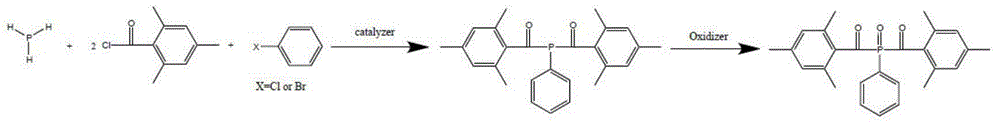
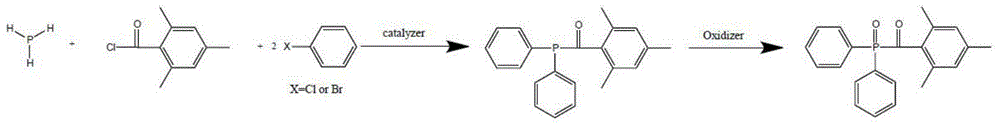
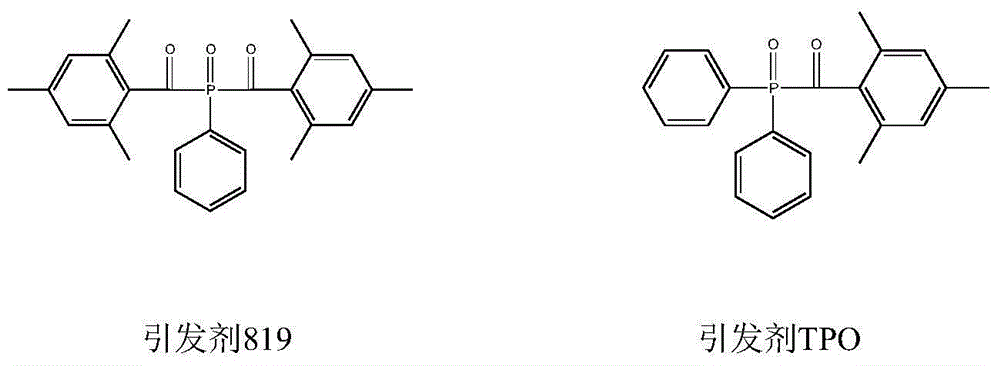
Preparation method of di (2,4,6-trimethylbenzoyl) phenyl phosphine oxide and (2,4,6-trimethylbenzoyl) diphenyl phosphine oxide
A technology of trimethylbenzoyl and diphenylphosphine oxide, which is applied in the fields of chemical instruments and methods, organic chemistry, and compounds of Group 5/15 elements of the periodic table, and can solve the problem that the by-product phenylphosphonic acid cannot be consumed, The theoretical yield can only reach 33% and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The preparation of two (2,4,6-trimethylbenzoyl) phenyl phosphine oxides, the steps are as follows:
[0046] (1) Add 150ml of re-distilled ether into a 250mL four-neck flask, purge and replace with nitrogen for 10 minutes, insert the gas inlet below the liquid level, and slowly introduce 6.8g of phosphine gas to make it fully absorbed in the solvent, Exhaust gas recycling.
[0047] (2) Add 23.7g of pyridine to the flask, control the temperature at 95-110°C, and add 24.8g of chlorobenzene dropwise into the flask while stirring. After the addition was complete, the mixture was incubated for 2 hours. 38.4g of 2,4,6-trimethylbenzoyl chloride was added dropwise at a controlled temperature of 45-50°C for reaction, and after the addition was complete, the mixture was kept warm for 2 hours.
[0048] (3) Control the temperature at 30-45°C, add 45g of 30% hydrogen peroxide dropwise, keep the reaction at 30-45°C for 2 hours after the addition, separate the liquid, wash the organi...
Embodiment 2
[0051] The preparation of (2,4,6-trimethylbenzoyl) diphenylphosphine oxide, the steps are as follows:
[0052] (1) Add 150ml of re-distilled ether into a 250mL four-neck flask, and blow nitrogen for replacement for 10 minutes. The air inlet is inserted below the liquid level, and 6.8g of phosphine gas is slowly introduced to make it fully absorbed in the solvent. Exhaust gas recycling.
[0053] (2) Add 23.7g of pyridine to the flask, control the temperature at 95-110°C, and add 49.6g of chlorobenzene dropwise into the flask while stirring. After the addition was complete, the mixture was incubated for 2 hours. 19.2 g of 2,4,6-trimethylbenzoyl chloride was added dropwise at a controlled temperature of 45-50° C. for reaction. After the addition was complete, the reaction was kept at a temperature for 2 hours.
[0054] (3) Control the temperature at 30-45°C, add 45g of 30% hydrogen peroxide dropwise, keep the reaction at 30-45°C for 2 hours after the addition, separate the liq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com