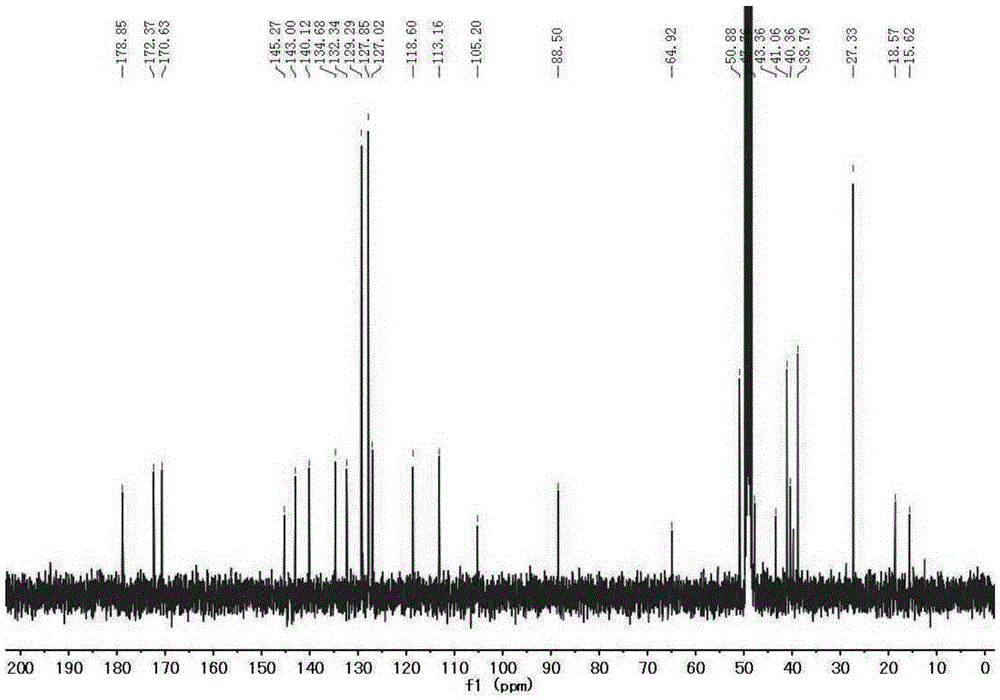
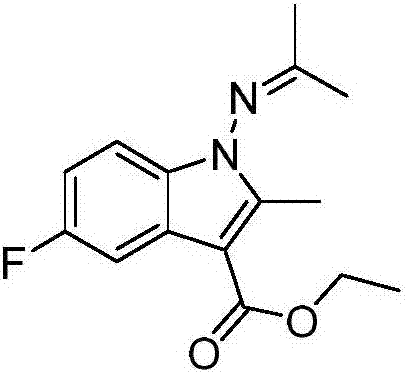
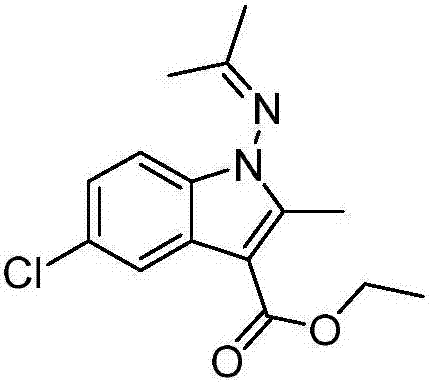
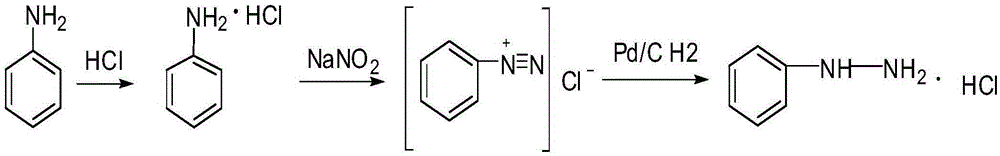
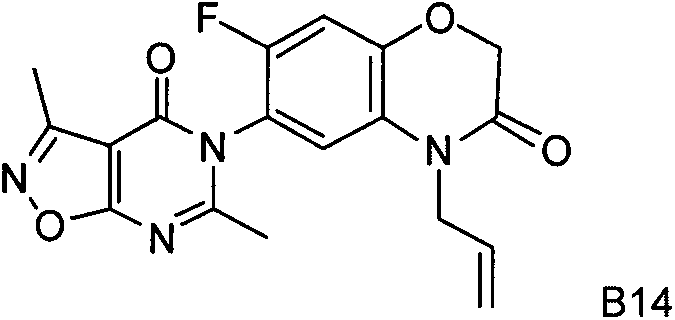
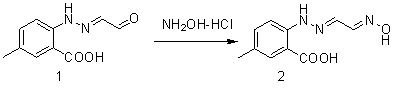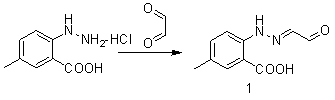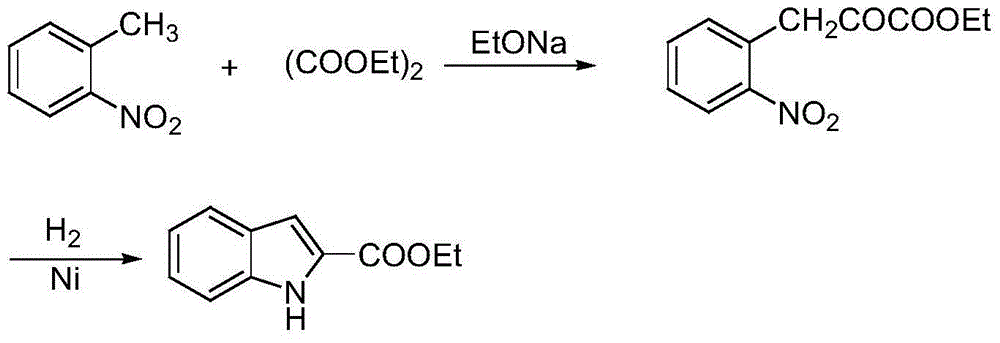Patents
Literature
85 results about "Phenylhydrazine hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phenylhydrazine hydrochloride is a hydrochloride resulting from the reaction of equimolar amounts of phenylhydrazine and hydrogen chloride. It contains a phenylhydrazine . Ontology Summary from ChEBI
Edaravone compound synthesized by new method
InactiveCN101830852AHigh purityReduce manufacturing costOrganic chemistryAcetic acidPhenylhydrazine hydrochloride
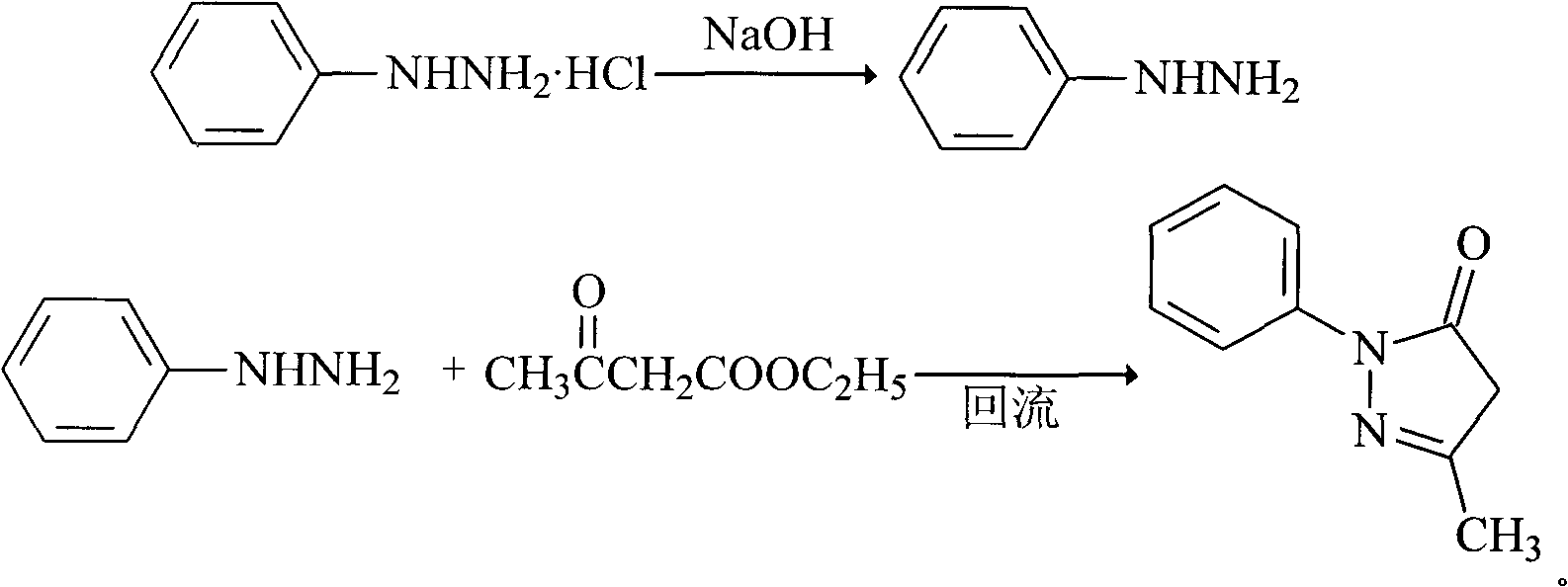
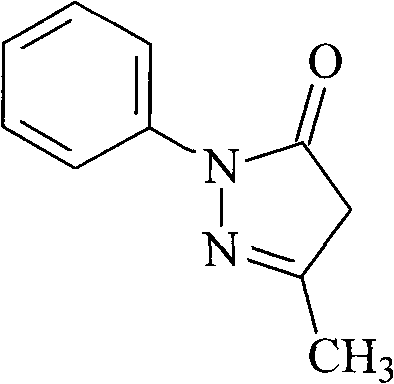
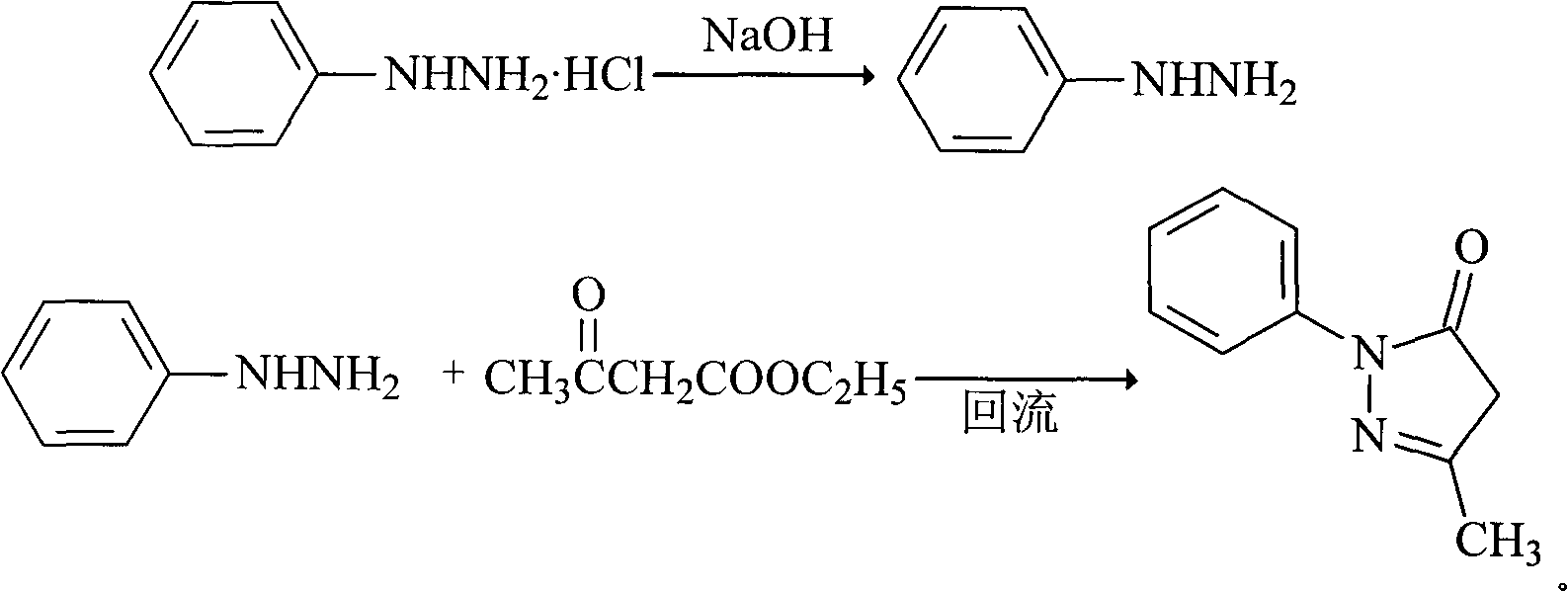
The invention relates to Edaravone synthesized by a new method. The method comprises the following steps of: reacting phenylhydrazine hydrochloride used as initial raw material with sodium hydroxide to generate phenylhydrazine; mixing the phenylhydrazine and ethyl acetoacetate to generate reflux reaction to prepare crude Edaravone; dissolving the crude Edaravone into an isopropanol water solution, adding active carbon for adsorbing and filtering to prepare fine white crystalloid powder. The invention has the advantages of low cost, high product yield and high purity.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Novel synthesis process of P-chlorophenylhydrazine hydrochloride
InactiveCN103848752ASimplified processing stepsGood processing effectHydrazine preparationP-chloroanilineReaction temperature
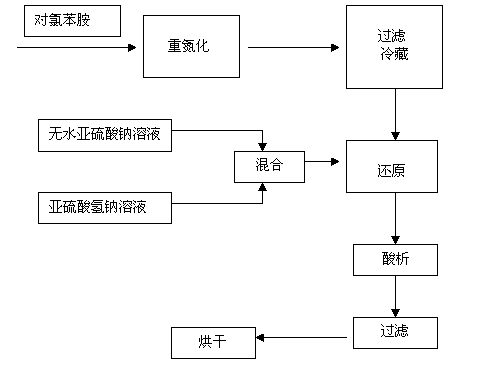
The invention discloses a preparation method of P-chlorophenylhydrazine hydrochloride. The method comprises the following steps: diazotizing chloroaniline taken as a raw material so as to prepare a corresponding diazonium salt; mixing anhydrous sodium sulfite with sodium hydrogen sulfite in a stirring manner, maintaining a pH (Power Of Hydrogen) value within 6 to 7, controlling a temperature below 40 DEG C, slowly adding the diazonium salt below a liquid surface and reacting at the temperature of 60 DEG C to 90 DEG C according to the molar ratio of the p-chloroaniline to the sodium hydrogen sulfite being 1:(1-1):3; maintaining the unchanged pH value in the step 2, slowly adding hydrochloric acid so as to maintain the pH value within 1 to 3, reacting for 1 to 3 hours at the reaction temperature of 40 DEG C to 90 DEG C, cooling to a room temperature, filtering and drying so as to obtain a reddish P-chlorophenylhydrazine hydrochloride solid. The pH value of the reaction system is maintained within 6 to 7 by taking a mixture of the sodium sulfite and the sodium hydrogen sulfite as a reducing agent, so that the phenomenon that the pH value is always regulated by NAOH (sodium hydroxide) or HCL (hydrogen chloride) in a reaction process is avoided; and the repeated heating during the reaction process is not required, so that the temperature is convenient to control; and the adding amount of water is reduced, so that the discharge of wastewater is greatly reduced, namely, the wastewater treatment at a later period is avoided. As a result, the cost is lowered.
Owner:NANJING UNIV OF SCI & TECH
Synthetic method for anti-sleeplessness medicine MK-4305 intermediate
InactiveCN103012293ASave raw materialsSimple and fast operationOrganic chemistryCarboxyl radicalPhenylhydrazine hydrochloride
The invention belongs to the field of organic synthesis and in particular relates to a synthesis method for an anti-sleeplessness medicine MK-4305 (suvorexant) intermediate. According to the synthesis method provided by the invention, 4-methyl-2-carboxyl phenylhydrazine hydrochloride is taken as substrate, hydrazone formation, oxime formation, loop closing and deoxidization are carried out, and 2-(4-methyl-2-carboxyl phenyl)-1,2,3-triazole can be conveniently and effectively synthesized. Compared with the existing method, the synthesis method provided by the invention has the advantages that raw materials are low in price, operation is easy, industrial production is easy to realize and no isomer is formed, so that the synthesis method provided by the invention has important application value. The compound synthesized by adopting the invention is a key intermediate for synthesizing anti-sleeplessness medicine MK-4305 (suvorexant) medicinal molecules.
Owner:TONGJI UNIV
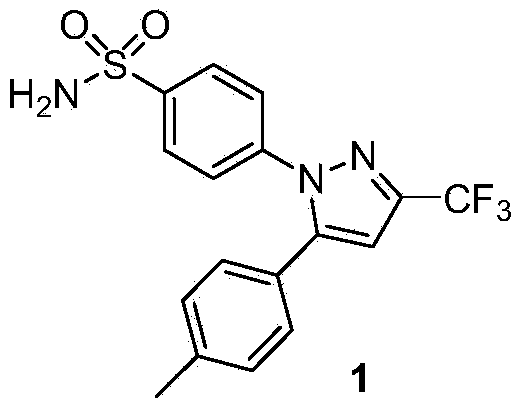
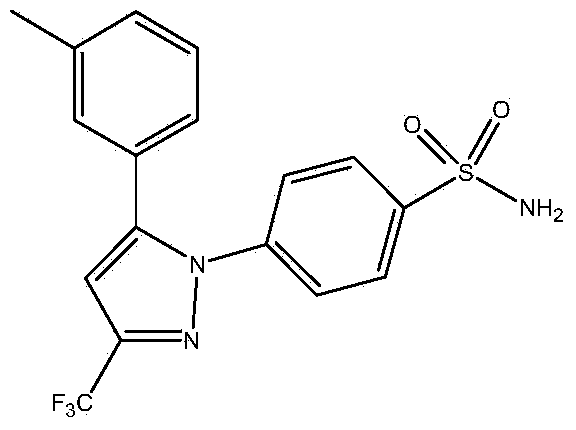
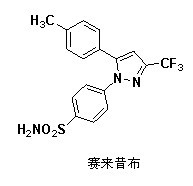
Synthesis method of celecoxib
InactiveCN102391184AHigh purityHigh yieldOrganic chemistryPhenylhydrazine hydrochlorideClaisen condensation
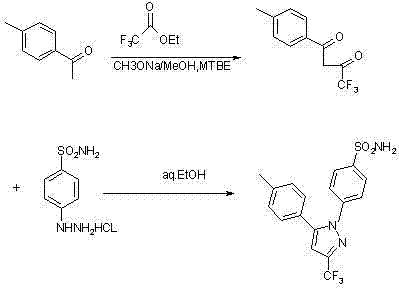
The invention relates to a synthesis method of celecoxib, which comprises the following specific steps of: 1, carrying out claisen condensation on p-methylacetophenone and trifluoroacetic acid ethyl esters in an aprotic organic solvent under the catalysis of alkali to obtain 1-(4-methylphenyl)-4,4,4-trifluoro-1,3-butanedione; and 2, reacting the obtained 1-(4-methylphenyl)-4,4,4-trifluoro-1,3-butanedione with sulfonamide-phenylhydrazine hydrochloride to obtain celecoxib, wherein in the step 1, the alkali for catalysis is selected from one or more of sodium hydride, potassium hydride, lithium hydride and calcium hydride. The synthesis method of celecoxib provided by the invention is easy to operate, high in yield, high in product purity and easy for industrial production.
Owner:JIANGXI SYNERGY PHARMA
Method for controlling and resource recovering waste water from production of phenylhydrazine
InactiveCN1401595AAchieve governanceImplement resourcesMultistage water/sewage treatmentPhenylhydrazine hydrochlorideResource recovery
A process for reclaiming the sewage generated during preparing phenyl hydrazine includes pretreating, passing through adsorption column filled by macroporous adsorption resin with polystyrene structure, and biochemical treating. Its advantages are high treating effect, high recovery yield of phenylhydrazine hydrochloride, and generating calcium sulfate as by-product.
Owner:NANJING UNIV
A kind of optimized Edaravone synthetic method
ActiveCN102285920ALow costTroubleshoot problems during synthesisOrganic chemistrySynthesis methodsFiltration
The invention belongs to the technical field of medical chemistry, and particularly relates to an optimal edaravone synthesis method. The method comprises the following steps in sequence: (1) adding phenyl hydrazine or phenylhydrazine hydrochloride serving as an initial raw material into a container in which water is held under a stirring condition and adjusting the pH value to be 6.0 by taking a proper amount of concentrated hydrochloric acid or ammonia water; (2) slowly dropwise adding ethyl acetoacetate into the solution obtained in the step (1), reacting and discharging heat, adding sodium hyposulfate after the solution is cooled to room temperature, heating the solution, performing reflux reaction for 2-5 hours, stopping heating, stirring, cooling, and performing vacuum filtration to obtain a light yellow granular edaravone rough product; and (3) recrystallizing the edaravone rough product obtained in step (2) by using absolute ethanol, performing vacuum filtration and drying to obtain white crystalline powder solid, namely an edaravone finished product. The method has the advantages of low cost, high yield and high purity; and the problems existing in the conventional edaravone synthesis process are solved.
Owner:JILIN BODA PHARMA
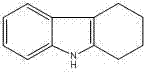
Method for synthesizing tetrahydrocarbazoles compound
InactiveCN102249983AReduce manufacturing costMild reaction conditionsOrganic chemistryCyclohexanoneHydrogen
The invention discloses a method for synthesizing a tetrahydrocarbazoles compound. The method is a green preparation method for synthesizing the tetrahydrocarbazoles compound by using phenylhydrazine hydrochloride or phenylhydrazine and cyclohexanone as raw materials and synthesizing 1,2,3,4-tetrahydrocarbazole in one step through condensation and ring-closing. The method provided by the invention comprises the following steps of: mixing the phenylhydrazine hydrochloride or phenylhydrazine, water and inorganic acid; dripping the cyclohexanone at the temperature in a range of 40-90 DEG C for 0.5-4 hours; and after dripping the cyclohexanone, keeping the heat for 0.5-3.0 hours to obtain the high-content 1,2,3,4-tetrahydrocarbazole. The method for synthesizing the 1,2,3,4-tetrahydrocarbazole is economical, simple and convenient and has the advantages of moderate reaction conditions, high yield and product purity, environmental friendliness and the like.
Owner:NANTONG HAIDI CHEM
Preparation method for celecoxib
The invention relates to a preparation method for celecoxib, and belongs to the field of chemical pharmaceuticals. The method comprises the step of performing cyclization reaction on 4, 4, 4-trifloro-1-(4-tolyl)-1, 3-butanedione and p-sulfamine phenylhydrazine or 4-sulfamine phenylhydrazine hydrochloride in a solvent to obtain a celecoxib coarse product, wherein the solvent for cyclization reaction is a low molecular organic acid or a low molecular organic acid aqueous liquor. According to the method, the product is high in yield, good in purity, easy to purity, good in quality and low in cost; and the method is environment-friendly in condensation process, and is suitable for large-scale industrial production.
Owner:HENAN DONGTAI PHARM
P-sulfonamide-phenylhydrazine hydrochloride and preparation process thereof
ActiveCN106631925AIncrease acidityReduce acidityPhysical/chemical process catalystsSulfonic acid amide preparationPhenylhydrazine hydrochlorideSulfite salt
The invention relates to a p-sulfonamide-phenylhydrazine hydrochloride and a preparation process thereof. Raw materials for preparing the p-sulfonamide-phenylhydrazine hydrochloride comprise 80-100 parts of p-aminobenzenesulfonamide, 150-220 parts of hydrochloric acid, 70-90 parts of sodium nitrate, 60-80 parts of caustic soda, 80-100 parts of sodium sulfite, 1-5 parts of phase-transfer catalyst and 0.1-0.5 part of strong acid catalyst, wherein the strong acid catalyst comprises the following components in parts by mass: 60-70 parts of picrolite, 120-130 parts of ammonia water, 40-50 parts of sulfuric acid and 10-20 parts of polyacrylamide. According to p-sulfonamide-phenylhydrazine hydrochloride and the preparation process thereof, the addition of the strong acid catalyst increases the diazo reaction yield; the finished product has favorable quality and high purity; the waste liquid treatment pressure is relieved; and the enterprise cost is saved.
Owner:平顶山奥思达科技有限公司
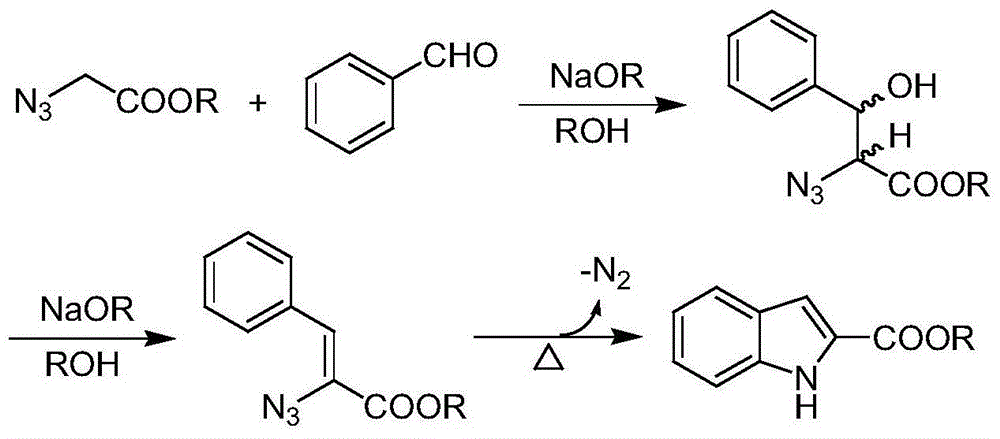
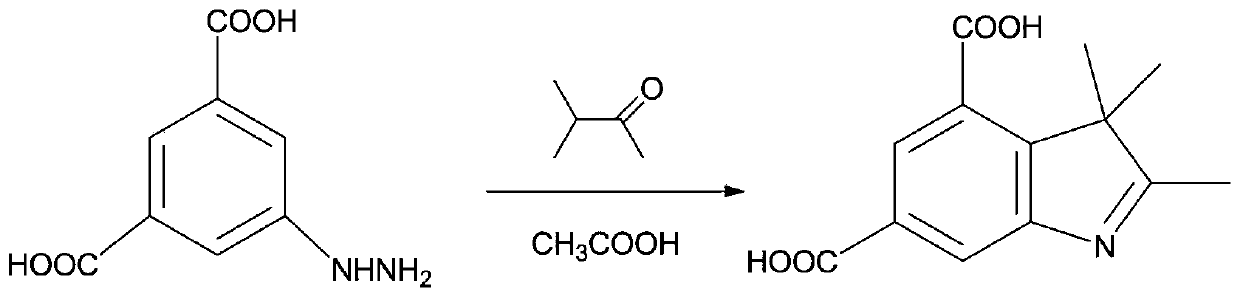
Synthetic method of substituted indol-2-formic acid
InactiveCN104402795ARaw materials are easy to getReduce generationOrganic chemistryHydrazonePhenylhydrazine hydrochloride
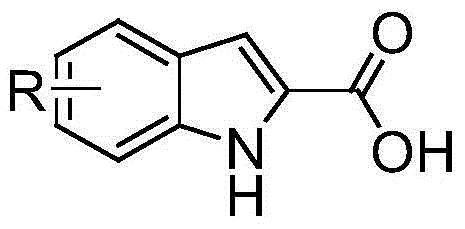
The invention discloses a synthetic method of substituted indol-2-formic acid. Substituted phenylhydrazine hydrochloride or phenylhydrazine is used as a raw material, hydrazone is formed through ethyl pyruvate, then substituted indol-2-ethyl formate is obtained through a Fischer indol synthetic reaction, and the substituted indol-2-formic acid is obtained after hydrolysis. The product purity is greater than 97%, and the reaction yield is 64%. The synthetic method disclosed by the invention has the characteristics of being free of severe conditions, simple to operate and environment-friendly, and has certain economic benefit. According to the synthetic method of the indol-2-formic acid, the raw materials are easily obtained, the operation is simple and convenient, three wastes are few, and the yield is high.
Owner:CHINA AGRI UNIV
Amphiphilic indole squarylium cyanine dye and application thereof in long-acting marking of lysosome
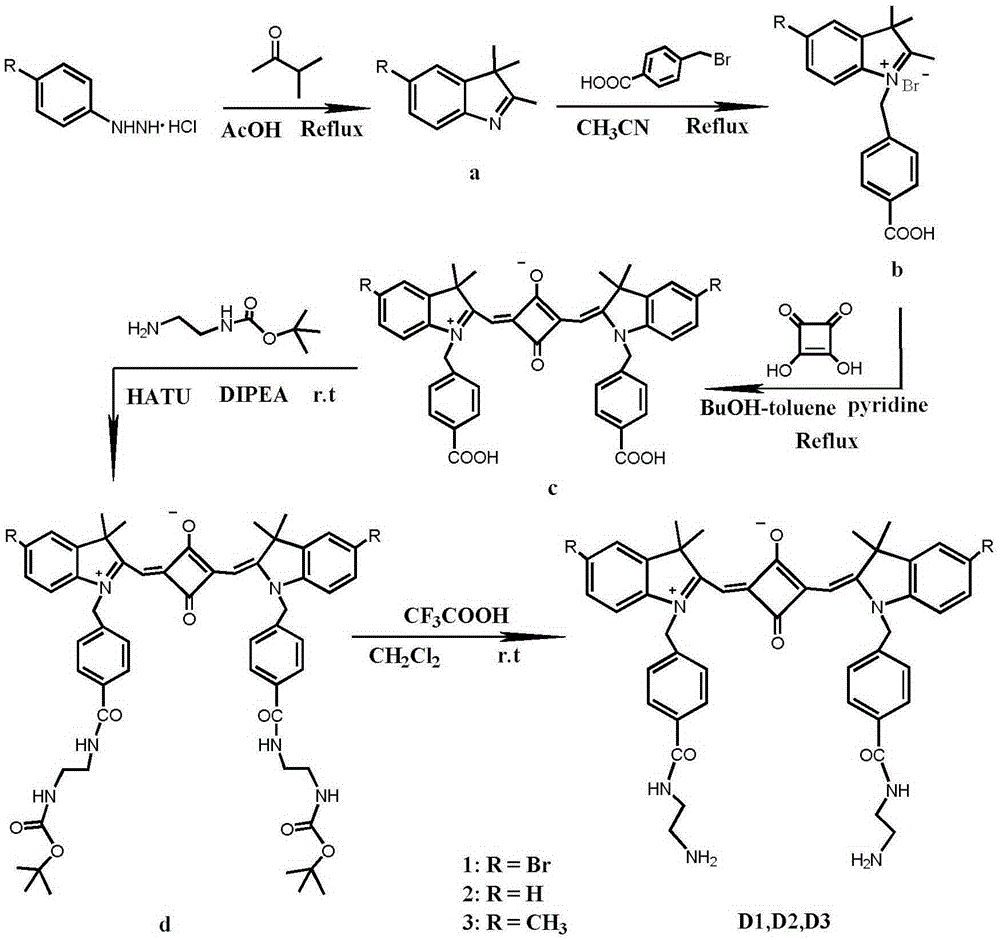
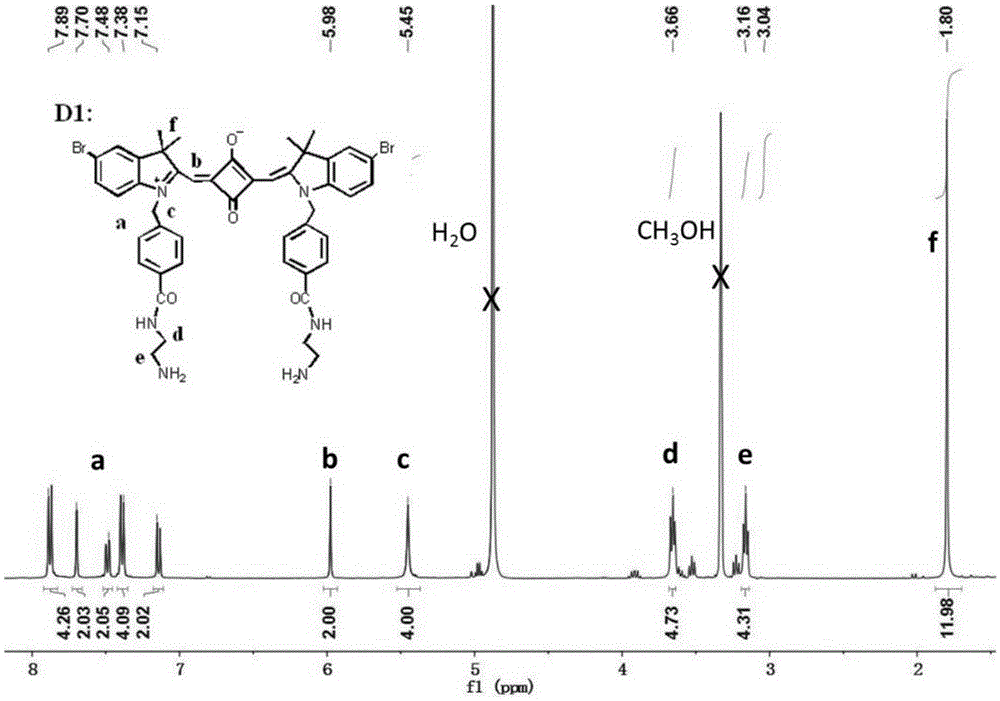
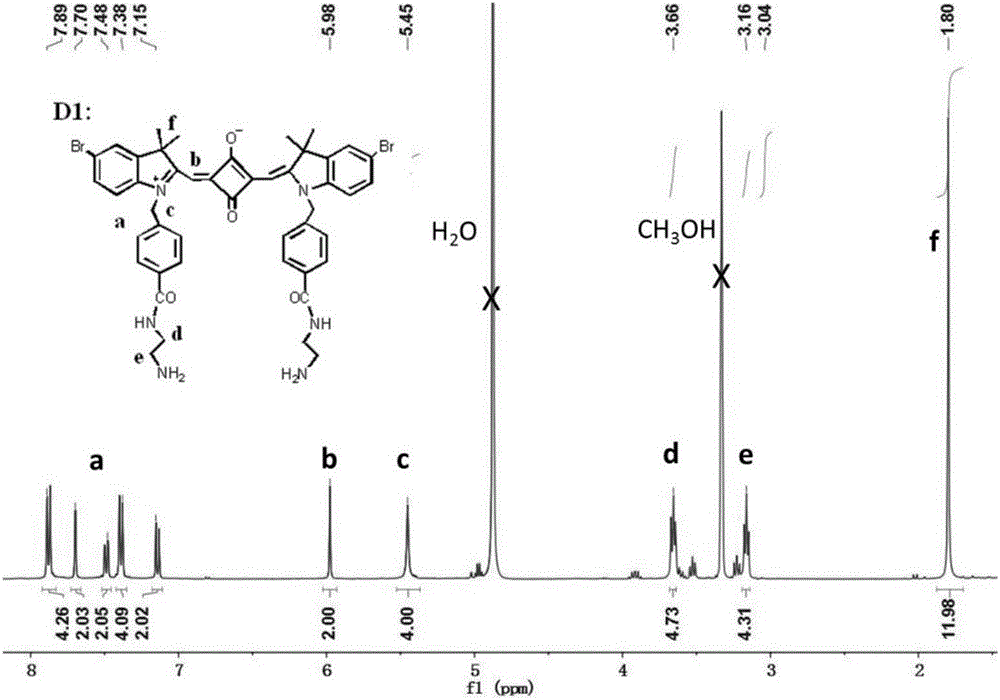
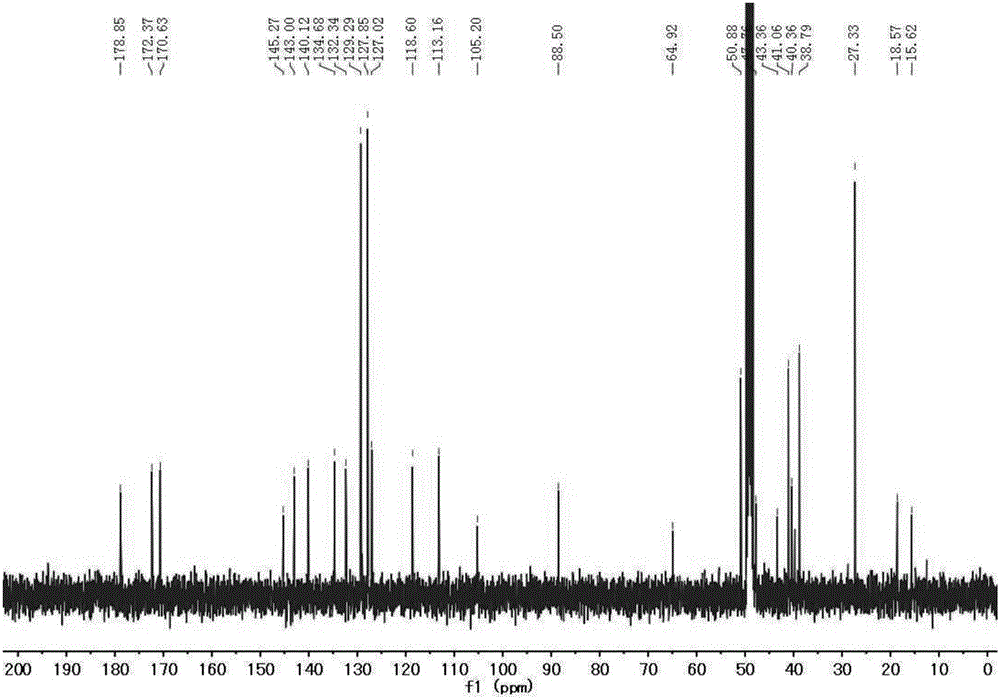
ActiveCN105238093AReduce distractionsSoluble in waterMethine/polymethine dyesFluorescence/phosphorescenceProtonationLysosome
The invention relates to synthesis of an amphiphilic indole squarylium cyanine dye and an application of the dye in the field of specific lysosome marking. In the invention, phenylhydrazine hydrochloride, of which a 4 position is substituted by bromine, hydrogen or methyl, is employed as a raw material to prepare the indole squarylium cyanine dye having the three substitutive groups and having carboxyl group functionalization, and then a condensation reaction between a carboxyl group and a primary amine is carried out to further introducing amino groups into the dye molecules. Ultraviolet absorption and fluorescent emission of the amphiphilic indole squarylium cyanine dye are both in near infrared region, so that interference due to background fluorescence in a bio-imaging process is reduced greatly. The dye is water-soluble so that the dye can be directly used in bio-imaging without any organic co-solvents, thereby achieving low bio-toxicity. The amino group in the dye is fully protonized under a lysosome acidic environment so that the electrostatic effect with lysosome membrane is more intensive. The dye can be used for marking the lysosome in living cells with marking time being capable of lasting for more than 48 h. It is the first time that the indole squarylium cyanine dye is applied in the field of lysosome marking.
Owner:BEIJING UNIV OF CHEM TECH
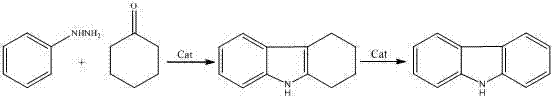
Green synthetic method of indole carbazole compounds for organic electroluminescent materials
The invention provides a green synthetic method of indole carbazole compounds for organic electroluminescent materials. The green synthetic material includes that ring adipic ketone compounds and phenylhydrazine hydrochloride are taken as raw materials reacting in one of decalin, diethylbenzene and diphenyl ether or every two mixed solvent and nonpolar solvent with boiling point exceeding 150DEG C, and the indole carbazole compounds are obtained after rapid reaction; after reaction is completed, a reaction system is cooled to indoor temperature to have products separated out, and only filtration is needed to obtain highly-purified indole carbazole compounds. With the method, the raw materials are easy to obtain and low in cost, the indole carbazole compounds can be obtained after rapid reaction without any acid and alkali and catalyst effect, high purity, less impurity and high yield are achieved, only cooling is needed in post treatment, and the highly-purified indole carbazole compounds can be obtained by filtration; easiness in operation is achieved, and the solvent can be recycled without treatment and cyclically used in the reaction process; discharge of wastewater and effluent of acid and alkali and the like is avoided, and green production is realized.
Owner:西安欧得光电材料有限公司
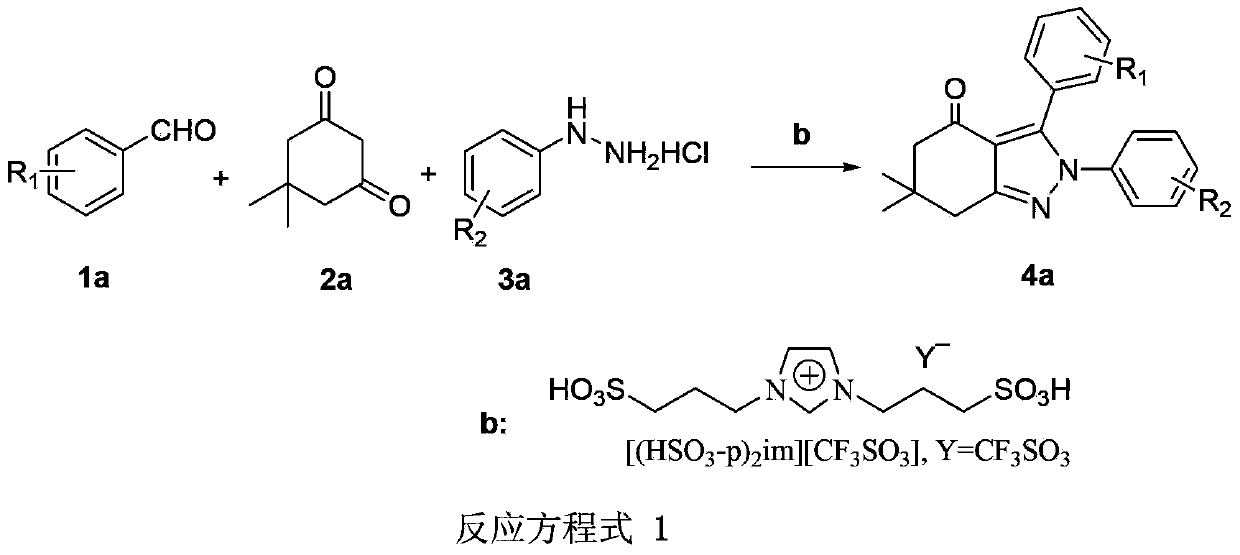
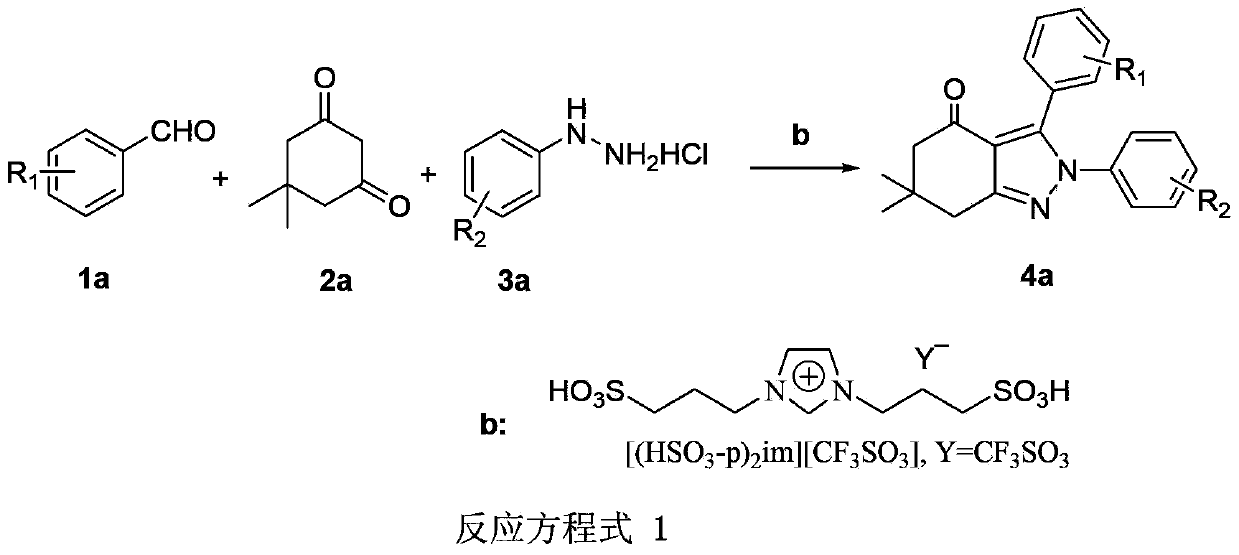
Synthesis method of tetrahydropyrazolone derivative
InactiveCN103387543AOrganic chemistryChemical recyclingPhenylhydrazine hydrochlorideSynthesis methods
The invention discloses a synthesis method of a tetrahydropyrazolone derivative. The structural formula of the tetrahydropyrazolone derivative is shown by a formula (4a) in a reaction equation 1. The synthesis method comprises the following steps of: by taking aromatic aldehyde shown by a structural formula (1a) in the reaction equation 1, 5,5-dimethylcyclohexanedione shown by a formula (2a) and phenylhydrazine hydrochloride shown by a formula (3a) as substrates and taking an ionic liquid shown by a formula (b) as a catalyst and ethanol as a reaction solvent, performing microwave reaction at 70-80 DEG C for 15-25 minutes; after the reaction, evaporating the solvent, washing solids with water and filtering; and drying filter cakes, and then re-crystallizing by use of ethanol to obtain the tetrahydropyrazolone derivative. The method disclosed by the invention has the characteristics of mild reaction conditions, simple and convenient operation, short reaction time, recyclable ionic liquid, environment-friendly synthesis process, high yield and purity and the like. The reaction equation is shown in the specification.
Owner:李佰林 +1
Preparation method of 1-amino indole derivative
ActiveCN107098847ARaw materials are easy to getHigh yieldOrganic chemistryOrganic solventPhenylhydrazine hydrochloride
The invention discloses a preparation method of a 1-amino indole derivative. The method comprises the steps of mixing a phenylhydrazine hydrochloride derivative, a diazo compound, an aldehyde or ketone compound, a catalyst, alkali and an organic solvent evenly; and carrying out condensation reaction in air or nitrogen at 80-100 DEG C for 2-12h to obtain the 1-amino indole derivative. The 1-amino indole derivative which cannot be synthesized by other others can be synthesized, and the used raw materials are available, high in yield, mild in reaction condition, wide in substrate range, high in reaction specificity, and simple and green in aftertreatment.
Owner:HUAQIAO UNIVERSITY
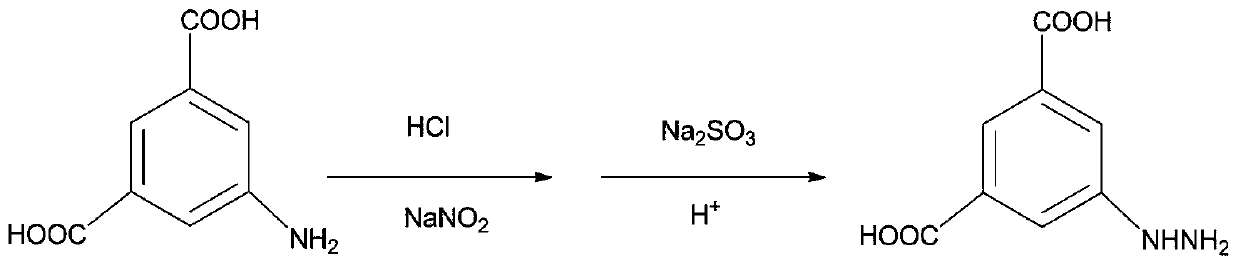
Synthetic method of phenylhydrazine hydrochloride
InactiveCN105348140AAvoid it happening againThe reaction process is simpleHydrazine preparationHydrogenPhenylhydrazine hydrochloride
The invention discloses a method for synthesizing phenylhydrazine hydrochloride through catalytic hydrogenation. The method is characterized in that benzene diazonium chloride and a hydrogenation catalyst are contacted in the presence of hydrogen and the phenylhydrazine hydrochloride is prepared. With the adoption of the method, the solid catalytic hydrogenation catalyst is used, the reaction process is simplified, and the economy is substantially improved; meanwhile, the catalytic hydrogenation method is adopted, production of sulfur dioxide is avoided, the method is environment-friendly, less salt wastewater is produced and comprises the single component, and recovery and treatment are facilitated.
Owner:WUHAN WUYAO PHARMA
Method for continuously preparing aniline diazonium salt and phenylhydrazine hydrochloride on basis of microreactor
InactiveCN109503417AHigh yieldShort reaction timeHydrazine preparationChemical/physical/physico-chemical microreactorsMicroreactorFiltration
The invention relates to a method for continuously preparing aniline diazonium salt and phenylhydrazine hydrochloride on the basis of a microreactor. An aniline salt and acid mixture and an nitrite aqueous solution which are prepared at a normal temperature are continuously injected into the microreactor to carry out diazotization reaction, and by the steps of reduction, acid precipitation and filtration, the phenylhydrazine hydrochloride is obtained. The method is easy to operate, the temperature of the diazotization reaction is high, the dosages of the reactants approximate the stoichiometric ratio, the time of the diazotization reaction is short, the yield is high, the aniline diazonium salt and the phenylhydrazine hydrochloride can be continuously produced, the energy consumption is reduced, the safety of the process is increased, and the method is applicable to industrial production.
Owner:SHANGHAI JIAO TONG UNIV
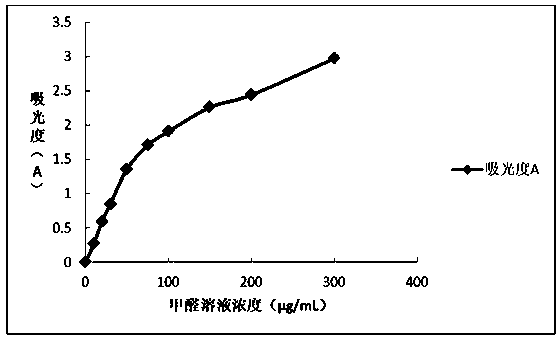
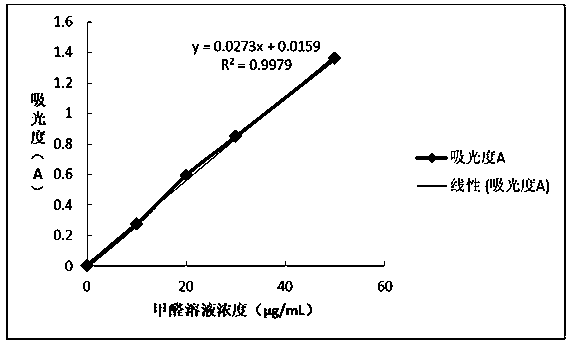
Quick formaldehyde testing method for textiles
PendingCN110018157AQuick testEasy to operateChemical analysis using titrationMaterial analysis by observing effect on chemical indicatorPhenylhydrazine hydrochlorideChloride
The invention belongs to the field of textile safety, and discloses a quick formaldehyde testing method for textiles. The method, in which color shades are compared to quickly determine whether a tested textile contains formaldehyde or not and whether the formaldehyde content complies with standard limit requirements or not, is established according to formaldehyde and phenylhydrazine hydrochloride color developing technologies, and has the advantages of high convenience in operation, low cost and high detection speed. The method comprises the following steps: (a) sampling, wherein a sample isweighed and placed in a sampling barrel, and the sampling barrel is lidded; (b) extraction, wherein pure water is added, vibration is carried out, the mixture is kept in a static state for 120 minutes after sufficient soaking, and a test solution is taken out; (c) color developing, wherein a certain amount of a solution to be tested is accurately taken out of the test solution, a phenylhydrazinehydrochloride solution with an equal volume and a ferric chloride solution with the equal volume are added in sequence, and the mixture is shaken well through vibration; and (d) color comparison and determination, wherein the solution color is compared with a standard color chart after 10 minutes, so that the content and concentration range of the formaldehyde in the sample can be determined.
Owner:中华人民共和国昆山海关
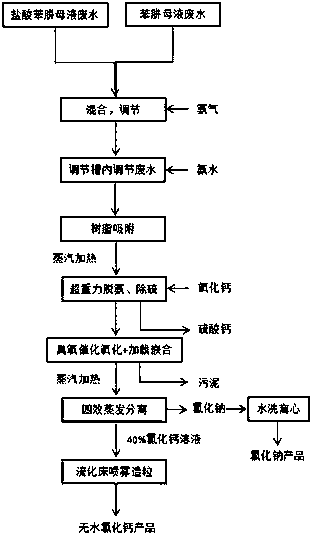
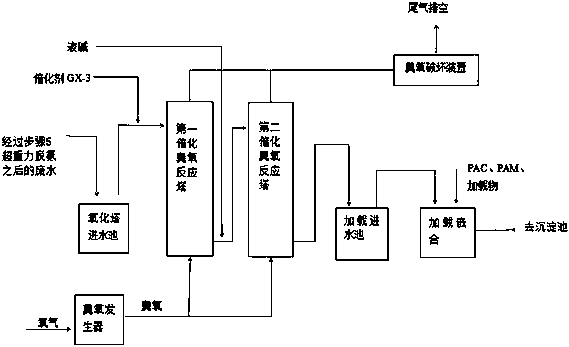
Treatment technology for high-concentration production wastewater
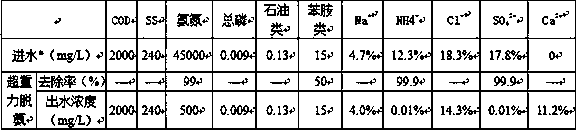
ActiveCN109534570ARealize comprehensive utilizationImprove ammonia removal efficiencyCalcium/strontium/barium chloridesSpecific water treatment objectivesHigh concentrationPhenylhydrazine hydrochloride
The invention relates to a treatment technology for high-concentration production wastewater. The high-concentration production wastewater comprises phenylhydrazine hydrochloride mother liquor wastewater and phenylhydrazine mother liquor wastewater, and the phenylhydrazine hydrochloride mother liquor wastewater and the phenylhydrazine mother liquor wastewater are treated by adopting the process flow comprising macro-porous resin adsorption, super-gravity stripping deamination, sulfur removal, ozone catalytic oxidation, loading chimerism, four-effect downstream evaporation concentrating separation and fluidized bed spray granulation. The treatment technology can reduce the treatment cost, and can recycle effective ingredients in the production wastewater.
Owner:QIDONG A&P CHEM FACTORY
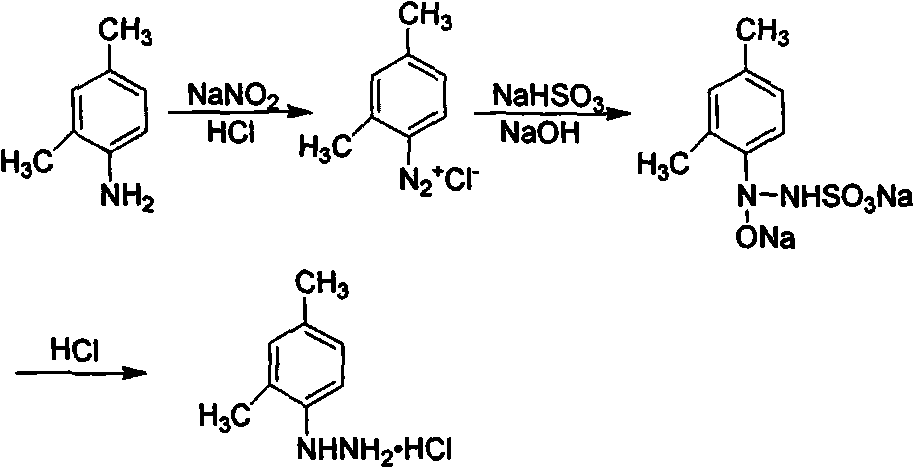
Method for synthesizing 2,4-dimethyl phenylhydrazine hydrochloride
InactiveCN102070486AHigh yieldShort reaction timeHydrazine preparationDimethylaniline N-oxideSulfite salt
The invention discloses a method for synthesizing 2,4-dimethyl phenylhydrazine hydrochloride, which is characterized in that the material adding proportion of raw materials of 2,4-dimethylaniline to sodium nitrite to sodium sulfite is 1 / 1.05 / 2.5, the zinc powder consumption is 0.05 times of the 2,4-dimethylaniline consumption; the diazo reaction is carried out at the temperature between 0 DEG C and 5 DEG C, the sodium nitrite is added within 10 minutes, and the diazotization is completed within 10 to 15 minutes; the reduction reaction is carried out at 100 DEG C when the pH value is about 6; and the acidulation temperature is 80 DEG C. The reaction is carried out under the process condition, the yield of obtained products is higher than 90 percent, and the purity is higher than 99 percent.
Owner:TIANJIN POLYTECHNIC UNIV
Kaolin-based immobilization carrier applied to methylparathion-degrading bacteria
InactiveCN106834265AHigh mechanical strengthEnhanced mass transferWater contaminantsOn/in inorganic carrierPhenylhydrazine hydrochlorideAmmonium nitrate
The invention discloses a kaolin-based immobilization carrier applied to methylparathion-degrading bacteria. A preparation method of the kaolin-based immobilization carrier applied to the methylparathion-degrading bacteria comprises the following steps: modifying kaolin via a mixed solution prepared from ammonium nitrate, 1,4-diamino-anthraquinone, phenylhydrazine hydrochloride, 2,3,4-trimethoxybenzaldehyde and 2-thiopheneacetyl chloride, and then preparing the kaolin into a material B; modifying the material B via a mixed solution prepared from tannic acid, pyrazoloanthrone, octamethylcyclotetrasiloxane and 2-chloro-4-fluorobenzoic acid, and then preparing the material B into a material C; modifying the material C via a mixed solution prepared from 3-amino-4-ethoxycarbonyl pyrazole, N,N-diisopropylethylamine and 3-(ethylamino)-4-methylphenol, and then preparing the material C into a material D; and modifying the material D via a mixed solution prepared from p-chlorobenzyl cyanide, benzimidazole-2-formaldehyde and acetone phenylhydrazone to obtain a material which is the kaolin-based immobilization carrier applied to the methylparathion-degrading bacteria.
Owner:光合强化(北京)生物科技有限公司
1-substitution phenyl-4-polysubstitution phenyl-5-methylmercapto-1H pyrazole compound with anti-hepatoma activity
The invention relates to the synthesis and anti-hepatoma activity study of a 1-substitution phenyl-3-methyl-4-(2-fluorine-4-chlorine-5-propargyloxyphenyl)-5-methylmercapto-1H pyrazole compound (A). The compound (A) is prepared through the following steps of reducing 2-chlorine-4-fluorine-5-nitro phenyl ethyl carbonate with iron powder, carrying out diazotization and Meerwein arylation reaction and reacting with carbon disulfide and dimethyl sulfate to obtain 2-chlorine-4-fluorine-5-(1,1-dimethylthio-3-oxo-1-alkene-2-group) phenyl ethyl carbonate (5), hydrolyzing the (5), then reacting with propargyl bromide to obtain 4,4-dimethylthio-3-(2-fluorine-4-chlorine-5-propargyloxyphenyl) butane-3-alkene-2-ketone (6), and finally with ethyl alcohol as a solvent, reacting (6) with different substituted phenylhydrazine hydrochlorides under reflux condition to generate the compound (A). Test results indicate that both the degrees of the compound A for inhibiting HepG2 cancer cells and promoting cell apoptosis are greater than or equal to control drug cyclophosphamide.
Owner:NANKAI UNIV
N-(substituted benzene)-based pyrazolyl ketone derivatives, and preparation method and application thereof
InactiveCN106749288AEnhanced inhibitory effectHigh activityBiocideOrganic chemistryPositive controlNatural product
The invention belongs to the field of organic chemistry, and discloses a series of new N-(substituted benzene)-based pyrazolyl ketone derivatives, and a preparation method and application thereof. The series of compounds are prepared through the steps of taking fraxinellone as a raw material, carrying out a series of reactions to obtain fraxinellone ketone, then reacting the fraxinellone ketone with different substituted phenylhydrazine hydrochloride to obtain the series of N-(substituted benzene)-based pyrazolyl ketone derivatives, and the structural general formula of the N-(substituted benzene)-based pyrazolyl ketone derivatives is shown in the description. The compounds disclosed by the invention have very strong growth inhibition and poisonous activity against three-year-old armyworms, part of the compounds are better than parent fraxinellone and positive control toosendanin, and are expected to be used to prepare new-style natural product insecticides, in addition, the compounds disclosed by the invention exhibit better bacteriostatic activity against some bacteria, and are expected to be developed into potential natural product bacteriostats.
Owner:ZHENGZHOU UNIV
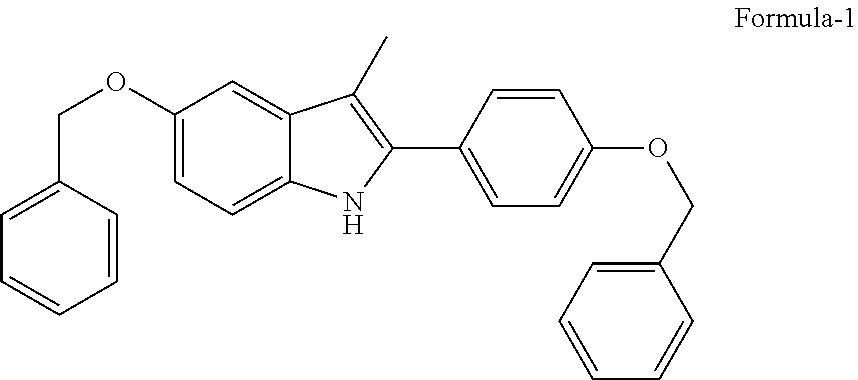
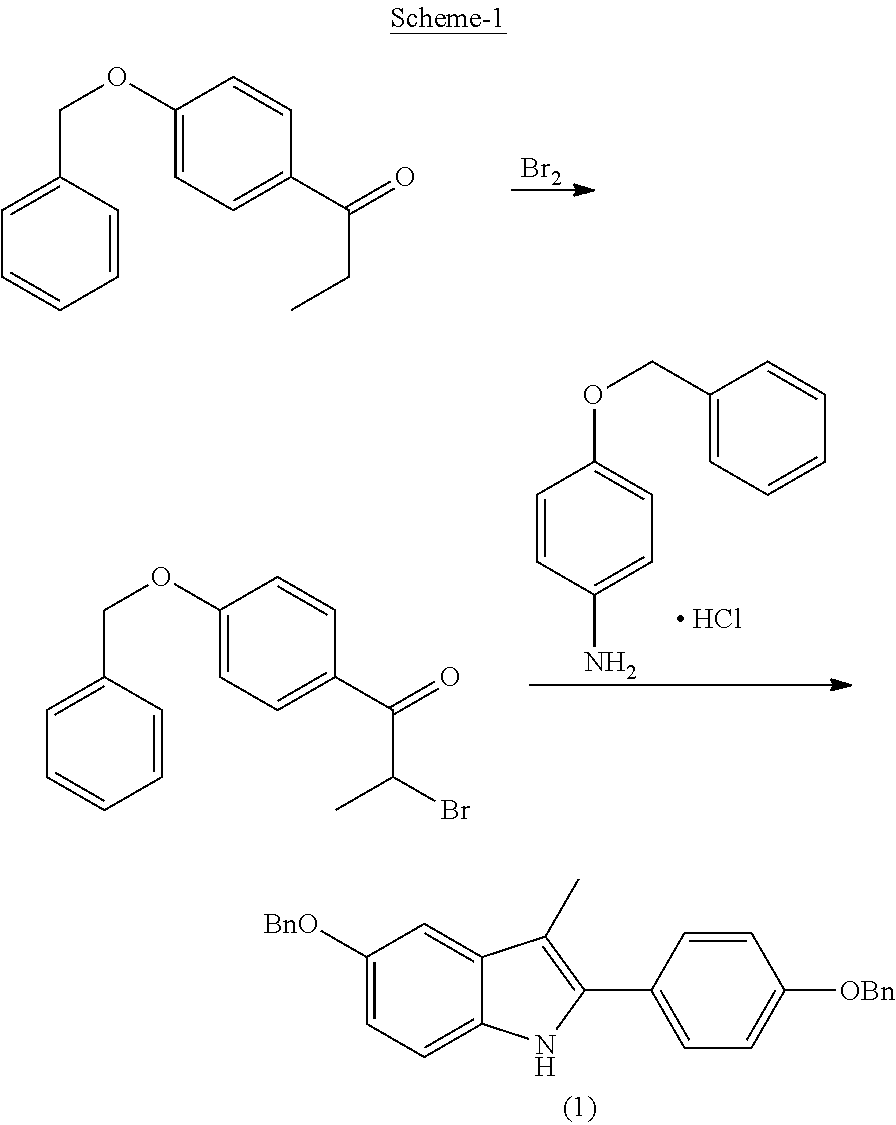
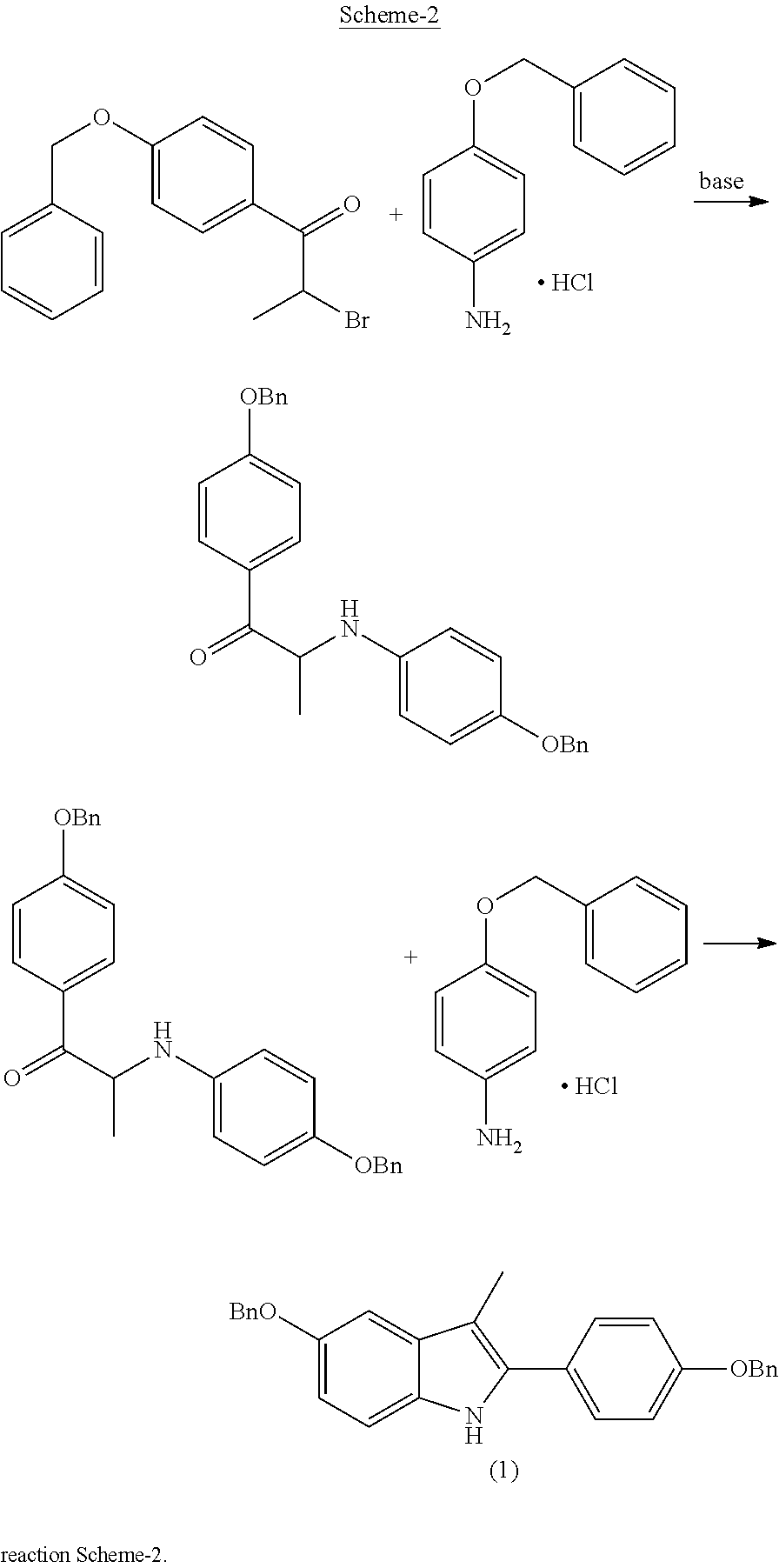
Process for the preparation of 5-benzyloxy-2-(4-benzyloxyphenyl)-3-methyl-1H-indole
The present invention is related to a process for the preparation of 5-benzyloxy-2-(4-benzyloxyphenyl)-3-methyl-1H-Indole (formula-1, a useful intermediate for the synthesis of bazedoxifene) using 4-benzyloxy propiophenone and 4-benzyloxy phenyl hydrazine hydrochloride.
Owner:DIVI S LAB LTD
Writing brush cleaning solution and preparation method thereof
InactiveCN107841190AEasy to cleanQuick wash suctionChemical paints/ink removersBetaineSodium phosphates
The invention discloses a writing brush cleaning solution and a preparation method thereof, and relates to the technical field of writing brush cleaning. The writing brush cleaning solution is prepared from the following components in parts by weight: 30 to 50 parts of sodium sulfonate phenylhydrazine hydrochloride, 20 to 35 parts of styryl p-chlorobenzophenone, 12 to 20 parts of polyoxyethylene lauryl ether, 8 to 15 parts of coconut oil oleamidopropyl betaine, 2 to 10 parts of sodium citrate, 8 to 10 parts of triphosphoric acid, 4 to 10 parts of sodium phosphate, 10 to 30 parts of potassium lactate, 30 to 40 parts of ethanol, 20 to 40 parts of acetone and 100 to 120 parts of water. The writing brush cleaning solution is a cleaning agent for cleaning dirt conveniently, is easy in composition, and has good cleaning effect.
Owner:大竹县第十小学
A class of amphiphilic indole squaraine dyes and their application in long-term labeling of lysosomes
ActiveCN105238093BReduce distractionsSoluble in waterMethine/polymethine dyesFluorescence/phosphorescenceProtonationLysosome
The invention relates to synthesis of an amphiphilic indole squarylium cyanine dye and an application of the dye in the field of specific lysosome marking. In the invention, phenylhydrazine hydrochloride, of which a 4 position is substituted by bromine, hydrogen or methyl, is employed as a raw material to prepare the indole squarylium cyanine dye having the three substitutive groups and having carboxyl group functionalization, and then a condensation reaction between a carboxyl group and a primary amine is carried out to further introducing amino groups into the dye molecules. Ultraviolet absorption and fluorescent emission of the amphiphilic indole squarylium cyanine dye are both in near infrared region, so that interference due to background fluorescence in a bio-imaging process is reduced greatly. The dye is water-soluble so that the dye can be directly used in bio-imaging without any organic co-solvents, thereby achieving low bio-toxicity. The amino group in the dye is fully protonized under a lysosome acidic environment so that the electrostatic effect with lysosome membrane is more intensive. The dye can be used for marking the lysosome in living cells with marking time being capable of lasting for more than 48 h. It is the first time that the indole squarylium cyanine dye is applied in the field of lysosome marking.
Owner:BEIJING UNIV OF CHEM TECH
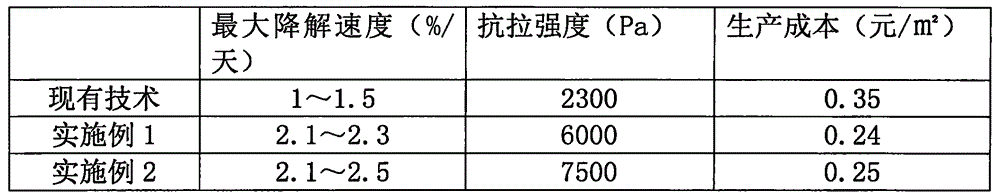
Degradable composite tourist commodity packaging film and preparation method thereof
InactiveCN105017569AFast degradationPromote degradationFlexible coversWrappersDiphenyl phosphatePhenylhydrazine hydrochloride
The invention discloses a degradable composite tourist commodity packaging film, which comprises the following components in parts by weight: 12 to 16 parts of phenylhydrazine hydrochloride, 5 to 8 parts of 2-amino-5-chloropyrazine, 4 to 8 parts of S-benzylthiourea hydrochloride, 3 to 6 parts of 3-cyano-5-fluoro-borophenylic acid, 4 to 6 parts of 2-bromo-3-nitrotoluene, 11 to 15 parts of 3-amino-2, 2-dimethyl acrylamide, 10 to 16 parts of acetylated konjac glucomannan, 7 to 10 parts of p-cresyl benzyl acetate, 2 to 5 parts of tetraethyl orthosilicate and 6 to 10 parts of diphenyl phosphate. The invention also discloses a preparation method of the degradable composite tourist commodity packaging film. According to the invention, the defects of the prior art can be solved and the degradation speed of the film is increased.
Owner:JILIN TECH COLLEGE OF ELECTRONICS INFORMATION
Novel dicarboxyl spiropyrane derivative and preparation method thereof
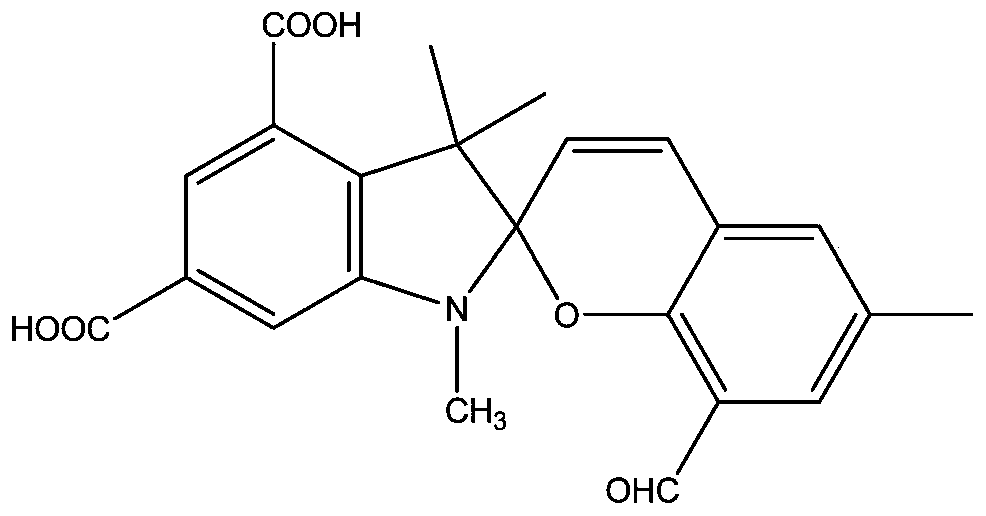
ActiveCN110590794AElectron-withdrawingLow costOrganic chemistryFluorescence/phosphorescenceOrganic synthesisPhenylhydrazine hydrochloride
The invention discloses a novel dicarboxyl spiropyrane derivative and a preparation method thereof in the technical field of organic synthesis. The preparation method comprises the following steps: S1, synthesizing 3,5-dicarboxyl phenylhydrazine hydrochloride; S2, synthesizing 2,3,3-trimethyl-3H-indole-4,6-dioctyl phthalate; S3, synthesizing 1,3,3-trimethyl-2-methylene-4,6-dicarboxyl-3H-indole; and S4, synthesizing 6-methyl-8-aldehyde-1',3',3'-trimethyl spiro[benzopyran-2,2'-indole]-4',6'-dioctyl phthalate. By introducing two carboxyl groups to an indoline part of spiropyrane, and introducingaldehyde to the site 8 on a pyranoid ring, the spiropyrane both has an electron absorption function and can form a hydrogen bond stable open-loop cyanine (SP) together with oxygen, has excellent chemical stability and photochromic performance, in addition, has a very good metal coordination capability, and can be matched with most metals without affecting open-loop capabilities of the metals.
Owner:HUNAN UNIV
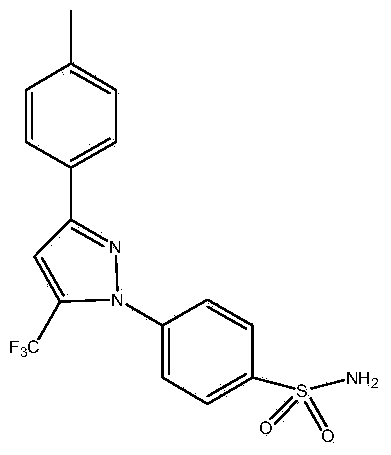
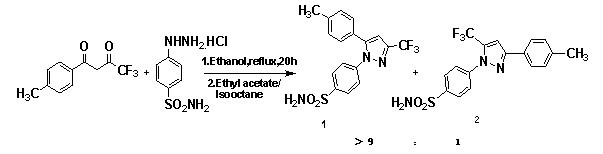
Synthetic method of celecoxib
InactiveCN103923011AImprove solubilityImprove recycling effectOrganic chemistryPhenylhydrazine hydrochlorideFiltration
The invention discloses a synthetic method of celecoxib. The synthetic method is characterized by comprising the following steps: firstly, adding sodium methylate as an aldol condensation catalyst and methylbenzene as a reaction solvent into a reaction container, adding p-methylacetophenone and trifluoroacetic acid ethyl ester, fully reacting, adding diluted hydrochloric acid, and separating out a water layer to obtain a methylbenzene solution of an intermediate-diketone; secondly, adding water, a phase transfer catalyst and 4-aminosulfophenyl hydrazine hydrochloride into the methylbenzene solution of the intermediate-diketone, and performing dehydration cyclization reaction to obtain a celecoxib reaction solution; thirdly, replenishing methylbenzene, standing, separating out the water layer and remaining an organic layer; cooling the organic layer, preserving heat and crystallizing to obtain a crystal product, and performing suction filtration, methylbenzene washing and water washing in sequence to obtain a celecoxib crude product; drying the crude product in vacuum, decolorizing and re-crystallizing to obtain a celecoxib raw material. According to the synthetic method, the celecoxib yield is increased, the reaction time is shortened, the celecoxib purity is high, the three-waste discharge in the whole production process is reduced, and the production cost is low.
Owner:SUZHOU TIANMA SPECIALTY CHEM
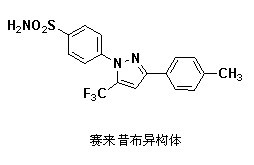
Preparation method for celecoxib isomer
InactiveCN102617474ALow costSimple post-processingOrganic chemistryPhenylsulfonamidePhenylhydrazine hydrochloride
The invention discloses a preparation method for celecoxib isomer, which includes the steps: firstly, preparing a crude product of 4, 4, 4-trifluoro-3-methoxyimino-1-(4-cresyl)-1-butanone from 4, 4, 4-trifluoro-1-(4-cresyl)-1, 3-butanedione with methoxylamine hydrochloride serving as ammoniation protective reagent; and secondly, adding p-amino sulfonyl phenylhydrazine hydrochloride into the prepared crude product, dissolving with ethyl acetate and acetic acid, obtaining a crude product after heating and backflow, and recrystallizing to obtain a pure product of 4-[3-(4-methylphenyl)-5-(trifluoromethyl)-1H-pyrazolyl] benzene sulfonamide, namely, the celecoxib isomer, which is white or almost white crystalline powder in appearance. The preparation method has the advantages of low cost, simplicity in post-processing, high yield and high selectivity.
Owner:STONE LAKE PHARMA TECH
Construction composite material with strong stability
InactiveCN104628310AChange the original activityImpermeableCement productionPhenylhydrazine hydrochloridePolyvinyl chloride
The invention discloses a construction composite material with a strong stability. The material is characterized by being composed of the following components in parts by weight: 25 to 30 parts of polyvinyl chloride, 8 to 15 parts of fly ash, 10 to 15 parts of cement clinker, 3 to 4 parts of protective colloid, 10 to 13 parts of hydroxymethyl cellulose, 3 to 5 parts of vinyl acetate, 3 to 4 parts of ethylene, 0.6 to 0.7 part of processing aid ACR401, 0.8 to 1 part of lubricating property modifier LS-303, 0.2 to 0.3 part of polyethylene wax, 0.3 to 0.4 part of paraffin, 0.12 to 0.15 part of stearic acid, 3 to 4 parts of calcium lignin sulfonate, 0.5 to 0.7 part of sodium methyl phenylhydrazine hydrochloride, and 0.5 to 0.8 part of foaming agent. The obtained construction composite material has the characteristics of penetration resistant property, freeze-resistant performance, environment-friendliness, no pollutant discharge, and long service life. The toughness of the construction material is high, during the using process, the adhesive force is strong, the falling ash is reduced, no precipitation is generated, the operation is simple and labor-saving, and the construction material is nontoxic, odorless, and pollution-free and is an environment-friendly construction material.
Owner:青岛诚运建筑工程有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com