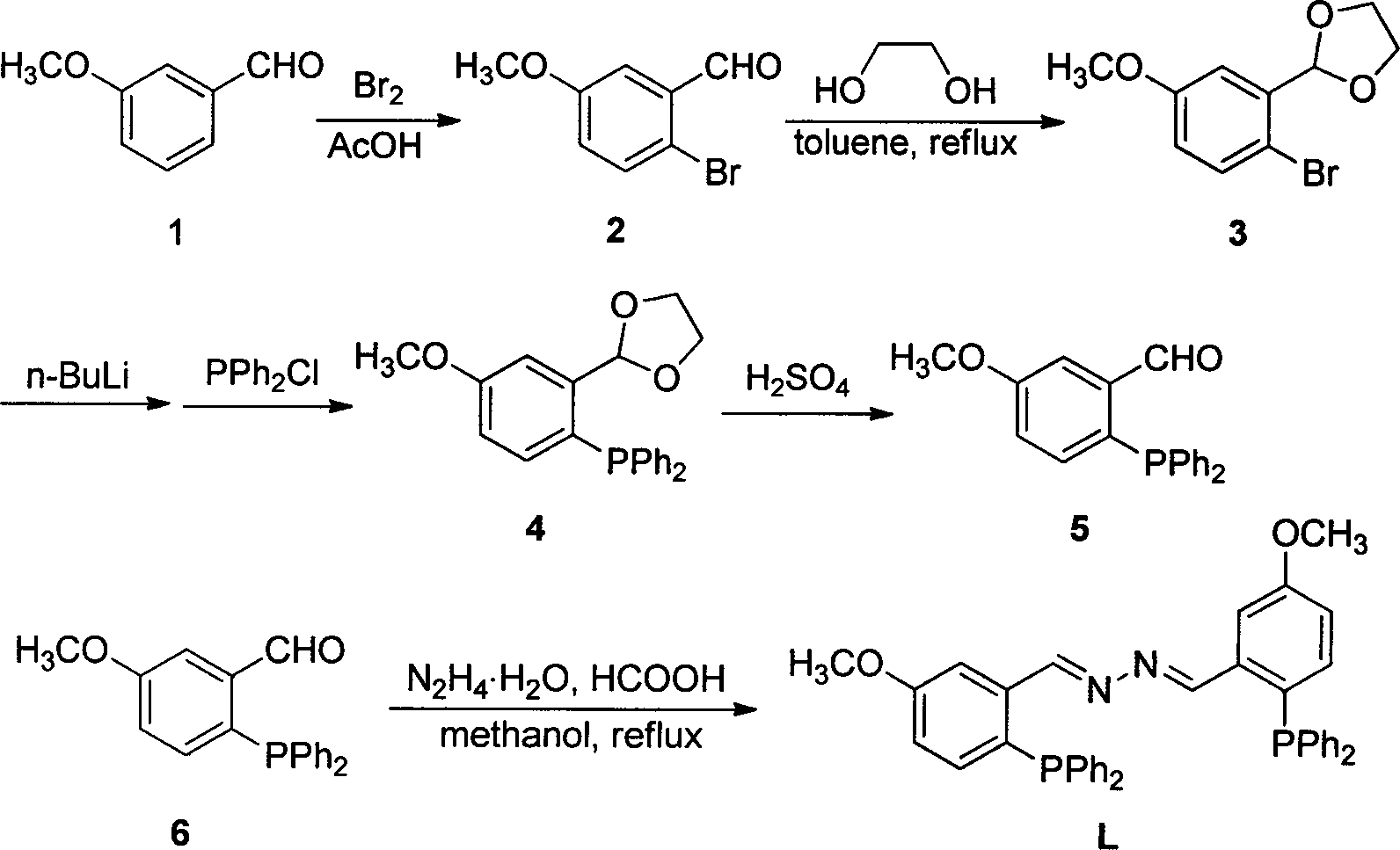
Synthetic method and application of (1E, 2E)-1,2-bi(5-methoxyl-2-diphenylphosphine benzylidene) hydrazine
A technology of diphenylphosphinobenzylidene and diphenylphosphino, which is applied in the field of preparation of imidophosphorus ligand-1 and catalyst ligand, and can solve the problem of high price, product residue, alkyne-alkyne self-coupling The problem of joint reaction and other problems is achieved, the reaction system is simple, the post-treatment is convenient, and the effect of avoiding alkyne self-coupling products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0033] A kind of synthetic method of described (1E, 2E)-1,2-bis(5-methoxy-2-diphenylphosphinobenzylidene)hydrazine is as follows:
[0034] (1) Preparation of 5-methoxy-2-diphenylphosphinobenzaldehyde
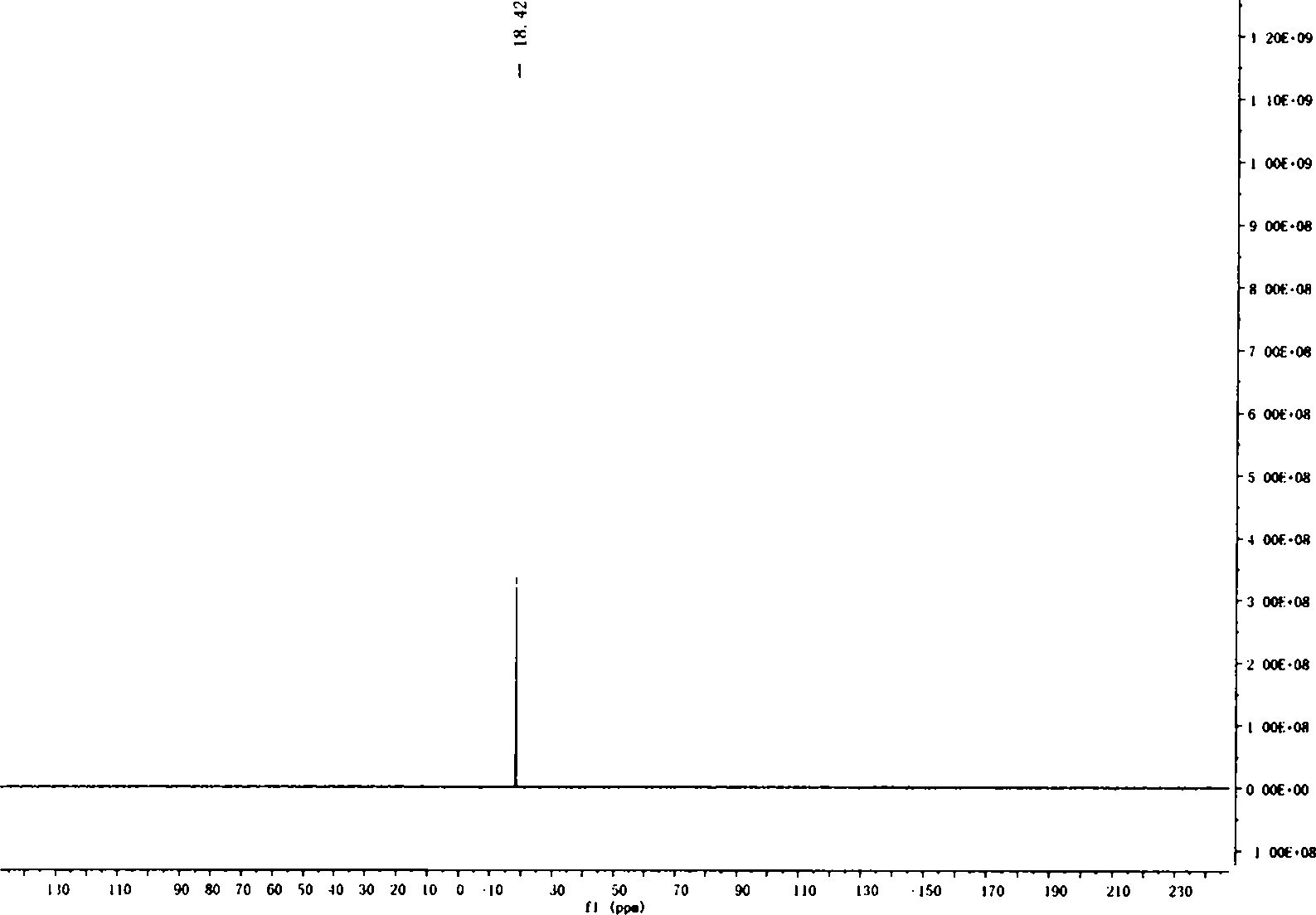
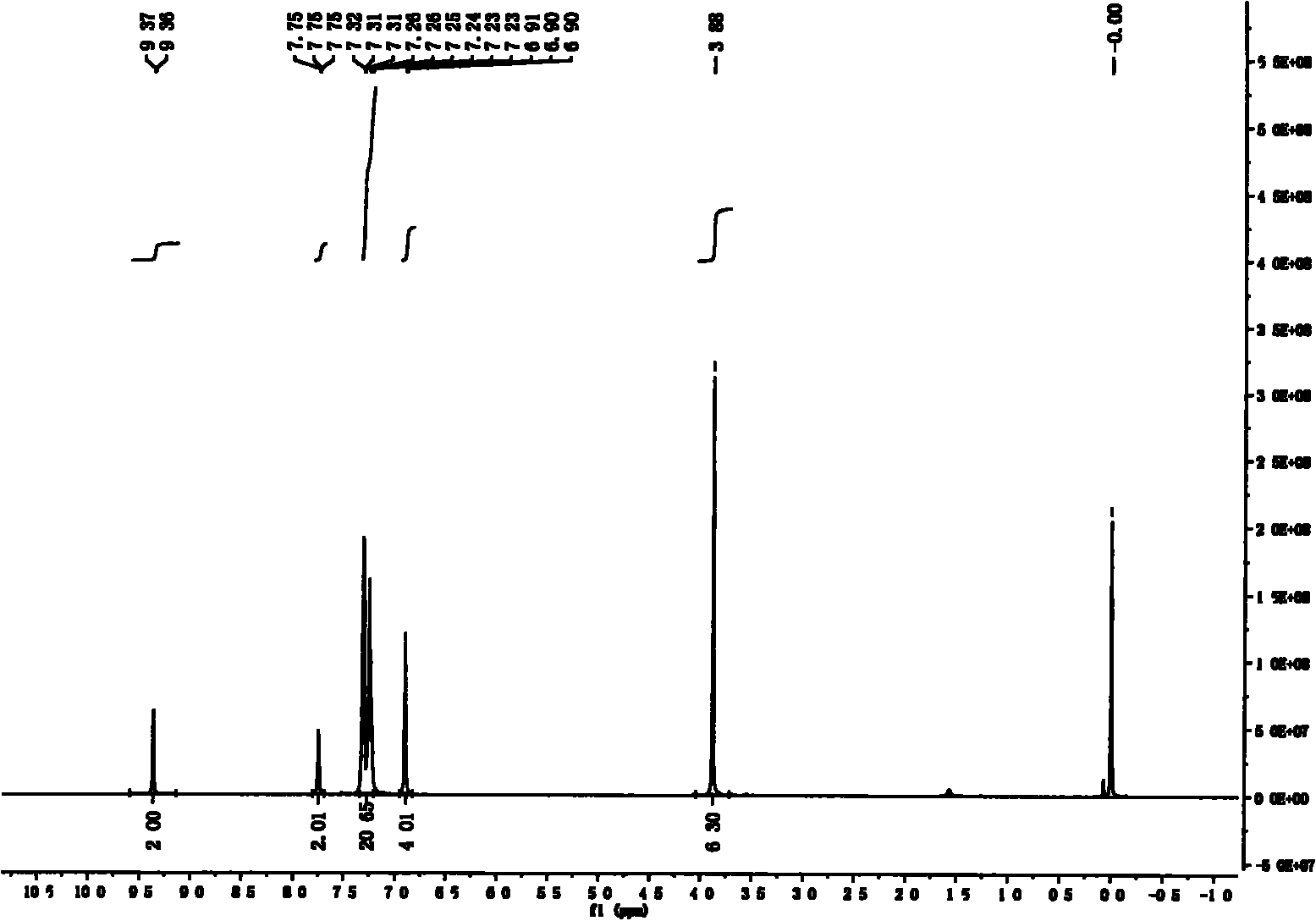
[0035] 1) At room temperature, in a 500ml three-neck round bottom flask, add m-methoxybenzaldehyde (60.6g, 446mmol), glacial acetic acid (100ml), after stirring evenly, slowly drop liquid bromine (27.5ml, 536mmol) into the solution ), dropwise, stirred at room temperature for 36 hours. After the reaction, the reaction liquid was quenched with 500 ml of saturated sodium sulfite solution, then poured into 200 ml of water, extracted with ether, the combined organic phase was washed with water and saturated brine, separated, and the organic phase was dried with anhydrous magnesium sulfate. Filtration and removal of the solvent gave 81.48 g (85%) of a dark yellow solid. The melting point is 71-76°C. 1 HNMR (400MHz, CDCl 3 )δ10.32(s, 1H, CHO), 7.53(d, J=8.8Hz, 1H, Ph-H), 7.42(d, J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com