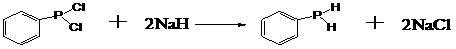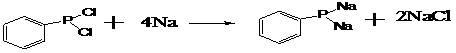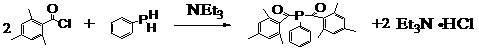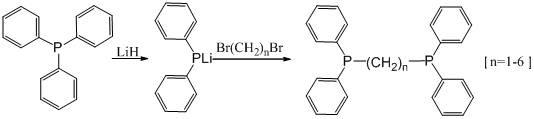Patents
Literature
330 results about "Phenylphosphine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
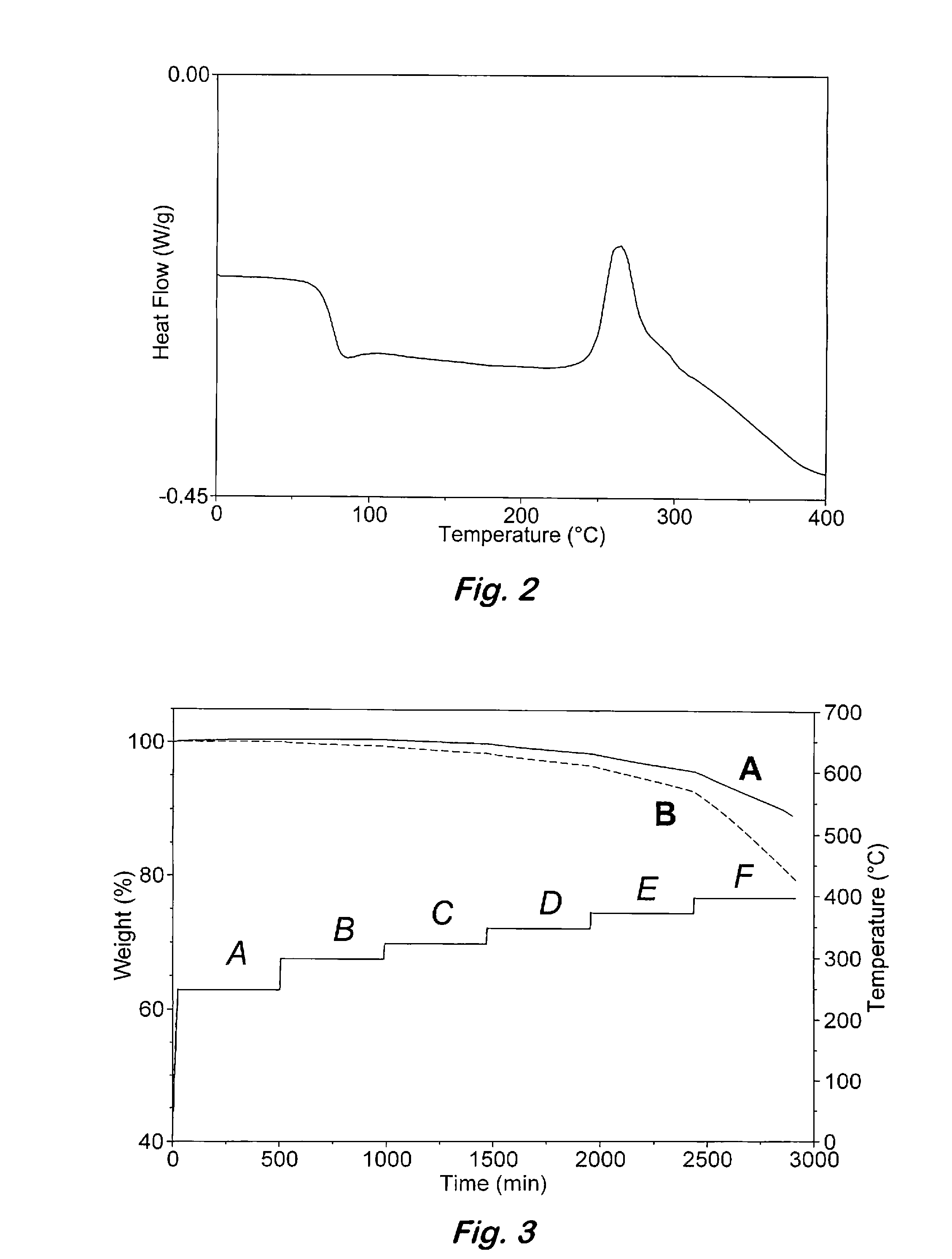
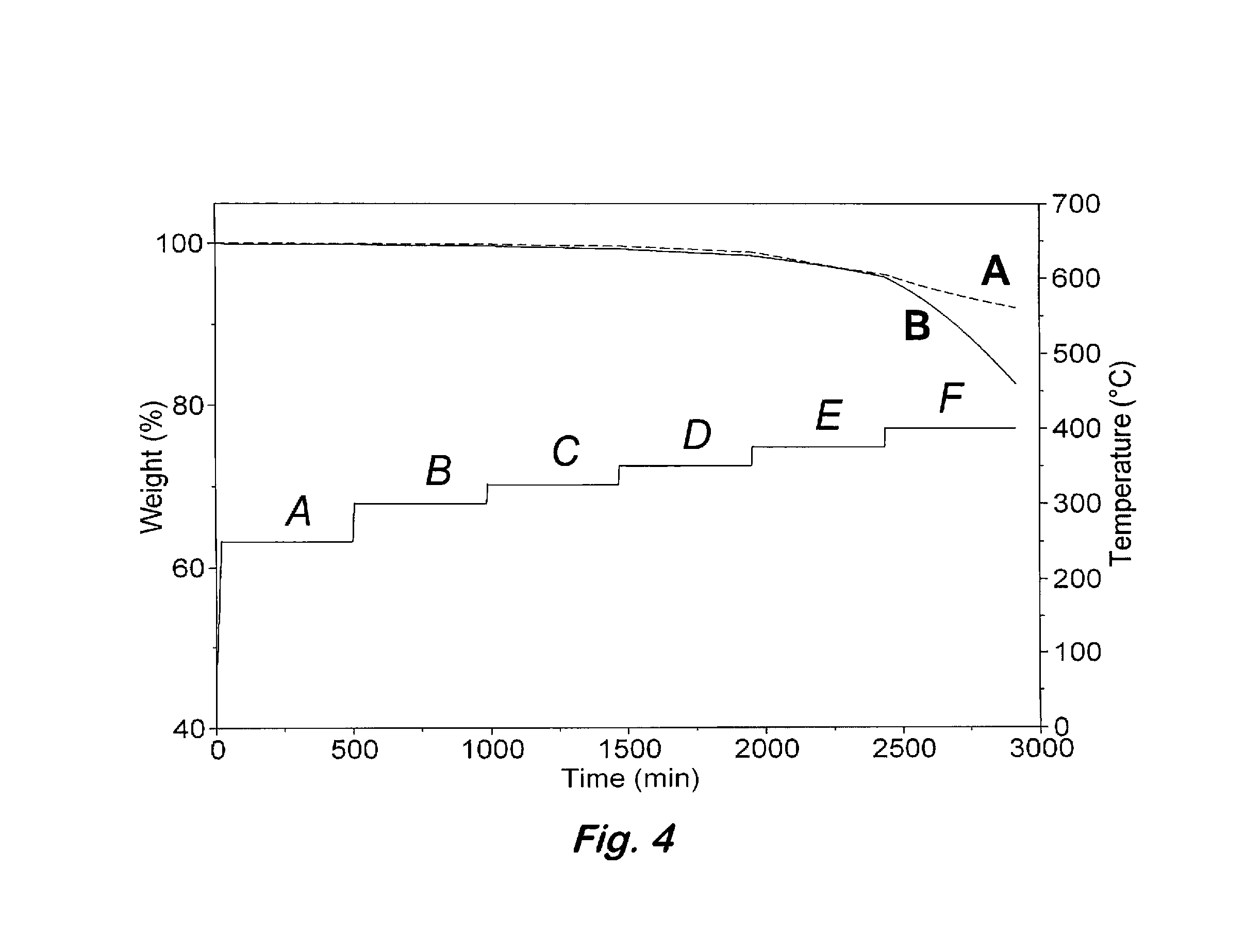
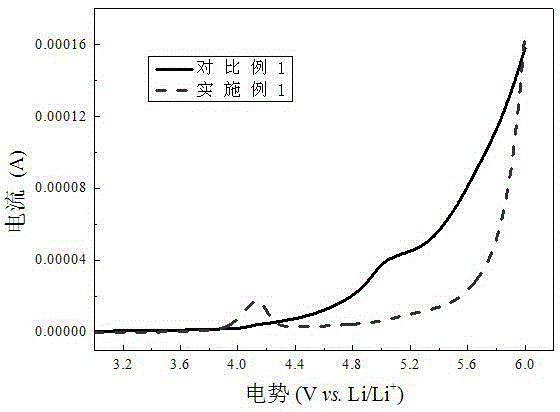
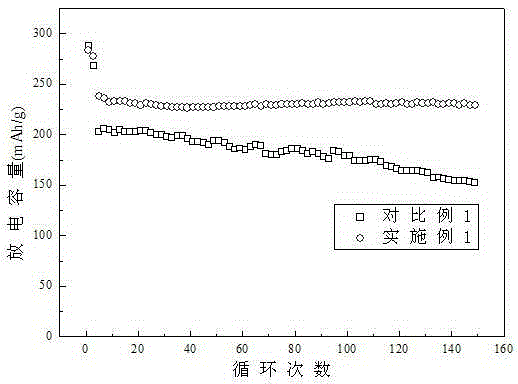
Phenylphosphine is an organophosphorus compound with the chemical formula C₆H₅PH₂. It is the phosphorus analog of aniline. Like other primary phosphines, phenylphosphine has an intense penetrating odor and is highly oxidizable. It is mainly used as a precursor to other organophosphorus compounds. It can function as a ligand in coordination chemistry.
Catalyst system and catalyzing method of propylene hydrogenation and formylation
ActiveCN1986055AReduce dosageImprove stabilityOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by carbon monoxide reactionFormylation reactionTriphenylphosphine
The present invention relates to catalyst system for propylene hydrogenation and formylation and process of catalytically synthesizing butyl aldehyde. The catalyst system is triaryl phosphine-Rh(I) catalyst system with proper additive, such as bisphosphite ester, in proper amount. Compared with similar available catalyst, the catalyst system has obviously higher catalytic acitivity, higher selectivity, higher stability and raised n-butyl aldehyde / isobutyl aldehyde ratio in the catalytically synthesized product.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI +1
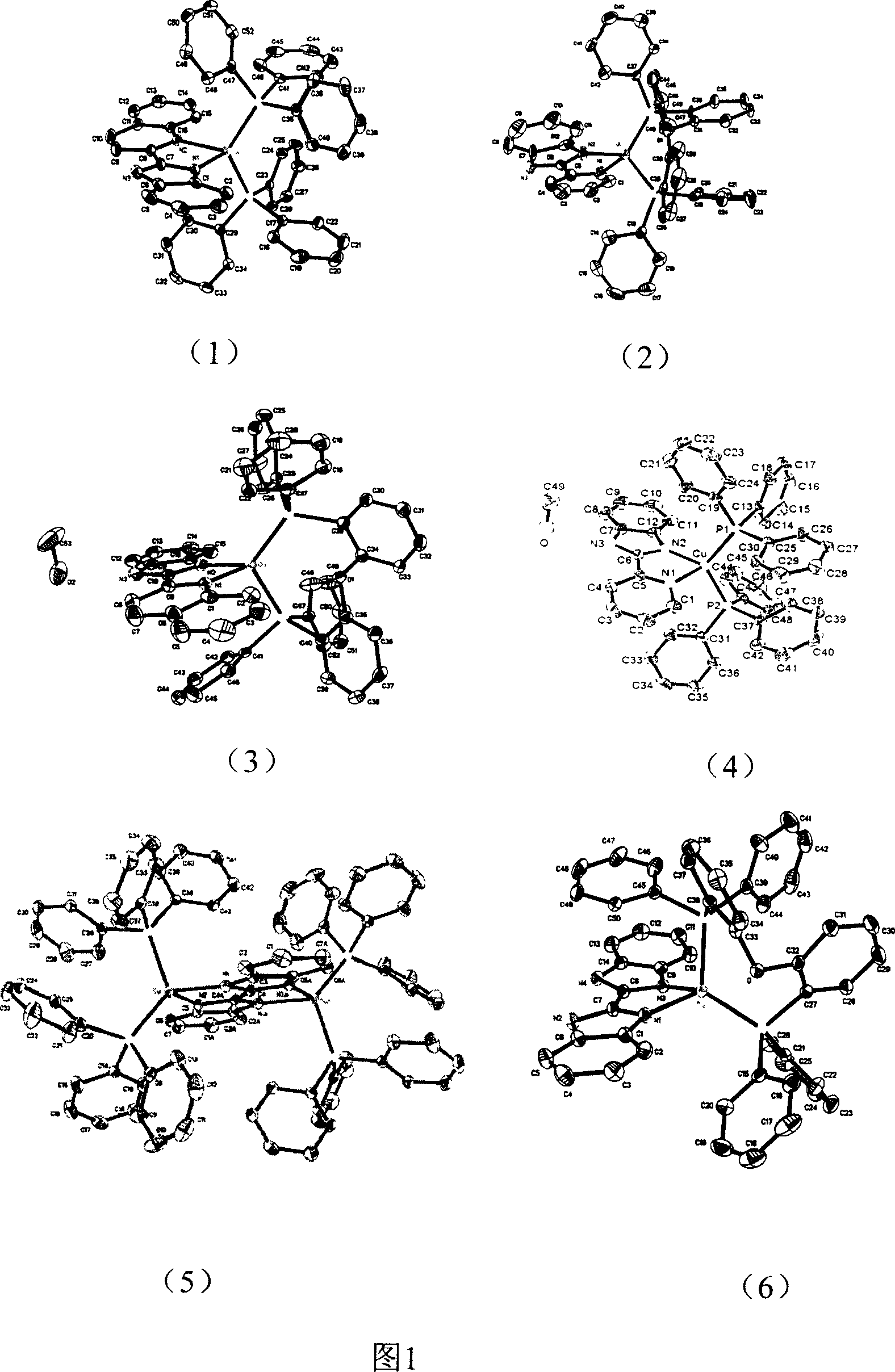
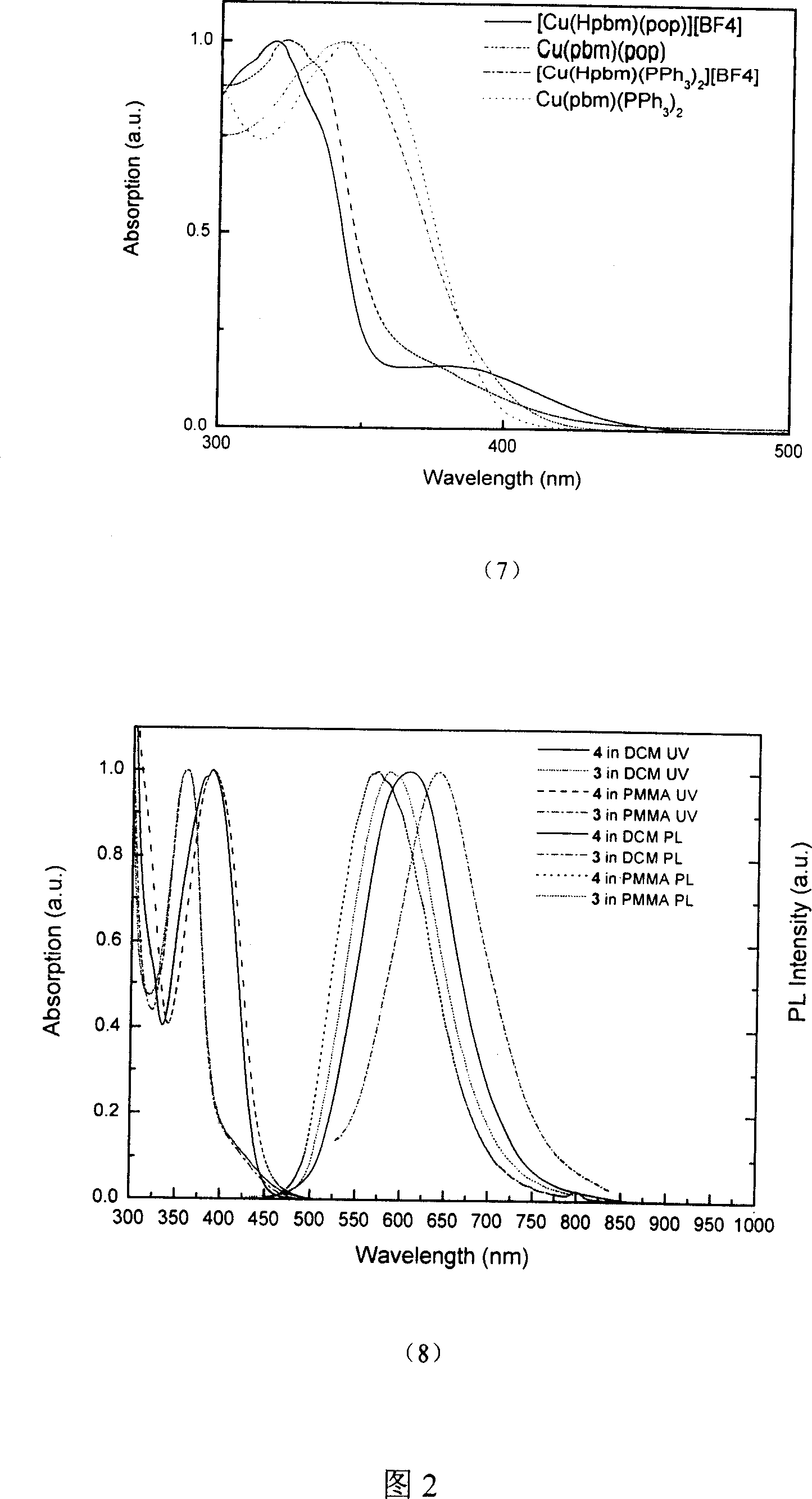
Copper (1) compound with imidazole derivate as compounding body
InactiveCN100999528AEasy to synthesizeEasy to purifyCopper organic compoundsLuminescent compositionsRoom temperatureTriphenylphosphine
The present invention relates to copper complex with imidazole as ligand. Ionic one-valent copper complex is prepared with pyridyl imidazole, pyridyl benzimidazole or quinolyl benzimidazole as the first ligand, triphenyl phosphine or bridged phenyl phosphine as the second ligand and one-valent copper ion. Neutral copper complex may be further obtained under the action of alkali. The ionic copper complex and the neutral copper complex may convert mutually under the action of acid and alkali. This kind of complex possesses strong room temperature phosphorescence emission and may be used as light emitting material.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Surface treatment of nanoparticles to control interfacial properties and method of manufacture
InactiveUS20050222325A1Material nanotechnologySynthetic resin layered productsPersonal care3-mercaptopropyltrimethoxysilane
A surface treated particle comprising a plurality of inorganic, metallic, semi-metallic, and / or metallic oxide particles and a star-graft copolymer with looped and / or linear polymeric structure on a star-graft copolymer, obtainable by a heterogeneous polymerization reaction in the particle surface proximity, encapsulating at least a portion of said particles and a method for making the same. The surface treatment comprises: Si (w, x, y, z), where: w, x, y, and z are mole percent tetrafunctional, trifunctional, difunctional, and monofunctional monomeric units, respectively; w, x, y, and z are about 0-50, 0-50, 5-99, and 0-5, respectively; w is tetraethylorthosilicate; x is selected from the group consisting of γ-glycidoxypropyltrimethoxysilane, γ-methacryloxypropyltrimethoxysilane, methyltrimethoxysilane, n-propyltrimethoxysilane, isobutyltrimethoxysilane, n-hexyltrimethoxysilane, n-octyltrimethoxysilane, n-octadecyltrimethoxysilane, phenyltrimethoxysilane, 3-(trimethoxysilyl)propylsuccinic anhydride, heptadecafluorotrimethoxysilane, 3-isocyanatopropyltrimethoxysilane, 2-(diphenylphosphino)ethyltrimethoxysilane, 3-aminopropyltrimethoxysilane, 3-mercaptopropyltrimethoxysilane, n-(trimethoxysilylpropyl)EDTA, pentafluorophenylpropyltrimethoxysilane, trifluoropropyltrimethoxysilane, and the triethoxy-containing counterparts of these monomers; y is selected from the group consisting of dicyclohexyldimethoxysilane, diethyldiethoxysilane, dimethyldichlorosilane, dimethyldiethoxysilane, dimethyldimethoxysilane, diphenyldiethoxysilane, diphenyldimethoxysilane, di-n-hexyldichlorosilane, n-hexylmethyldichlorosilane, methyldodecyldiethoxysilane, n-octylmethyldimethoxysilane, and the diethoxy-containing counterparts of these monomers; and z is selected form the group consisting of n-octadecyldimethylmethoxysilane, triethylsilanol, trimethylethoxysilane, trimethylmethoxysilane, and the ethoxy-containing counterparts of these monomers. Product(s) per se, defined as surface treated ZnO and / or TiO2, and the use of the product(s) per se in personal care formulations are excluded.
Owner:NANOPHASE TECH CORP
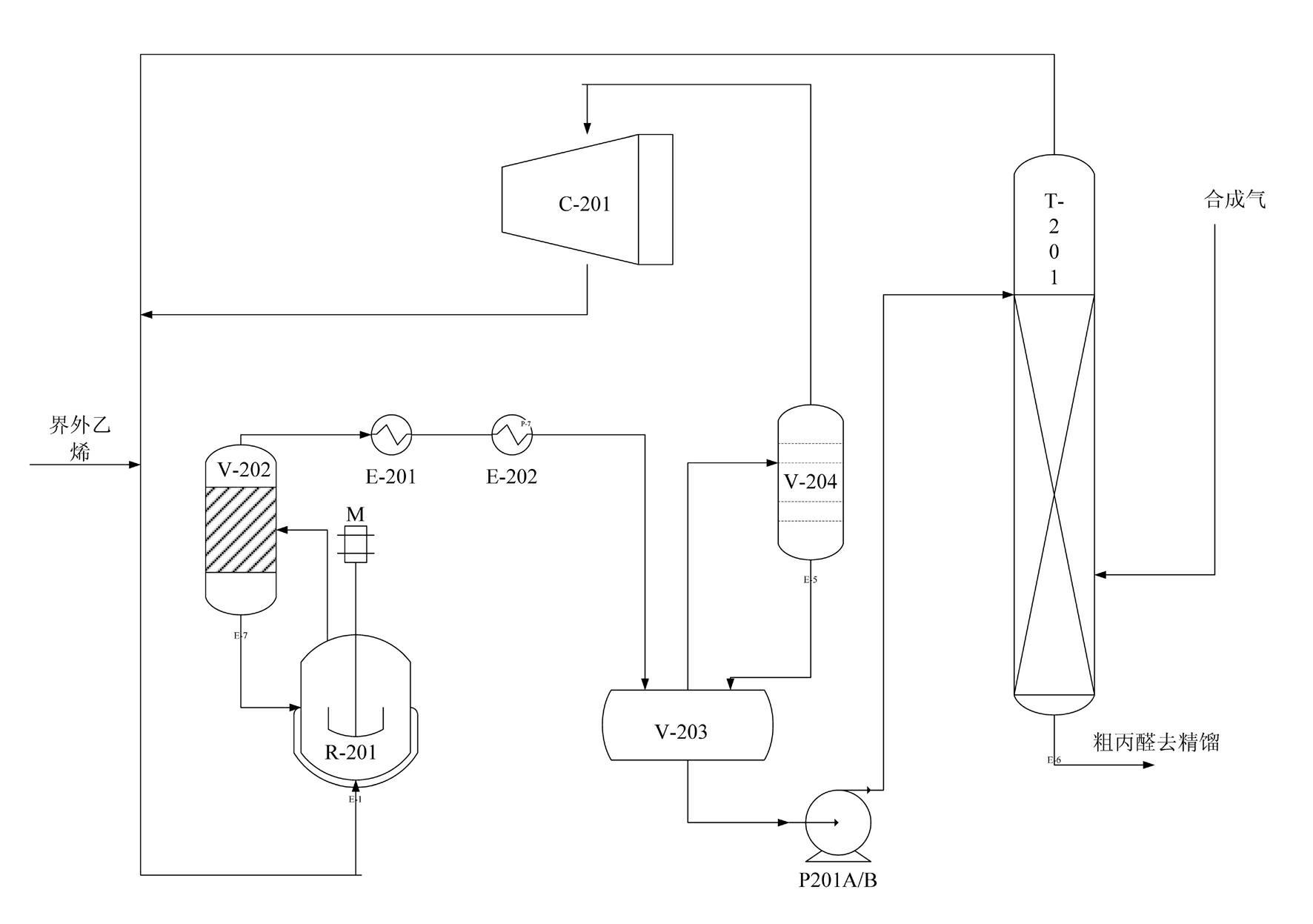
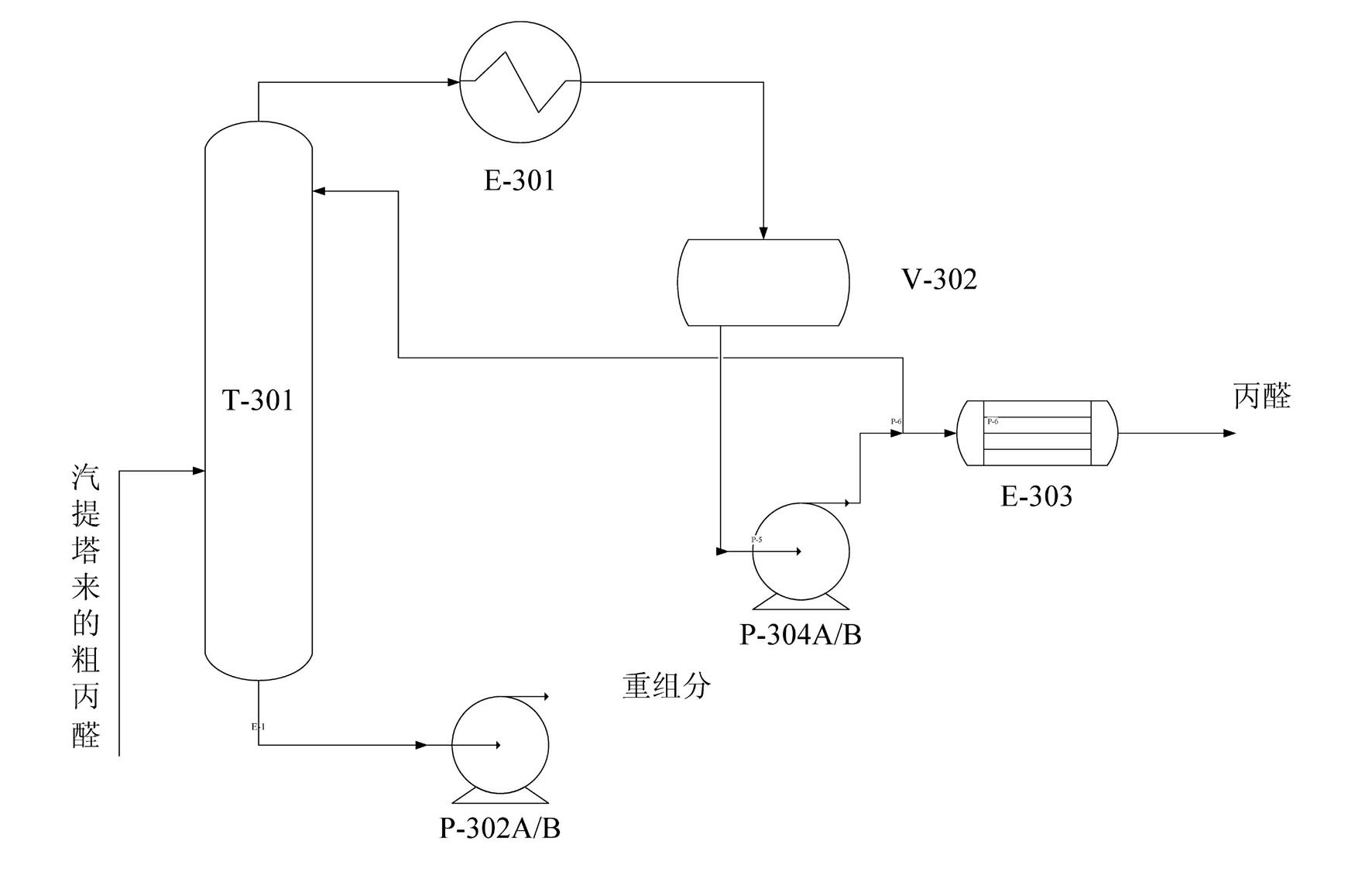
Synthesis method of propionaldehyde by low-pressure carbonyl of ethylene
ActiveCN102115433AReduce consumptionLess investmentPreparation by carbon monoxide reactionPhosphoniumFormylation reaction
The invention provides a synthesis method of propionaldehyde by low-pressure carbonyl of ethylene, belonging to a preparation method of saturated compound which contains -CHO group and is connected with a noncyclic carbon atom. The synthesis method comprises the steps of: taking ethylene, carbon monoxide and hydrogen as raw materials, adopting a rhodium / phosphonium complex catalyst system, and synthesizing the propionaldehyde by ethylene hydrogen formylation reaction. The synthesis method is characterized by comprising a synthesis operating unit and a rectification operating unit, wherein thesynthesis operating unit comprises a compressor, a synthesis reactor, a coarse propionaldehyde collecting tank and a gas stripping column; an entrainment separator is respectively arranged above the synthesis reactor and the coarse propionaldehyde collecting tank; and an iron-free propionaldehyde solution catalyst system of metal rhodium catalyst and ligand triphenylphosphine is used, the rhodiumconcentration in the catalyst solution is 80ppm-150ppm, and the weight percentage concentration of the triphenylphosphine is 1.5%-3%. The synthesis method of the propionaldehyde by the low-pressure carbonyl of the ethylene is simple in equipment, less in investment, simple in technological operation, and small in catalyst consumption. Counting as the ethylene, the raw material conversion rate is more than or equal to 90%, and the propionaldehyde product content is more than or equal to 99.5%.
Owner:南京诺奥新材料有限公司
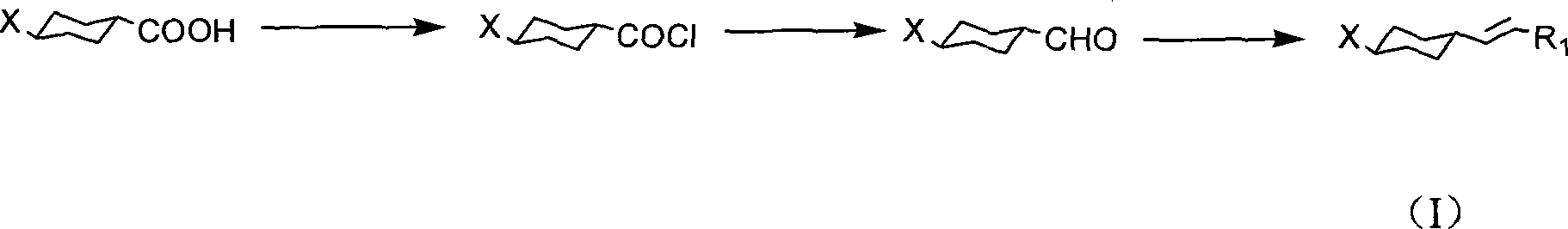
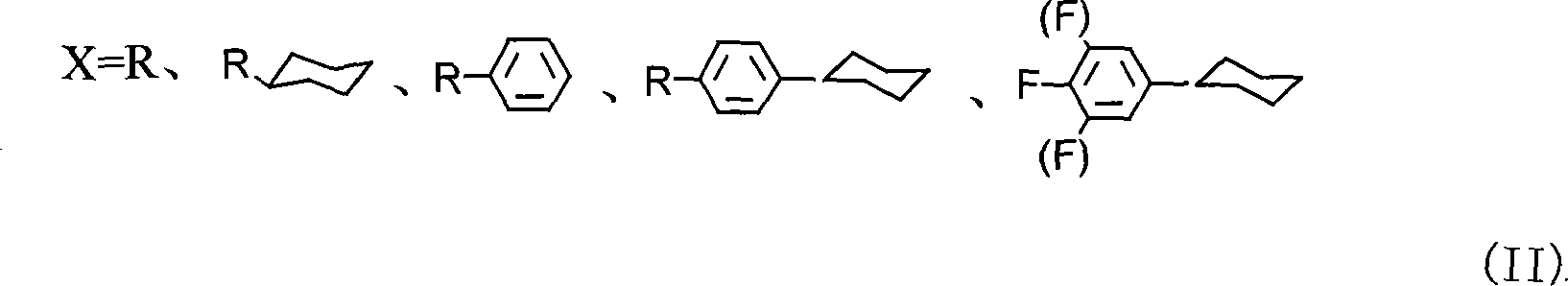
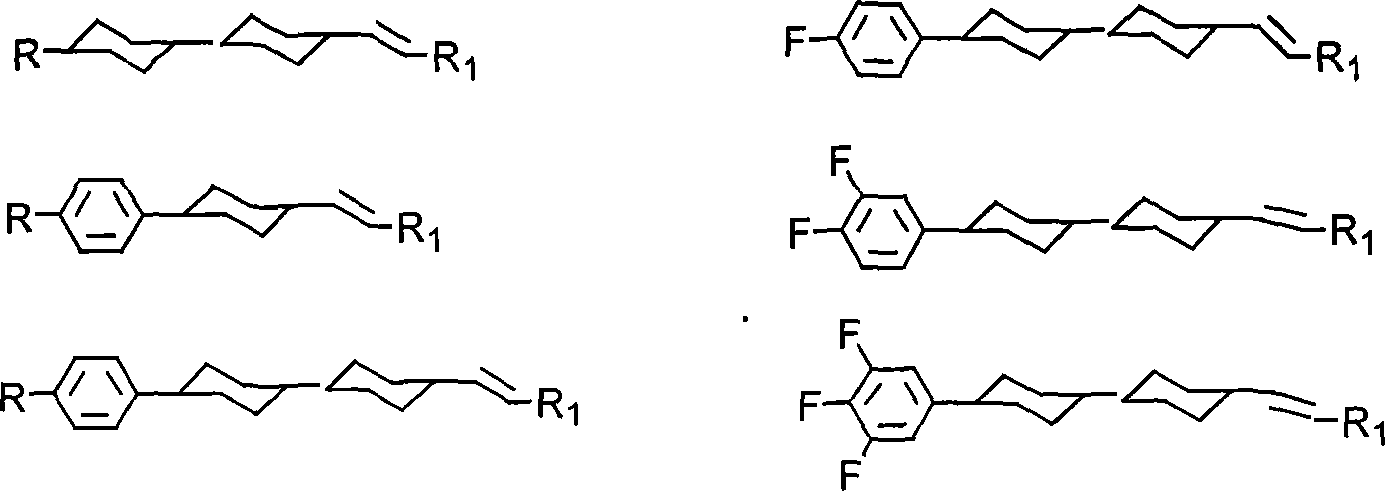
Method for producing cyclohexyl group olefin hydrocarbon liquid crystal material
ActiveCN101244977ASmall optical anisotropyLow viscosityLiquid crystal compositionsHydrocarbonsAlkanePhosphonium
The invention discloses a preparation method of trans-4-replacing cyclohexyl formaldehyde and trans-4-replacing cyclohexyl olefin, which uses trans-4-replacing cyclohexyl formic acid as raw material, and prepares the trans-4-cyclohexyl olefin by acyl chloride, silicon hydrogen alkane reduction, and reaction with methyl bromide triphenylphosphine quarter phosphonium. The preparation method of the trans-4-replacing cyclohexyl formaldehyde and the trans-4-replacing cyclohexyl olefin is simple in method, clean in using, and has wide application prospect.
Owner:内蒙古永太化学有限公司
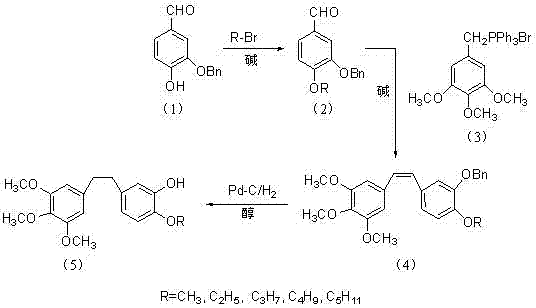
Method for preparing 3,4,5-trimethoxy-3'-hydroxy-4'-alkoxy diphenylethane
InactiveCN103539642AImprove protectionSignificant technological progressOrganic compound preparationCarbonyl compound preparationPalladium on carbonBenzaldehyde
Owner:SHANGHAI INST OF TECH
Adhesive compositions
A two part curable composition is provided, where the composition comprises: (a) a first part comprising: (i) a (meth)acrylate component; (ii) 1,4-quinones, such as napthoquione or benzoquinone and derivatives thereof in an amount less than or equal to about 0.05 weight percent; (iii) triaryl or alkaryl phenylphosphine in an amount greater than or equal to about 0.5 weight percent; and (iv) an amine; and (b) a second part comprising: (i) benzoyl peroxide in an amount greater than about 1.0 weight percent.
Owner:HENKEL KGAA
Reaction-type halogen-free flame retardant bis-(p-aminocarboxyphenyl)phenylphosphine oxide and synthetic method thereof
InactiveCN104945658APollution is notReduce production stepsGroup 5/15 element organic compoundsBenzoic acidDichlorophenylphosphine
The invention relates to reaction-type halogen-free flame retardant bis-(p-aminocarboxyphenyl)phenylphosphine oxide and its synthetic method and application and belongs to the field of preparation of a flame retardant. The synthetic method comprises the following steps: using glacial acetic acid as a solvent, adding para amino benzoic acid into glacial acetic acid, slowly dropwise adding dichlorophenylphosphine oxide into an acetic acid solution of para aminobenzoic acid, continuously stirring and reacting at 85-95 DEG C for 4-6 h, and absorbing hydrogen chloride discharged by the reaction by the use of aqueous alkali; and cooling a reaction product after the end of the reaction, carrying out suction filtration, washing and drying to obtain white powdery bis-(p-aminocarboxyphenyl)phenylphosphine oxide. The bis-(p-aminocarboxyphenyl)phenylphosphine oxide flame retardant has advantages of low toxicity, low smoke, innocuousness, high efficiency and the like, is a reactive flame retardant integrating a carbon source, an acid source and a gas source, and is applied in inherent flame retarding of nylon or thermoplastic polyester copolymer.
Owner:NANJING LIHAN CHEM CO LTD
Preparation method for POSS/PDMAEMA organic/inorganic hybrid material according to thiol-ene click chemistry method
The invention relates to a preparation method for a POSS / PDMAEMA organic / inorganic hybrid material according to a thiol-ene click chemistry method. The preparation method comprises the following steps: obtaining PDMAEMA through synthesis according to an RAFT polymerization method; taking dimethyl phenylphosphine as a catalyst, taking n-hexylamine as a reducer, and conducting a reduction reaction on a dithioester group at the tail end of PDMAEMA to generate sulfydryl; conducting thiol-Michael addition reactions on the double bonds of PDMAEMA containing sulfydryl and POSS containing ethenyl according to the thiol-ene click chemistry method to generate a POSS / PDMAEMA organic / inorganic hybrid material with a temperature / pH dual response. The preparation method has the advantages that the prepared POSS / PDMAEMA organic / inorganic hybrid material is small in size, stable in chemical bond, and uniform in particle diameter; due to a click reaction to PDMAEMA, the hydrophilic performance of POSS is effectively improved; in a water solution, the POSS / PDMAEMA organic / inorganic hybrid material is excellent in temperature sensibility and pH sensibility; due to a special structure and the stimulus response performance, the POSS / PDMAEMA organic / inorganic hybrid material is a new-generation environment-friendly new material product, and can be applied to drug carrying, targeted release, metal ion recovery, environmental pollutant adsorption and other fields.
Owner:TONGJI UNIV
Polycaprolactone polyol as well as preparation method and application thereof
The invention relates to polycaprolactone polyol as well as a preparation method and application thereof. The method comprises the following steps that S1, under the protection of the water-free and oxygen-free conditions and inert gas, an initiator, a catalyst, an inhibitor and caprolactone are uniformly mixed; and S2, a system obtained in the step S1 is heated to 120-230 DEG C for reaction for 10-180 minutes so as to carry out ring-opening polymerization reaction, vacuum defoamation is carried out, and cooling and discharging are carried out so as to obtain the polycaprolactone polyol, wherein the inhibitor is one or more of organic phosphine, glyphosate-sodium, ethyldiphenylphosphine or tribenzylphosphine. According to the preparation method, the specific side reaction inhibitor is added, so that the polycaprolactone polyol which is controllable in molecular weight, low in acid value, few in metal residues, narrow in polymer molecular weight distribution and capable of keeping the original excellent physicochemical properties is obtained, and the acid value of the polycaprolactone polyol prepared through the preparation method is less than 0.5 mg KOH / g.
Owner:GUANGDONG BOSSIN NOVEL MATERIALS TECH CO LTD
Cycloolefin addition polymer and making method
InactiveUS20100190950A1Improve breathabilityHigh thermal stability (or heat resistance)Semi-permeable membranesPolymer sciencePtru catalyst
A cycloolefin addition polymer is prepared by addition polymerization of a cycloolefin-functionalized siloxane and optionally norbornene in the presence of catalyst A which is a nickel or palladium complex having a cyclopentadienyl ligand and a methyl, triphenylphosphine or allyl ligand and co-catalyst B, typically tris(pentafluorophenyl)boron or trityltetra(pentafluorophenyl)borate. The polymer is easy to manufacture and has high thermal stability and mechanical strength as well as good gas permeability.
Owner:SHIN ETSU CHEM IND CO LTD +1
Space environmentally durable polyimides and copolyimides
InactiveUS6841652B2Low color requirementIncrease temperaturePhosphorus organic compoundsSolubilityFiber
Polyimides displaying low color in thin films, atomic oxygen resistance, vacuum ultraviolet radiation resistance, solubility in organic solvents in the imide form, high glass transition (Tg) temperatures, and high thermal stability are provided. The poly(amide acid)s, copoly(amide acid)s, polyimides and copolyimides are prepared by the reaction of stoichiometric ratios of an aromatic dianhydride with diamines which contain phenylphosphine oxide groups in polar aprotic solvents. Controlled molecular weight oligomeric (amide acid)s and imides can be prepared by offsetting the stoichiometry according to the Carothers equation using excess diamine and endcapping with aromatic anhydrides The polyimide materials can be processed into various material forms such as thin films, fibers, foams, threads, adhesive film, coatings, dry powders, and fiber coated prepreg, and uses include thin film membranes on antennas, second-surface mirrors, thermal optical coatings, and multi-layer thermal insulation (MLI) blanket materials.
Owner:NASA
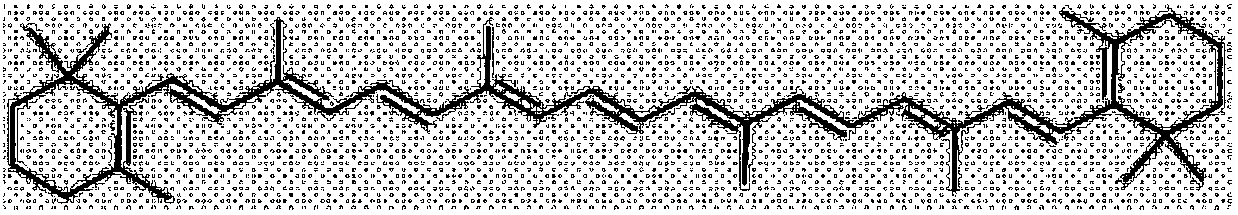
Method for synthesizing beta-carotene from vitamin A through one-pot method
The invention discloses a method for synthesizing beta-carotene from vitamin A through a one-pot method. The synthesis method comprises the following steps that firstly, a vitamin A triphenyl phosphine salt reaction solution is obtained, wherein triphenyl phosphine is added in an organic solvent, acid is slowly dropwise added, after dropwise adding is completed, heat preservation is conducted, then vitamin A or a derivative of the vitamin A and alkali 1 are added, a heat preservation reaction continues, and the vitamin A triphenyl phosphine salt reaction solution is obtained; secondly, all-trans beta-carotene is obtained, wherein an acidic medium oxidizing agent and alkali 2 are dropwise added under a low-temperature condition, after the heat preservation reaction is completed, the reaction temperature is increased, the reaction continues, a beta-carotene reaction solution is obtained, a beta-carotene crude product is obtained after post-treatment, and the all-trans beta-carotene is obtained after the beta-carotene crude product is subjected to thermal isomerization. The obtained beta-carotene is crystal solid from red to dark red, the content is larger than 95%, and the yield is 55% or above.
Owner:XIAMEN KINGDOMWAY VI TAMIN INC +1
Benzocyclobutene polysiloxane polymer monomer or resin and preparation method thereof
The invention relates to a benzocyclobutene polysiloxane polymer monomer or resin and a preparation method thereof. The invention discloses a dimethyl-4-benzocyclobutene silicon-based ethylmethyl cyclotetrasome disclosed as Formula (III) and a preparation method thereof. The preparation method of the polysiloxane polymer monomer comprises the following steps:Sequentially adding a tetramethyl-tetravinyl-cyclosiloxane solution diluted by solvent methylbenzene and 4-(1-H-1,1-methyl)silicon benzocyclobutene into a reactor, adding a platinum catalyst after applying a nitrogen atmosphere, and reacting at 60-90 DEG C for 20-40 hours; and adding triphenylphosphine, heating under reflux for 3 hours, cooling, filtering, concentrating the filtrate by distillation, adding methanol, mixing, precipitating, filtering, repeatedly precipitating the solid with methanol, and drying the solid to obtain the polysiloxane polymer monomer product. The product has excellent thermal properties, mechanical properties, electric properties and film-forming properties, and has wide development and application prospects as a high-performance dielectric film material or packaging material in the fields of micro-electronics, aerospace, national defence and the like. (III).
Owner:BEICHUAN RUIHUI SCI & TECH CO LTD
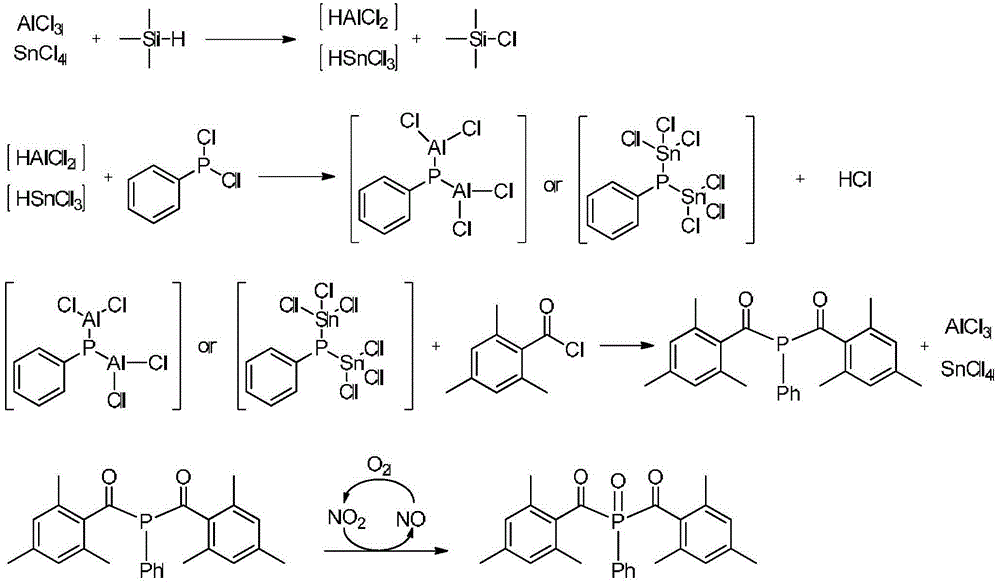
Preparation method for photoinitiator bis(2,4,6-trimethylbenzoyl)phenylphosphine oxide
InactiveCN104151358ALess prone to fireHigh reaction safety factorGroup 4/14 element organic compoundsGroup 5/15 element organic compoundsPotassiumPhenylphosphine
The invention discloses a preparation method for a photoinitiator bis(2,4,6-trimethylbenzoyl)phenylphosphine oxide. The preparation method comprises the synthesis steps: under the protection of inert gas, carrying out a reaction of aluminium trichloride or tin tetrachloride and trimethylsilane in the temperature range of 0-100 DEG C to obtain aluminium dichloride ions, and rapidly acting with dichlorophenyl phosphine in the reaction system, to obtain a bis(aluminium trichloride or tin tetrachloride)phosphine salt intermediate; then carrying out a reaction of the intermediate and 2,4,6-trimethyl benzoyl chloride, to obtain bis(2,4,6-trimethylbenzoyl)phenyl phosphine; and under the condition of the temperature of 20-50 DEG C, oxidizing by nitrogen dioxide, to obtain the target product. The method avoids use of a dangerous active metal potassium or sodium, and adopts aluminium trichloride or tin tetrachloride with relatively low price and trimethylsilane for on-site generation of the active intermediate to achieve the reaction. The method has the characteristics of low preparation cost, simple and safe operation process, easy control of the reaction process, high product yield, good purity, easy realization of mass production and the like.
Owner:ZHANGJIAGANG JIMUTE CHEM TECH
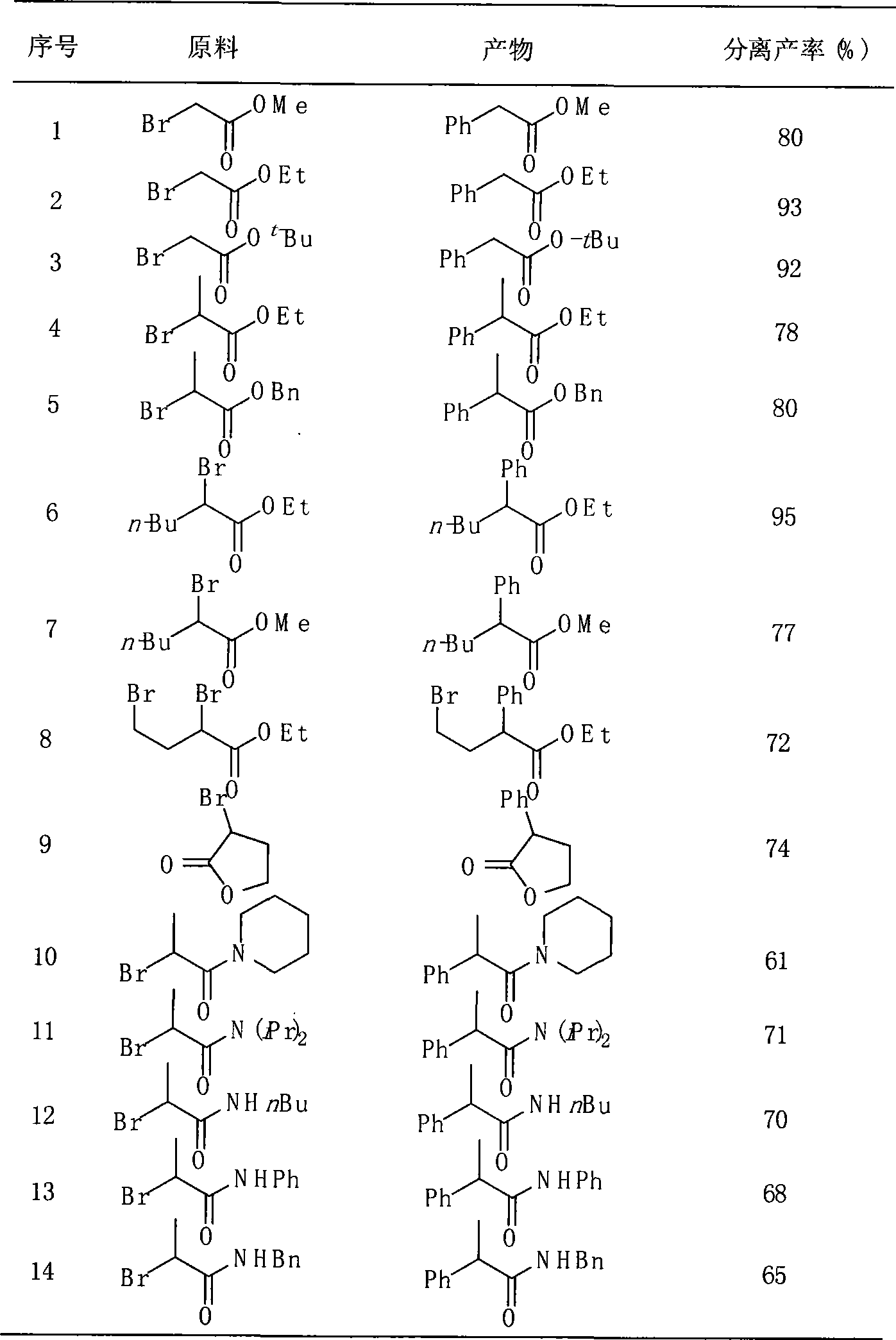
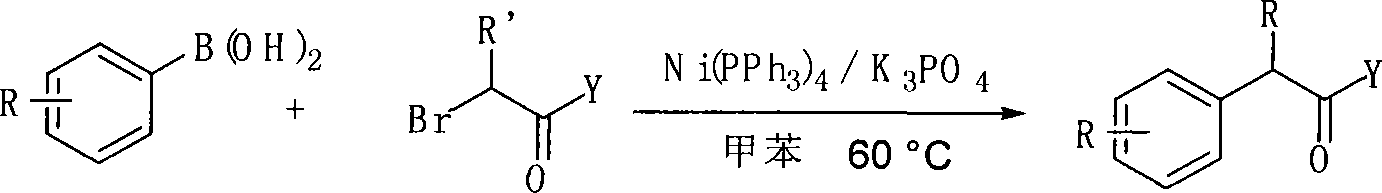
Method for preparing alpha-aryl carbonyl compound
InactiveCN101157590ASubstrate stabilizationCheap and easy to getOrganic-compounds/hydrides/coordination-complexes catalystsCarbonyl group formation/introductionKetonePhenylphosphine
The invention relates to a method of preparing alpha-aryl carbonyl compound. With the catalyst of four triphenylphosphine nickel or acetylacetone nickel ligand, the aryl boric acid, the alpha-halogenated carbonyl compound, the ligand and the additive are dissolved in toluene, and after reaction for 10 to 24 hours in 50 to 100 DEG C, the alpha-aryl carbonyl compound is obtained, wherein, the ligand is single-tooth phosphine ligand or double-tooth phosphine ligand; and the additive is absolute potassium phosphate or tri-potassium phosphate. The invention, which utilizes the catalyst with low cost, stable substrate, easy obtaining and gentle reaction conditions, can not only catalyze arylation of ester, amide and ketone, but also can be compatible with beta hydrogen, thereby greatly expanding the range of the substrate. Moreover, the invention possesses great application potential and can directly constitute the main framework of the steride.
Owner:WUHAN UNIV
New application of benzo [C] phenanthridine and protopine in producing overriding resistant bacterium medicament
InactiveCN101195627AEnhanced inhibitory effectHigh activityAntibacterial agentsOrganic active ingredientsBenzo(c)phenanthreneDouble bond
The invention relates to a new application of benzo [C] phenanthridine as formula I and original tropine alkaloid for preparing anti-drug-resistant bacterial drug, wherein R1-R10, R12-R15 are hydrogen and hydroxyl groups, cycloalkyl or alkyl groups with 1-12 carbon atoms, alkox or acyloxy groups, benzyloxy, chlorine or other halide atoms, amido, methylol, aldehydo, aldehydo, acetonyl, carboxy group, mesyloxy, 4-methyl-benzene sulfonyl oxygen group, aryl sulfonyl oxygen group, biphenyl phosphine acyloxy and -OCONH2, R11 is hydrogen, methyl or oxygen atom, R5 and R6 (or R15 of the formula I), R14 and R15 are formed with double bonds, R12 or R13 and nearby N atoms are formed with double bonds, N atom can be tertiary or quaternary N type, the substituents of nearby carbons of R1-R10 can have dioxolane structure. The inventive alkaloid can effectively restrain and kill drug-resistant bacterials as MRSA and ESBLs.
Owner:成都军区昆明总医院
Synthesis method for ultraviolet photoinitiator XBPO
InactiveCN102675365AHigh priming activityHigh activityGroup 5/15 element organic compoundsPhenacylPtru catalyst
The invention provides a synthesis method for ultraviolet photoinitiator XBPO, which comprises the following steps of: putting sodium hydride and solvent benzene into a stirring reaction kettle, dropwise adding dichlorophenyl phosphine, putting catalyst organic base, dropwise adding 2, 4, 6-trimethyl benzoyl chloride, slowly dropwise adding pure water, slowly dropwise adding oxidant, dropwise adding 2% sodium hydroxide, washing with water, concentrating, crystallizing, filtering, and drying to obtain the ultraviolet photoinitiator. The synthesis method has the advantages that the photolytic product of the ultraviolet photoinitiator XBPO has two trimethyl benzoyl chloride and one phenyl phosphine acyl free radical, which are higher in initiation activity, compared with the photo initiation activity of TPO. The wide wave band has light sensitivity, so that the GR-XBPO not only can be independently used, but also can be used in cooperation with the other ultraviolet absorbers.
Owner:HUBEI GURUN TECH CO LTD
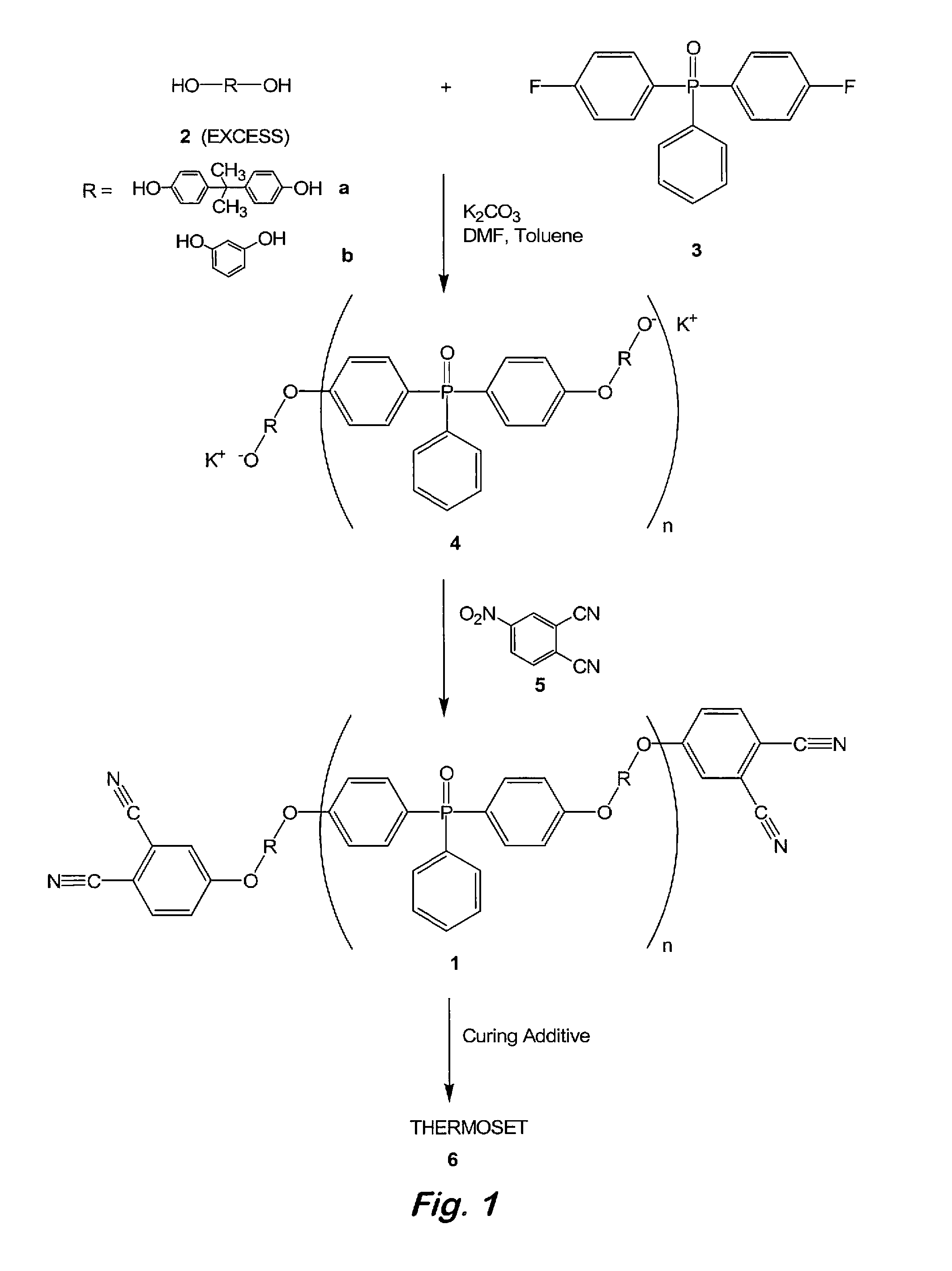
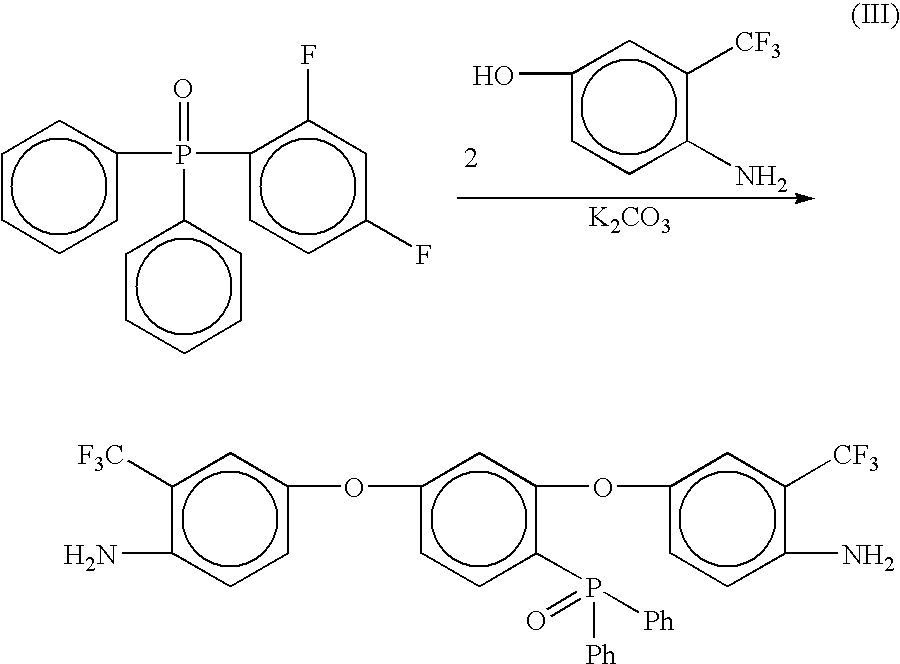
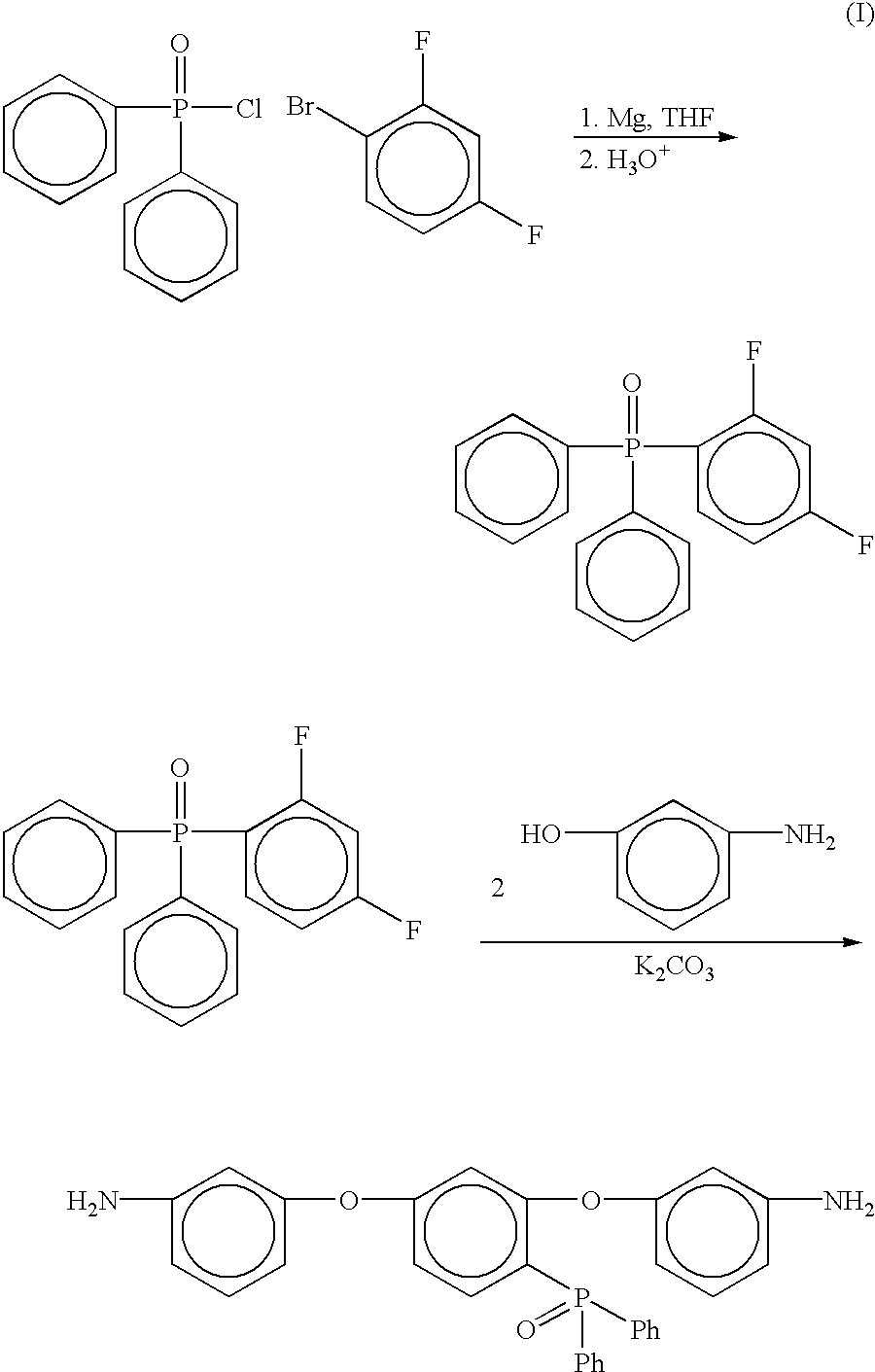
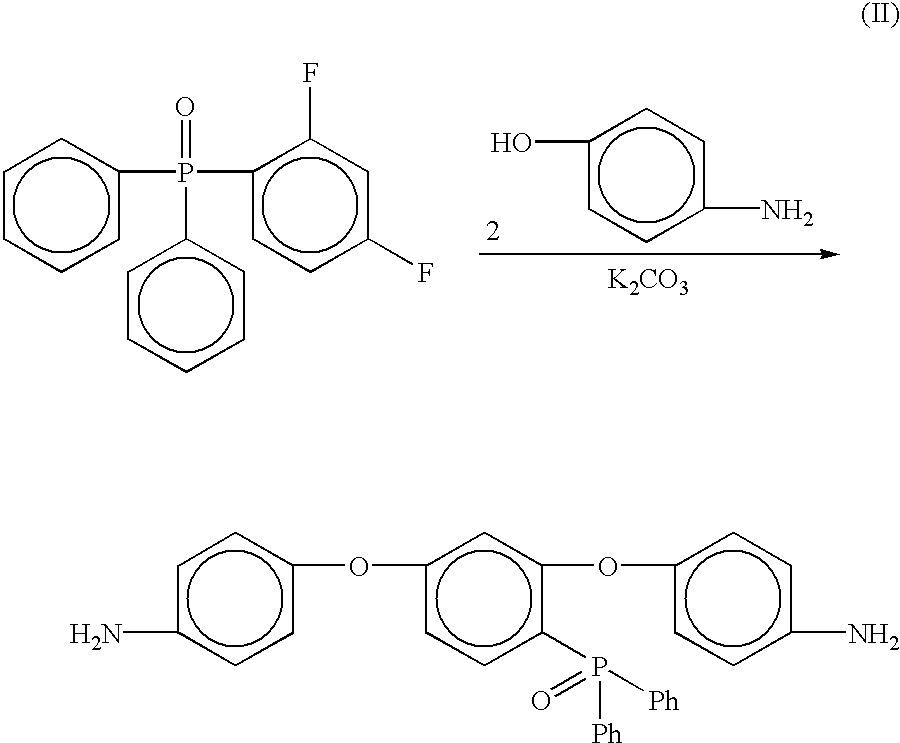
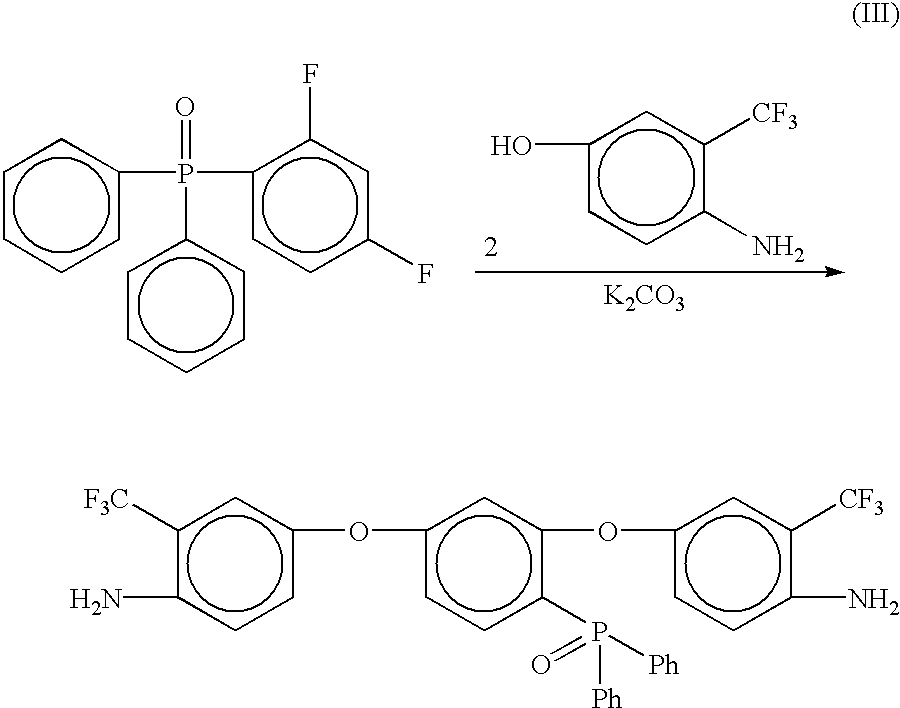
Phosphine oxide containing phthalonitriles
Compounds having the formulas below. Each R1 is an aromatic-containing group. Each R2 is methyl, phenyl, alkyl, perfluoroalkyl, and trifluoromethyl. M is an alkali metal, and n is a positive integer. A thermoset made by curing a mixture comprising a curing agent and the below phthalonitrile monomer. A method of: reacting a bis(fluorophenyl)phenylphosphine oxide with an excess of an aromatic diol in the presence of an alkali metal carbonate to form the oligomer below.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
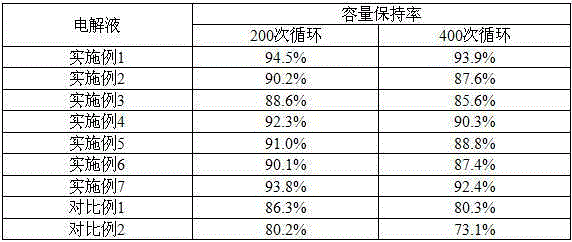
Electrolyte for improving high temperature and high voltage performance of lithium ion battery and application thereof in lithium ion battery
ActiveCN105406124AAvoid decompositionImprove stabilitySecondary cells servicing/maintenanceElectrolytic agentOrganic solvent
The invention discloses an electrolyte for improving high temperature and high voltage performance of a lithium ion battery and application thereof in the lithium ion battery. The electrolyte comprises lithium salt, organic solvent and additive, wherein the additive is composed of methoxyl diphenylphosphine and cathode surface film-forming additive, content of the methoxyl diphenylphosphine in the electrolyte is 0.01-10.0wt.%, and content of the cathode surface film-forming additive in the electrolyte is 0.02-5wt.%; the organic solvent is composed of 10-50wt.% of cyclic carbonate and 30-70wt.% of linear carbonate; and concentration of the lithium salt in the electrolyte is 0.5-2.5mol / L. According to the electrolyte provided by the invention, MDP and cathode surface film-forming additive are used simultaneously as combined additive, stability of a positive electrode material of the lithium ion battery under high voltage can be improved, decomposition of the electrolyte on surface of the positive electrode is inhibited, and cycle performance of the high-voltage lithium ion battery under normal temperature and high temperature is improved.
Owner:YICHUN JINHUI NEW ENERGY MATERIALS
Synthesis method of polysulfone-polyimid copolymer
InactiveCN1493603AReduce dosageOmit the step of synthesizing biphenyl dianhydrideSynthesis methodsPhosphine
A polysulfone-polyimide copolymer is prepared from polysulfone, polyimide, Ni as catalyst, triphenyl phosphine as coordinate agent, Zn powder as reducer and non-protonic solvent as reaction medium through reacting at 60-120 deg.C, adding non-protonic solvent, dichlorodiphthaleine imide prepared from chlorophenyl anhydride and diamine, and the dichloroaryl compound and its derivative, and continuously reacting for 2-8 hr. Its advantages are high output rate (91-100%), high performance, and low cost.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Photocurable and thermosetting resin composition, cured product thereof, and printed circuit board
ActiveUS20080308301A1Improve abilitiesSufficient deep curabilityPhotosensitive materialsRadiation applicationsResistPhosphine oxide
The present invention provides a photocurable and thermosetting resin composition having excellent surface curability and deep curability, allowing pattern formation with a laser beam having a wavelength of 350 to 410 nm, and being useful as a solder resist for laser direct imaging, the composition including a carboxylic resin (A), an oxime ester-based photopolymerization initiator (B) such as 2-(acetyloxyiminomethyl)thioxanthene-9-one, and another photopolymerization initiator than (B) such as 2,4,6-trimethylbenzoyl-diphenyl-phosphine oxide, and a sulfur compound (E) such as 2-mercaptobenzothiazole.
Owner:TAIYO INK MFG
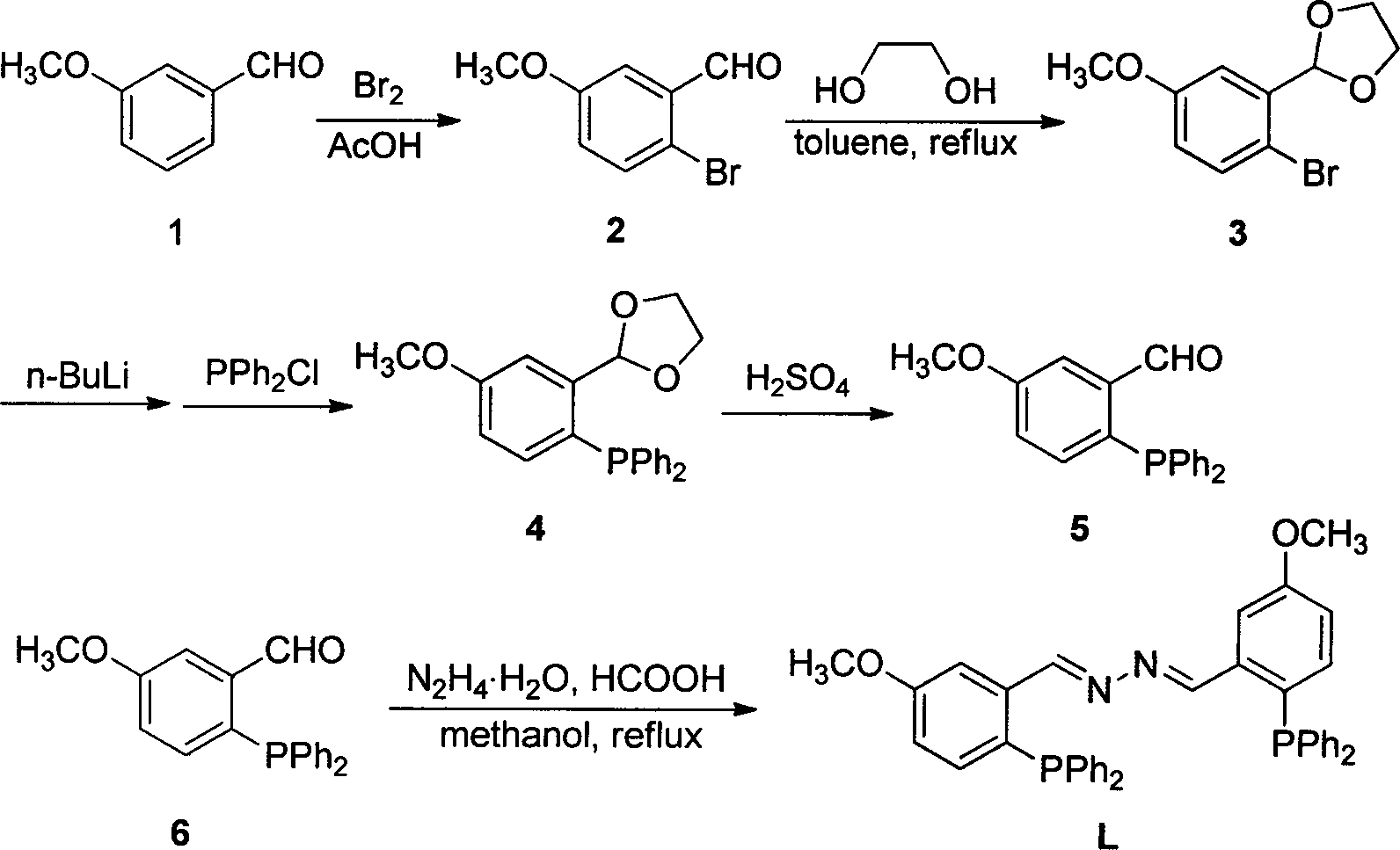
Synthetic method and application of (1E, 2E)-1,2-bi(5-methoxyl-2-diphenylphosphine benzylidene) hydrazine
InactiveCN103483383AHigh reactivityAvoid formingOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalysts2-DiphenylphosphinobenzaldehydeBenzaldehyde
(1E, 2E)-1,2-bi(5-methoxyl-2-diphenylphosphine benzylidene) hydrazine is a dual-phosphine ligand containing an imine group. An aromatic ring has methoxyl which supplies electrons so that double metal has the stronger coordination capability. According to the synthetic method, intermediate 5-methoxyl-2-(diphenylphosphino)benzaldehyde and hydrazine hydrate serve as raw materials, formic acid serves as a catalyst, and the raw materials and the formic acid flow back in methyl alcohol to obtain bis-imine bis-phosphine four-tooth ligand L. Bis-imine bis-phosphine four-tooth ligand L and Cu(CH3CN)4CIO4 in-situ catalytic Sonogashira cross coupling reaction is carried out, the catalytic system is very high (the yield reaches to 99%) in reactivity to aryl iodide and is active to aryl bromide to some degree, alkyne self-coupling products can be well avoided, the reactive system is simple, and post-processing is convenient. Adopted Cu is low in cost, easy to obtain, economical and clean, and takes part in the Sonogashira coupled reaction instead of expensive and toxicant Pd. No rganic amine used as a solvent or alkali is used. The (1E, 2E)-1,2-bi(5-methoxyl-2- diphenylphosphine benzylidene) hydrazine has very good application prospects in industrial production in the future.
Owner:NANKAI UNIV
Graphene reinforced and toughened biodegradable polyester compound and foaming material thereof
The invention discloses a graphene reinforced and toughened biodegradable polyester compound and a foaming material thereof. The preparation method comprises the following steps: A, preparing GO / DMF dispersion liquid; B, dissolving a polyfunctional epoxy chain extender in DMF, adding a triphenylphosphine catalyst, and inducing a chemical reaction to obtain GO-epoxy powder; and C, carrying out meltblending on the dried PBAT, PLA and GO-epoxy powder through a circulating twin-screw internal mixer, and carrying out mold pressing to obtain the graphene reinforced and toughened biodegradable polyester composite sheet. According to the invention, epoxy resin grafted graphene oxide is used as a compatilizer and a reinforcing agent; the compatibility of the PBAT / PLA compound is improved, the tensile strength and elongation at break of the PBAT / PLA compound are improved, the tensile strength of the obtained graphene reinforced and toughened biodegradable polyester compound is 25-55 MPa, and the elongation at break of the obtained graphene reinforced and toughened biodegradable polyester compound is 100-600%. And high-pressure CO2 foaming is carried out to prepare the low-density, high-hardness, high-strength and high-toughness graphene reinforced and toughened biodegradable polyester composite foaming material.
Owner:SUN YAT SEN UNIV
Preparation method of zinc oxide quantum dot modified polyfluorene blue polymer luminescent material
InactiveCN102295745AImprove luminous efficiencyImprove thermal stabilitySolid-state devicesSemiconductor/solid-state device manufacturingHeat stabilityPolyfluorene
The invention relates to a preparation method of a polyfluorene blue high-molecular luminescent material modified by zinc oxide quantum dots. The method comprises the synthesis of 1,4-di-n-butoxybenzene, the synthesis of 1,4-dibutoxy-2, 5-bis(bromomethyl)benzene, the synthesis of 1,4-dibutoxy phenyl-2,5-tri phenylphosphine bromine, the synthesis of 1,4- dibutoxy-2,5-divinyl benzene, the synthesisof 2,7-dibromofluorene, the synthesis of 2,7-dibromo-9,9-dioctyl benzene and the synthesis of a polymer-based nanocomposite. Compared with the prior art, the present invention has the following advantages that the preparation technology is simple and the cost is low; the heat stability of the polymer is excellent; the unique quantum confinement effect and the fluorescent characteristic of the added ZnO quantum dots enable the polymer fluorescent emission spectrum to make an obvious blue shift, and the fluorescent strength is obviously enhanced; in addition, ZnO is a good electron transport material, ZnO introduced into a luminescent layer polymer is capable of effectively balancing the ratio of electron and hole for forming exciton and increasing the luminescent efficiency of the devices.
Owner:SHANGHAI UNIV
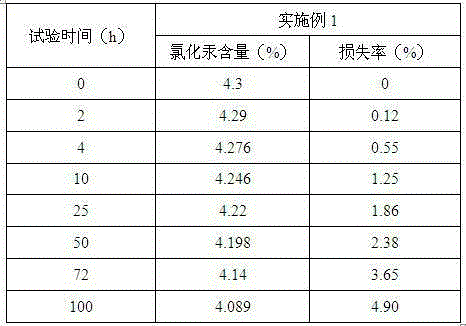
Method for low-mercury catalyst with good heat stability
ActiveCN105195224AImprove thermal stabilityPreparation by halogen halide additionOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystPhenylphosphine
A provided method for low-mercury catalyst with good heat stability comprises preparation of active carbon, preparation of an adsorption solution, and adsorption processing. The adsorption processing steps comprises ultrasonic assisted adsorption, microwave assisted adsorption and vacuum penetration adsorption. The prepared low-mercury catalyst comprises, in percent by weight, 4.3% of mercuric chloride, 8.6% of zinc chloride, 8.6% of palladium chloride, 2.87% of platinum chloride, 2.87% of tetrabutyl ammonium chloride, 2.87% of tripotasium triphenylphosphine-3,3',3''-trisulfonate, and 0.18% of water. The low-mercury catalyst possesses the mechanical strength of 98.6%, the granularity of 3-6 mm (98%), the bulk density of 460 g / L, and the mercuric chloride ignition loss of 0.39%. The prepared low-mercury catalyst is good in heat stability and low in mercuric chloride loss rate. When the low-mercury catalyst is applied to vinyl chloride synthesis reaction, the acetylene conversion rate is 99.93%, the vinyl chloride selectivity is close to 100%, and the vinyl chloride yield is 95.2%.
Owner:NINGXIA JINHAI CHUANGKE CHEM TECH
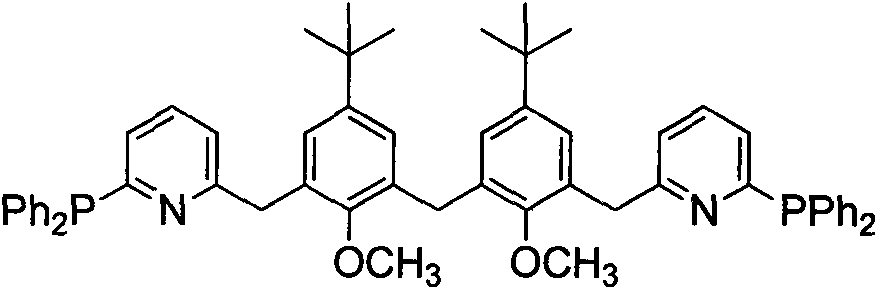
Synthetic method and application of novel multi-aryl bridged long-chain diphosphine ligand
InactiveCN103554183AHigh reactivityReduce dosageOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsOrganic synthesisCarbon–carbon bond
A novel multi-aryl bridged long-chain diphosphine ligand is disclosed. A semi-calix[4]arene is taken as a structural skeleton and subjected to double-side decoration, so that a novel phosphine-nitrogen ligand is designed and synthesized, and also the novel ligand and a palladium salt are used for in-situ catalysis of a Suzuki coupling reaction. The preparation method of the ligand comprises: synthesizing a semi-calix intermediate bis(5-tert-butyl-3-hydroxymethyl-2-methoxyphenyl)methane, brominating the intermediate and bridging with an intermediate 2-bromo-6-diphenylphosphanylpyridine to obtain the multi-aryl bridged long-chain diphosphine ligand. The advantages comprise that the novel diphosphine ligand employs the calixarene fragment as the main body, pyridine structure is used to stabilize the molecule and to adjust the molecular cavity, and the ligand is used to catalyze the Suzuki coupling reaction in the presence of trace palladium for carbon-carbon bond construction in organic synthesis. The ligand is stable in structure; the synthesis reaction is short in time, mild in condition, high in yield, easy for mass production and commercialization; and the catalyst use amount is small when the ligand is used for Suzuki coupling reactions, and it is expected that functional groups with specific function can be introduced for promoting synthesis of multiple medicaments.
Owner:NANKAI UNIV
Method for synthesizing bis(diphenylphosphino)-alkane
ActiveCN102633836ANo impact on industrial productionImprove responseGroup 5/15 element organic compoundsAlkaneLithium diphenylphosphide
The invention discloses a method for synthesizing bis(diphenylphosphino)-alkane, belonging to the field of organic synthesis. The method comprises the following steps of: enabling triphenylphosphine to react with lithium hydride to generate a lithium diphenylphosphide intermediate under anhydrous and anoxybiotic conditions, and then directly reacting with dibromoalkane to synthesize a bis(diphenylphosphino)-alkane compound. According to the method disclosed by the invention, the intermediate generated during the preparation of lithium diphenylphosphide by using the lithium hydride has no affects on follow-up reaction, the reaction process is optimized, the reaction is easier to control, the separation cost is reduced, and the method is more suitable for industrial production.
Owner:河南惠成新材料有限公司
Phenyl phosphine diamide derivative as well as synthesis method and application thereof
InactiveCN108659040AFair priceLow boiling pointGroup 5/15 element organic compoundsEpoxySynthesis methods
The invention provides a phenyl phosphine diamide derivative as well as a synthesis method and application thereof. The synthesis method comprises the following steps: by taking phenyl phosphine oxalyl chloride and primary amine as reaction raw materials, tetrahydrofuran or diethyl ether as reaction solvents, and triethylamine as a reaction catalyst, dissolving the primary amine into ethyl ether or tetrahydrofuran, and by taking triethylamine as an equal mass as a substrate, slowly dropping a phenyl phosphine oxalyl chloride containing ethyl ether or tetrahydrofuran solution while stirring ata uniform speed, wherein the dropping time is longer than 2 hours; controlling a reaction temperature to be below 5 DEG C, and continuously stirring for more than 5 hours at the room temperature afterdropping is completed; filtering a product to remove triethylamine hydrochloride, carrying out vacuum distillation, and washing an obtained product with deionized water, so as to obtain a crude product. The product can be applied to flame-retardant modification on an epoxy resin. The product provided by the invention can be used as a flame-retardant modifier, meanwhile is low in synthesis raw material price, simple and gentle in synthesis process and low in solvent melting point in a reaction process and can be recycled and repeatedly used, and energy consumption is correspondingly reduced.
Owner:FUJIAN CONSTR ENG GRP BUILDING MATERIAL SCI & TECH DEV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
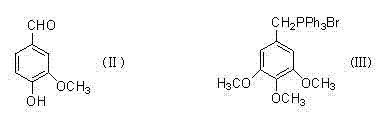
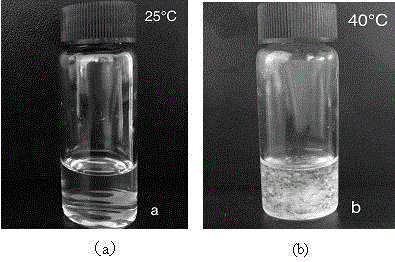
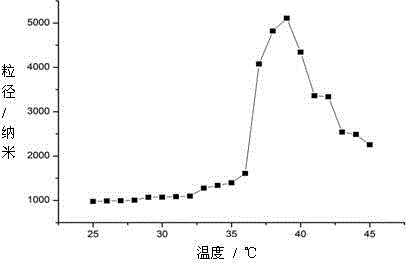
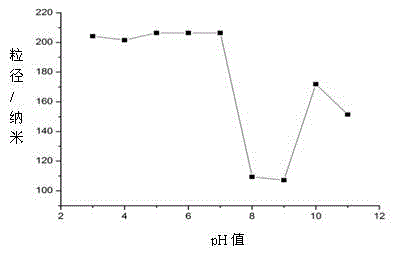
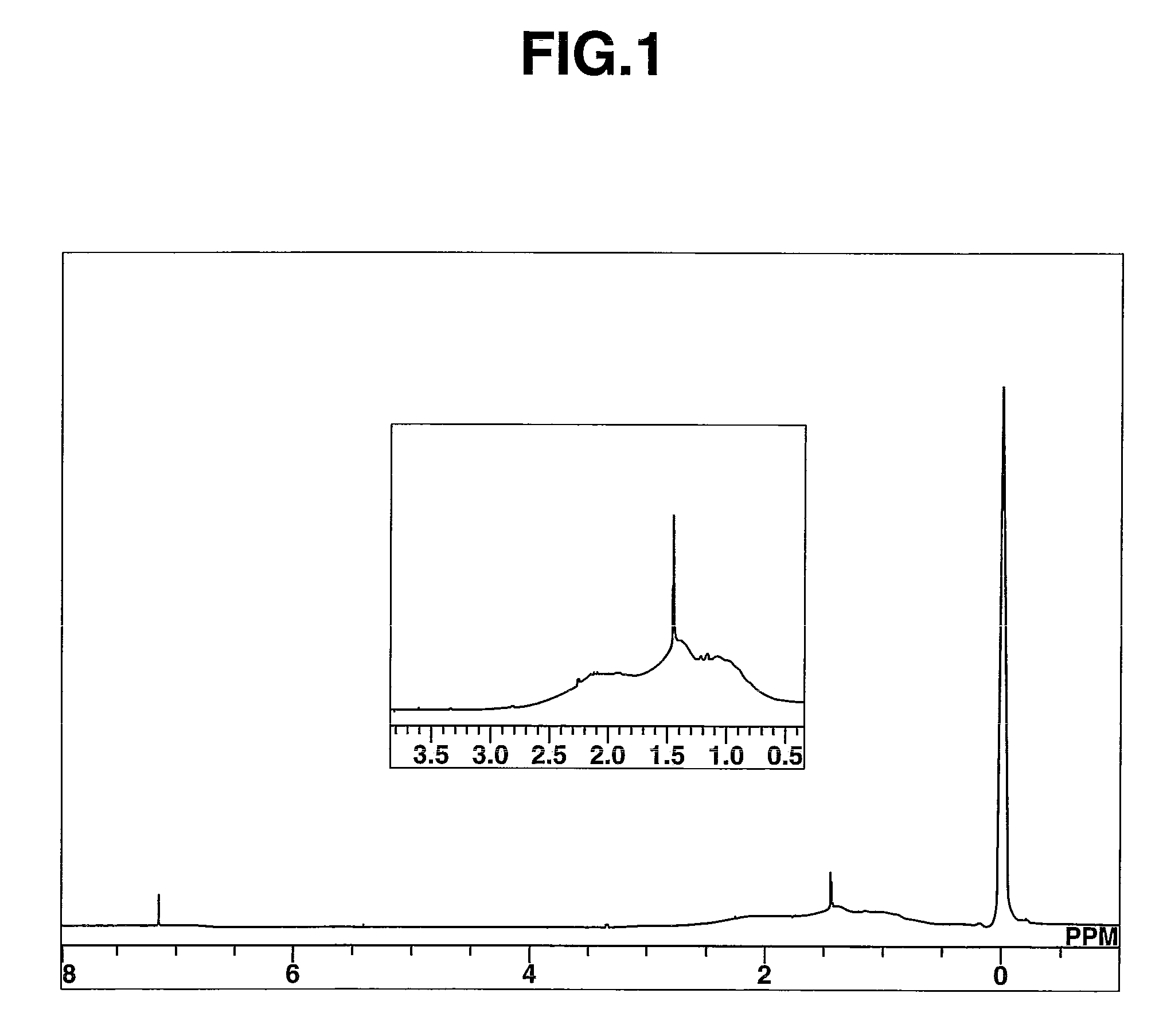
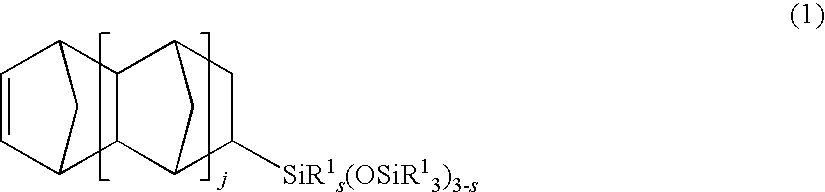
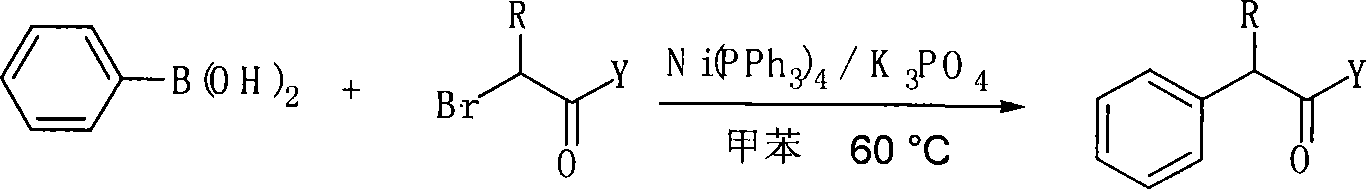
![New application of benzo [C] phenanthridine and protopine in producing overriding resistant bacterium medicament New application of benzo [C] phenanthridine and protopine in producing overriding resistant bacterium medicament](https://images-eureka.patsnap.com/patent_img/1952f98d-dba1-4c2a-9709-072b88d30fd4/s2007100664977e00011.PNG)
![New application of benzo [C] phenanthridine and protopine in producing overriding resistant bacterium medicament New application of benzo [C] phenanthridine and protopine in producing overriding resistant bacterium medicament](https://images-eureka.patsnap.com/patent_img/1952f98d-dba1-4c2a-9709-072b88d30fd4/s2007100664977c00011.PNG)
![New application of benzo [C] phenanthridine and protopine in producing overriding resistant bacterium medicament New application of benzo [C] phenanthridine and protopine in producing overriding resistant bacterium medicament](https://images-eureka.patsnap.com/patent_img/1952f98d-dba1-4c2a-9709-072b88d30fd4/s2007100664977d00021.PNG)