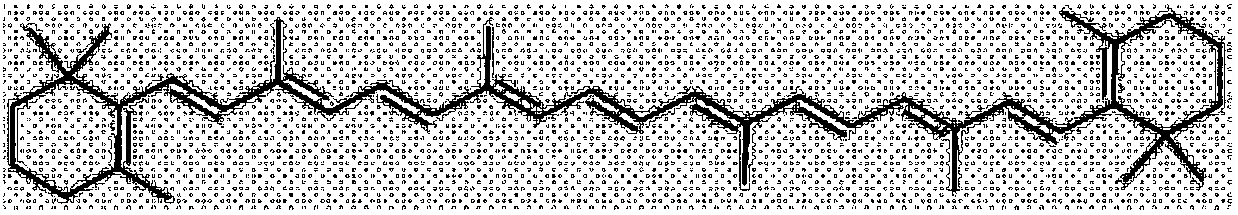
Method for synthesizing beta-carotene from vitamin A through one-pot method
A carotene and vitamin technology, applied in the field of β-carotene synthesis, can solve the problems of difficulty in application, high cost of divalent titanium, and difficulty in realizing industrial production, and achieve simple post-processing, high production efficiency, and reduced yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Take 90.96g vitamin A alcohol (2.9497 million IU / g, 0.281mol) and 15.4ml pyridine (0.191mol) and dissolve it in 500ml methanol to prepare vitamin A alcohol-methanol solution. Add 77.4g (0.295mol) of triphenylphosphine and 1000ml methanol respectively in the three-necked flask, cool to 0°C, slowly add 45.4ml HBr (mass concentration is 47%, 0.393mol) dropwise through the dropping funnel, and control the dropping temperature to 0°C, continue stirring for 2 hours after the dropwise addition, add vitamin A alcohol-methanol solution, react at 10°C for 24 hours, and obtain vitamin A triphenylphosphine bromide reaction solution.
[0048] Take 104.8g of sodium persulfate (0.440mol) and add water to 350g, the above-mentioned vitamin A triphenylphosphine bromide reaction liquid is evacuated and supplemented with nitrogen three times, the temperature is lowered to 5°C, and the sodium persulfate solution is started to be added dropwise. Add 10% w / w sodium carbonate solution (814 g, ...
Embodiment 2
[0053] Replace vitamin A alcohol with vitamin A alcohol mother liquor 210.5g (1,275,000 IU / g, 0.281mol), and the rest is the same as in Example 1 to obtain 48.83g of beta-carotene, the appearance of which is a positive red crystalline solid. The method of GB 5009.83-2016 was used for analysis, and the results showed that the content of β-carotene was 97.04%, and the yield was 62.82%.
Embodiment 3
[0055] Replace sodium persulfate with ammonium persulfate (109.7g, 0.481mol) and add water to 500g. After adding vitamin A alcohol-methanol solution, the temperature for continuing the heat preservation reaction is changed to 20°C. The remainder is the same as in Example 2 to obtain β-carotene 47.63g, the appearance is red crystalline solid. The method of GB 5009.83-2016 was used for analysis, and the results showed that the content of β-carotene was 95.81%, and the yield was 60.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com