Patents
Literature
736 results about "Phenacyl" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
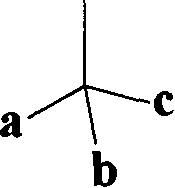
In organic chemistry, a phenacyl group is an aromatic substituent that consists of a phenyl group attached to an acyl group. A molecule containing a phenacyl group has the formula RCH₂(CO)C₆H₅ and the structure shown to the right. Here, R denotes the remainder of the molecule; for instance, if R is Br, then the compound could be called "phenacyl bromide". Note however that in the standard IUPAC nomenclature this compound would instead be called "2-bromo-1-phenylethanone".
Slow release of fragrant compounds in perfumery using 2-benzoyl benzoates, 2-alkanoyl benzoates or alpha -keto esters
A fragrance delivery system which releases fragrant alcohols upon exposure to light. The system comprises 2-benzoyl benzoates of general formulae which can comprise various subtituents R1-R5 as defined in the application and a substituted R* which is the organic part of a fragrant alcohol.
Owner:FIRMENICH SA
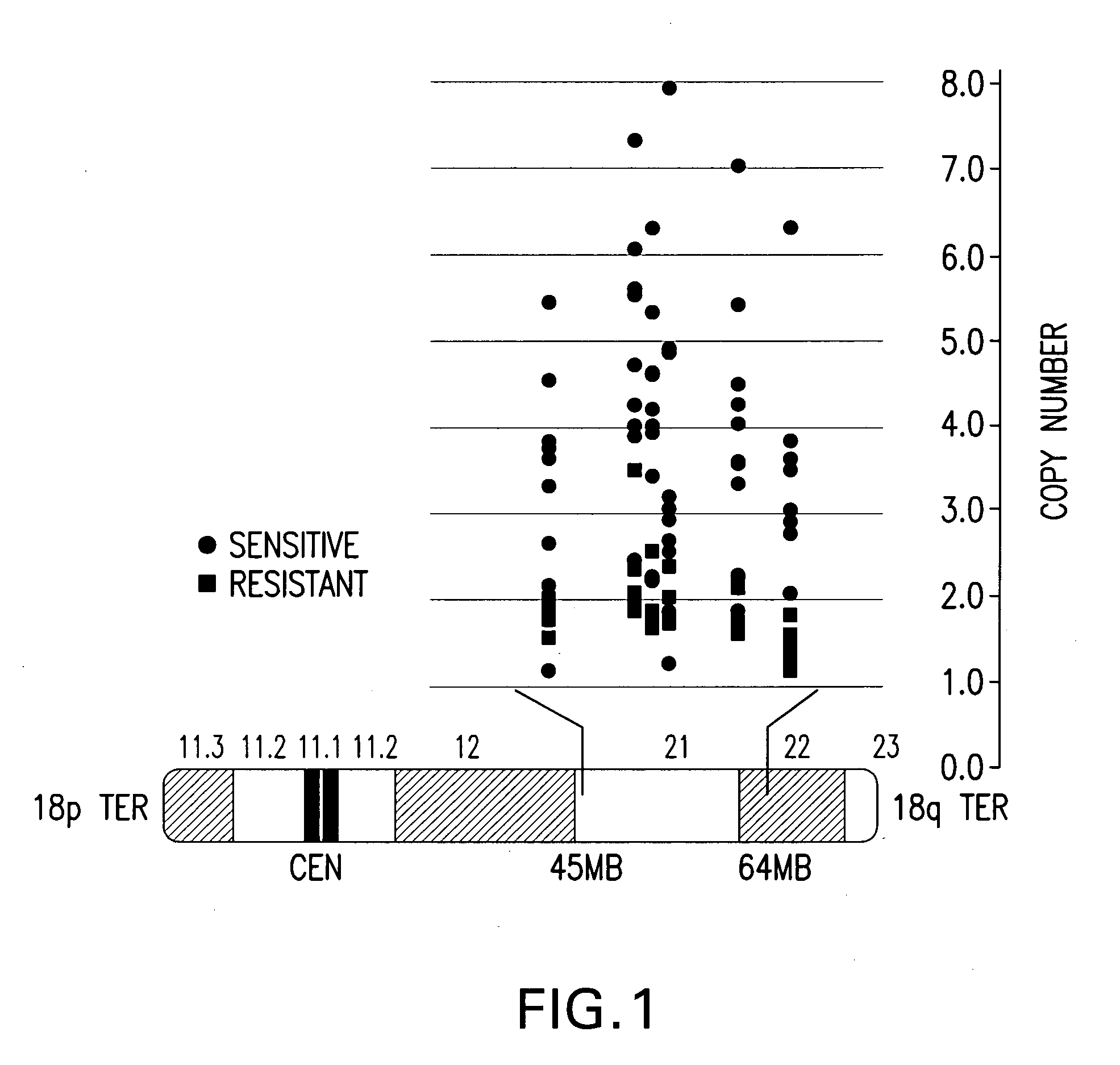
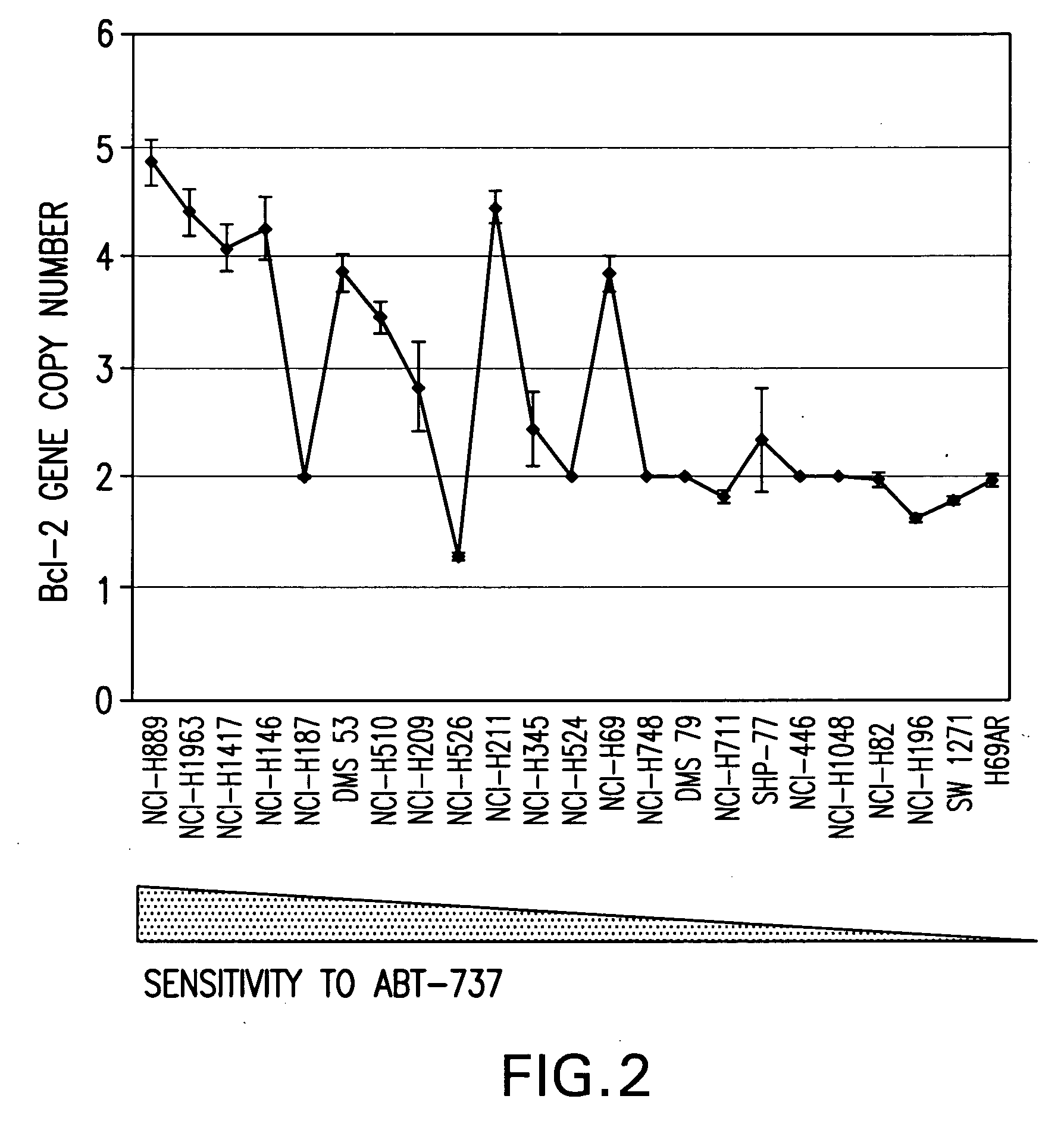
Companion diagnostic assays for cancer therapy
InactiveUS20080193943A1Promote stratificationParticular utilitySugar derivativesMicrobiological testing/measurementPhenacylOncology
A method for classifying cancer patients as eligible to receive cancer therapy with a small molecule inhibitor of Bcl-2 comprising determination of the presence or absence in a patient tissue sample of chromosomal copy number status at the chromosomal locus 13q14 comprising the microRNA's miR-15a and miR-16-1 or at the chromosomal locus 11q23.1 comprising the microRNA miR-34c. The classification of cancer patients based upon the presence or absence of 13q14 loss or gain allows better selection of patients to receive chemotherapy with a small molecule Bcl-2 inhibitor such as N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-1-cyclohex-1-en-1-yl) methyl)piperazin-1-yl)benzoyl)-4-(((1R)-3-(morpholin-4-yl)-1-((phenylsulfanyl) methyl)propyl)amino)-3-((trifluoromethyl)sulfonyl)benzenesulfonamide, and for monitoring patient response to this therapy.
Owner:ABBOTT LAB INC
Antifungal compound of alkyl substitutional triazole class
An alkyl substituted triazazole type antifungal compound for preparing the antifungal medicines, and its various enantiomers, diaisomers, Nox and precursors are disclosed.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Pharmaceutical compositions
The present invention relates to a pharmaceutical composition comprising primarily amorphous 2-[4-(4-chlorobenzoyl)phenoxy]-2-methyl-propanoic acid, 1-methylethyl ester.
Owner:ROSENBERG JOERG +5
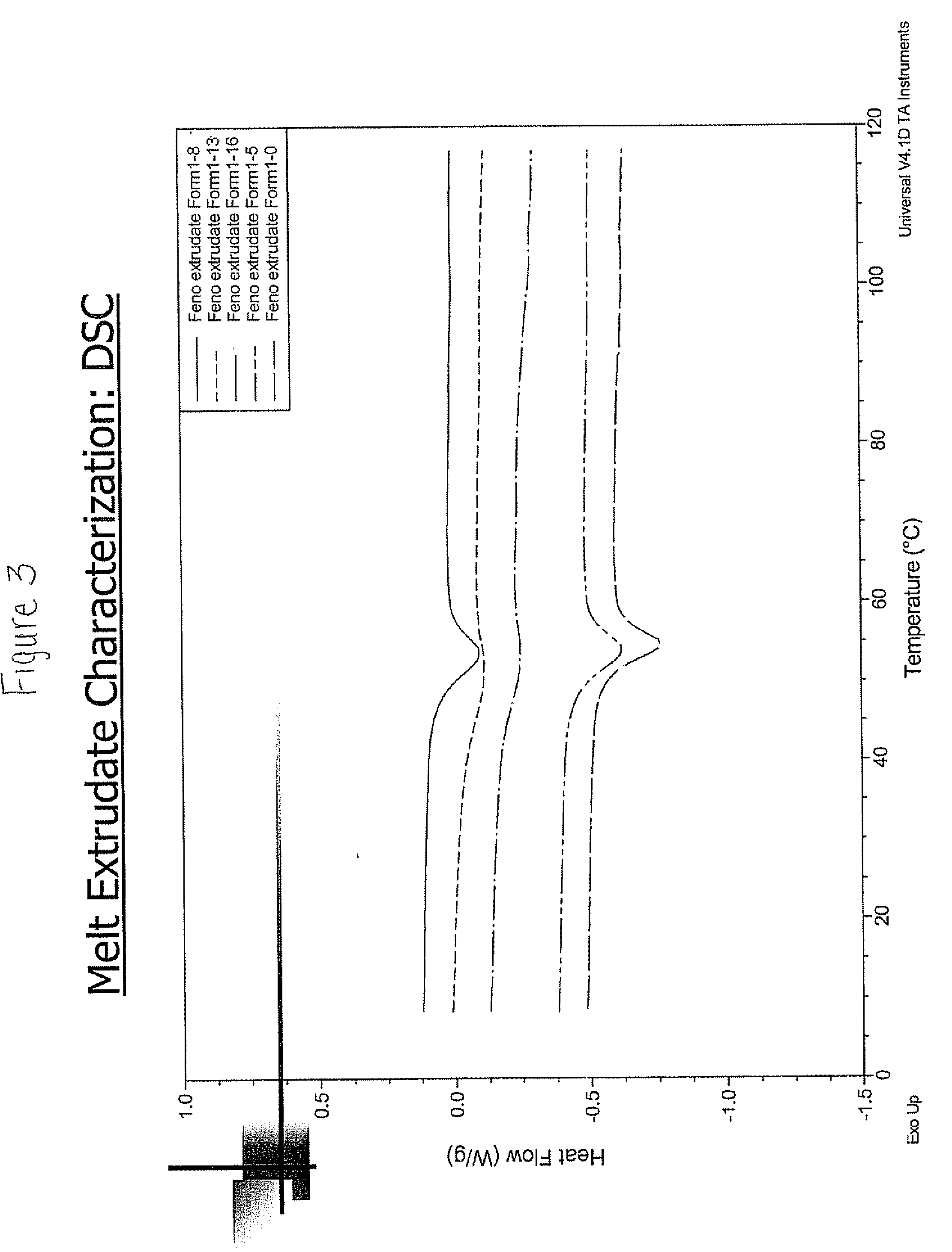
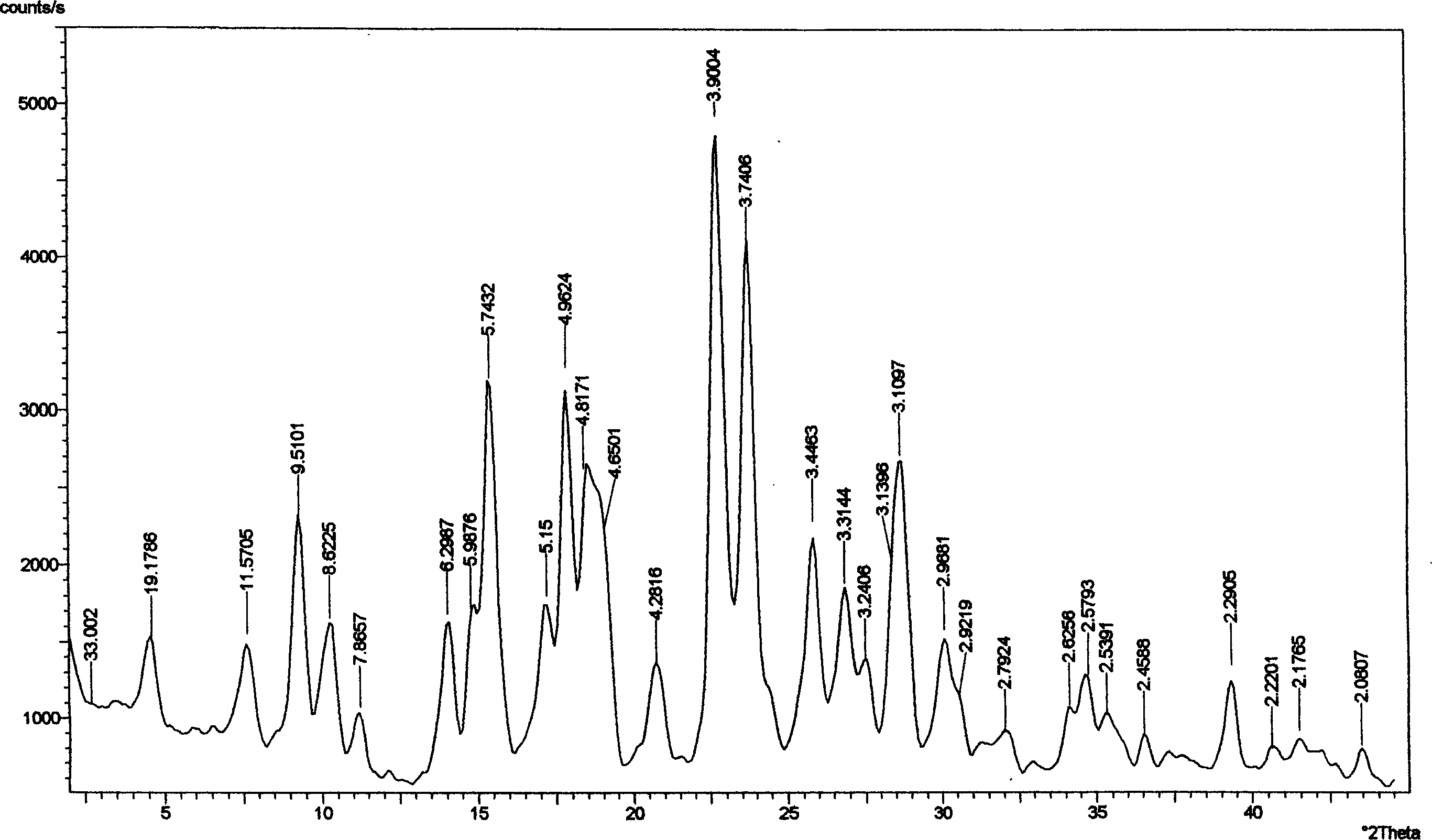
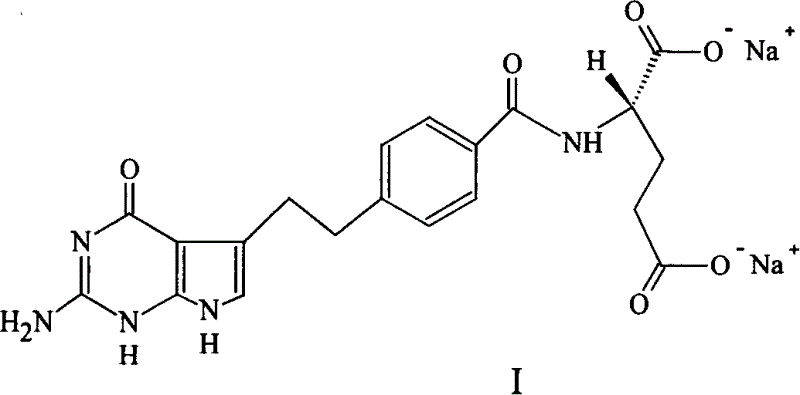
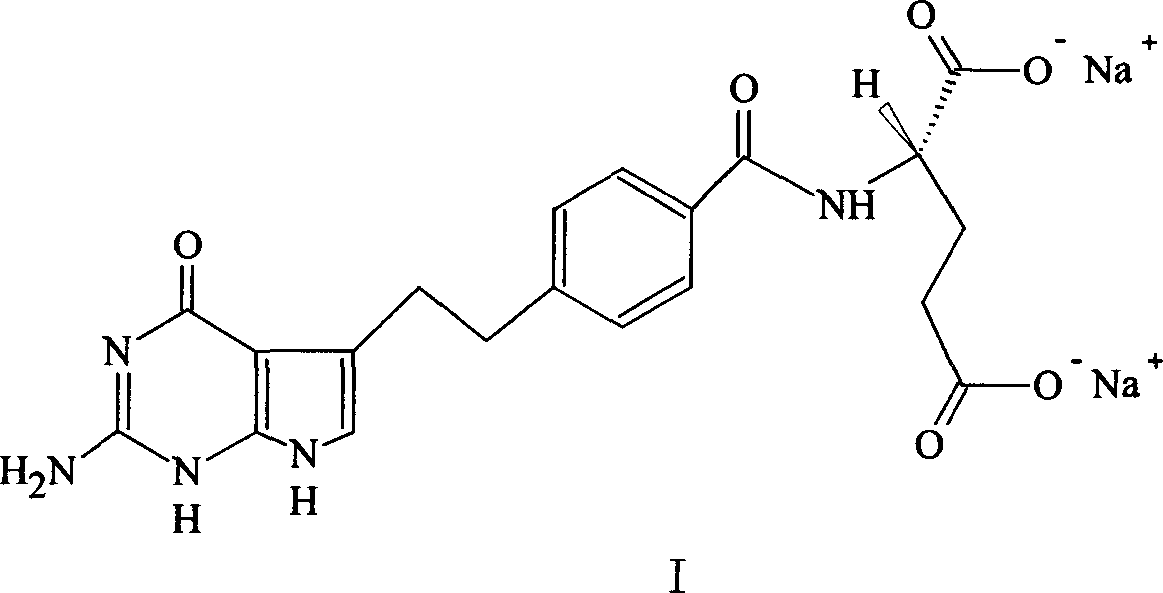
Crystal form of Peimeiqusai disodium and its preparation
ActiveCN1778802AImprove controllabilitySimple and fast operationOrganic active ingredientsOrganic chemistryPhenacylPemetrexed disodium
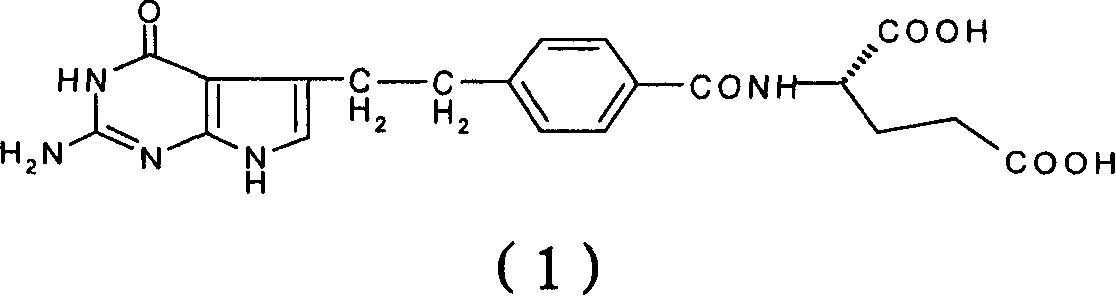
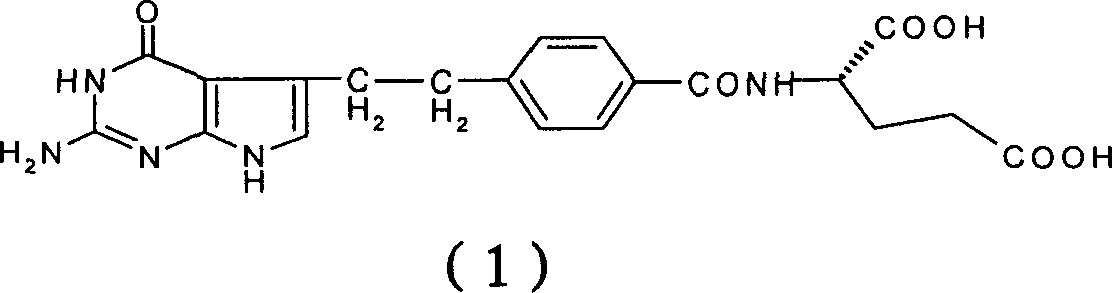
A crystal form of folic acid antagonistic N-(4-(2-(2-amino-4,7-methyl-4-oxo-1H-pyrrolo(2,3-d)pyrimidine-5-radical)ethyl)benzoyl)-L-glutamic acid disodium salt and its production are disclosed.
Owner:重庆凯林制药有限公司 +2
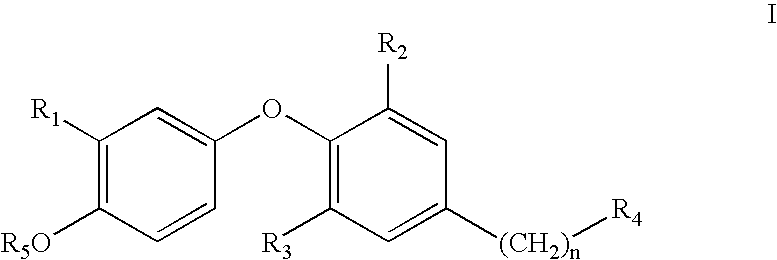
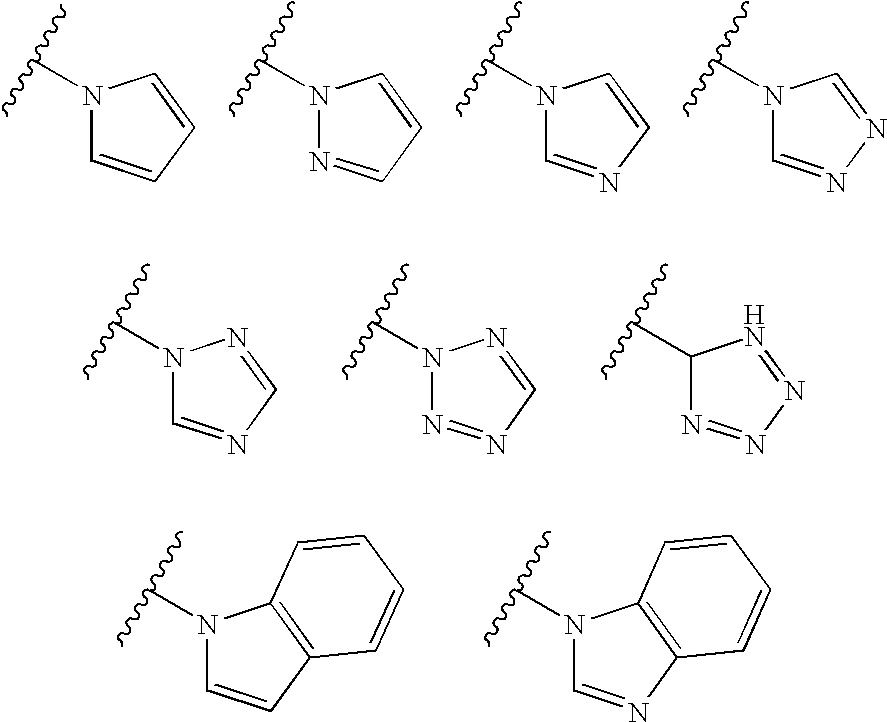
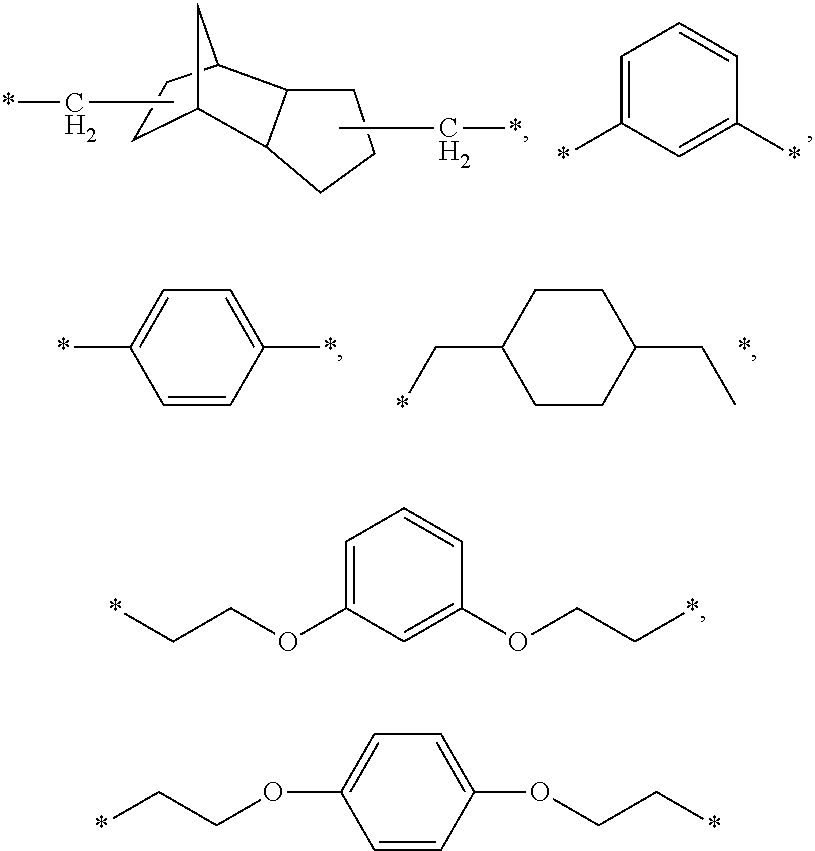
Thyroid receptor ligands and method II
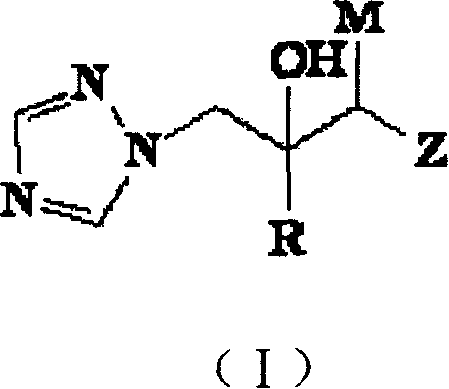
New thyroid receptor ligands are provided which have general formula (I) in which: n is an integer from 0 to 4; R1 is halogen, trifluoromethyl, or alkyl of 1 to 6 carbons or cycloalkyl of 3 to 7 carbons; R2 and R3 are the same or different and are hydrogen, halogen, alkyl of 1 to 4 carbons or cycloalkyl of 3 to 5 carbons, at least one of R2 and R3 being other than hydrogen; R4 is a carboxylic acid amide (CONR′R″) or an acylsulphonamide (CONHSO2R′) derivative, or a pharmaceutically acceptable salt thereof, and all stereoisomers thereof; or when n is equal to or greater than one, R4 may be a heteroaromatic moiety which may be substituted or unsubstituted, or an amine (NR′R″). R5 is hydrogen or an acyl (such as acetyl or benzoyl) or other group capable of bioconversion to generate the free phenol structure (wherein R5=H). In addition, a method is provided for preventing, inhibiting or treating a disease associated with metabolism dysfunction or which is dependent upon the expression of a T3 regulated gene, wherein a compound as described above is administered in a therapeutically effective amount. Examples of such diseases associated with metabolism dysfunction or are dependent upon the expression of a T3 regulated gene include obesity, hypercholesterolemia, atherosclerosis, cardiac arrhythmias, depression, osteoporosis, hypothyroidism, goiter, thyroid cancer as well as glaucoma, congestive heart failure and skin disorders.
Owner:KARO BIO AB
Dental composition, method of producing and use thereof
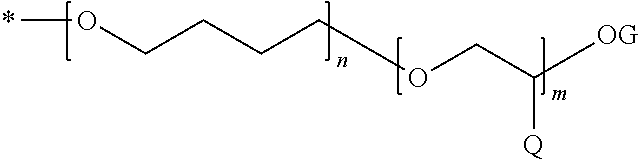
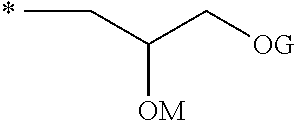
The invention relates to a dental composition havinga) compound (A) with the following features:only one backbone unit (U) with 6 to 20 carbon atoms, at least 6 carbon atoms thereof forming an aromatic or an aliphatic cyclic moiety, the remaining carbon atoms either being part of substituents pending from the cyclic moiety or being part of bridging groups to spacer units, wherein one or more of the remaining carbon atoms can be replaced by an oxygen atom, the backbone unit not comprising a bisphenol structure,one or two spacer unit(s) (S) being connected to the backbone unit (U) via an ether linkage, at least one spacer unit (S) having a —CH2-CH2-CH2-CH2-O-CH2-CH(Q)-OG chain or a —CH2-CH(OG)-CH2-OM residue or a mixture of these two types of spacers within one spacer unit,with G having at least one group selected from acroyl, methacroyl, acetyl, benzoyl, and combinations thereof, M having at least one aryl group, and combinations thereof, Q having at least one group selected from hydrogen, methyl, phenyl, phenoxymethyl, and combinations thereof,b) filler (B) and c) initiator (C).
Owner:3M INNOVATIVE PROPERTIES CO
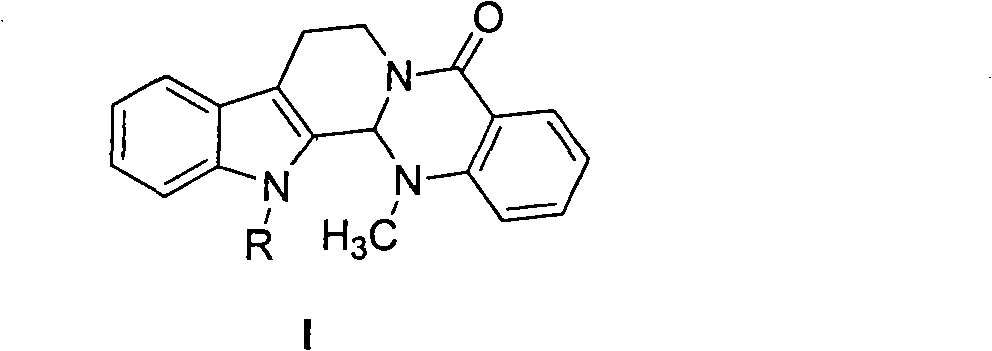
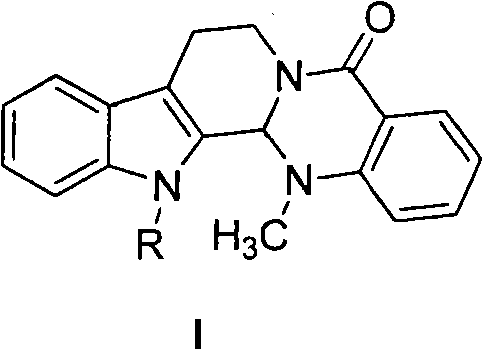
Substituted-evodiamine anti-tumor and antifungal compounds and preparation method thereof
The invention discloses substituted-evodiamine anti-tumor and antifungal compounds and a preparation method thereof. The compounds have a general structural formula (I), wherein R represents: (1) benzyl or substituted benzyl; (2) benzoyl or substituted benzoyl; (3) linear or branched alkyl having 2 to 6 carbon atoms; and (4) an ester group. The substituted-evodiamine compounds of the invention have good anti-tumor activities for various tumor cells, provide a new way for deep research and development of new anti-tumor medicaments and can be used for preparing new anti-tumor medicaments.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Novel compound and preparation method and use thereof
InactiveCN101891715AEasy to operateMild responseGroup 4/14 element organic compoundsOrganic reductionMedicinal chemistrySilicon based
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
Positive photosensitive composition
InactiveUS6858370B2Increase exposureAvoid sensitivityPhotosensitive materialsRadiation applicationsActinic RaysAlicyclic Hydrocarbons
A positive photosensitive composition comprises: (A) an acid generator capable of generating an acid upon irradiation with one of an actinic ray and a radiation; and (B) a resin having a monocyclic or polycyclic alicyclic hydrocarbon structure and capable of decomposing by the action of an acid to increase the solubility in an alkali developer, wherein the acid generator (A) comprises at least two compounds of a sulfonium salt compound not having an aromatic ring, a triarylsulfonium salt compound, and a compound having a phenacylsulfonium salt structure.
Owner:FUJIFILM HLDG CORP +1
Preparation method of 3,5-dibenzoyl-2-deoxy-2-fluoro-2-methyl-D-ribo-gamma-lactone
InactiveCN103804446AMild reaction conditionsEasy to operateSugar derivativesSugar derivatives preparationAlkanePhenacyl
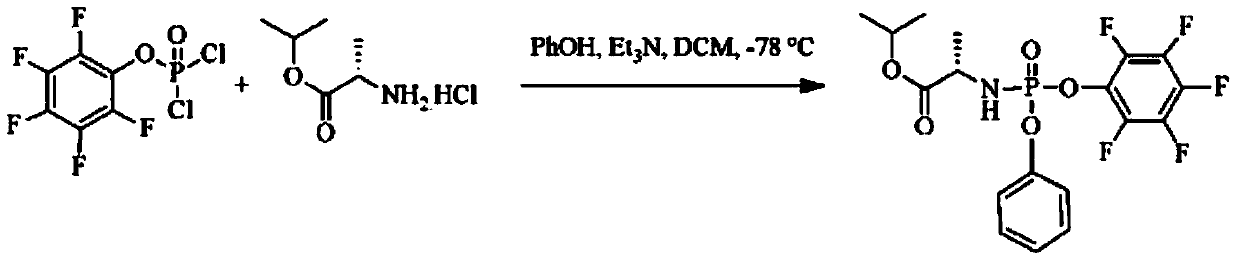
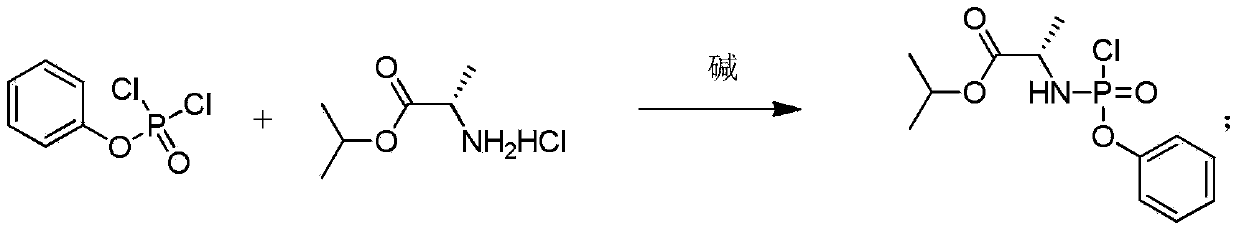
The invention provides a preparation method of 3,5-dibenzoyl-2-deoxy-2-fluoro-2-methyl-D-ribo-gamma-lactone (sofosbuvir). The method comprises the following steps: adding alkali into a mixed solution of L-alanine isopropyl ester or an acid salt thereof and phenyl dichlorophosphate for reacting to obtain (S)-2-phenoxy-chloro-phosphoryl amino isopropyl propionate, reacting the (S)-2-phenoxy-chloro-phosphoryl amino isopropyl propionate with 4-trifluoromethylphenol in the presence of alkali at the temperature of 0-10 DEG C to obtain racemic (S)-2-[(4-trifluoromethyl-phenoxyl)-phenoxy-phosphoryl amino)]alanine isopropyl ester, dissolving the racemic (S)-2-[(4-trifluoromethyl-phenoxyl)-phenoxy-phosphoryl amino)]alanine isopropyl ester into an ether or alkane solvent at the normal temperature, cooling to 50-10 DEG C below zero, and separating (S)-2-[(S)-(4-trifluoromethyl-phenoxy)]-phenoxy-phosphoryl amino)]alanine isopropyl ester out; and reacting the (S)-2-[(S)-(4-trifluoromethyl-phenoxy)]-phenoxy-phosphoryl amino)]alanine isopropyl ester with (2'R)-2'-deoxy-2'-fluoro-2'-methyluridine in the presence of organic alkali with high steric hindrance to obtain the 3,5-dibenzoyl-2-deoxy-2-fluoro-2-methyl-D-ribo-gamma-lactone. The method has the advantages of simple steps, mild reaction and suitability for industrial production.
Owner:苏州东南药业股份有限公司
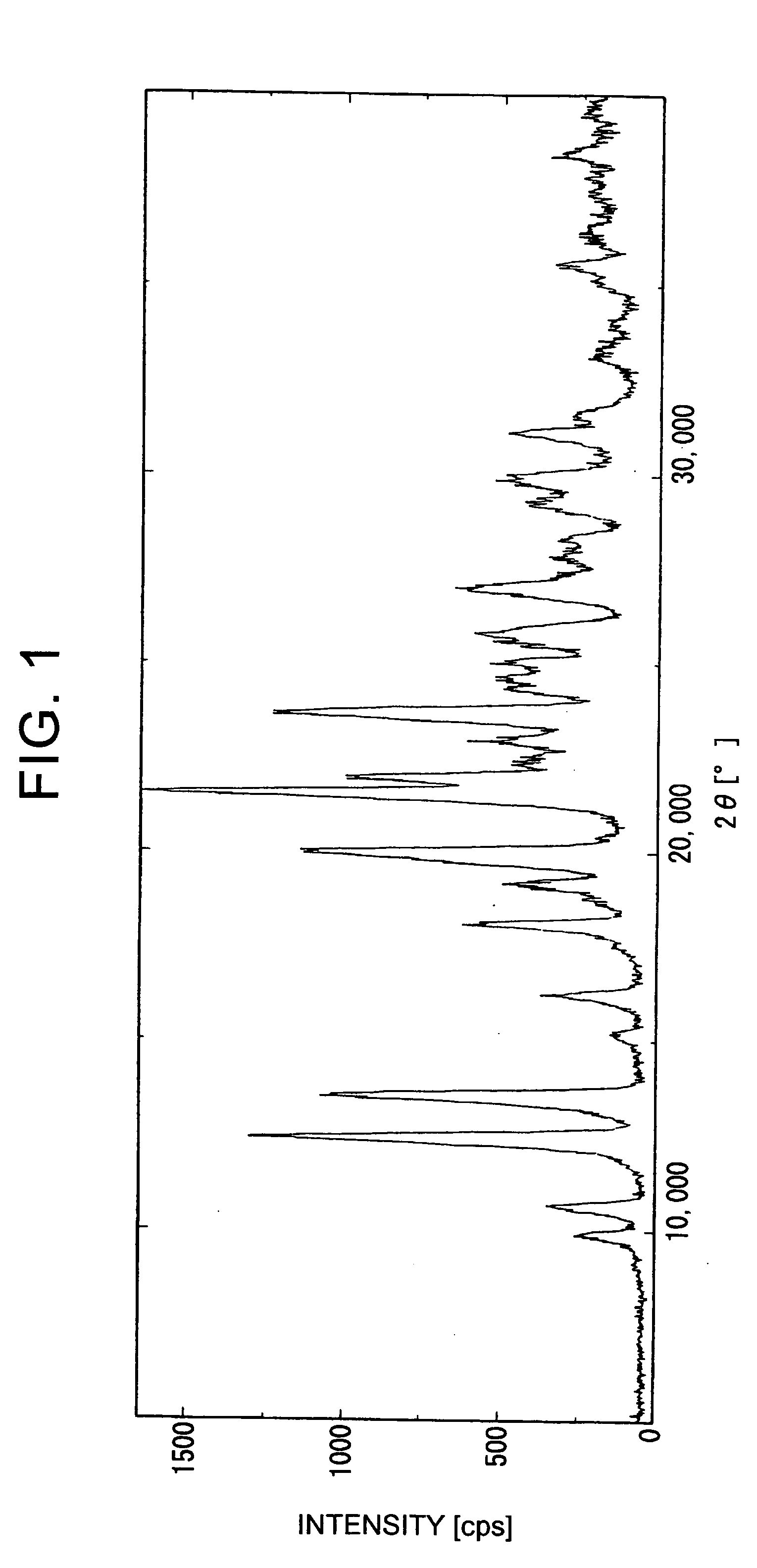
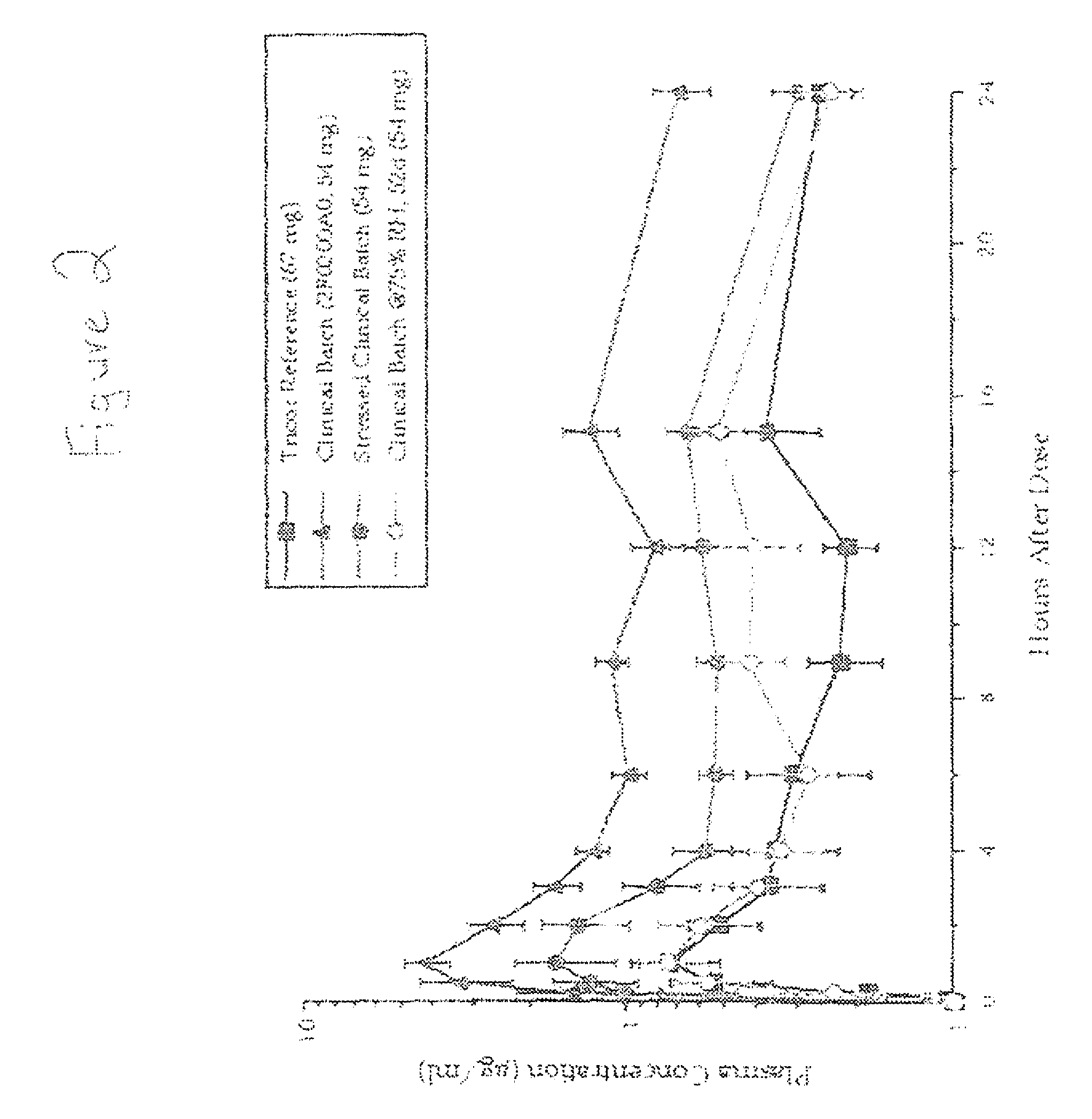
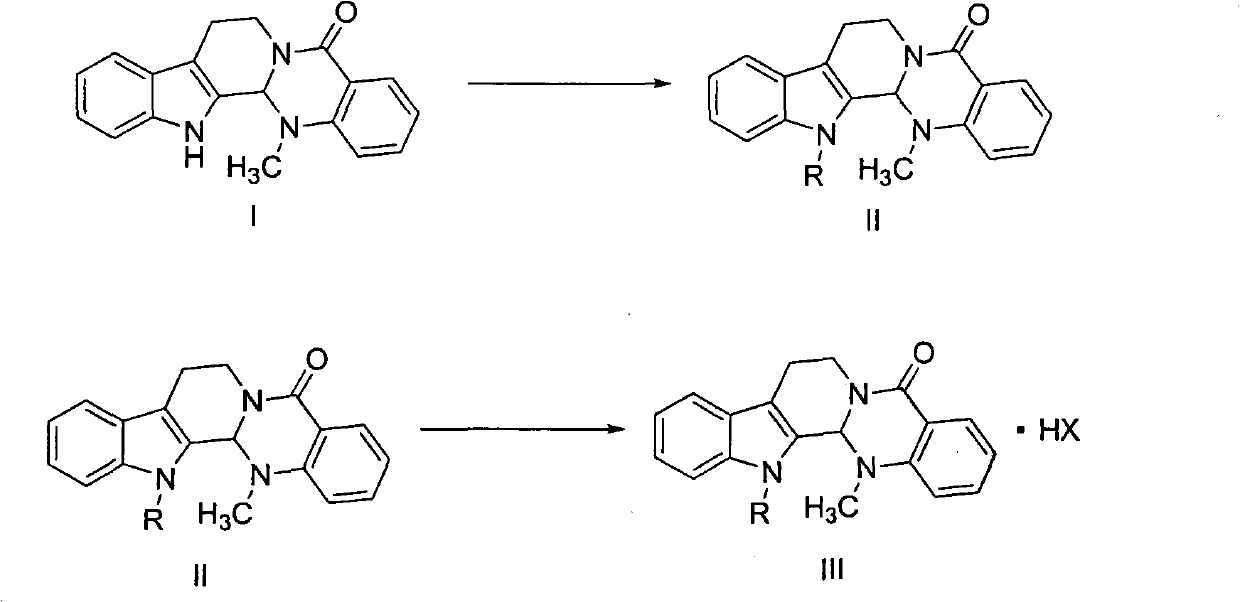
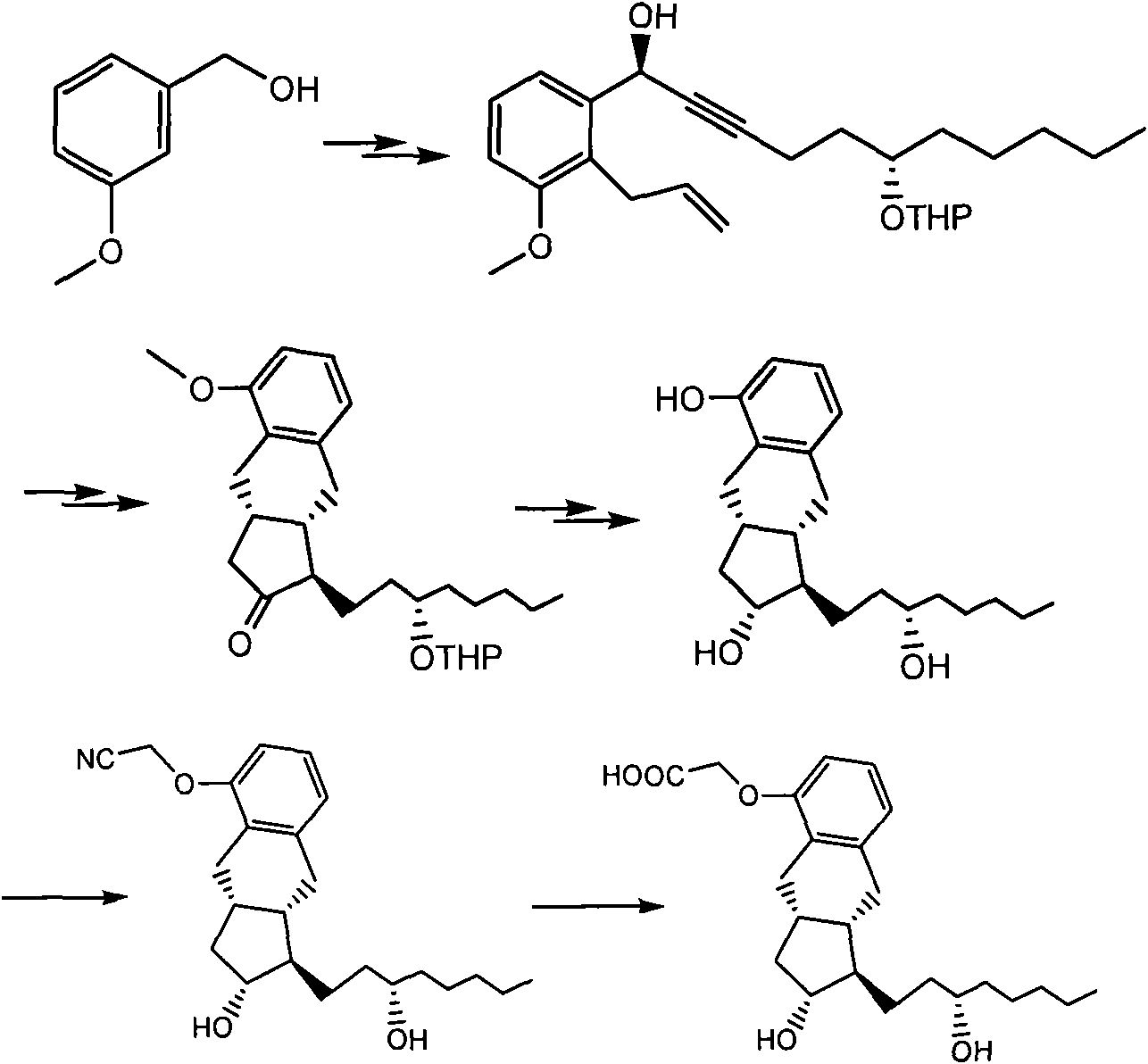
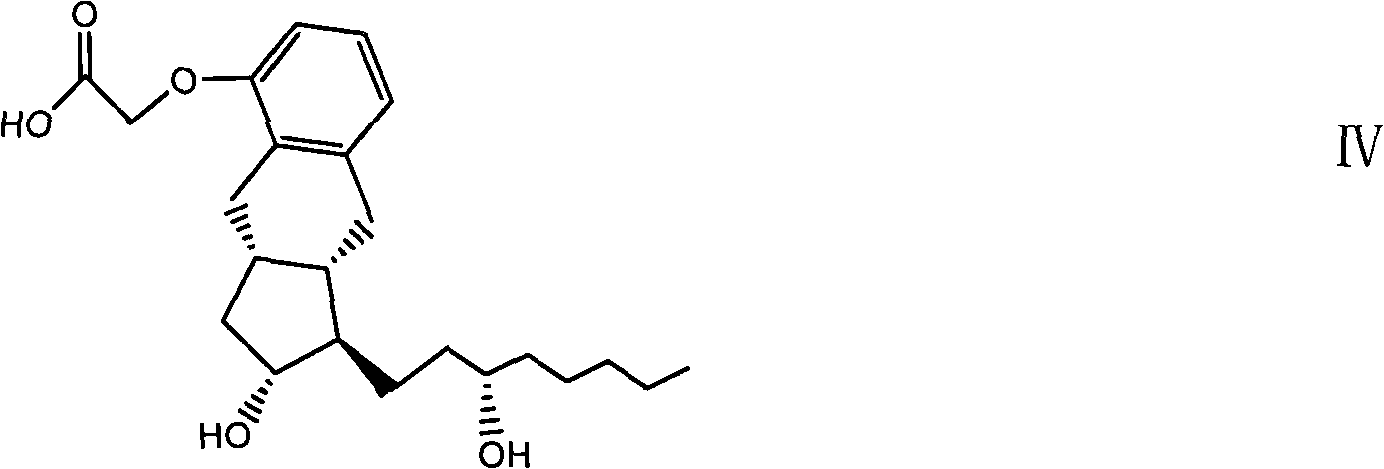
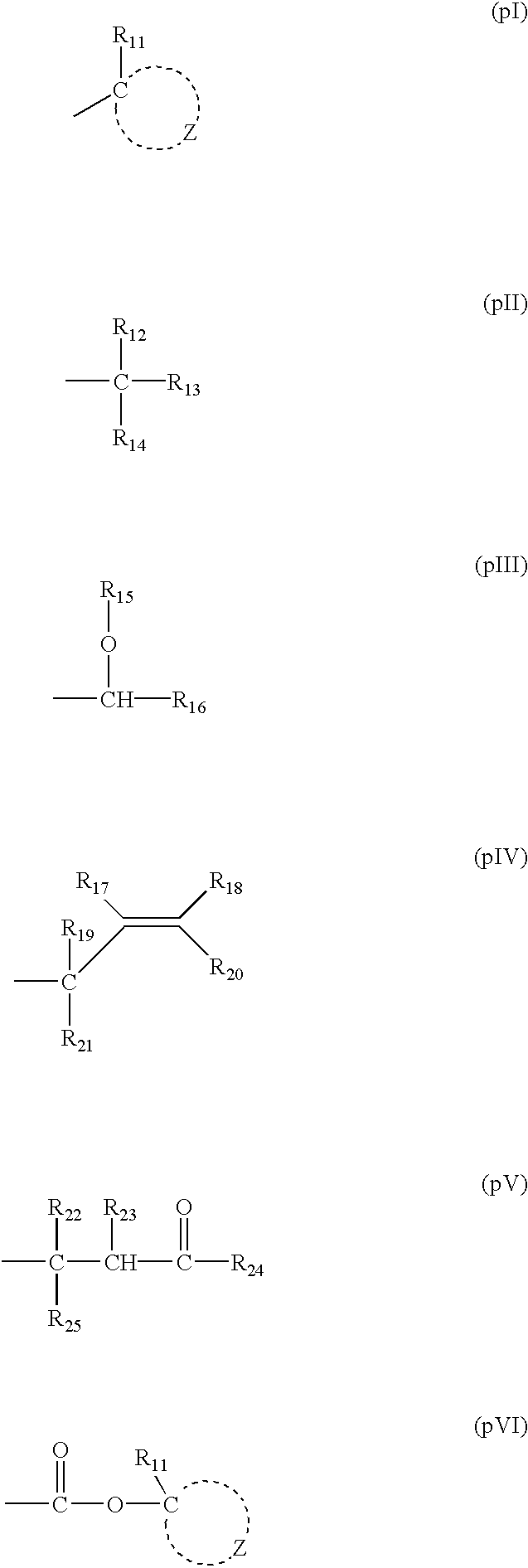
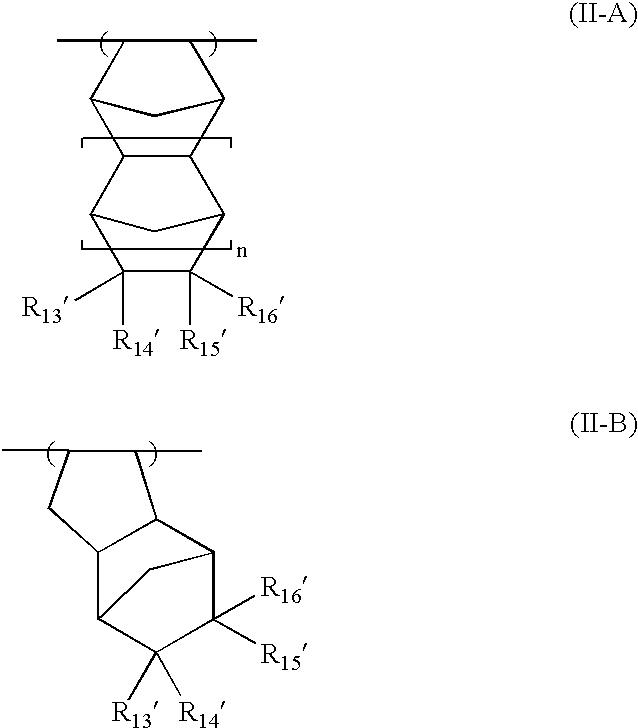
Crystalline forms of 1-benzoyl-4-[2-[4-methoxy-7-(3-methyl-1H-1,2,4-triazol-1-yl)-1-[(phosphonooxy)methyl]-1H-pyrrolo[2,3-c]pyridin-3-yl]-1,2-dioxoethyl]-piperazine
The instant disclosure provides crystalline forms of 1-benzoyl-4-[2-[4-methoxy-7-(3-methyl-1H-1,2,4-triazol-1-yl)-1-[(phosphonooxy)methyl]-1H-pyrrolo[2,3-c]pyridin-3-yl]-1,2-dioxoethyl]-piperazine, salts and solvates thereof. The present disclosure also generally relates to pharmaceutical compositions comprising the crystalline form(s), as well of methods of using the crystalline form(s) in the treatment of HIV and / or AIDS, and methods for obtaining such crystalline form(s).
Owner:VIIV HEALTHCARE UK (NO 5) LTD
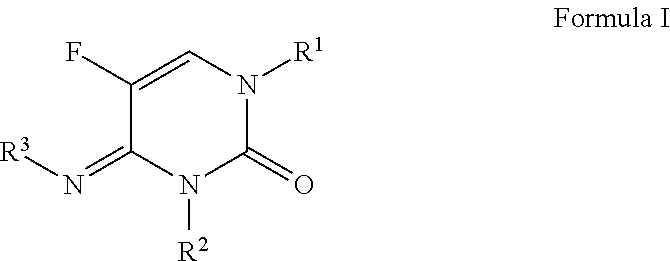
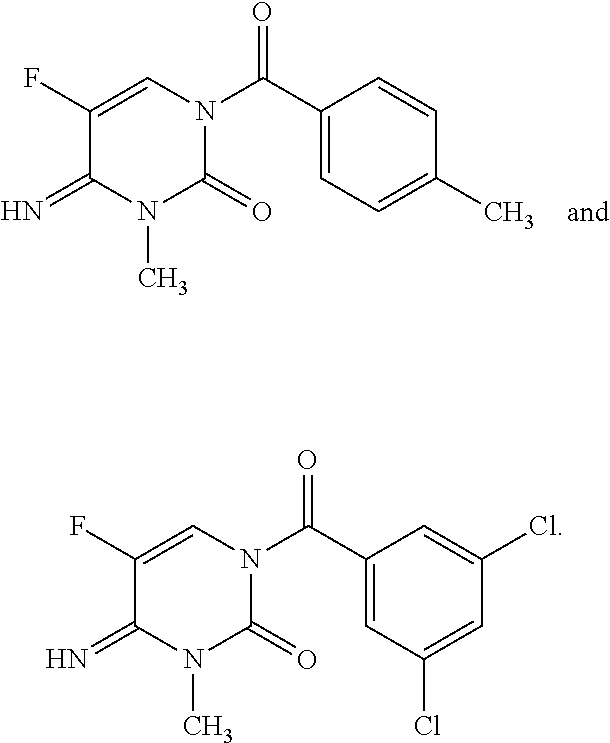
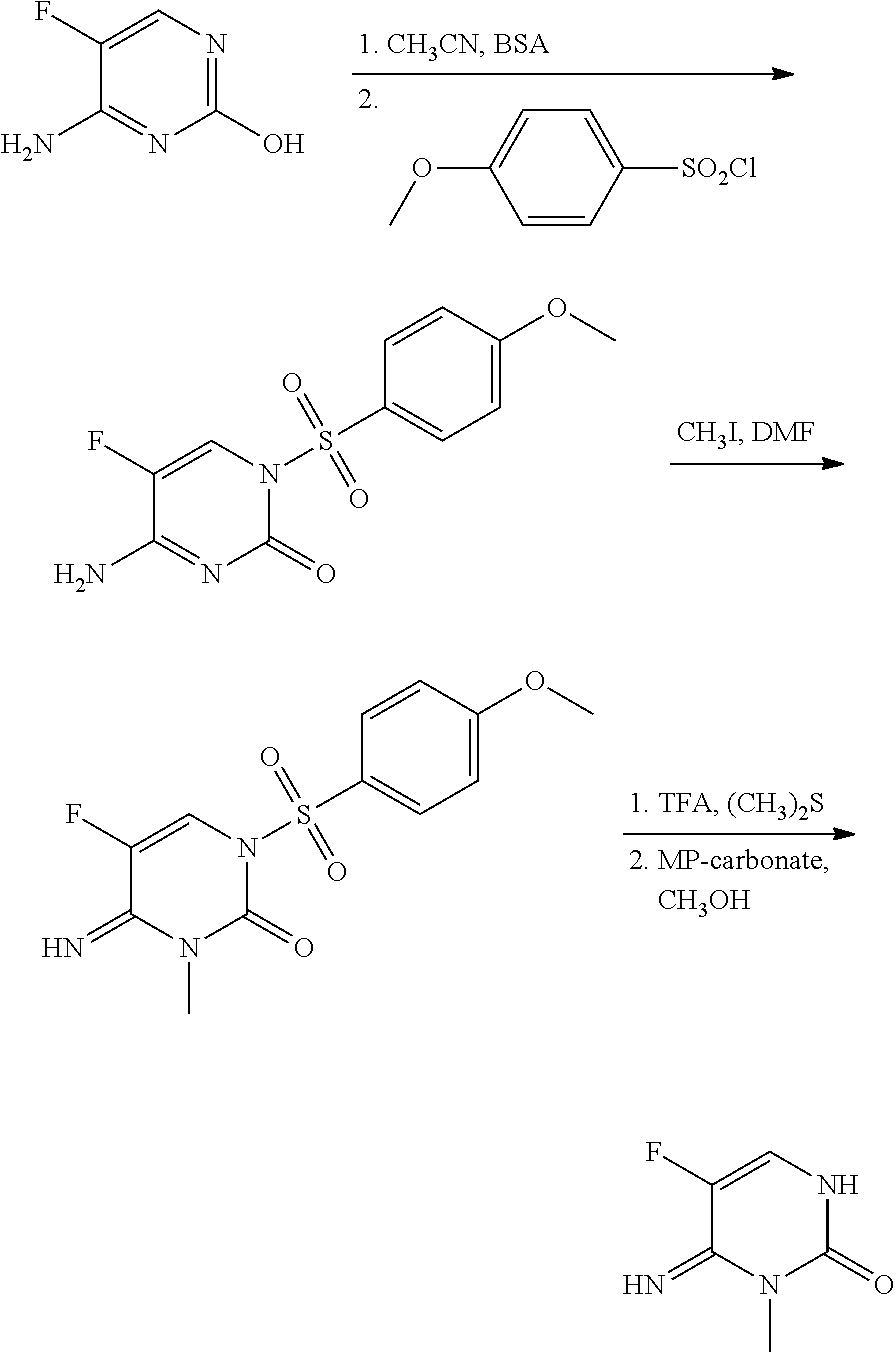
1-(substituted-benzoyl)-5-fluoro-4-imino-3-methyl-3,4-dihydropyrimidin-2(1H)-one derivatives
This present disclosure is related to the field of 1-(substituted-benzoyl)-5-fluoro-4-imino-3-methyl-3,4-dihydropyrimidin-2(1H)-ones and their derivatives and to the use of these compounds as fungicides. Fungicides are compounds, of natural or synthetic origin, which act to protect and / or cure plants against damage caused by agriculturally relevant fungi. Generally, no single fungicide is useful in all situations. Consequently, research is ongoing to produce fungicides that may have better performance, are easier to use, and cost less. The present disclosure relates to 1-(substituted-benzoyl)-5-fluoro-4-imino-3-methyl-3,4-dihydropyrimidin-2(1H)-one compounds and their use as fungicides. The compounds of the present disclosure may offer protection against fungi and fungi like organisms including ascomycetes, basidiomycetes, deuteromycetes, and oomycetes.
Owner:ADAMA MAKHTESHIM LTD
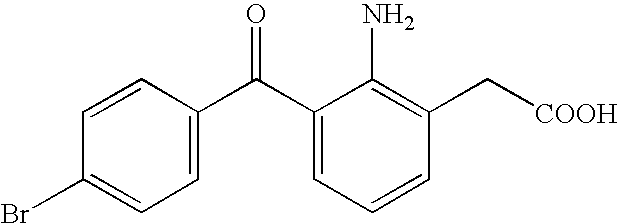
Aqueous liquid preparation containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid
An aqueous liquid preparation of the present invention containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid or its pharmacologically acceptable salt or a hydrate thereof, an alkyl aryl polyether alcohol type polymer such as tyloxapol, or a polyethylene glycol fatty acid ester such as polyethylene glycol monostearate is stable. Since even in the case where a preservative is incorporated into said aqueous liquid preparation, the preservative exhibits a sufficient preservative effect for a long time, said aqueous liquid preparation in the form of an eye drop is useful for the treatment of blepharitis, conjunctivitis, scleritis, and postoperative inflammation. Also, the aqueous liquid preparation of the present invention in the form of a nasal drop is useful for the treatment of allergic rhinitis and inflammatory rhinitis (e.g. chronic rhinitis, hypertrophic rhinitis, nasal polyp, etc.).
Owner:SENJU PHARMA CO LTD
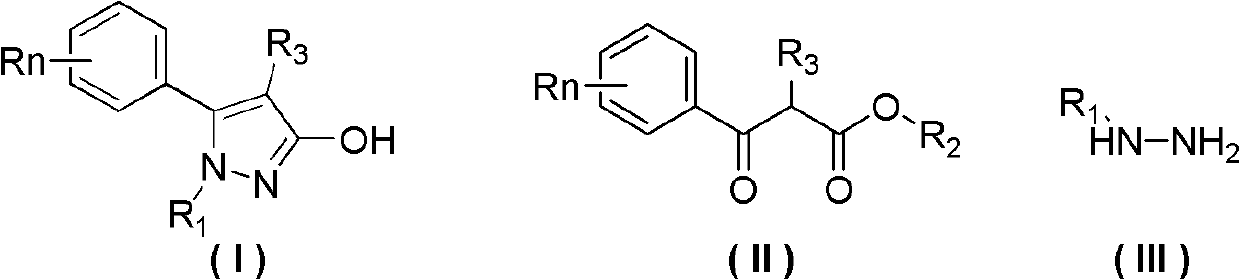
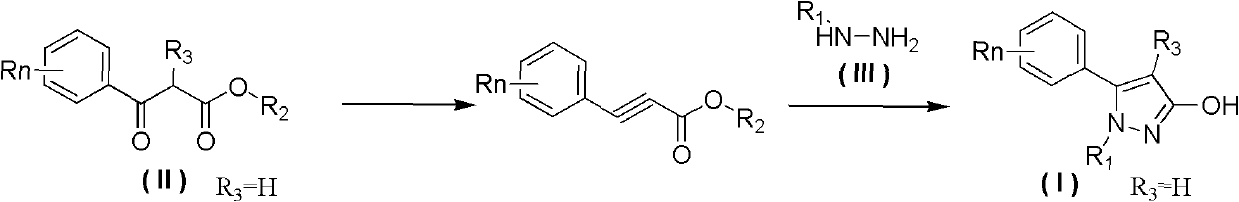
Preparation method of 3-hydroxy-substituted pyrazol
ActiveCN102584705ARaw materials are easy to getEasy to operateOrganic chemistryInorganic saltsPhenacyl
The invention belongs to the field of organic synthesis, and discloses a preparation method of 3-hydroxy-substituted pyrazol as shown in the formula (I). The response equation is : (a chemical formula), and definition of each substituent group in the formula is shown in a instruction book. Substituted benzene formoxyl ethyl carboxylic acid ester as shown in the formula (II) and substituted hydrazine as shown in the formula (III) serve as raw materials, simultaneously the substituted hydrazine as shown in the formula (III) serves as a solvent, and reaction is carried out at 0-40 DEG C for 5-10 hours. When the substituted hydrazine with low concentration is adopted, inorganic salt is added in a reaction system, and charging ratio of (II), (III) and the inorganic salt is 1: (3-10): (0-1). The preparation method is easy in raw material acquisition, simple in synthetic route, simple and convenient in operation, high in product yield, easy to purify and the like, and applicable to laboratory synthetics and industrial production.
Owner:SHENYANG SINOCHEM AGROCHEMICALS R&D CO LTD
Disperse die, disperse die composition, ink for ink-jet printing, and staining method and stained material using the die, composition or ink
InactiveCN101309975AImprove dyeing propertiesExcellent sublimation fastnessMonoazo dyesIsothiazolanthrone/isoxazolanthrone/isoselenazolanthrone dyesDisperse dyePolymer science
Disclosed are: a novel orange-colored disperse dye which has excellent staining properties onto a hydrophobic fiber material, a high light-resistant color-fastness, an excellent sublimation fastness, and a good buildup property, and is useful as a dye for use in an automotive interior sheet; a disperse dye composition for yellow-to-orange, brown, dark blue and black colors which comprises the disperse dye; an ink for ink-jet printing; a staining method or a stained material using the disperse die, the composition or the ink. The orange-colored disperse dye is represented by the formula (1) below and can stain a hydrophobic fiber material (e.g., a polyester) into orange color. [Chemical formula] (1) wherein X1 represents a chlorine or bromine atom; R1 represents a benzoyl group, a cyanoethyl group, a C1-C4 alkyl group, a phenyl group or a phenyl group substituted by at least one C1-C4 alkyl group.
Owner:KIWA CHEM IND CO LTD
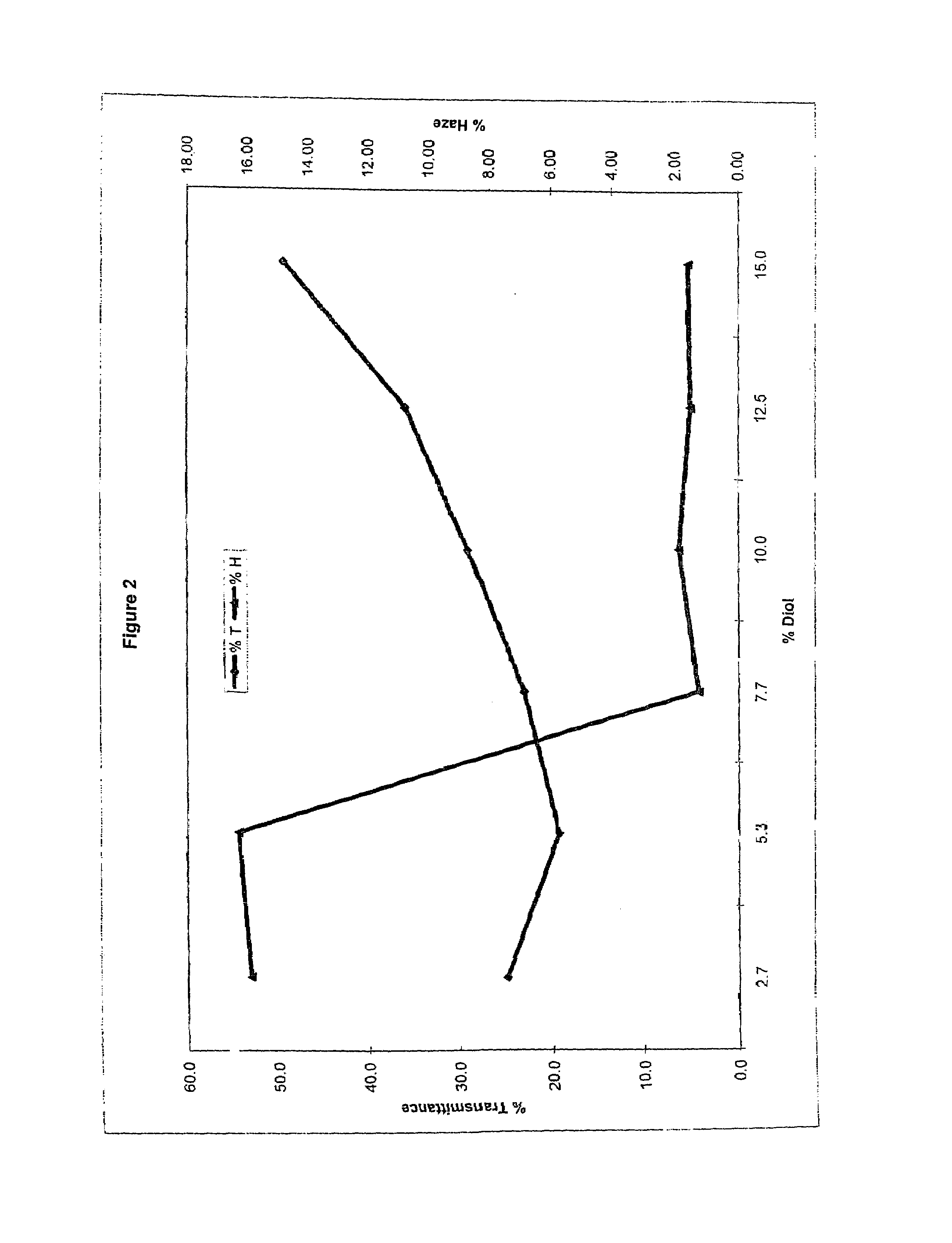
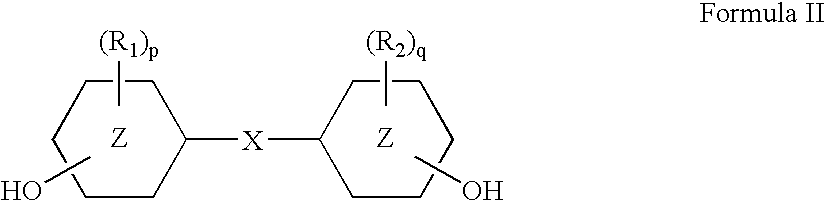
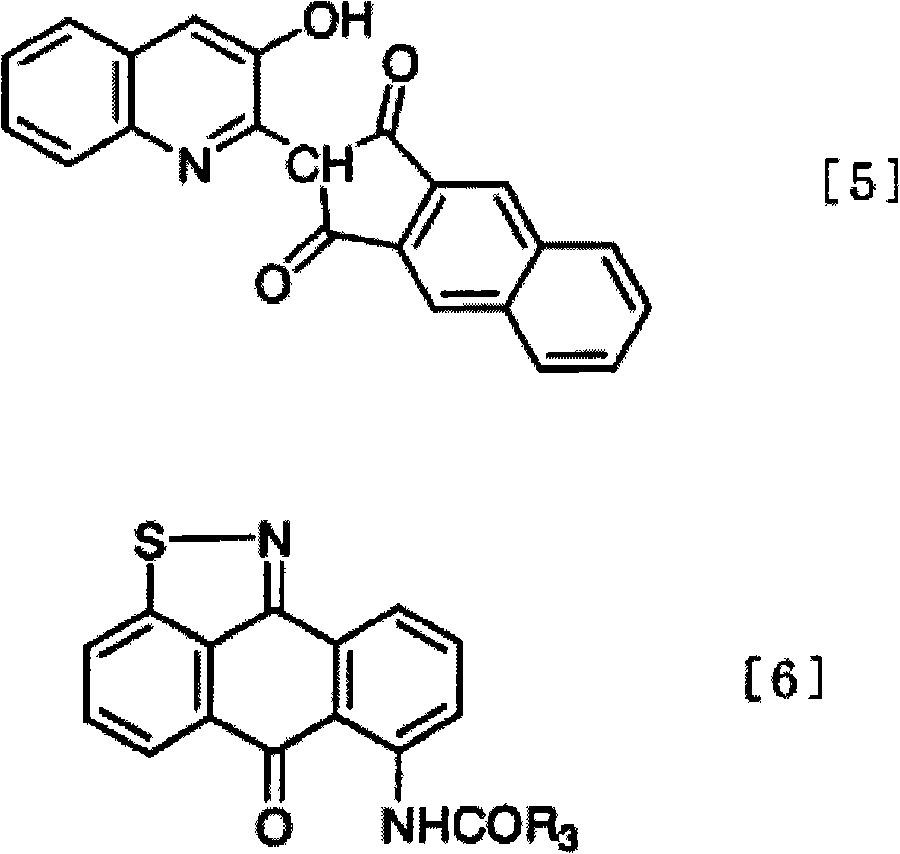
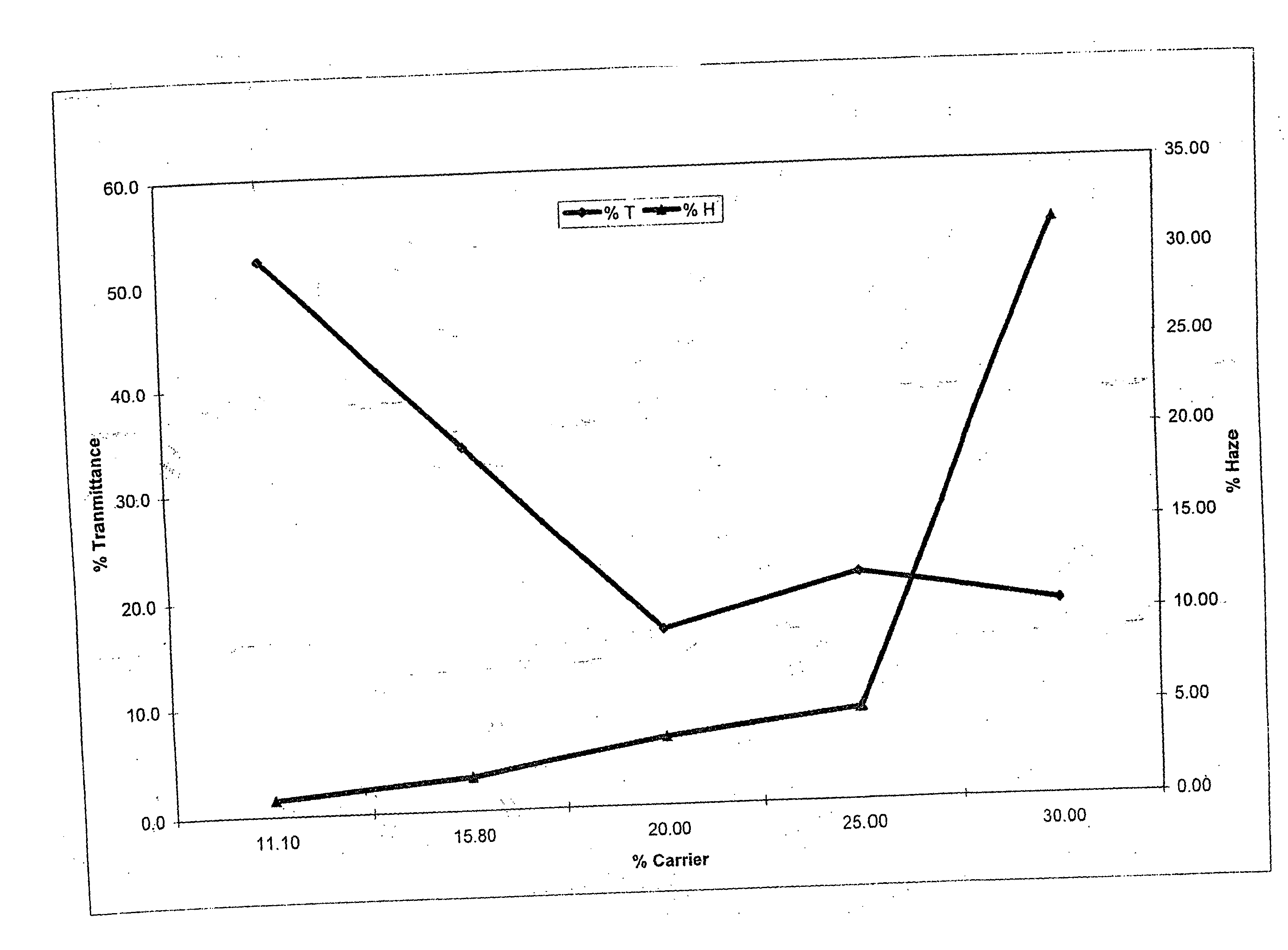
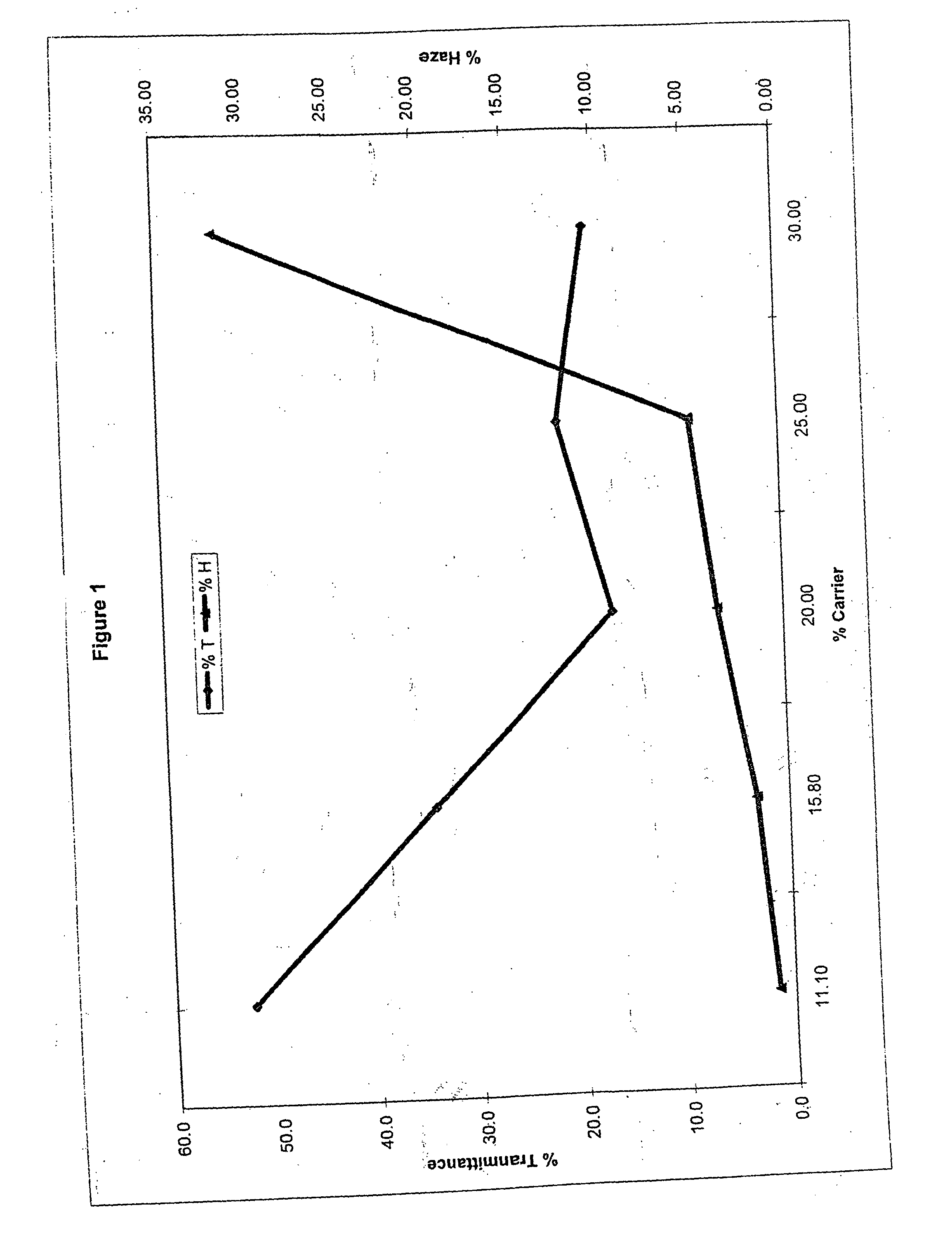
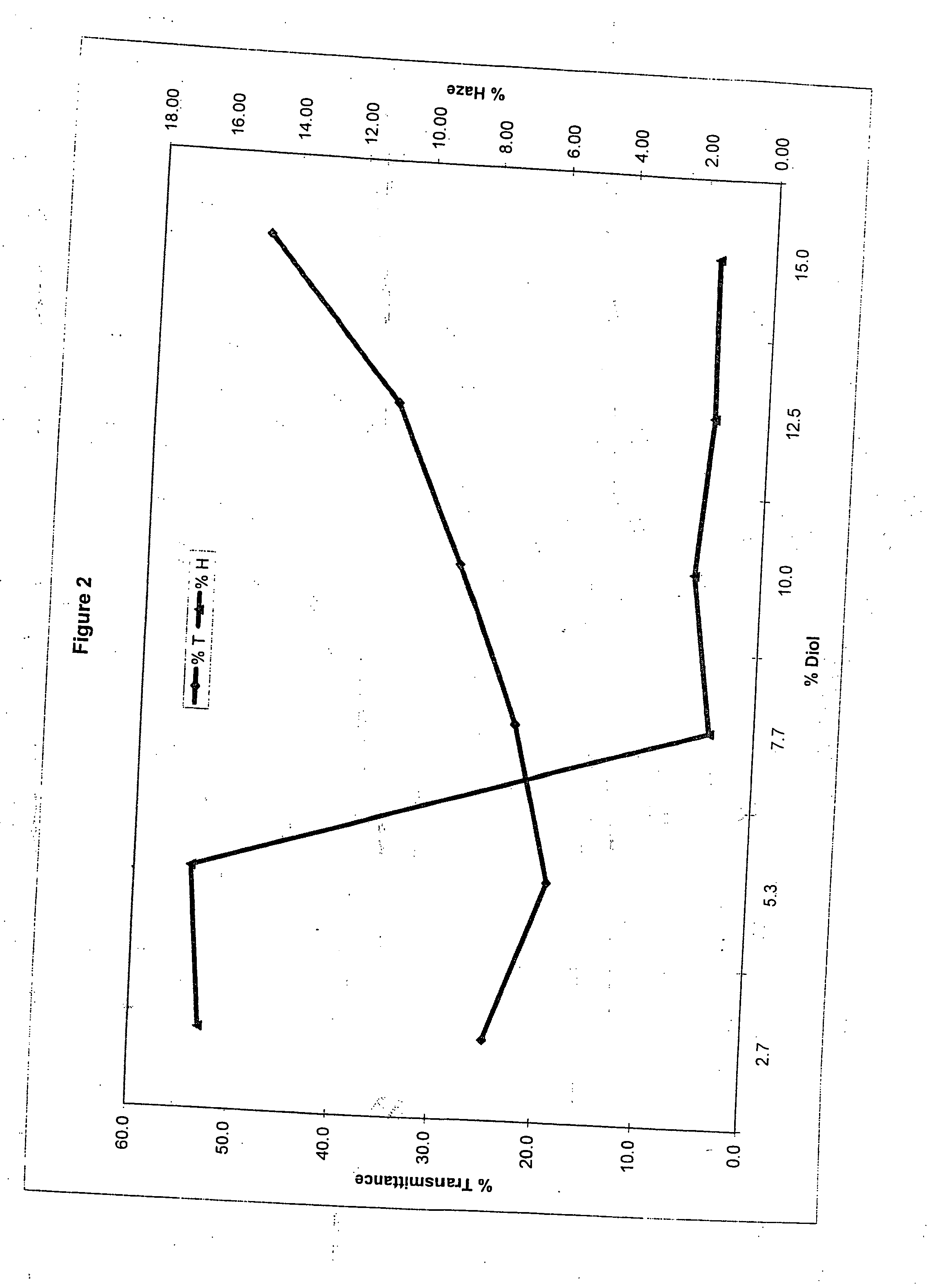
Method of dyeing a plastic article
In the method of the present invention a plastic article (e.g., a molded article of thermoplastic polycarbonate) is immersed at least partially in a dye bath which includes one or more dyes, water, at least one carrier, and at least one diol. The dye bath contains: (i) at least one dye (e.g., a static and / or photochromic dye); (ii) water; (iii) at least one carrier represented by the following general formula I, R—O—(CH2)n—OH I wherein R is a radical selected from linear or branched C1-C18 alkyl, benzyl, benzoyl and phenyl, and n is 2 or 3; and (iv) a diol selected from at least one of linear or branched C2-C20 aliphatic diols, poly(C2-C4 alkylene glycol), cycloaliphatic diols having from 5 to 8 carbon atoms in the cyclic ring, monocyclic aromatic diols, bisphenols and hydrogenated bisphenols. In an embodiment of the present invention, the carrier is ethyleneglycol butyl ether, and the diol is diethylene glycol. The present invention also relates to a method of separating the dye from the water, carrier and diol components of the dye bath, by contacting the dye bath with particulate activated carbon.
Owner:COVESTRO LLC
Optical film containing polymer having naphtyl group
InactiveUS20090116109A1Easy to processIncrease resistanceLiquid crystal compositionsSynthetic resin layered productsCarbon numberPhenacyl
The object of the present invention is to provide an optical film excellent in transparency, heat resistance and processability. An optical film containing a polymer having at least a repeating unit represented by following general formula (I):wherein in the general formula (I), R1 and R3 each independently denote a hydrogen atom, a straight-chain or branched alkyl group with a carbon number of 1 to 4, or a substituted or unsubstituted phenyl group; R2, A and B each independently denote a hydrogen atom, a halogen atom, a straight-chain or branched alkyl group with a carbon number of 1 to 4, a straight-chain or branched alkyl halide group with a carbon number of 1 to 4, a straight-chain or branched alkoxy group with a carbon number of 1 to 4, an alkoxycarbonyl group, an acyloxy group, an amino group, an azide group, a nitro group, a cyano group or a hydroxyl group (however, R2 is not a hydrogen atom); R4 denotes a hydrogen atom, a straight-chain or branched alkyl group with a carbon number of 1 to 4, a substituted or unsubstituted cycloalkyl group with a carbon number of 5 to 10, a substituted or unsubstituted phenyl group, a substituted or unsubstituted naphthyl group, or a substituted or unsubstituted heterocyclic group; R5 denotes a hydrogen atom, a straight-chain or branched alkyl group with a carbon number of 1 to 4, a benzyl group, a silyl group, a phosphoric acid group, an acyl group, a benzoyl group or a sulfonyl group; l, m and n denote an integer of 2 or more.
Owner:NITTO DENKO CORP
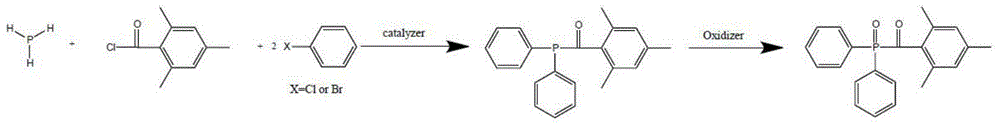
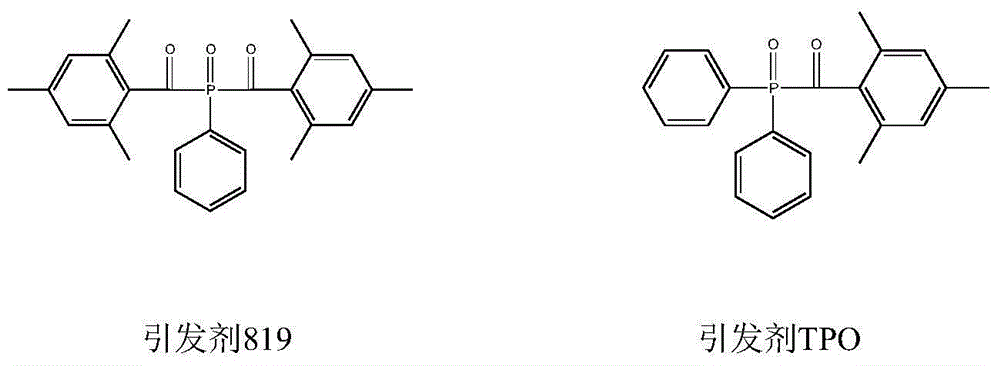
Preparation method of di (2,4,6-trimethylbenzoyl) phenyl phosphine oxide and (2,4,6-trimethylbenzoyl) diphenyl phosphine oxide
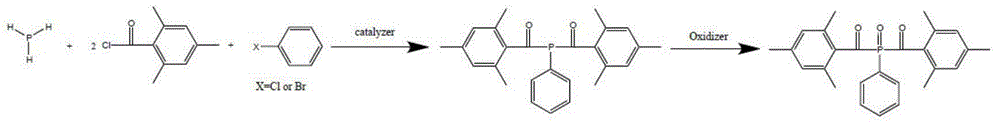
The invention relates to a preparation method of di (2,4,6-trimethylbenzoyl) phenyl phosphine oxide and (2,4,6-trimethylbenzoyl) diphenyl phosphine oxide used in the technical field of radiation polymerization curing new materials, hydrogen phosphide as a raw material is reacted with chlorobenzene or bromobenzene and 2,4,6-trimethylbenzoyl chloride, and an acyl oxygen phosphonic compound is obtained by oxidizing with an oxidant. By adjusting the feed ratio, the di (2,4,6-trimethylbenzoyl) phenyl phosphine oxide and the (2,4,6-trimethylbenzoyl) diphenyl phosphine oxide are respectively obtained, and the purpose for simultaneously producing the two target products of di (2,4,6-trimethylbenzoyl) phenyl phosphine oxide and the (2,4,6-trimethylbenzoyl) diphenyl phosphine oxide can be achieved by use of same material and device, and compared with known technology paths reported in literatures, the preparation method has the significant advantages of novelty of the chemical reaction technology, cost economic competitiveness and environmental friendliness.
Owner:TIANJIN MOSEN TECH CO LTD
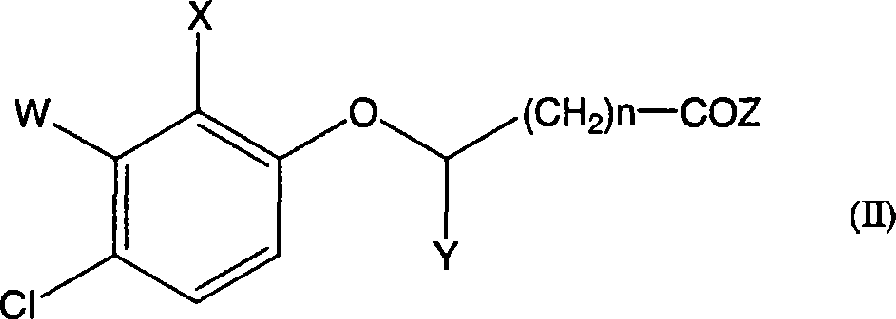
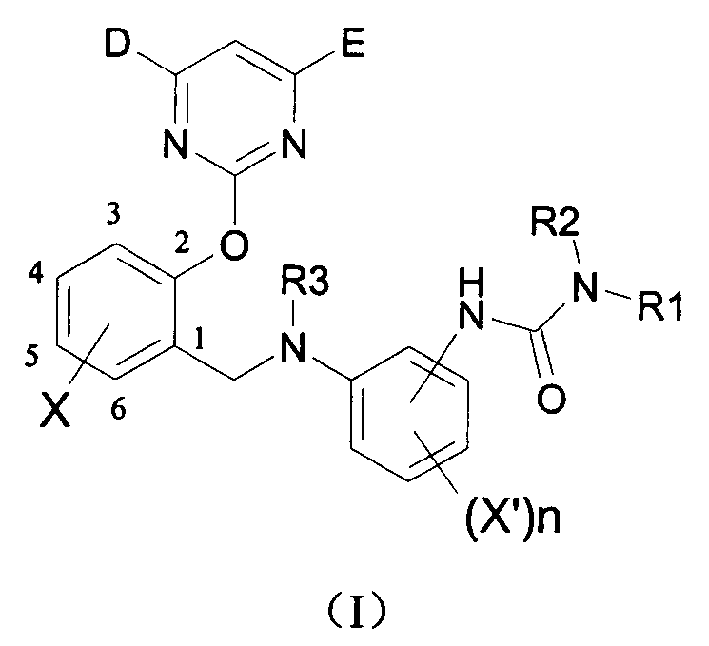
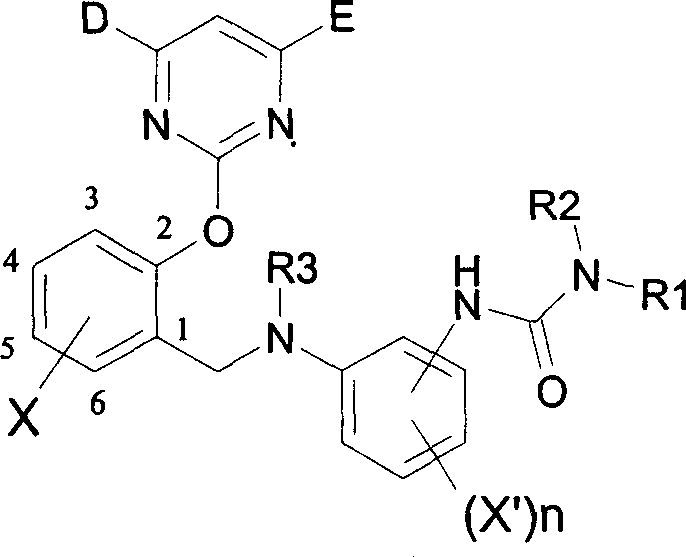
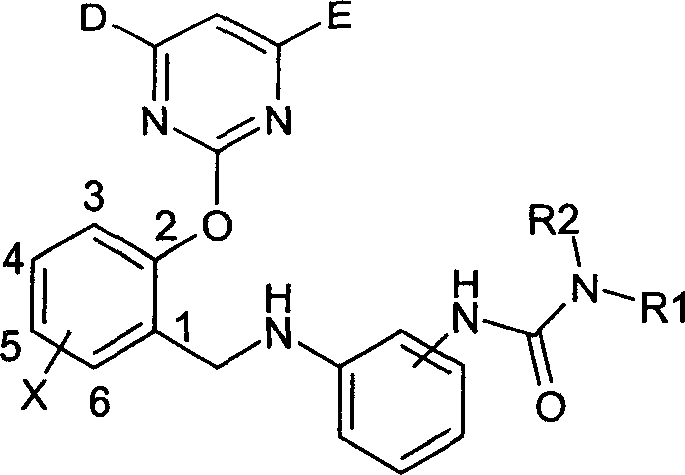
2-pyrimidine oxy-n-ureido phenyl-benzyl amide compound, preparing method and use thereof
InactiveCN1488626AGood weeding effectImprove herbicidal activityBiocideOrganic chemistryAlkaneCarboxyl radical
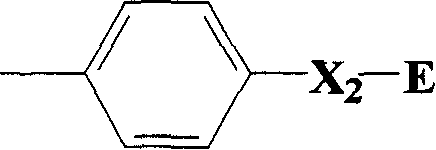
The invention is a kind of 2-pyrimidine oxo-N-urea phenyl benzyl amine compound, the manufacturing method. The structural formula is shown in the picture. D or E = hydrogen, halogen, C1-4C alkyl, C1-C4 alkoxy, C1-C4 halogenated alkyl or C1-C4 alkylogen oxide; X=hydrogen, halogen, nitro, cyan, carboxy, ester group, sulfonyl, C1-C8 alkane acyl amido, C1-C8 halogeno alkane acyl amido, C1-C8 alkane acyl group, C1-C8 alkyl, C1-C8 halogeno alkyl, C1-C8 alkoxy, phenyl etc; R1, R2=hydrogen, ester group, sulfonyl, C1-C8 alkyl, C1-C8 substituted alkyl, C1-C8 alkyl acyl, phenyl, etc; Xí» is H, urea, halogen atom, carboxy, ester group, C1-C8 alkane acyl, C1-C8 alkyl, C1-C8 halogeno alkyl, C1-C8 alkoxy, phenyl, substitution phenyl or heterocyclic radical; R3=hydrogen, C1-C8 alkane acyl, C1-C8 halogeno alkyl, benzene formyl, substitution benzene formyl, C1-C8 alkyl.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI +1
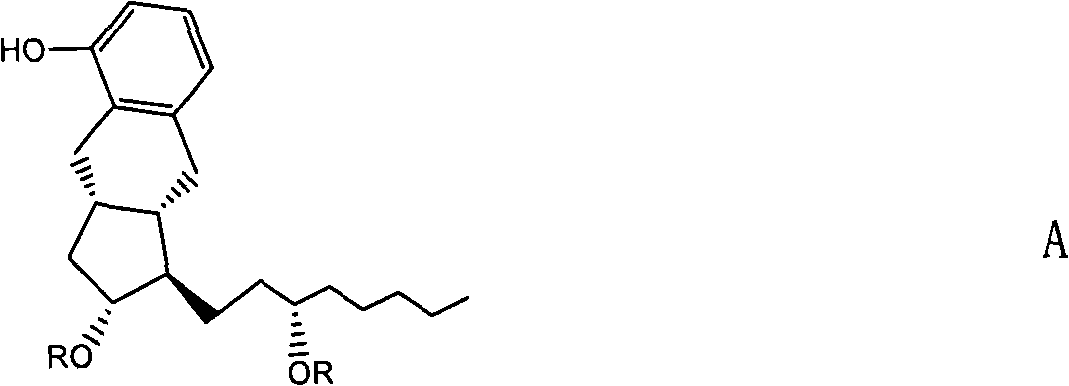
Crystalline forms of 1-benzoyl-4-[2-[4-methoxy-7-(3-methyl-1H-1,2,4-triazol-1-YL)-1-[(phosphonooxy)methyl]-1H-pyrrolo[2,3-C]pyridin-3-YL]-1,2-dioxoethyl]-piperazine
Owner:VIIV HEALTHCARE UK (NO 5) LTD
Process for preparing pemetrexed
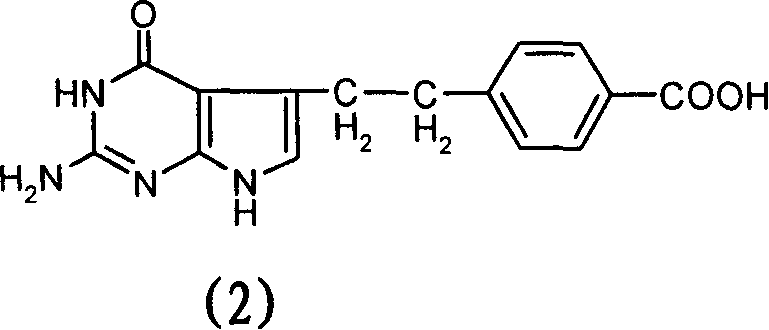
The related preparation method in hydrosolvent for N-[4-]2-(2-amido-4, 7-dihydro-4-oxo-3H- pyrrolo[2, 3-d]pyrimidine-5-radical)ethyl]benzoyl]-L-glutacid (Pemetrexed) and its acceptable salt in pharmacy is to react the 4-[2-(2-amido-4, 7-dihydro-4-oxo-3H-pyrrolo[2, 3-d] pyrimidine- 5-radical)ethyl]benzoic acid with L-glutacid dissolved in organic solvent and water. This invention is more simple than prior art and fit to industrial production.
Owner:QILU PHARMA CO LTD +1
Method of dyeing a plastic article
In the method of the present invention a plastic article (e.g., a molded article of thermoplastic polycarbonate) is immersed at least partially in a dye bath which includes one or more dyes, water, at least one carrier, and at least one diol. The dye bath contains: (i) at least one dye (e.g., a static and / or photochromic dye); (ii) water; (iii) at least one carrier represented by the following general formula I,R—O—(CH2)n—OHIwherein R is a radical selected from linear or branched C1–C18 alkyl, benzyl, benzoyl and phenyl, and n is 2 or 3; and (iv) a diol selected from at least one of linear or branched C2–C20 aliphatic diols, poly(C2–C4 alkylene glycol), cycloaliphatic diols having from 5 to 8 carbon atoms in the cyclic ring, monocyclic aromatic diols, bisphenols and hydrogenated bisphenols. In an embodiment of the present invention, the carrier is ethyleneglycol butyl ether, and the diol is diethylene glycol. The present invention also relates to a method of separating the dye from the water, carrier and diol components of the dye bath, by contacting the dye bath with particulate activated carbon.
Owner:COVESTRO LLC
Herbicidal composition
Owner:ISHIHARA SANGYO KAISHA LTD
Anticancer taxanes such as paclitaxel, docetaxel and their structural analogs, and a method for the preparation thereof
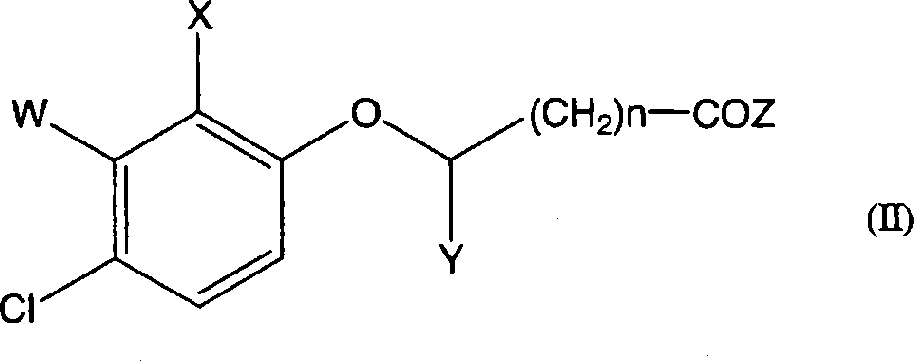
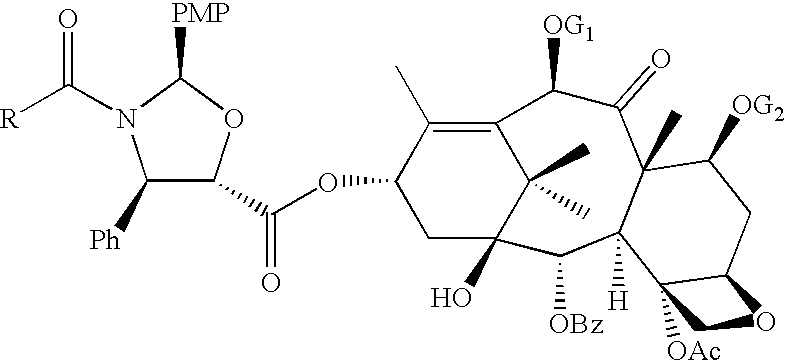
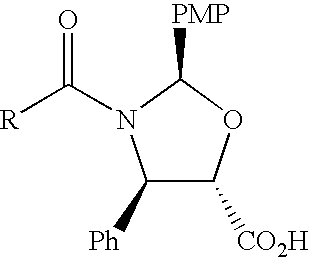
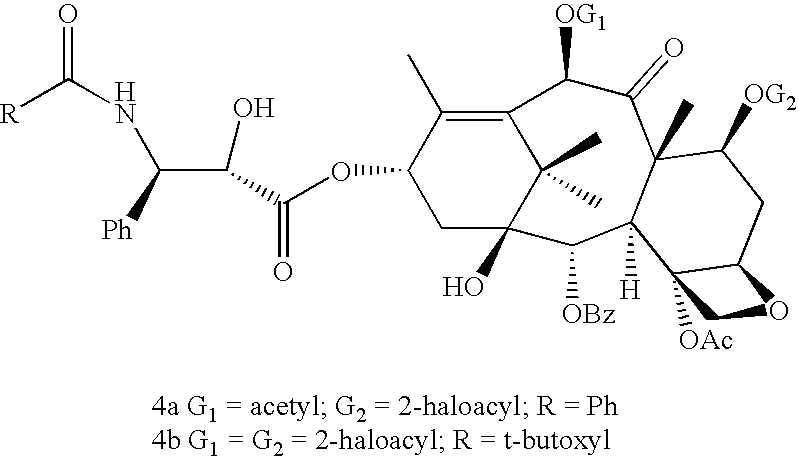
A process for the preparation of taxanes comprising wherein R is a tert. butoxycarbonyl or benzoyl group; PMP is p-methoxyphenyl group; G1 is acetyl group; G2 is haloacetyl group comprisinga) protecting the C-7 hydroxyl group of 10-deacetylbaccatin III with haloacetyl chlorides and then acetylating the C-10 hydroxyl group with acetyl chloride to obtain a protected 10-deacetylbaccatin III (1);b) subjecting the protected 10-deacetylbaccatin III (1) to coupling with an oxazolidine-5-caboxylic acid of formula 2 wherein R is tert. butoxycarbonyl or benzoyl; PMP is p-methoxyphenyl group in the presence of a condensation agent and an activating agent to obtain C-13 esters of formula 3;c) treating the coupled products 3 with weak acidic medium to open the oxazolidine ring to obtain intermediates of formula 4; wherein R is a tert. butoxycarbonyl or benzoyl group; G1 is acetyl group; G2 is haloacetyl groupd) subjecting the intermediates of compound 4 to selective deprotection of haloacyl group in the presence of acetyl group under mild alkaline condition at −20 to +40° C. for 6-24 h in the presence of ammonia or aliphatic amine or aromatic amines or their combination to obtain paclitaxel or docetaxel.
Owner:DABUR PHARM LTD
Methods for producing cyclic benzamidine derivatives
InactiveUS20060058370A1Operational securityQuality improvementBiocideSulfonic acid esters preparationPhenacylMorpholine
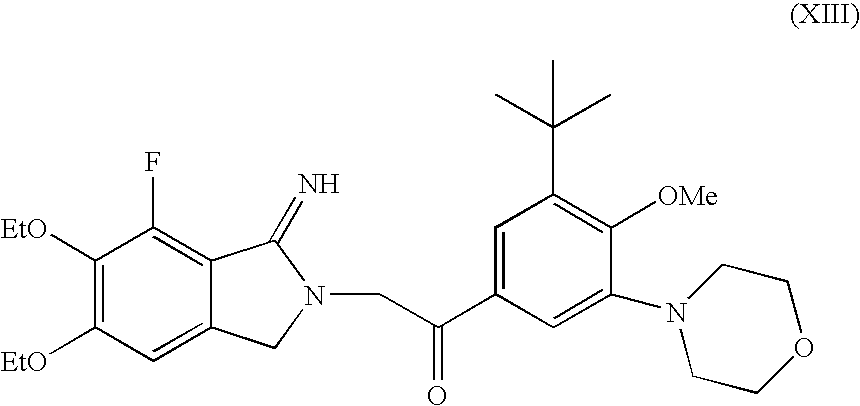
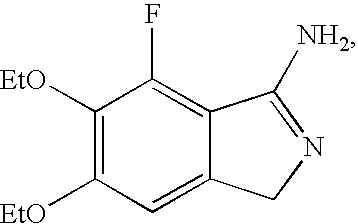
In the present invention, the methods of producing a fluorinated cyclic benzamidine derivative (A), or a salt thereof, comprise the step of reacting a specific novel compound with ammonia or imide. The methods of this invention for producing a morpholine-substituted phenacyl derivative (B), or a salt thereof, comprise reaction of a specific novel compound with morpholine, reaction of the product with a halogenating reagent, and deketalization of the product. The methods of this invention for a producing cyclic benzamidine derivative (C), or a salt thereof, comprise the step of coupling compound (A), or a salt thereof, with compound (B), or a salt thereof, in the presence of an ether or a hydrocarbon. The methods of this invention for recrystallizing a cyclic benzamidine derivative (C), or a salt thereof, comprise the steps of dissolving compound (C), or the salt thereof, in a mixed solvent comprising an alcohol and water, or a mixed solvent comprising an ether and water, and after dissolution, adding additional water to precipitate crystals of compound (C), or the salt thereof.
Owner:EISIA R&D MANAGEMENT CO LTD
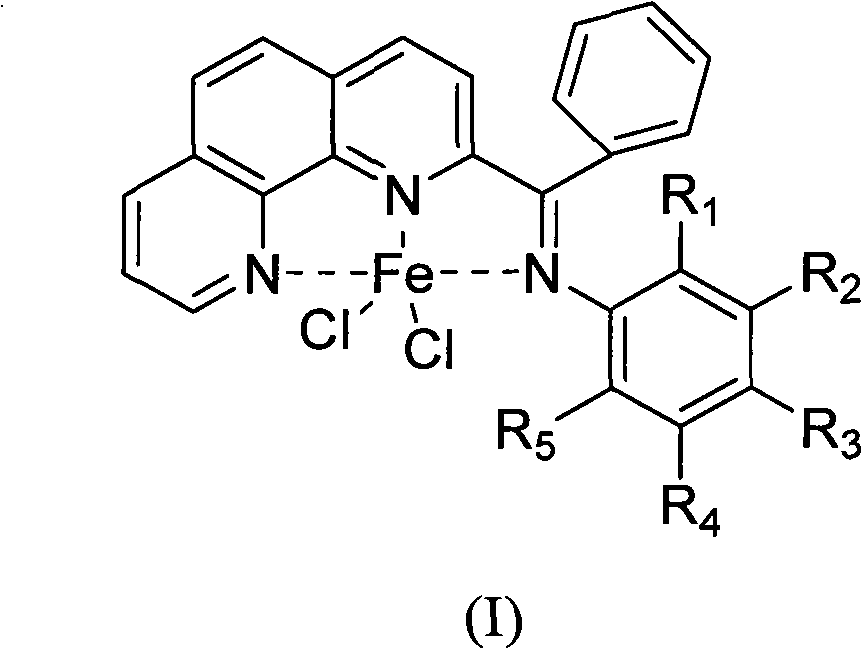
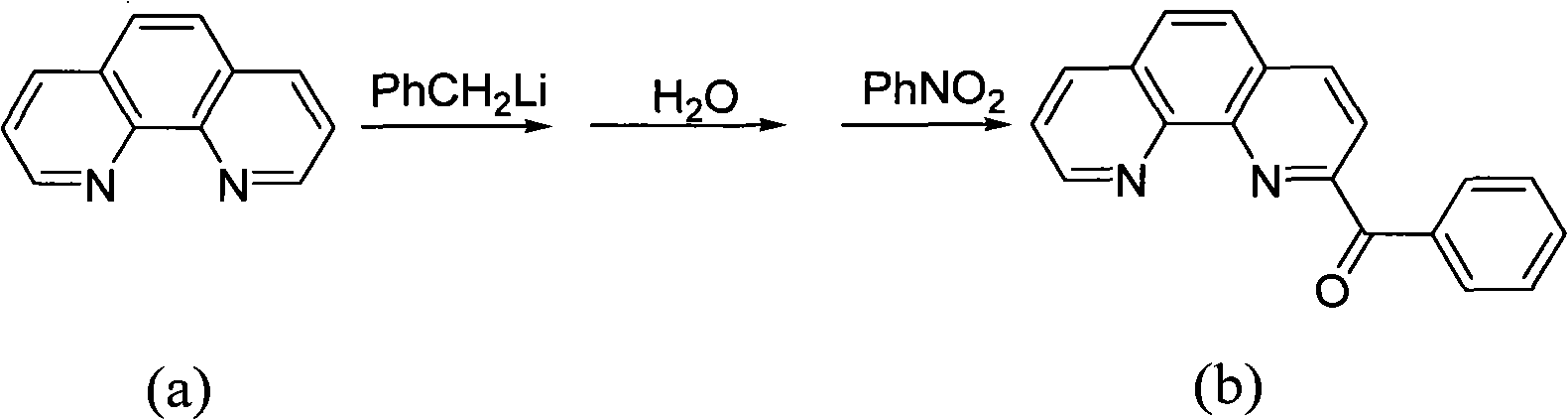
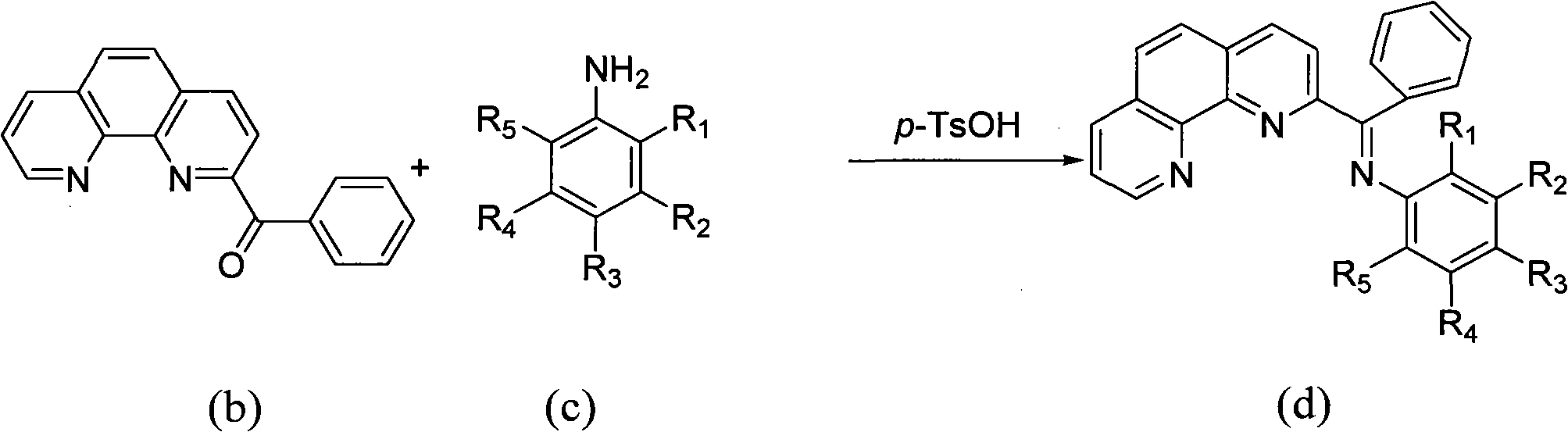
Preparation method of benzoyl-substituted 1,10-phenanthroline complex and catalytic application in ethylene oligomerization
ActiveCN102964388AReduce manufacturing costLow priceOrganic-compounds/hydrides/coordination-complexes catalystsIron organic compoundsPotassium cyanidePhenanthroline
The invention provides a method for preparing a chlorinated 2-benzoyl-1,10-phenanthroline condensed amine iron (II) complex shown in formula I, and its catalytic application in ethylene oligomerization. The preparation method comprises the following steps: orderly performing hydrolysis and an oxidation reaction with nitrobenzene of initial reaction raw materials of 1,10-phenanthroline and benzyl lithium to obtain 2-benzoyl-1,10-phenanthroline, then performing condensation of 2-benzoyl-1,10-phenanthroline with substituted aniline to obtain 2-benzoyl-1,10-phenanthroline condensed amine ligand, and reacting the ligand with ferrous chloride to obtain a target product I. The synthetic method provided by the invention is few in steps, and simple in process, substitutes nitrobenzene for selenium dioxide in the prior art for the oxidation reaction, substitutes nontoxic benzyl lithium for hypertoxic potassium cyanide in the prior art, reduces the preparation cost of the catalyst, and has wide industrialization prospects.
Owner:CHINA PETROLEUM & CHEM CORP +1
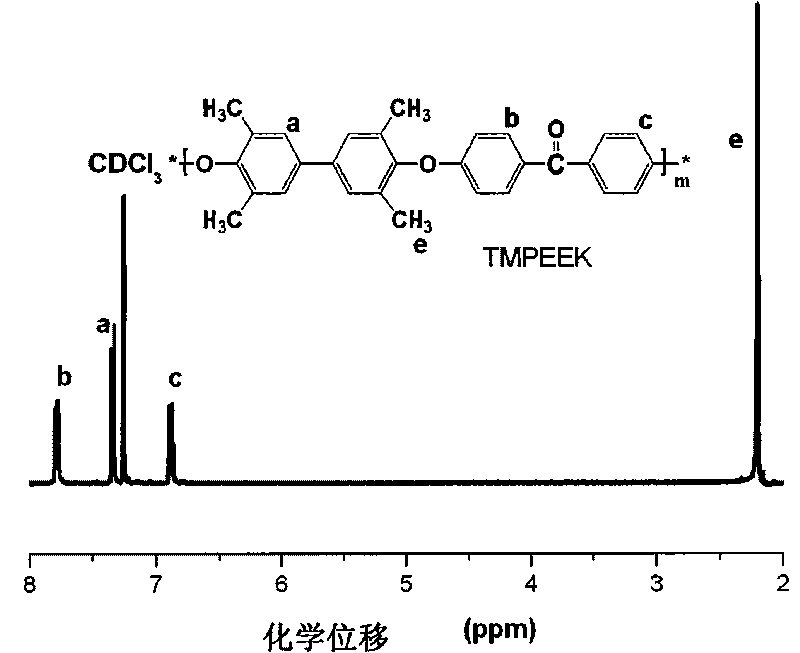
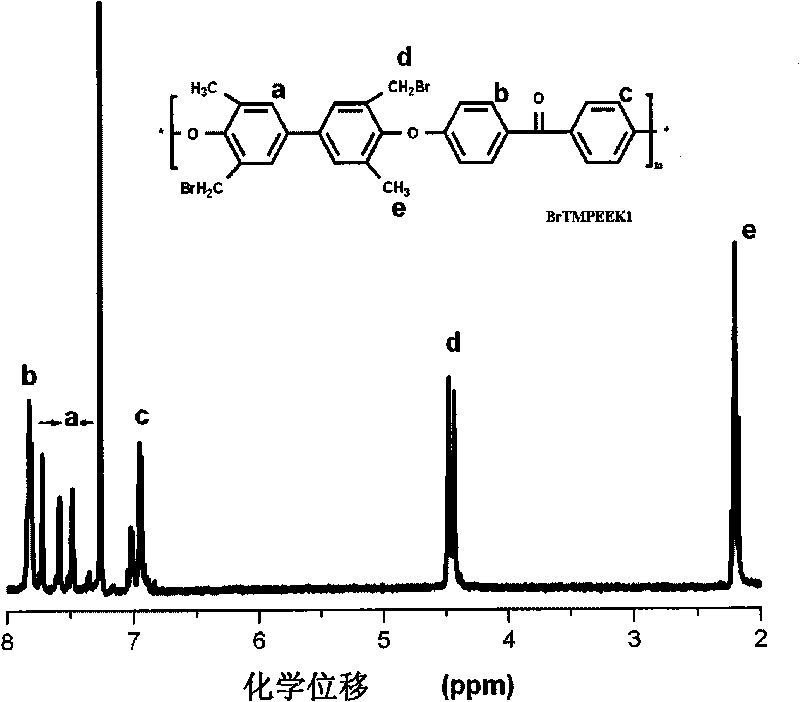
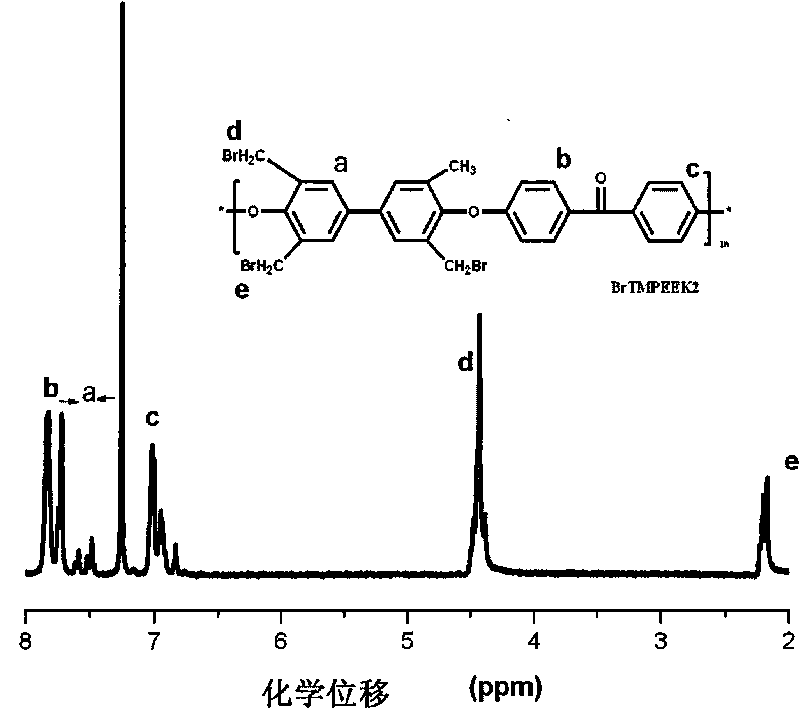
Tetramethyl-diphenol type polyarylether ketone (polyarylether sulphone) containing bromine at phenmethyl position and preparation method thereof
The invention belongs to the field of high-molecular materials, in particular to bromo-polyarylether ketone / polyarylether sulphone the phenmethyl position of which is substituted by bromine and a preparation method thereof. The bromo-polyarylether ketone / polyarylether sulphone is prepared by taking tetramethyl-diphenol type polyarylether ketone (polyarylether sulphone) containing phenacyl as a polymer main body and N-bromosuccinimide as raw materials and introducing a bromine group to the main chain of the polymer. The bromination degree can be effectively controlled by regulating reaction time and the ratio of monomer to the polymer, laying foundations for further functionalizing the polymer. Meanwhile, the introduction of a polar functional group can reduce crystals of the polymer, thereby effectively improving the processability of the polymer.
Owner:JILIN UNIV
Substituted benzoylcyclohexandiones
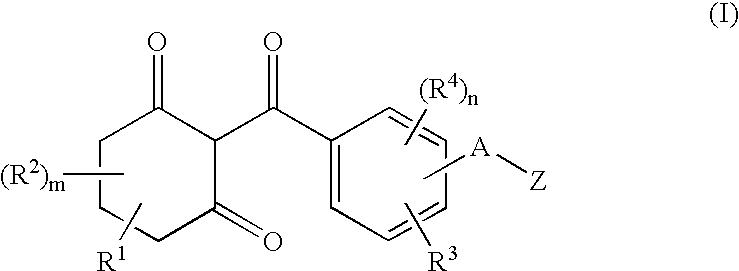
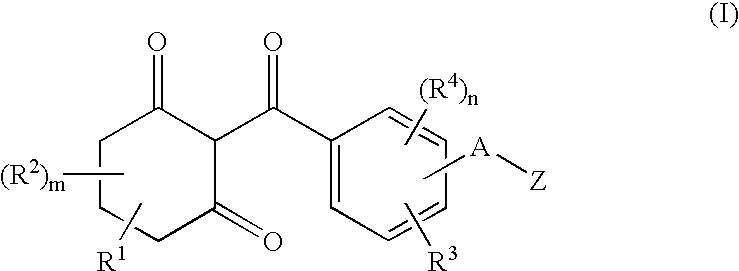
InactiveUS6924251B1Improve herbicidal activityImprove soil structureBiocideOrganic chemistryPhenacylCombinatorial chemistry
Owner:BAYER AG
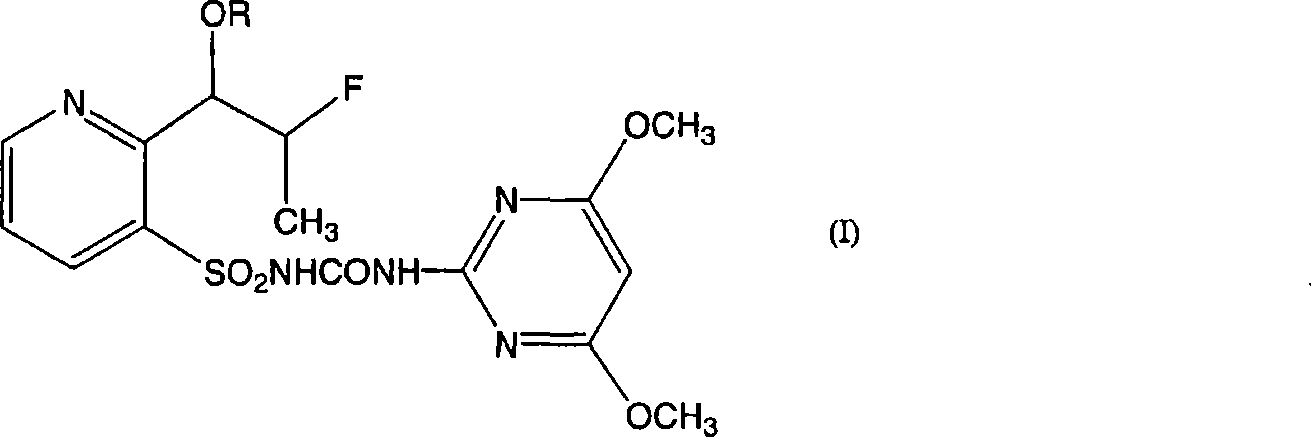
5-Substituted picolinic acid compounds and their production process
The present invention provides novel 5-substituted picolinic acid compounds of formula (I) or a pharmaceutically acceptable salt thereof: wherein R1 and R2 are independently H, C2-C6 acyl or halo-substituted benzoyl; and R3 is -C(O)O-C1-C6 alkyl, C(O)OH, CN, CONH2, CONHCH3, CON(CH3)2, 1-methyltetrazole or 2-methyltetrazole, with the proviso that when R2 is acetyl and R3 is methoxycarbonyl, R1 is not H; and that when R3 is CN, CONH2, CONHCH3, CON(CH3)2, 1-methyltetrazole or 2-methyltetrazole, R1 and R2 are H. The present invention also relates to a pharmaceutical composition comprising compound of the present invention, which is useful in the treatment of IL-1 and TNF mediated diseases or the like. The present invention further relates to a process for producing the compounds of the formula (I).
Owner:PFIZER INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
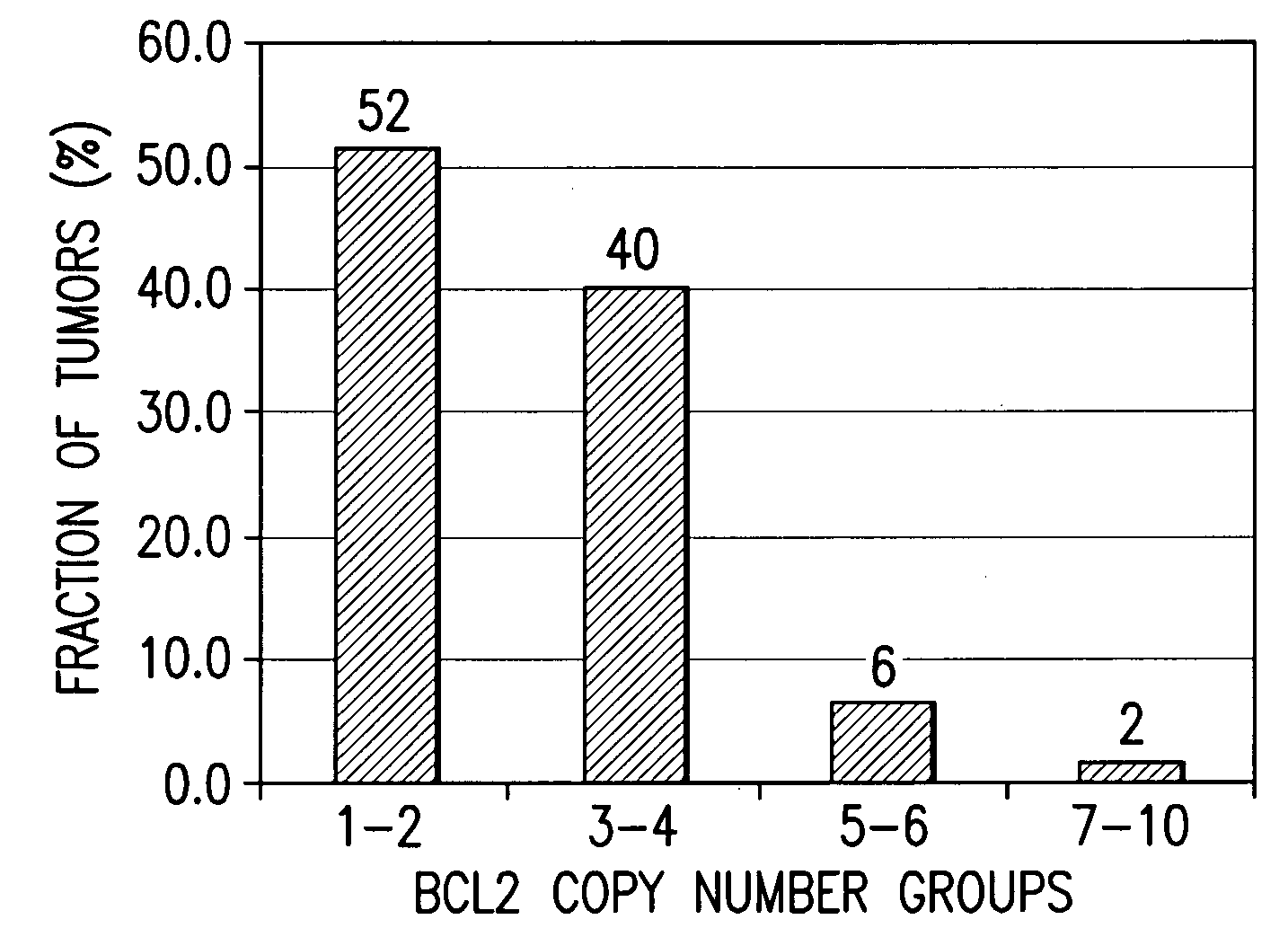
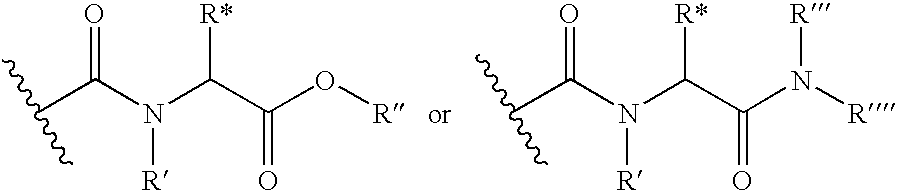
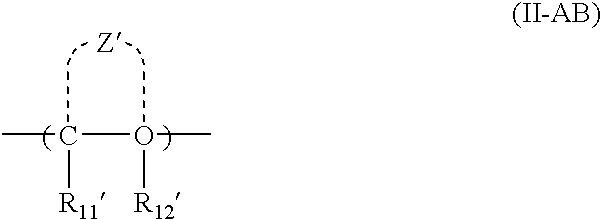
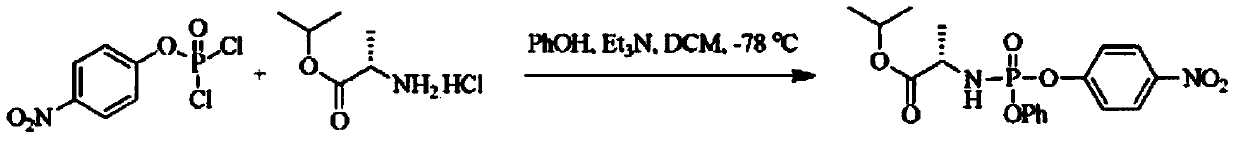
![Crystalline forms of 1-benzoyl-4-[2-[4-methoxy-7-(3-methyl-1H-1,2,4-triazol-1-yl)-1-[(phosphonooxy)methyl]-1H-pyrrolo[2,3-c]pyridin-3-yl]-1,2-dioxoethyl]-piperazine Crystalline forms of 1-benzoyl-4-[2-[4-methoxy-7-(3-methyl-1H-1,2,4-triazol-1-yl)-1-[(phosphonooxy)methyl]-1H-pyrrolo[2,3-c]pyridin-3-yl]-1,2-dioxoethyl]-piperazine](https://images-eureka.patsnap.com/patent_img/5d171be7-790e-4d37-9468-b34d12641a8a/US20070155702A1-20070705-D00001.png)
![Crystalline forms of 1-benzoyl-4-[2-[4-methoxy-7-(3-methyl-1H-1,2,4-triazol-1-yl)-1-[(phosphonooxy)methyl]-1H-pyrrolo[2,3-c]pyridin-3-yl]-1,2-dioxoethyl]-piperazine Crystalline forms of 1-benzoyl-4-[2-[4-methoxy-7-(3-methyl-1H-1,2,4-triazol-1-yl)-1-[(phosphonooxy)methyl]-1H-pyrrolo[2,3-c]pyridin-3-yl]-1,2-dioxoethyl]-piperazine](https://images-eureka.patsnap.com/patent_img/5d171be7-790e-4d37-9468-b34d12641a8a/US20070155702A1-20070705-D00002.png)
![Crystalline forms of 1-benzoyl-4-[2-[4-methoxy-7-(3-methyl-1H-1,2,4-triazol-1-yl)-1-[(phosphonooxy)methyl]-1H-pyrrolo[2,3-c]pyridin-3-yl]-1,2-dioxoethyl]-piperazine Crystalline forms of 1-benzoyl-4-[2-[4-methoxy-7-(3-methyl-1H-1,2,4-triazol-1-yl)-1-[(phosphonooxy)methyl]-1H-pyrrolo[2,3-c]pyridin-3-yl]-1,2-dioxoethyl]-piperazine](https://images-eureka.patsnap.com/patent_img/5d171be7-790e-4d37-9468-b34d12641a8a/US20070155702A1-20070705-D00003.png)
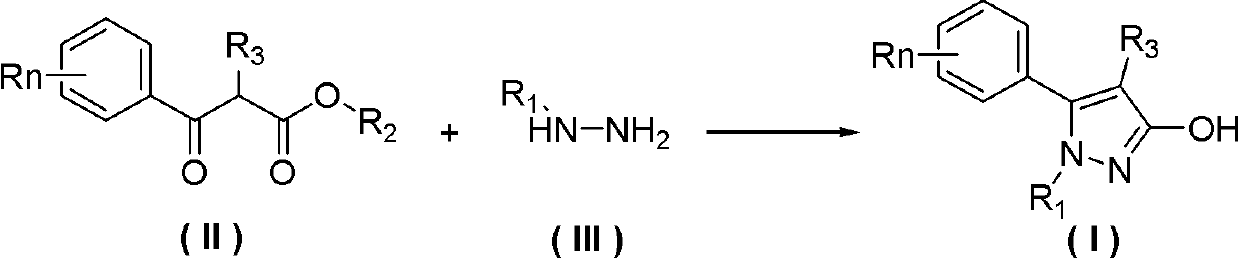
![Crystalline forms of 1-benzoyl-4-[2-[4-methoxy-7-(3-methyl-1<i>H</i>-1,2,4-triazol-1-YL)-1-[(phosphonooxy)methyl]-1<i>H</i>-pyrrolo[2,3-<i>C</i>]pyridin-3-YL]-1,2-dioxoethyl]-piperazine Crystalline forms of 1-benzoyl-4-[2-[4-methoxy-7-(3-methyl-1<i>H</i>-1,2,4-triazol-1-YL)-1-[(phosphonooxy)methyl]-1<i>H</i>-pyrrolo[2,3-<i>C</i>]pyridin-3-YL]-1,2-dioxoethyl]-piperazine](https://images-eureka.patsnap.com/patent_img/a8c04695-fa65-4ab7-85ba-6a86597aa650/US07851476-20101214-D00001.png)
![Crystalline forms of 1-benzoyl-4-[2-[4-methoxy-7-(3-methyl-1<i>H</i>-1,2,4-triazol-1-YL)-1-[(phosphonooxy)methyl]-1<i>H</i>-pyrrolo[2,3-<i>C</i>]pyridin-3-YL]-1,2-dioxoethyl]-piperazine Crystalline forms of 1-benzoyl-4-[2-[4-methoxy-7-(3-methyl-1<i>H</i>-1,2,4-triazol-1-YL)-1-[(phosphonooxy)methyl]-1<i>H</i>-pyrrolo[2,3-<i>C</i>]pyridin-3-YL]-1,2-dioxoethyl]-piperazine](https://images-eureka.patsnap.com/patent_img/a8c04695-fa65-4ab7-85ba-6a86597aa650/US07851476-20101214-D00002.png)
![Crystalline forms of 1-benzoyl-4-[2-[4-methoxy-7-(3-methyl-1<i>H</i>-1,2,4-triazol-1-YL)-1-[(phosphonooxy)methyl]-1<i>H</i>-pyrrolo[2,3-<i>C</i>]pyridin-3-YL]-1,2-dioxoethyl]-piperazine Crystalline forms of 1-benzoyl-4-[2-[4-methoxy-7-(3-methyl-1<i>H</i>-1,2,4-triazol-1-YL)-1-[(phosphonooxy)methyl]-1<i>H</i>-pyrrolo[2,3-<i>C</i>]pyridin-3-YL]-1,2-dioxoethyl]-piperazine](https://images-eureka.patsnap.com/patent_img/a8c04695-fa65-4ab7-85ba-6a86597aa650/US07851476-20101214-D00003.png)