Novel dicarboxyl spiropyrane derivative and preparation method thereof
A technology of spiropyran derivatives and dicarboxylic groups, which is applied in the field of novel dicarboxyspiropyran derivatives and its preparation, can solve the problems of thermal stability, poor fatigue resistance, instability of cyanine body at the ring-opening part, and limited application fields etc. to achieve the effects of easy reaction conditions, excellent photochromic properties, and a wide range of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
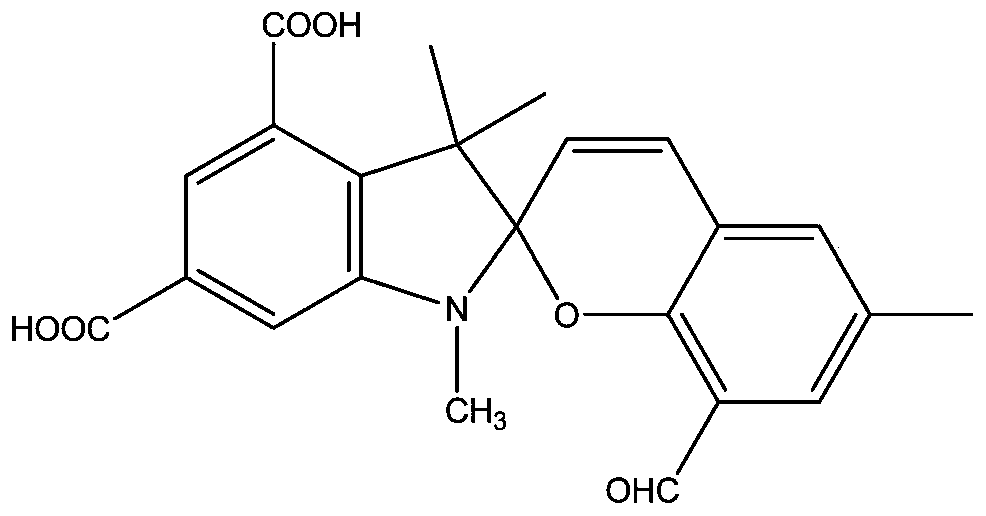
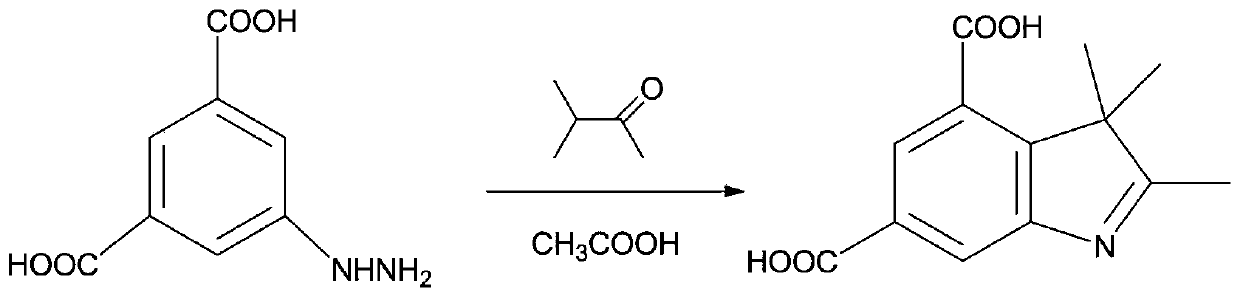
[0039] as attached Figure 2-5 As shown, the present invention proposes a novel biscarboxy spiropyran derivative and a preparation method thereof, specifically comprising the following steps:
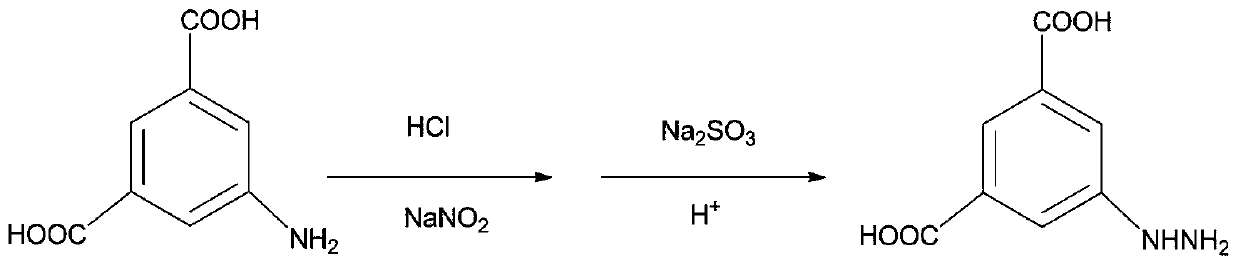
[0040] S1, synthesis of 3,5-dicarboxyphenylhydrazine hydrochloride;
[0041] Add 8.0g of aminoisophthalic acid and 50mL of water into a 150mL round bottom flask, add 10mL of concentrated hydrochloric acid to dissolve it, keep the temperature below 5°C in an ice-salt bath, stir the solution into a slurry, and slowly add 3.0 6mL aqueous solution of sodium nitrite, stop adding until the starch potassium iodide test paper turns slightly blue, and keep magnetic stirring throughout the whole process to form reaction solution A; weigh 18.0g sodium sulfite and add it to a 300mL round-bottomed flask, then add 65mL water and stir to dissolve, dissolve and pour into Slowly add the above reaction solution A dropwise and keep stirring. After stirring for 1 hour, add 22mL of concentrated hydrochlori...
Embodiment 2
[0049] as attached Figure 2-5 As shown, the present invention proposes a novel biscarboxy spiropyran derivative and a preparation method thereof, specifically comprising the following steps:
[0050] S1, synthesis of 3,5-dicarboxyphenylhydrazine hydrochloride;
[0051] Add 9.0g of aminoisophthalic acid and 50mL of water into a 150mL round bottom flask, add 13mL of concentrated hydrochloric acid to dissolve it, keep the temperature below 5°C in an ice-salt water bath, stir the solution into a slurry, and slowly add 3.5 6mL of aqueous solution of sodium nitrite, stop adding until the starch potassium iodide test paper turns slightly blue, and keep magnetic stirring throughout the whole process to form reaction solution A; weigh 18.9g of sodium sulfite and add it to a 300mL round bottom flask, then add 65mL of water and stir to dissolve, dissolve and pour into Slowly add the above-mentioned reaction solution A and keep stirring. After stirring for 1.5 hours, add 25mL of concent...
Embodiment 3
[0062] as attached Figure 2-5 As shown, the present invention proposes a novel biscarboxy spiropyran derivative and a preparation method thereof, specifically comprising the following steps:
[0063] S1, synthesis of 3,5-dicarboxyphenylhydrazine hydrochloride;
[0064] Add 10.0g of aminoisophthalic acid and 50mL of water into a 150mL round bottom flask, add 15mL of concentrated hydrochloric acid to dissolve it, keep the temperature below 5°C in an ice-salt water bath, stir the solution into a slurry, and slowly add 4.0 6mL of aqueous solution of sodium nitrite, stop adding until the starch potassium iodide test paper turns slightly blue, and keep magnetic stirring throughout the whole process to form reaction solution A; weigh 20.0g of sodium sulfite and add it to a 300mL round bottom flask, then add 65mL of water and stir to dissolve, dissolve and pour into Slowly add the above-mentioned reaction solution A and keep stirring. After stirring for 2 hours, add 30mL of concentr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com