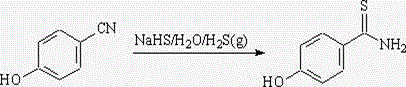Patents
Literature
76 results about "Phosphorus pentasulphide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phosphorus pentasulfide is the inorganic compound with the formula P2S5 or dimer P4S10. This yellow solid is the one of two phosphorus sulfides of commercial value. Samples often appear greenish-gray due to impurities.
Solid electrolyte sheet
InactiveUS20100151335A1Safety and proccessabilityNeither oxidized nor reducedSolid electrolytesConductive materialElectrolysisSulfur
A solid electrolyte sheet including: 80 to 99 wt % of an inorganic solid electrolyte, and 1 to 20 wt % of a binder; the inorganic solid electrolyte being obtainable by firing a raw material containing lithium sulfide (Li2S) with phosphorus pentasulfide (P2S5), or elemental phosphorus and elemental sulfur.
Owner:IDEMITSU KOSAN CO LTD
Acid-resistance promoting composition
ActiveUS20090294294A1Less susceptible to chemical attackReduce the amount requiredPaper/cardboard articlesDecorative surface effectsPhosphorus pentasulphideSulfide compound
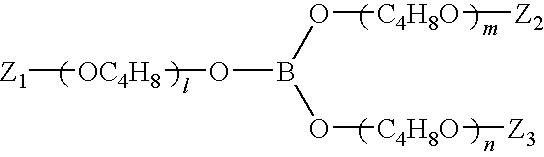
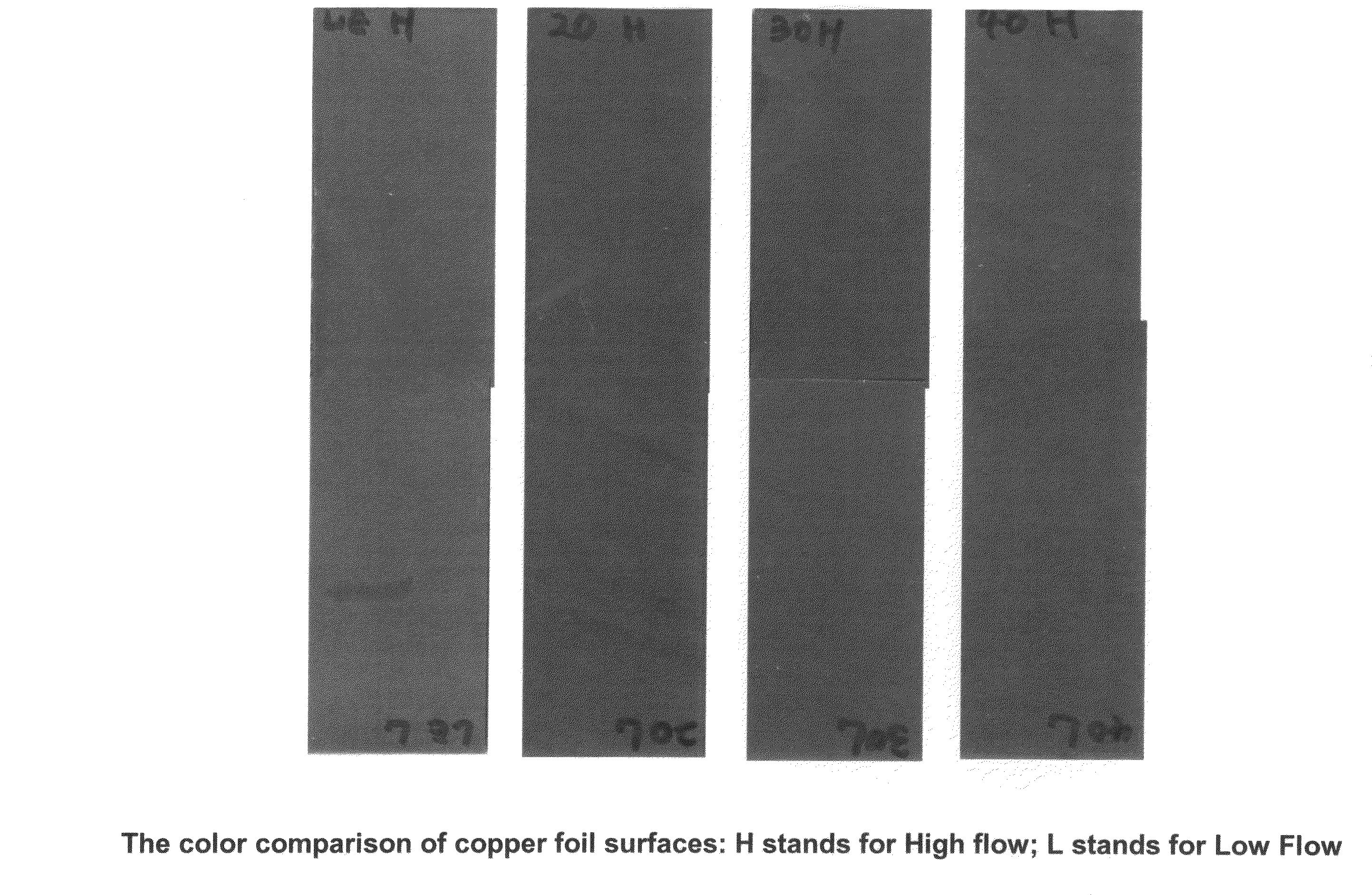
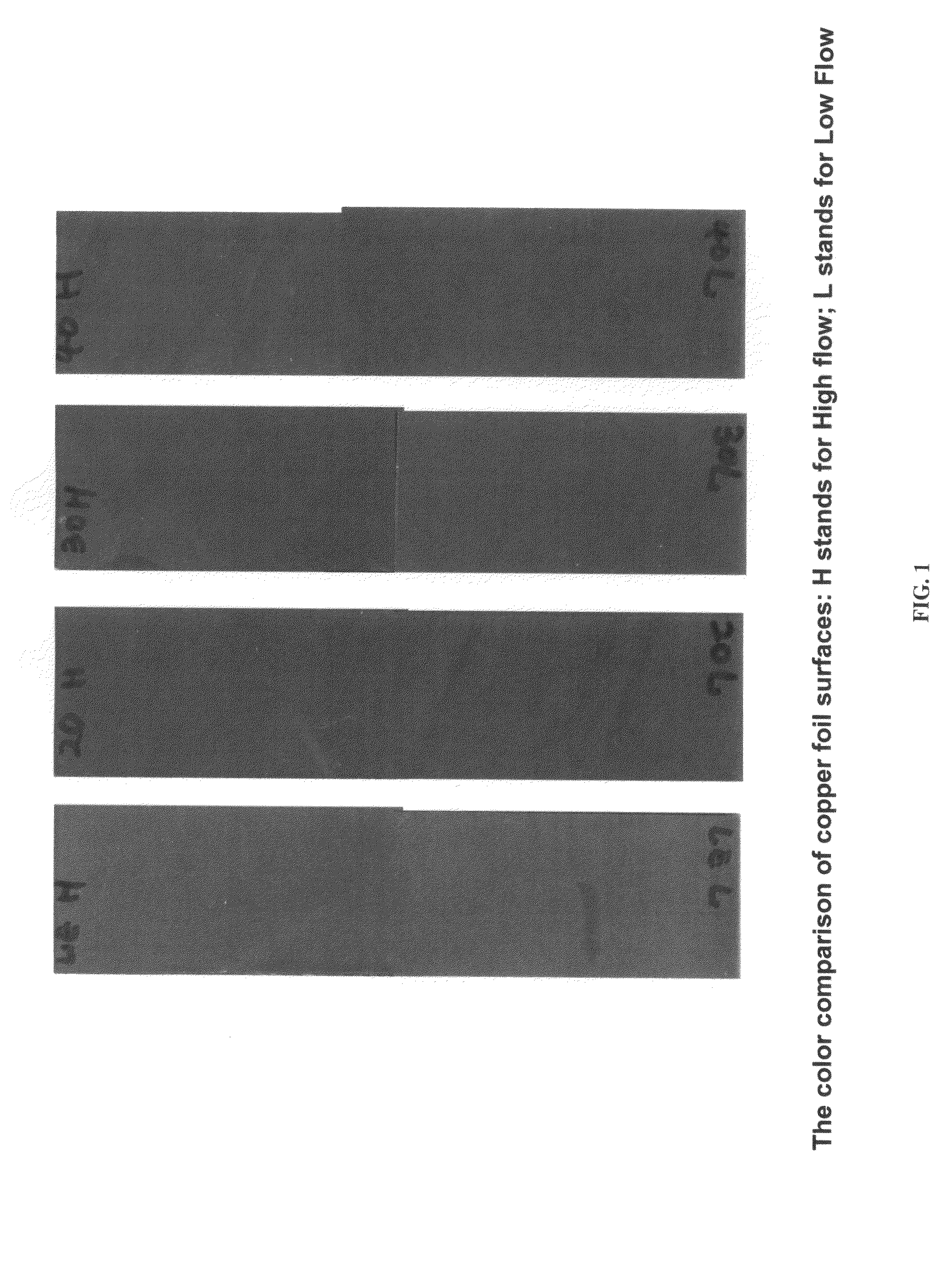
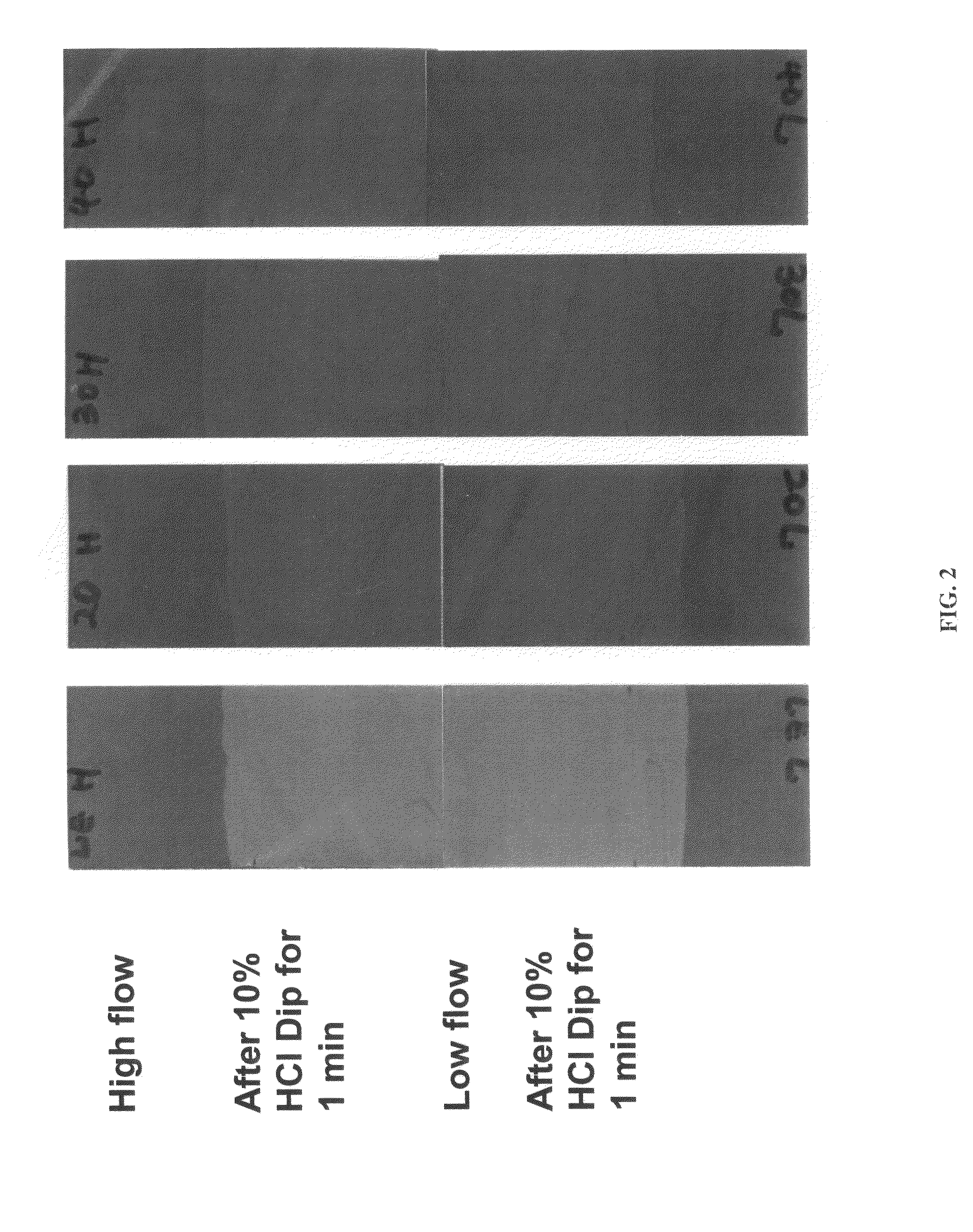
A composition for providing acid resistance to copper surfaces in the production of multilayered printed circuit boards. The composition comprises an acid, an oxidizer, a five-membered heterocyclic compound and a thiophosphate or a phosphorous sulfide compound. In a preferred embodiment, the phosphorous compound is phosphorus pentasulfide. The composition is applied to a copper or copper alloy substrate and the copper substrate is thereafter bonded to a polymeric material.
Owner:MACDERMID ACUMEN INC
Preparation method and application of non-molybdenum sulfide mineral flotation and separation inhibitor
The invention discloses a preparation method and application of a non-molybdenum sulfide mineral flotation and separation inhibitor. The preparation method for the flotation and separation inhibitor comprises the step of enabling phosphorus pentasulfide and alkaline compounds to react with water-soluble macromolecules, and thus the flotation and separation inhibitor can be obtained. According to the preparation method for the non-molybdenum sulfide mineral flotation and separation inhibitor, operation is easy, the process conditions are mild, the raw material cost is low, and the industrial production requirements are met; and moreover, the prepared product directly serves as the non-molybdenum sulfide mineral inhibitor applied to flotation and separation of molybdenum sulfide minerals and non-molybdenum sulfide minerals, the molybdenum sulfide minerals and the non-molybdenum sulfide minerals can be effectively separated, the grade of molybdenum concentrates is improved, and the flotation and separation inhibitor is particularly suitable for flotation and separation of molybdenite, copper sulfide ore, galena, pyrite, jamesonite, arsenopyrite, bismuth sulfide ore and the like.
Owner:CENT SOUTH UNIV
Process for the preparation of sulfur- and phosphorus-containing organosilicon compounds
Process for the preparation of sulfur- and phosphorus-containing compounds of the formula:in which halogen-containing silanes of the formula R1n(R2O)3-n-Si-Alk-Hal are reacted with phosphorus pentasulfide (P4S10) and a metal sulfide.
Owner:DEGUSSA AG
Preparation method of sulfide solid electrolyte
InactiveCN108336400ALow priceEase of industrial implementationSolid electrolytesSecondary cellsSulfidePhosphorus pentasulphide
The invention provides a preparation method of sulfide solid electrolyte and belongs to the field of solid electrolyte. The preparation method comprises the following steps: (1) putting raw materialsincluding sulfur powder, lithium hydride, phosphorus pentasulfide and lithium phosphate into a vacuum drying box and drying; (2) under the protection of an inert atmosphere and weighing the dried rawmaterials in percentage by mass respectively: 15 percent to 40 percent of the sulfur powder, 5 percent to 20 percent of the lithium hydride, 50 percent to 70 percent of the phosphorus pentasulfide and0 to 10 percent of the lithium phosphate; pre-grinding in a mortar for 5 to 20min; adding the raw materials into a sealed ball milling pot; carrying out ball milling at room temperature and at a rotary speed of 200 to 600r / min for 24 to 60h; (3) after ball milling reaction is finished, taking the powder out from the ball milling pot under the inert atmosphere; adding the powder into a crucible and sintering in a high-temperature tubular furnace under a nitrogen atmosphere, wherein the sintering temperature is 200 to 400 DEG C and the sintering time is 2 to 6h; taking the powder out from the crucible to obtain the sulfide solid electrolyte. The preparation method of the sulfide solid electrolyte, provided by the invention, has the characteristics of simple technology, low raw material costand easiness for industrialized production.
Owner:UNIV OF SCI & TECH BEIJING
Sulfide ore floatation composite collecting agent and preparation method and application thereof
ActiveCN107252735APromote resource utilizationEnhanced harvesting capacityFlotationPhosphorus pentasulphideSolvent
The invention discloses a sulfide ore floatation composite collecting agent and a preparation method and application thereof. The sulfide ore floatation composite collecting agent is composed of modified X oil and hydrocarbon-type oil. The preparation method of the sulfide ore floatation composite collecting agent comprises the steps that the hydrocarbon-type oil is used as a solvent; and the X oil and phosphorus pentasulfide react, and thus the composite collecting agent composed of the modified X oil and the hydrocarbon-type oil is obtained. The preparation method is wide in raw material source, low in cost and simple in process operation, the prepared floatation collecting agent has high collecting capacity and selectivity to sulfide ores such as copper sulfide ores, copper-molybdenum ores and lead-zinc ores are achieved, and good floatation indexes in a floatation test are obtained.
Owner:CENT SOUTH UNIV
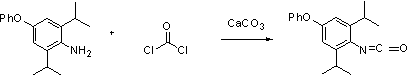
Synthesis process for diafenthiuron as thiourea insecticide and acaricide
InactiveCN102993075AMild reaction conditionsEasy to operateOrganic chemistryThioureaSynthesis methods
The invention discloses a synthesis research method for diafenthiuron as a novel thiourea insecticide and acaricide. The synthesis method comprises the following steps of: firstly, synthesizing an N-2,6-diisopropyl-4-phenoxylphenyl)isocyanate intermediate by taking 2,6-diisopropyl-4-aminobiphenyl ether and triphosgene as raw materials; then, subjecting the generated N-2,6-diisopropyl-4-phenoxylphenyl)isocyanate intermediate and tert-butylamine to a reaction to generate 3-(2,6-diisopropyl-4-phenoxylphenyl)-1-tert-butyl urea; and then, subjecting the 3-(2,6-diisopropyl-4-phenoxylphenyl)-1-tert-butyl urea and phosphorus pentasulfide to a reaction under the action of potassium carbonate to finally generate diafenthiuron. The synthesis process is a more effective method for synthesizing the diafenthiuron, which has the advantages of mild reaction conditions, simplicity in operation, cheaper raw materials, lower toxicity and the like.
Owner:JIANGSU CHANGQING AGROCHEMICAL CO LTD
Technology for removing arsenic in polyphosphoric acid
The invention discloses a technology for removing arsenic in polyphosphoric acid, comprising the following steps: introducing hydrogen sulfide or sulfide (such as sodium sulfide, phosphorus pentasulfide and the like) into phosphoric acid solution; generating precipitate at a certain temperature; and obtaining low-arsenic phosphoric acid after filtering and separating. The low-arsenic phosphoric acid can satisfy the requirements of market and environment protection, is a key for expanding polyphosphoric acid application, and has wide market prospect.
Owner:胡金来
Method for directly utilizing waste liquor containing phosphoric acid and phosphorous acid
InactiveCN101786744ASimple production equipmentEasy to operateWater contaminantsMultistage water/sewage treatmentPhosphorous acidSolubility
The invention discloses a method for directly utilizing waste liquor containing phosphoric acid and phosphorous acid. In the method, yellow phosphorus refine dearsenization waste liquor containing the phosphorous acid and the orthophosphoric acid is dearsenized by phosphorus pentasulfide and is directly neutralized to obtain the mixed solution of phosphate and phosphite; and according to the solubility difference of the phosphate and phosphite, a fractional crystallization method is adopted to concentrate, crystallize and separate to obtain the product of orthophosphate and the phosphite. The process has the characteristics of simple treatment process, easy and safe operation, high phosphorus recovery rate, capability of comprehensively utilizing wastes and the like.
Owner:KUNMING UNIV OF SCI & TECH
Probenazole production process
ActiveCN101712677AReduce pollutionMake up for the lack ofOrganic chemistryHydrobromidePhosphorus pentasulphide
The invention discloses a probenazole production process, comprising the steps of: carrying out a condensation reaction on lactic acid and o-phenylenediamine in hydrochloric acid water solution; regulating pH to obtain 2-alpha-hydroxyethyl benzimidazole; putting into a container using acetone and sulfuric acid as solvents for oxidization with potassium permanganate; extracting, desolventizing and drying to obtain 2- acetyl benzimidazole; putting into a container using glacial acetic acid as a solvent to be brominated with bromne; drying a filtering cake to make 2- Dibromo-acetyl benzimidazole hydrobromide; finally cyclize with formamide and phosphorus pentasulfide in a container using ethyl acetate as a solvent; purifying; and regulating the pH to obtain probenazole, the content of which is larger than or equal to 99%. The production process reduces the cost and risks of safety environmental protection, reduces environmental pollution, enhances production efficiency, enables the whole reaction process to execute at normal temperature and pressure, and has mild reaction condition and stable reaction yield of each step. The total yield counted by the o-phenylenediamine is above 75%.
Owner:JIANGSU NOON CROP SCI CO LTD
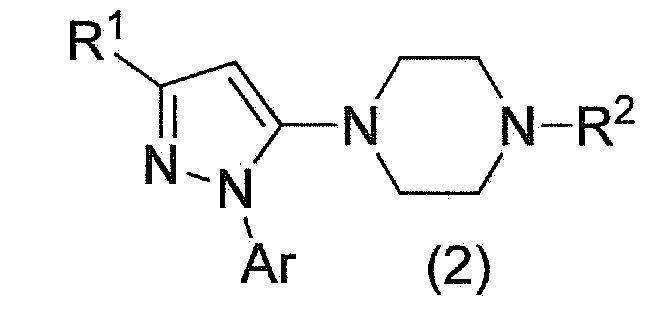
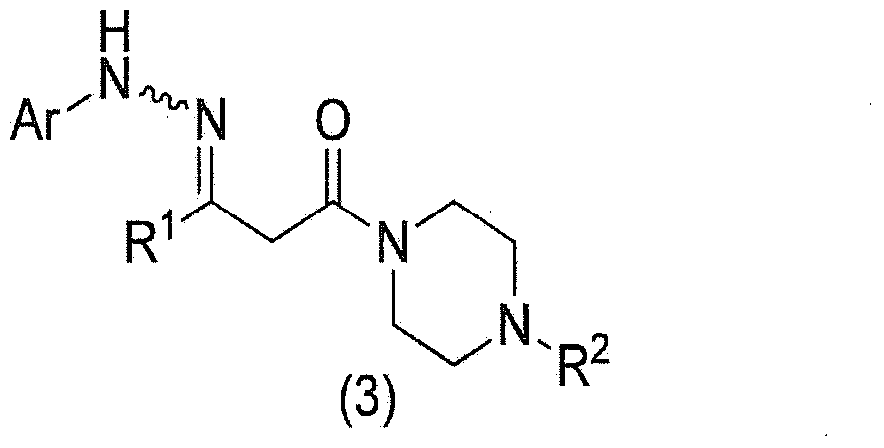
Method for manufacturing pyrazole derivative
ActiveCN103649055AEfficient preparationHigh yieldOrganic active ingredientsOrganic chemistryCarboxylic saltPhosphorus pentasulphide
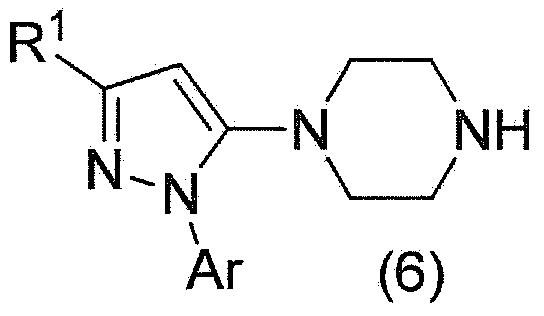
The present invention provides a prolinamide compound useful as a drug or the like and an industrially advantageous method for producing a piperazinyl pyrazole compound that is a production intermediate of the prolinamide compound. Specifically, the invention is a method for producing a compound represented by general formula (1) as represented by the following formula (where the symbols in the formula are the same as defined in the specification) or a salt thereof, wherein a compound represented by general formula (2) is produced, a pyrazole ring being formed via a reaction between a compound represented by general formula (3) and phosphorus pentasulfide; and, once the compound represented by general formula (2) is deprotected, a carboxylate of a compound represented by general formula (6) is produced, the compound obtained by forming a carboxylic acid and a salt.
Owner:MITSUBISHI TANABE PHARMA CORP
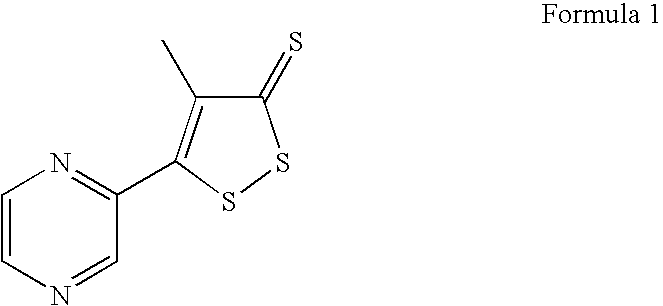
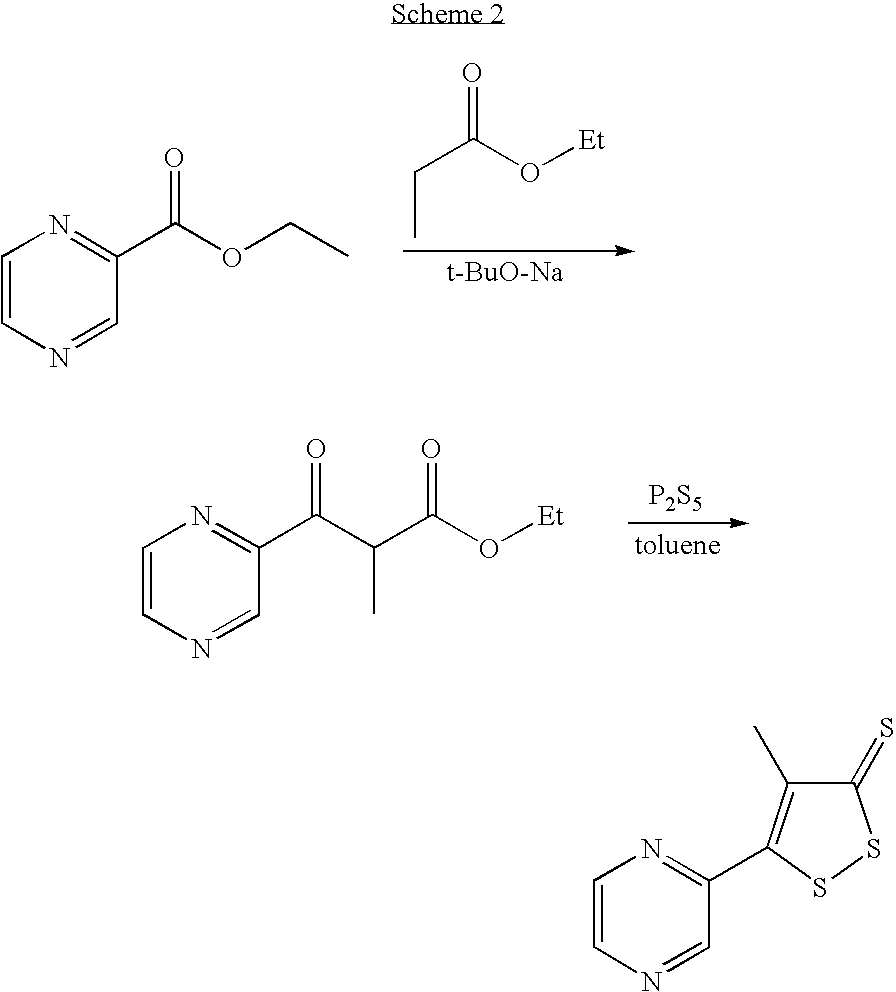
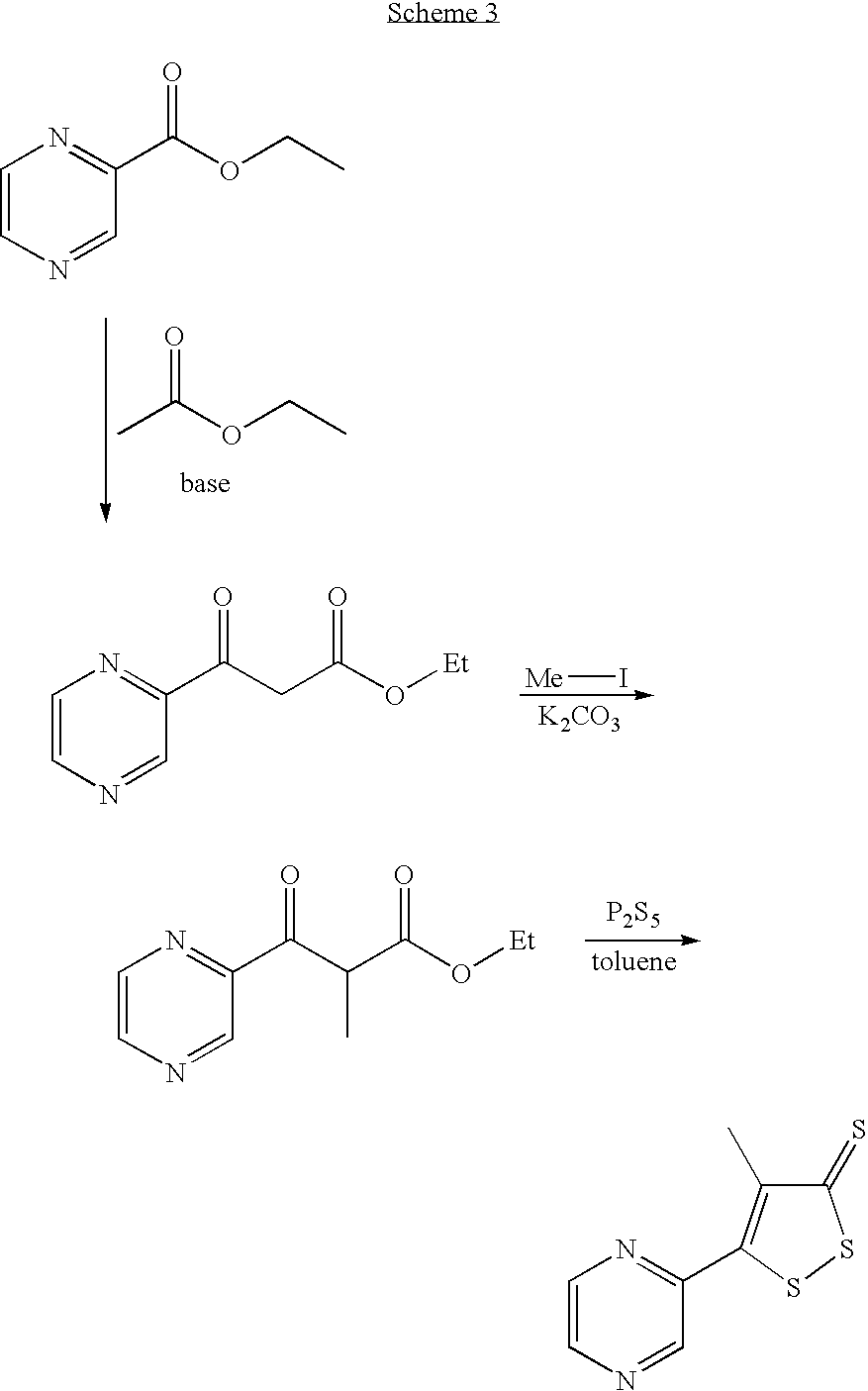
Method for preparing oltipraz
InactiveUS7288652B2High yieldShorten the construction periodBiocideOrganic chemistryPhosphorus pentasulphideSolvent
Provided is a method for preparing oltipraz. The method includes reacting methyl 2-methyl-3-(pyrazin-2-yl)-3oxopropionate with phosphorus pentasulfide in the presence of a mixed solvent of toluene and xylene, followed by recrystallization.
Owner:PROTHERAPEUTICS INC
Novel process for producing thiabendazole
InactiveCN106349235AReduce pollutionImprove efficiencyOrganic chemistryHydrobromidePhosphorus pentasulphide
The invention discloses a process for producing thiabendazole. The process includes carrying out condensation reaction on lactic acid and o-phenylenediamine in acidic aqueous solution(with, but not limited to, hydrochloric acid); regulating pH (potential of hydrogen) values to obtain 2-alpha-hydroxyl ethyl benzimidazole; placing the 2-alpha-hydroxyl ethyl benzimidazole in a container with acetone and sulfuric acid (but not limited to sulfuric acid, or hydrochloric acid and other inorganic acid and strong acid and weak alkali salt) and carrying out oxidation reaction on the 2-alpha-hydroxyl ethyl benzimidazole and potassium permanganate (but not limited to potassium permanganate, or hydrogen peroxide and organic peroxide); carrying out extraction, desolvation and drying to obtain 2-acetyl benzimidazole; placing the 2-acetyl benzimidazole in a container with glacial acetic acid and carrying out halogenations reaction on the 2-acetyl benzimidazole and bromine (but not limited to, or chlorine and other halogens); drying filter cake to obtain 2-dibromomethane acetyl benzimidazole hydrobromide; carrying out reaction on formamide and phosphorus pentasulfide in a container with ethyl acetate to obtain thioformamide; removing phosphorus pentoxide by means of filter-press by the aid of nitrogen to obtain thioformamide / ethyl acetate solution and carrying out cyclization reaction on the thioformamide / ethyl acetate solution and 2-dibromomethane acetyl benzimidazole; purifying reaction products and regulating pH (potential of hydrogen) values to obtain the thiabendazole with the content higher than or equal to 99%. The acetone and the sulfuric acid in the corresponding container are used as solvents. The glacial acetic acid in the corresponding container is used as a solvent. The ethyl acetate in the corresponding container is used as a solvent. The process has the advantages that the cost can be lowered, risks in the aspects of safety and environmental protection can be reduced, environmental pollution can be abated, and the production efficiency can be improved; integral reaction procedures are carried out at the normal temperatures under the normal pressures, accordingly, reaction conditions are mild, the yield of the reaction at each step is quite stable, and the overall yield measured by the aid of the o-phenylenediamine can reach 75% at least.
Owner:孟宪锋
Process of preparing multi-functional additive for lubricant composition form saturated cashew nut shell liquid
InactiveUS20020128159A1Reduce coefficient of frictionImprove the lubrication effectAdditivesPhosphorus organic compoundsPolymer sciencePhosphorus pentasulphide
A process for the preparation of multi-functional additive, amino di(alkylcyclohexyl0) phosphorodithioate, for use as an additive in a lubricant composition so as to impart improved coefficient of friction, wear reduction, antioxidant and extreme pressure properties, including the steps of (a) hydrogenating distilled technical cashew nut shell liquid with palladium or nickel or platinum catalyst; to fully hydrogenate the olefinic chain and aromatic ring of the precursor; (b) reacting fully hydrogenated technical cashew nut shell liquid with phosphorus pentasulfide to obtain unpolymerized hydrogenated cashew nut shell liquid phosphorodithioic acid, the reaction being carried out at a temperature ranging from 20 to 140.degree. C. ; and (c) condensing the unpolymerized fully saturated cashew nut shell liquid phosphorodithioic acid with at least one amine to obtain the amino di(alkylcyclohexyl) phosphorodithioate. A lubricant containing a major proportion of a material selected from the group consisting of an oil of lubricating viscosity and a grease; and remainder an additive including amino di(alkylcyclohexyl)phosphorodithioate prepared by the foregoing process.
Owner:INDIAN OIL CORPORATION
Boron magnetic rare earth lubricating oil additive and preparation method thereof
ActiveCN104031721AEnergy saving and environmental protectionObvious fuel-saving effectAdditivesRare-earth elementPhosphate
The invention discloses a boron magnetic rare earth lubricating oil additive and a preparation method thereof. The additive is prepared from mixed sulfur amine phosphate, lanthanum oxide, rare earth carbonate, boric acid and calcium sulfonate, wherein the weight ratio of the mixed sulfur amine phosphate to the lanthanum oxide, rare earth carbonate, boric acid and calcium sulfonate is 100:(4.5-5):(4.5-5):(12-17):(12-17); the mixed sulfur amine phosphate is prepared from ricinoleic acid and triethanolamine reacting with phosphorus pentasulfide and octanol. The additive disclosed by the invention is an oil-soluble boron magnetic rare earth complex which is used as a friction improver, an antiwear agent and a cleaning agent for the lubricating oil and particularly the lubricating oil for the engine of an internal combustion engine, and the energy saving effect is remarkable; with the rare earth element, low sulfur and low phosphorous, the additive also has the advantages of energy conservation and environmental protection.
Owner:纳米克斯(辽宁)能源有限公司
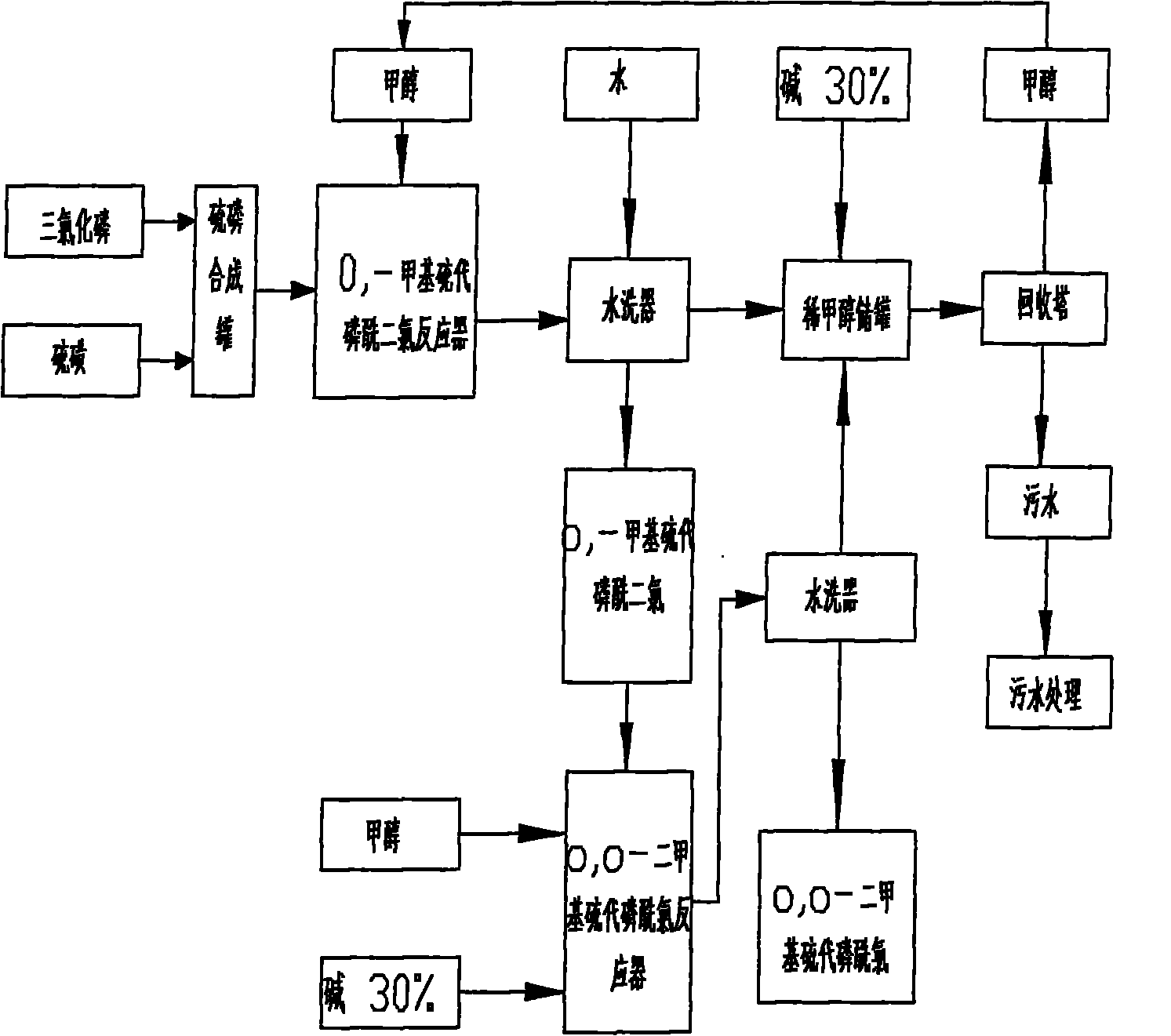
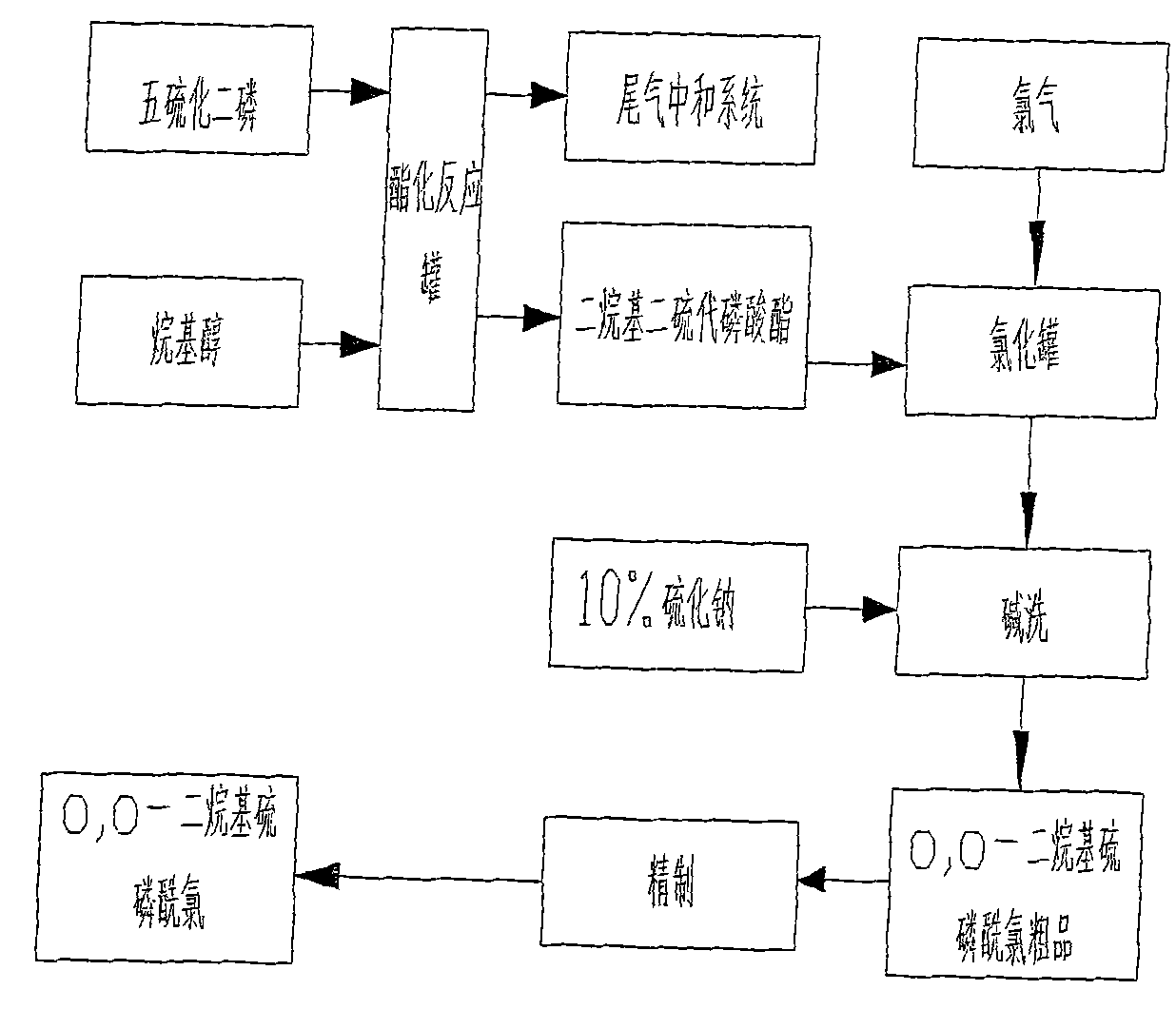
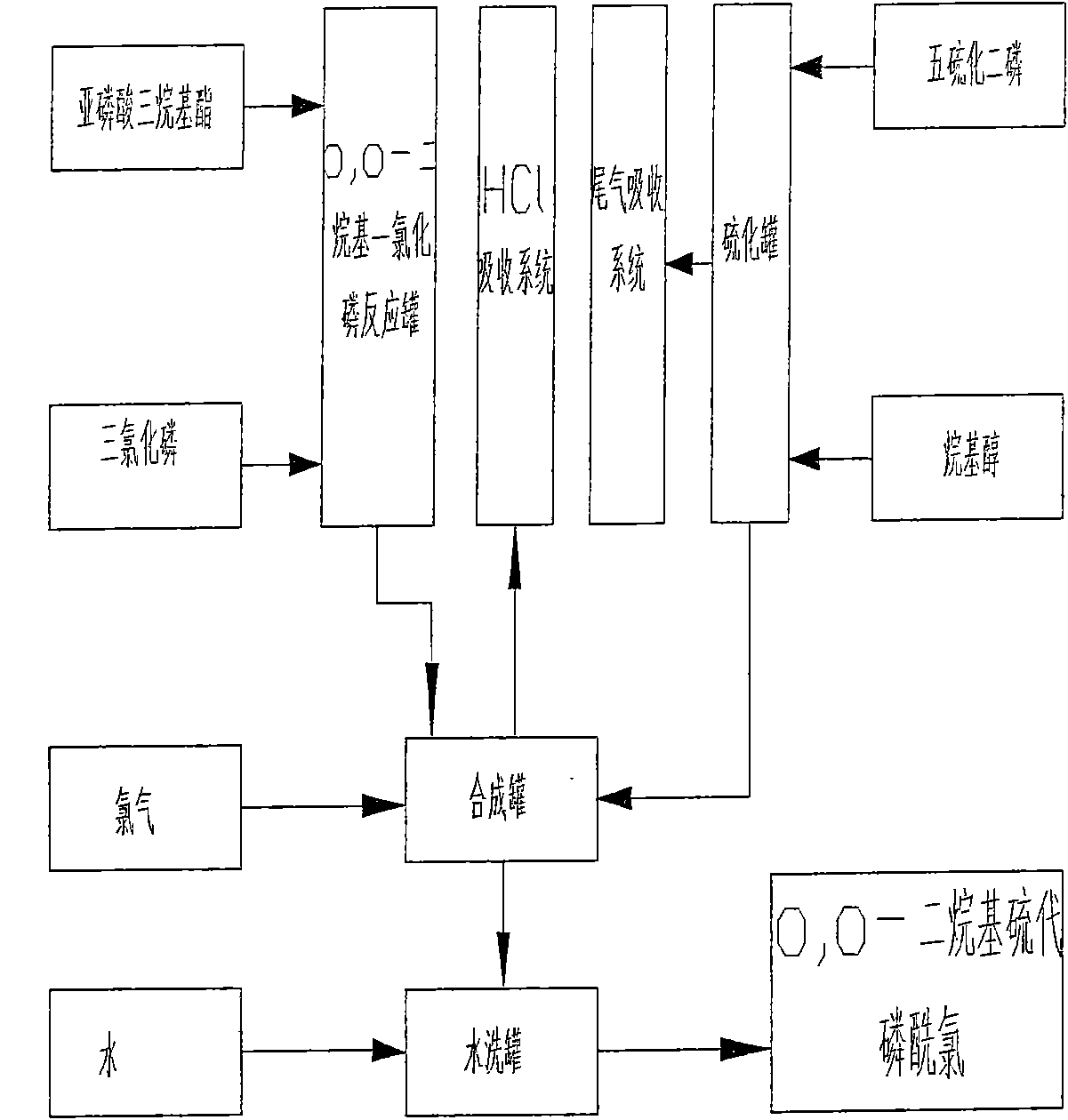
Production process of O, O-dialkyl thiophosphoryl chloride
ActiveCN101830925AReduce energy consumptionIncrease consumptionGroup 5/15 element organic compoundsPhosphorus pentasulphidePhosphorus trichloride
The invention relates to a production process of O, O-dialkyl thiophosphoryl chloride, which improves the production process of the O, O-dialkyl thiophosphoryl chloride produced by a phosphorus pentasulfide method. An O, O-dialkyl thiophosphoryl chloride product is synthesized by adding a by-product obtained by reacting phosphorus pentasulfide with alkanol and introducing chlorine to O, O-dialkyl phosphorus chloride obtained by reacting trialkyl phosphite with phosphorus trichloride. The invention can achieve the total yield of the trialkyl phosphite by 94 percent and the total yield of the phosphorus pentasulfide more than 95 percent and has safe production, little energy consumption and pollution without generating sulphur residues.
Owner:荆州三才堂化工科技有限公司
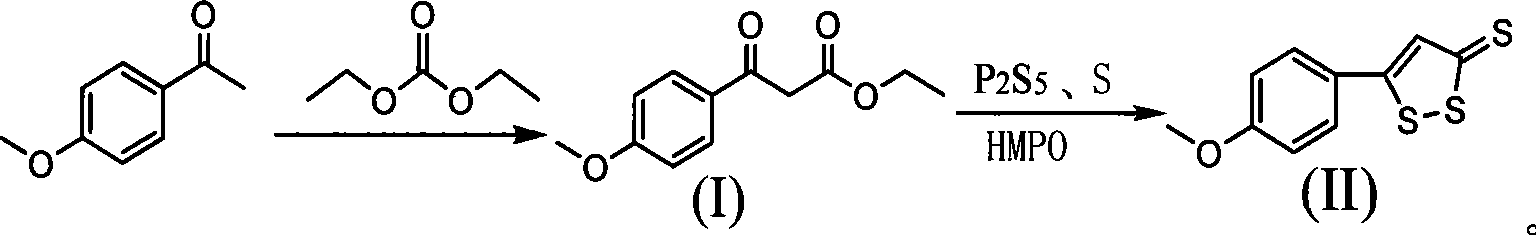
Method for preparing anethol trithione
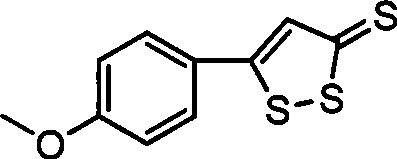
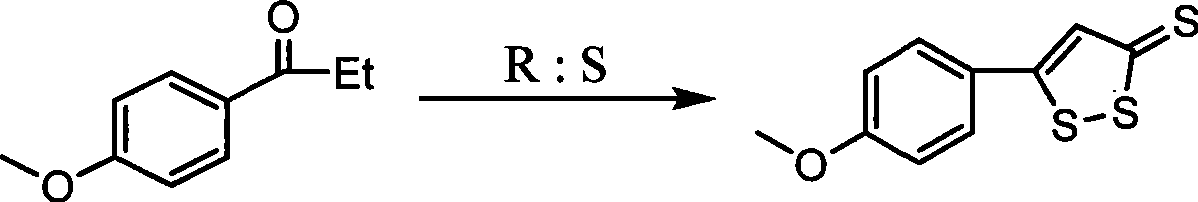
ActiveCN101463030ASave raw materialsRaw materials are easy to getOrganic chemistryDigestive systemSulfurPhosphorus pentasulphide
The invention discloses a preparation method of anethol trithione, relates to an organic compound, in particular to a method for preparing the anethol trithione by taking p-methoxy acetophenone and diethyl carbonate as raw materials to prepare p-methoxybenzoyl ethyl acetate under the action of alkali,and performing sulfur cyclization; and provides the preparation method of the anethol trithione with available and cheap raw materials, simple process, easy control, good product quality and high yield. The preparation method comprises the following steps: taking the p-methoxy acetophenone and the diethyl carbonate as the raw materials, and preparing a compound (I) ( the p-methoxybenzoyl ethyl acetate) by heating reaction under the action of the alkali; mixing the p-methoxybenzoyl ethyl acetate with phosphorus pentasulfide, sulfur, hexamethyldisiloxane and a solvent to obtain a mixture which is refluxed for reaction, and neutralized with potassium carbonate to obtain the product anethol trithione.
Owner:福建深纳生物工程有限公司
Method for preparing dialkyl dithiophosphate
InactiveCN101921293AImprove responseShort reaction timeGroup 5/15 element organic compoundsAlcoholPhosphorus pentasulphide
The invention discloses a method for preparing dialkyl dithiophosphate, comprising the following steps: (1) continuously adding phosphorus pentasulfide in an alcohol solution under stirring when the pressure is minus 0.01-0.05MPa and the temperature is 30-100 DEG C, and preserving the temperature and reacting for 2-5h to obtain dialkyl phosphorodithioicacid, wherein the weight ratio of the added phosphorus pentasulfide to the alcohol solution is 1: 4.0-4.8; and (2) under the temperature of 20-60 DEG C, adding a sodium hydroxide solution with the concentration of 15-25wt% in the dialkyl phosphorodithioicacid based on the weight ratio of 1: 1.0-1.1, and performing neutralization reaction to obtain the dialkyl dithiophosphate. In the invention, the reaction of the phosphorus pentasulfide and the alcohol solution has good effect, short reaction time, short production cycle and high product yield, and the waste gas generated by the reaction can be recycled, thus the invention is an environment-friendly and efficient production process.
Owner:WEIFANG SHIHUA CHEM
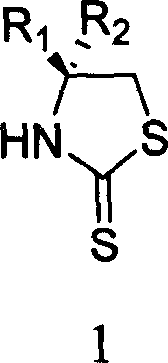
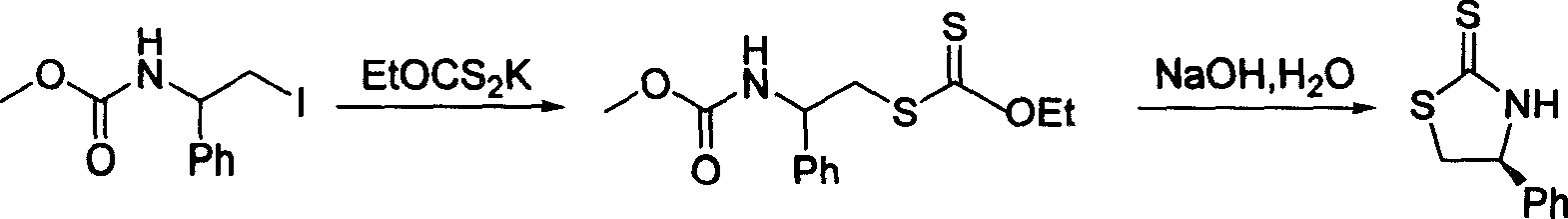
Process for synthesizing chiral thiazolidine-2-thioketone
The invention discloses a synthesizing method of chiral thiazolidine-2-thionone compound with structure formula 1, which is characterized by the following: refluxing chiral thiazolidine ketone and phosphorus pentasulfide in the organic solvent; simplifying the synthesizing method; controlling reacting condition easily; fitting for industrial manufacturing.
Owner:DONGHUA UNIV +1
Process for synthesizing O,O'-alkyl thiophosphoric acid
InactiveCN1702073ALess side effectsReduce the likelihood of accidentsGroup 5/15 element organic compoundsAlcoholOrganic solvent
The invention discloses a method for synthesizing O, O'-dialkyl phosphorothioic acid, which takes phosphorothioic acid and low-carbon alcohol as materials to synthesize O, O'-dialkyl phosphorothioic acid with the existing of catalyst and organic solvent. The invention chooses organic amine and tervalence organo-phosphines compound as catalyst and choose non-polar organics such as aromatics, alkyl halide and esters as organic solvent. The invention has the advantages of little side reaction, simple removal of reaction heat, low likelihood of accident and high yield.
Owner:姚文刚
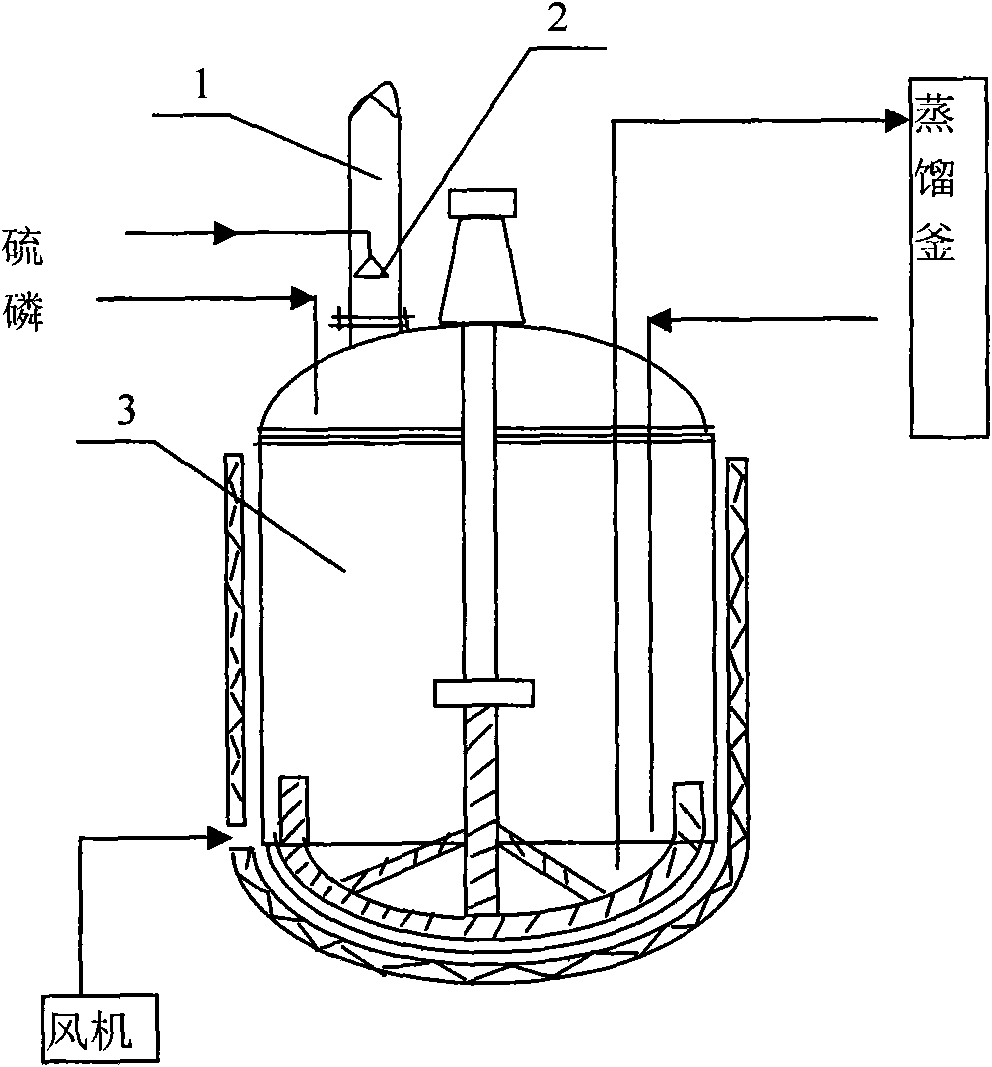
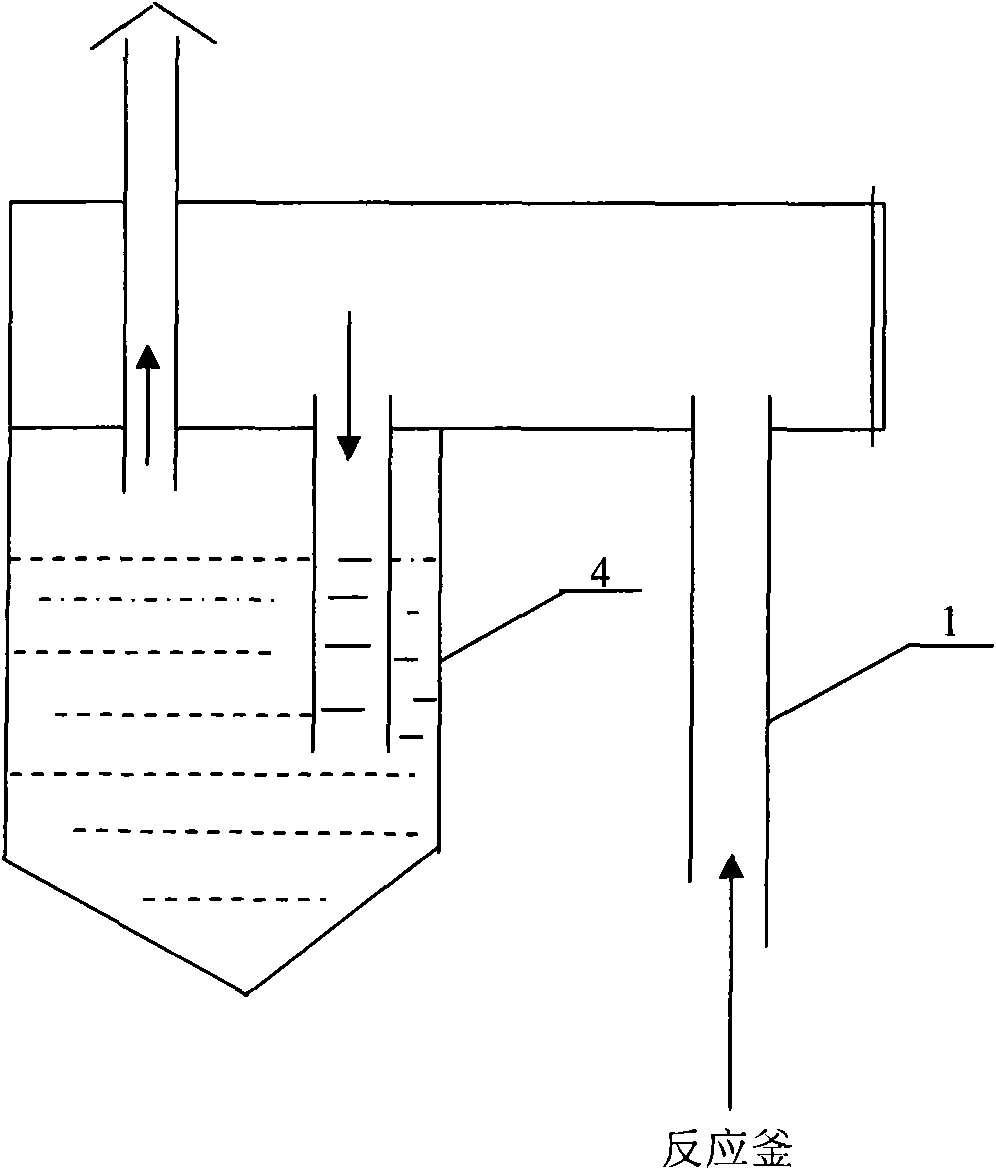
Preparation method of phosphorus pentasulfide
ActiveCN101844755AReduce generationReduce dosagePhosphorus sulfur/selenium/tellurium compoundsDistillationPositive pressure
The invention relates to a preparation method of phosphorus pentasulfide by a liquid phase method, and the preparation method comprises the following steps: 1) molten sulphur and molten yellow phosphorus are continuously conveyed into a reaction kettle according to stoichiometric proportion, liquid phase reaction is carried out under stirring, the constant reaction temperature in the reaction kettle is 410-430 DEG C, the constant reaction pressure is 17-21 millibars (gauge pressure, micro positive pressure), and the pressure is generated by introduced nitrogen; and 2) after the reaction, the materials are continuously pumped into a distillation kettle, reduced pressure distillation is carried out in the distillation kettle by utilizing reaction heat, the constant distillation temperature is 390-395 DEG C, and the constant distillation vacuum degree is 80-95 millibars (absolute pressure). By using the preparation method, purer products can be obtained, and the energy consumption is reduced.
Owner:辽宁瑞兴化学有限公司
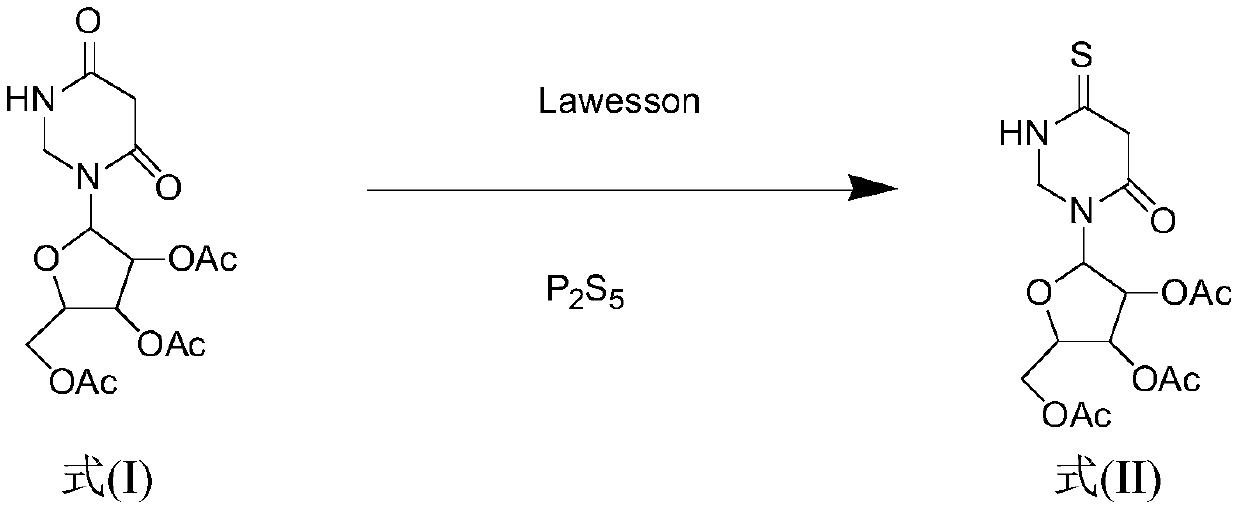
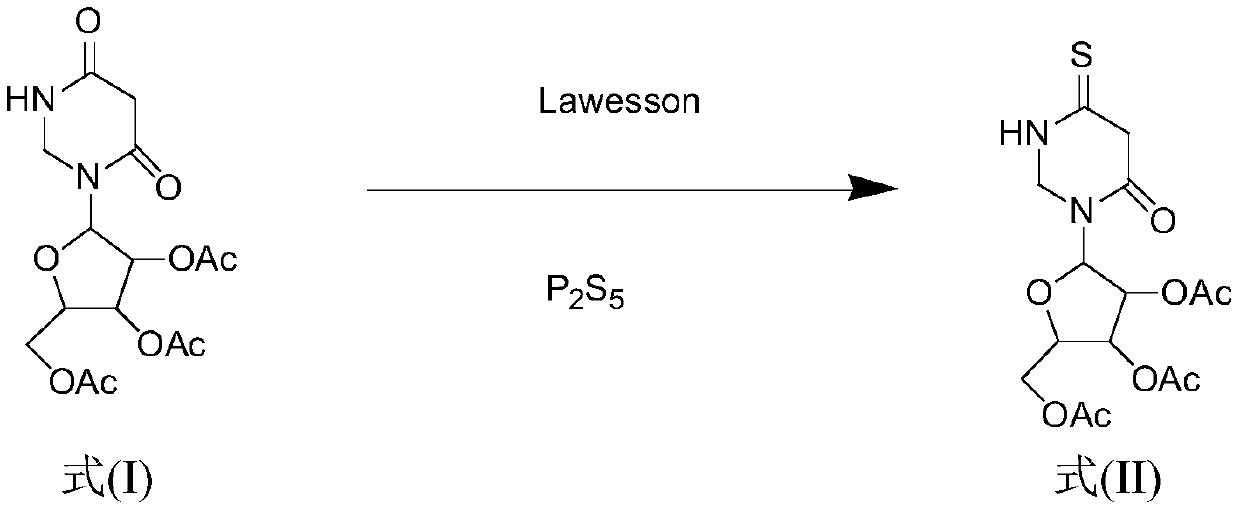
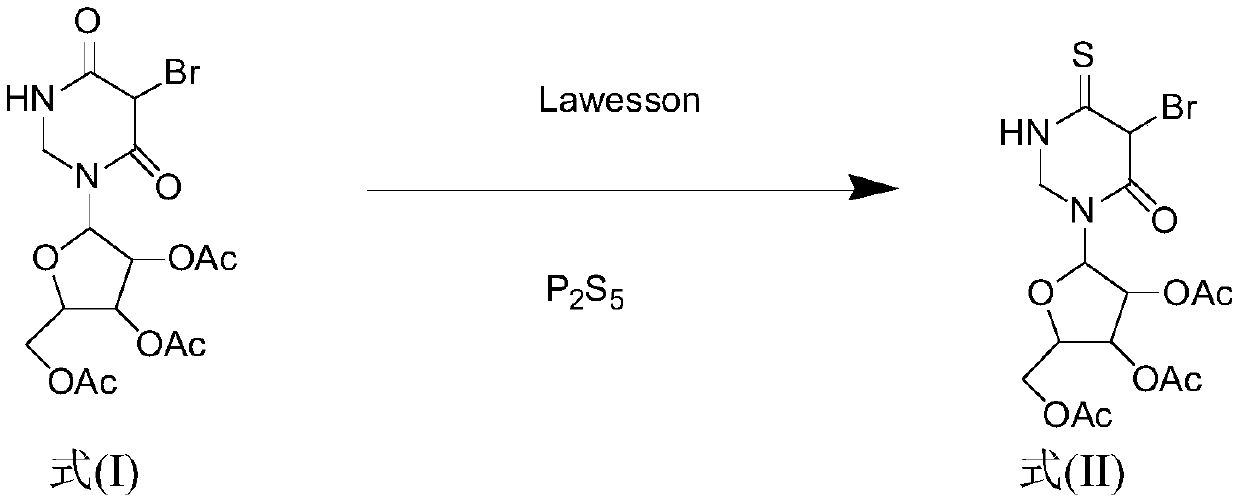
Method for synthesizing 4-S-2', 3', 5'-O-triacetyluridine
ActiveCN110204584AIncrease costShort reaction timeSugar derivativesSugar derivatives preparationChemical synthesisVulcanization
The invention belongs to the field of chemical synthesis, and discloses a method for synthesizing 4-S-2', 3', 5'-O-triacetyluridine. According to the synthesis method, a compound 2', 3', 5'-O-triacetyluridine is used as a raw material, phosphorus pentasulfide and a Lawesson's reagent are combined to be used as a vulcanizing agent, 1,4-dioxane is used as a solvent for chemical reaction, and finally4-S-2', 3', 5'-O-triacetyluridine is prepared. By the method for synthesizing 4-S-2', 3', 5'-O-triacetyluridine provided by the invention, the reaction time is shortened, and moreover, the vulcanization efficiency is greatly improved. According to the synthesis method provided by the invention, the cost is reduced, the reaction aftertreatment is simple and convenient, and meanwhile, the yield ofa reaction thionucleoside compound is improved.
Owner:DALIAN UNIV
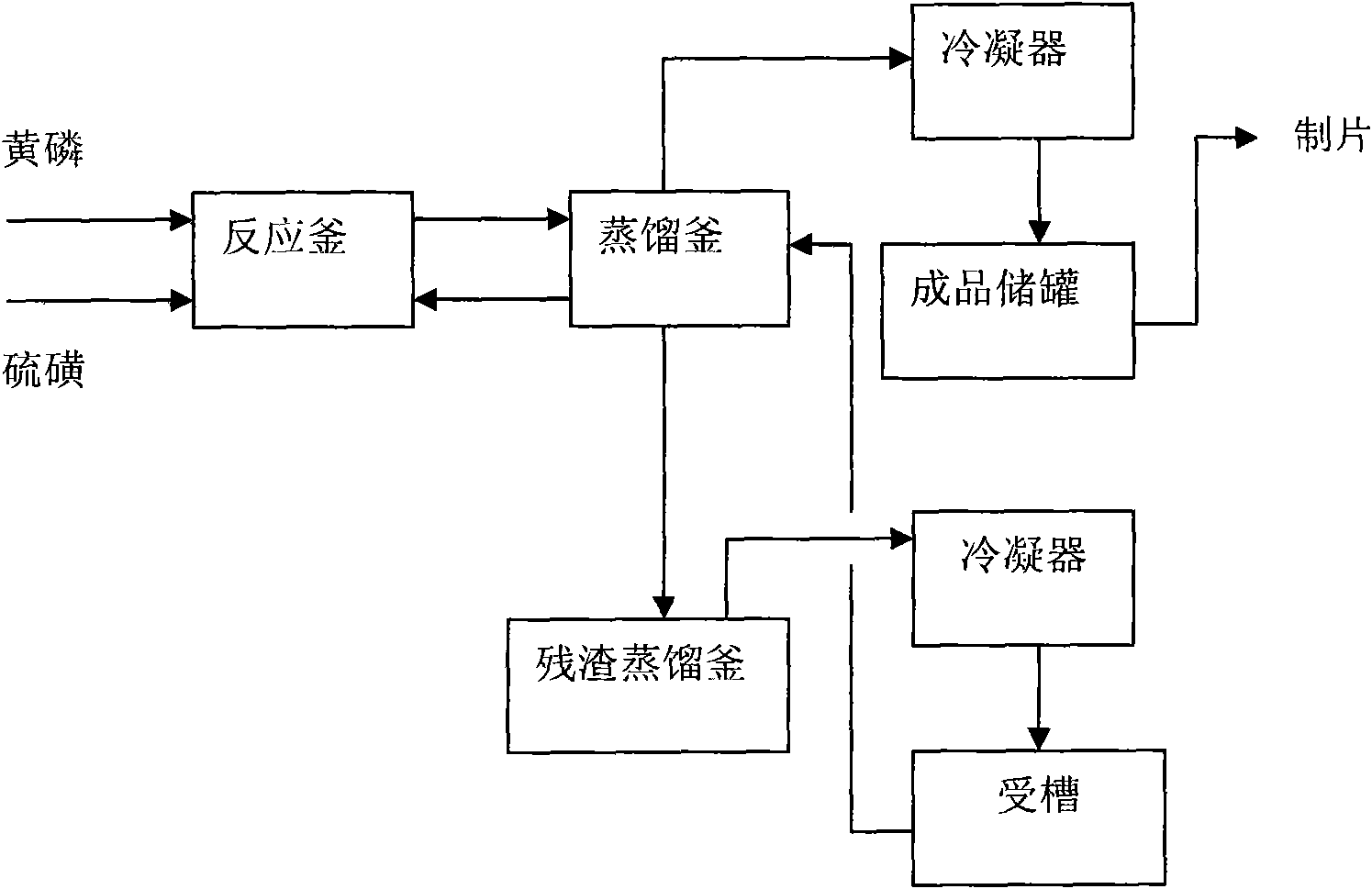
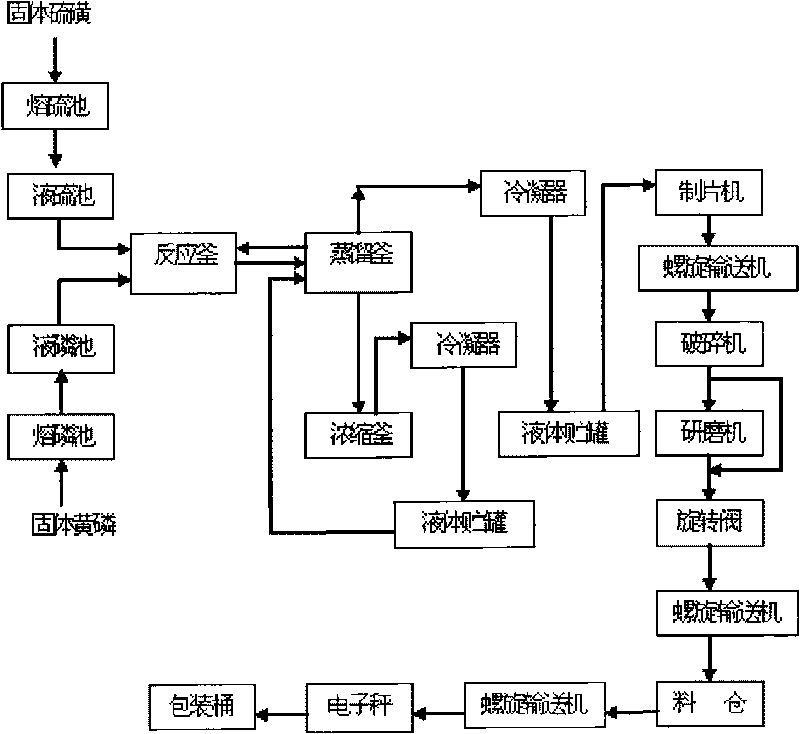
Production technology for synthesizing high-purity phosphorus pentasulfide by liquid-phase continuous process
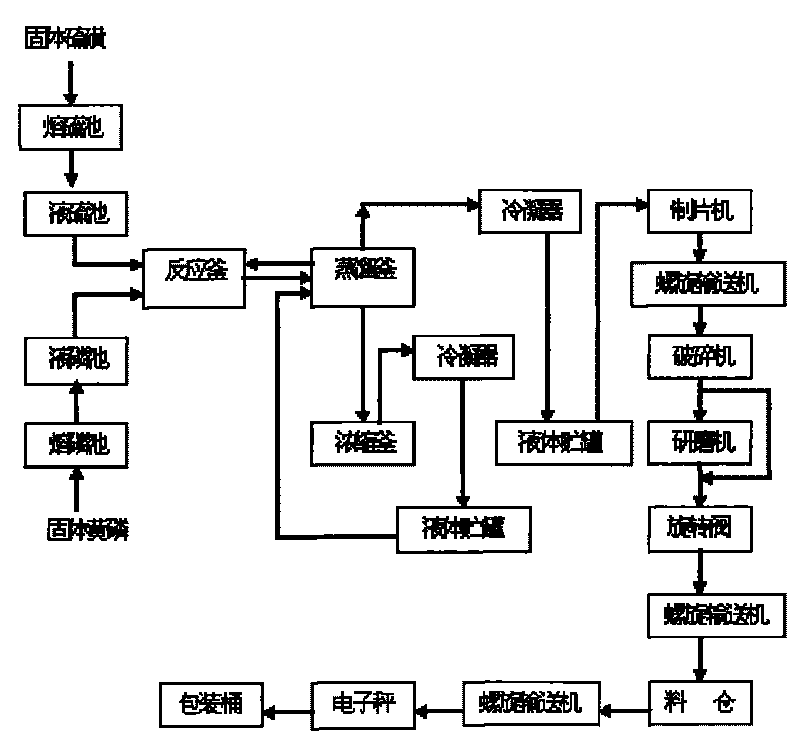
InactiveCN101712464AQuality improvementOperational securityPhosphorus sulfur/selenium/tellurium compoundsChemical industryDistillation
The invention relates to production technology for synthesizing high-purity phosphorus pentasulfide by liquid-phase continuous process, which belongs to the field of preparation of fine phosphorus chemical products in chemical industry. The production technology for synthesizing high-purity phosphorus pentasulfide by liquid-phase continuous process comprises the following steps: after melting and precipitating solid yellow phosphorus and solid sulphur, continuously conveying the melted and precipitated solid yellow phosphorus and solid sulphur into a reaction kettle with a pump in the ratio of 1 to 2.6, stirring under the condition of 400 DEG C and 0.15 MPa to perform liquid phase reaction, continuously pumping reacted raw materials into a distillation still, performing negative pressure distillation in the distillation still by using reaction heat, condensing formed phosphorus pentasulfide steam by reduced pressure distillation into liquid phosphorus pentasulfide by using a condenser, concentrating the liquid phosphorus pentasulfide into a phosphorus pentasulfide product storage tank, conveying the product into a pelleter with the pump, processing the product into sheet, crushing, grinding and finally packaging into finished products. The production technology for synthesizing high-purity phosphorus pentasulfide by liquid-phase continuous process has the advantages of simple operation, stability and safety, low energy consumption, high degree of automation and excellent product quality.
Owner:LIAONING PROVINCE PETROLEUM CHEM IND PLANNING&DESIGNING INST
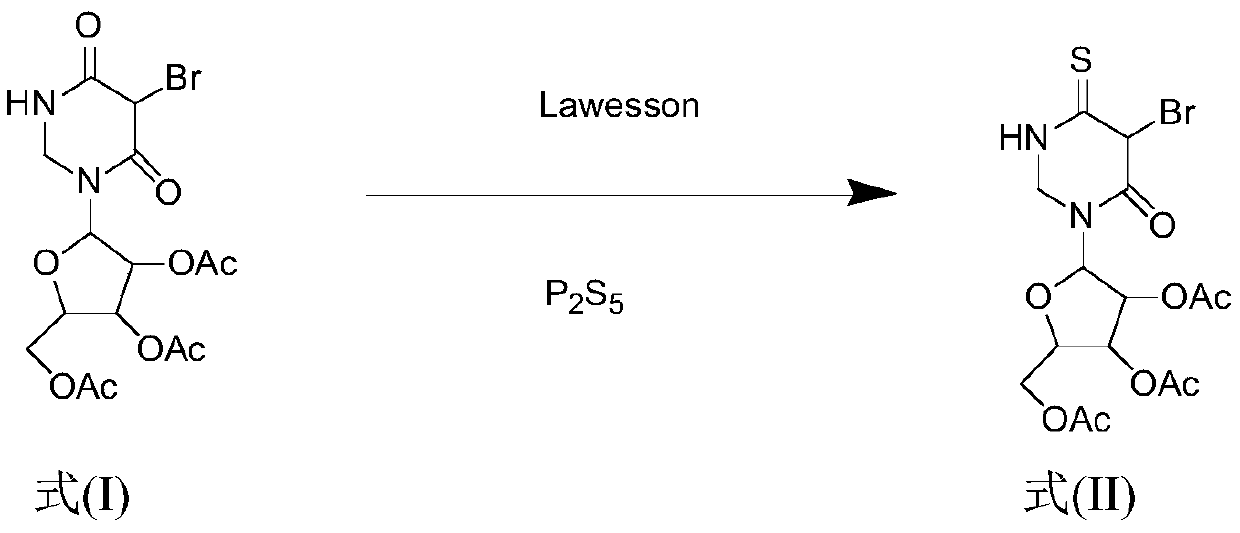
Synthesis method for 4-S-5-Br-2',3',5'-O-triacetyluridine
ActiveCN110105416AShort reaction timeImprove vulcanization efficiencySugar derivativesSugar derivatives preparationChemical synthesisChemical reaction
The invention belongs to the field of chemical synthesis and discloses a synthesis method for 4-S-5-Br-2',3',5'-O-triacetyluridine. The synthesis method comprises the steps that a compound 5-Br-2',3',5'-O-triacetyluridine serves as a raw material, phosphorus pentasulfide and a Lawesson reagent are combined to serve as a vulcanization reagent, 1,4-dioxane serves as a solvent, and a chemical reaction is carried out to finally prepare the 4-S-5-Br-2',3',5'-O-triacetyluridine. The synthesis method for the 4-S-5-Br-2',3',5'-O-triacetyluridine has the advantages that the reaction time is shortened,and the vulcanization efficiency is greatly improved. The synthesis method reduces the cost, the treatment after the reaction is simple and convenient, and meanwhile the yield of the thionucleoside compound after the reaction is increased.
Owner:DALIAN UNIV
Naphthenic acid corrosion inhibition using new synergetic combination of phosphorus compounds
ActiveUS9228142B2Low acid valueDistillation corrosion inhibitionOther chemical processesPhosphorous acidPhosphoric Acid Esters
The present invention relates to the field of processing hydrocarbons which causes corrosion in the metal surfaces of processing units. The invention addresses the technical problem of high temperature naphthenic acid corrosion and sulphur corrosion and provides a solution to inhibit these types of corrosion. The three combination compositions are formed by three mixtures separately, with one mixture obtained by mixing compound A, which is obtained by reacting high reactive polyisobutylene (HRPIB) with phosphorous pentasulphide in presence of catalytic amount of sulphur with compound B such as trialkyl phosphate and second mixture obtained by mixing compound A with compound C such as phosphite like di-isodecyl phenyl phosphite, and third mixture obtained by mixing compound A with compound D such as a phosphonate, wherein each of these three mixtures independently provide high corrosion inhibition efficiency in case of high temperature naphthenic acid corrosion inhibition and sulphur corrosion inhibition. The invention is useful in all hydrocarbon processing units, such as, refineries, distillation columns and other petrochemical industries.
Owner:DORF KETAL CHEM (I) PTE LTD
Tricyclic furo[2, 3-d]pyrimidone compounds and application thereof
The invention relates to compounds. Cyanoethyl acetate and chloroacetone with different substituent groups are used as initial raw materials, a cyanoethyl ester compound is generated under the action of sodium ethoxide, then furan compounds are generated through intramolecular ring closing under the action of trifluoroacetic acid, the furan compounds respectively react with 2-pyrrolidone, valerolactam and caprolactam under the action of phosphorus oxychloride to form a ring, so as to obtain furo[2, 3-d]pyrimidinone compounds, and then oxygen-sulfur exchange is performed under the action of phosphorus pentasulfide, so as to obtain the furo[2, 3-d]pyrimidinethione compounds. The 54 kinds of obtained tricyclic furo[2, 3-d]pyrimidone compounds are used as positive control of DOX, the inhibitory activity of Hela cervical cancer cells, MCF-7 breast cancer cells and A549 lung cancer cells is achieved, and results show that the compounds C11 and C17 have the inhibitory activity of the Hela cervical cancer cells; the compounds D5 and D7 have inhibitory activity on A549 lung cancer cells; and the compound C14 has inhibitory activity on MCF-7 breast cancer cells.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
A kind of boron magnetic rare earth lubricating oil additive and preparation method thereof
ActiveCN104031721BEnergy saving and environmental protectionObvious fuel-saving effectAdditivesRare-earth elementPhosphate
The invention discloses a boron magnetic rare earth lubricating oil additive and a preparation method thereof. The additive is prepared from mixed sulfur amine phosphate, lanthanum oxide, rare earth carbonate, boric acid and calcium sulfonate, wherein the weight ratio of the mixed sulfur amine phosphate to the lanthanum oxide, rare earth carbonate, boric acid and calcium sulfonate is 100:(4.5-5):(4.5-5):(12-17):(12-17); the mixed sulfur amine phosphate is prepared from ricinoleic acid and triethanolamine reacting with phosphorus pentasulfide and octanol. The additive disclosed by the invention is an oil-soluble boron magnetic rare earth complex which is used as a friction improver, an antiwear agent and a cleaning agent for the lubricating oil and particularly the lubricating oil for the engine of an internal combustion engine, and the energy saving effect is remarkable; with the rare earth element, low sulfur and low phosphorous, the additive also has the advantages of energy conservation and environmental protection.
Owner:纳米克斯(辽宁)能源有限公司
Process for producing power shaped phosphorus pentasulfide
InactiveCN101402451AAvoid it happening againAvoid Burning HazardsPhosphorus sulfur/selenium/tellurium compoundsChemical industryPhysical chemistry
The invention relates to a method for preparing powdery phosphorus pentasulfide, belongs to the field of preparing a fine phosphorus chemical product in chemical industry, and in particular relates to a method for preparing powdery phosphorus pentasulfide by sheet phosphorus pentasulfide. The method comprises the following steps: the sheet phosphorus pentasulfide is put into grinding equipment and is ground under the protection of an inert solvent; and the phosphorus pentasulfide and the inert solvent are filtered and separated to obtain a powdery phosphorus pentasulfide product. The process of the method has the characteristics of simple processing, short processing time, easy and safe operation, high product yield and the like; and the yield of the powdery product can reach 99 percent.
Owner:KUNMING UNIV OF SCI & TECH
All-solid sodium secondary battery electrolyte and preparation method and application thereof
The invention provides an all-solid sodium secondary battery electrolyte and a method and application thereof. The all-solid sodium secondary battery electrolyte is prepared from a first component anda second component, wherein the first component is selected from Na2S (sodium sulfide) and / or Na2Se (disodium selenide); the second component is selected from one or multiple of Sb2S3 (antimony trisulfide), Sb2Se3 (antimony triselenide), Sb2S5 (antimony pentasulfide), Sb2Se5 (antimony pentaselenide), P2S5 (phosphorus pentasulfide), P2S3 (phosphorus trisulfide), As2S3 (arsenic trisulfide), As2S5 (arsenic pentasulfide), S (sulfur), Se (selenide), SnS2 (selenide disulfide), SiS2 (silicon disulfide), SnS (selenide sulfur), GeS2 (germanium disulfide) and GeS (germanium sulfide); the granularity ofthe all-solid sodium secondary battery electrolyte is 10nm to 10mum; the raw materials also comprise doping matters, and the doping matters are selected from one or multiple of a third component, oxide and halide; the third component and the second component are different. The all-solid sodium secondary battery electrolyte has the advantages that the sodium ion conductivity is higher in an all-solid sodium secondary battery; the electrochemical window is broader; the all-solid sodium secondary battery prepared by the electrolyte has good rate and cycle properties.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
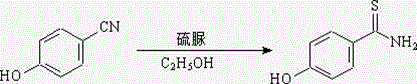
Synthesis method of 4-Hydroxythiobenzamide
The invention discloses a synthesis method of 4-Hydroxythiobenzamide. The method comprises the following steps: mixing methyl p-hydroxybenzoate with stronger ammonia water, carrying out high-temperature reaction inside a reaction kettle, and then carrying out vacuum concentration on the mixture, so as to obtain p-hydroxybenzene formamide; and refluxing p-hydroxybenzene formamide and phosphorus pentasulfide in a solvent, and obtaining the 4-Hydroxythiobenzamide. The method disclosed by the invention is mild in reaction, safe to operate, low in raw material price, easy to obtain, high in product purity and yield, and low in handling expense, a novel method is provided for obtaining the 4-Hydroxythiobenzamide, and the method disclosed by the invention is applicable to industrial mass production.
Owner:UNIV OF JINAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
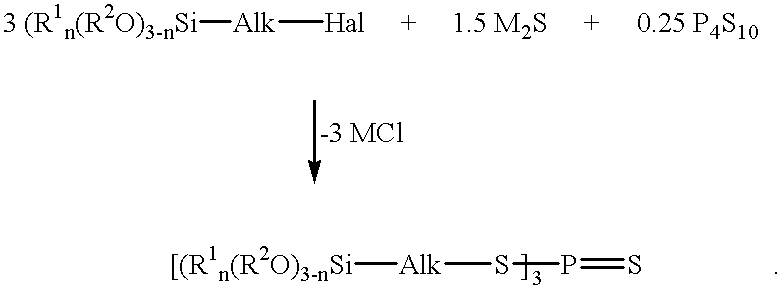
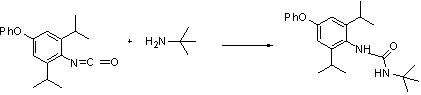

![Tricyclic furo[2, 3-d]pyrimidone compounds and application thereof Tricyclic furo[2, 3-d]pyrimidone compounds and application thereof](https://images-eureka.patsnap.com/patent_img/b8bdec14-229b-4335-aaf5-8d8349d7fe39/FDA0003018062530000011.png)
![Tricyclic furo[2, 3-d]pyrimidone compounds and application thereof Tricyclic furo[2, 3-d]pyrimidone compounds and application thereof](https://images-eureka.patsnap.com/patent_img/b8bdec14-229b-4335-aaf5-8d8349d7fe39/BDA0003018062540000071.png)
![Tricyclic furo[2, 3-d]pyrimidone compounds and application thereof Tricyclic furo[2, 3-d]pyrimidone compounds and application thereof](https://images-eureka.patsnap.com/patent_img/b8bdec14-229b-4335-aaf5-8d8349d7fe39/BDA0003018062540000281.png)