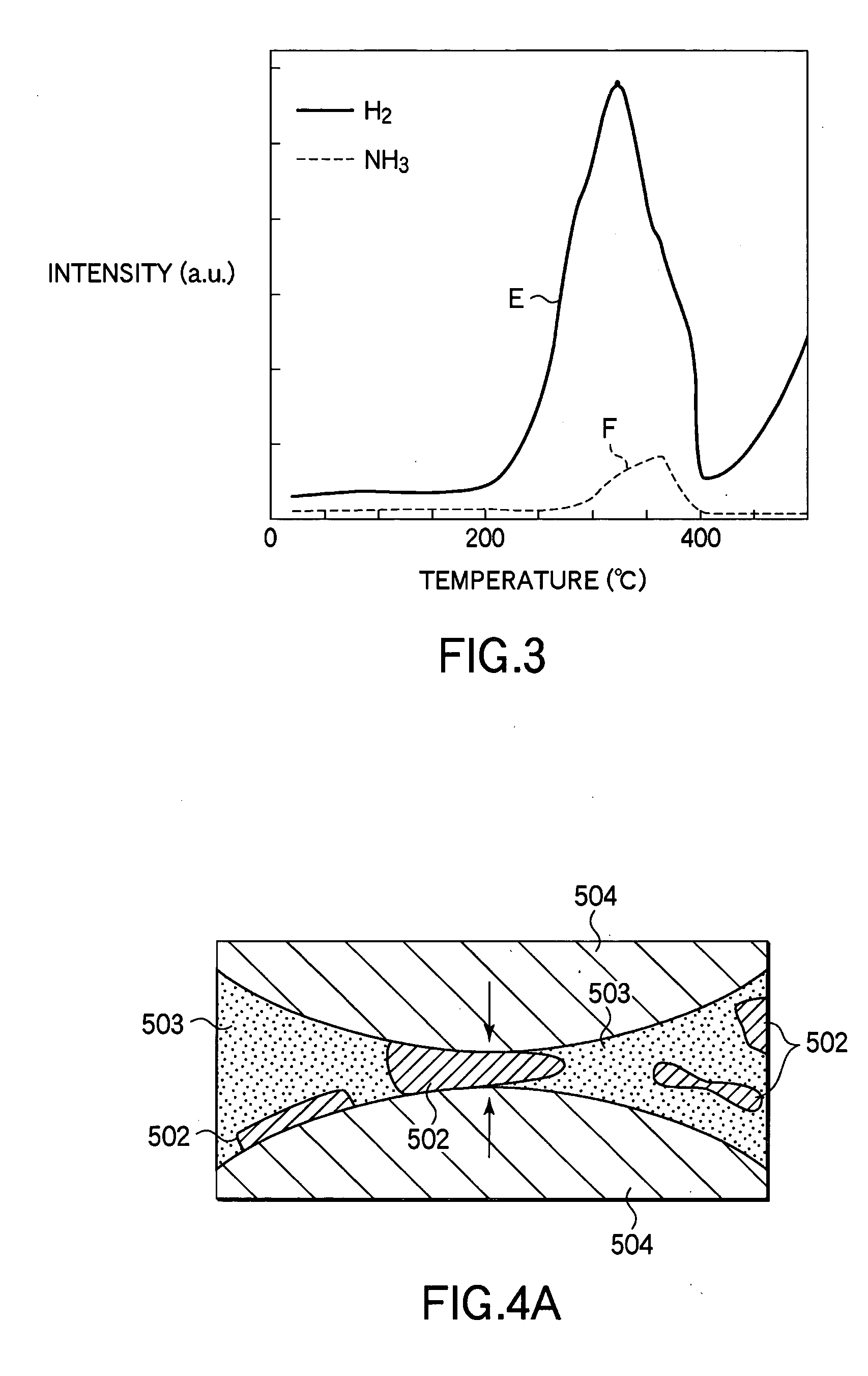
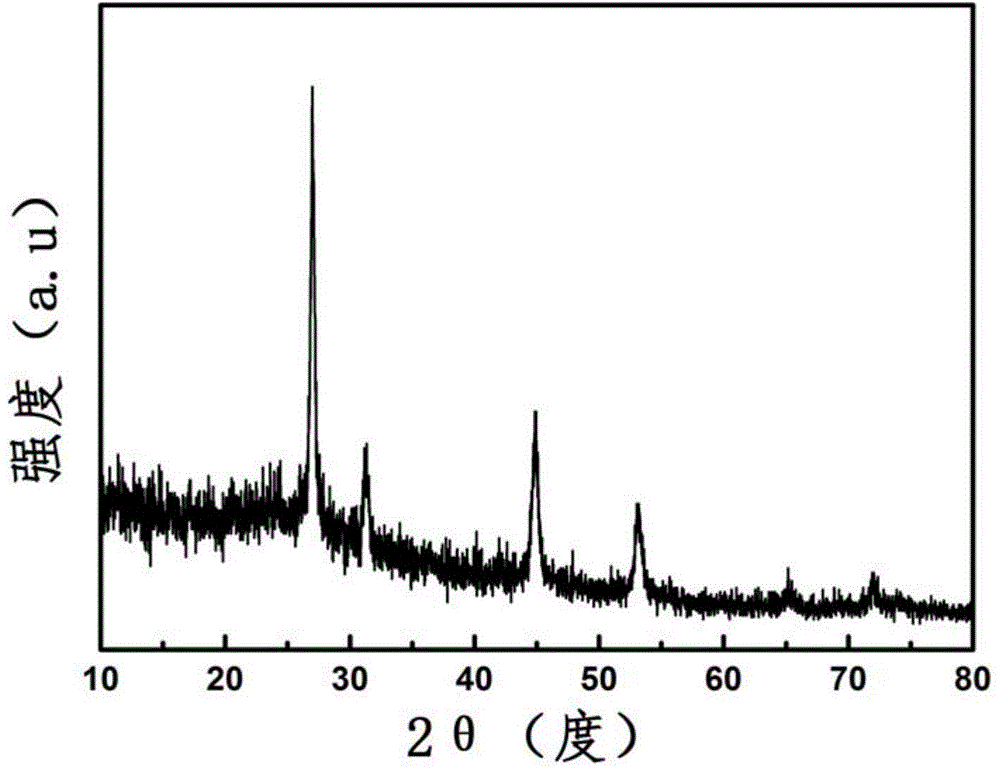
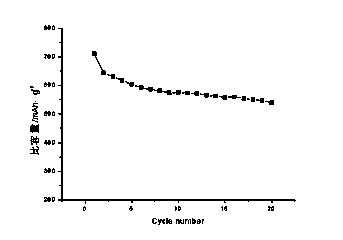
Patents
Literature
229 results about "Lithium hydride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lithium hydride is an inorganic compound with the formula LiH. This alkali metal hydride is a colorless solid, although commercial samples are grey. Characteristic of a salt-like (ionic) hydride, it has a high melting point, and it is not soluble but reactive with all organic and protic solvents. It is soluble and nonreactive with certain molten salts such as lithium fluoride, lithium borohydride, and sodium hydride. With a molecular mass of slightly less than 8.0, it is the lightest ionic compound.
Method for the selective hydrogenation of polymer containing conjugated diene
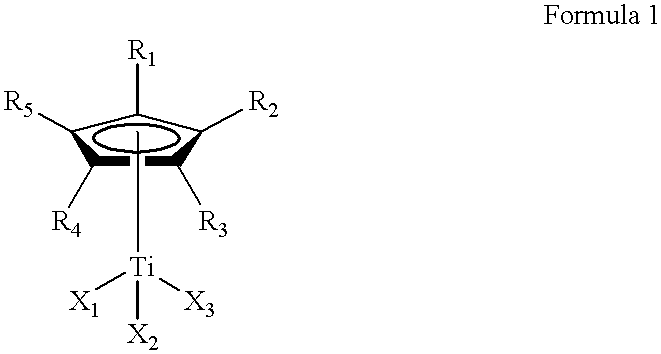
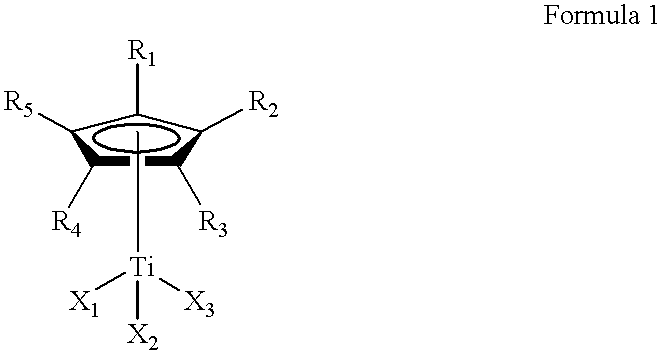
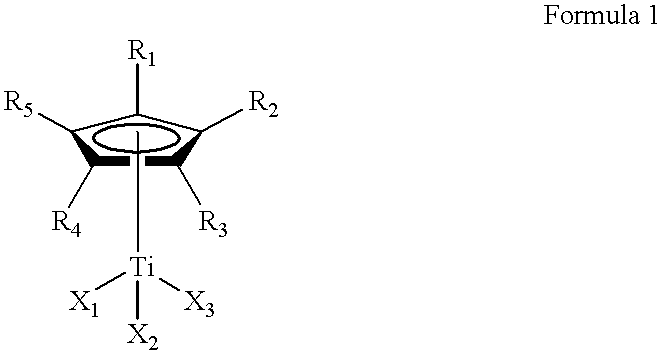
This invention relates to a method for the selective hydrogenation of the unsaturated double bonds in the conjugated diene units of a homopolymer or copolymer in the presence of a novel homogeneous organotitanium-based catalyst. Also, the process of this invention can demonstrate a high yield of hydrogenation and hydrogenation reproducibility using a novel catalyst, so prepared from a mixture consisting of a substituted or unsubstituted monocyclopentadienyl titanium compound expressed by the following formula 1 and lithium hydride derived from a reaction of both alkyl lithium and hydrogen in solution. In particular, this invention also relates to a novel method for the selective hydrogenation of unsaturated double bonds in the conjugated diene units of a conjugated diene polymer or copolymer, so prepared via the reaction between a conjugated diene monomer and vinyl-substituted aromatic monomerwherein, R1, R2, R3, R4 and R5, which may be same or different, are selected from hydrogen atoms and alkyl groups of 1~5 carbon atoms; X1, X2 and X3, which may be same or different, are selected from halogen atoms.
Owner:KOREA KUMHO PETROCHEMICAL CO LTD
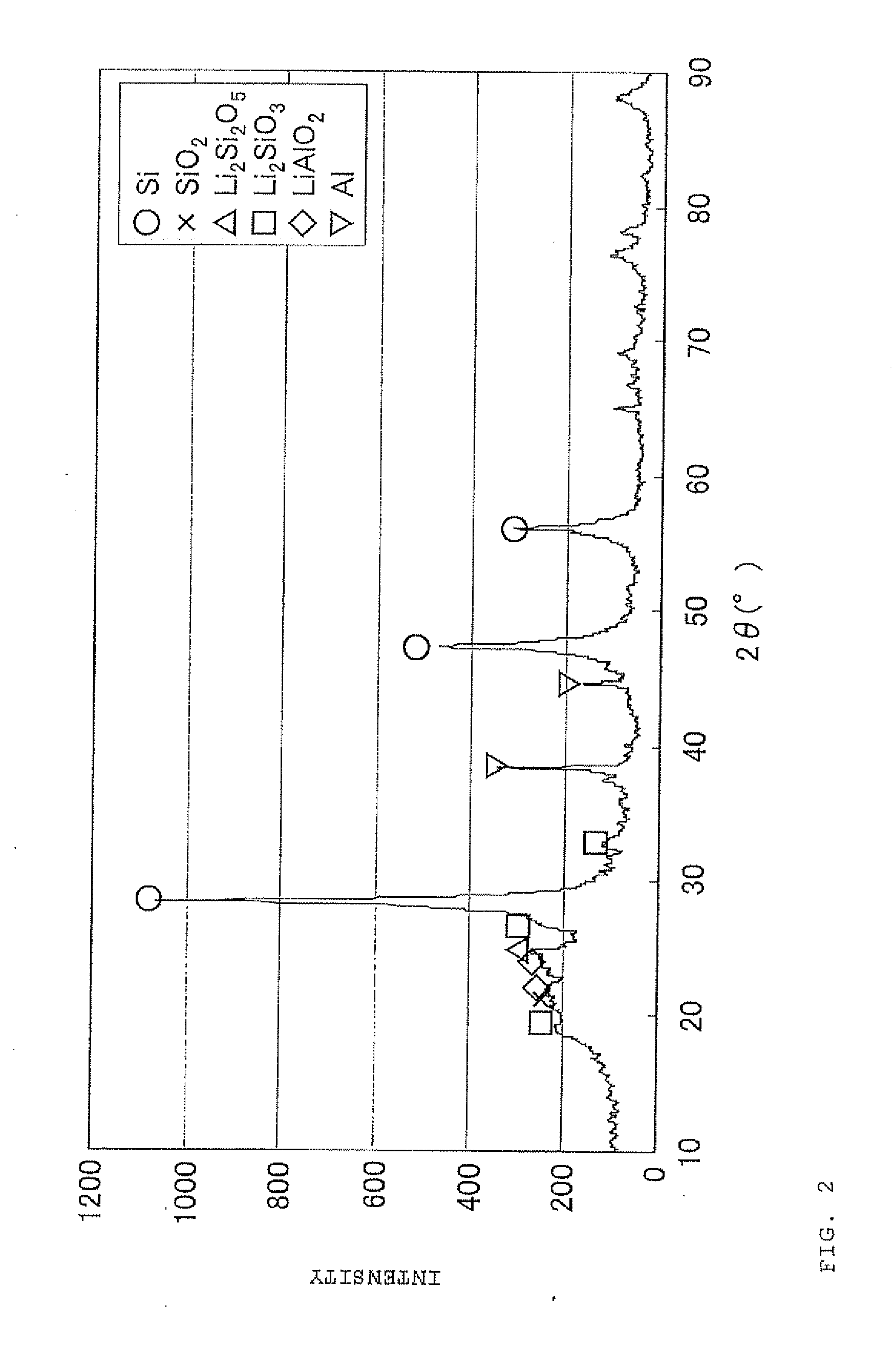
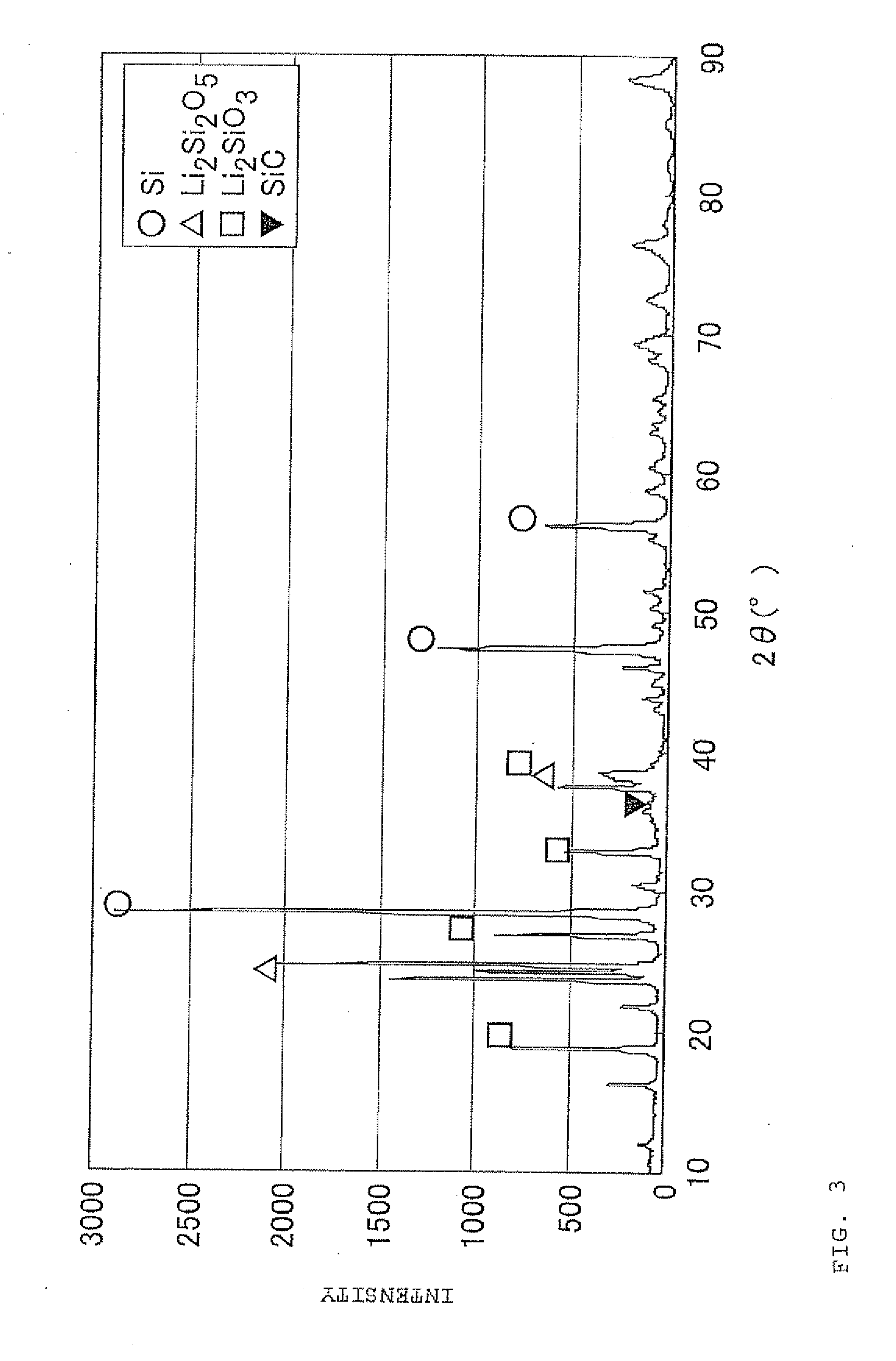
Negative electrode material for secondary battery with non-aqueous electrolyte, method for manufacturing negative electrode material for secondary battery with non-aqueous electrolyte, and lithium ion secondary battery
ActiveUS20110244333A1Cycle durability of negativeElectronic conductivity of negativeMaterial nanotechnologyElectrode thermal treatmentOxide compositeAtomic order
The present invention is a method for manufacturing a negative electrode material for a secondary battery with a non-aqueous electrolyte comprising at least: coating a surface of powder with carbon at a coating amount of 1 to 40 mass % with respect to an amount of the powder by heat CVD treatment under an organic gas and / or vapor atmosphere at a temperature between 800° C. and 1300° C., the powder being composed of at least one of silicon oxide represented by a general formula of SiOx (x=0.5 to 1.6) and a silicon-silicon oxide composite having a structure that silicon particles having a size of 50 nm or less are dispersed to silicon oxide in an atomic order and / or a crystallite state, the silicon-silicon oxide composite having a Si / O molar ratio of 1 / 0.5 to 1 / 1.6; blending lithium hydride and / or lithium aluminum hydride with the powder coated with carbon; and thereafter heating the powder coated with carbon at a temperature between 200° C. and 800° C. to be doped with lithium at a doping amount of 0.1 to 20 mass % with respect to an amount of the powder. As a result, there is provided a method for manufacturing a negative electrode material for a secondary battery with a non-aqueous electrolyte that enables a silicon oxide negative electrode material superior in first efficiency and cycle durability to conventional ones to be mass-produced (manufactured) readily and safely even in an industrial scale.
Owner:SHIN ETSU CHEM IND CO LTD
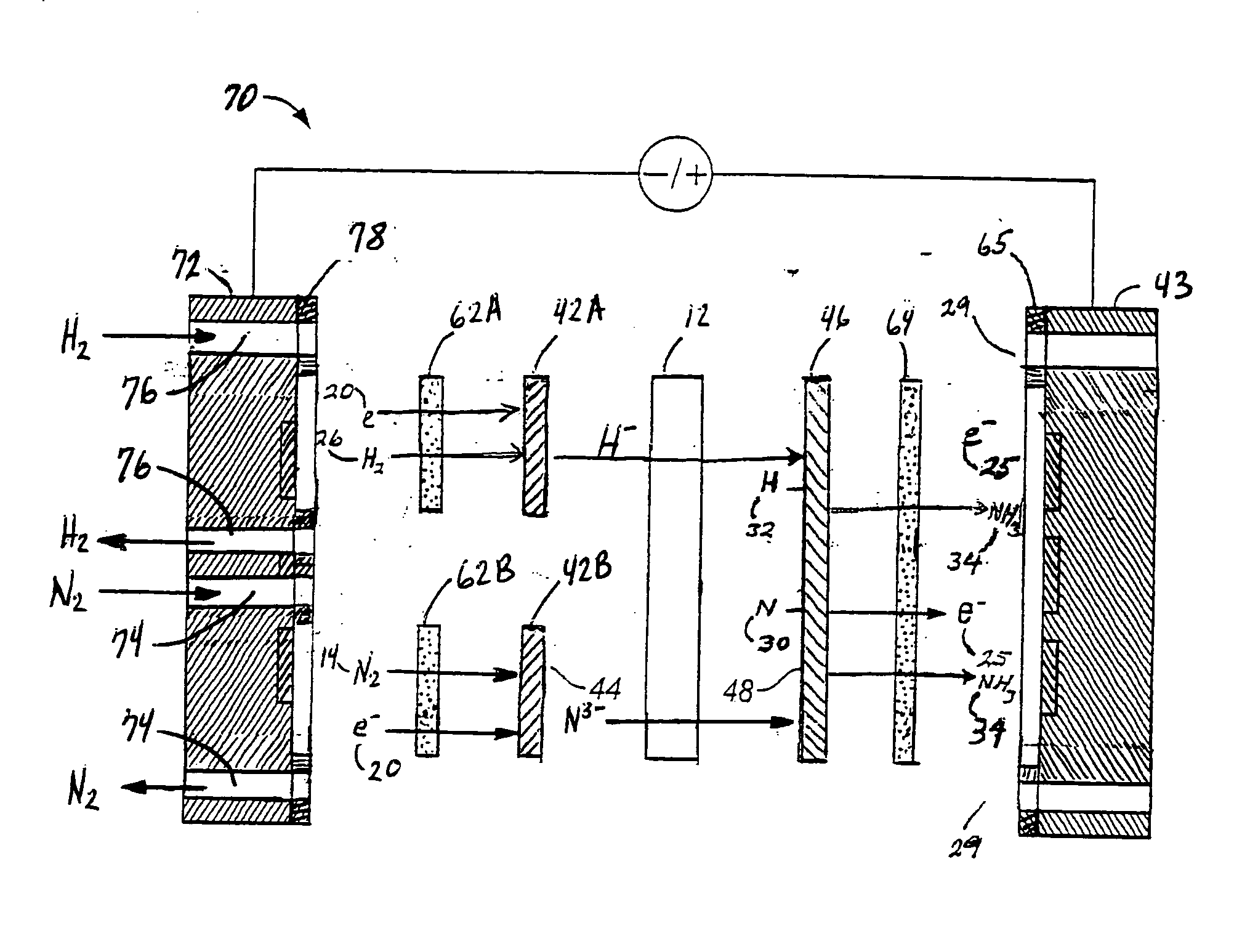
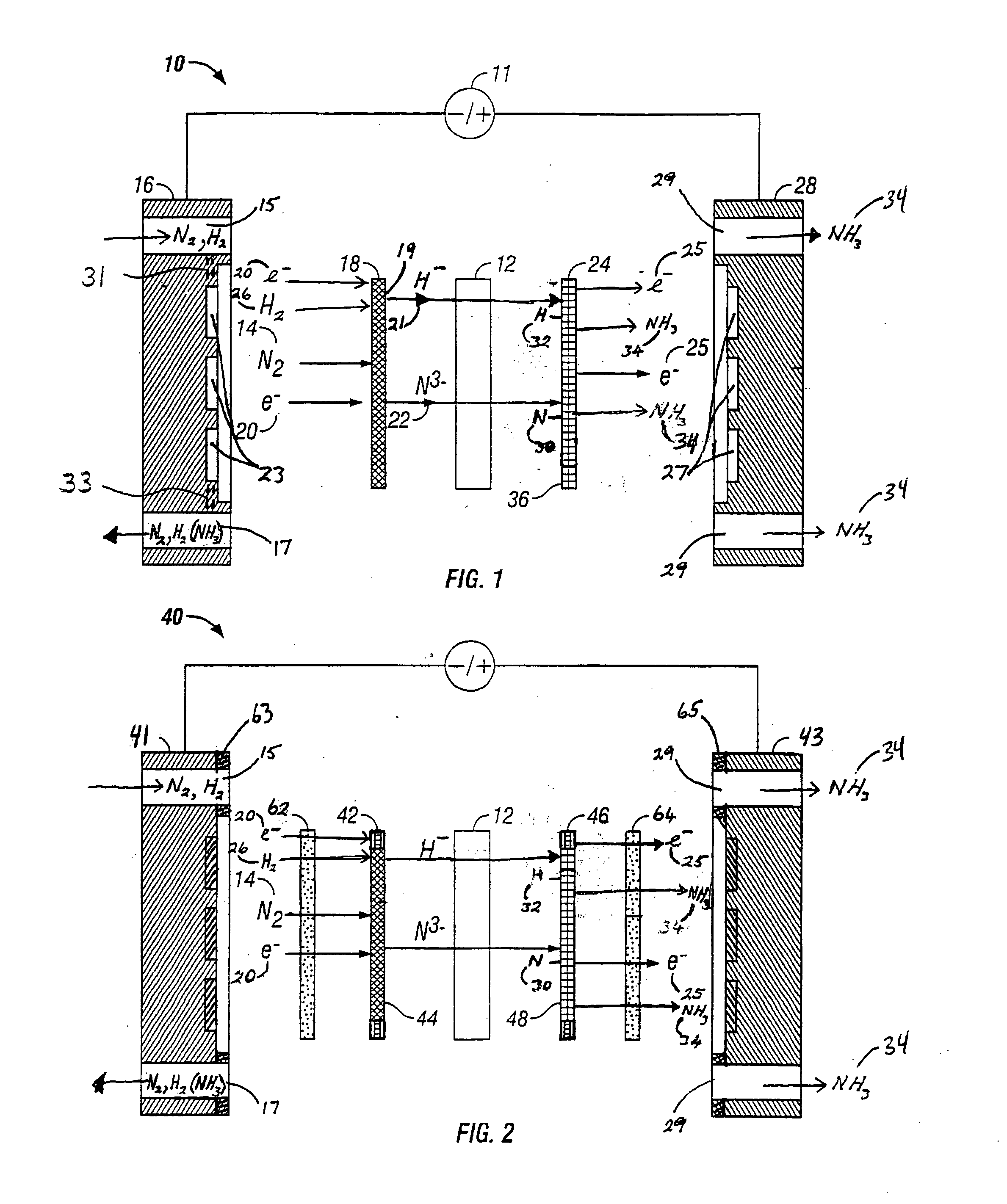
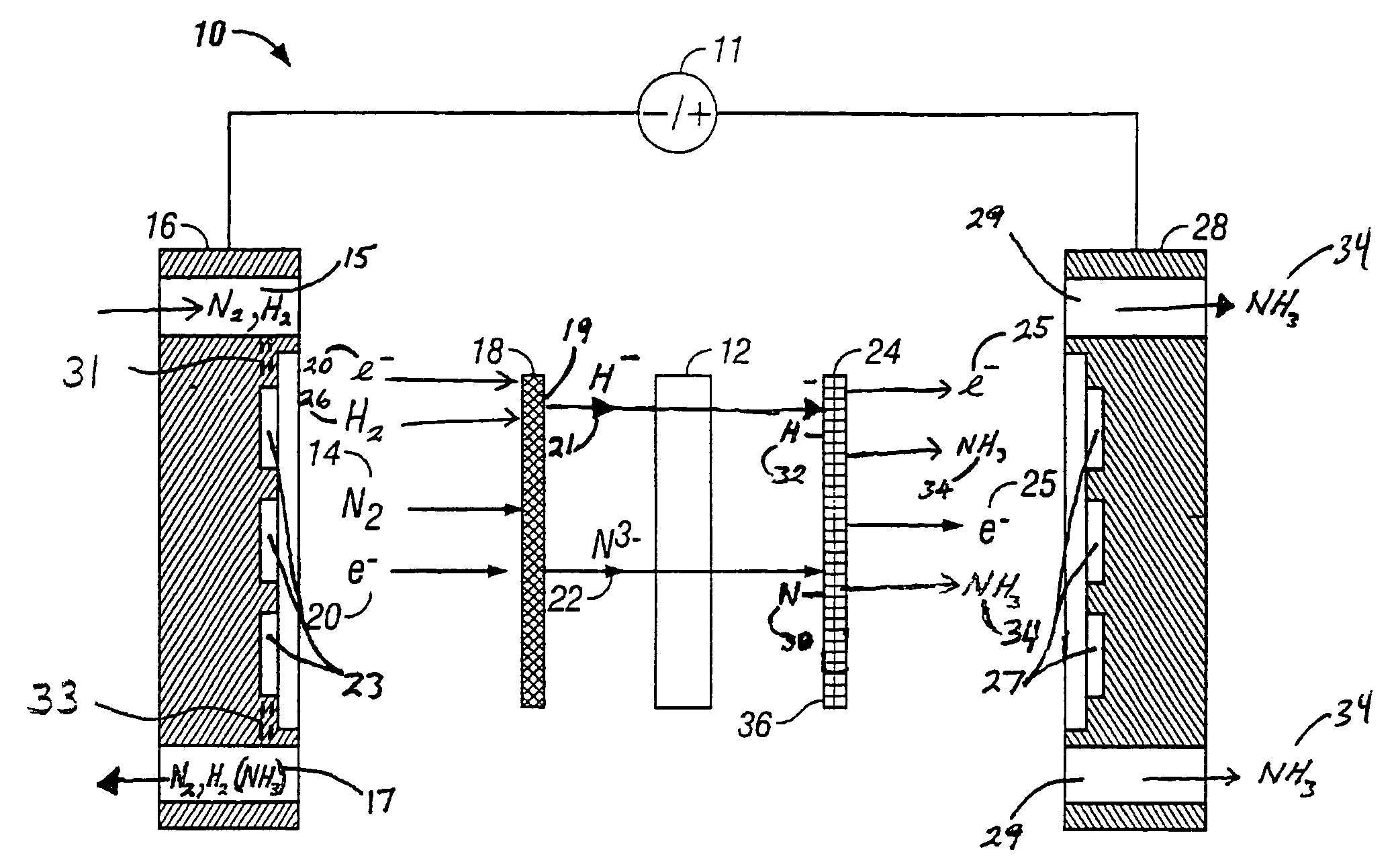
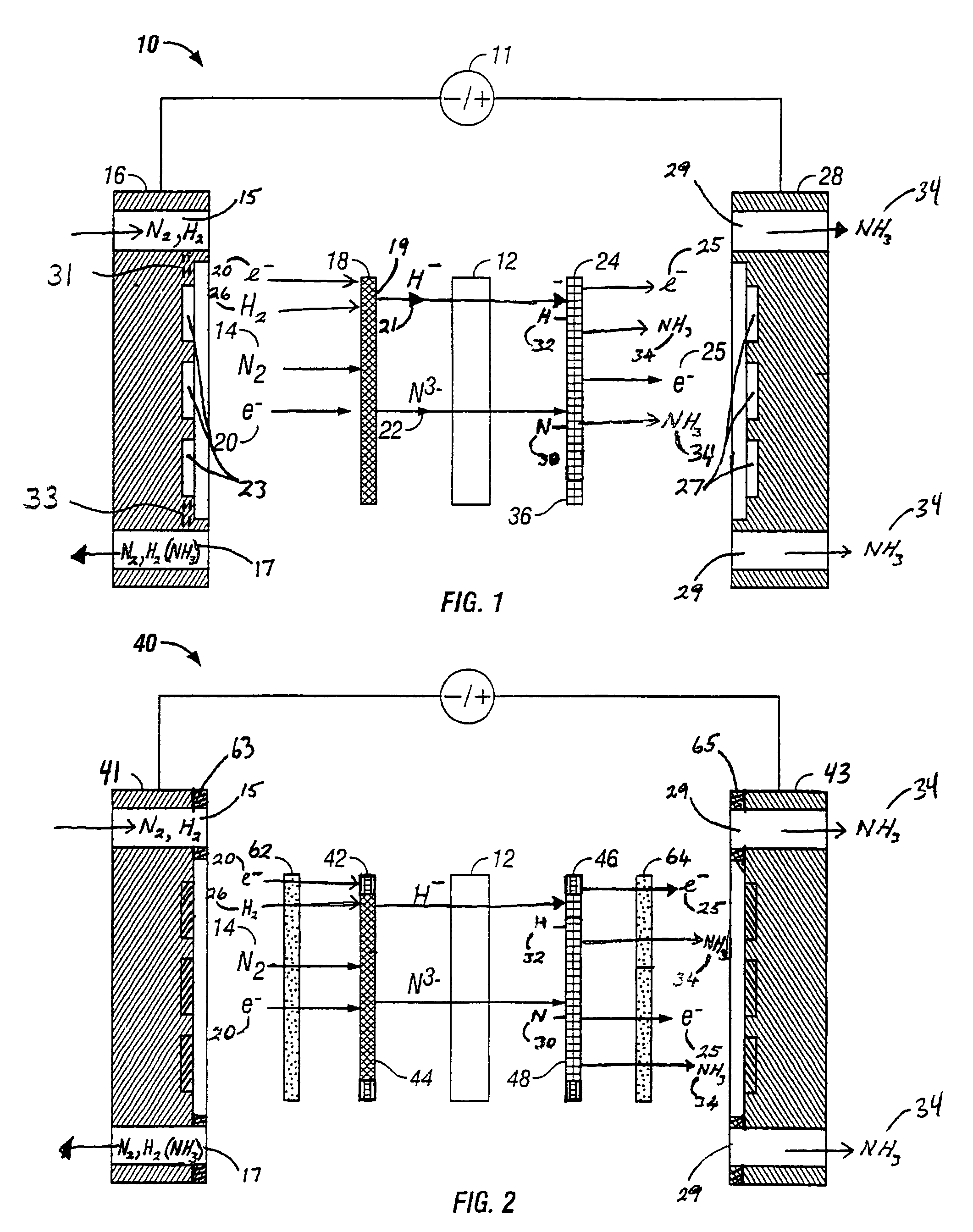
Electrochemical synthesis of ammonia
A method for the anodic electrochemical synthesis of ammonia gas. The method comprises providing an electrolyte between an anode and a cathode, providing nitrogen and hydrogen gases to the cathode, oxidizing negatively charged nitrogen-containing species and negatively charged hydrogen-containing species present in the electrolyte at the anode to form adsorbed nitrogen species and adsorbed hydrogen species, respectively, and reacting the adsorbed nitrogen species with the adsorbed hydrogen species to form ammonia. Nitrogen and hydrogen gases may be provided through a porous cathode substrate. The negatively charged nitrogen-containing species in the electrolyte may be produced by reducing nitrogen gas at the cathode and / or by supplying a nitrogen-containing salt, such as lithium nitride, into the molten salt electrolyte. Similarly, the negatively charged hydrogen-containing species in the electrolyte may be produced by reducing hydrogen gas at the cathode and / or by supplying a hydrogen-containing salt, such as lithium hydride, into the molten salt electrolyte.
Owner:LYNTECH INC
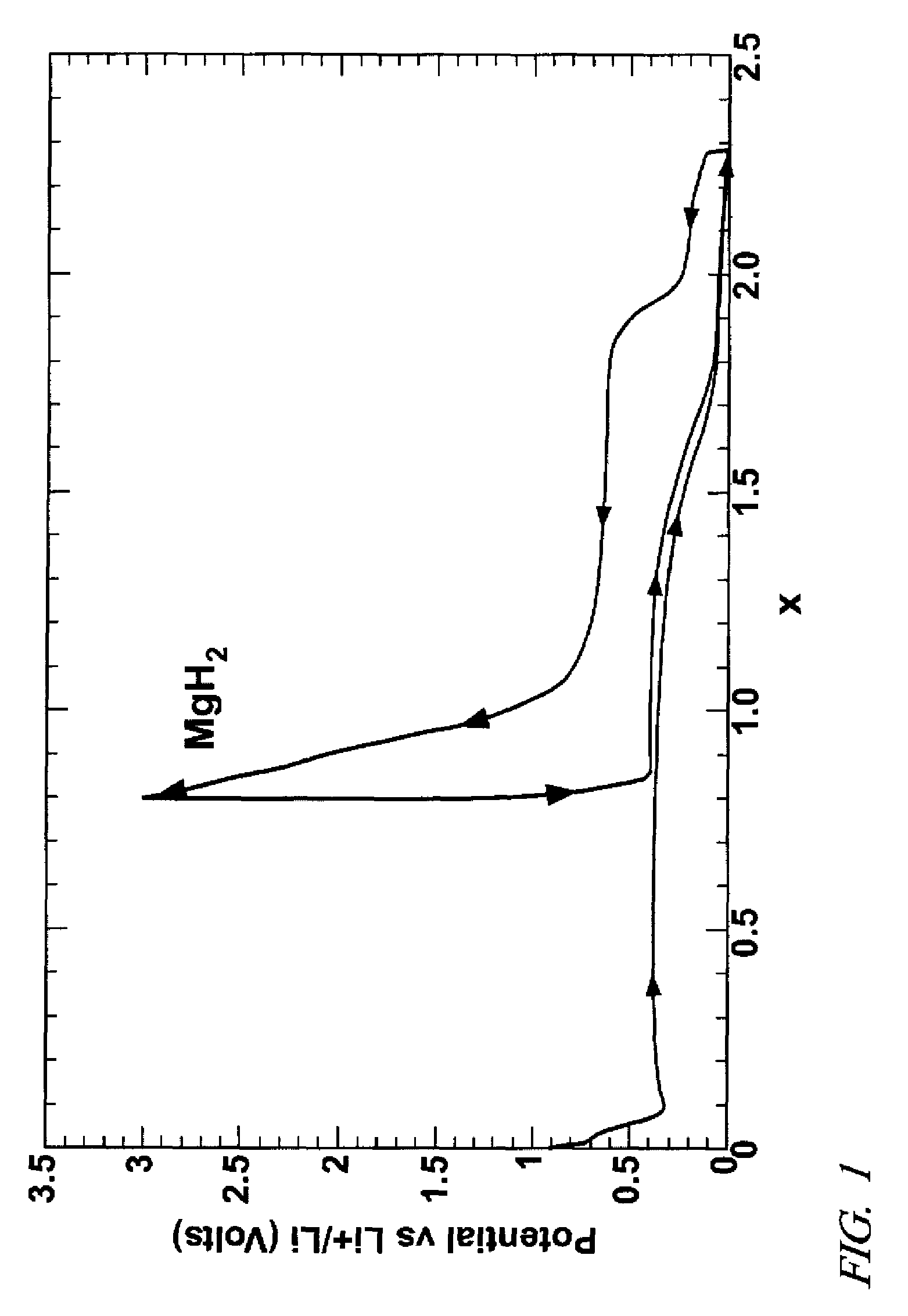
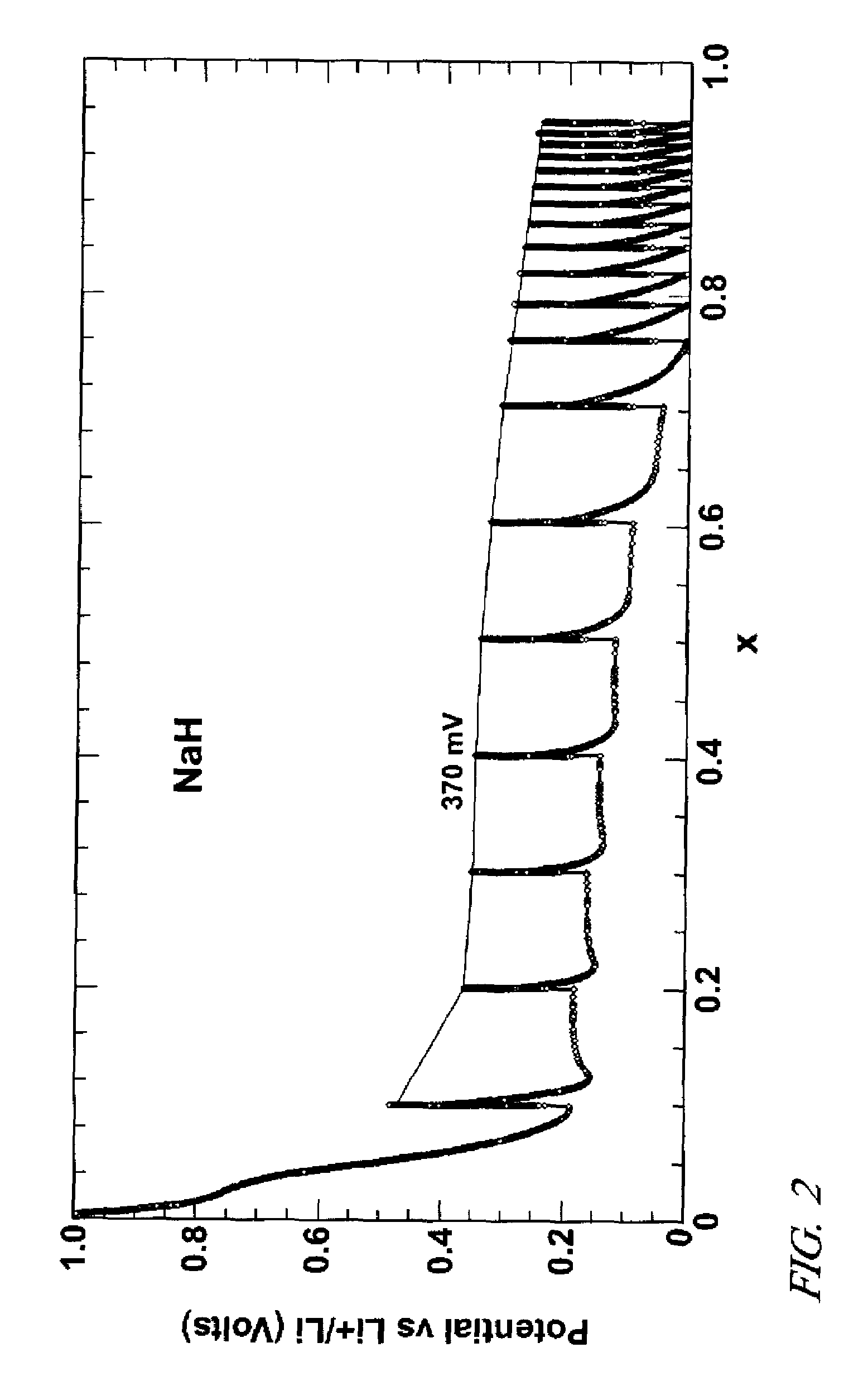
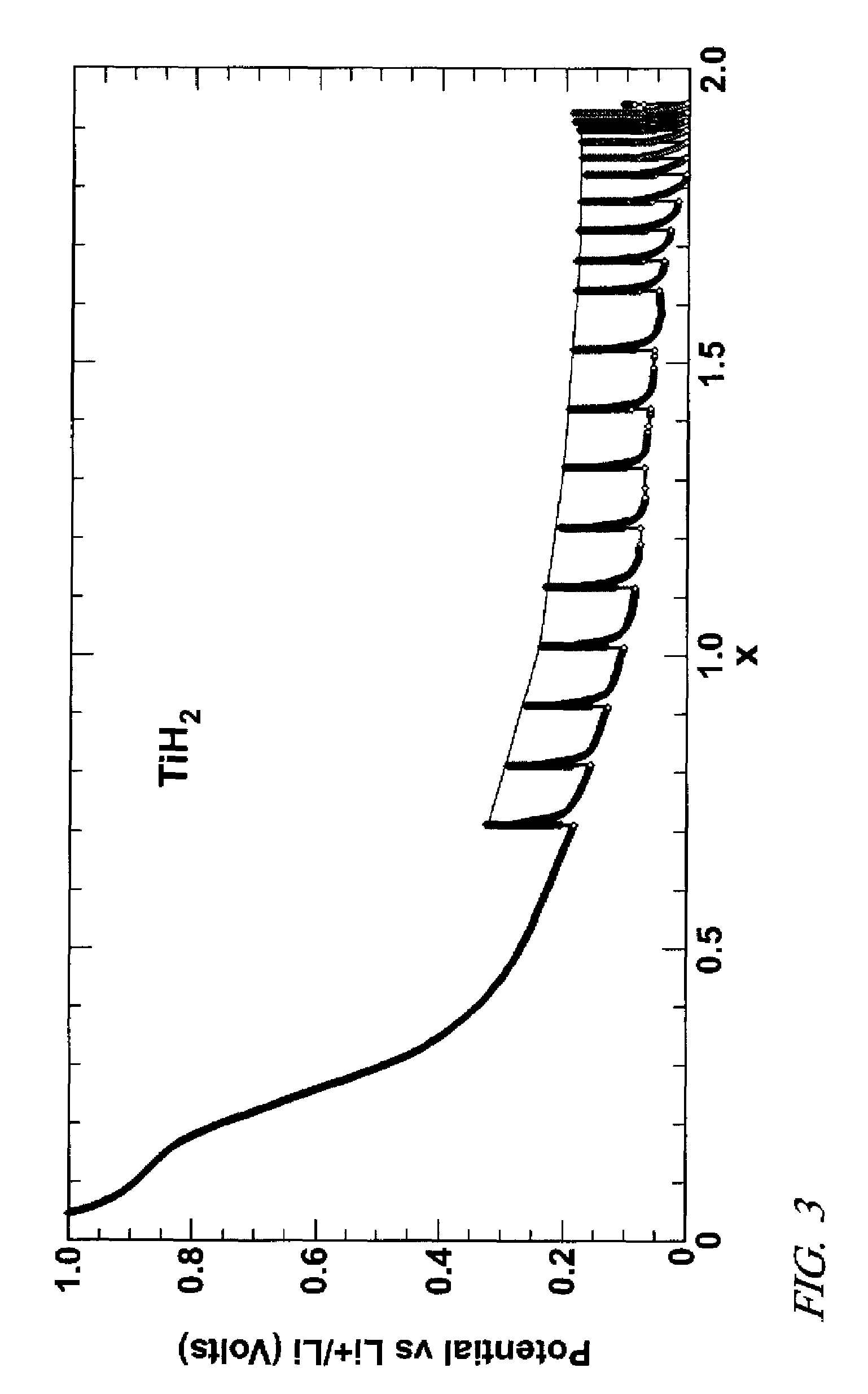
Lithium hydride negative electrode for rechargeable lithium batteries
A lithium battery comprises a negative electrode composition that uses lithium hydride and a second metal. The negative electrode composition is activated by infusing lithium into particles of the second metal hydride to form lithium hydride and the second metal. As the battery is discharged lithium is released from the electrode and the second metal hydride formed. Charging of the battery re-infuses lithium into the negative electrode composition with the re-formation of lithium hydride.
Owner:GM GLOBAL TECH OPERATIONS LLC +1
Lithium hydride negative electrode for rechargeable lithium batteries
ActiveUS7736805B2Negative electrodesNon-aqueous electrolyte accumulator electrodesPhysical chemistryLithium-ion battery
A lithium battery comprises a negative electrode composition that uses lithium hydride and a second metal. The negative electrode composition is activated by infusing lithium into particles of the second metal hydride to form lithium hydride and the second metal. As the battery is discharged lithium is released from the electrode and the second metal hydride formed. Charging of the battery re-infuses lithium into the negative electrode composition with the re-formation of lithium hydride.
Owner:GM GLOBAL TECH OPERATIONS LLC +1
Electrochemical synthesis of ammonia
A method for the anodic electrochemical synthesis of ammonia gas. The method comprises providing an electrolyte between an anode and a cathode, providing nitrogen and hydrogen gases to the cathode, oxidizing negatively charged nitrogen-containing species and negatively charged hydrogen-containing species present in the electrolyte at the anode to form adsorbed nitrogen species and adsorbed hydrogen species, respectively, and reacting the adsorbed nitrogen species with the adsorbed hydrogen species to form ammonia. Nitrogen and hydrogen gases may be provided through a porous cathode substrate. The negatively charged nitrogen-containing species in the electrolyte may be produced by reducing nitrogen gas at the cathode and / or by supplying a nitrogen-containing salt, such as lithium nitride, into the molten salt electrolyte. Similarly, the negatively charged hydrogen-containing species in the electrolyte may be produced by reducing hydrogen gas at the cathode and / or by supplying a hydrogen-containing salt, such as lithium hydride, into the molten salt electrolyte.
Owner:LYNTECH INC
Hydrogen storage matter and manufacturing method and apparatus for the same
InactiveUS20060127304A1Improve efficiencyIncrease storage capacityMaterial nanotechnologyPhysical/chemical process catalystsLithium compoundNanostructure
A hydrogen storage matter contains at least a nano-structured and organized lithium imide compound precursor complex. In the hydrogen stroge matter, the lithium imide compound precursor complex has been nano-structured and organized by mixing fine powder lithium amide with fine powder lithium hydride at a predetermined ratio to prepare a mixture as a starting material, and then processing the mixture by a predetermined complex formation processing method.
Owner:HIROSHIMA UNIVERSITY +1
Preparation method of lithium sulfide powder
InactiveCN104609376ARational use of resourcesEase of industrial implementationAlkali metal sulfides/polysulfidesSulfurRoom temperature
The invention discloses a preparation method of lithium sulfide powder. The preparation method comprises the following steps: (1) drying sulfur powder; (2) under inert atmosphere protection, mixing the dried sulfur powder with lithium hydride powder in a mole ratio of 1:(1-3), adding the obtained mixture into a sealed ball-milling tank, and carrying out ball-milling on the mixture for 1-24 h under the condition that the rotating speed is 100-500 r / min at room temperature; and (3) after the ball-milling reaction is completed, under inert atmosphere, taking the powder out of the ball-milling tank, so that the lithium sulfide powder is obtained. The preparation method of lithium sulfide powder disclosed by the invention has the characteristics of simple process, low cost, and easiness for industrialized production.
Owner:ZHEJIANG UNIV OF TECH
Lithium ion battery composite anode material and preparation thereof
The invention relates to a lithium ion battery composite anode material and a preparation thereof. The lithium ion battery composite anode material comprises a base and a carbon-containing layer formed on the surface of the base; the base comprises nanometer silicon, a lithium-containing compound and a carbon micro powder mixture; the carbon-containing layer is an amorphous carbon layer covering on the surface of the base particles and playing the role of connecting different base compositions. The preparation method of the lithium ion battery composite anode material is characterized by comprising: blending nanometer silicon, lithium hydride, carbon micro powder, an organic carbon source precursor, a surfactant and the like, performing low-temperature setting and then crushing, and performing high-temperature carbonization treatment under an oxygen isolation condition. The preparation method is simple in technology and low in cost, and the prepared composite material has high capacity, good cycle performance and a wide application prospect.
Owner:北京赛德美资源再利用研究院有限公司
Foamed neutron absorber material
The invention discloses a foamed neutron absorber material. The foaming neutron absorber material is prepared from the mixture of 10-60% of neutron absorber and 40-90% of matrix material through foaming, wherein the neutron absorber is one or more than one of boron carbide, ferrite, rare earth compound, lithium hydride and paraffin wax, and the matrix material is cement, or macromolecular polymer or their mixture. 'Foaming' process is innovatively applied, massive synthesized neutron absorber are dispersed on walls of the pores formed by foaming. The matrix cellular caves greatly expand capacity for capturing neutron rays, neutron rays in the interior of foamed body are diffused by virtue of electromagnetic vortex pore wall, and the scattered neutron waves are gradually attenuated and absorbed.
Owner:李勇
Hydrogen storage material and method for manufacturing same
InactiveUS20090121184A1Decrease hydrogen generation temperatureRelease peak temperatureAlkali/alkaline-earth/beryllium/magnesium hydridesNitrogen compoundsImideNitride
[Problem] To provide a hydrogen storage material which decreases the hydrogen release start temperature and the hydrogen release peak temperature, and to provide a method for manufacturing thereof.[Solution] Provided is a hydrogen storage material which contains: a mixture and a reaction product of lithium hydride and magnesium amide, wherein the lithium hydride and the magnesium amide are prepared by combining as the raw materials: one or more substance selected from the group consisting of an amide compound, an imide compound, and a nitride of magnesium, and an amide compound, an imide compound, and a nitride of lithium; and one or more substance selected from the group consisting of an amide compound, an imide compound, a nitride, a hydride, and a metal of magnesium, and an amide compound, an imide compound, a nitride, a hydride, and a metal of lithium, with the raw materials contains both magnesium and lithium metallic species.
Owner:TAIHEIYO CEMENT CORP +1
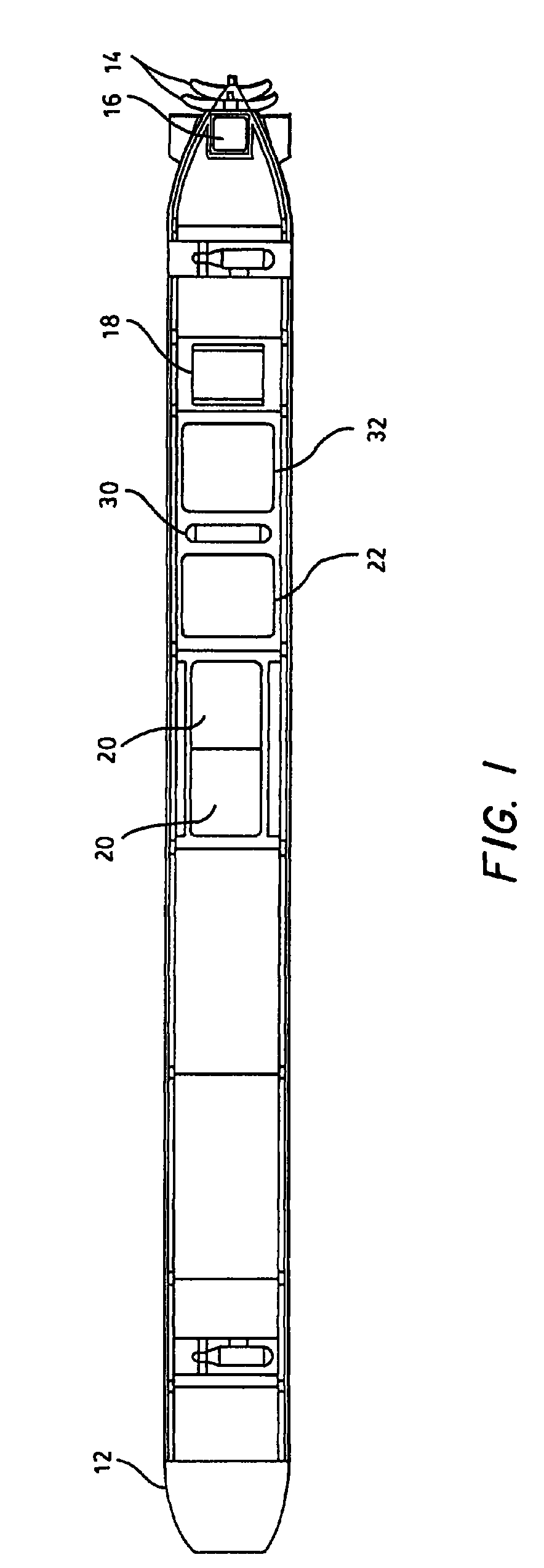
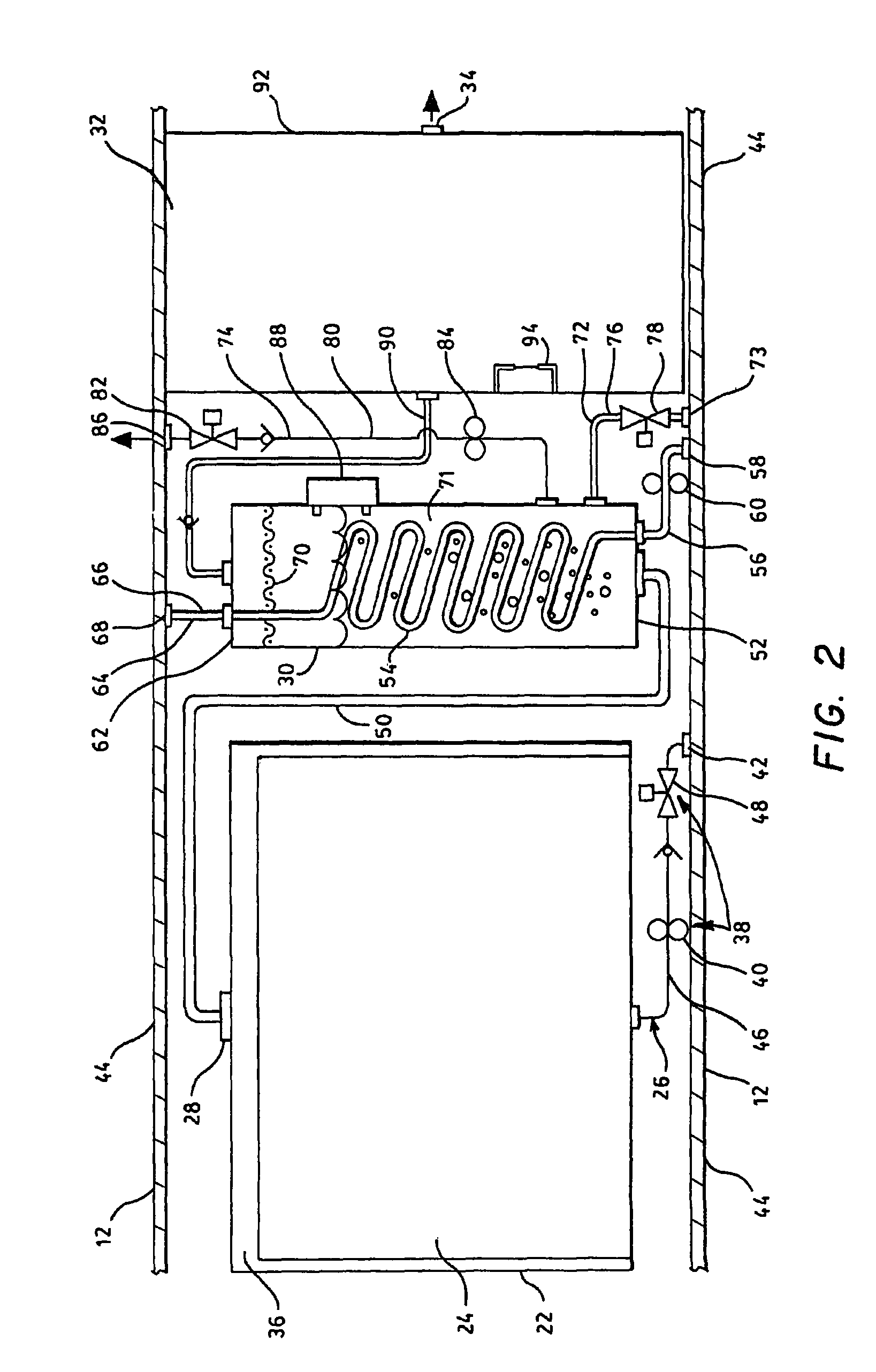
Hydrogen generation apparatus for an underwater vehicle
InactiveUS7938077B1Improve staminaEnabling quieter operationHydrogenPropulsion based emission reductionCalcium hydroxideHydrogen
A hydrogen generation apparatus for an underwater vehicle is presented, the apparatus including a hydrolysis reaction compartment, a mass of solid lithium hydride disposed in the compartment, inlet and outlet structure for passing sea water through the compartment to generate steam, lithium hydroxide and hydrogen gas, a condenser for condensing out the steam and retaining the condensate and lithium hydroxide, and a tank for collecting the hydrogen gas, the tank having outlet structure for discharging the hydrogen gas to a vehicle propulsion system.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Nuclear reactor for lunar surface
ActiveCN103258576AThe module mass is smallEasy to transportNuclear energy generationCooling arrangementNuclear reactor coreNuclear reactor
Owner:CHINA INSTITUTE OF ATOMIC ENERGY
Method for generating hydrogen through lithium borohydride-porous carbon hydrolysis and reaction system
InactiveCN101841048AReduce volumeHigh hydrogen release efficiencyFuel cell detailsLiquid waterChemistry
The invention relates to a method for generating hydrogen through lithium borohydride-porous carbon hydrolysis, and a reaction system, which belongs to a hydrogen producing-storing technique for fuel cell hydrogen sources. The method is characterized in that hydrolysis material is prepared by mechanically ball-milling LiBH4 and porous carbon material, and the influent water rate of liquid water reacting with the hydrolysis material and the saturated steam pressure of steam are controlled so as to effectively control the hydrogen-releasing amount, hydrogen-releasing speed and the like of reaction. The method precisely combines hydrolysis hydrogen production with a hydrogen production system, and the hydrolysis hydrogen production system needs no catalyst for acceleration, can continuously and stably release hydrogen, and is convenient to control and higher in hydrogen-releasing efficiency than a NaBH4 hydrolysis hydrogen production system. The method completely meets the requirements of hydrogen fuel cells for hydrogen sources. The application of the technique of the invention has important and profound significance for promoting the progress of industries related to new energy, achieving the aim of saving energy and reducing emission and promoting economic development.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI
P-aramid nanofiber solution and preparation method thereof
The invention relates to a novel method for preparing a p-aramid nanofiber solution, belongs to the field of macromolecules and also belongs to the field of nano-materials. According to the solution, p-aramid nanofibers have the diameter of 20-50nm and the length of about 10 microns. The method comprises the steps: purging a dry three-mouthed bottle with nitrogen gas for 10 minutes under the protection of nitrogen gas, adding a deprotonation reagent (sodium hydride or potassium hydride or lithium hydride) and anhydrous dimethyl sulfoxide under the protection of nitrogen gas, mechanically stirring, heating to the temperature of 70 DEG C within 20 minutes, continuously reacting for 40 minutes, and then, cooling to the temperature of 30 DEG C; and then, slowly adding poly-p-phenylene terephthalamide staple fibers, and reacting for 6-72 hours at the temperature of 30 DEG C, thereby obtaining an orange-red solution containing the p-aramid nanofibers, wherein the weight percent of the p-aramid nanofibers is 0.05-3.6%. The method disclosed by the invention is simple and is strong in operability, and a feasible method is provided for the low-cost and large-scale preparation of p-aramid nanofiber materials.
Owner:LUDONG UNIVERSITY
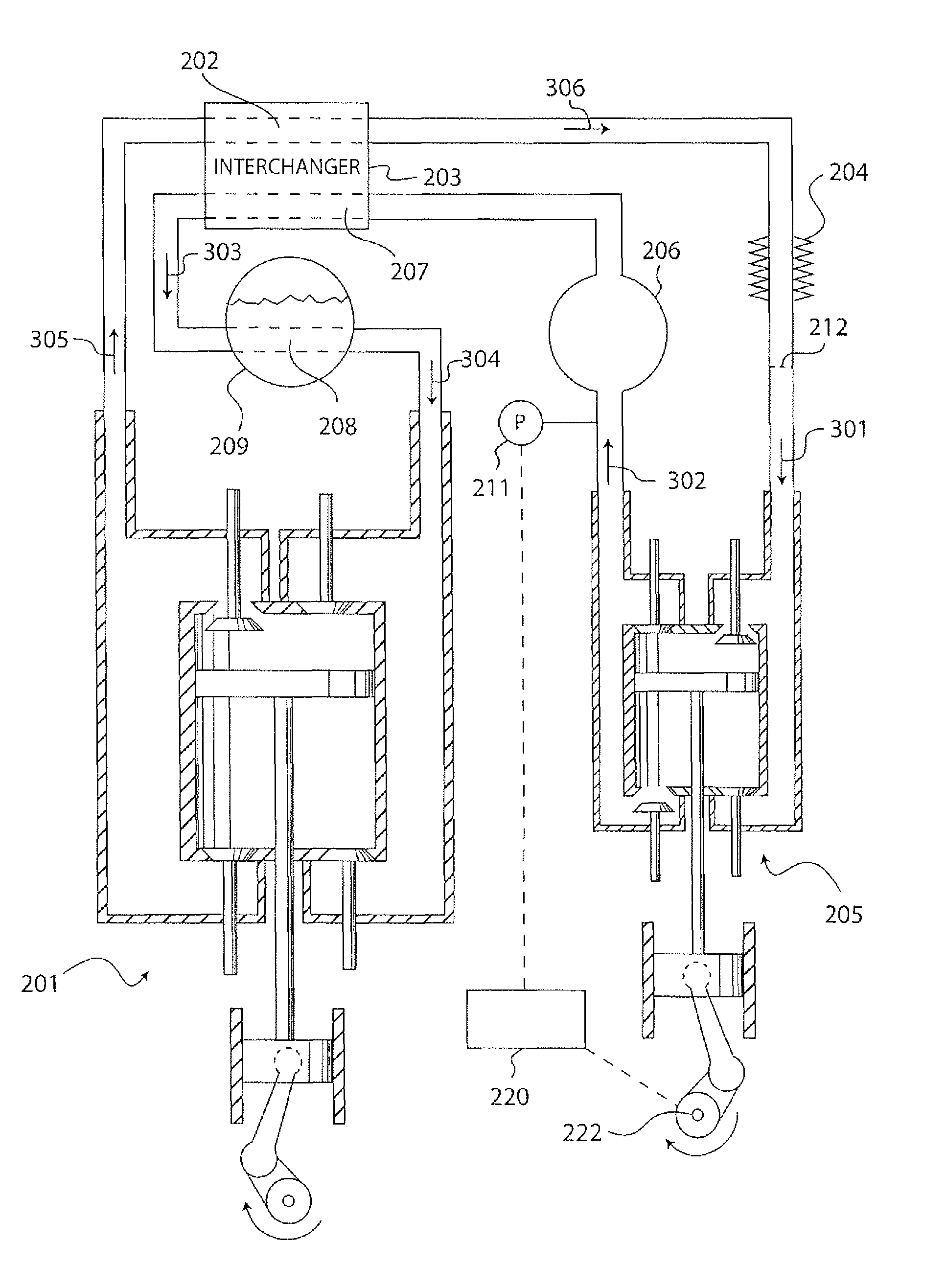
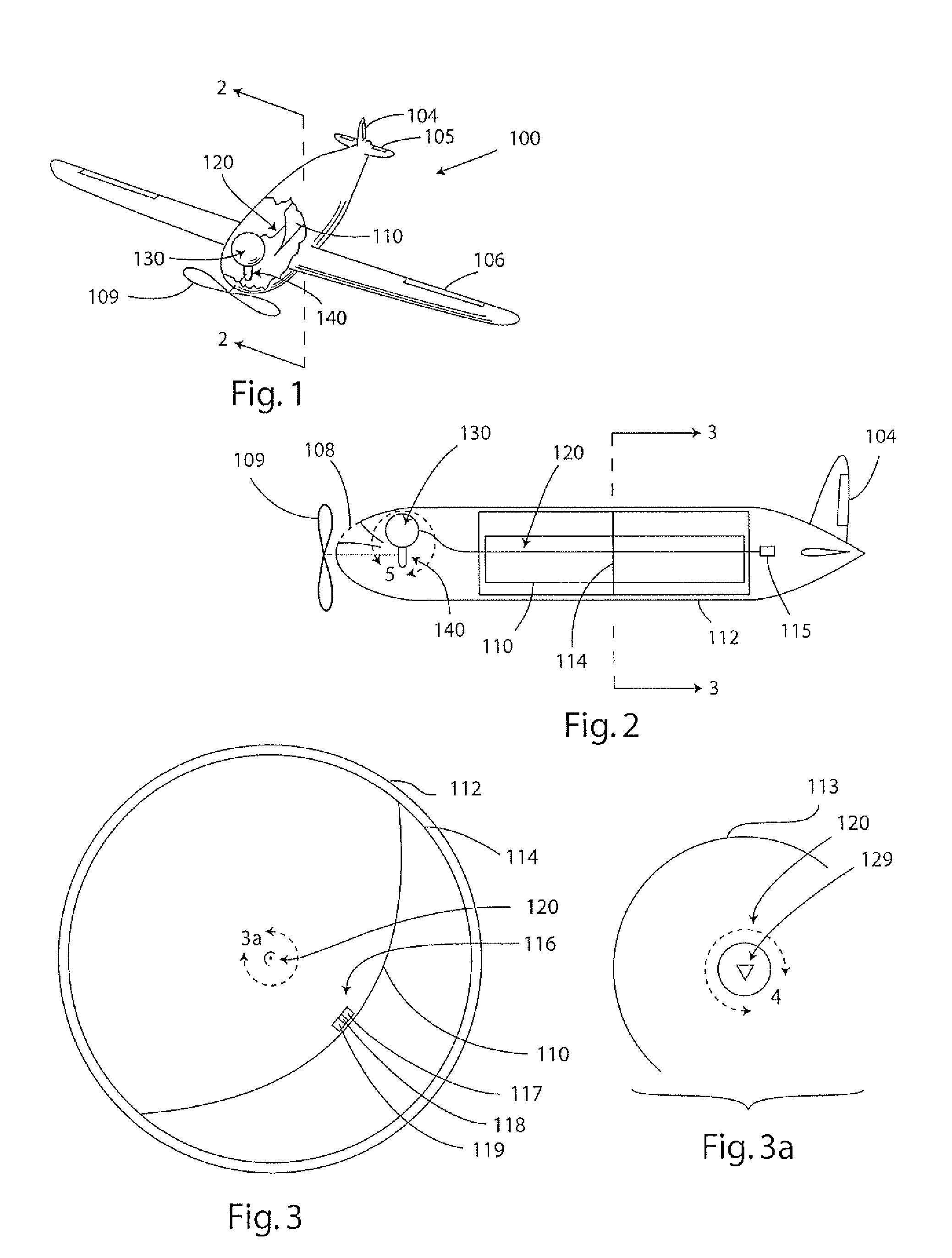
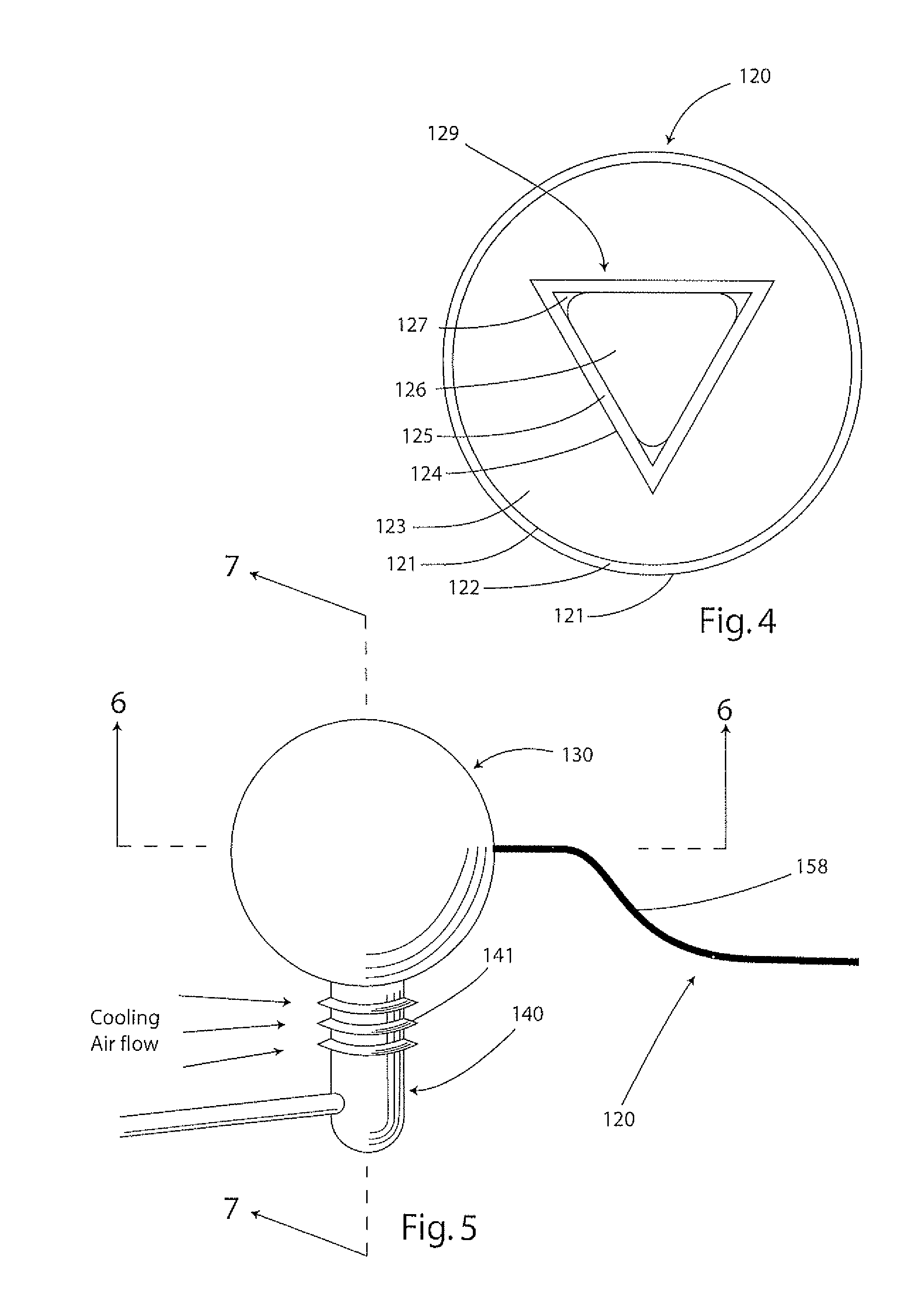
Rankline-Brayton engine powered solar thermal aircraft
InactiveUS8132412B2Significant pressure dropHigh thermal efficiencyRocket type power plantsFrom solar energyThermal energyWorking fluid
Owner:LAWRENCE LIVERMORE NAT SECURITY LLC
Scaffolded borazane-lithium hydride hydrogen storage materials
InactiveUS7166150B2Alkali/alkaline-earth/beryllium/magnesium hydridesReversible hydrogen uptakeChemistryLithium hydride
In one aspect, the invention provides a hydrogen storage composite formed of a mesoporous scaffolding material and a hydrogen storage composition comprising precursors that react to form quarternary B—H—Li—N composition. In another aspect, the invention provides a process for forming hydrogen storage material. In each aspect, a high portion of hydrogen is released as hydrogen gas and a lesser portion of hydrogen is released as a hydrogen-containing byproduct.
Owner:GM GLOBAL TECH OPERATIONS LLC
Gradient type macromolecule-based neutron absorption grid tray material and preparation method thereof
ActiveCN104228268AEffective absorptionEnsure safetySynthetic resin layered productsLaminationHigh absorptionGadolinium oxide
The invention discloses a gradient type macromolecule-based neutron absorption grid tray material which comprises a fast neutron slow absorption layer, an intermediate energy neutron absorption layer and a heat neutron absorption layer, wherein the three absorption layers are formed by gradient lamination; a neutron absorption body material doped in the fast neutron slow absorption layer is a lithium fluoride, lithium hydride or boron carbide absorption body material; a neutron absorption body material doped in the intermediate energy neutron absorption layer is a samarium oxide neutron absorption body material; a neutron absorption body material doped in the heat neutron absorption layer is a gadolinium oxide or cadmium oxide neutron absorption body material. The invention also discloses a preparation method of the gradient type macromolecule-based neutron absorption grid tray material. The gradient type macromolecule-based neutron absorption grid tray material has the characteristics of high absorption efficiency, long service life, high mechanical property, simple technology and the like, has a certain absorption effect on gamma photons, can be used for spent fuel storage and neutron sources of other types and has a wide application prospect.
Owner:ZHONGXING ENERGY EQUIP
Preparation method of sulfide solid electrolyte
InactiveCN108336400ALow priceEase of industrial implementationSolid electrolytesSecondary cellsSulfidePhosphorus pentasulphide
The invention provides a preparation method of sulfide solid electrolyte and belongs to the field of solid electrolyte. The preparation method comprises the following steps: (1) putting raw materialsincluding sulfur powder, lithium hydride, phosphorus pentasulfide and lithium phosphate into a vacuum drying box and drying; (2) under the protection of an inert atmosphere and weighing the dried rawmaterials in percentage by mass respectively: 15 percent to 40 percent of the sulfur powder, 5 percent to 20 percent of the lithium hydride, 50 percent to 70 percent of the phosphorus pentasulfide and0 to 10 percent of the lithium phosphate; pre-grinding in a mortar for 5 to 20min; adding the raw materials into a sealed ball milling pot; carrying out ball milling at room temperature and at a rotary speed of 200 to 600r / min for 24 to 60h; (3) after ball milling reaction is finished, taking the powder out from the ball milling pot under the inert atmosphere; adding the powder into a crucible and sintering in a high-temperature tubular furnace under a nitrogen atmosphere, wherein the sintering temperature is 200 to 400 DEG C and the sintering time is 2 to 6h; taking the powder out from the crucible to obtain the sulfide solid electrolyte. The preparation method of the sulfide solid electrolyte, provided by the invention, has the characteristics of simple technology, low raw material costand easiness for industrialized production.
Owner:UNIV OF SCI & TECH BEIJING
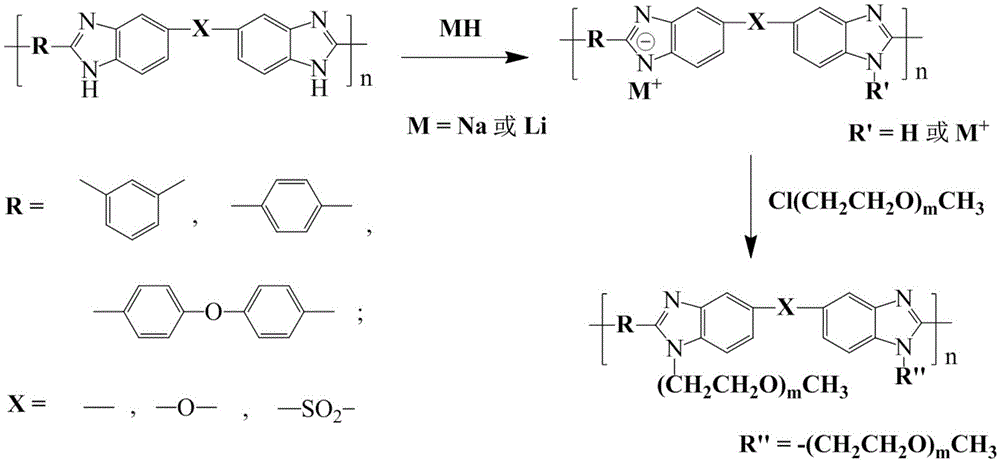
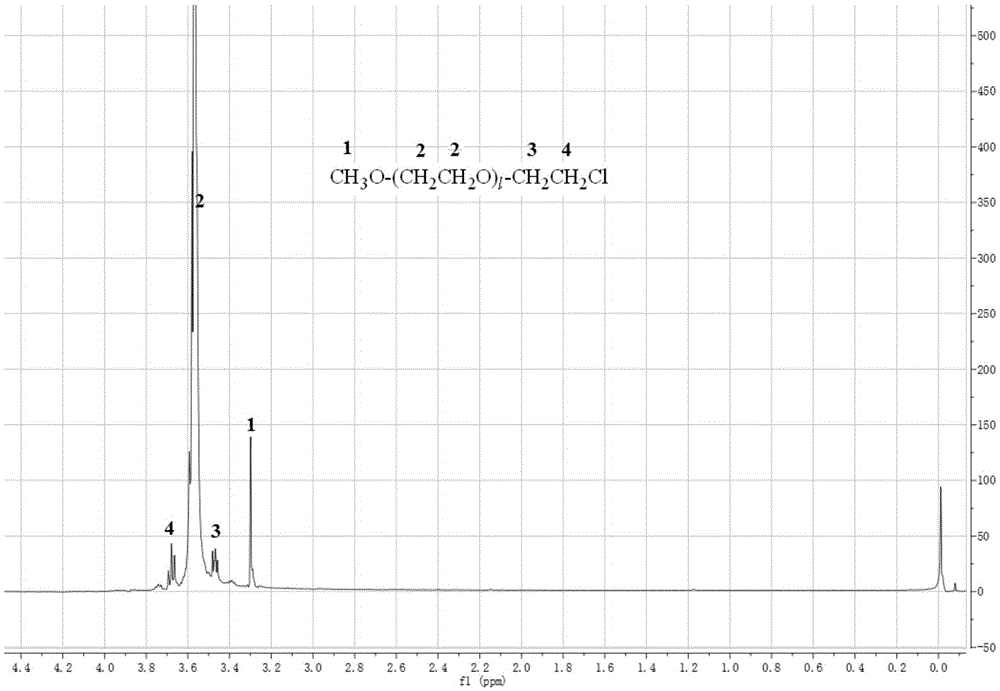
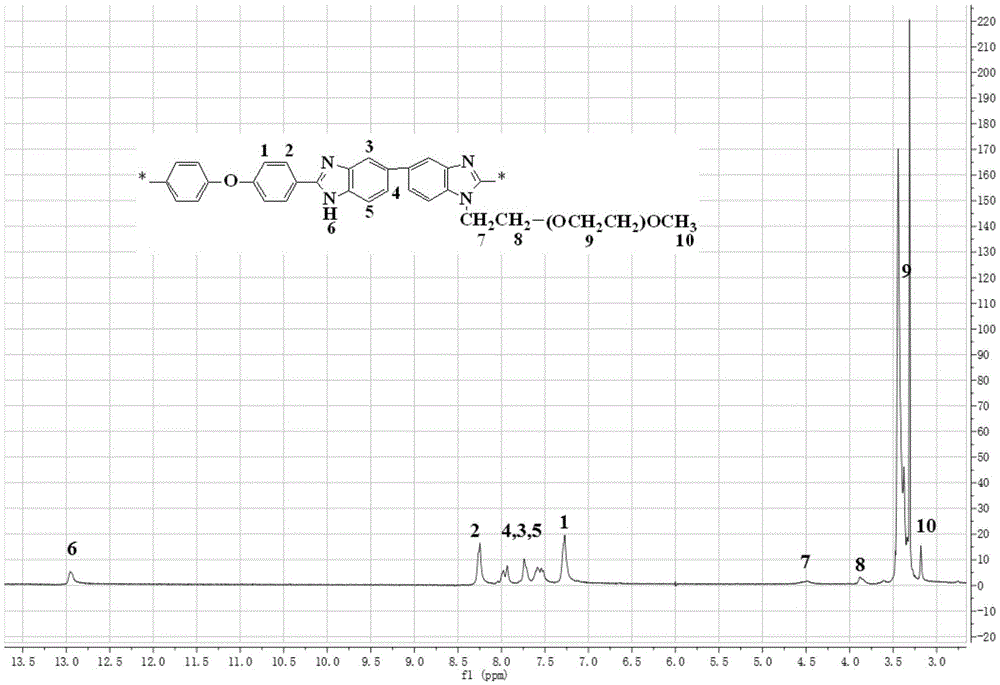
Polybenzimidazole-polyethylene glycol grafted copolymer and preparation and application thereof
The invention discloses synthesis of a polybenzimidazole-polyethylene glycol grafted copolymer and a preparation method of a crosslinking membrane of the copolymer.The preparation method comprises the steps that under protection of nitrogen or argon, a secondary amino group (N-H) in a polybenzimidazole structure reacts with sodium hydride or lithium hydride to generate polybenzimidazole polyanions, then the polybenzimidazole polyanions and polyethylene glycol monomethyl ether chloride are subjected to a grafting reaction to obtain the polybenzimidazole-polyethylene glycol grafted copolymer, and the polybenzimidazole-polyethylene glycol grafted copolymers with the different polyethylene glycol chain lengths and contents can be prepared by controlling the using amount of sodium hydride or lithium hydride and adopting polyethylene glycol monomethyl ether chloride with the different average molecular weights; a lithium salt electrolyte, butanedinitrile and the polybenzimidazole-polyethylene glycol grafted copolymer are dissolved into organic solvent, casting is performed for membrane preparing, and then the all-solid-state electrolyte membrane with the good mechanical property and the higher lithium ion conductivity is obtained.The membrane has the potential application prospect in the fields of lithium ion batteries and the like.
Owner:SHANGHAI JIAO TONG UNIV
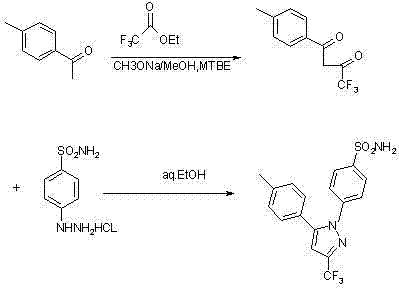
Synthesis method of celecoxib
InactiveCN102391184AHigh purityHigh yieldOrganic chemistryPhenylhydrazine hydrochlorideClaisen condensation
The invention relates to a synthesis method of celecoxib, which comprises the following specific steps of: 1, carrying out claisen condensation on p-methylacetophenone and trifluoroacetic acid ethyl esters in an aprotic organic solvent under the catalysis of alkali to obtain 1-(4-methylphenyl)-4,4,4-trifluoro-1,3-butanedione; and 2, reacting the obtained 1-(4-methylphenyl)-4,4,4-trifluoro-1,3-butanedione with sulfonamide-phenylhydrazine hydrochloride to obtain celecoxib, wherein in the step 1, the alkali for catalysis is selected from one or more of sodium hydride, potassium hydride, lithium hydride and calcium hydride. The synthesis method of celecoxib provided by the invention is easy to operate, high in yield, high in product purity and easy for industrial production.
Owner:JIANGXI SYNERGY PHARMA
Preparation method of multi-element polyphase composite lithium ion battery negative material
ActiveCN104993104AHigh reversible capacityImprove the first Coulombic efficiencyCell electrodesSecondary cellsChemical reactionProtection layer
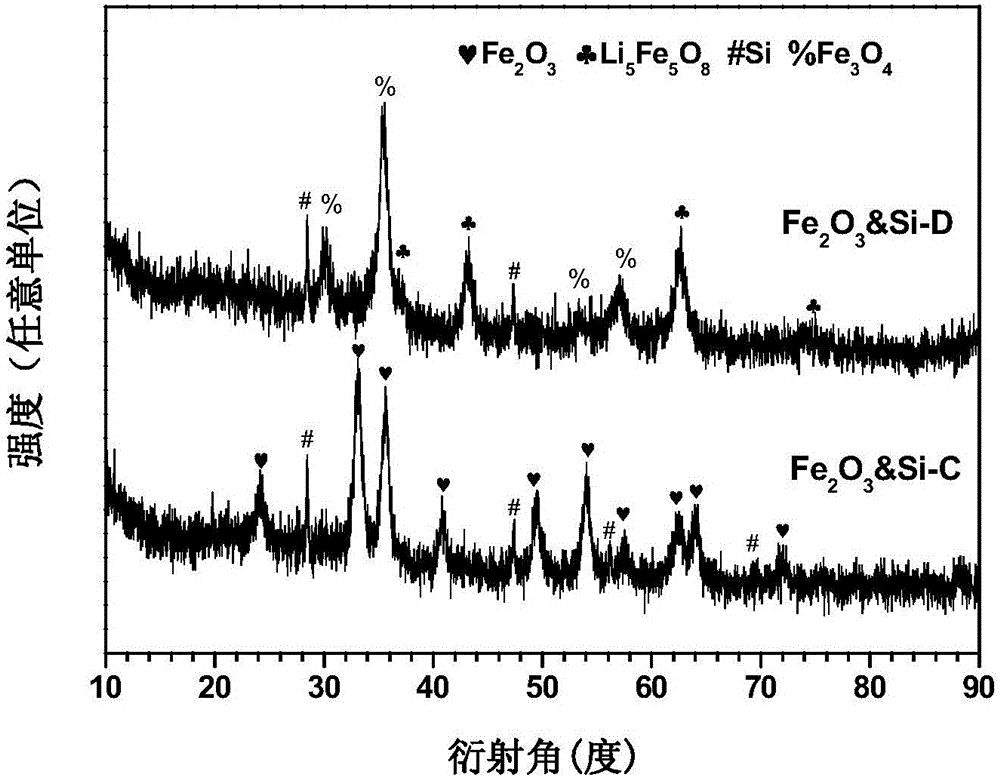
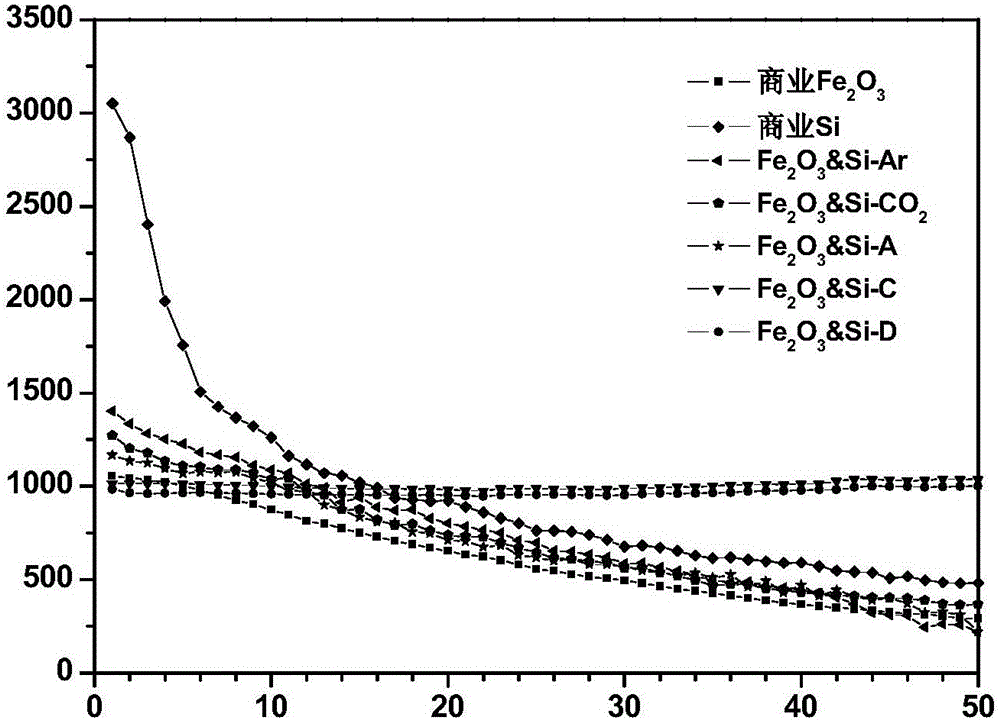
The invention discloses a preparation method of a multi-element polyphase composite lithium ion battery negative material. The method is characterized in that ball milling of metal oxide, a silicon-based material, lithium hydride and CO2 gas is carried out to obtain the multi-element polyphase composite material; and the silicon-based material and lithium hydride cannot be added after the CO2 gas. The metal oxide is compounded with the silicon-based material and are pre-lithiated in the ball milling process of the metal oxide, the silicon-based material and lithium hydride, the CO2 gas chemically reacts with the above obtained pre-lithiated product to form a protection layer on the surface of the pre-lithiated product, and the obtained multi-element polyphase composite material has the advantages of high reversible capacity, long cycle life and high initial coulomb efficiency as a lithium ion battery negative electrode; and electrodes can be directly produced by using an aqueous binder in air through adopting a smearing technology, so the negative material is convenient for popularization and application.
Owner:ZHEJIANG UNIV
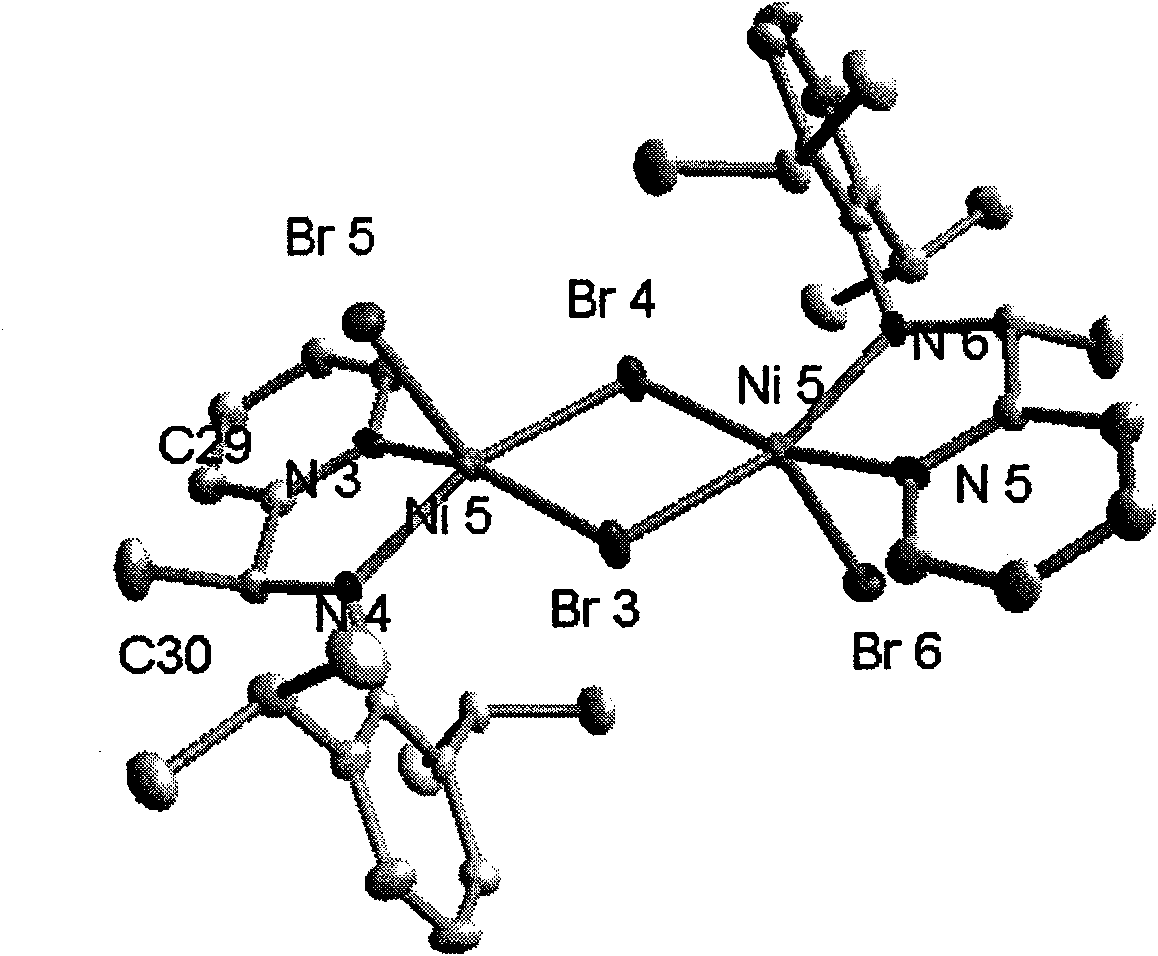
2-ammonia methyl-pyridine nickel composition, preparation method and application thereof
InactiveCN101607934AInhibits chain transfer and chain terminationImprove linearityNickel organic compoundsKetoneChemistry
The invention discloses a 2-ammonia methyl-pyridine nickel composition, a preparation method and the application thereof. The composition has a structure of a formula I, wherein R1 stands for hydrogen or alkyl, R2 stands for hydrogen or alkyl, R3 stands for hydrogen or alkyl, and X stands for halogen. The preparation method of the composition comprises the following steps: (1) generating 2-pyridine imine compound through the ketone-ammonia condensation reaction of an aromatic ketone compound and 2,6-diisopropyl aniline; then, reacting with trimethylbenzene magnesium bromide, trimethylaluminium or lithium aluminum hydride to obtain 2-ammonia methyl-pyridine ligand; and (2) under a waterless and anoxic condition, obtaining a 2-ammonia methyl-pyridine nickel composition I by the complexation reaction of the 2-ammonia methyl-pyridine ligand III and (DME) NiX2. The 2-ammonia methyl-pyridine nickel composition has a specific ligand substitute structure, can catalyze ethylene to actively polymerize so as to obtain polyethylene with high molecular weight, narrow distribution and a certain branching degree.
Owner:SUN YAT SEN UNIV
Method for synthesizing lithium borohydride
ActiveCN103922285ASimple reaction conditionsShort reaction timeMonoborane/diborane hydridesMagnesium diborideHydrogen absorption
The invention discloses a method for synthesizing lithium borohydride. The method aims at solving the problems that an existing method for preparing lithium borohydride is complex in technology, strict in synthesis condition and the like. The method comprises the following steps that in a protective atmosphere, after lithium hydride, magnesium diboride and a catalyst are mixed according to a ratio, ball-milling treatment is conducted, and a product is obtained after ball-milling treatment; a hydrogen absorption reaction is conducted on the product which is obtained after ball-milling treatment, so that a mixture of the lithium borohydride and magnesium hydride is obtained and is recorded as a reaction product; the magnesium hydride and the lithium borohydride in the reaction product are separated, so that the lithium borohydride is obtained. The method overcomes the shortages that a traditional method for preparing the lithium borohydride is complex in technology and strict in synthesis condition and the purity of the product is low and the like. The method is simple, gentle in reaction condition and short in reaction time. Synthesized LiBH4 is high in yield and purity. Meanwhile, through the method, the production period of the LiBH4 can be shortened, the production cost is reduced, the lithium borohydride can be produced in a large scale industrially, and application of the lithium borohydride is facilitated.
Owner:SICHUAN INST OF MATERIALS & TECH
Rankline-Brayton Engine Powered Solar Thermal Aircraft
InactiveUS20100162702A1Significant pressure dropHigh thermal efficiencyRocket type power plantsFrom solar energyThermal energyWorking fluid
A solar thermal powered aircraft powered by heat energy from the sun. A Rankine-Brayton hybrid cycle heat engine is carried by the aircraft body for producing power for a propulsion mechanism, such as a propeller or other mechanism for enabling sustained free flight. The Rankine-Brayton engine has a thermal battery, preferably containing a lithium-hydride and lithium mixture, operably connected to it so that heat is supplied from the thermal battery to a working fluid. A solar concentrator, such as reflective parabolic trough, is movably connected to an optically transparent section of the aircraft body for receiving and concentrating solar energy from within the aircraft. Concentrated solar energy is collected by a heat collection and transport conduit, and heat transported to the thermal battery. A solar tracker includes a heliostat for determining optimal alignment with the sun, and a drive motor actuating the solar concentrator into optimal alignment with the sun based on a determination by the heliostat.
Owner:LAWRENCE LIVERMORE NAT SECURITY LLC
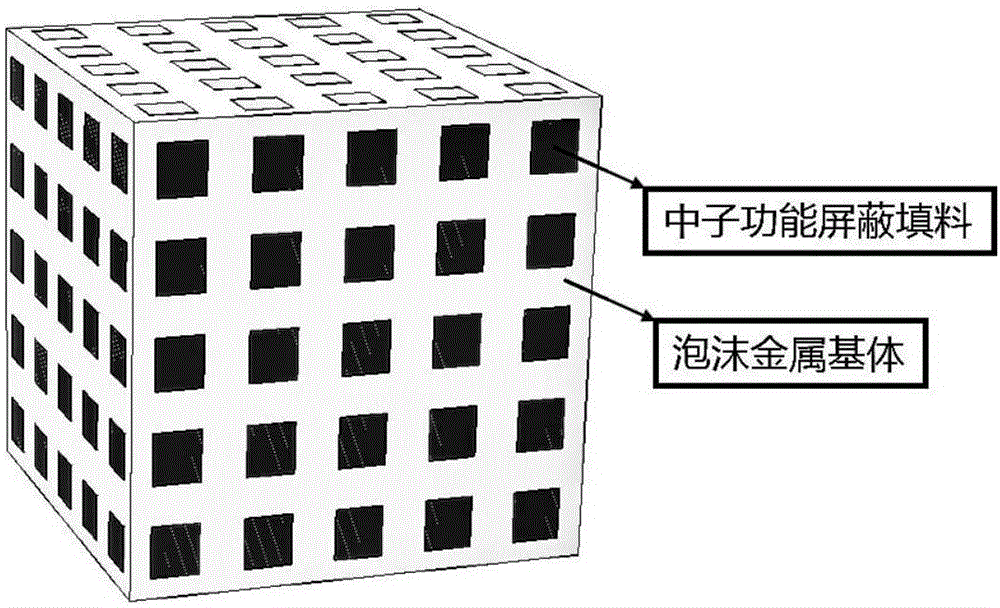
Neutron shield composite material taking foam metal as matrix and preparation method thereof
InactiveCN106280501AHigh neutron shielding performanceImproved Radiation Shielding PerformanceLithium hydridePolypropylene
The invention discloses a neutron shield composite material taking foam metal as a matrix and a preparation method thereof. The material comprises a foam metal matrix and a neutron shield functional filler, wherein the neutron shield functional filler comprises a neutron absorption functional filler and a neutron moderation functional filler; the neutron moderation functional filler is one or a mixture of several materials in resins such as polyethylene, polypropylene, polystyrene and the like or paraffin and water; the neutron absorption functional filler is one or a mixture of several materials in borax, boric acid, boron carbide, lithium hydride and the like; the foam metal is foam of metals such as perforated lead, aluminum, iron, copper, nickel and the like. According to the invention, the preparation method comprises the following steps of: sequentially performing high polymer heating and melting on raw materials, mixing organic fillers with inorganic fillers, pretreating the foam metal, filling the perforated foam metal with fillers, cooling, curing, and finally obtaining the product.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS
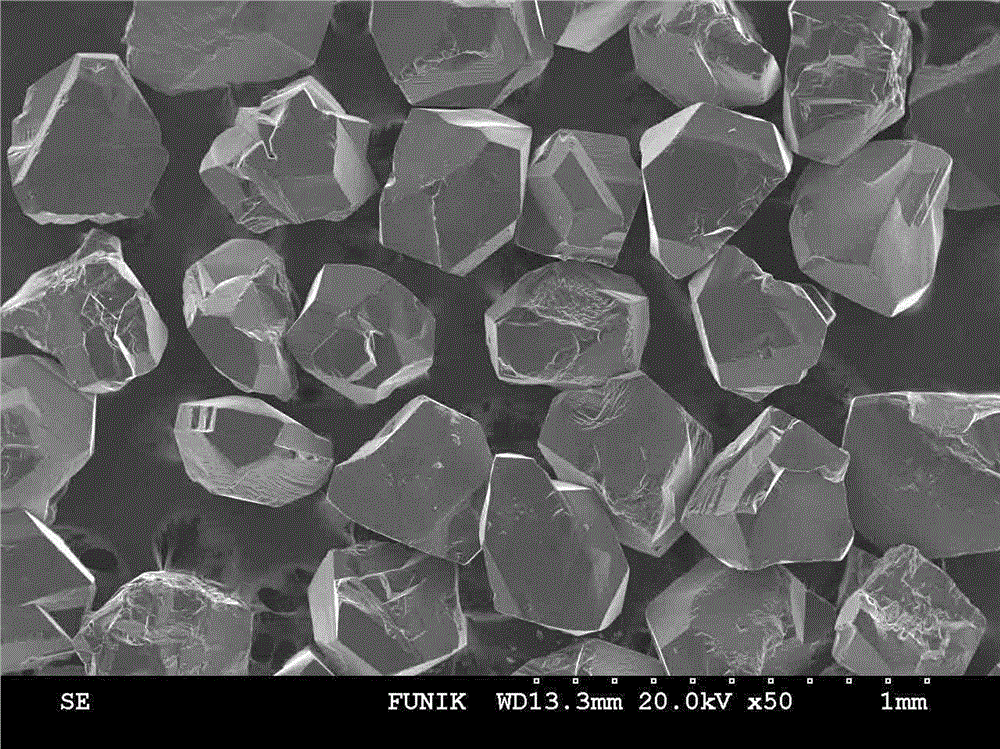
Manufacturing method for cubic boron nitride crystal
ActiveCN104607110AHigh strengthImprove thermal stabilityUltra-high pressure processesHexagonal boron nitrideTitanium nitride
The invention provides a manufacturing method for a cubic boron nitride crystal. The manufacturing method comprises the following steps: uniformly mixing hexagonal boron nitride powder, catalyst powder and additives, and pressing the mixture into a synthesis stick, wherein the catalyst powder comprises lithium nitride and lithium hydride, and the additives comprise ammonium fluoride, titanium nitride and aluminium nitride; carrying out high-pressure high-temperature synthesis on the synthesis stick to obtain a cubic boron nitride crystal crude product; crushing the cubic boron nitride crystal crude product to obtain a massive cubic boron nitride crystal crude product; sequentially soaking the massive cubic boron nitride crystal crude product by water and acid-base to obtain a massive cubic boron nitride crystal finished product. The cubic boron nitride crystal obtained by the manufacturing method disclosed by the invention is an equiareal golden crystal, has the characteristics of high strength, high thermal stability, high wear resistance, high crack resistant capability, high thermal stability and the like, is suitable for long-life electroplated tools and ceramic bond systems, as well as is applied to processing hardened carbon tool steel, hard alloys, alloy steel, and nickel-based and cobalt-based high-temperature alloy materials.
Owner:FUNIK ULTRAHARD MATERIAL
Lithium-containing hydrogen-storing alloy electrode material and its prepn
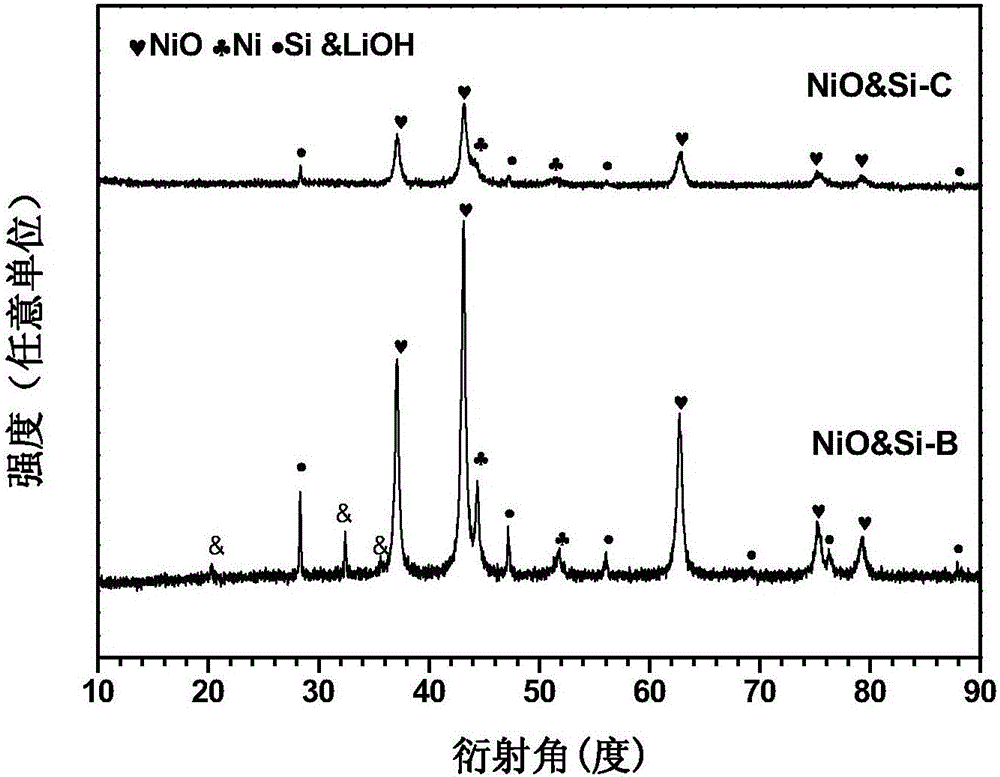
In the present invention, during the ball milling of AB5 type hydrogen-storing alloy is a special high-energy ball mill, LiH or Li is made to form alloy mechanically with AB5 type hydrogen-storing alloy to prepare composite hydrogne-storing alloy electrode material with Li content of 0.1-2.0 wt%. The present invention is also suitable for preparing composite lithium-containing hydrogen-storing alloy electrode material with AB, A2B and AB2 type hydrogen-storing alloy. The present invention utilizes the leaching of Li in alkali solution of make hydrogen-storing alloy form pores and to raise theelectric catalytic activity of Ni / MH cell negative pole. In addition, Li dissolved in the alkali solutio to form LiOH can protect the positive pole of the cell, and raise the circulation life and discharge capacity of cell.
Owner:NANKAI UNIV
Preparation of hydrogen storage materials
InactiveUS20090127129A1Rapidly and effectively convertingQuick releaseHydrogenElectrolysis componentsLithium hydrideNanometer size
A candidate hydrogen storage material, M, capable of reaction with hydrogen to form a hydride, MHm (m=number of H atoms per formula unit), and to subsequently release hydrogen on demand, is processed electrochemically to enhance its absorption / desorption properties. For example, a magnesium hydride (MgH2) composition, arranged as a positive electrode, is reduced with lithium ions in a direct current electrolytic cell to form nanometer-size particles of magnesium (and lithium hydride). The cell operation may be reversed to oxidize magnesium to nanometer size particles of magnesium hydride. Thereafter, the nanometer-size particles of M / MHm adsorb and desorb hydrogen at higher yields and under more moderate storage processing conditions.
Owner:GM GLOBAL TECH OPERATIONS LLC +1
Lithium borohydride/rare earth magnesium base alloy composite hydrogen storage material and preparation method thereof
ActiveCN103101880AHigh quality hydrogen storage densitySimple manufacturing methodHydrogen productionFreeze-dryingAlloy composite
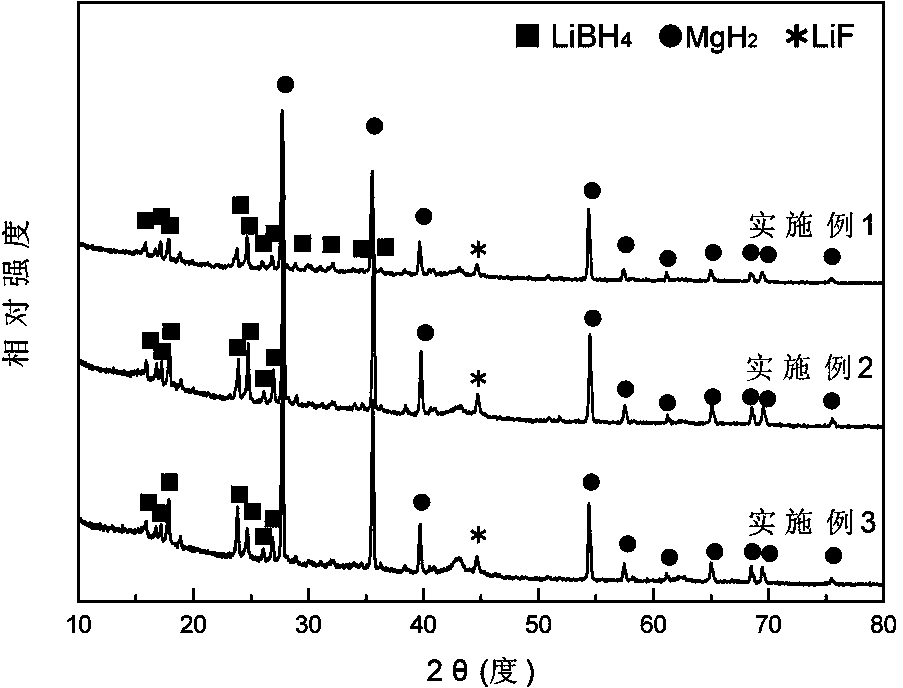
The invention discloses a lithium borohydride / rare earth magnesium base alloy composite hydrogen storage material which consists of lithium borohydride and rare earth magnesium base alloy, wherein the general formula is LiBH4 / La(1-x)MgxNiaCobMncAld; the rare earth magnesium base alloy accounts for 10-80% of the composite material by mass; and x is 0.1-0.8, a is 2.7-3.2, b is 0.1-0.8, c is 0.1-0.4 and d is 0.05-0.5. The lithium borohydride / rare earth magnesium base alloy composite hydrogen storage material is prepared by the following steps of: smelting according to the proportion of the components and tossing to obtain an alloy sheet; after heat treatment and cooling, performing ball milling and sieving to obtain tossing-state alloy powder; performing hydrogen treatment of the tossing-state alloy powder to obtain hydrogenated-state alloy powder; mixing LiBH4 and alloy powder according to a mass proportion; adding heptane, hexane or tetrahydrofuran, and performing ball milling; and freeze-drying to obtain the lithium borohydride / rare earth magnesium base alloy composite hydrogen storage material. The lithium borohydride / rare earth magnesium base alloy composite hydrogen storage material disclosed by the invention has high mass hydrogen storage density, and the preparation method is simple and easy to implement. The composite material can be widely applied to the fields such as large-scale transportation of hydrogen, hydrogen supply source of fuel cell, purification of hydrogen and the like.
Owner:GUANGDONG INST OF RARE METALS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com