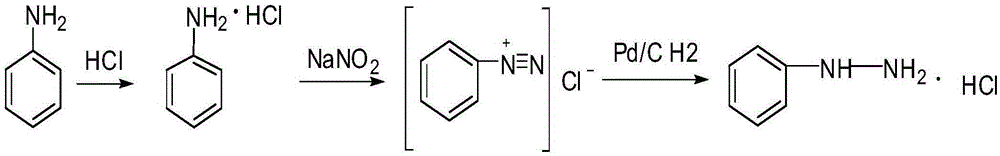
Synthetic method of phenylhydrazine hydrochloride
A technology of phenylhydrazine hydrochloride and diazobenzene chloride, applied in the direction of hydrazine preparation and the like, can solve problems such as aggravating the economic burden of enterprises, affecting the growth of crops, damaging buildings and other facilities, etc. The effect of less waste water
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 1 benzenediazonium chloride
[0041] Put 100 g of aniline (1.07 mol) and 326 g of 30% by mass hydrochloric acid (2.68 mol of HCl) into a 1 L flask, stir, and cool to 0° C. with an ice-water bath. The 406g mass percentage composition is 20% NaNO 2 Solution (1.18mol NaNO 2 ) is slowly poured into the solution in the above-mentioned flask, stirred, and incubated for 1.5 hours. Gained solution containing benzene diazonium chloride is designated as solution 1 # , analyzed with chromatographic detection, solution 1 # The benzenediazonium chloride yield obtained in is 142.7g.
[0042] Solution 2 # ~Solution 6 # The preparation operation is the same as solution 1 # , the difference lies in the ratio of raw materials and reaction conditions, solution 2 # With methanol as solvent, solution 3 # With ethanol as solvent. Solution 1 # ~Solution 6 # The ratio of raw materials and reaction conditions are shown in Table 2.
[0043] Table 2 prep...
Embodiment 2
[0045] Catalytic hydrogenation of benzenediazonium chloride in embodiment 2 tank reactor
[0046] Solution 1 # ~Solution 6 # The catalytic hydrogenation reaction of:
[0047] With the solution 1 that obtains in embodiment 1 # Added to the 5L tank type hydrogenation reactor. Add 9g of palladium-carbon catalyst into the kettle-type hydrogenation reactor, the hydrogenation pressure is 0.4MPa, and the reaction temperature is controlled at 30°C. After 2 hours of reaction, the material is discharged, and the filtrate is obtained by suction filtration under reduced pressure. Detected by chromatography, the total yield of phenylhydrazine hydrochloride reached 94%. Raw materials, reaction conditions and catalysts are as reaction number 1 in table 3 # shown.
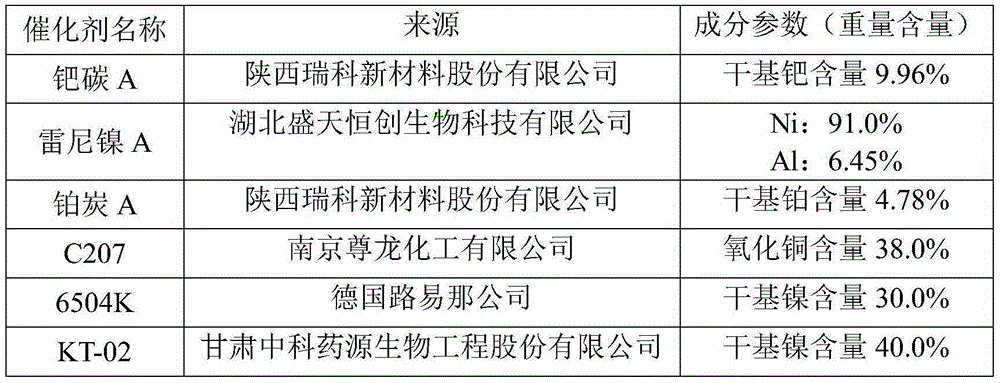
[0048] Solution 2 # ~Solution 6 # Operation process of catalytic hydrogenation reaction with solution 1 # The difference is the type and amount of catalyst, and the reaction conditions are different. Its raw mat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com