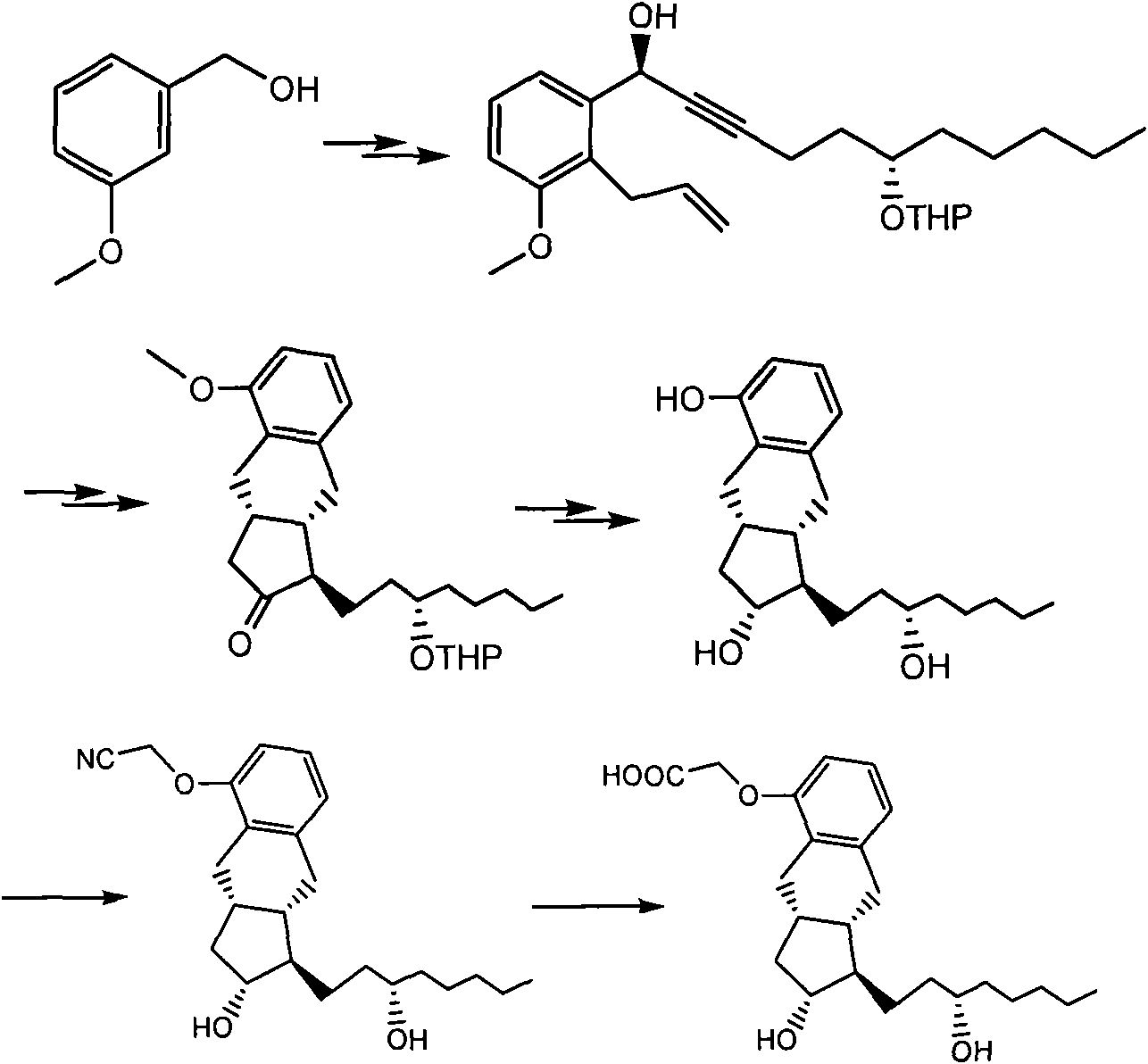
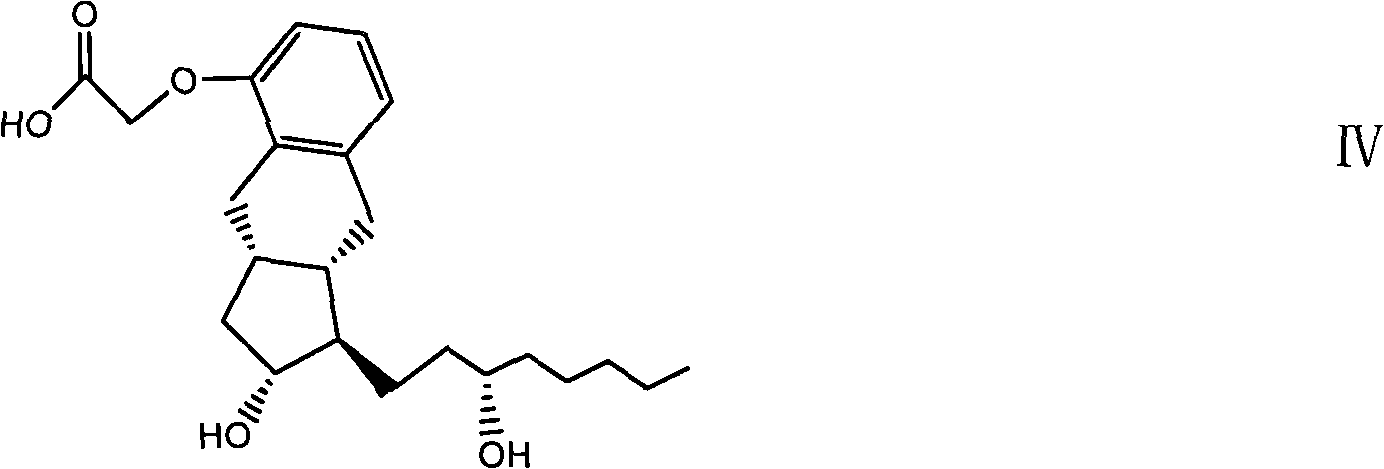
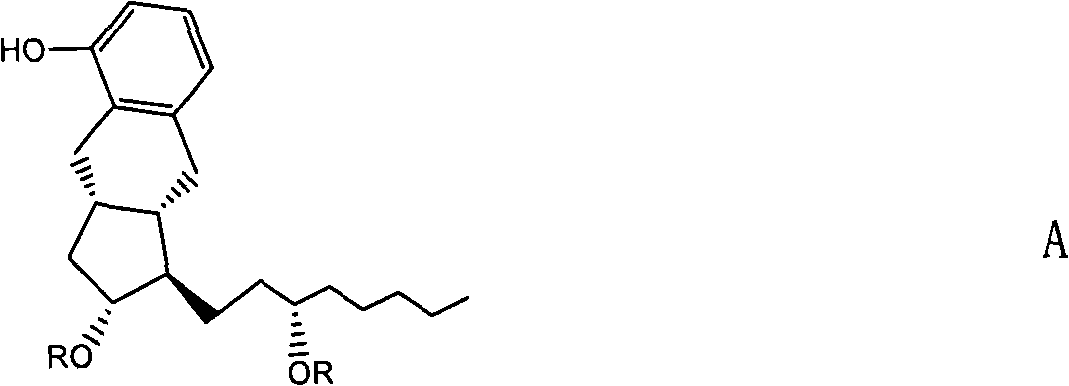
Novel compound and preparation method and use thereof
A compound and application technology, applied in the field of chemical synthesis, can solve problems such as complicated operation, and achieve the effect of less by-products and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0097] The prostacyclin UT15 obtained by the above-mentioned preparation method of the present invention can be used to prepare a medicine for treating pulmonary hypertension, and the medicine contains an effective amount of prostacyclin UT15 and a pharmaceutically acceptable carrier.
[0098] As used herein, the terms "comprising" or "including" include "comprising," "consisting essentially of," and "consisting of."
[0099] As used herein, the term "consisting essentially of" means that the composition may contain, in addition to essential ingredients or essential ingredients, minor amounts of minor ingredients and / or impurities that do not affect the active ingredient. For example, sweeteners to improve taste, antioxidants to prevent oxidation, and other additives commonly used in the art may be included.
[0100] As used herein, the term "effective amount" refers to an amount that produces function or activity in humans and / or animals and is acceptable to humans and / or ani...
Embodiment 1
[0115] Preparation of compound I
[0116] Compound II (2.80g) and ethyl acetate (56ml) were added to a 1L hydrogenation shaker flask, 10% palladium on carbon (0.28g) was added, and the mixture was shaken for 8h at 20-25°C under a hydrogen pressure of 50-60Psi. A small amount of celite was added for filtration, and the filtrate was concentrated to dryness under reduced pressure to obtain compound I. m / z: 523.3 (M+Na + ); 1 H NMR (MeOD, 500MHz) δ 0.96 (t, 3H), 1.25-1.81 (m, 29H), 2.68-2.93 (m, 6H), 3.55-3.60 (m, 4H), 4.95 (m, 2H), 6.45(d, 1H), 6.71(d, 1H), 6.92(t, 1H); 13 CNMR (MeOH, 125MHz) δ 14.3, 20.1, 20.2, 23.8, 25.2, 25.4, 26.9, 29.9, 30.8, 30.9, 33.5, 34.3, 34.7, 38.4, 41.7, 42.2, 52.2, 63.1, 63.3, 73.1, 76.9, 105.5, 105.7, 113.9, 120.5, 126.2, 127.2, 132.4, 133.5, 141.2, 155.2.
Embodiment 2
[0118] Preparation of compound III
[0119] Compound I obtained in Example 1 was dissolved in 14 ml of tetrahydrofuran, 21 ml of water and 42 ml of glacial acetic acid were added, and the mixture was stirred to dissolve. N 2 Under protection, it was heated to 45 °C and stirred for 4 h. Slowly cooled to room temperature, half-saturated brine (120ml) was added, ethyl acetate (120ml) was stirred and the layers were separated, the aqueous layer was back-extracted with ethyl acetate (60ml), the ethyl acetate layers were combined, saturated sodium bicarbonate (300ml*2 ), washed with saturated sodium chloride (100 ml), dried over anhydrous sodium sulfate, and concentrated to dryness under reduced pressure to give a colorless oily compound. The oil was purified by silica gel column chromatography to obtain compound III (1.2 g) as a white solid product. mp113-115℃; [a] 25 D+50.8 (c 0.324, MeOH). IR 3415, 3060, 2932, 753, and 702 cm-1; m / z: 355.3 (M+Na + ); 1 H NMR (MeOD, 500MHz)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com