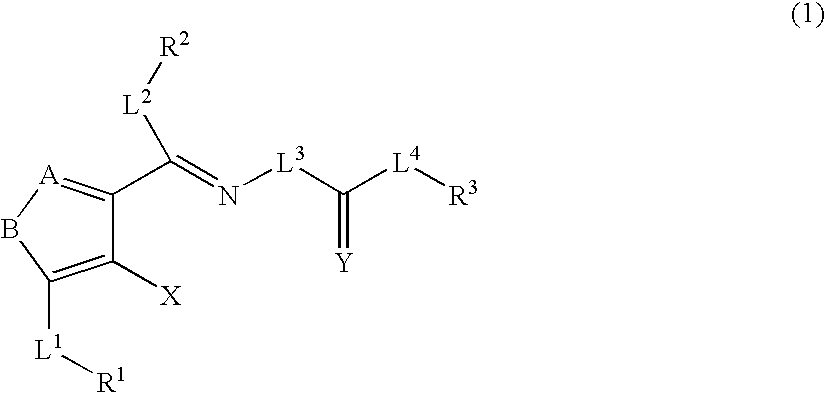
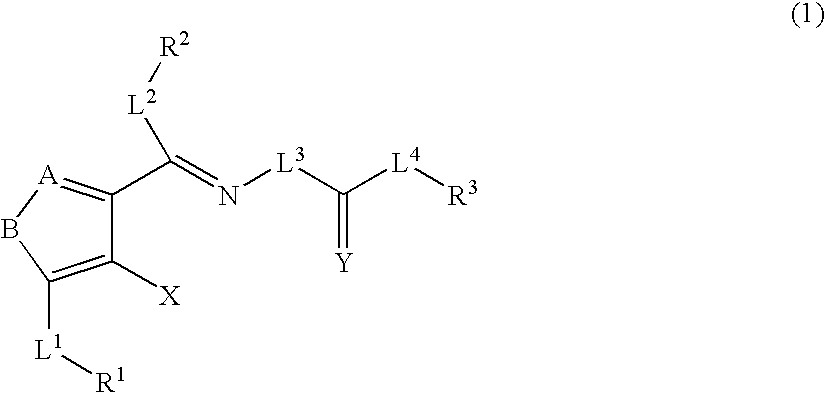
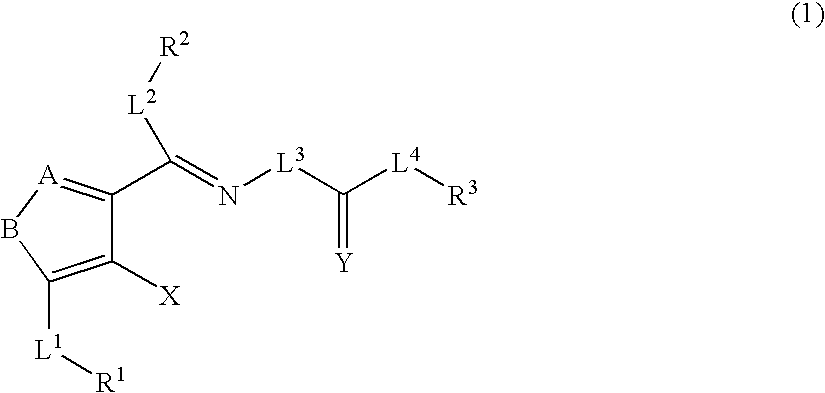
Heterocyclic compounds and thrombopoietin receptor activators
A compound represented by the formula (1): wherein A is a nitrogen atom or CR4, B is an oxygen atom, a sulfur atom or NR9 (provided that when A is a nitrogen atom, B is not NH), R1 is a C2-14 aryl group, L1 is a bond, CR10R11, an oxygen atom, a sulfur atom or NR12, X is OR13 SR13 or NR14NR15, R2 is a hydrogen atom, a formyl group, a C1-10 alkyl group or the like, L2 is a bond or the like, L3 is a bond, CR17R18, an oxygen atom, a sulfur atom or NR19, L4 is a bond, CR20R21, an oxygen atom, a sulfur atom or NR22, Y is an oxygen atom, a sulfur atom or NR23, and R3 is a C2-14 aryl group, a tautomer, prodrug or pharmaceutically acceptable salt of the compound or a solvate thereof.
Owner:NISSAN CHEM IND LTD
Carboxylic acid derivative and salt thereof
The present invention provides a novel carboxylic acid compound, a salt thereof or a hydrate of them useful as an insulin sensitizer, and a medicament comprising the compound as an active ingredient. That is, the present invention provides a carboxylic acid compound represented by the following formula, a salt thereof, an ester thereof or a hydrate of them. Wherein R<1 >represents a hydrogen atom, hydroxyl group, halogen, carboxyl group, or a C1-6 alkyl group etc., each of which may have one or more substituents; L represents a single bond, or a C1-6 alkylene group, a C2-6 alkenylene group or a C2-6 alkynylene group, each of which may have one or more substituents; M represents a single bond, or a C1-6 alkylene group, a C2-6 alkenylene group or a C2-6 alkynylene group, each of which may have one or more substituents; T represents a single bond, or a C1-3 alkylene group, a C2-3 alkenylene group or a C2-3 alkynylene group, each of which may have one or more substituents; W represents a carboxyl group; represents a single bond etc.; X represents a single bond, oxygen atom, a group represented by -NR<X1>CQ<1>O- (wherein Q<1 >represents an oxygen atom or sulfur atom; and R<X1 >represents a hydrogen atom, formyl group, or a C1-6 alkyl group etc., each of which may have one or more substituents), -OCQ<1>NR<X1>- (wherein Q<1 >and R<X1 >are as defined above), -CQ<1>NR<X1>O- (wherein Q<1 >and R<X1 >are as defined above), ONR<X1>CQ<1>- (wherein Q<1 >and R<X1 >are as defined above), -Q<2>SO2- (wherein Q<2 >is an oxygen atom or -NR<X10>- (wherein R<X10 >represents a hydrogen atom, formyl group, or a C1-6 alkyl group etc., each of which may have one or more substituents)) or -SO2Q<2>- (wherein Q<2 >is as defined above), (wherein, provided that R<X2 >and R<X3>, and / or R<X4 >and R<X5 >may together form a ring, Q<3 >and Q<4 >are the same as or different from each other and each represents an oxygen atom, (O)S(O) or NR<X10 >< /
Owner:EISIA R&D MANAGEMENT CO LTD
Dental composition, method of producing and use thereof
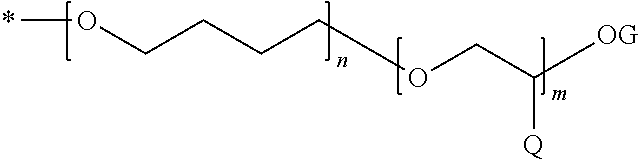
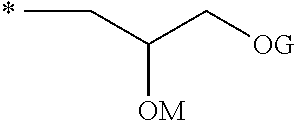
The invention relates to a dental composition havinga) compound (A) with the following features:only one backbone unit (U) with 6 to 20 carbon atoms, at least 6 carbon atoms thereof forming an aromatic or an aliphatic cyclic moiety, the remaining carbon atoms either being part of substituents pending from the cyclic moiety or being part of bridging groups to spacer units, wherein one or more of the remaining carbon atoms can be replaced by an oxygen atom, the backbone unit not comprising a bisphenol structure,one or two spacer unit(s) (S) being connected to the backbone unit (U) via an ether linkage, at least one spacer unit (S) having a —CH2-CH2-CH2-CH2-O-CH2-CH(Q)-OG chain or a —CH2-CH(OG)-CH2-OM residue or a mixture of these two types of spacers within one spacer unit,with G having at least one group selected from acroyl, methacroyl, acetyl, benzoyl, and combinations thereof, M having at least one aryl group, and combinations thereof, Q having at least one group selected from hydrogen, methyl, phenyl, phenoxymethyl, and combinations thereof,b) filler (B) and c) initiator (C).
Owner:3M INNOVATIVE PROPERTIES CO
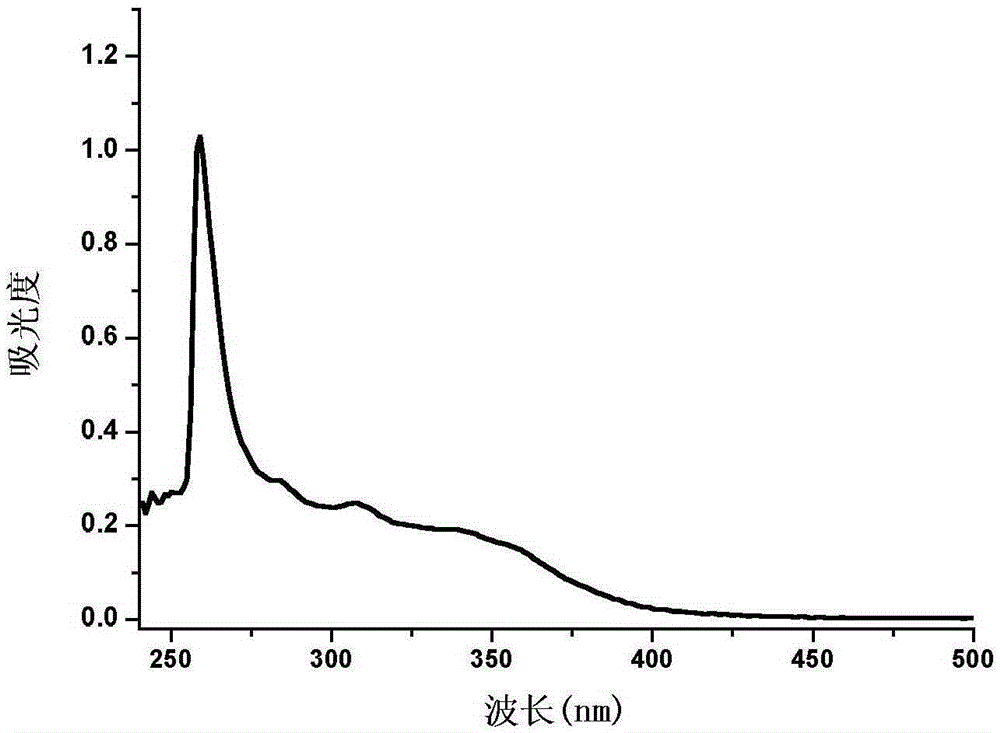
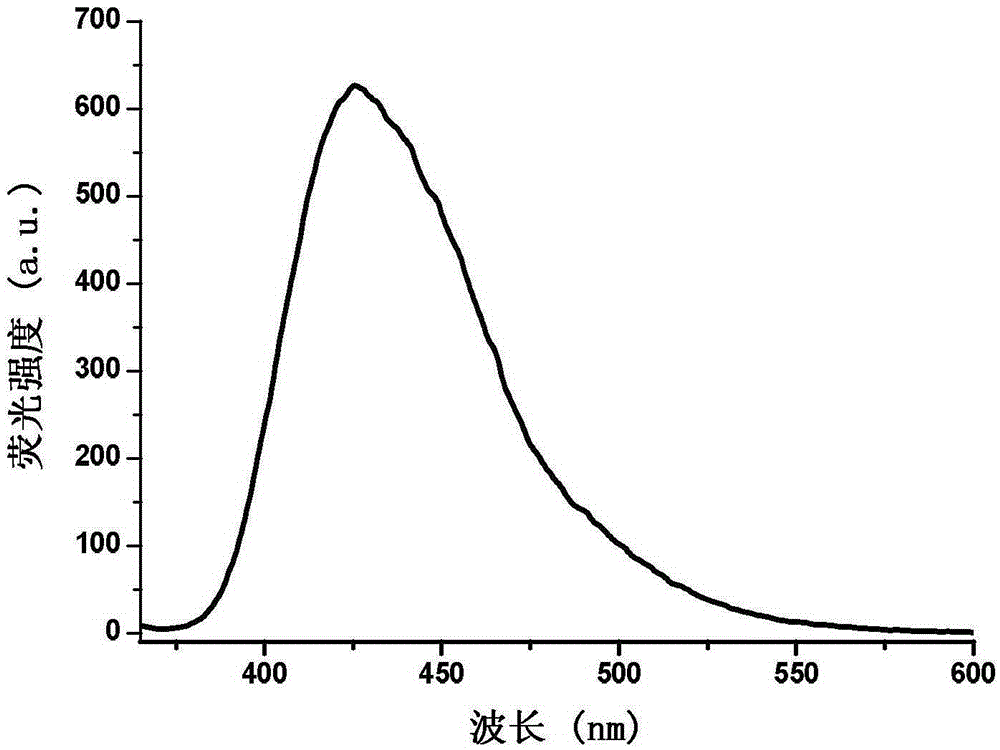
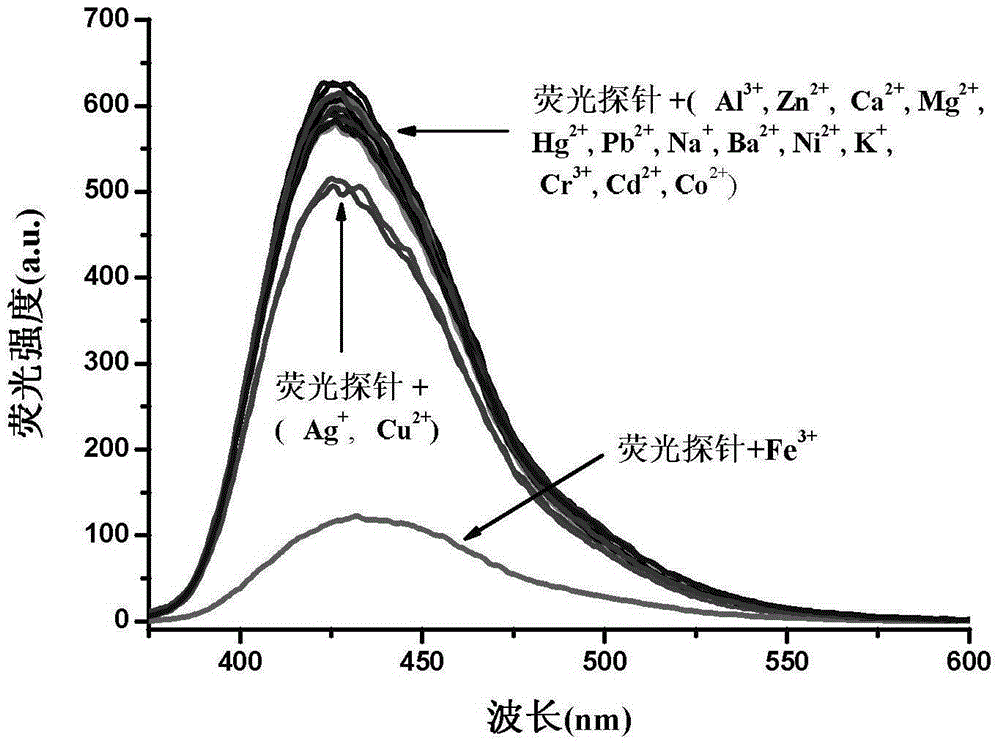
Phenanthrene and imidazole-coumarin double-fluorescent group ratio fluorescent molecular probe for iron ion detection and synthesis and use methods thereof
InactiveCN105255481AHigh fluorescence intensityExcellent fluorescence performanceOrganic chemistryFluorescence/phosphorescenceKetonePhenyl group
The invention provides a phenanthrene and imidazole-coumarin double-fluorescent group ratio fluorescent molecular probe for iron ion detection and synthesis and use methods thereof, relates to fluorescent molecular probes and synthesis and application thereof and aims to solve the problem that an existing Fe<3+> fluorescent probe is prone to being interfered by pH, concentration and other metal ions. The fluorescent molecular probe is 4-methyl-7-hydroxide radical-8-[2-(1- phenyl group-1H-phenanthrene and [9, 10-d] imidazole-2-)benzene ammonia methylene]-2H-pyran-2-ketone. The phenanthrene and imidazole-coumarin double-fluorescent group ratio fluorescent molecular probe is formed by conducting condensation on 1-N-phenyl group-2-(2-aminophenyl)-1H-phenanthrene and [9, 10-d] imidazole and 4-methyl-7-hydroxide radical-8- formyl group coumarin, and the yield is 75-85%. The fluorescent molecular probe is dissolved in mixed liquid of N, N- dimethylformamide and an HEPES buffering solution, existence of iron ions is judged through the absorbance value or fluorescence intensity change before and after adding of test samples, and the fluorescent molecular probe can be used for detection of Fe<3+> pollution in water.
Owner:QIQIHAR UNIVERSITY
Heterocyclic compounds and thrombopoietin receptor activators
Owner:NISSAN CHEM CORP
Novel compound and preparation method and use thereof
InactiveCN101891715AEasy to operateMild responseGroup 4/14 element organic compoundsOrganic reductionMedicinal chemistrySilicon based
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
Method for the conversion of a Z-isomer into E-isomer
InactiveUS20040015020A1Simple processOrganic compound preparationOrganic chemistry methodsArylSilylene
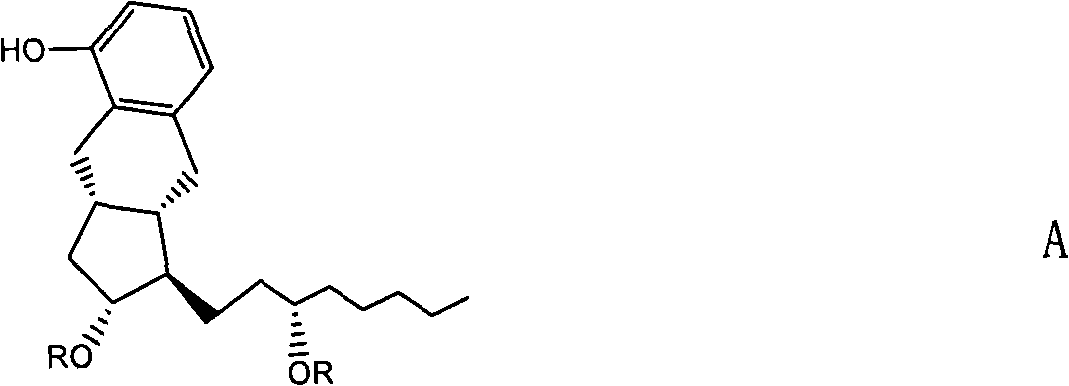
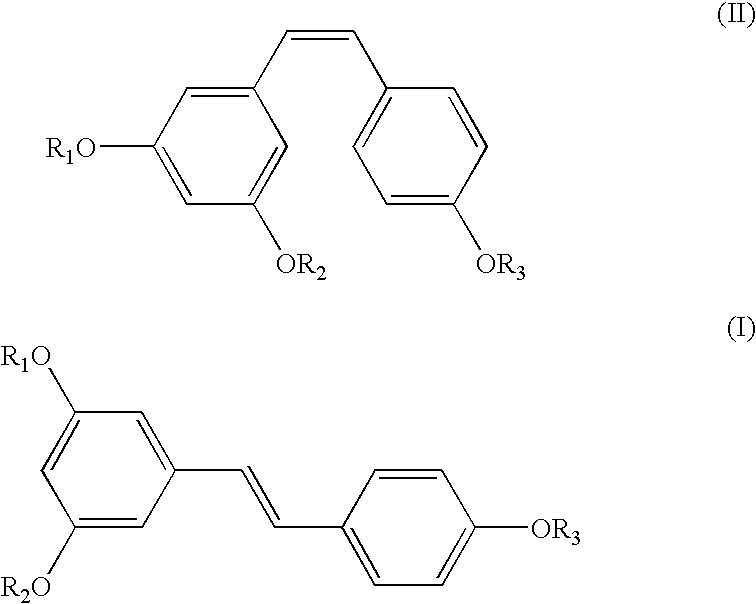
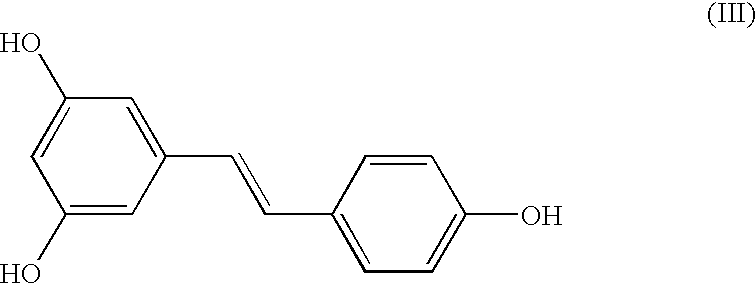
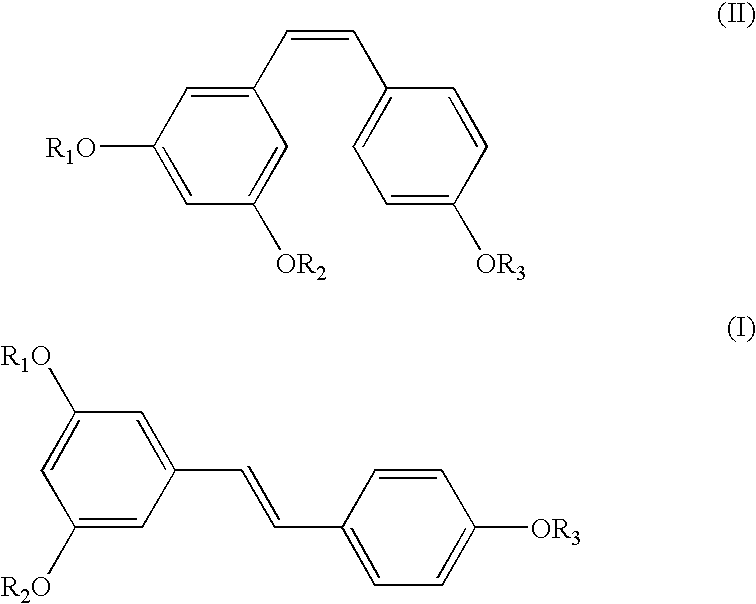
A method of converting (Z)-1-(3,5-disubstituted phenyl)-2-(4-substituted phenyl)ethene of general formula (II) to (E)-1-(3,5-disubstituted phenyl)-2-(4-substituted phenyl)ethene of general formula (I) wherein R1, R2 and R3 are same or different and independently represent (C1-C4)alkyl, (C1-C4)alkoxy(C1-C4)alkyl, (C1-C4)alkoxy(C1-C4)alkoxy(C1-C4)alkyl, allyl, vinyl, silyl, formyl, acyl, aryl(C1-C4)alkyl or substituted aryl(C1-C4)alkyl group. The present invention also provides a process for the conversion of (E)-1-(3,5-disubstituted phenyl)-2-(4-substituted phenyl)ethene of general formula (I) prepared by a process described above to E-resveratrol of the formula (III).
Owner:ORCHID CHEM & PHARM LTD
Amide derivative
InactiveUS20060128755A1Good anti-inflammatory activityHigh activityBiocideNervous disorderArylHalogen
Amide derivatives represented by the formula (I): wherein: A is a cycloalkyl group, an aryl group or a heteroaryl group; X is a nitrogen atom or CR17; Y is —NRa—, —(CRbRb′)m—, and the like; m is 0 to 4; and R1 to R17 may be the same or different and each is a hydrogen atom, a halogen atom, a cyano group, a nitro group, a carboxyl group, a formyl group, a hydroxyl group, an ammonium group, an alkyl group optionally having one or more substituents, ZR18 and the like, Z is —O—, —S(O)p—, —S(O)pO—, —NH—, —NR19—, and the like; or R1 and R2 may in combination form a ring, a pharmaceutically acceptable salt thereof, a hydrate thereof, or a solvate thereof may be applied to pharmaceutical use such as anti-inflammatory and analgesic action and the like.
Owner:EA PHARMA CO LTD
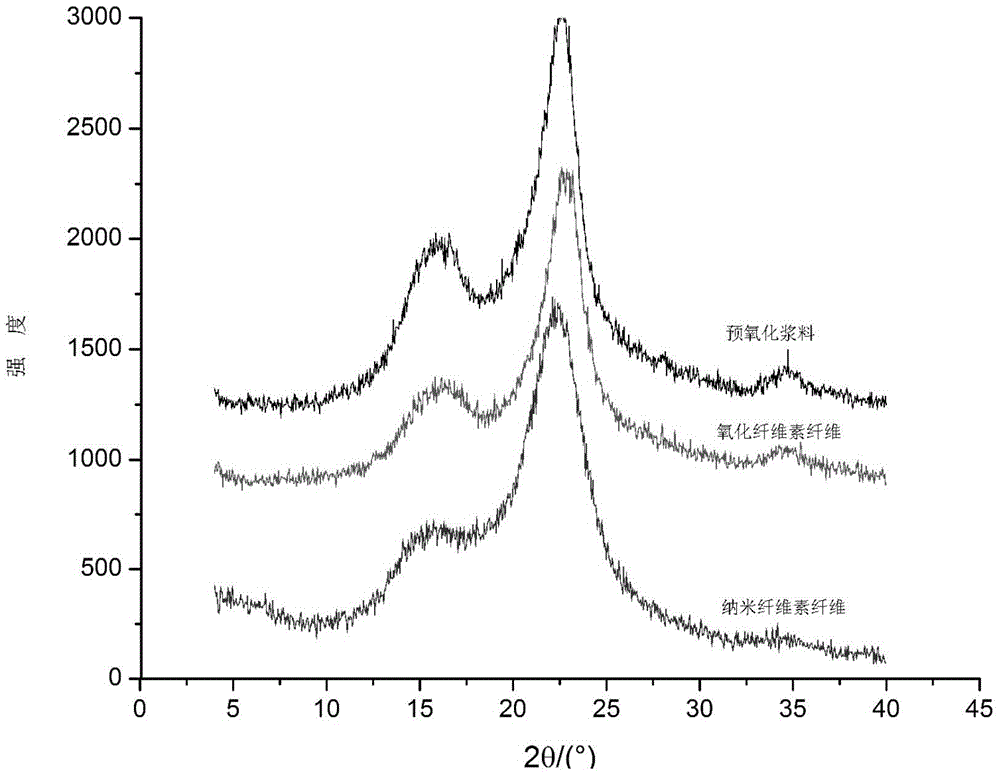
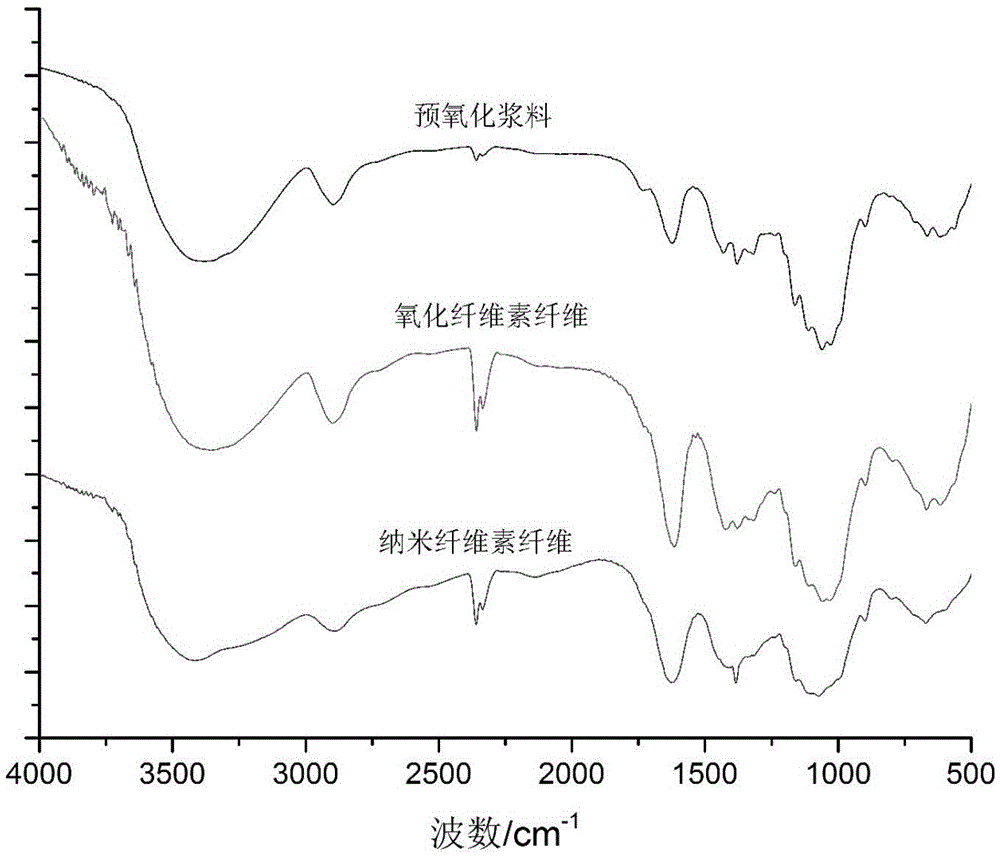
Nano-crystalline cellulose fiber high in carboxyl group content and preparation and application thereof
InactiveCN105463606AIncrease the total carboxyl contentGuaranteed crystallinityOther chemical processesArtificial filaments from cellulose derivativesPolymer scienceFreeze-drying
The invention belongs to the technical field of biomass nanometer materials and discloses a nano-crystalline cellulose fiber high in carboxyl group content and preparation and application thereof. A preparation method comprises the steps of preheating fiber slurry for 0.5-1.5h with sodium periodate at the temperature of 50-60 DEG C; adding a sodium chlorite solution and acetum into the slurry to be reacted for 1-3h at the temperature of 30-50 DEG C, adding TEMPO, NaClO2 and NaClO, and performing microwave heating to the temperature of 55-65 DEG C to enable the mixture to be reacted for 1-3h; performing ultrasonic dispersion and freeze drying to obtain the nano-crystalline cellulose fiber high in carboxyl group content. According to the nano-crystalline cellulose fiber, hydroxyl at the positions of C2 and C3 of cellulose is oxidized into a formyl group which is oxidized into carboxyl by sodium chlorite, hydroxyl at the position of C6 is selectively oxidized into carboxyl through a TEMPO neutral oxidation system, and accordingly the total carboxyl group content of the fiber is greatly increased, and obtained products can be used for advanced treatment of waste water at the middle section of paper making.
Owner:SOUTH CHINA UNIV OF TECH
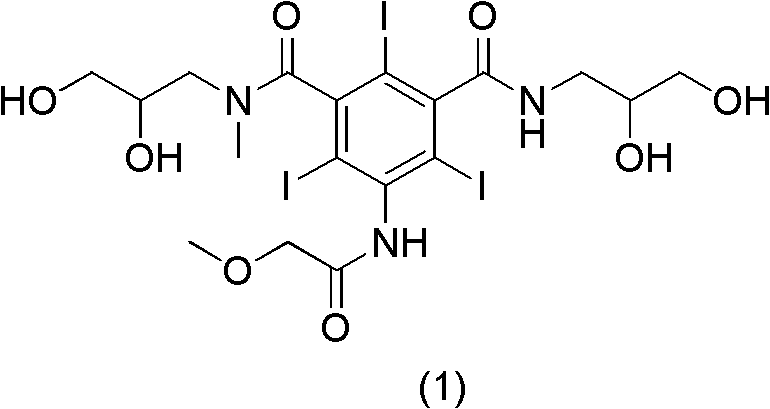
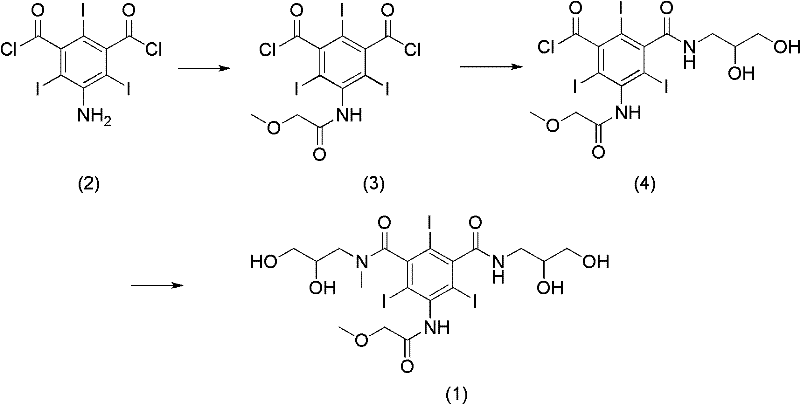
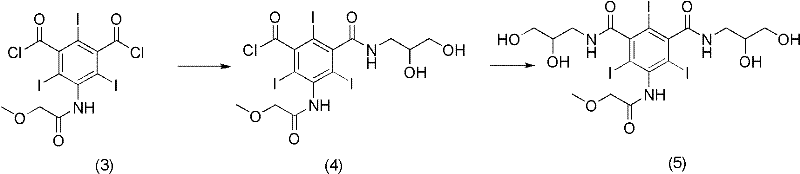
Preparation method of Iopromide
ActiveCN102351735AIncrease productionHigh purityOrganic compound preparationCarboxylic acid amides preparationBenzoic acidTriiodide
The invention relates to a preparation method of Iopromide. 3-methoxy acetamino-5-(2,3-dihydroxy n-propylamine formyl)-2,4,6-triiodide benzoic acid shown as a formula (9) is used as an intermediate to provide a cheaper and more reasonable method for synthesizing Iopromide with high yield and purity.
Owner:ZHEJIANG STARRY PHARMA
N-heteroarylnicotinamide derivatives
InactiveUS20050004368A1High insecticidal activityInexpensively and simply producingBiocideOrganic chemistryHydrogen atomPhenyl group
An N-heteroaryl-4-(haloalkyl)nicotinamide derivative represented by formula (I): [wherein R represents a C1-C6 haloalkyl group; R1 represents a hydrogen atom, a C1-C6alkyl group which may be substituted a C2-C6 alkenyl group or an acyl group; X represents a group represented by formula C—R2, or a nitrogen atom; R2 and R3 each independently represent a hydrogen atom, a halogen atom, a C1-C6 alkyl group which may be substituted, a C3-C7 cycloalkyl group, a C2-C6 alkenyl group, a C3-C7 cycloalkenyl group, a formyl group, a group represented by formula CH═NOR4, a cyano group, a phenyl group which may be substituted, a heterocyclic group which may be substituted, a C1-C6 alkoxy group which may be substituted, a C1-C6 alkylthio group or a phenoxy group]; or a salt thereof, a pesticide containing it as an active component, a method for producing it and intermediates thereof.
Owner:SANKYO AGRO
Cellulose fibers and process for producing the same, cellulose fiber assembly, and cellulose-fiber composite material
ActiveUS20130338250A1High production costGood fibrillationImpression capsArtificial filaments from cellulose derivativesCellulose fiberChemical modification
The present invention relates to cellulose fibers wherein a part of the hydroxyl groups of the cellulose have been substituted with at least one of a carboxy group and formyl group of 0.1 mmol / g or larger based on the weight of the cellulose fibers, and have been further substituted with a chemical modification group other than the carboxy and formyl groups.
Owner:OJI HLDG CORP
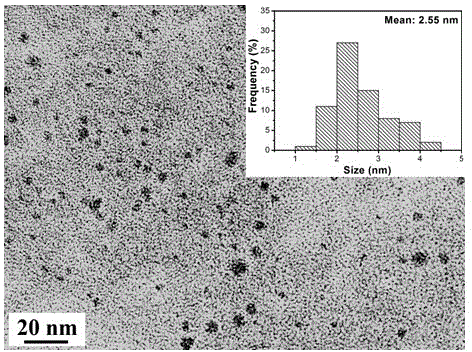
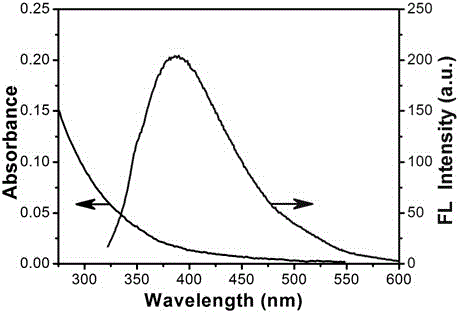
Amphipathy carbon quantum dot and preparation method thereof
ActiveCN104789217AStrong fluorescenceExcitation wavelength dependentNanoopticsFluorescence/phosphorescenceAir atmosphereQuantum yield
The invention discloses am amphipathy carbon quantum dot and a preparation method thereof. The amphipathy carbon quantum dot is prepared by virtue of the following steps: carrying out pyrolysis on chili powder which is adopted as a carbon source in an air atmosphere, and then carrying out the extraction and purification by virtue of ethanol. The particle size of the carbon quantum dot prepared in the method is 0.5nm to 4.5nm, and the surface of the carbon quantum dot is not only provided with hydrophilic groups such as hydroxyl, carboxyl, formyl groups and amino groups but also provided with hydrophobic groups such as methyl, methylene and phenyl, so the carbon quantum dot is amphipathic. The fluorescent quantum yield of the carbon quantum dot is 70 to 75 percent. The preparation method is simple to operate, and raw materials are low in price and easy to obtain and can be used for preparing the amphipathy carbon quantum dots in a large scale. Compared with other single hydrophilic and single hydrophobic carbon quantum dots, the prepared amphipathy carbon quantum dot is better in biological compatibility, the cell developing effect is better, and the amphipathy carbon quantum dot can be well applied to the fields such as biological developing, biological medical imaging and fluorescent detection.
Owner:ANHUI UNIVERSITY
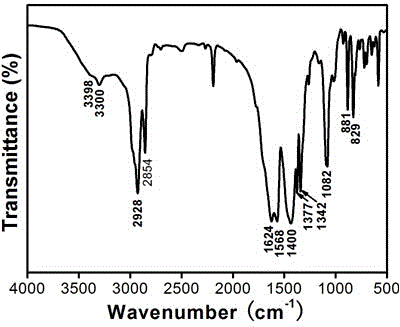
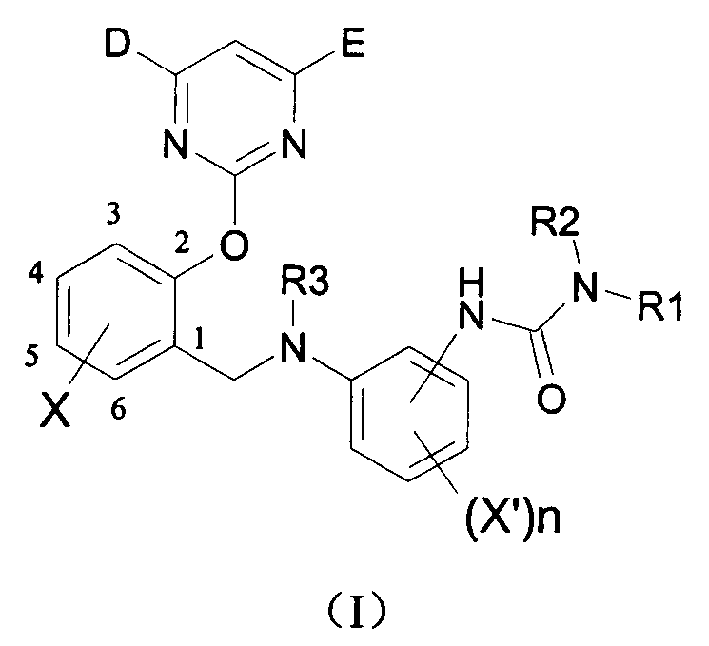
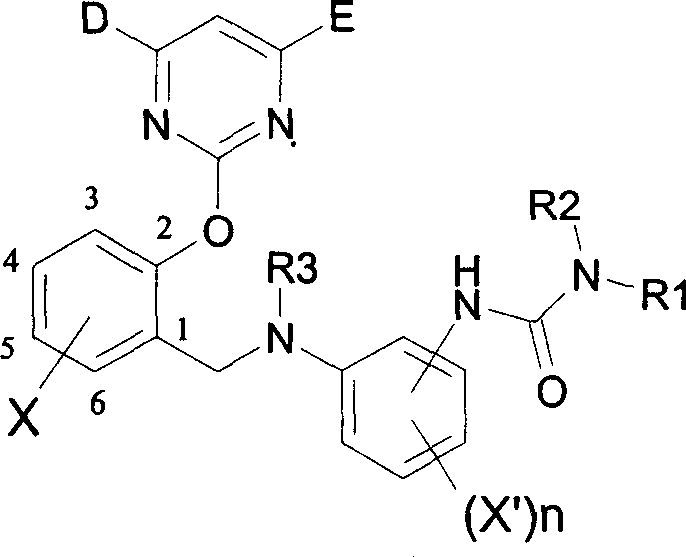
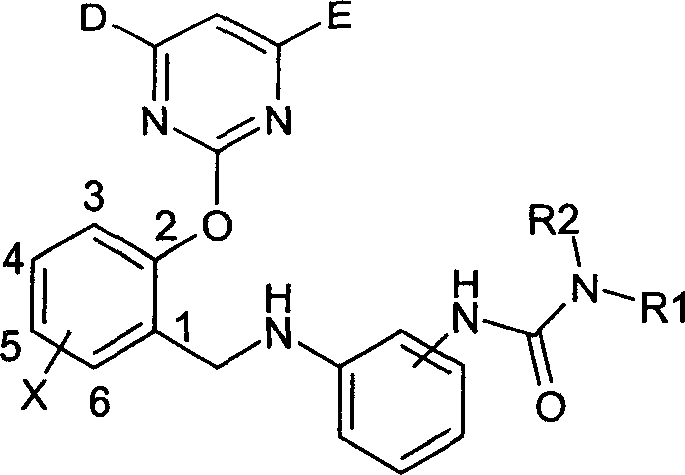
2-pyrimidine oxy-n-ureido phenyl-benzyl amide compound, preparing method and use thereof
InactiveCN1488626AGood weeding effectImprove herbicidal activityBiocideOrganic chemistryAlkaneCarboxyl radical
The invention is a kind of 2-pyrimidine oxo-N-urea phenyl benzyl amine compound, the manufacturing method. The structural formula is shown in the picture. D or E = hydrogen, halogen, C1-4C alkyl, C1-C4 alkoxy, C1-C4 halogenated alkyl or C1-C4 alkylogen oxide; X=hydrogen, halogen, nitro, cyan, carboxy, ester group, sulfonyl, C1-C8 alkane acyl amido, C1-C8 halogeno alkane acyl amido, C1-C8 alkane acyl group, C1-C8 alkyl, C1-C8 halogeno alkyl, C1-C8 alkoxy, phenyl etc; R1, R2=hydrogen, ester group, sulfonyl, C1-C8 alkyl, C1-C8 substituted alkyl, C1-C8 alkyl acyl, phenyl, etc; Xí» is H, urea, halogen atom, carboxy, ester group, C1-C8 alkane acyl, C1-C8 alkyl, C1-C8 halogeno alkyl, C1-C8 alkoxy, phenyl, substitution phenyl or heterocyclic radical; R3=hydrogen, C1-C8 alkane acyl, C1-C8 halogeno alkyl, benzene formyl, substitution benzene formyl, C1-C8 alkyl.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI +1
Active oxygen scavengers containing pterin derivatives
PCT No. PCT / JP97 / 02649 Sec. 371 Date Mar. 31, 1998 Sec. 102(e) Date Mar. 31, 1998 PCT Filed Jul. 30, 1997 PCT Pub. No. WO98 / 04558 PCT Pub. Date May 2, 1998An active oxygen scavenger comprising as an active ingredient a pterin derivative of the formula (I): wherein R1 and R2 are independently a hydrogen atom, an alkyl group of 1 to 4 carbon atoms, or an acyl group of the formula R3-CO-, R3 is an alkyl group of 1 to 4 carbon atoms, X is a formyl group or hydroxymethyl group, A is a group of the formula (Ia): and n is 0 or an integer of 1 or more, with the proviso that when X is a hydroxymethyl group, n is 0, or when n is an integer of 1 or more, each of R1 and R2 is a hydrogen atoms, and X is a formyl group, or a cyclic compound thereof, or a salt thereof is disclosed.
Owner:CMIC
Aromatic triamide-lanthanide complexes
ActiveUS20090036537A1Easily preparedGood yieldUltrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsChemistryMetal chelate
The present invention provides luminescent lanthanide metal chelates comprising a metal ion of the lanthanide series and a complexing agent comprising at least one phthalamidyl moiety. Also provided are probes incorporating the phthalamidyl ligands of the invention and methods utilizing the ligands of the invention and probes comprising the ligands of the invention.
Owner:RGT UNIV OF CALIFORNIA
Method for preparing adsorbable oxycellulose through ramie oxidation degumming process
InactiveCN105061606AAdsorptiveImprove adsorption capacityOther chemical processesPaper material treatmentOxidized cellulosePeroxide
The invention provides a method for preparing adsorbable oxycellulose through a ramie oxidation degumming process. The method includes the steps that ramie original hemp serves as a raw material, the original hemp is smashed into short fibers through a smashing machine, and the short fibers are added with deionized water and soaked for pretreatment; a degumming solution is prepared from an oxidizing agent, a stabilizing agent, a fiber expanding agent, a penetrating agent, a chelating agent and water, the ramie original hemp and the degumming solution are mixed, gum in the ramie original hemp is fully removed by means of oxidizability of hyperoxide, and cellulose with active hydroxyl groups is oxidized into oxycellulose containing a great number of formyl groups; the oxycellulose is separated from water and dried to obtain the oxycellulose in the powder state. The method is simple in flow path, environmentally friendly, low in requirement for equipment and beneficial for large-scale production and has good economic and social benefits, the total reaction time is shortened, chemicals and cost are saved, efficiency is greatly improved, reaction conditions are moderate, and additional value of the cellulose is substantially increased.
Owner:DONGHUA UNIV
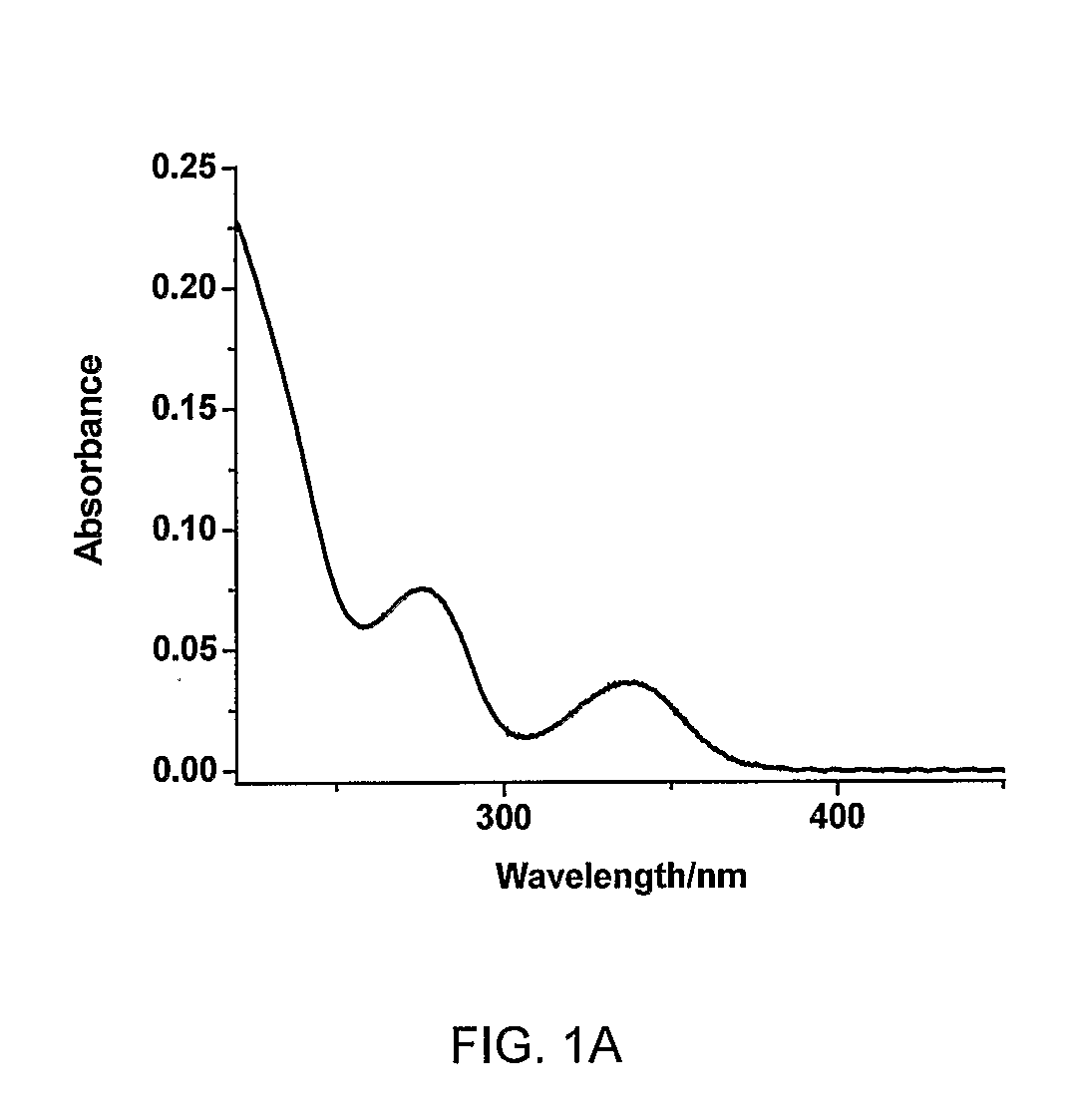
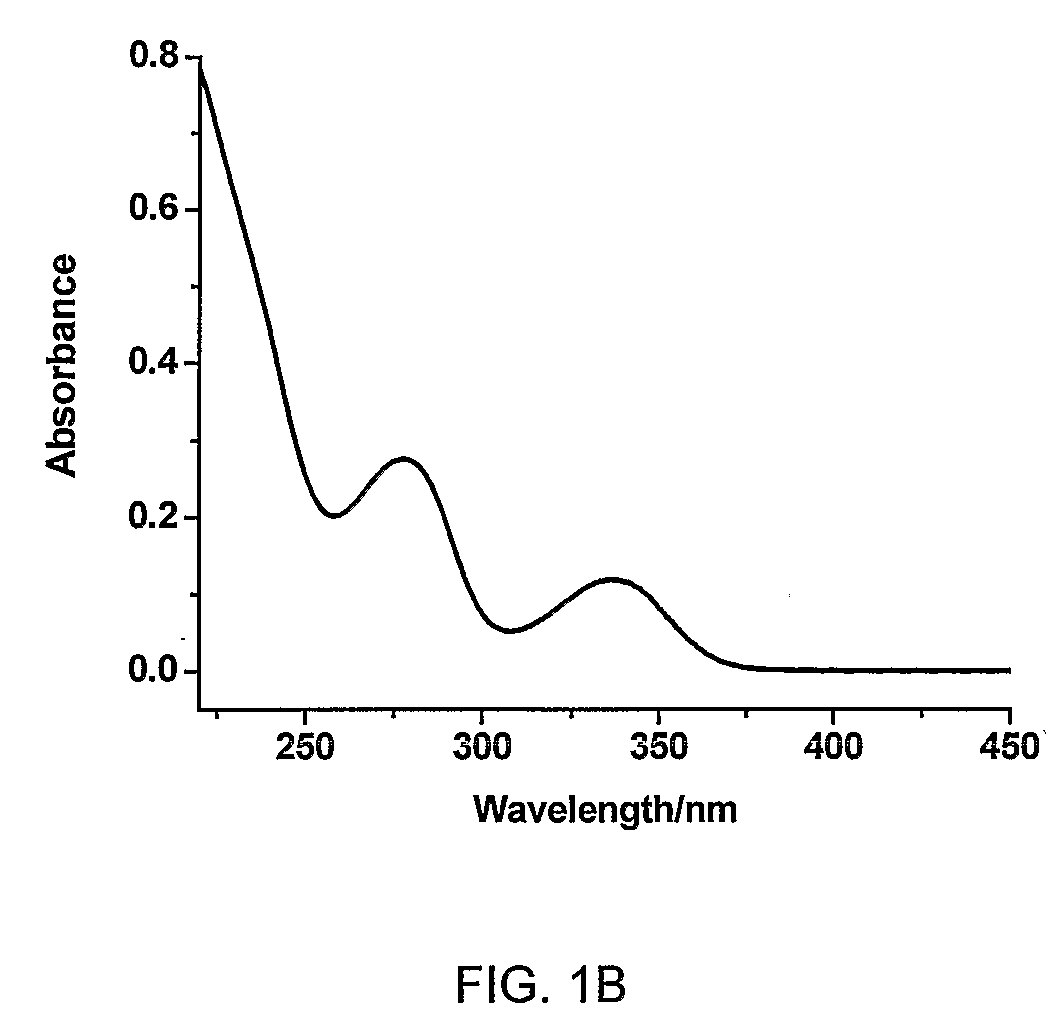
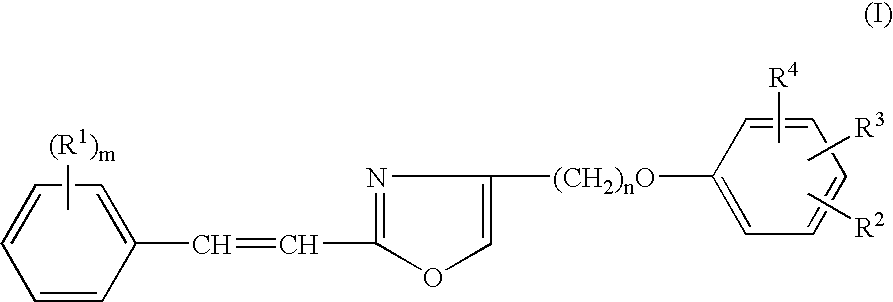
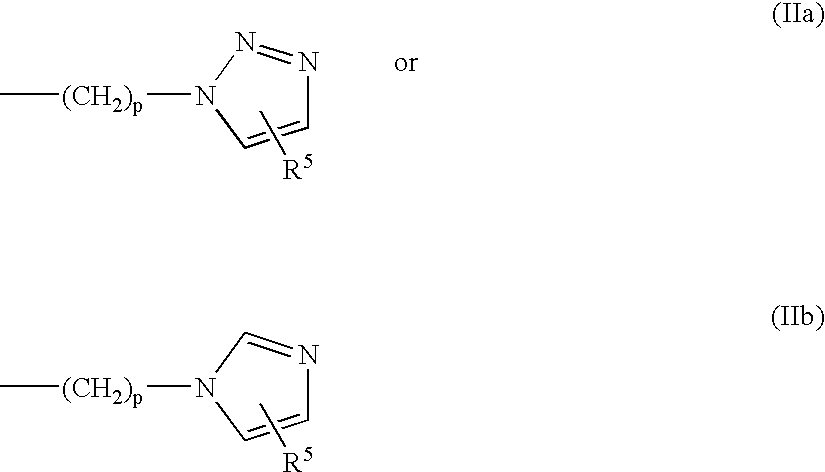
Heterocyclic compounds, oxazole derivatives, process for preparation of the same and use thereof
This invention provides a heterocyclic compound having potent tyrosine kinase-inhibiting activity represented by the formula: wherein m is an integer of 1 to 3; n is an integer of 1 or 2; R<1 >is a halogen atom or an optionally halogenated C1-2 alkyl group; each of R<2 >and R<3 >is, same or different, a hydrogen atom, a halogen atom, a lower alkyl group or a lower alkoxy group; R<4 >is a group represented by the formula: wherein p is an integer of 2 to 5; R<5 >is a C1-4 alkyl group substituted by alkoxycarbonyl group, carbamoyl group, carbamoyloxy group, alkylsulfonyl group, alkylsulfinyl group, sulfamoyl group, carbamoylamino group, alkylsulfonylamino group, acylamino group, and the like; or a salt thereof and a pharmaceutical composition comprising thereof.
Owner:TAKEDA PHARMA CO LTD
Preparation method of Niraparib
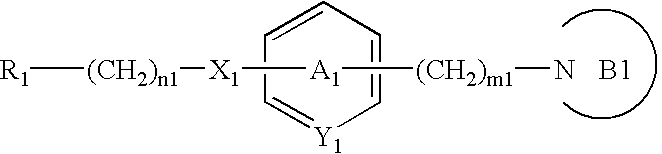
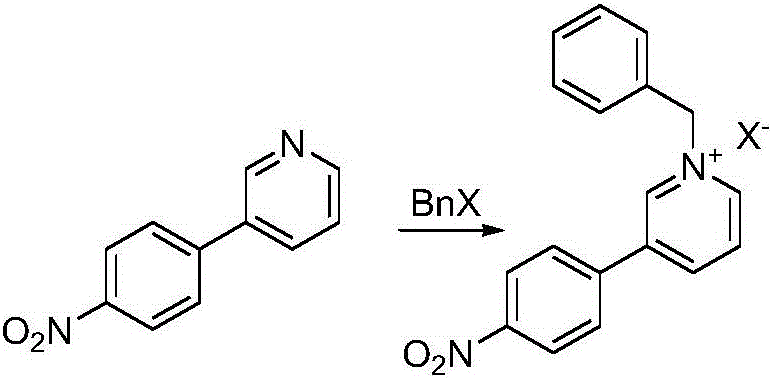
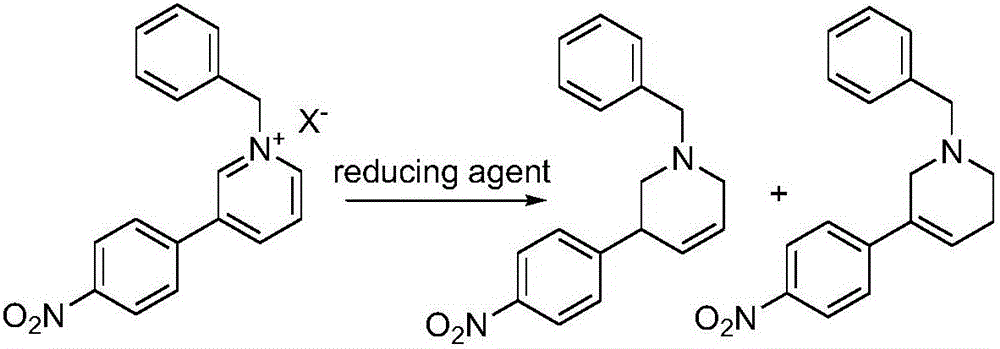
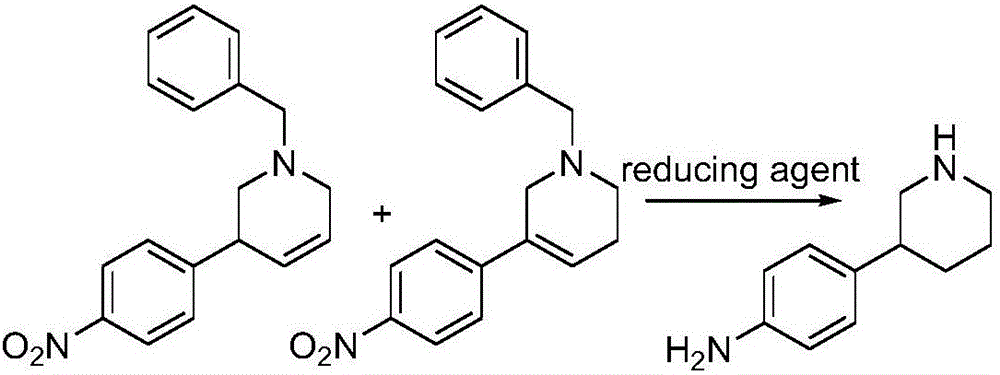
The invention discloses a preparation method of a compound 2-[4-((3S)-3-piperidin) phenyl]-2H-indazole-7-carboxamide; through the reaction of 4-nitryl phenylpyridine and benzyl halides, the benzyl quaternary ammonium salt is generated; the pyridine quaternary ammonium salt is restored selectively through sodium borohydride; under the effect of palladium reagent, the 3-(4- aminophenyl)- piperidine is obtained the (S)-3-(4- halogenated phenyl) piperidine is obtained through manually splitting the reagent, and then condensed with 3- formyl group-2- nitrobenzene methyl benzoate and forms pyrazol ring under the effect of sodium azide; through aminolysis, the Niraparib (molecule entity is: 2-[4-((3S)-3-piperidin) phenyl]-2H-indazole-7-carboxamide) is prepared.
Owner:NANJING CORE TECH CO LTD
Fused heterocyclic derivative, medicinal composition containing the same, and medicinal use thereof
ActiveUS20090325900A1Excellent GnRH antagonistic activityControl productionBiocideNervous disorderDiseaseCarboxyl radical
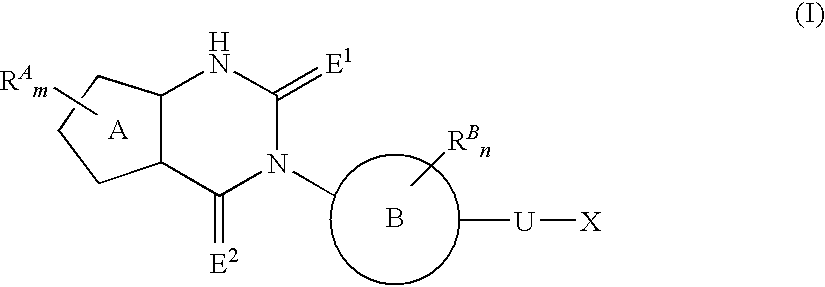
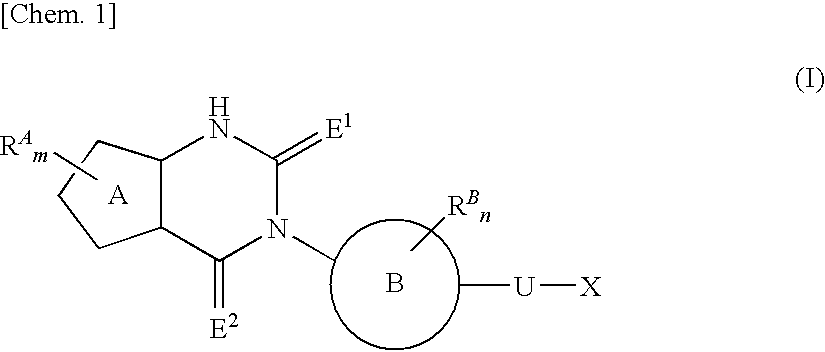
The present invention provides a compound useful as an agent for the prevention or treatment of a sex hormone-dependent disease or the like. That is, the present invention provides a fused heterocyclic derivative represented by the following general formula (I), a pharmaceutical composition containing the same, a medicinal use thereof and the like. In the formula (I), ring A represents 5-membered cyclic unsaturated hydrocarbon or 5-membered heteroaryl; RA represents halogen, alkyl, alkenyl, alkynyl, carboxy, alkoxy, carbamoyl, alkylcarbamoyl or the like; ring B represents aryl or heteroaryl; RB represents halogen, alkyl, carboxy, alkoxy, carbamoyl, alkylcarbamoyl or the like; E1 and E2 represent an oxygen atom or the like; U represents a single bond or alkylene; X represents a group represented by Y, —SO2—Y, —O— (alkylene)-Y, —O-Z in which Y represents Z, amino or the like; Z represents cycloalkyl, heterocycloalkyl, aryl, heteroaryl or the like; or the like.
Owner:KISSEI PHARMA
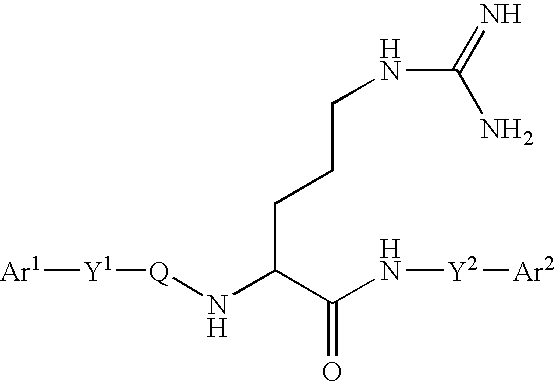
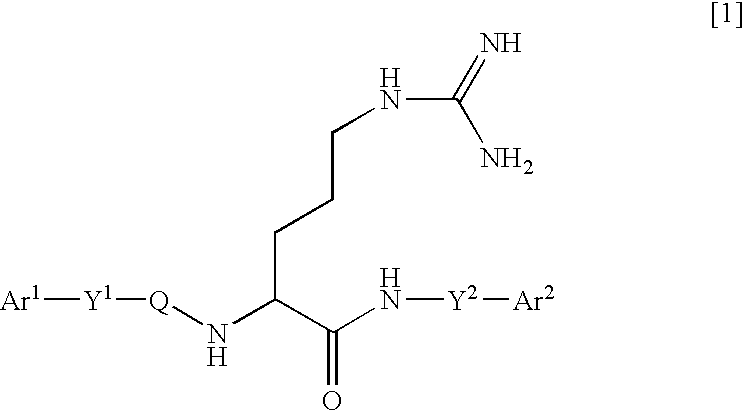
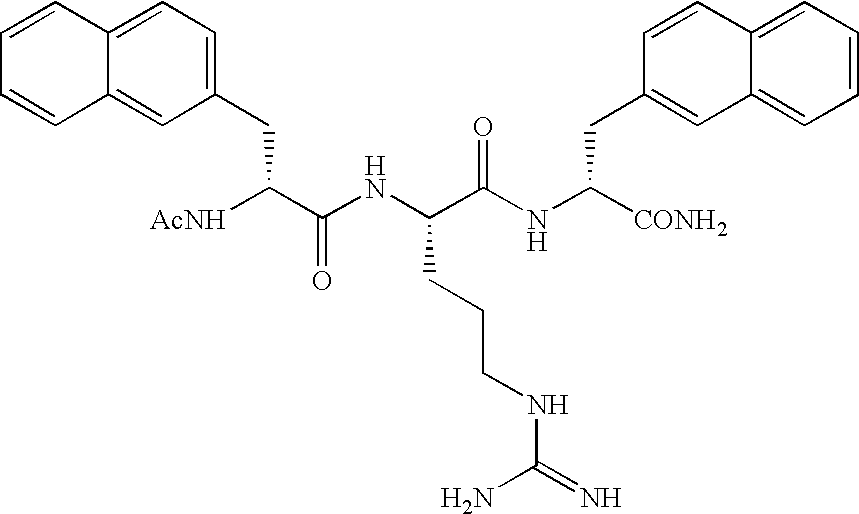
Arginine derivatives
An arginine derivative represented by the formula: [wherein Ar<1 >and Ar<2 >may be the same or different, and are each a phenyl group, a substituted phenyl group, a naphthyl group, a substituted naphthyl group or a heteroaromatic ring group containing one or more of nitrogen, oxygen and sulfur atoms; Y<1 >is a C1-5 alkylene group, a C2-5 alkenylene group or a single bond; and the C1-5 alkylene group optionally contains a carbon atom substituted with a phenyl group, a substituted phenyl group, a naphthyl group, a substituted naphthyl group or a C1-10 acylamino group; Q is a carbonyl group or a sulfonyl group; Y<2 >is a C1-5 alkylene group; the C1-5 alkylene group optionally contains a carbon atom substituted with a phenyl group, a substituted phenyl group, a naphthyl group, a substituted naphthyl group, a hydroxyl group, a carbamoyl group, a mono-C1-5 alkylamide group or a di-C1-5 alkylamide group], or a pharmaceutically acceptable salt thereof. There are provided peptidergic ligands which have the affinity and specificity to MC4 receptor.
Owner:TAISHO PHARMACEUTICAL CO LTD
Anthrapyridone compound or salt thereof, magenta ink composition and colored product
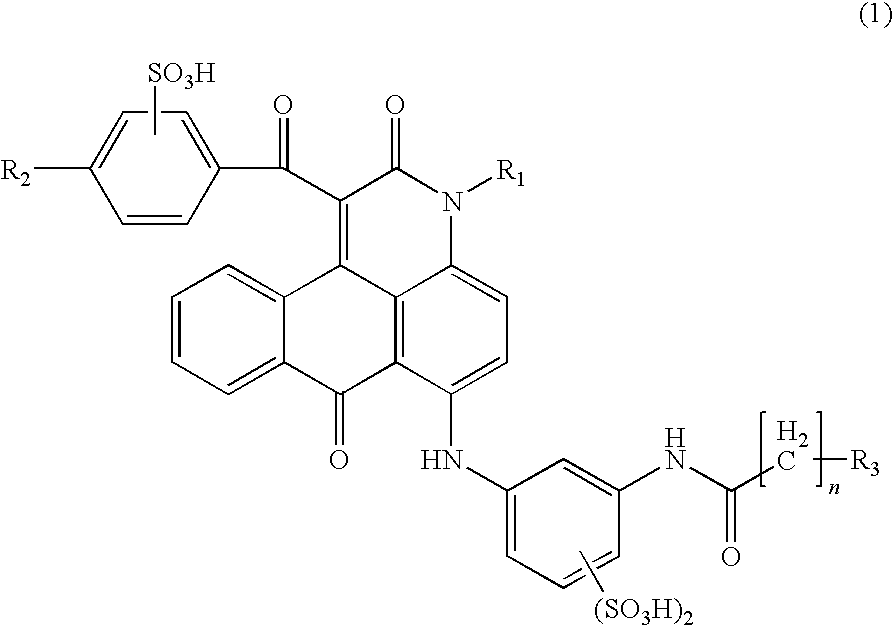
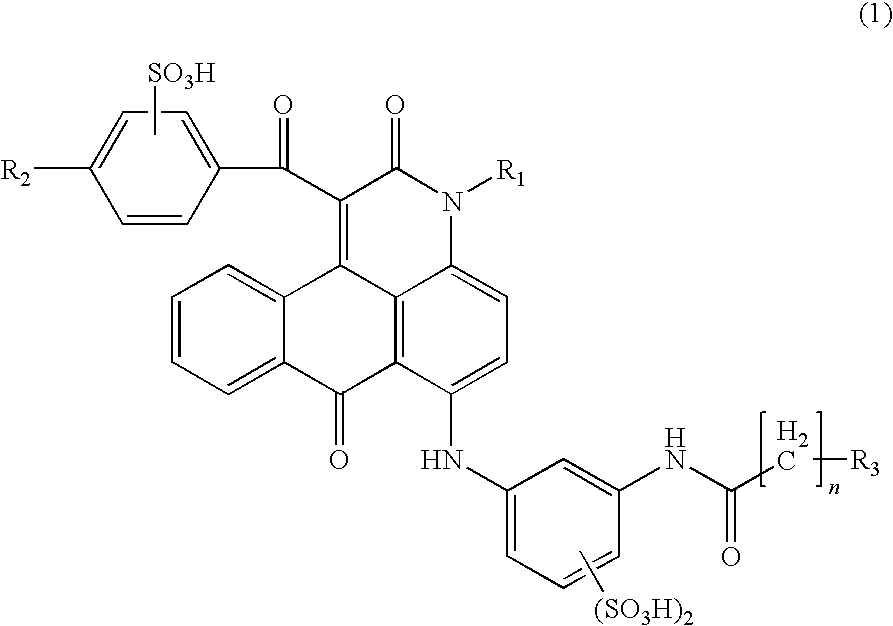
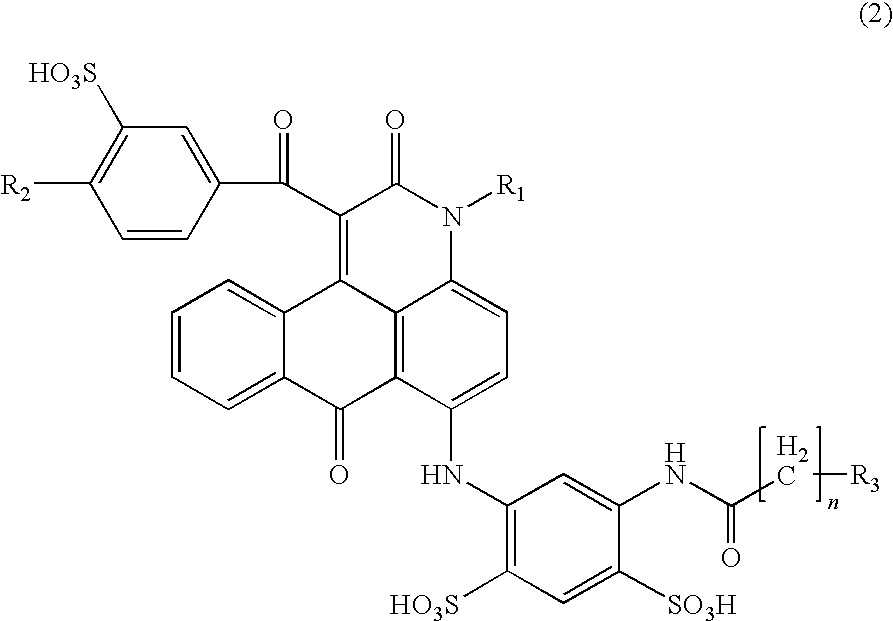
ActiveUS7871464B2Vivid and highly bright hueGood water solubilityOrganic chemistryMeasurement apparatus componentsCarboxyl radicalAcyl group
The present invention relates to an anthrapyridone compound represented by the following formula (1):wherein, n represents an integer number of 1 to 3, R1 represents a hydrogen atom, an alkyl group or the like, R2 represents a hydrogen atom or a methoxy group, R3 represents an anilino group having, as a substituent, at least one group selected from the group consisting of sulfo group, a carboxy group, an alkoxy group, a carbamoyl group, an cyano group, an alkyl group, an anilino group, a phenoxy group, an amino group, a hydroxy group and a mercapto group, an unsubstituted anilino group or the like, or a salt thereof, and provides a magenta coloring matter (compound) having high solubility in water and a hue and vividness which are suitable for inkjet recording and being excellent in fastnesses such as light fastness, moisture fastness and ozone gas fastness on recorded matter; and a magenta ink composition containing it.
Owner:NIPPON KAYAKU CO LTD
Azo dye, colored curable composition, color filter and producing method therefor
InactiveUS20050175908A1High resolutionHigh light transmittanceMonoazo dyesOperating means/releasing devices for valvesArylHalogen
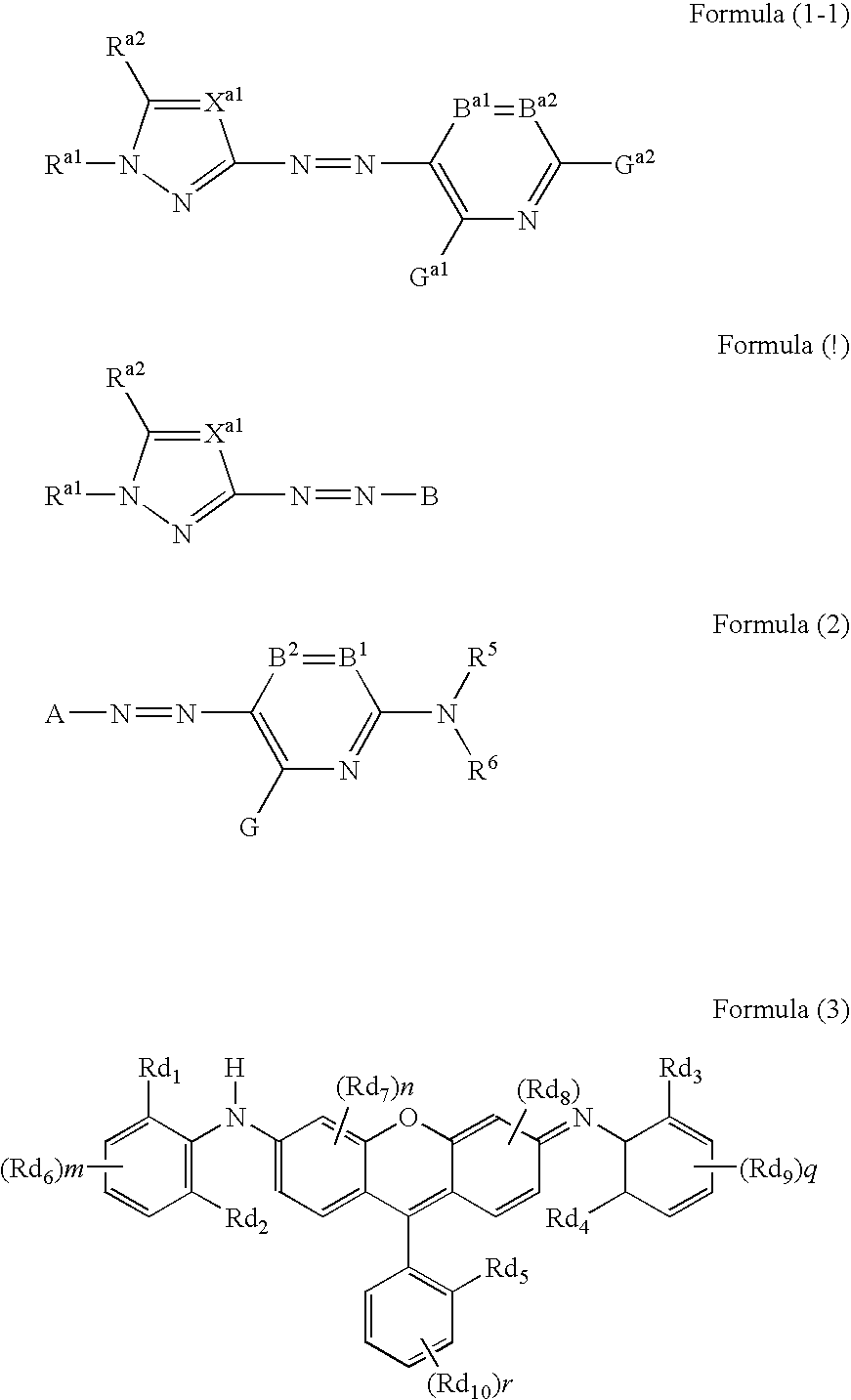
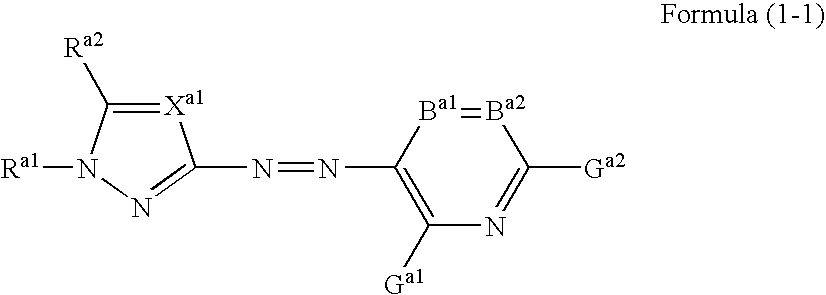
A dye represented by the following formula (1-1), a colored curable composition using the same, and a colored curable composition including a dye represented by the following formula (1) and at least one dye represented by the following formula (2) or (3): wherein Ra1 is an aliphatic group, an aryl group, a heterocyclic group, an acyl group or the like; Xa1 is —CRa3═ or N; Ra2 and Ra3 are each H or a substituent; B is a coupler residue; A is a residue of a 5-membered heterocyclic diazo component A-NH2; B1 and B2 is —CR1═, —CR2═ or N; R5 and R6 is H, an aliphatic group, an aromatic group, a heterocyclic group or the like; G, R1 and R2 is H, halogen, an aliphatic group, an aromatic group, a heterocyclic group, a cyano group, a carboxyl group, a carbamoyl group, an alkoxycarbonyl group or the like; Rd1 to Rd4 is H or an aliphatic group; Rd5 is a sulfo group or a sulfamoyl group; Rd6 to Rd10 is a substituent; m, n, p and q=0 to 3; r=0 to 4
Owner:FUJIFILM HLDG CORP +1
Preparation method of covalent organic framework/carbon nitride composite material and application thereof
ActiveCN108246339ASimple preparation processShort synthesis timeWater treatment compoundsWater contaminantsNano catalystCarbon composites
The invention discloses a preparation method of a covalent organic framework / carbon nitride composite material and application thereof. Firstly, a covalent organic framework material is synthesized byusing p-phenylenediamine and triformyl phloroglucinol as synthetic monomers through a room-temperature solid-phase method, then the covalent organic framework material and carbon nitride are mixed, stirred and dried in methyl alcohol, and pyrolysis is carried out under the protection of inert gas to obtain a target product. The covalent organic framework / carbon nitride composite material disclosed by the invention is large in specific surface area, high in nitrogen doping amount and rich in pore structure and can effectively remove toxic organic pollutants in water by constructing a novel Fenton-like system, and new application of the covalent organic framework material in the field of catalysis is expanded; unavoidable metal ion leaching in the activating process of a metal nano catalystis overcome; and the preparation method has the advantages of simple process, easiness for repeated operation, controllable structure, suitability for large-scale production and the like.
Owner:HEFEI UNIV OF TECH
Preparation method of conjugated three-dimensional porphyrin-based covalent organic framework material
ActiveCN110294843AImprove biomimetic catalytic performanceOrganic-compounds/hydrides/coordination-complexes catalystsFiltrationPorphyrin
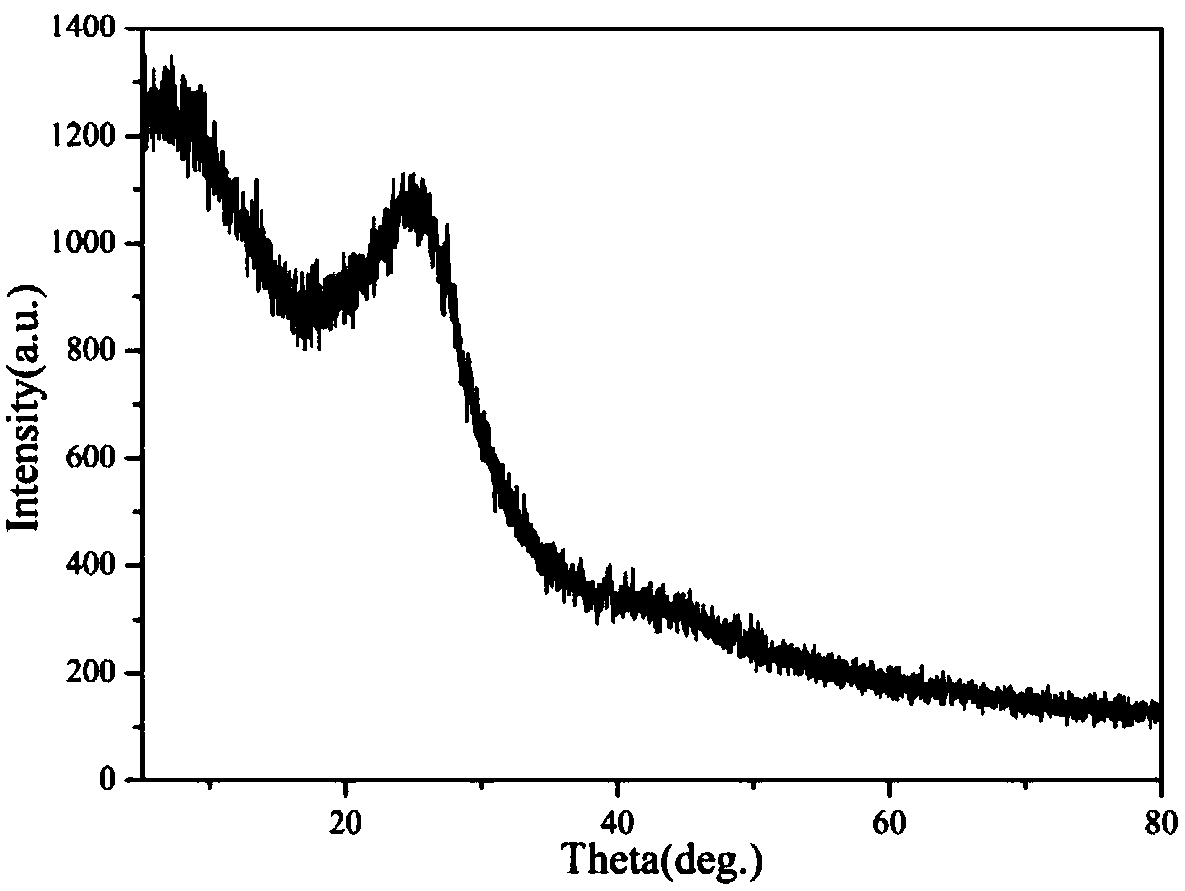
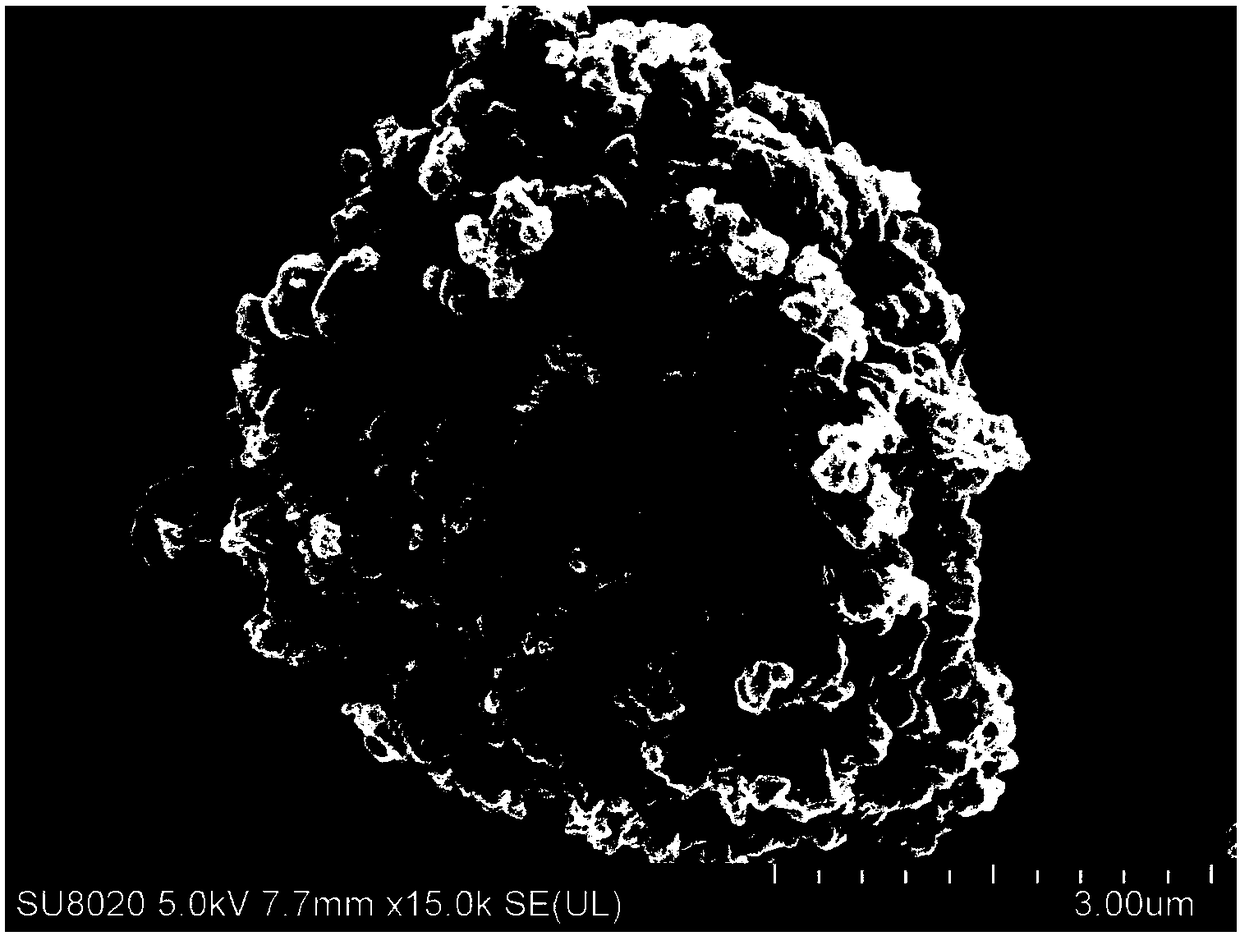
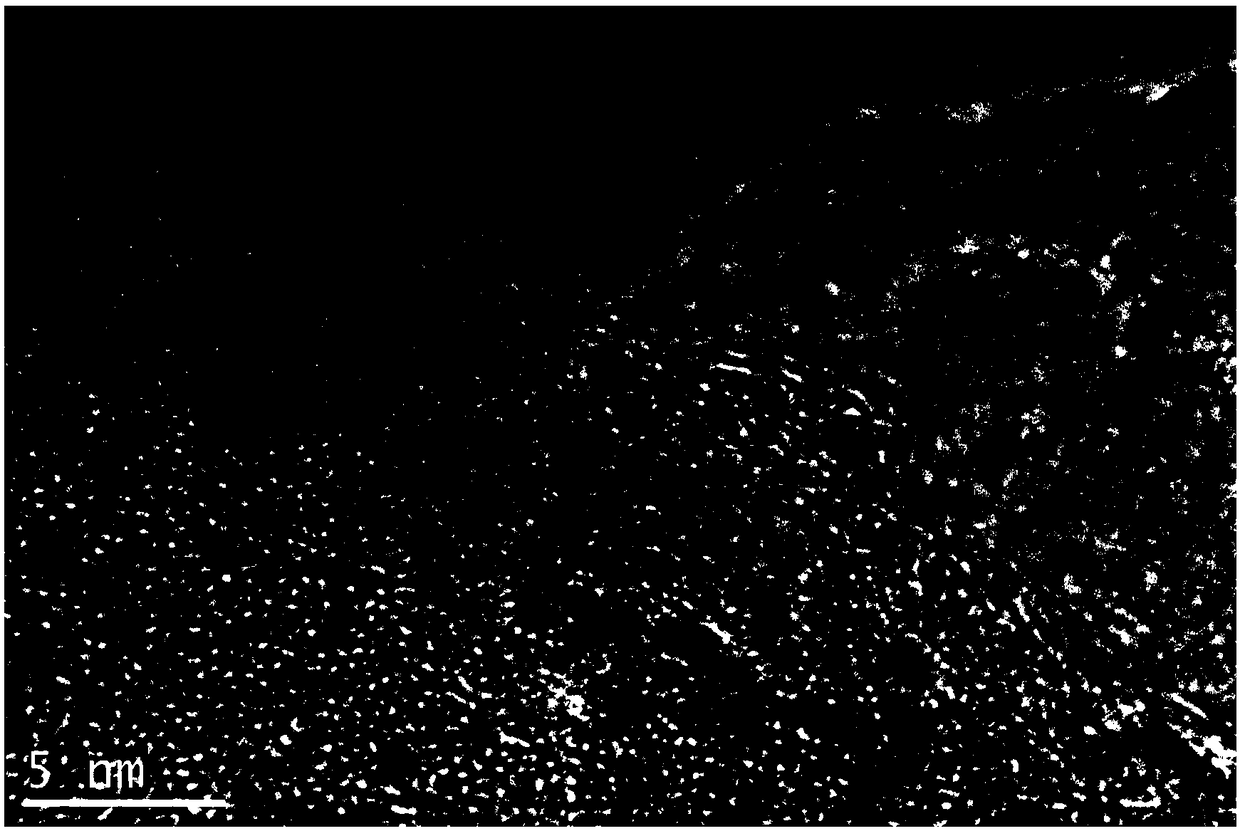
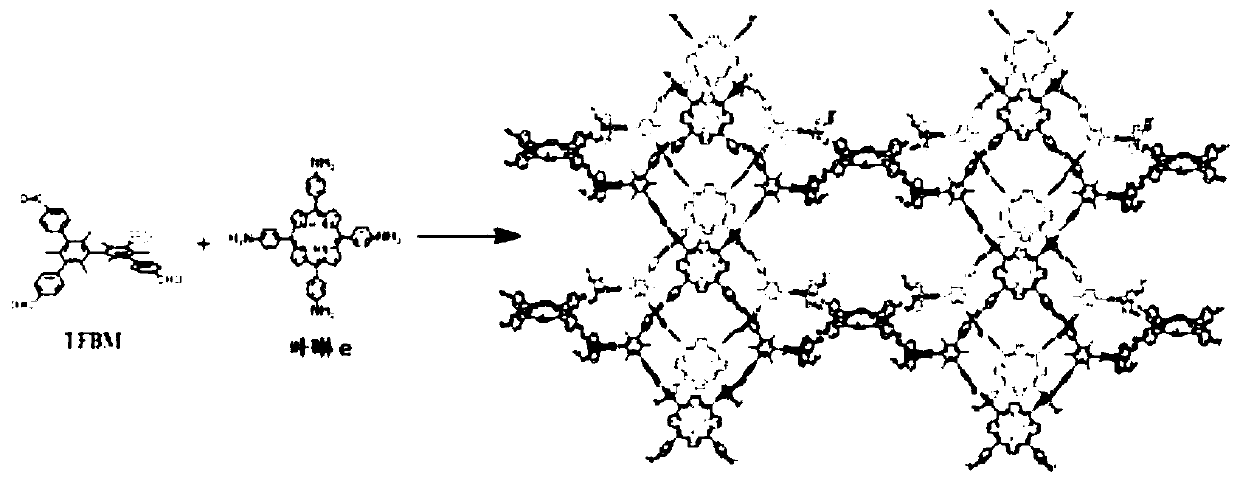
The invention discloses a preparation method of a conjugated three-dimensional porphyrin-based covalent organic framework material. The preparation method comprises the following steps: taking 3,3', 5,5'-tetra (4-formylphenyl) hexamethylbiphenyl (TFBM) and a series of porphyrin derivatives as raw materials, o-dichlorobenzene and n-butanol as solvents and acetic acid as a catalyst, and carrying outsolvothermal reaction for several days; after the reaction is finished, sequentially using DMF and THF for suction filtration and washing, carrying out soxhlet extraction for 24 hours, and carrying out vacuum drying to obtain purple black powder, namely the target product. For the first time, tetrahedral tetraaldehyde based on steric hindrance effect is used as a building block, so that the material has a fully conjugated three-dimensional skeleton structure, good thermal stability and chemical stability, and porphyrin units facing three-dimensional pore channels are used as single active catalytic sites, so that the material shows good application prospects in the field of bionic catalysis.
Owner:JIANGNAN UNIV
Polyacetal resin composition
The present invention provides a polyacetal resin composition having a metallic appearance, suppressing the generation of volatile organic compound (VOC), in particular formaldehyde from the molded article thereof to an extremely low level and giving excellent weathering (light) resistance. Specifically, (A) 100 parts by weight of a polyacetal copolymer containing 1.0 mmol / kg or smaller of a hemiformal terminal group, 2.0 mmol / kg or smaller of a formyl terminal group, 0.5 wt % or smaller of an unstable terminal group, (B) 0.03 to 0.30 part by weight of a hindered phenol-based antioxidant, (C) 0.01 to 1 part by weight of a guanamine compound, (D) 0.2 to 1 part by weight of a hindered amine-based stabilizer, and (E) 0.1 to 1 part by weight of an ultraviolet absorber, and (F) 1 to 20 parts by weight of a metallic pigment are blended together.
Owner:POLYPLASTICS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com