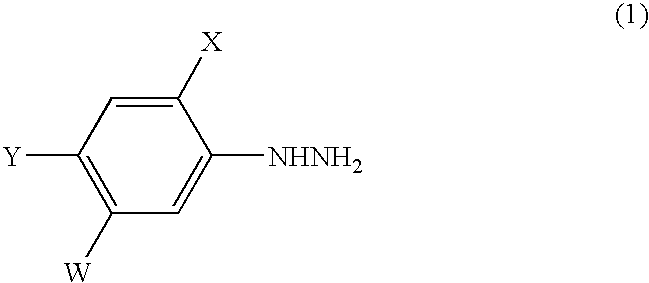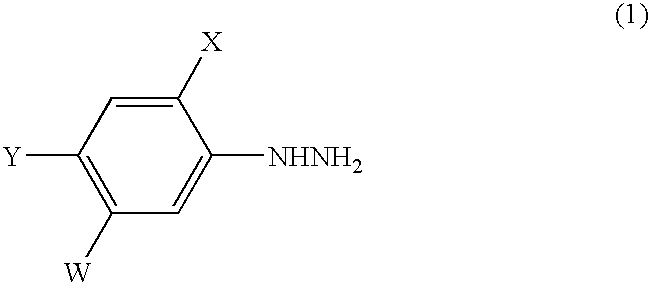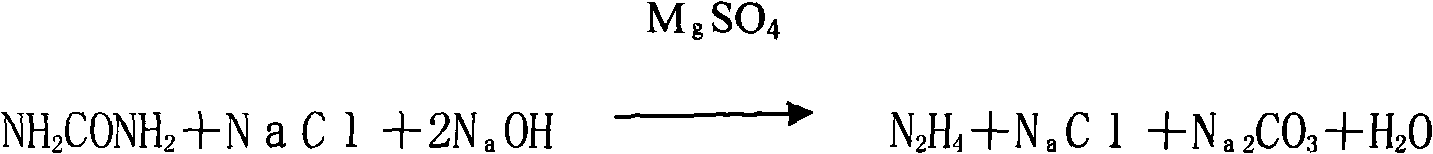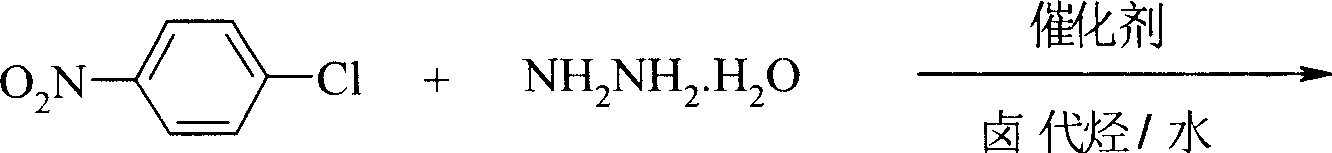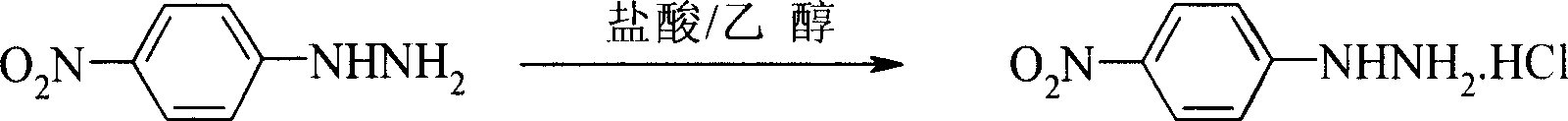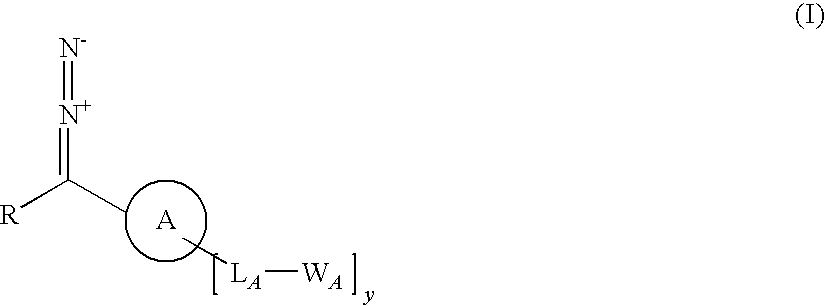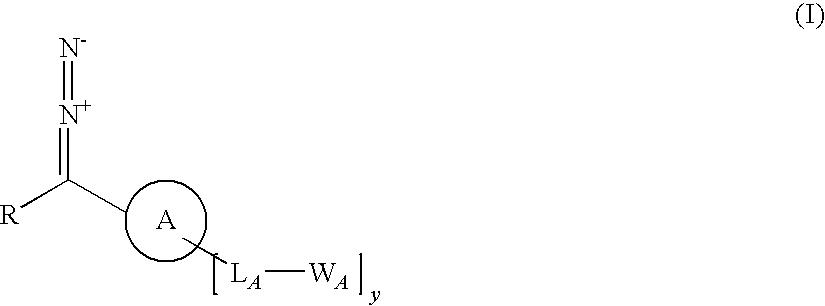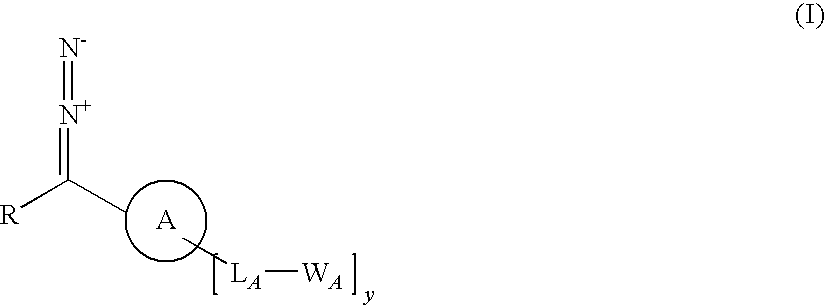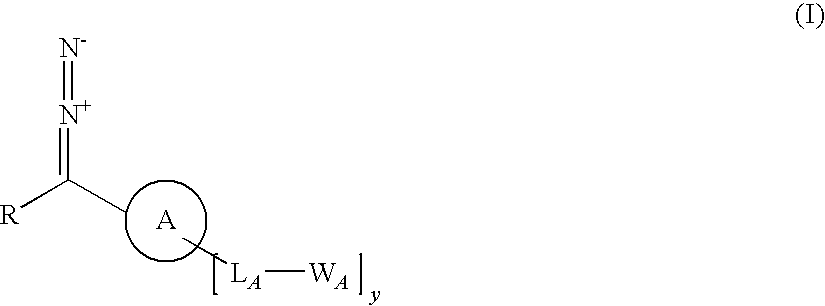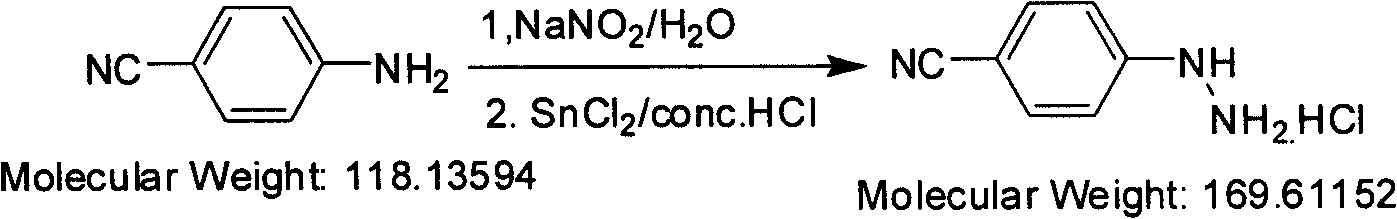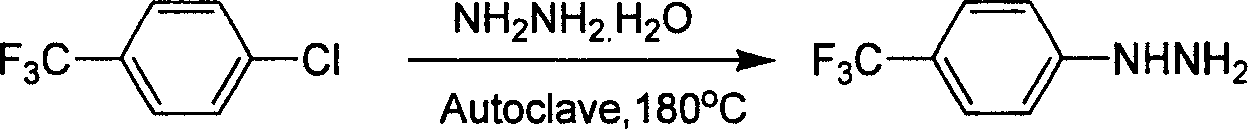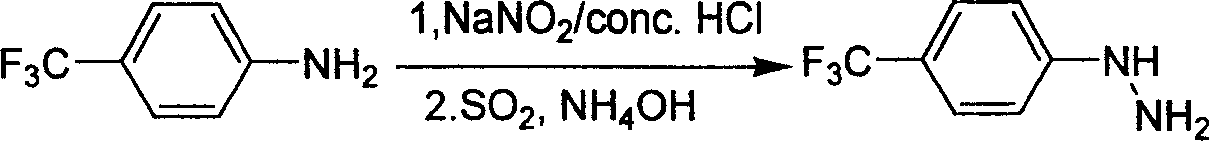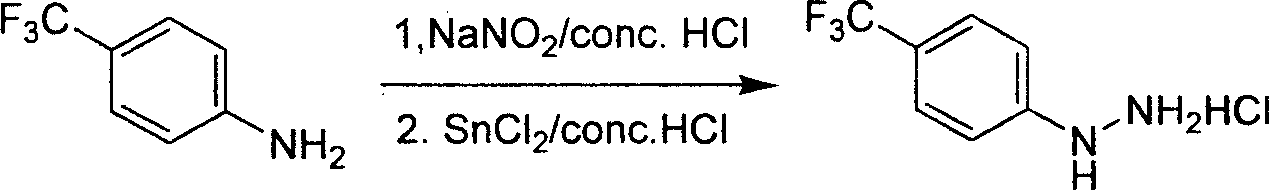Patents
Literature
429results about "Hydrazine preparation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for preparing hydrazinobenzene in continuous micro-channel reactor
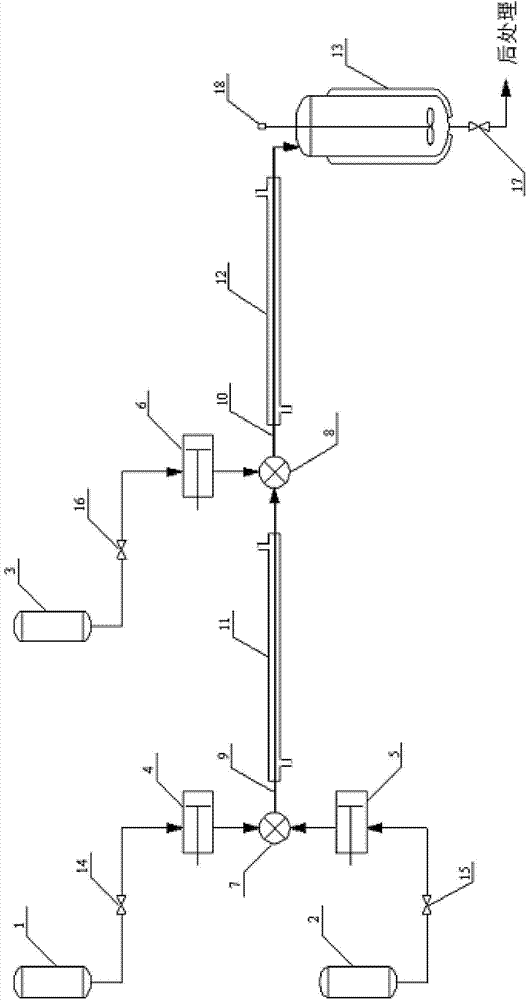
InactiveCN106316879AHigh mixing mass transfer effectImprove thermal conductivityHydrazine preparationToxic gasContinuous flow
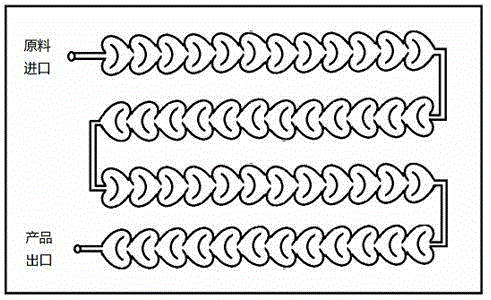
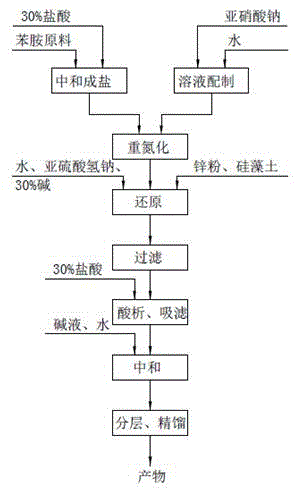
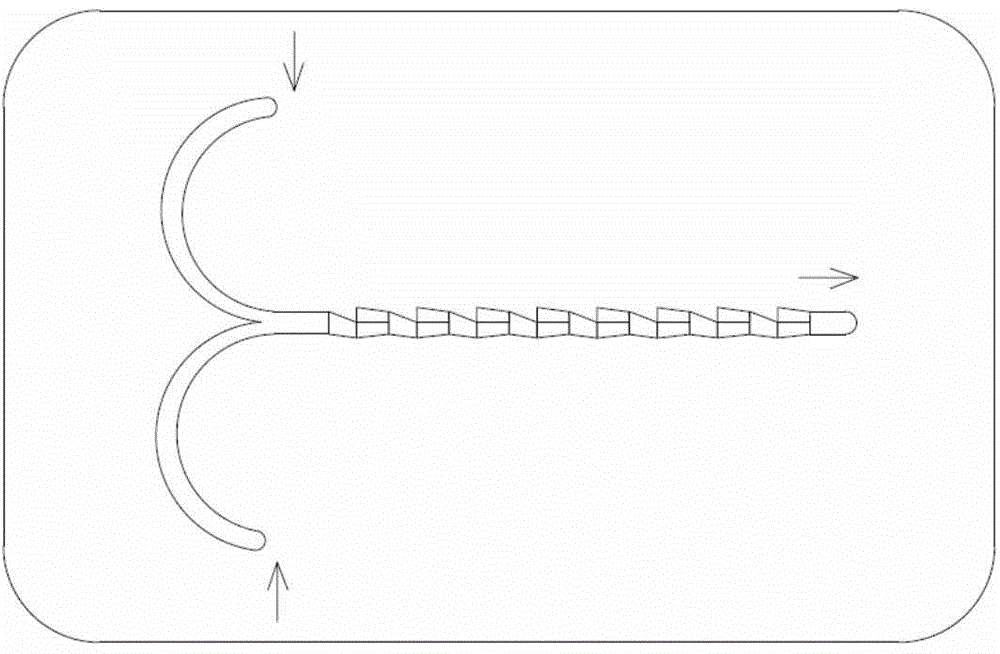
The invention relates to a method for preparing highly pure hydrazinobenzene by using continuous flow micro-channel reactor. The method concretely comprises the following steps: preparing aniline hydrochloride from hydrochloric acid and aniline, respectively pumping the aniline hydrochloride and a sodium nitrite solution into the micro-channel reactor by two metering pumps to obtain a diazo salt solution, reducing the obtained reaction solution, carrying out acid separation on the reduced solution, filtering the obtained solution, neutralizing the filtered solution, and distilling the neutralized solution to obtain the highly pure hydrazinobenzene. The diazotization process is a strong exothermic reaction, and the generated diazo salt easily decomposes after standing at a high temperature for a long time, and generates toxic gases which pollute environment and even blast. The mixing effect of the heart-shaped micro-channel reactor is far better than the mass transfer effect generated by stirring, so the aniline conversion rate can reach 99%, the mixing mass transfer effect is good, and heat conduction is fast to avoid local overheating phenomenon; and the reaction can be carried out at constant temperature conditions, so the temperature runaway blast danger of general reactors is eliminated, and the safety is improved.
Owner:CHINA PETROLEUM & CHEM CORP +1
Processes for the preparation of tetrakis(Faryl)borate salts
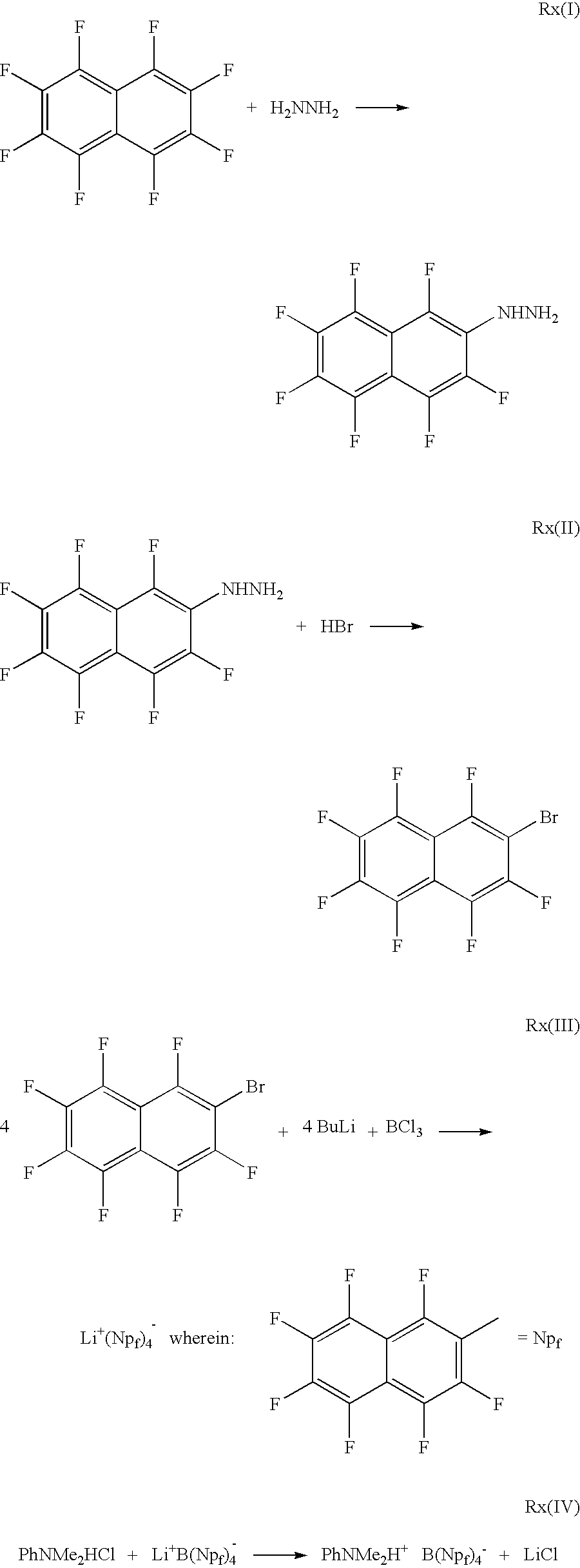
InactiveUS8642497B2Reduce probabilityReasonable timeHydrazine preparationPhysical/chemical process catalystsArylBorate salt
Owner:WR GRACE & CO
Method and device for preparing ethylphenylhydrazine hydrochloride by pipelines
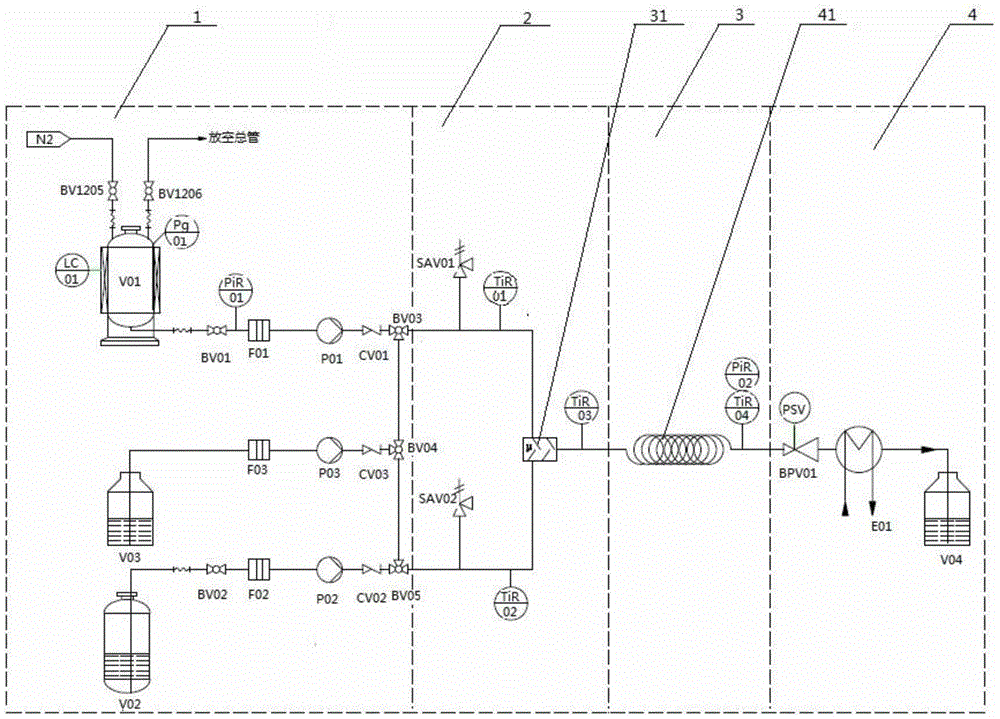
The invention provides a specific device for preparing ethylphenylhydrazine hydrochloride by pipelines. The device comprises two tubular reactors with jackets, two mixers, three containers, three metering pumps and a reaction kettle, wherein the first mixer, the first tubular reactor, the second mixer, the second tubular reactor and the reaction kettle are sequentially connected by virtue of conduits; the first metering pump and the second metering pump are respectively arranged on the first container and the second container by virtue of conduits which are provided with stop valves and are connected with the first mixer; the three metering pumps are arranged on the third container by virtue of a conduit which is provided with a stop valve and is connected with the second mixer; the inlet of the reaction kettle is communicated with the outlet of the second tubular reactor; a pipeline is arranged at the outlet of the reaction kettle, is communicated with a posttreatment system and is provided with a stop valve. The invention further provides a method for preparing ethylphenylhydrazine hydrochloride by using the device. The method is convenient to operate and simple in posttreatment, and the product has good yield and high purity and is suitable for industrilization.
Owner:ZHEJIANG UNIV OF TECH
Microreactor device for producing 2-hydroxyethylhydrazine and preparation process
ActiveCN104876833AEthylene oxide accumulationNo accumulationHydrazine preparationMicroreactor2-hydroxyethylhydrazine
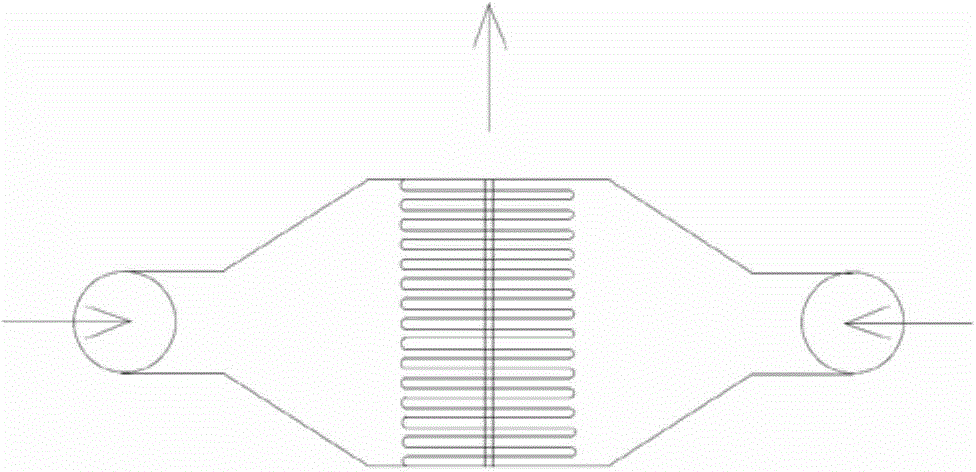
The invention discloses a microreactor device for producing 2-hydroxyethylhydrazine and preparation process. The device comprises a feeding system, a mixing system, a reaction system, a backpressure valve, a cooling receiving system, a washing system and an external circulating bathing system, wherein the feeding system comprises a hydrazine hydrate storing tank, an oxirane storing tank and a feeding pump; the mixing system comprises a CPMM or SIMM or Starlam serial micro mixer; the reaction system comprises a rimule reactor section; the pressure of the backpressure valve ranges from 0 to 15MPa; the cooling receiving system comprises a CRMH serial micro heat exchanger and a receiving storing tank; the washing system comprises a washing storing tank and a washing pump which are connected in series; the hydrazine hydrate storing tank or oxirane storing tank, the feeding pump, the micro mixer, the rimule reactor, the backpressure valve, the micro heat exchanger and the receiving storing tank are connected in series; the mixing system, the reaction system and the cooling receiving system are respectively arranged at the circulating bathing system.
Owner:大连微凯化学有限公司
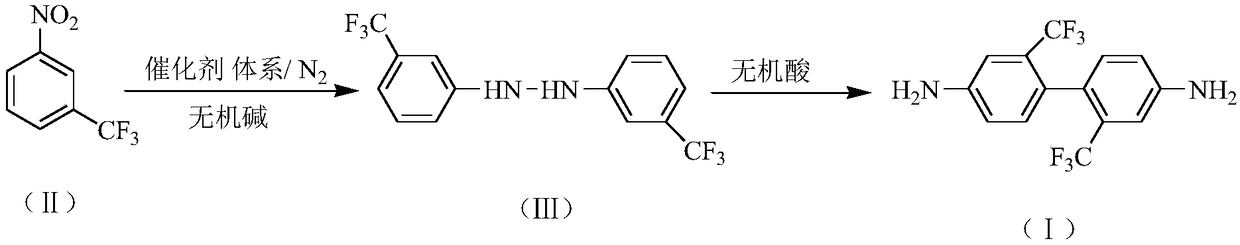
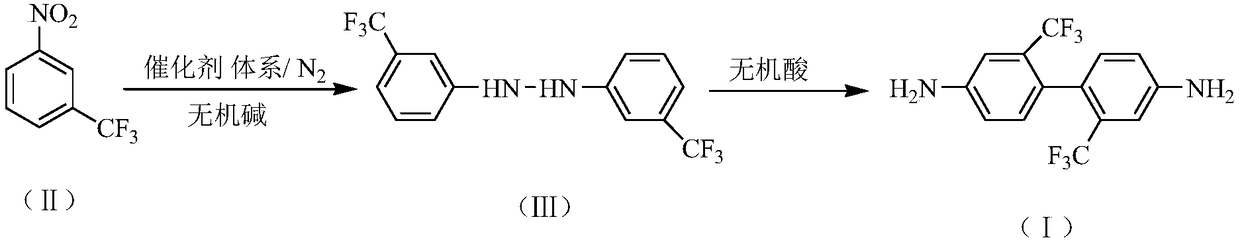
Preparation method of 2,2'-bis(trifluoromethyl)-4,4'-diaminodiphenyl
InactiveCN109232273AReduce generationHigh purityHydrazine preparationPreparation by rearrangement reactionsHydroxyanthraquinoneDodecylsulfonic acid
The invention relates to a preparation method of 2,2'-bis(trifluoromethyl)-4,4'-diaminodiphenyl. The preparation method comprises the following steps: synthesizing 3,3'-bis(trifluoromethyl)hydrazo-benzene in an inorganic alkaline aqueous solution by adopting nitrobenzotrifluoride as a raw material, adopting a phase transfer catalyst, a co-catalyst and Pd / C as a catalytic system and adopting aromatic hydrocarbon as a solvent, and performing the re-arrangement reaction on the 3,3'-bis(trifluoromethyl)hydrazo-benzene in an inorganic acid aqueous solution, thus obtaining 2,2'-bis(trifluoromethyl)-4,4'-diaminodiphenyl, wherein the phase transfer catalyst is one or a mixture of more of sodium dodecyl benzene sulfonate, sodium dodecyl sulfate and cetyl trimethyl ammonium bromide, and the co-catalyst is one or a mixture of more of 2,3-dichloro-1,4-naphthoquinone, 2-hydroxyanthraquinone and 2,6-dioxyanthraquinone. The preparation method has the advantages of mild reaction condition, simple process, high product quality and yield, low production cost, environmental friendliness, suitability for continuous production and the like.
Owner:烟台海川化学制品有限公司
Treatment method for acyl-chlorination reaction tail gas
InactiveCN103908870ASafe and easy production operationDoes not affect product qualityHydrazine preparationDispersed particle separationP-chloroanilineAcyl group
The invention relates to a treatment method for acyl-chlorination reaction tail gas. The reaction tail gas successively enters a three-step series hydrogen chloride falling-film absorption tower and a two-step series ammonium hydroxide absorption tower; p-chloroaniline and recovered hydrochloric acid are taken as raw materials and subjected to diazotization reaction for generating a diazo salt; and an aqueous solution of ammonium sulfite is taken as a reducing agent, after the diazo salt is dropwise added at room temperature, the recovered hydrochloric acid is dropwise added for acidification hydrolysis and further for preparing p-chlorophenylhydrazine hydrochloride. By employing the method to treat the acyl-chlorination reaction tail gas, tedious technologies for discharging and drying solid materials and selecting low-temperature equipment for liquefying sulfur dioxide are avoided, and the production operation is safe and convenient; and the produced tail gas absorption solution can be used as a raw material for preparing organic chemical products, is environment-friendly and is capable of creating economic benefit.
Owner:TIANJIN QIDONG CHEM PLANT
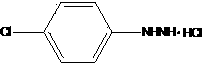
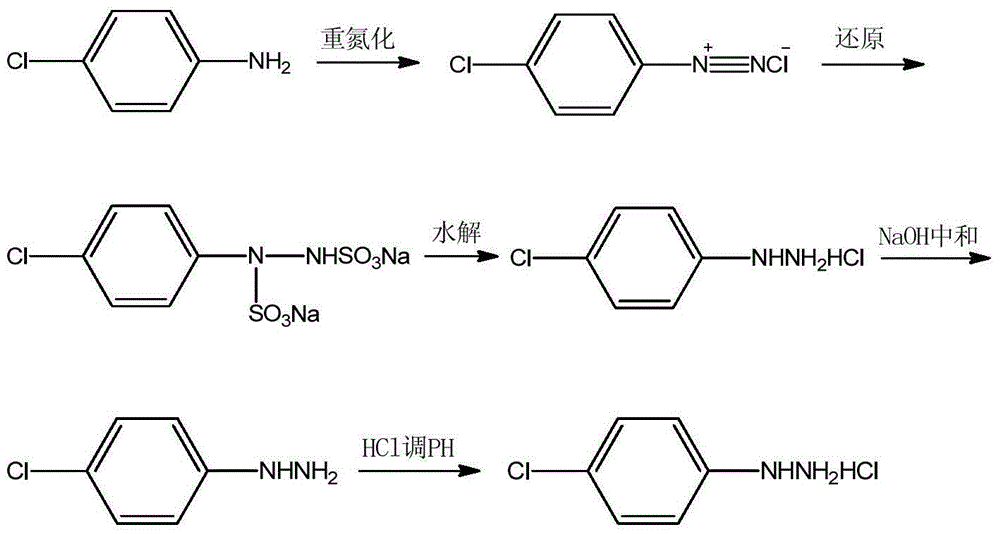
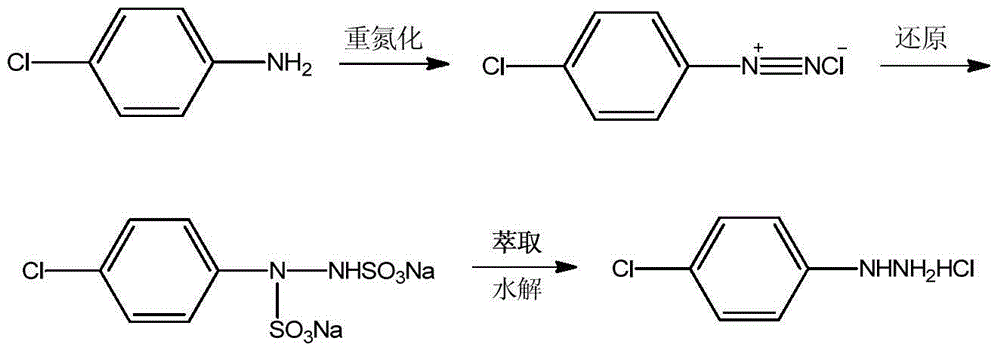
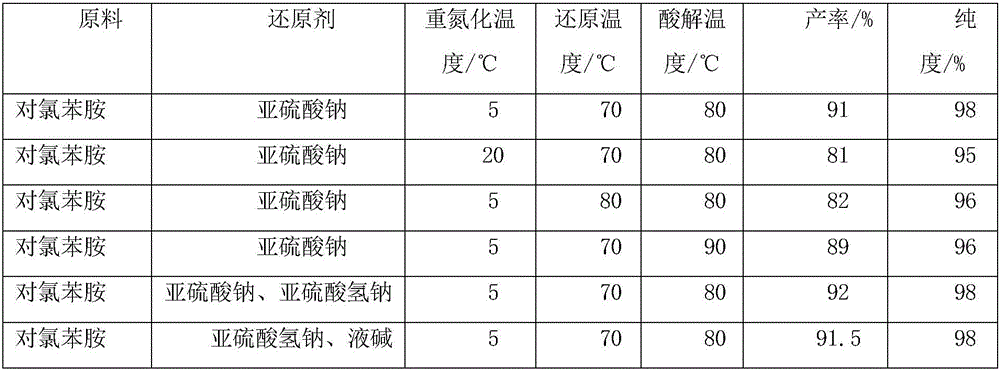
Preparation method of 4-chlorophenylhydrazine hydrochloride
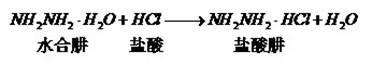
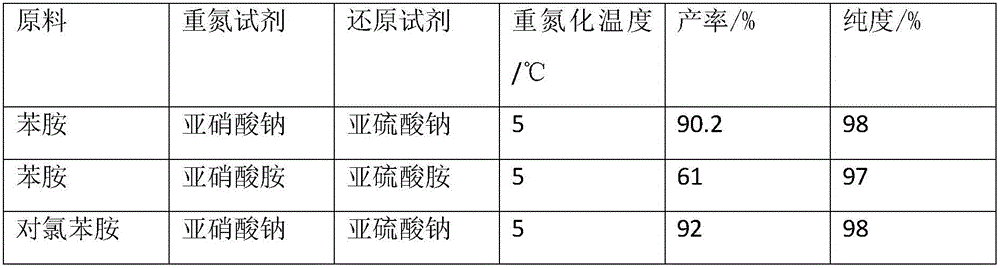
The invention provides a preparation method of 4-chlorophenylhydrazine hydrochloride. The preparation method of the 4-chlorophenylhydrazine hydrochloride includes a diazotization reaction, a reduction reaction and an acidizing reaction. The diazotization reaction includes throwing 4-chlorophenylhydrazine to water; dropping hydrochloric acid to enable the 4-chlorophenylhydrazine hydrochloride to dissolve completely; and dropwise adding a sodium nitrite aqueous solution to obtain a chlorination diazonium parachloroaniline aqueous solution. The reduction reaction includes dropwise adding the chlorination diazonium parachloroaniline aqueous solution to an ammonium sulfite aqueous solution and obtaining diazonium parachloroaniline disulfonate. The acidizing reaction includes dropping the hydrochloric acid to the diazonium parachloroaniline disulfonate; filtering; washing; discharging; stoving; and obtaining a 4-chlorophenylhydrazine hydrochloride end product. The preparation method of the 4-chlorophenylhydrazine hydrochloride is easy to operate by using the sodium nitrite aqueous solution as a reducing agent; and the product seeds out late and side reactions are reduced due to the fact that ammonium chloride and ammonium bisulfate generated in the acidizing reaction have high solubility in the water; and furthermore product quality and product yields are improved effectively due to the fact that the product is incompact in crystallization, good in flow-ability and easy to discharge and wash.
Owner:天津市天川化工有限公司
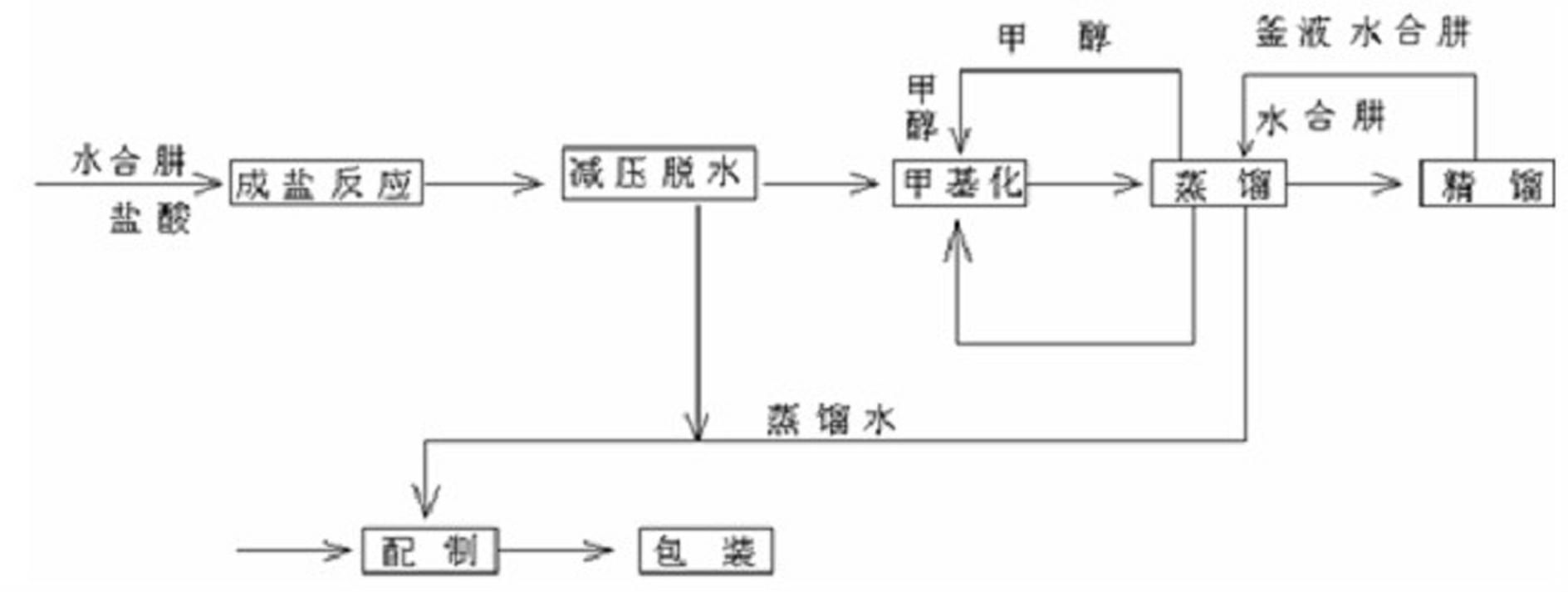
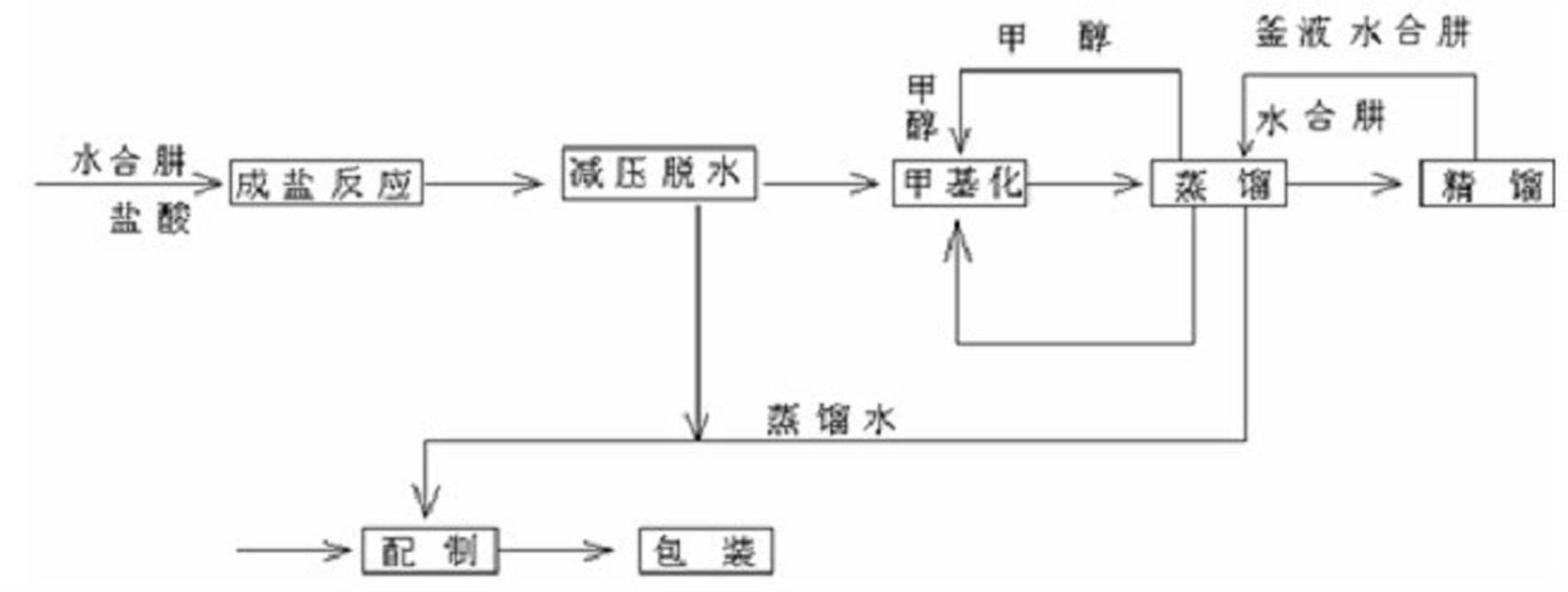
Process for producing methyl hydrazine with hydrazine hydrate method
ActiveCN102516117AEmission reductionReduce consumptionHydrazine preparationChemical oxygen demandHydrazine compound
The invention discloses a process for producing methyl hydrazine with a hydrazine hydrate method. The process is characterized by comprising the following steps of: reacting hydrazine chloride with methanol under the protection of an inert gas; isolating methyl hydrazine hydrochloride by using a hydrazine hydrate; and undergoing distilling and rectifying processes to obtain the methyl hydrazine. The process has the advantages of saving in energy, reduction in the emission of waste water, waste gas, waste residues and COD (Chemical Oxygen Demand) emission and high environmental friendliness. Internal circulation and continuous production is realized, the labor intensity of workers is lowered, reaction pressure is lowered from 0.7-1.3 MPa to 0.5-0.7 MPa, and the safe coefficient is increased.
Owner:DONGLI NANTONG CHEM
Novel synthesis process of P-chlorophenylhydrazine hydrochloride
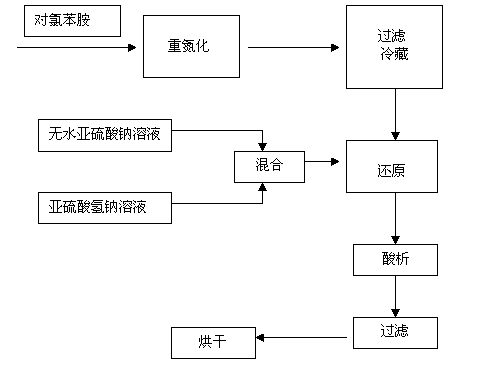
InactiveCN103848752ASimplified processing stepsGood processing effectHydrazine preparationP-chloroanilineReaction temperature
The invention discloses a preparation method of P-chlorophenylhydrazine hydrochloride. The method comprises the following steps: diazotizing chloroaniline taken as a raw material so as to prepare a corresponding diazonium salt; mixing anhydrous sodium sulfite with sodium hydrogen sulfite in a stirring manner, maintaining a pH (Power Of Hydrogen) value within 6 to 7, controlling a temperature below 40 DEG C, slowly adding the diazonium salt below a liquid surface and reacting at the temperature of 60 DEG C to 90 DEG C according to the molar ratio of the p-chloroaniline to the sodium hydrogen sulfite being 1:(1-1):3; maintaining the unchanged pH value in the step 2, slowly adding hydrochloric acid so as to maintain the pH value within 1 to 3, reacting for 1 to 3 hours at the reaction temperature of 40 DEG C to 90 DEG C, cooling to a room temperature, filtering and drying so as to obtain a reddish P-chlorophenylhydrazine hydrochloride solid. The pH value of the reaction system is maintained within 6 to 7 by taking a mixture of the sodium sulfite and the sodium hydrogen sulfite as a reducing agent, so that the phenomenon that the pH value is always regulated by NAOH (sodium hydroxide) or HCL (hydrogen chloride) in a reaction process is avoided; and the repeated heating during the reaction process is not required, so that the temperature is convenient to control; and the adding amount of water is reduced, so that the discharge of wastewater is greatly reduced, namely, the wastewater treatment at a later period is avoided. As a result, the cost is lowered.
Owner:NANJING UNIV OF SCI & TECH
Process for the preparation of phenylhydrazines
InactiveUS6852890B1Efficient preparationHydrazine preparationOrganic compound preparationOxygenHydrolysis
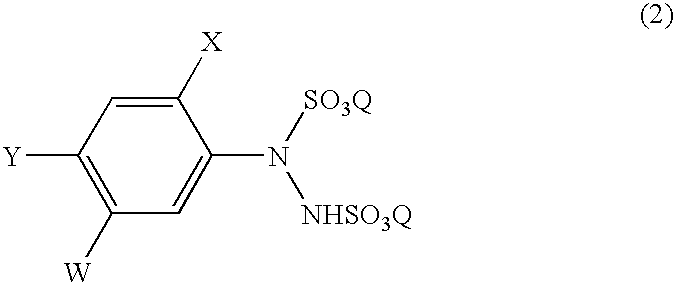
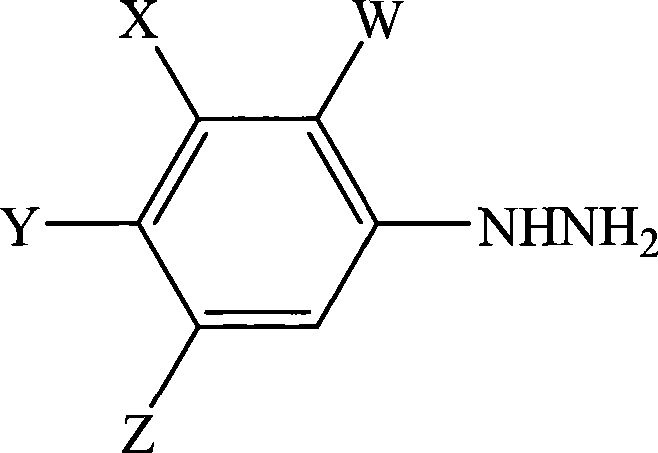
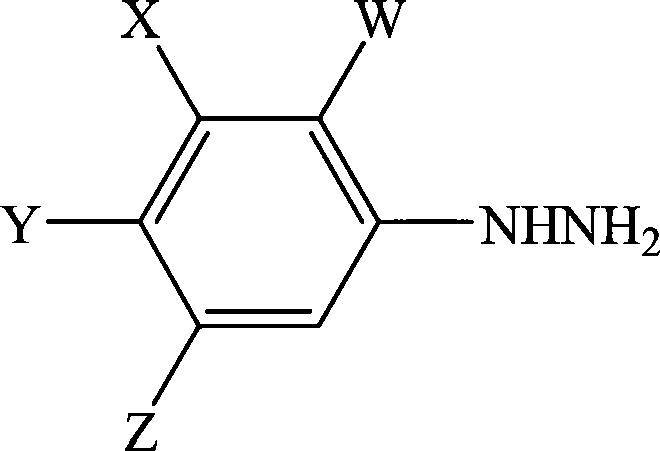
A process for the preparation of a phenylhydrazine or an inorganic acid salt thereof of the formula (1): wherein X is a hydrogen or halogen atom; Y is a halogen atom; and W is a hydrogen atom or —ZR in which Z is an oxygen or sulfur atom, and R is a hydrogen atom, an alkyl group, a haloalkyl group, and so on, by the hydrolysis of a phenylhydrazine derivative of the formula (2): where X, Y and W are the same as defined above, and the Q groups are a hydrogen atom, an ammonium group or an alkali metal atom in the presence of water and an inorganic acid, in which the concentration of the inorganic acid is at least 6 moles per 1 kg of water in a reaction system.
Owner:SUMITOMO CHEM CO LTD
Treatment method for tail gas produced by reaction of sulfonyl chlorination
InactiveCN103071365AReasonable process designSimple and safe operationHydrazine preparationChlorine/hydrogen-chlorideSodium nitriteStorage tank
A treatment method for tail gas produced by reaction of sulfonyl chlorination comprises the following steps: (1), the tail gas produced by the reaction of sulfonyl chlorination is injected into a tertiary series hydrogen chloride falling-film absorption tower, and absorption liquid in a primary hydrogen chloride falling-film absorption tower becomes hydrochloric acid to enter a hydrochloric acid storage tank; (2), tail gas after the hydrogen chloride gas is absorbed by the tertiary hydrogen chloride falling-film absorption tower and then passes through a secondary series aqua ammonia absorption tower, sulfur dioxide gas in the tail gas is absorbed by aqua ammonia in a primary aqua ammonia absorption tower, and the residual tail gas enters a secondary aqua ammonia absorption tower; (3), ammonium sulfite water solution is prepared; (4), parachloroaniline and recovered hydrochloric acid in the primary hydrogen chloride falling-film absorption tower are taken as raw material, and sodium nitrite water solution is added into the raw materials to produce parachlorobenzene diazonium chloride; (5), parachlorobenzene diazonium chloride is dripped into the ammonium sulfite water solution to produce diazonium parachlorobenzene disulfonate; and (6), recovered hydrochloric acid is dripped into the diazonium parachlorobenzene disulfonate to prepare parachlorobenzene hydrazine dihydrochloride. The treatment method effectively separates the tail gas, utilizes hydrochloric acid and ammonium sulfite, which are recovered by the tail gas, to prepare the parachlorobenzene hydrazine dihydrochloride, thereby being environment-friendly and economical.
Owner:天津市天川化工有限公司
Synthesis of bis(thio-hydrazide amide) salts
InactiveUS7709683B2Easy to disassembleLower levelHydrazine preparationOrganic compound preparationThio-Potassium hydroxide
Owner:SYNTA PHARMA CORP
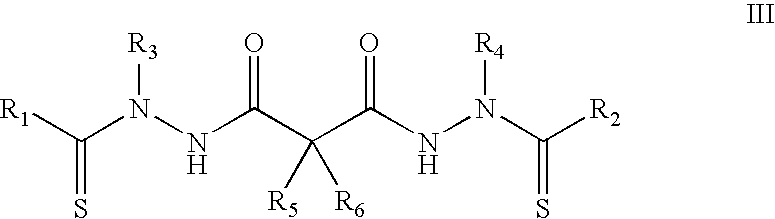
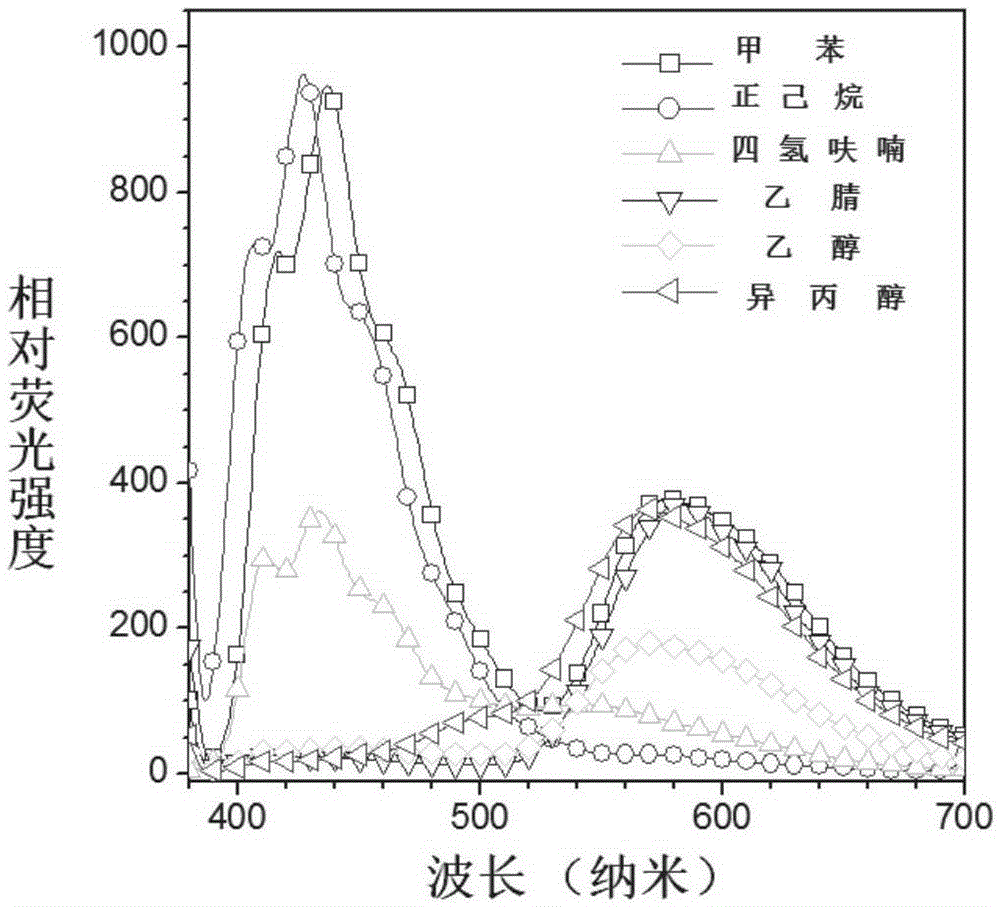
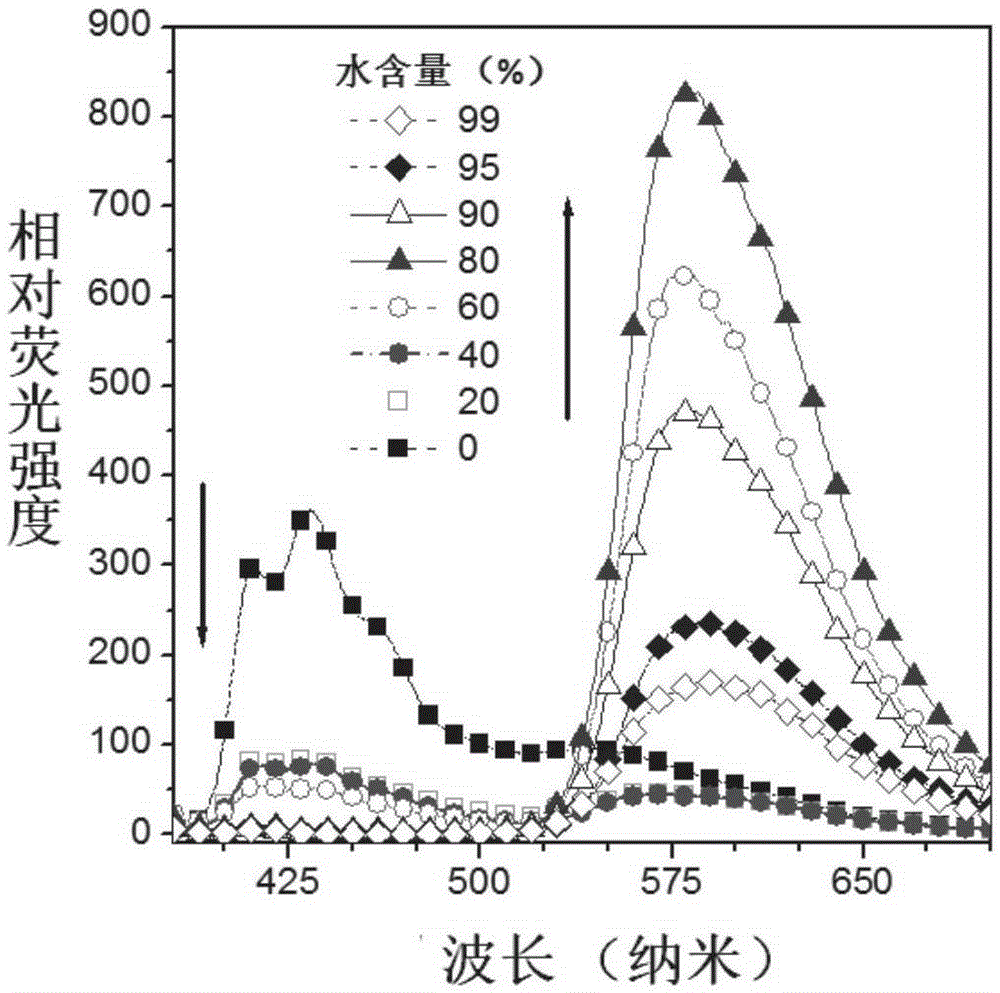
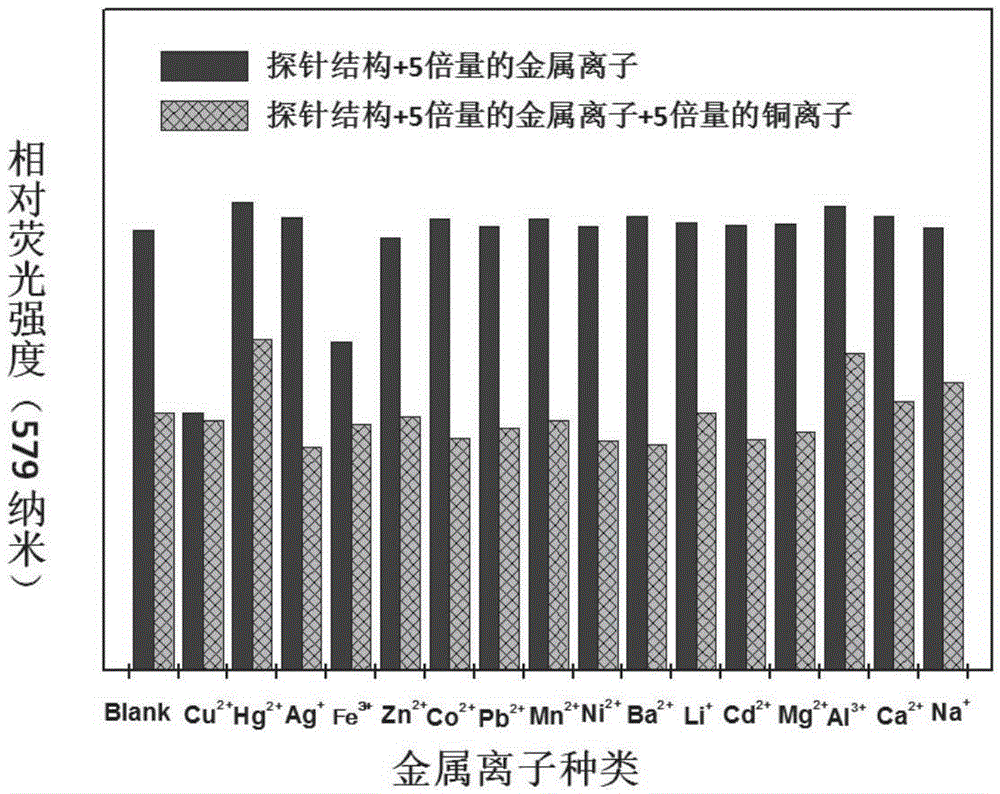
Buzane-based azine structure compound based on meta-position linkage and preparation method and application thereof
ActiveCN106496067AExcellent aggregation-induced luminescent propertiesProportional adjustmentHydrazine preparationCarboxylic acid nitrile preparationIonAggregation-induced emission
The invention relates to a buzane-based azine structure compound based on meta-position linkage and a preparation method and application thereof. The compound can serve as a red probe, has excellent aggregation-induced emission characteristics, and can be used for selectively and quantitatively recognizing metal ions under the water system condition. Effective red shift of solid fluorescence and adjustment of the ESIPT type molecular alcohol ketone proportion, ion selectivity and the detection limit are achieved through the capacity change of pushing-drawing substitutes with substituent groups, and the red fluorescent probe has important application and significance in the fields of chemical sensing, bioanalysis, ion detection and the like.
Owner:HKUST SHENZHEN RES INST
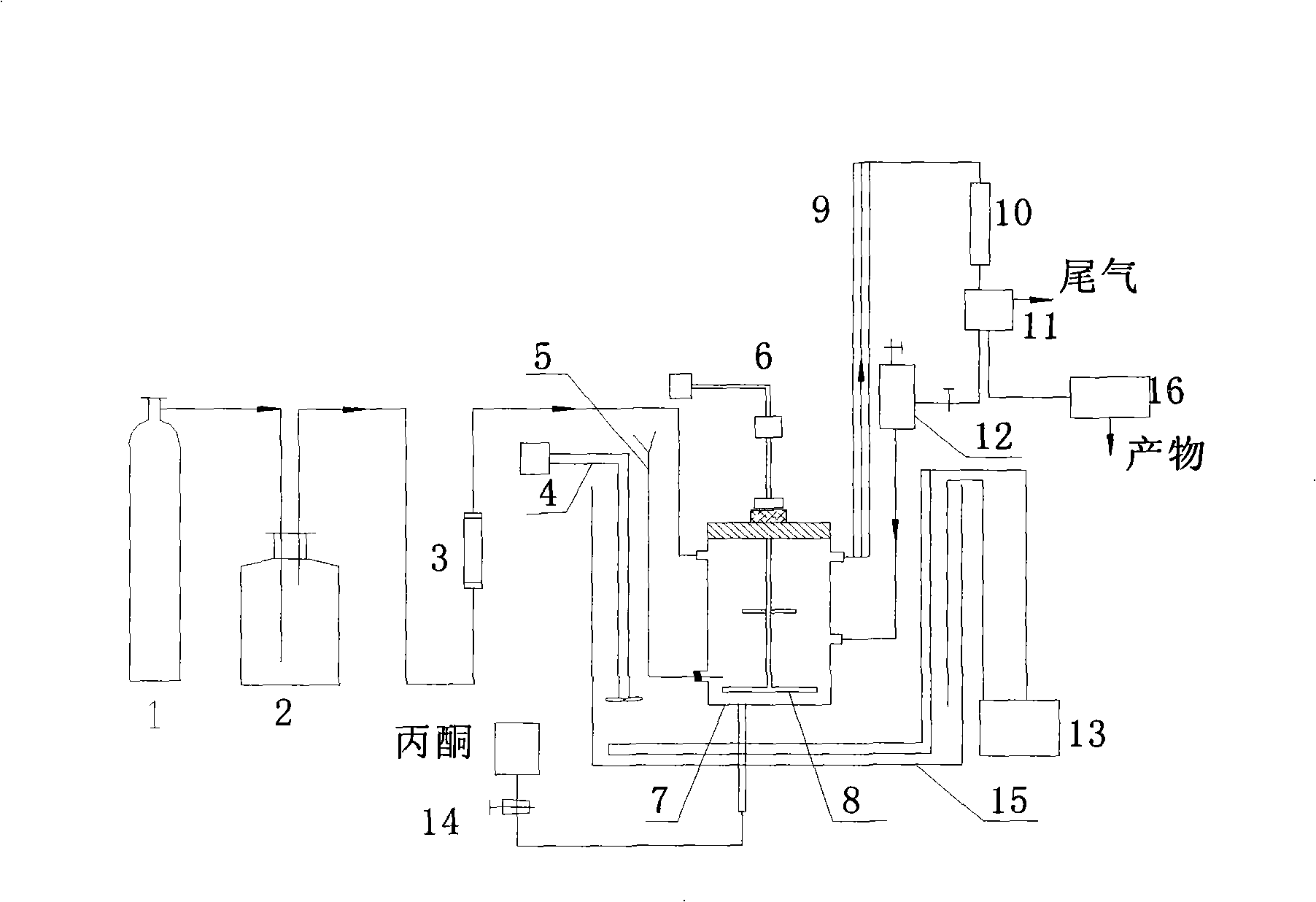
Method for gas stripping and separating hydrazine from hydrazine-containing solution using acetone and use thereof
ActiveCN101311107ASolving the Condensation Wastewater ProblemHydrazine preparationHydrazineGas phaseHydrazine compound
The invention relates to a method for obtaining pure hydrazine hydrate by isolating and purifying from solution containing hydrazine. The preparation method is that gas-phase acetone and the hydrazine which is contained in oxidation liquid are directly reacted and stripped under the boiling condition; or liquid-phase acetone and the solution are reacted first and then gas stripping is carried out, after the solution is hydrolyzed, the pure hydrazine hydrate is obtained; and the obtained product is sent into a general hydrolyzing tower, in which hydrolysis is carried out to produce the pure hydrazine hydrate solution, and acetone produced by the hydrolysis is sent back to a system for recycling, wherein, the mole ratio of the acetone and the hydrazine is 2 to 3.6 and the reaction temperature is 100 DEG C to 130 DEG C. By adopting the invention, the hydrazine produced by urea-chlorine method can be stripped by acetone azine (CH3)2C=N-N=C-(CH3)2, which causes the recycling of the inorganic materials such as sodium carbonate, sodium chloride, and the like, in the solution to be possible, thereby thoroughly solving the problem of waste water condensation of the ADC processing technique in the urea-chlorine method.
Owner:EAST CHINA UNIV OF SCI & TECH +2
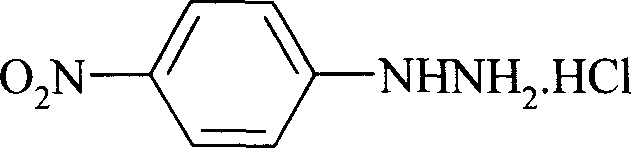
Preparation process of p-nitro phenyl hydrazine hydrochloride
InactiveCN100999483AMild reaction conditionsSimple and safe operationHydrazine preparationHydrazine compoundNitrobenzene
The invention relates to a p-Nitrophenylhydrazine muriate preparation methods. In halogenated hydrocarbonand water two-phase system, take p-cl-nitrobenzene as raw material to take reaction with hydrazine hydrate under catalyst, obtain p-Nitrophenylhydrazine, then through hydrochloride to obtain product of the invention. With this method, the purity of p-Nitrophenylhydrazine muriate can achieve more than 99%, 80 to 85% yield.
Owner:SHANGHAI CHEM REAGENT RES INST
Tailored control of surface properties by chemical modification
InactiveUS20100068783A1Big economySignificant technical bulk advantageHydrazine preparationGroup 5/15 element organic compoundsArylReactive intermediate
A process for producing a substrate having an adhesive surface, which process comprises: (a) contacting the substrate with a carbene precursor, which carbene precursor is a compound of the following formula (1): whose substituent groups are SP defined herein, provided that when R is aryl or heteroaryl, said aryl or heteroaryl may be substituted by one, two, three, four or five groups, which groups are independently selected from various groups including -LB-WB; and (b) either: (i) when WA or WB comprises an adhesive functional group, generating a carbene reactive intermediate from the carbene precursor so that it reacts with the substrate to functionalise the surface, thereby yielding said substrate having an adhesive surface; or (ii) when WA or WB comprises a group which is a precursor of an adhesive functional group, generating a carbene reactive intermediate from the carbene precursor so that it reacts with the substrate to functionalise the surface, and (c) converting said group which is a precursor into an adhesive functional group thereby yielding said substrate having an adhesive surface. The invention further relates to carbene precursor compounds for use in the process, substrates produced by the process and to processes for preparing certain precursor compounds.
Owner:OXFORD UNIV INNOVATION LTD
Method for preparing phenylhydrazine derivant
InactiveCN101134734AReduce manufacturing costShort reaction timeHydrazine preparationAromatic amineReducing agent
The present invention relates to phenyl hydrazine derivative preparing process. By using aromatic amine as the initial material, and through diazo reaction, reduction reaction with sodium pyrosulfite as reductant at 10-35 deg.c and in pH 7-9 condition, purifying and drying, phenyl hydrazine derivative product of high quality is produced in low production cost.
Owner:太仓市华联化工实业有限公司
Carbene precursor compound for producing an adhesive surface on a substrate
InactiveUS8530212B2Big advantageHydrazine preparationGroup 5/15 element organic compoundsArylReactive intermediate
Owner:OXFORD UNIV INNOVATION LTD
Pt-load catalyst taking mesoporous carbon as carrier, as well as preparation method and usage thereof
InactiveCN102671656AAdvantages of preparation methodHigh reactivityHydrazine preparationMetal/metal-oxides/metal-hydroxide catalystsO-nitrochlorobenzenePretreatment method
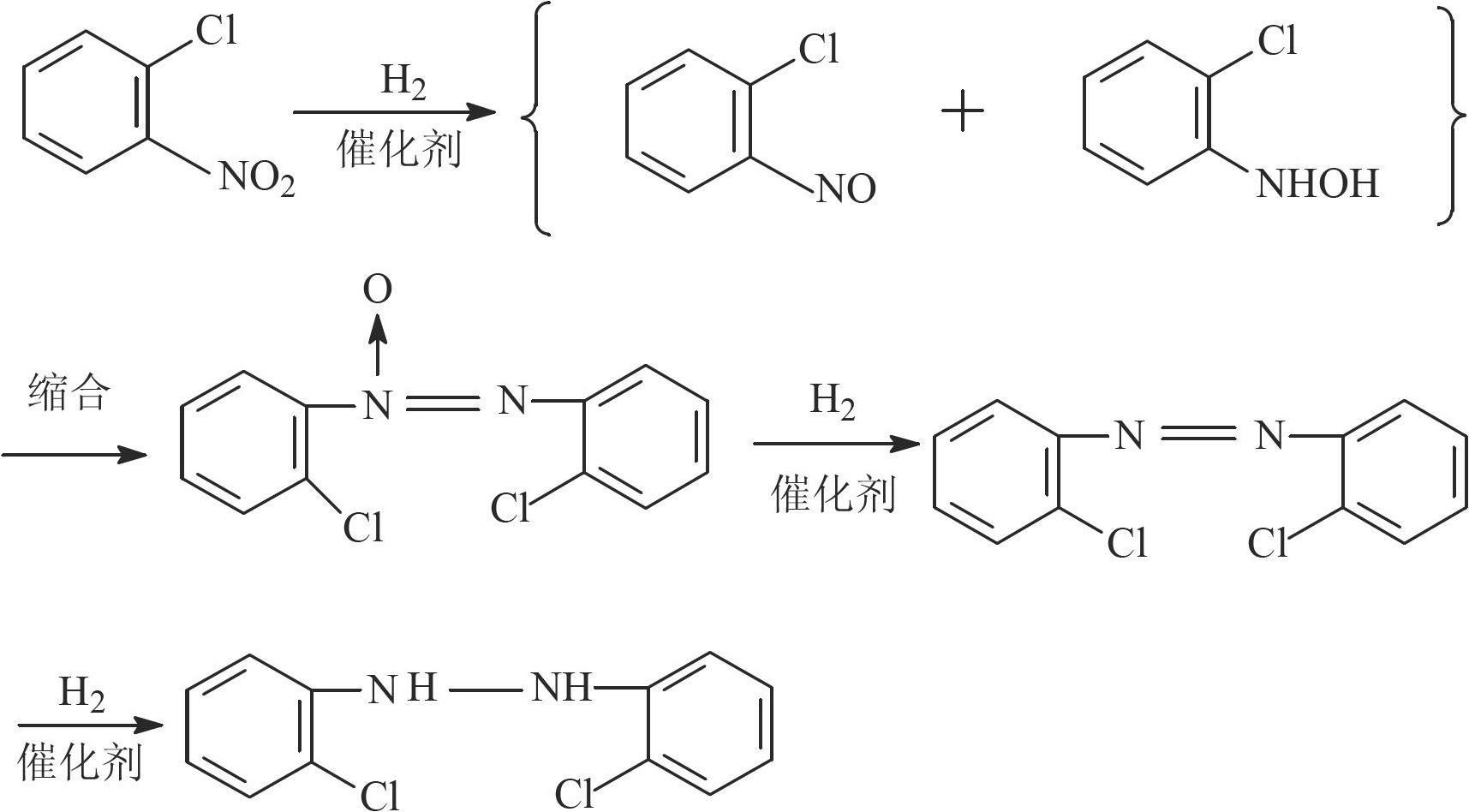
The invention relates to a Pt-load catalyst taking mesoporous carbon as a carrier, which is characterized in that the mesoporous carbon is pretreated before Pt is loaded; the pretreatment method comprises one in the following steps of: (1) adding the mesoporous carbon into a 10-30% hydrogen dioxide solution, soaking for 1-24hours, washing and removing hydrogen peroxide; (2) adding the mesoporous carbon into a 1-6M hydrochloric acid or nitric acid solution, soaking for 1-24hours, washing and removing hydrochloric acid or nitric acid; and (3) adding the mesoporous carbon into a 1-6M sodium hydroxide solution, soaking for 1-24hours, washing and removing alkali, wherein the load quantity of Pt is 0.5-5%. The invention also relates to the application of the Pt-load catalyst in preparation of 2, 2'-dichloro hydroazobenzene (DHB) from ortho-nitrochlorobenzene through hydrogenation. The catalyst uses the mesoporous carbon as the carrier, so that the internal diffusion resistance can be better reduced, and the reaction speed is accelerated. The Pt-load catalyst is high in reaction activity and selectivity, and can be repeatedly used for more than eight times, so that the using cost of the catalyst is lowered.
Owner:CHANGZHOU UNIV +1
Preparation method for 4-chlorophenylhydrazine hydrochloride
InactiveCN103910650AEliminates the problem of easy agglomeration and difficult operation of feedingEasy dischargeHydrazine preparationSolubilityHydrogen Sulfate
A provided preparation method for 4-chlorophenylhydrazine hydrochloride comprises the following steps: 1) under an acidic condition, at 5-10 DEG C, dropwise adding a sodium nitrite aqueous solution with a concentration of 20% into 4-chloroaniline to perform a diazo reaction and to generate a diazo salt; 2) under a room temperature condition, dropwise adding an ammonium sulfite aqueous solution into a solution of the diazo salt obtained in the step 1, heating to 50-60 DEG C and keeping the temperature for 3-4 h; and 3) at 50-70 DEG C, dropwise adding a hydrochloric acid solution with a concentration of 20% into the solution obtained in the step 2, keeping the temperature for 1-2 h, reducing the temperature, filtering, washing, discharging the material, and drying to obtain a finished product. The beneficial effects comprise that by using ammonium sulfite aqueous solution as a reducing agent in the reduction reaction, the problems are solved that sodium sulfite solid is easy to cake during material charging in a conventional technology and material charging is not easy to operate; ammonium chloride and ammonium hydrogen sulfate which are generated in the acidification reaction have high solubility in water, and crystallization precipitation is late, thereby facilitating performing of the acidification reaction in the solution and reducing side reactions; and the product crystallizes loosely, is good in fluidity, easy for material discharging and washing, and product quality and yield are both better than conventional production levels.
Owner:TIANJIN QIDONG CHEM PLANT
Synthesis method of parachlorophenylhydrazine hydrochloride
InactiveCN105837466AHigh yieldThe synthesis process is simpleHydrazine preparationAfter treatmentSynthesis methods
The invention provides a synthesis method of parachlorophenylhydrazine hydrochloride. The synthesis method comprises the following steps: 1. diazotization of parachloroaniline; 2. reduction; 3. acidification; and 4. after-treatment. The synthesis method has the advantages of environment-friendly raw materials, simple synthesis technique, environment friendliness, low cost, high product yield, energy saving and consumption reduction, effectively lowers the production cost, and can enhance the economic benefit of the enterprise.
Owner:JIANGSU TUOQIU AGRI CHEM CO LTD
P-chlorophenylu hydrazine hydrochloride preparation method
The invention discloses a p-chlorophenylu hydrazine hydrochloride preparation method. P-chloroaniline serves as a raw material, after diazotization and reduction, non-polar solvent toluene or benzene, dichloromethane, trichloromethane and dichloroethane are used for extracting an aqueous phase to remove impurities, and then hydrochloric acid is added into the aqueous phase for hydrolysis to obtain p-chlorophenylu hydrazine hydrochloride through cooling, filtering and during. The chemical equation is shown as the following (please see the formula in the specification). Compared with the prior art, the non-polar solvents are used for extraction and impurity removal, the yield and the purity of an obtained product are high, the purity of the p-chlorophenylu hydrazine hydrochloride is larger than 99%, the yield is larger than 86%, filtering only needs to be carried out once, and operation is easy. Waste water amount is reduced by half compared with the prior art, and environmental protection pressure is reduced.
Owner:HUNAN HAILI CHEM IND
Preparation method of paracyano-group phenyl hydrazine hydrochloride
InactiveCN101550091ALower requirementReasonable choice of preparation processHydrazine preparationHydrazine compoundAniline
The invention relates to a preparation method for compounding fragrant hydrazine, in particular to a preparation method of paracyano-group phenyl hydrazine hydrochloride, which mainly solves the technical problems of poor crystal shape and low yield of the prior preparation method. The adopted technical scheme is as follows: the preparation method of the paracyano-group phenyl hydrazine hydrochloride is characterized in that: paracyano-group aniline is used as a raw material and is prepared into diazo hydrochloride in a hydrochloric water solution by the diazotizing reaction of sodium nitrite in the mode of one-step continuous reacting operation, the diazo hydrochloride is reduced by a tin-protochloride hydrochloric solution, filtered and washed to obtain paracyano-group phenyl hydrazine hydrochloride, and the paracyano-group phenyl hydrazine hydrochloride is recrystallized by the mixed solution of methanol and water to obtain a white crystal target product. The paracyano-group phenyl hydrazine hydrochloride obtained by the invention is an important normal medical intermediate product.
Owner:上海药明康德新药开发有限公司
Method for producing 3-(2,2,2-trimethylhydrazinium)propionate dihydrate
A method for producing 3-(2,2,2-trimethylhydrazinium)propionate dihydrate by saponification of salts of 3-(2,2,2-trimethylhydrazinium)propionate esters with subsequent purification step using saturation with carbon dioxide or sulphur dioxide in alcoholic solution.
Owner:GRINDEKS
Industrial synthesis method for 4-trifluoromethylphenylhydrazine Hydrochloride
InactiveCN101209980ALower requirementReasonable choice of preparation processHydrazine preparationHydrazine compoundFiltration
The invention relates to a preparation method for composing aromatic hydrazine hydrochloride, in particular to an industrial preparation method of 4-trifluoromethyl phenylhydrazine hydrochlorid. The invention mainly aims at solving the technical problems that low yield of the existing synthetic methods has low yield and industrial production can not be realized. The invention takes 4-trifluoromethyl aniline as raw material, successive reactions 'in one pot' happen, diazotization and reduction are carried out successively, diazonium hydrochloride is prepared after diazo reaction of sodium nitrite in aqueous hydrochloric acid solution, then hydrochloric acid solution of reducer stannous chloride is used for achieving reduction reaction, finally, filtration and washing are carried out and the 4-trifluoromethyl phenylhydrazine hydrochlorid is prepared. The invention can be used for the industrial production of the 4-trifluoromethyl phenylhydrazine hydrochlorid, and the yield can be up to more than 75 percent.
Owner:上海药明康德新药开发有限公司
Synthetic method of phenylhydrazine
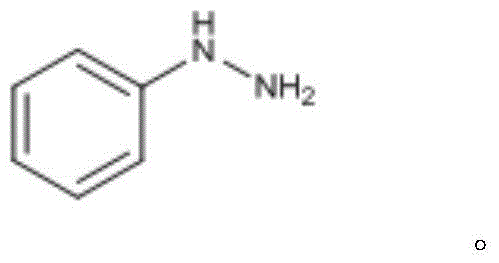
ActiveCN104610093AReduce water consumptionHigh recycling valueHydrazine preparationDistillationAniline
The invention discloses a synthetic method of phenylhydrazine. The synthetic method comprises the following steps: adding dilute sulfuric acid into aniline, dropwise adding ammonium nitrite at 0-5 DEG C, and then performing thermal reaction for 4-6 minutes; then cooling to a temperature between -5 and -10 DEG C, adding a solid reducing agent, and stirring violently; when the temperature of the reaction system naturally rises to be more than 40 DEG C, adding dilute sulfuric acid to adjust the pH value to be 2-4, and heating to 70-100 DEG C to react for 4-6 hours; and separating the obtained reaction product to obtain phenylhydrazine. According to the synthetic process of phenylhydrazine, disclosed by the invention, aniline is used as a raw material, dilute sulfuric acid is used as salt forming and acidifying reagents, ammonium nitrite is used as a diazotization reagent, ammonium sulfite and / or ammonium hydrogen sulfite is used as the reducing agent, ammonia gas is used for neutralizing a reaction solution, phenylhydrazine is finally obtained by virtue of extraction and distillation, and a byproduct salt is single ammonium sulfate.
Owner:江苏艾科维科技有限公司
Synthetic method for 4,4'-dihalogenated-3,3'-dialkyl(alkoxyl) biphenyl compounds
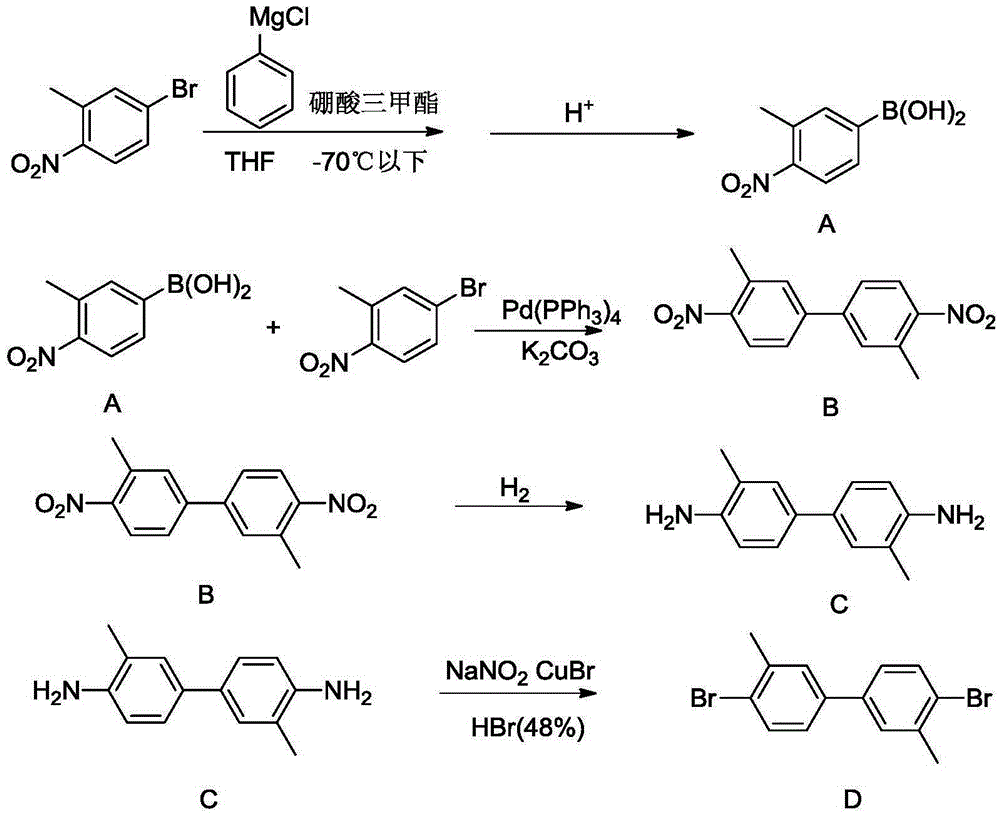
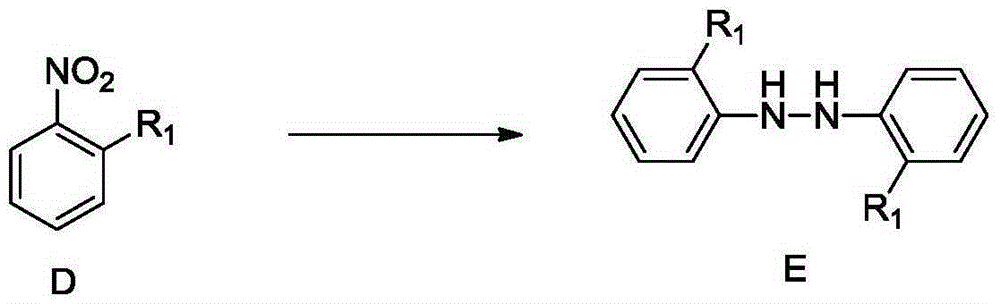
InactiveCN105348038AMild reaction conditionsEasy to implementHydrazine preparationOrganic compound preparationBenzeneHydrazobenzene
The invention relates to a synthetic method for 4,4'-dihalogenated-3,3'-dialkyl(alkoxyl) biphenyl compounds. The method employs o-nitro alkyl (alkoxyl) benzene as an initial raw material, 2,2'-dialkyl(alkoxyl) hydrazobenzene is prepared through catalysis hydrogenation in an alkaline environment, hydrochloric acid acidifying rearrangement is carried out, 4,4'-diamino-3,3'-dialkyl(alkoxyl) biphenyl is prepared, finally, a diazotization reaction is carried out and 4,4'-dihalogenated-3,3'-dialkyl(alkoxyl) biphenyl compounds are prepared. The synthetic method is advantaged by cheap and easily available raw materials, mild reaction conditions, simple operation, high safety coefficient, high yield and low cost.
Owner:烟台九目化学股份有限公司
Method for preparing 4-chlorine phenylhydrazine
InactiveCN101157634AShort reaction timeReduce manufacturing costHydrazine preparationSodium metabisulfiteHydrolysis
The invention relates to a preparative method of 4-chlorophenyl hydrazi. By using 4-chloraniline as the initial raw material, the preparative method is capable of producing the 4-bromophenylhydrazine after the diazotization reaction, the reduction reaction and hydrolysis, wherein, the reduction reaction which utilizes sodium metabisulfite as the reduction agent is carried out under the conditions that the temperature is 10 to 35 DEG C and the PH is 7 to 9. The 4- chlorophenyl hydrazi produced by the preparative method of the invention is characterized in high product purity and low production cost.
Owner:太仓市华联化工实业有限公司
Carbocyclic hydrazino inhibitors of copper-containing amine oxidases
InactiveUS6982286B2Inhibits adhesion of leukocyteUseful in treatmentBiocideNervous disorderHydrazine compoundOxidative enzyme
The present invention is directed to carbocyclic hydrazino compounds that function as inhibitors of copper-containing amine oxidases commonly known as semicarbazide-sensitive amine oxidases (SSAO), including the human SSAO known as Vascular Adhesion Protein-I (VAP-1). These SSAO inhibitors have therapeutic utility as drugs to treat conditions and diseases including, but not limited to, a number of inflammatory conditions and diseases (in particular chronic inflammatory conditions such as chronic arthritis, inflammatory bowel diseases, and chronic skin dermatoses), diseases related to carbohydrate metabolism and to aberrations in adipocyte differentiation or function and smooth muscle cell function, and vascular diseases. The novel compounds have the general formula: or a pharmaceutically acceptable solvate, hydrate, or salt thereof wherein R1 to R11 are as defined herein.
Owner:BIOTIE THERAPIES CORP
Synthesis method for aromatic hydrazine sulfate
The invention discloses a synthesis method for aromatic hydrazine sulfate. The synthesis method includes the steps that firstly, aromatic amine is added in sulfuric acid with the mass fraction ranging from 15% to 65%, the temperature is lowered to -5-15 DEG C, a diazotization reagent is dropwise added in the reaction liquid, and diazonium salt is obtained after reaction; secondly, a reducing agent is prepared into a solution, a reducing solution is obtained, the diazonium salt obtained in the first step is dropwise added in the reducing solution, heating reduction is conducted after drop-by-drop addition is completed, and reducing material liquid is obtained through filtration; and thirdly, the reducing material liquid obtained in the second step is heated, dilute sulphuric acid is dropwise added in the reducing material liquid to conduct the acidolysis reaction, the temperature is lowered after the reaction is finished, the aromatic hydrazine sulfate is separated out, a filter cake and filtrate are obtained through filtration, the filter cake is washed with dilute sulphuric acid, and an aromatic hydrazine sulfate product is obtained, and sodium sulfate salt is recycled after the filtrate is preprocessed. Compared with a traditional process, the obtained aromatic hydrazine sulfate is high in yield and purity, the purity reaches 97% or above, the yield is higher than 91%, salt in the wastewater obtained through the reaction is the simplex sodium sulfate salt, and the recycling value is high.
Owner:ZHEJIANG QICAI ECO TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com