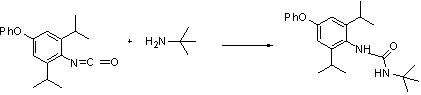Synthesis process for diafenthiuron as thiourea insecticide and acaricide
A technology for the synthesis of diafenthiuron, an acaricide diafenthiuron, is applied in the field of synthesis technology of diafenthiuron, an insecticide and acaricide based on thiourea, which can solve the problems of easy coking, difficulty in heating, high energy consumption, etc. The effect of mild reaction conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
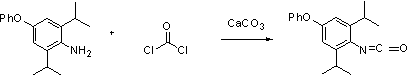
[0027] (1) Take a 250 mL dry round bottom flask, add 7.65 g trimer phosgene and 13.7 g calcium carbonate to a mixed system consisting of 60 mL methyl chloride and 35 mL water under stirring. Then 17.4g of 2,6-diisopropyl-4-phenoxyaniline was slowly added dropwise to the above-mentioned mixed system, and the temperature of the system during the whole process was kept within the range of 0-5 degrees. The system was heated to reflux for 2 hours. After the reaction, the system was cooled and filtered, the organic phase was washed twice with 50 mL of water, and anhydrous Na 2 SO 4 After drying and concentrating, the oily crude product obtained will be directly used in the next reaction.
[0028] (2) Take a 50 mL dry round bottom flask, add 18.54 g of N-(2,6-diisopropyl-4-phenoxyphenyl) isocyanate and dilute with 10 mL of toluene, then add 13.8 g tert-butylamine. The reaction mixture was stirred at room temperature for 12 hours, and then the reaction system was concentrated and re...
Embodiment 2
[0031] (1) Take a 500 mL dry round bottom flask, add 15.3 g trimer phosgene and 27.4 g calcium carbonate to a mixed system consisting of 120 mL methyl chloride and 70 mL water under stirring. Then 34.8g of 2,6-diisopropyl-4-phenoxyaniline was slowly added dropwise to the above-mentioned mixed system, and the temperature of the system during the whole process was kept within the range of 0-5 degrees. The system is heated to reflux for 1-3 hours. After the reaction, the system was cooled and filtered, the organic phase was washed twice with 100 mL of water, and anhydrous Na 2 SO 4 After drying and concentrating, the oily crude product obtained will be directly used in the next reaction.
[0032] (2) Take a 100 mL dry round bottom flask, add 37.08g of N-(2,6-diisopropyl-4-phenoxyphenyl) isocyanate and dilute with 20mL of toluene, then add 27.6 g tert-butylamine. The reaction mixture was stirred at room temperature for 10-13 hours, and then the reaction system was concentrated an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com