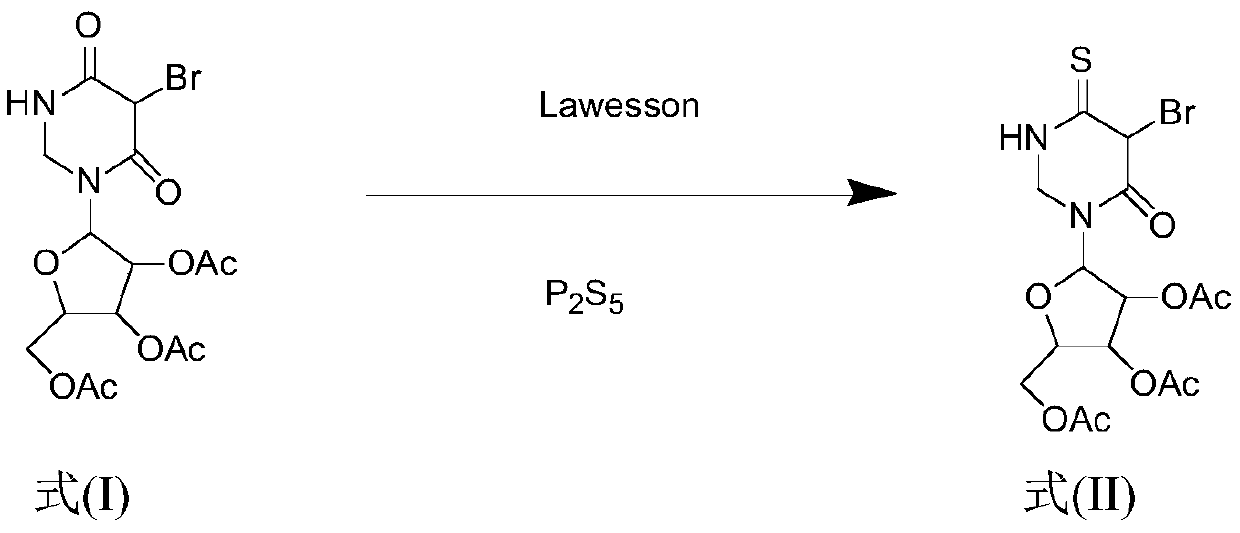
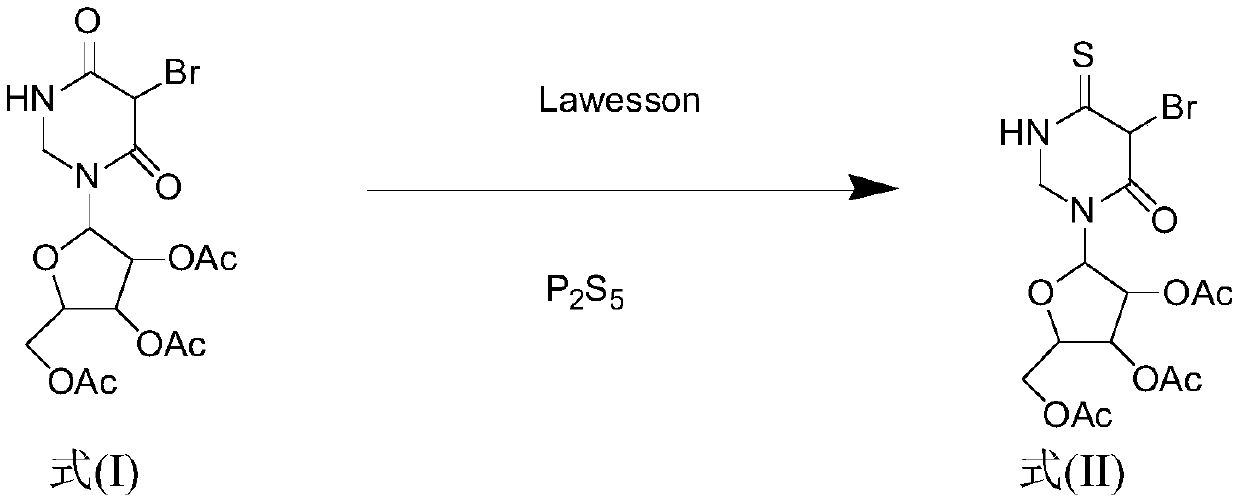
Synthesis method for 4-S-5-Br-2',3',5'-O-triacetyluridine
A 4-s-5-br-2, triacetyl uridine technology, applied in chemical instruments and methods, bulk chemical production, sugar derivatives, etc. Long time and other problems, to achieve the effect of simple and convenient post-reaction treatment, improved vulcanization efficiency, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Dissolve 5-Br-2',3',5'-O-triacetyluridine (1.00g, 2.20mmol) in 50mL of 1,4-dioxane, stir at room temperature to fully dissolve, Then adding different molar ratios of P 2 S 5 (0.391g, 1.76mmol) and Lawesson (0.356g, 0.88mmol) were used as vulcanizing agents, the reaction temperature was 95°C, the reaction was monitored by TLC, and the raw material point disappeared after 1.3h, which proved that the reaction was complete, and it was evaporated under reduced pressure at 55°C Solvent, the crude product is subjected to column separation (PE:EA=6:1; 4:1), after separation, it is mixed with absolute ethanol and petroleum ether for recrystallization, and the solid 4-S-5-Br-2' is obtained after suction filtration and drying ,3',5'-O-triacetyluridine 0.90g, the yield was 88%.
Embodiment 2
[0045] Dissolve 5-Br-2',3',5'-O-triacetyluridine (1.00g, 2.20mmol) in 50mL of 1,4-dioxane, stir at room temperature to fully dissolve, Then adding different molar ratios of P 2 S 5 (0.439g, 1.98mmol) and Lawesson (0.267g, 0.66mmol) were used as vulcanizing agents, the reaction temperature was 95°C, the reaction was monitored by TLC, and the raw material point disappeared after 1.3h, which proved that the reaction was complete, and it was evaporated under reduced pressure at 55°C Solvent, the crude product is subjected to column separation (PE:EA=6:1; 4:1), after separation, it is mixed with absolute ethanol and petroleum ether for recrystallization, and the solid 4-S-5-Br-2' is obtained after suction filtration and drying ,3',5'-O-triacetyluridine 0.92g, the yield was 90%.
Embodiment 3
[0047] Dissolve 5-Br-2',3',5'-O-triacetyluridine (1.00g, 2.20mmol) in 50mL of 1,4-dioxane, stir at room temperature to fully dissolve, Then adding different molar ratios of P 2 S 5 (0.488g, 2.2mmol) and Lawesson (0.178g, 0.44mmol) were used as vulcanizing agents, the reaction temperature was 95°C, the reaction was monitored by TLC, the raw material point disappeared after 1.3h, which proved that the reaction was complete, and it was evaporated under reduced pressure at 55°C Solvent, the crude product is subjected to column separation (PE:EA=6:1; 4:1), after separation, it is mixed with absolute ethanol and petroleum ether for recrystallization, and the solid 4-S-5-Br-2' is obtained after suction filtration and drying ,3',5'-O-triacetyluridine 0.93g, the yield was 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com