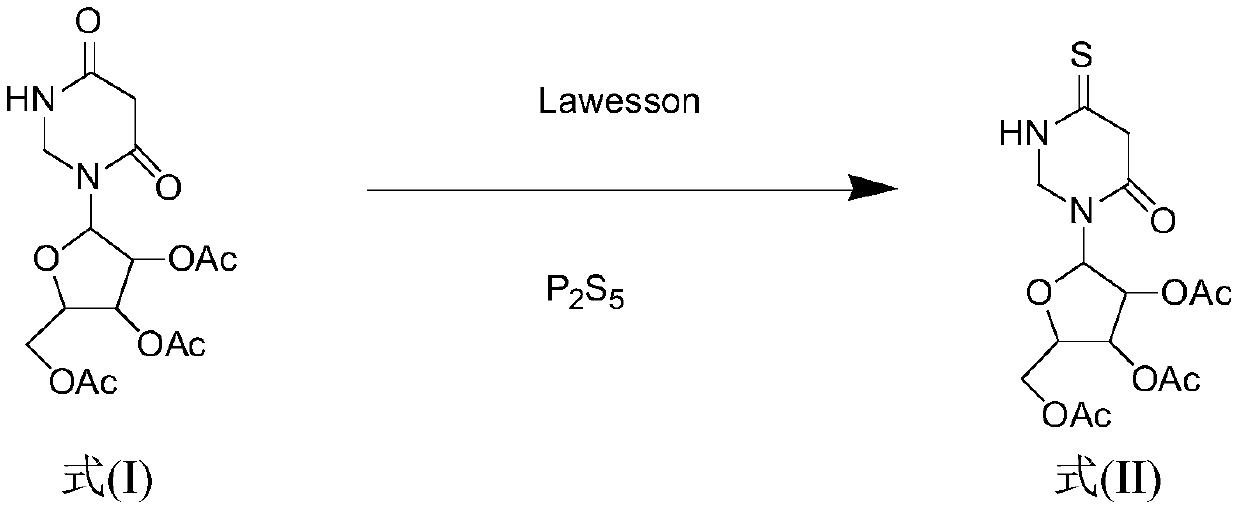
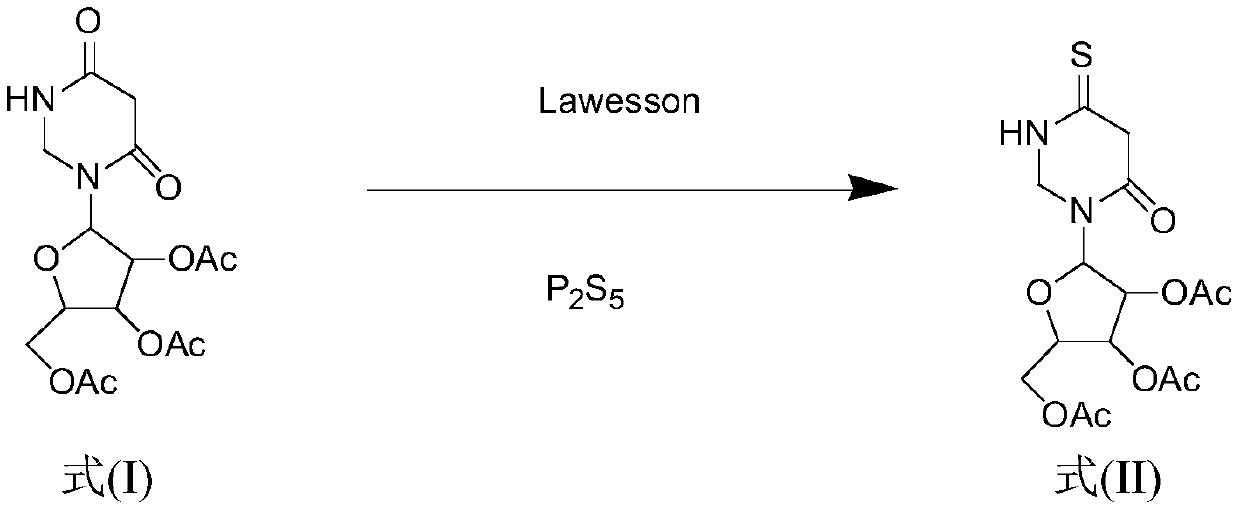
Method for synthesizing 4-S-2', 3', 5'-O-triacetyluridine
A technology of triacetyl uridine and 4-S-2, which is applied in chemical instruments and methods, bulk chemical production, sugar derivatives, etc., and can solve the problems of phosphorus pentasulfide being highly toxic, long reaction time, perishable price, etc. , to achieve the effect of simple and convenient post-reaction treatment, easy operation, and improved vulcanization efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Dissolve 2',3',5'-O-triacetyluridine (1.00g, 2.70mmol) in 50mL of 1,4-dioxane, stir to fully dissolve, then add different molar ratios P 2 S 5 (0.479g, 2.16mmol) and Lawesson (0.436g, 1.08mmol) were used as vulcanizing agents, heated to 95°C for reaction, and the reaction was monitored by TLC. The disappearance of the raw material point in 20min proved that the reaction was complete, and the solvent was evaporated under reduced pressure at 55°C , the crude product is subjected to column separation (PE:EA=6:1; 4:1), after separation, it is recrystallized by mixing absolute ethanol and petroleum ether, and the solid 4-S-2',3',5 '-O-triacetyluridine 0.938g, the yield is 90%.
Embodiment 2
[0041] Dissolve 2',3',5'-O-triacetyluridine (1.00g, 2.70mmol) in 50mL of 1,4-dioxane, stir to fully dissolve, then add different molar ratios P 2 S 5 (0.539g, 2.43mmol) and Lawesson (0.327g, 0.810mmol) were used as vulcanizing agents, heated to 95°C to react, and the reaction was monitored by TLC. The raw material point disappeared after 20min, which proved that the reaction was complete, and the solvent was evaporated under reduced pressure at 55°C , the crude product is subjected to column separation (PE:EA=6:1; 4:1), after separation, it is recrystallized by mixing absolute ethanol and petroleum ether, and the solid 4-S-2',3',5 '-O-triacetyluridine 0.969g, the yield is 93%.
Embodiment 3
[0043] Dissolve 2',3',5'-O-triacetyluridine (1.00g, 2.70mmol) in 50mL of 1,4-dioxane, stir to fully dissolve, then add different molar ratios P 2 S 5 (0.599g, 2.70mmol) and Lawesson (0.218g, 0.540mmol) were used as vulcanizing agents, heated to 95°C to react, and the reaction was monitored by TLC. The raw material point disappeared after 20min, which proved that the reaction was complete, and the solvent was evaporated under reduced pressure at 55°C , the crude product is subjected to column separation (PE:EA=6:1; 4:1), after separation, it is recrystallized by mixing absolute ethanol and petroleum ether, and the solid 4-S-2',3',5 '-O-triacetyluridine 1.001g, the yield is 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com