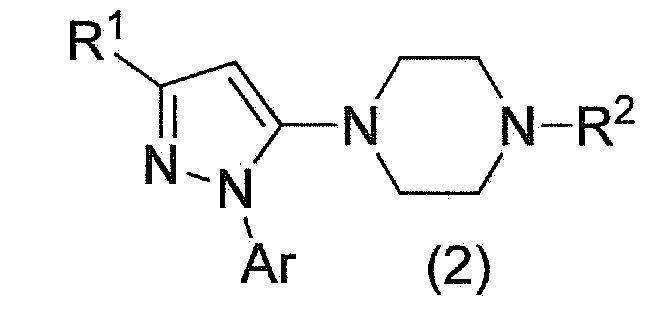
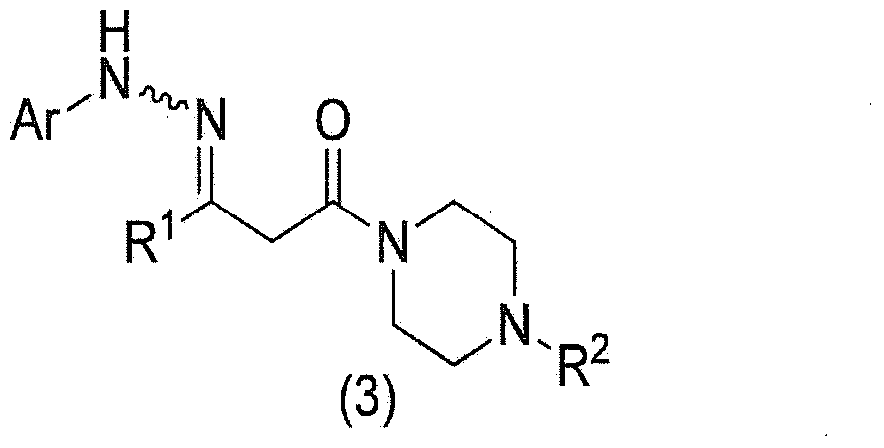
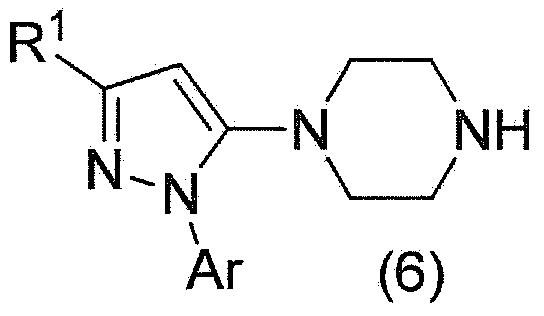
Method for manufacturing pyrazole derivative
A compound and production formula technology, applied in the field of piperazinyl pyrazole compounds, achieves the effect of superior storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] Preparation of 5-(4-tert-butoxycarbonylpiperazin-1-yl)-3-methyl-1-phenylpyrazole
[0124]
[0125] To a solution of 4-tert-butoxycarbonyl-1-[3-(2-phenylhydrazone)butyryl]piperazine (compound 3b) (721 mg) and sodium carbonate (254 mg) in THF (10 mL) was added phosphorus pentasulfide (267 mg), and the mixture was heated under reflux for 30 minutes. The solvent was evaporated under reduced pressure, diethyl ether (30 mL) was added to the residue, and the mixture was filtered. Water (20 mL) was added to the filtrate, and the mixture was separated. The obtained organic layer was washed with saturated brine and dried over anhydrous magnesium sulfate and filtered. The filtrate was concentrated under reduced pressure to produce 5-(4-tert-butoxycarbonylpiperazin-1-yl)-3-methyl-1-phenylpyrazole (compound 2b) (580 mg, yield 85%).
[0126] 1H-NMR (500MHz, DMSO-d 6 )Δ1.39(9H,s), 2.15(3H,s), 2.73(4H,m), 3.37(4H,m), 5.83(1H,s), 7.28(1H,t,J=7.4Hz), 7.46 (2H, t, J=7.4Hz), 7.76 (2H, d, J=...
Embodiment 2
[0128] Preparation of 3-methyl-1-phenyl-5-(1-piperazinyl)pyrazole acetate
[0129]
[0130] Dissolve 1-ethoxycarbonylpiperazine (Compound 4a) (37.5 kg) in THF (550 L). The solution was cooled, diketene (19.9 kg) was added dropwise at 0-7°C, and the mixture was stirred at 1°C-5°C for 1.5 hours. A solution of phenylhydrazine (25.6 kg) in THF (10 L) was added dropwise to the reaction mixture, and the mixture was stirred at 20°C-26°C for 7 hours. The reaction mixture was concentrated under reduced pressure until the residual amount became 100 L, and THF (260 L) was added to the remaining reaction mixture. To this reaction mixture were added sodium carbonate (25.1 kg) and phosphorus pentasulfide (26.3 kg), and the mixture was stirred at 36° C.-44° C. for 1.5 hours and at 45° C.-52° C. for 3 hours. The reaction mixture was cooled, phosphorus pentasulfide (5.3 kg) was added, and the mixture was stirred at 45°C-51°C for 1 hour and cooled. To this reaction mixture were added toluene (3...
Embodiment 3
[0133] Synthesis of 4-tert-Butoxycarbonyl-1-[3-(2-phenylhydrazone)butyryl]piperazine
[0134]
[0135] To a solution of 1-tert-butoxycarbonylpiperazine (compound 4b) (80 g) in N,N-dimethylformamide (256 mL) under ice cooling was added diketene (34.7 mL) dropwise, and the mixture Stir at room temperature for 1 hour. To this reaction mixture was added dropwise a mixed solution of phenylhydrazine (48.7 g) in ethanol (192 mL) and water (192 mL) under water cooling, and the mixture was stirred at room temperature for 1 hour. To this reaction mixture was added water (320 mL) dropwise, and the mixture was stirred at room temperature for 1 hour. The precipitate was collected by filtration, washed with a 1:1 mixture of methanol and water, and dried under vacuum to produce 4-tert-butoxycarbonyl-1-[3-(2-phenylhydrazone)butyryl]piperazine ( Compound 3b) (149 g, yield 97%, regioisomer ratio 1:1).
[0136] 1H-NMR (300MHz, CDCl 3 )Δ1.46, 1.48 (9H, s), 1.95 (1.5H, s), 2.11 (1.5H, s), 2.88 (0.5H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com