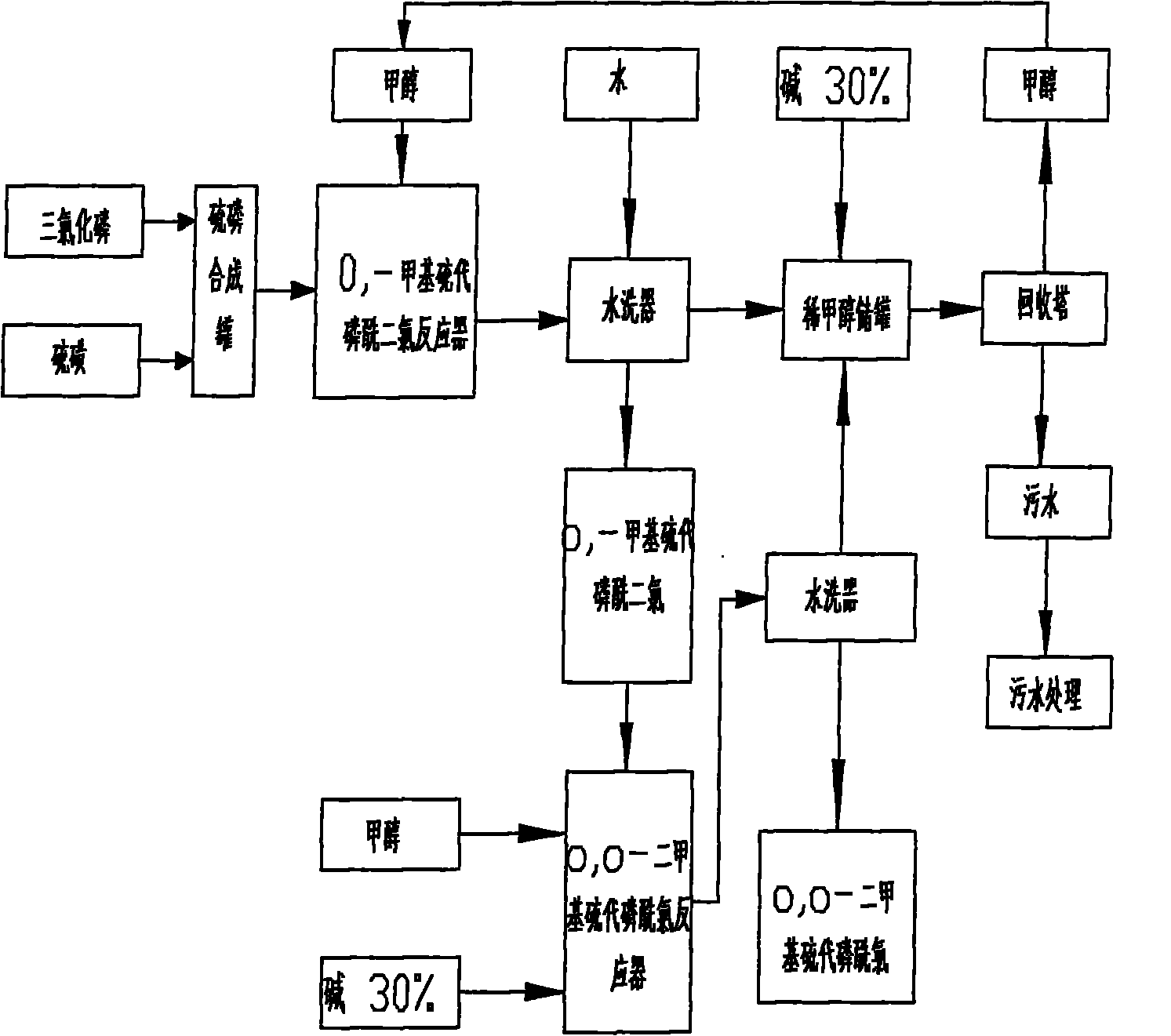
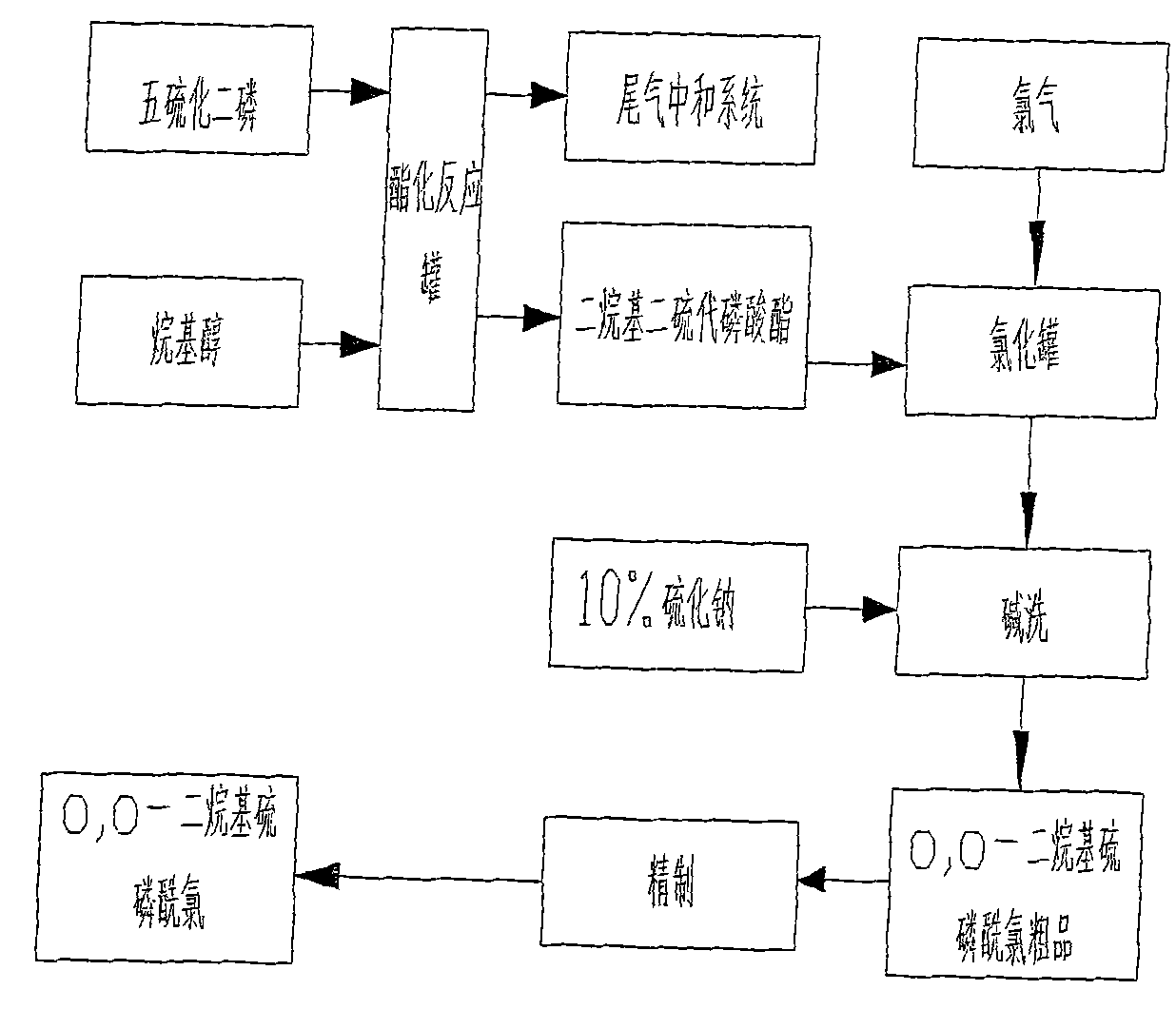
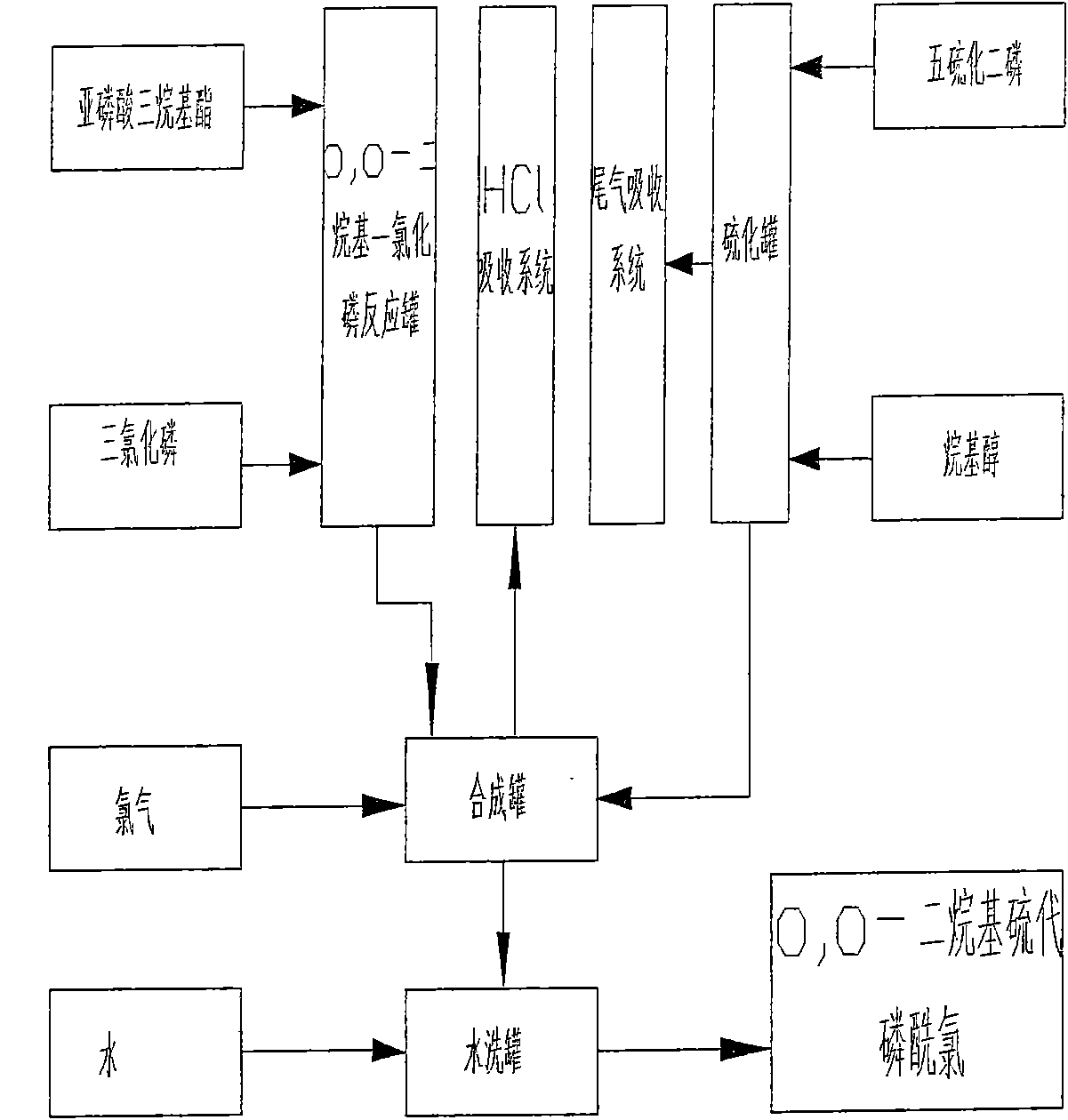
Production process of O, O-dialkyl thiophosphoryl chloride
A technology for the production of alkyl thiophosphoryl and chlorine, which is applied in chemical instruments and methods, organic chemistry, compounds of Group 5/15 elements of the periodic table, etc. and other problems, to achieve the effect of low cooling consumption, low energy consumption and high steam consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0048] Example 1: The raw materials used for the synthesis of this product are trimethyl phosphite, phosphorus pentasulfide, methanol, chlorine, and phosphorus trichloride. Trimethyl phosphite and phosphorus trichloride are reacted in the reactor to generate O, O-dimethyl phosphorus monochloride is added to the intermediate dichlorodisulfide after the reaction of phosphorus pentasulfide and methanol and chlorination, that is, O , O-dimethylphosphoryl thiochloride.
[0049]Phosphorus trichloride input amount 151Kg in the present embodiment, trimethyl phosphite 272Kg, phosphorus pentasulfide 364Kg, methyl alcohol 211Kg, chlorine 233Kg; The product O of producing, the weight of O-dimethylphosphoryl thiochloride is 1000Kg. The total yield of phosphorus trichloride is 94%, and its content is 99.1%.
example 2
[0050] Example 2: The raw materials used for the synthesis of this product are phosphorus trichloride, triethyl phosphite, dehydrated alcohol, phosphorus pentasulfide, and chlorine. The product O produced by the reaction of triethyl phosphite and phosphorus trichloride, O-diethyl phosphorus monochloride is added to the intermediate dichlorodisulfide after the reaction of phosphorus pentasulfide and absolute ethanol and chlorination, to obtain O, O-diethylthiophosphoryl chloride.
[0051] Phosphorus trichloride input amount 128Kg among the present example, triethyl phosphite 308Kg, phosphorus pentasulfide 309Kg, dehydrated alcohol 257Kg, chlorine 198Kg; The product O of producing, the weight of O-diethylphosphoryl thiochloride is 1000Kg. The total yield of phosphorus trichloride is 94%, the total yield of phosphorus pentasulfide is 94%, and its content is 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com