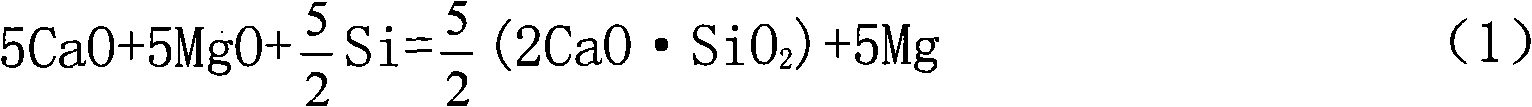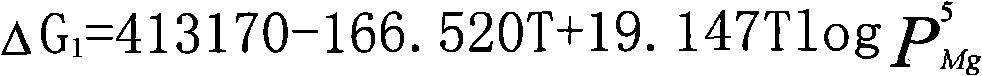Patents
Literature
136 results about "Chemical equation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and formulae, wherein the reactant entities are given on the left-hand side and the product entities on the right-hand side. The coefficients next to the symbols and formulae of entities are the absolute values of the stoichiometric numbers. The first chemical equation was diagrammed by Jean Beguin in 1615.
Automatic recognition method pf mathematical formula in image
InactiveCN101329731ARealize automatic identificationAutomate analysisCharacter and pattern recognitionChemical equationTheoretical computer science
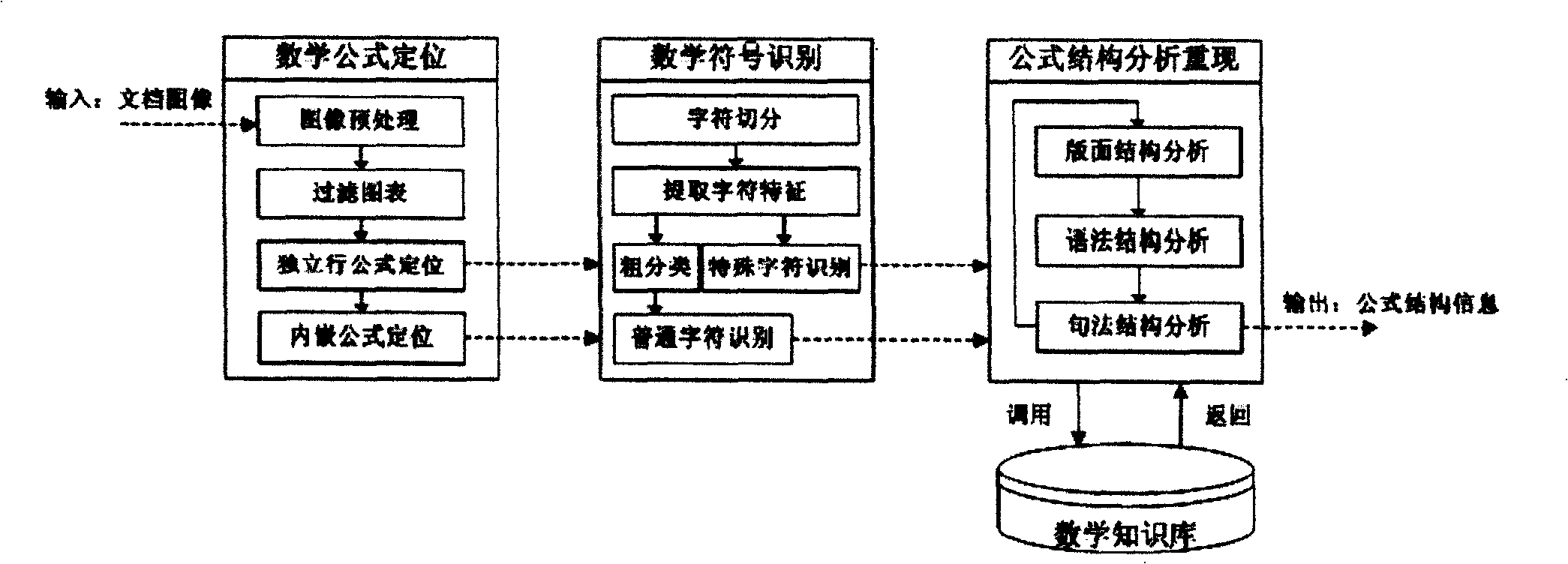
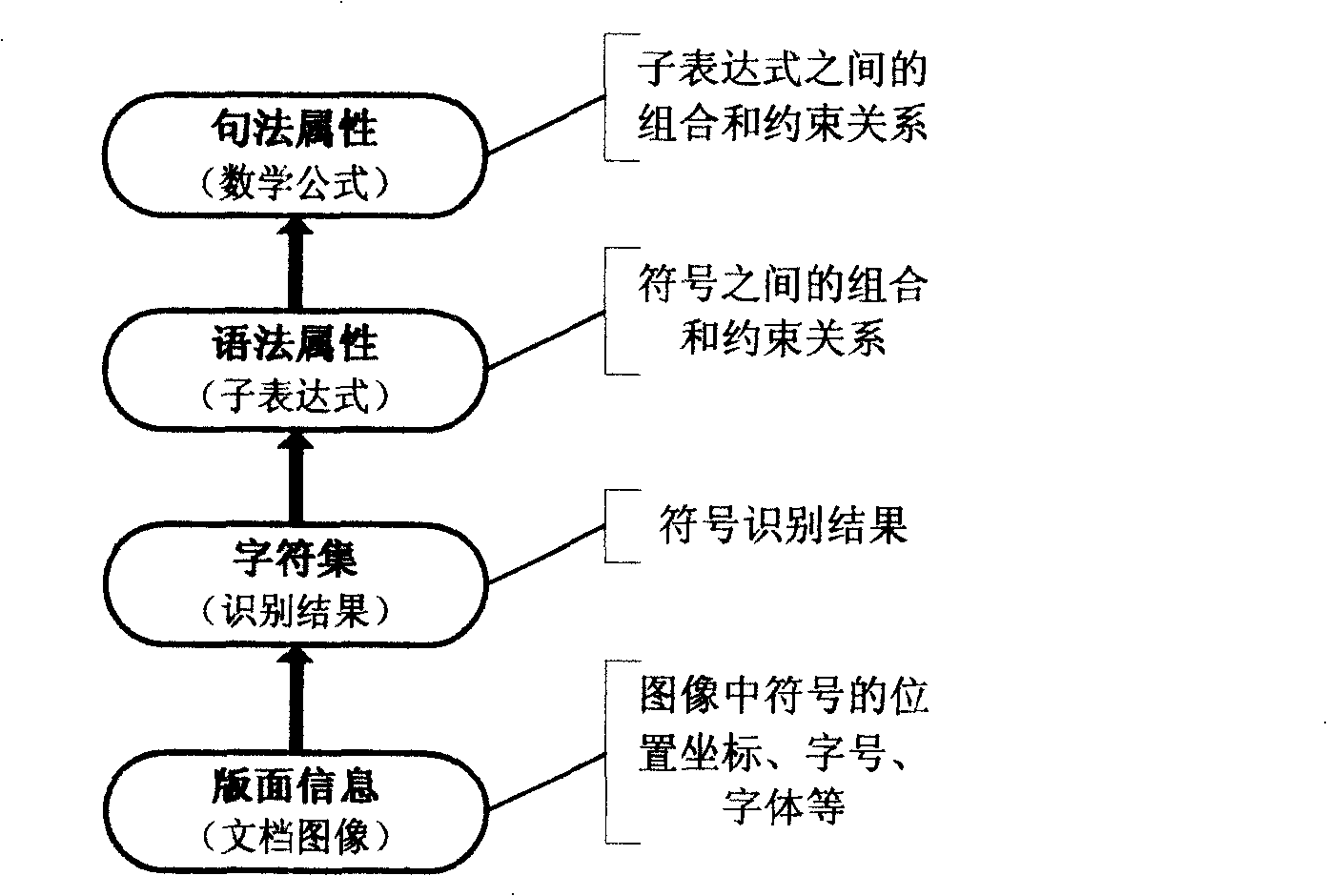
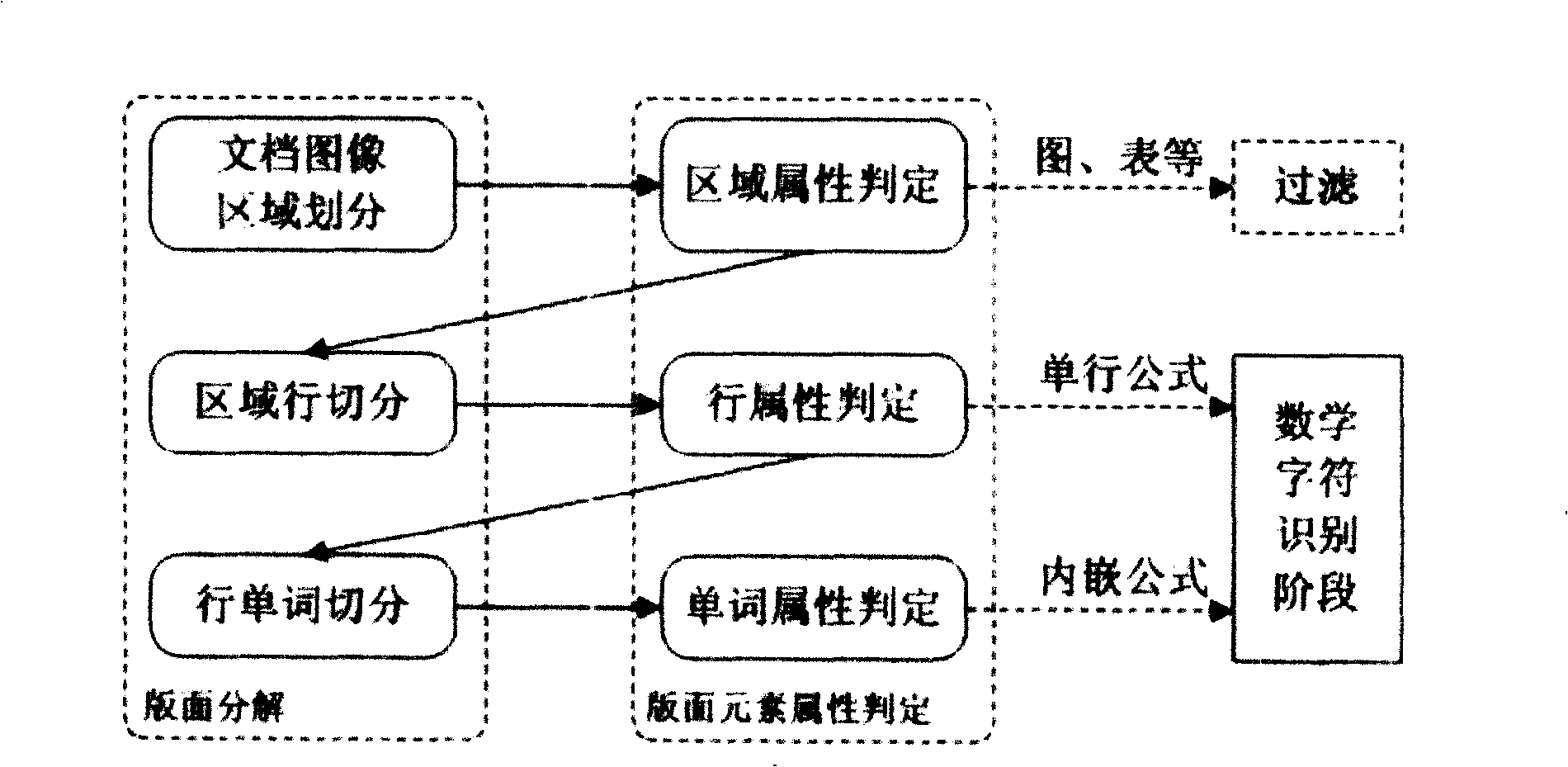
The invention relates to an automatic recognition method of a mathematical formula in an image, which comprises the steps that: a syntactic structure model of the mathematical formula is built, and a bottom knowledge base of the mathematical formula is built; the location of the mathematical formula in the image, the recognition of a mathematical symbol, the analysis and comprehension of a mathematical formula structure and the expression and formatted output of the mathematical formula structure are carried out. The automatic recognition method designs a complete set of method and model for solving the recognition and comprehension difficulties of an off-line mathematical formula image and forms a method for automatically processing the mathematical formula image in the whole process. The method can realize the automatic judgment and extraction of an individual-line / embedded mathematical formula in an image, thereby meeting the application requirements of the automatic inputting of the mathematical formula image and the comprehension and format recurrence of the mathematical formula structure. The method can be combined with the existing normal text OCR system to form a document image processing system with more complete functions and also can support the research on expression processing methods in other fields, such as automatic processing aiming at chemical equations, etc.
Owner:NANKAI UNIV
Preparation method of fipronil
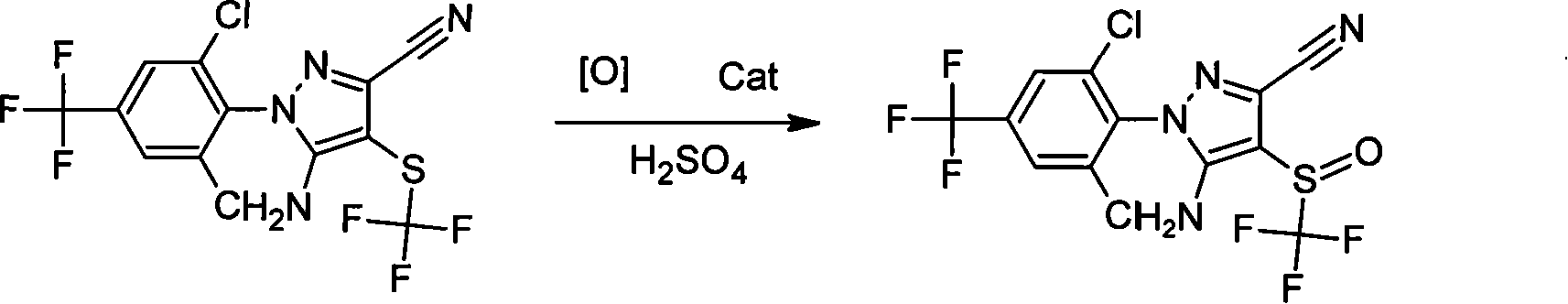
ActiveCN101250158ASimple processMild reaction conditionsOrganic chemistryArthropodicidesChemical reactionFipronil
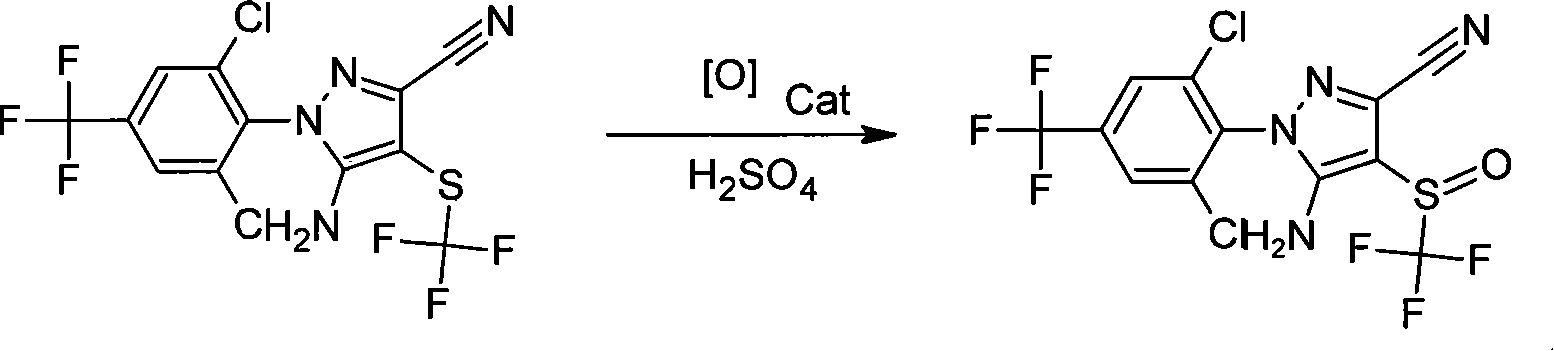
The invention discloses a method for using 5-amino-3-cyano-1-(2, 6-dichloro-4-trifluoromethylphenyl)-4-Trifluoromethylthio pyrazole as raw material to generate fipronil via catalysis and oxidation in solvent and sulfate medium. The chemical equation is represented as above. The invention oxidizes sulfate medium to generate fipronil, and the sulfate can be diluted by adding water and be layered to recover. The invention has the advantages of simple process, environment protection, mild reaction conditions and low production cost, with wide industrial application.
Owner:HUNAN CHEM RES INST
Preparation method for intermediate epichlorophdrin of herbicide pretilachlor
InactiveCN101284768AEmission reductionEliminate potential safety hazardsOrganic chemistryOrganic compound preparationChemical reactionOrganic solvent
The invention relates to a method for preparing the designed herbicide pretilachlor intermediate chloride ether. Under the action of organic amine catalyst, the chloride ether can be obtained by ethylene glycol monopropyl ether and bis (trichloromethyl) hydroxyphenyl in the organic solvent through the following chemical equation: HOC2H4OC3H7+Cl3COCOOCCl3 arrow ClC2H4OC3H7+CO2+HCl. The method replaces the traditional chlorination reagent with the bis (trichloromethyl) hydroxyphenyl, such as, thionly chloride, phosphorus oxychloride and hydrogen chloride, etc., and the potential safety hazard can be eliminated from the process source by using the novel chlorination reagent. Simultaneously, the emissions of pollutants are greatly reduced, and the method is a cleaning process with mild reaction conditions, safe and reliable operating process and high product yield, and has good industrialization implementation value and social economic benefits.
Owner:HANGZHOU VOCATIONAL & TECHN COLLEGE
Blood clotting predictor
A computer program product for predicting the speed and efficacy of a blood-clotting agent is disclosed. The product comprises a computer usable medium having computer readable program code means embodied in the medium for causing an application program to execute on a computer with a database for storing data therein. The computer readable program code means comprises a first computer readable program code means for causing the computer to enter data into the database from a user interface, a second computer readable program code means for causing the computer to enter chemical equations into the database according to a user's input, a third computer readable program code means for causing the computer to compile differential equations corresponding to the chemical equations, a fourth computer readable program code means for causing the computer to solve the differential equations, and a fifth computer readable program code means for causing the computer to display the results of the solution to the differential equations.
Owner:MANN KENNETH G +3
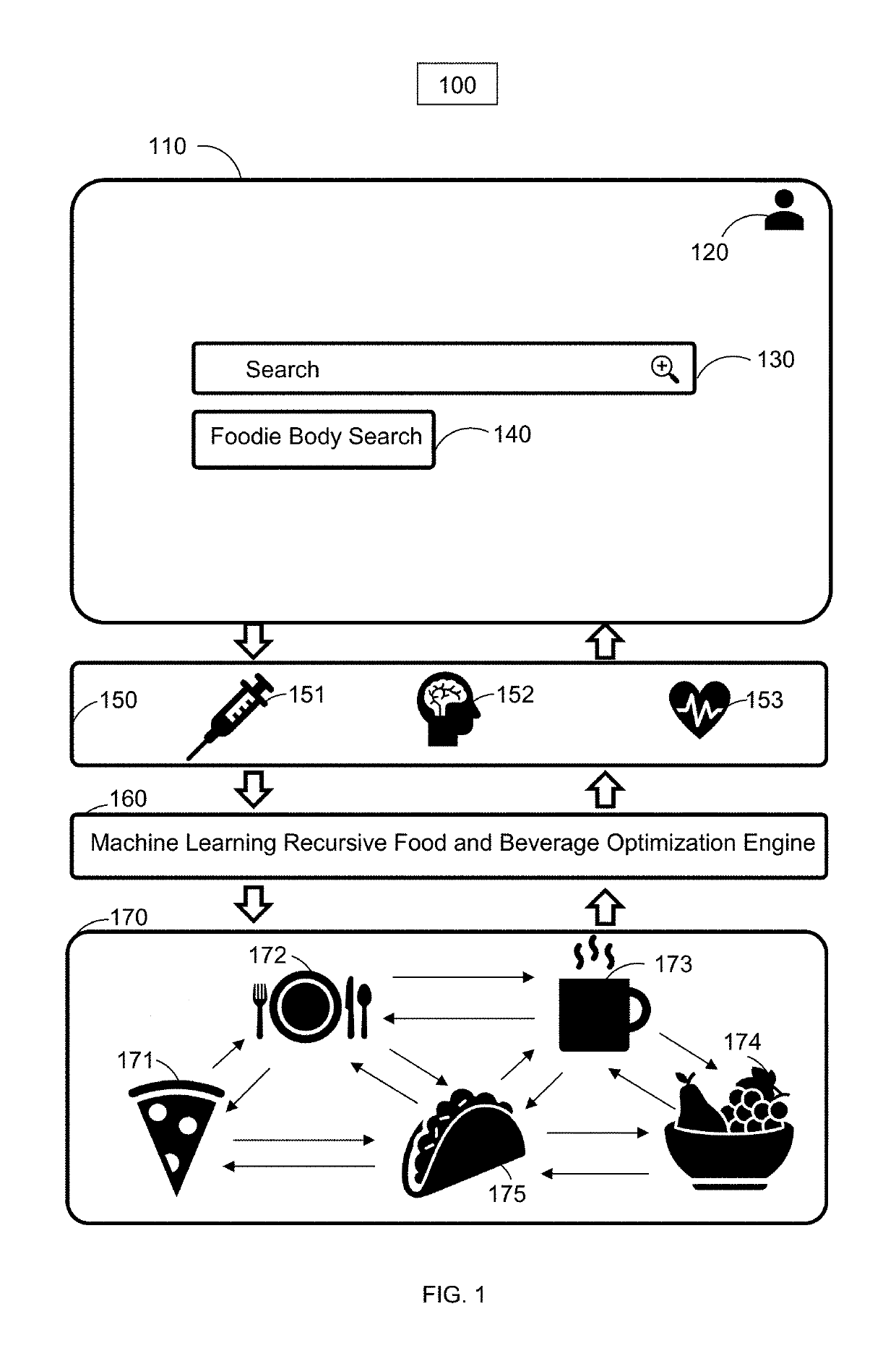
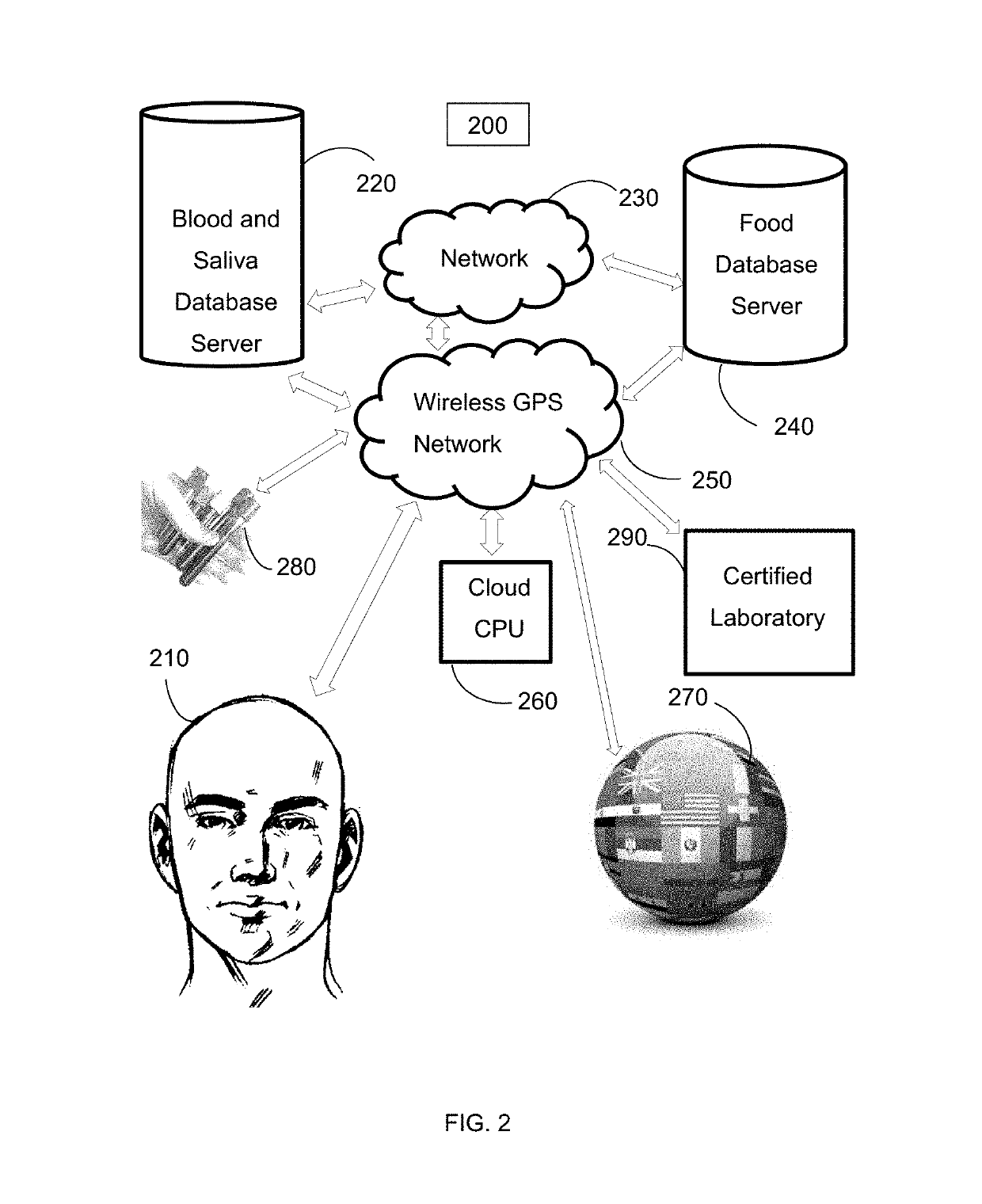
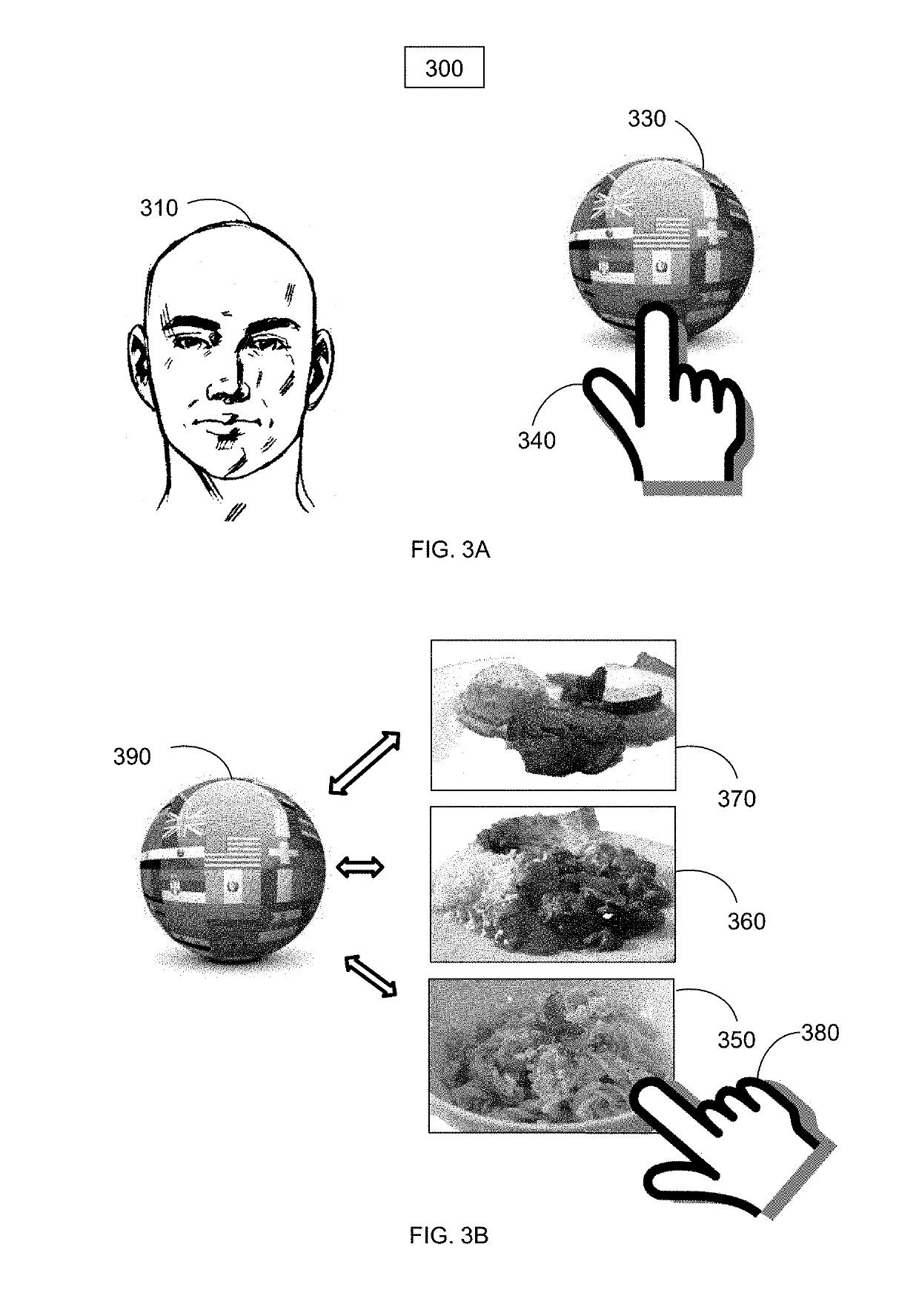
Online food and beverage search method based on food to biomarker optimization algorithms in a node ranked database
A computer implemented method for use in conjunction with a computing device, system, network, and cloud with touch screen two dimension display or augmented / mixed reality three dimension display comprising: obtaining, analyzing and detecting user blood, saliva, hair, urine, stool, fingernail, height, weight and skin sampling analysis chemistry data, mapping the blood, saliva, hair, urine, stool, fingernail, height, weight and skin data into a database associated with a specific user, applying the data with optimization equations, mapping equations to food and beverage chemistry, scoring or ranking a plurality of optimized results such that a user may order food and beverage from a food / beverage distribution point or have food / beverage delivered to the user which has been specifically optimized for their specific biochemistry characteristic target ranges. The method is particularly useful in enhancing online internet search engine results.
Owner:CIRCLESX LLC
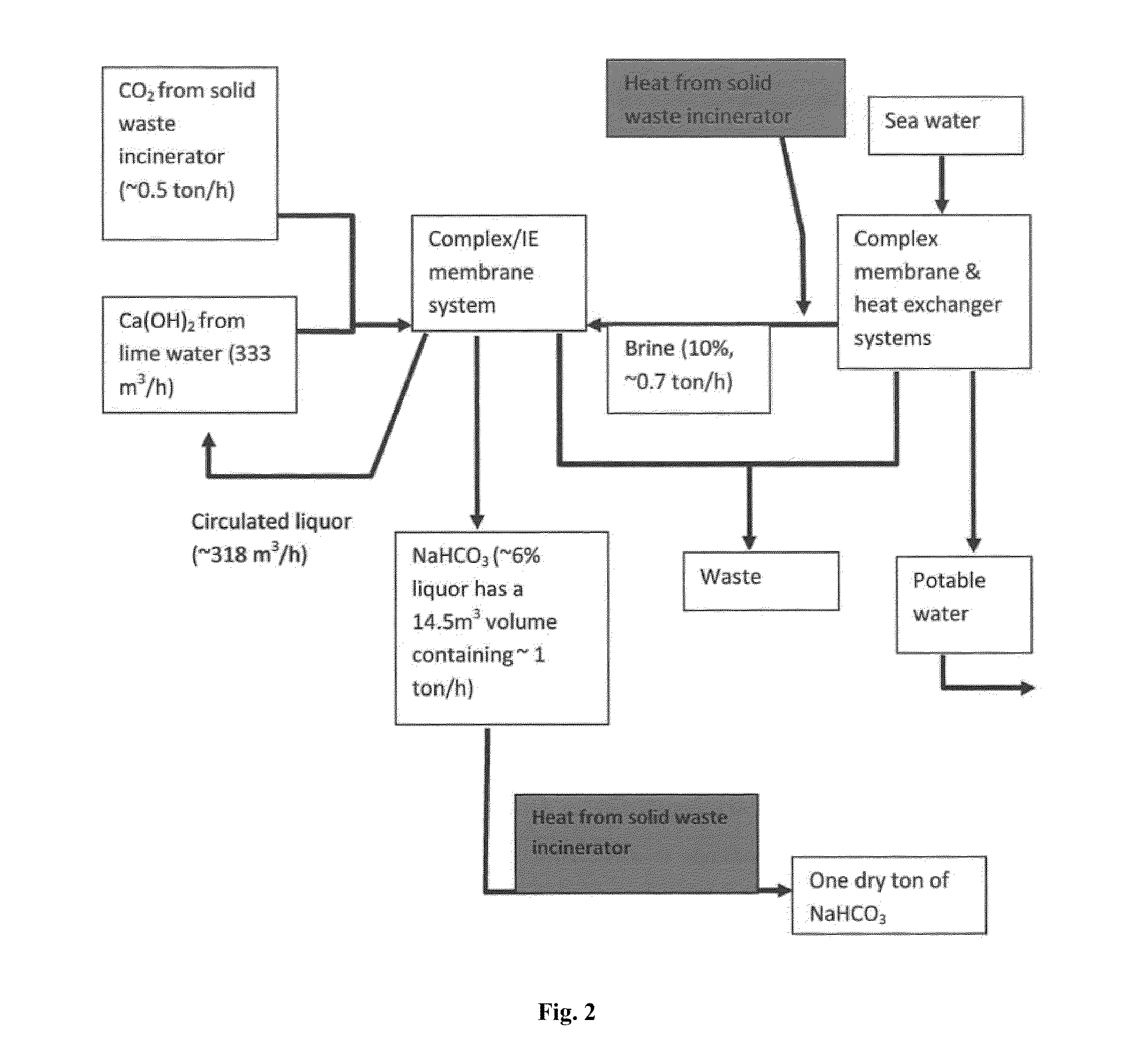
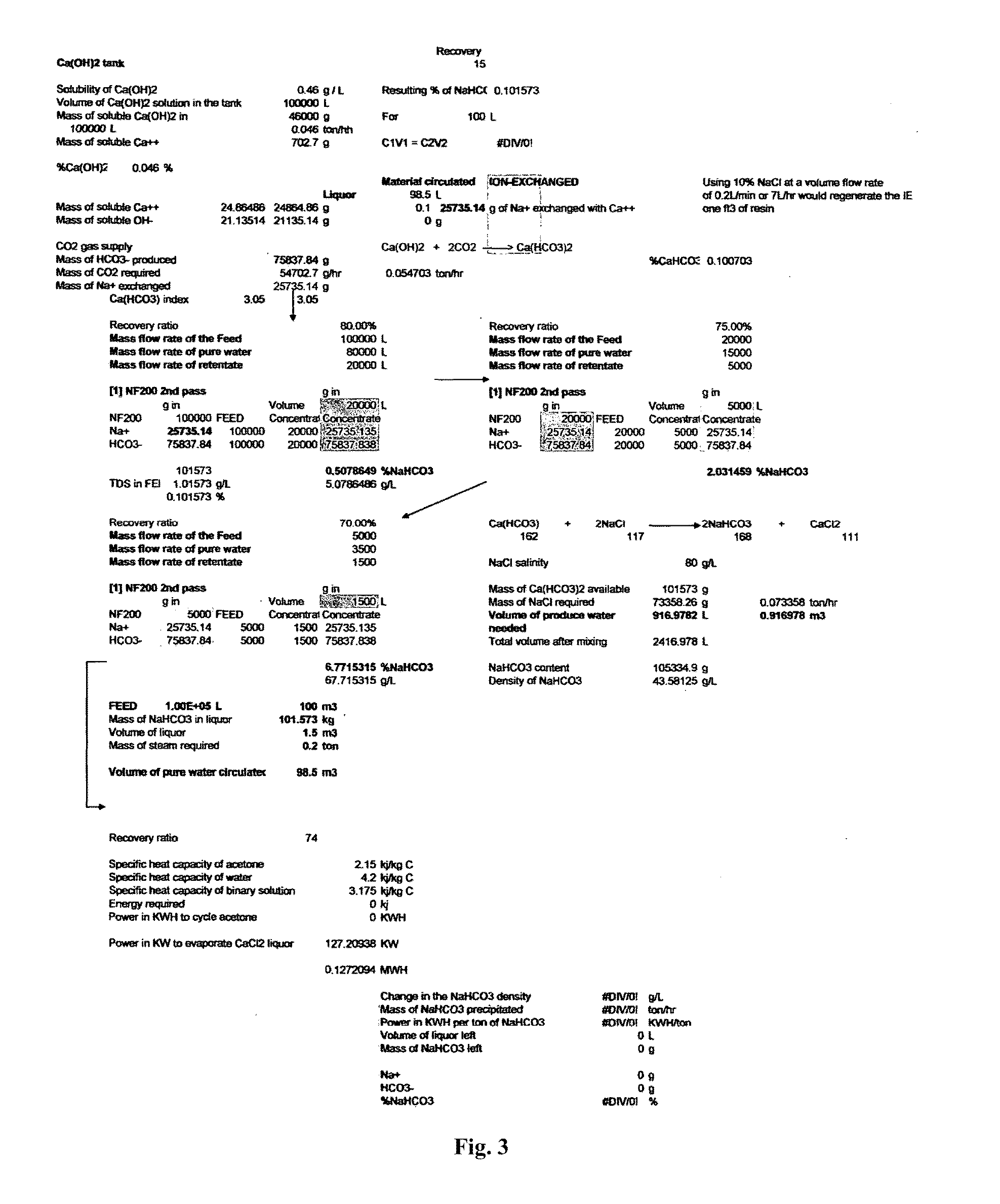
Combined solid waste, carbon dioxide quicklime sparging, brine water, and reverse osmosis/ion exchange processes for the production of soda chemicals
InactiveUS20110217227A1Calcium/strontium/barium carbonatesSemi-permeable membranesWaste processingChemical equation
The proposed invention uses a classical chemical equation where carbon dioxide CO2 is reacted with quick lime Ca(OH)2 to produce soda carb NaHCO3 and concentrating it to 6% using advanced membrane and resin technology. The invention requires three chemicals CO2, Ca(OH)2, and sodium chloride NaCl to produce NaHCO3. The output of many industrial processes lacks waste heat and in many instances CO2 and the present invention combines a solid waste processing unit to the above processes which allows the production of solid products or high % liquors. Availability of waste heat sources can lead to high efficiency in NaHCO3, Na2CO3, and NaOH production. The process is not chloro-alkali electrochemical or Solvay column ammonia processing technique. Advanced membrane uses technologies of reverse osmosis and nanofiltration systems while resin technology uses ion exchange systems. Therefore, we conveniently call it the solid waste-quicklime membrane SWQM process.
Owner:ENGSL FZE +1
Low-temperature coefficient permanent magnet ferrite material and its production method
InactiveCN101106001AImprove stabilityImprove reliabilityInorganic material magnetismMetallurgyChemical equation
The invention relates to a material used in the ferrite magnetic material industry and the production method thereof, in particular to a low-temperature coefficient permanent magnetic ferrite material and the production method thereof. Directed at the prior art's problems such as high temperature coefficient, the addition of expensive factors including La, Co, and Bi, and high production cost, the invention provides a low-temperature coefficient permanent magnetic ferrite material and the production method thereof without adding factors such as La, Co, and Bi. The materials of the invention area: The main phase is magnetoplumbite structure, and the chemical equation is: Sr1-xAxFe12-yMyO19, wherein: A=Ca, Na, Ba, Pb, K 1-2 types, x=0.05-0.5; M=Al, Cr, Zn2 / 3V1 / 3, Zn2 / 3Nb1 / 3, Cu2 / 3V1 / 3, Cu2 / 3Nb1 / 3, Zn1 / 2Ge1 / 2, Zn1 / 2Si1 / 2, Cu1 / 2Ga1 / 2, Cu1 / 2Si1 / 2 1-3 types.
Owner:李凌峰
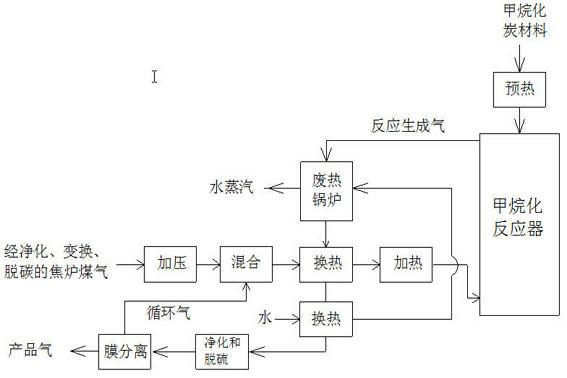
Process for preparing methane by utilizing coke oven gas
The invention relates to a process for preparing methane by utilizing coke oven gas. The adopted materials are methanation carbon materials and the coke oven gas with low oxygen content and high hydrogen content. The principle is that H2 in mixed raw material gas reactes with 30-70 percent of C with high hydrogenation activity in the methanation carbon materials to generate methane through the chemical equation that C and 2H2 generate CH4. The process flow comprises the following steps of: converting CO in the coke oven gas into H2; decarburizing and pressurizing; mixing the H2 and recycle gas according to a certain proportion to obtain mixed raw material gas rich in H2; reacting the H2 in the mixed raw material gas with 30-70 percent of C with high hydrogenation activity in the methanation carbon materials to generate reactive gas through the chemical equation that C and 2H2 generate CH4 in a methanation reactor under a certain temperature and pressure; separating the reactive gas through heat transfer, purification and desulfuration to obtain recycle gas and product gas; returning the recycle gas into the mixed raw material gas; and outputting the product gas. The process has the characteristics of low hydrogen consumption, large methane output, and environmental protection.
Owner:TAIYUAN UNIV OF TECH
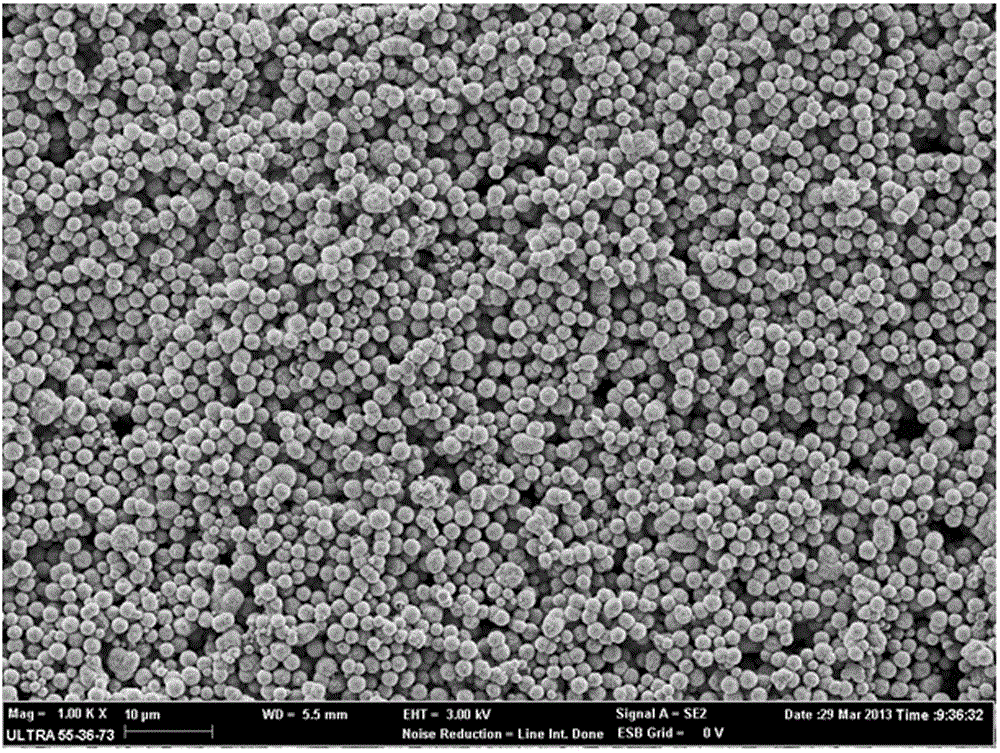
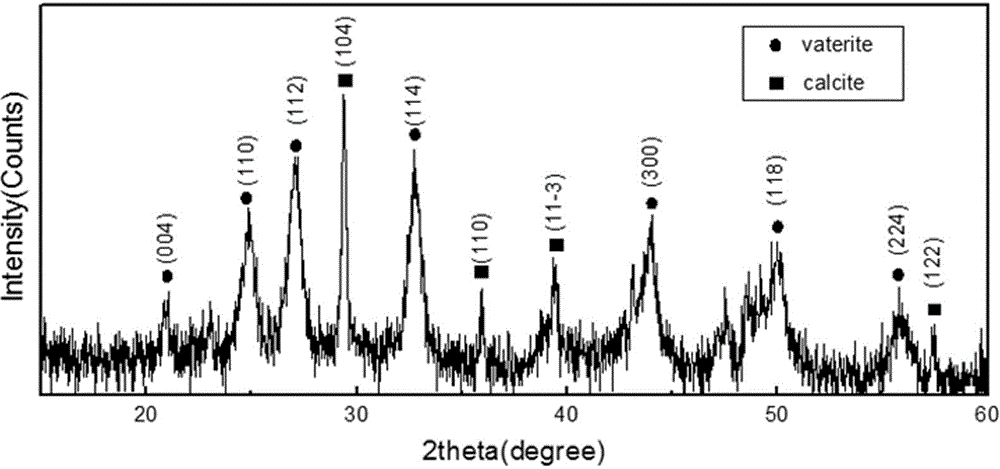
Vaterite calcium carbonate microspheres and preparation method thereof
InactiveCN104692439ARich sourcesSolve the problem of wastewater useCalcium/strontium/barium carbonatesCalcium crystalsChemical reaction
The invention discloses a method for simply preparing vaterite calcium carbonate microspheres. The preparation method comprises the steps of 1, preparing a sericin solution; 2, preparing a sericin solution with a certain concentration as a template regulation system through the dialyzed sericin solution according to the chemical equation shown in the specification, and dropping calcium salt and carbonate which have the same concentration into a reaction system in a certain proportion; 3, agitating the reaction system for a period of time, centrifuging to remove precipitate, and washing with water at three times; 4, drying the CaCO3 precipitate through a drying box after water washing, so as to obtain the finished product of vaterite calcium carbonate microspheres. The granularity of the CaCO3 microspheres is 1 to 1.5 microns; the vaterite calcium crystal form in the obtained material is more than 80%; the natural sericin solution is used as the template regulation system to regulate CaCO3 crystal form.
Owner:ZHEJIANG SCI-TECH UNIV
Method for growing calcium borate oxysalt crystal with frequency multiplication effect
InactiveCN101942699AEasy to processSimplify the Orientation ProgramPolycrystalline material growthBy pulling from meltCalcium borateX-ray
The invention relates to a method for growing a calcium borate oxysalt crystal with a frequency multiplication effect. The method comprises the following steps of: weighing raw materials according to a stoichiometric ratio of a chemical equation of the calcium borate oxysalt and performing crystal growth by the traditional pulling method, wherein a seed crystal is formed by finding the direction of the maximum nonlinear coefficient of the calcium borate oxysalt crystal which grows in the traditional direction through X-ray diffraction and cutting the crystal along the optimum phase matching direction; conveying the cut seed crystal slowly, vertically and downwards into polycrystal material melt to make the top of the seed crystal contact with the polycrystal material melt; maintaining the seed crystal to grow in the vertical direction of the polycrystal material liquid level of the calcium borate oxysalt; and obtaining the calcium borate oxysalt crystal with the frequency multiplication effect through three technological processes of necking, shouldering and ending. The method avoids the defects of complicated process, low utilization ratio, low properties of devices and the like caused by that the traditional calcium borate oxysalt crystal grows along the b direction. The method has the advantages of high optical homogeneity in the light-transmitting direction and high practical value.
Owner:SHANDONG UNIV +1
Technology for producing sodium fluosilicate
InactiveCN102849744ALight in massSilicon halogen compoundsSulfur-trioxide/sulfuric-acidChemical equationSewage
The invention discloses a technology for producing sodium fluosilicate. The technology comprises the following steps of 1, preparing a sodium sulfate solution having the mass content of 26 to 32%, 2, adding fluosilicic acid having the mass content of 8 to 14% into the sodium sulfate solution with stirring, 3, controlling a use amount of the sodium sulfate solution, adjusting a use amount of fluosilicic acid so that compared with a theoretical use amount calculated by a chemical equation, the use amount of fluosilicic acid is increased by 3 to 6%, 4, carrying out crystallization after a reaction of sodium sulfate and fluosilicic acid, 5, washing, separating sodium fluosilicate crystals, and carrying out centrifugal drying, and 6, recovering sulfuric acid and fluosilicic acid. The technology reduces unit consumption of sodium sulfate, reduces sodion content of sewage and realizes recovery of sulfuric acid in the sewage. Compared with the prior art, the technology has high efficiency, is environmentally friendly, simple and convenient, and has low salt consumption and a low cost. Sewage produced by the technology for producing sodium fluosilicate can be directly recovered and used.
Owner:贵州开磷氟硅化工有限责任公司
Polarizing plate protective film and method for manufacturing the same, polarizing plate and method for manufacturing the same, and liquid crystal display device
InactiveUS20090185112A1Liquid crystal compositionsOptical articlesTectorial membraneCellulose ester membrane
A protective film for polarizers which comprises a cellulose ester film and which, even when stored for long, suffers no film deformation failures such as ridging and protrusion failures; a process for producing the protective film; a polarizer; a process for producing the polarizer; and a liquid-crystal display employing the polarizer. The process for producing a protective film for polarizers is characterized by forming a melt comprising a cellulose ester and at least one member selected among compounds respectively represented by the following general formulae (1) to (3) into a continuous cellulose ester film by melt casting and winding the film into a roll.[Chemical formula 1] General formula (1) (In the formula, R1 to R5 each represents a substituent.) [Chemical formula 2] General formula (2) (In the formula, R1 to R6 each represents a substituent.) [Chemical formula 3] General formula (3) (In the formula, Rf represents perfluoroalkyl, Rc represents alkylene, Z represents a nonionic polar group, n is 0 or 1, and m is an integer of 1-3.)
Owner:KONICA MINOLTA OPTO
Method for preparing benzene end-capping polyaryletherketone polyme
ActiveCN102702459AAddress reactivitySolve technical problemsOrganic compound preparationCarbonyl compound preparation by condensationChemical reactionPhenyl Ethers
The invention relates to a method for preparing benzene end-capping polyaryletherketone polymers, which belongs to the technical field of high polymer materials. The preparation method comprises the steps as follows: using 4-(p fluorophenyl acyl) biphenyl or 4-(p fluorophenyl acyl) phenyl ether as an end-capping agent, using 4,4'-difluorobenzophenone and a bisphenol monomer as reactants, and using a nucleophilic route to prepare the benzene end-capping polyaryletherketone polymers with glass transition temperature of 147 DEG C and initial thermo-gravimetric temperature above 540 DEG C. Two detailed chemical equations describing the preparation process are attached. With two end-capping monomers having boiling points above 320 DEG C and suitable process conditions, the method for preparing the benzene end-capping polyaryletherketone polymers solves the technical problem caused by the fact that the p fluorophenyl acyl has low boiling point and volatilizes in an end-capping reaction in the prior art, and synthetizes polyaryletherketone with a benzene oxygen end group and polyaryletherketone with a biphenyl end group, so as to meet the requirement of continuous industrial production.
Owner:山东君昊高性能聚合物有限公司
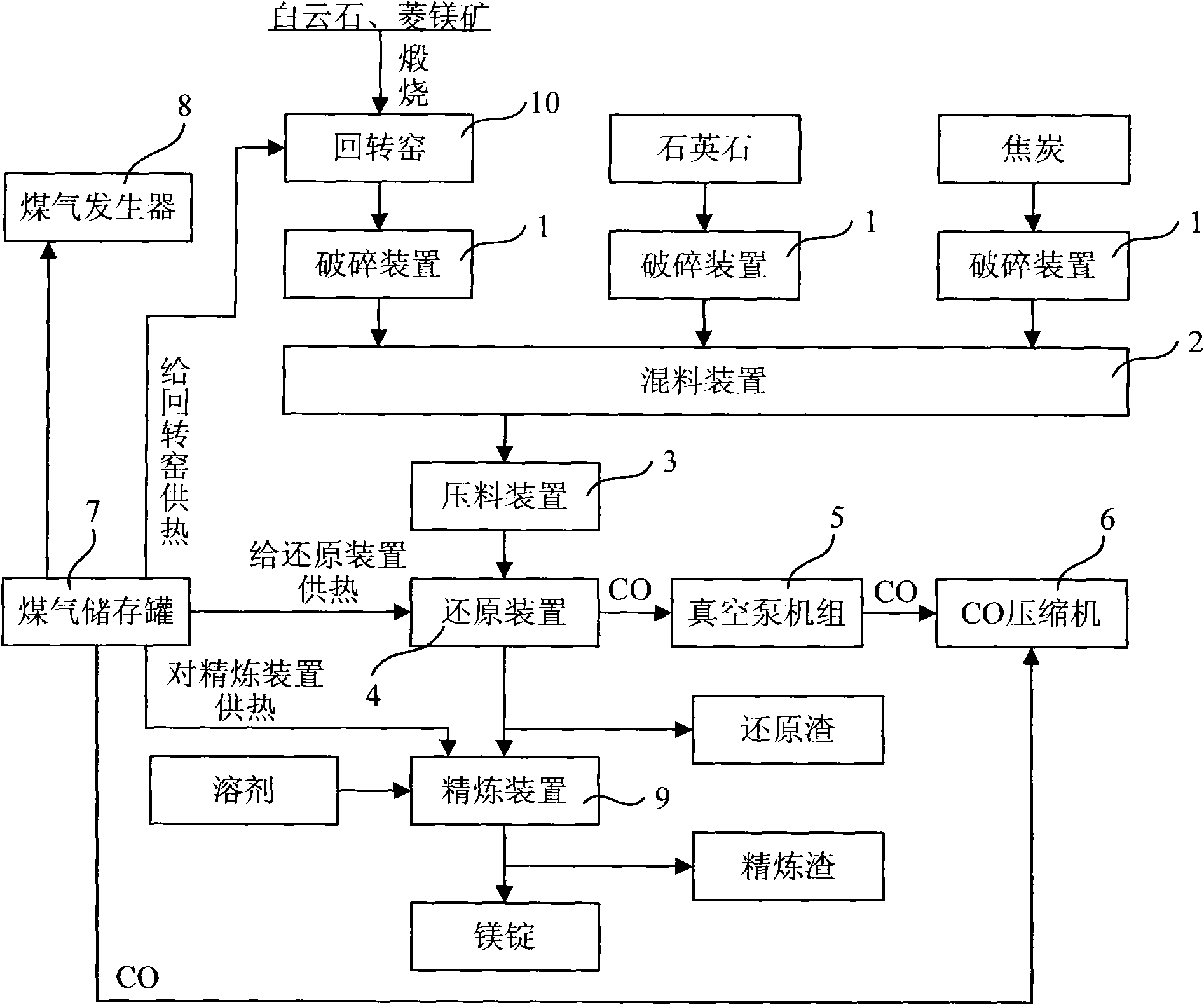
Method for preparing metallic magnesium by carbothermic reduction and device thereof
The invention discloses a method for preparing metallic magnesium by carbothermic reduction and a device thereof. The method comprises the following steps: crushing raw materials of calcined dolomite,quartzite and carbonaceous reducing agent and then mixing; pressing the above raw materials into bulk bodies; under a vacuum state, carrying out reduction reaction on the mixed material pressed intobulk bodies according to the chemical equation: 4CaO + 5MgO + 2SiO2 + 5C = 2(2CaO-SiO2)+ 5Mg + 5CO to prepare metallic magnesium, wherein, the raw materials are mixed according to the molar ratio of the reduction reaction, the carbonaceous reducing agent can be graphite, charcoal or coke, and the raw materials also can comprises calcined magnesite or magnesium oxide, at the moment, the calcined dolomite can be replaced by quick lime or calcined limestone. In the method, the CaSi2 production technology principle is introduced, carbonaceous reducing agent is utilized to replace FeSi, thereby greatly reducing production cost, reducing energy consumption, decreasing environmental pollution, and speeding up the preparation speed of metallic magnesium.
Owner:何锡钧 +1
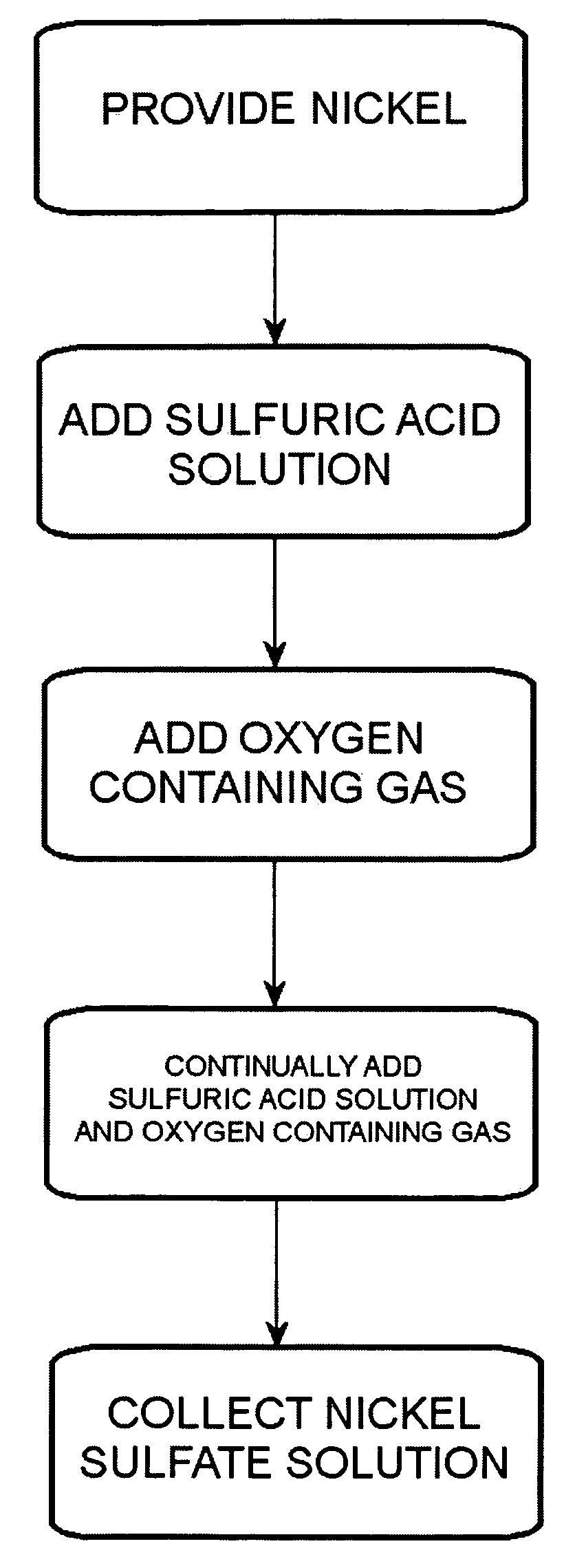
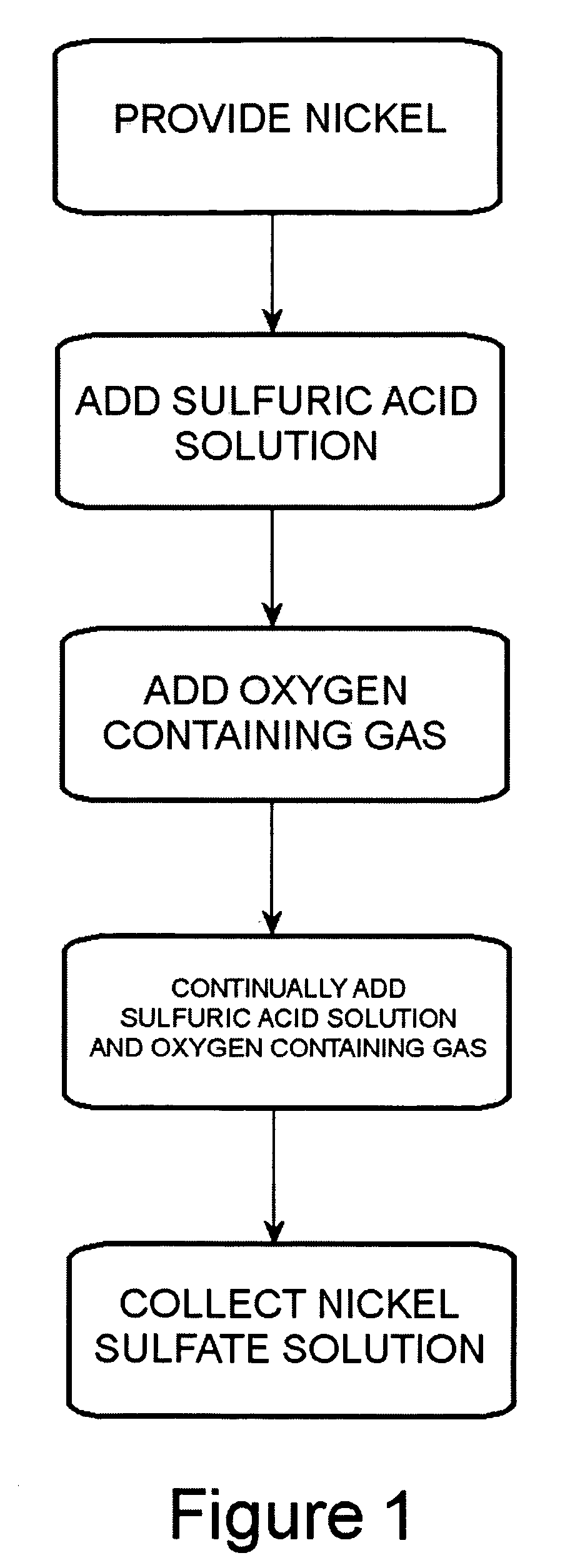
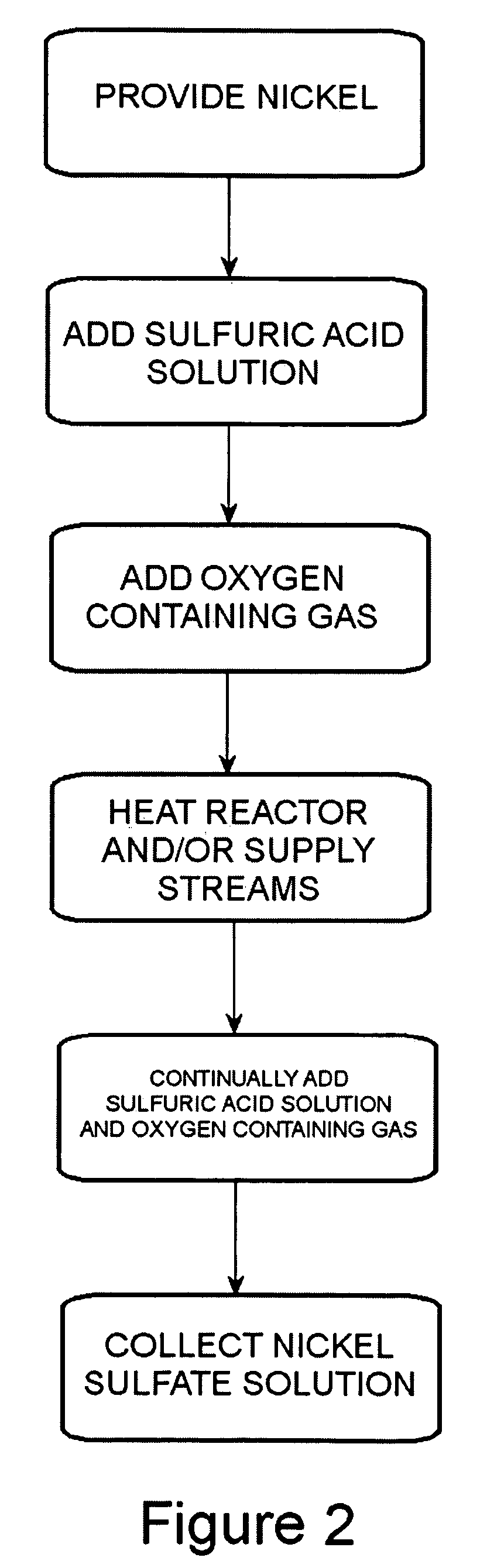
Process for converting nickel to nickel sulfate
A preferred embodiment of the present invention provides a process for making nickel sulfate by converting nickel metal into nickel sulfate, which may be converted to nickel hydroxide. Nickel metal is dissolved in sulfuric acid and oxygen containing gas is introduced to produce a nickel sulfate solution having nickel sulfate and water as illustrated in the following chemical equation.Ni+H2SO4+½O2→NiSO4+H2OThe nickel sulfate is filtered and sulfuric acid is continually added to maintain stoichiometry within a reactor until the nickel metal is dissolved. The sulfuric acid, oxygen containing gas and nickel metal may be heated to facilitate the desired reaction. Then, the nickel sulfate may be utilized to produce nickel hydroxide.
Owner:CHEVRON TECH VENTURES +1
Method for producing trichloromethoxybenzene by using chlorobenzene as solvent
ActiveCN102120717ALow priceConveniently preparedOrganic chemistryOrganic compound preparationOrganic solventChemical reaction
The invention relates to a production method of an aromatic compound, in particular to a method for producing trichloromethoxybenzene by using chlorobenzene as solvent. The method comprises the following steps: using anisole as starting raw material and chlorobenzene as solvent to perform photo-chlorination reaction and recycling chlorobenzene to prepare trichloromethoxybenzene. The chemical equation is shown as below. In the method, chlorobenzene is used as the organic solvent of the photo-chlorination reaction for preparing trichloromethoxybenzene; as chlorobenzene is cheaper and can be better used in the photo-chlorination reaction, photo-chlorination reaction can be performed according to a proper molar ratio of chlorobenzene to the raw material anisole in the presence of proper catalyst under proper reaction conditions and trichloromethoxybenzene can be prepared better. Therefore, the method is suitable for industrial large-scale production.
Owner:KINGCHEM LIAONING CHEMICAL CO LTD
Method for obtaining sulfur from sulfur compounds in coal chemical plant and electric power plant
ActiveCN103318846AHigh purityChange the phenomenon of being unable to driveSulfur preparation/purificationGas passingElectric power
The invention relates to a method for obtaining sulfur from sulfur compounds in a coal chemical plant and an electric power plant. The method comprises the following steps of: (1) dedusting exhaust gas from a coal burning boiler system, and then concentrating the exhaust gas to improve the content of sulfur dioxide in the exhaust gas; (2) heating the concentrated exhaust gas through an exhaust gas preheater, then introducing the exhaust gas into a constant temperature selective reduction reactor, and enabling the exhaust gas to react under the action of a selective reduction catalyst by adding hydrogen as a reducing agent so as to generate elemental sulfur, wherein the chemical equation is H2+SO2=S+H2O (1), and the heat generated by the reaction is taken away by a cooling coil which is arranged in the constant temperature selective reduction reactor to ensure that the temperature in the constant temperature selective reduction reactor is maintained at around 210-240 DEG C; (3) removing liquid sulfur from the exhaust gas subjected to catalytic reduction through a sulfur condenser, and introducing the exhaust gas into a Claus reaction device, wherein the liquid sulfur enters a liquid sulfur tank; and (4) burning the sulfur and sulfide contained in the tail gas passing through the Claus reaction device by using a combustion furnace so as to convert the sulfur and sulfide into sulfur dioxide, delivering the sulfur dioxide into the boiler system to mix with a dedusted process gas, and concentrating the mixture, wherein the gas discharged during concentration is mainly carbon dioxide.
Owner:JIANGSU HENGXIN ENERGY TECH
Network editor capable of inputting chemical equation and chemical structural formula and system
InactiveCN101770451AShorten the timeNatural language data processingSpecial data processing applicationsChemical structureData information
The invention discloses a network editor capable of inputting a chemical equation and a chemical structural formula and a network information inputting and editing system. The editor can be totally or partially arranged in a server, and the server is connected with a client via the network and processes data information sent by the client; and the editor can also be partially or totally arranged in the client, and the client is connected with the server via the network and executes codes sent by the server. The editor also comprises a chemical equation inputting module which is used for inputting the chemical equation information in the editor; and the chemical equation inputting module comprises a second test inputting unit, a subscript processing unit and a second picture forming unit, wherein the second picture forming unit is used for forming the inputted reactants, products, reaction conditions and arrows into picture formats. The editor enables users to efficiently express frequently-used academic information such as formulas, symbols, functional images, figures and the like with strong academic nature in network communication, and can carry out on-line editing, viewing and modification.
Owner:施昊 +2
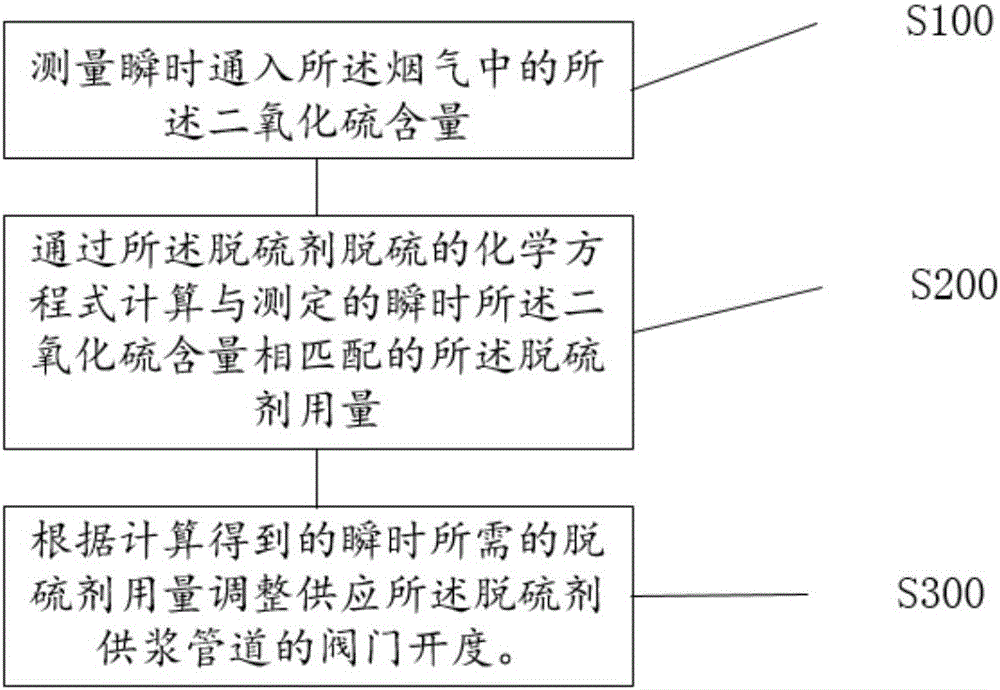
Method and control system for controlling supply of desulfurizing agent for flue gas
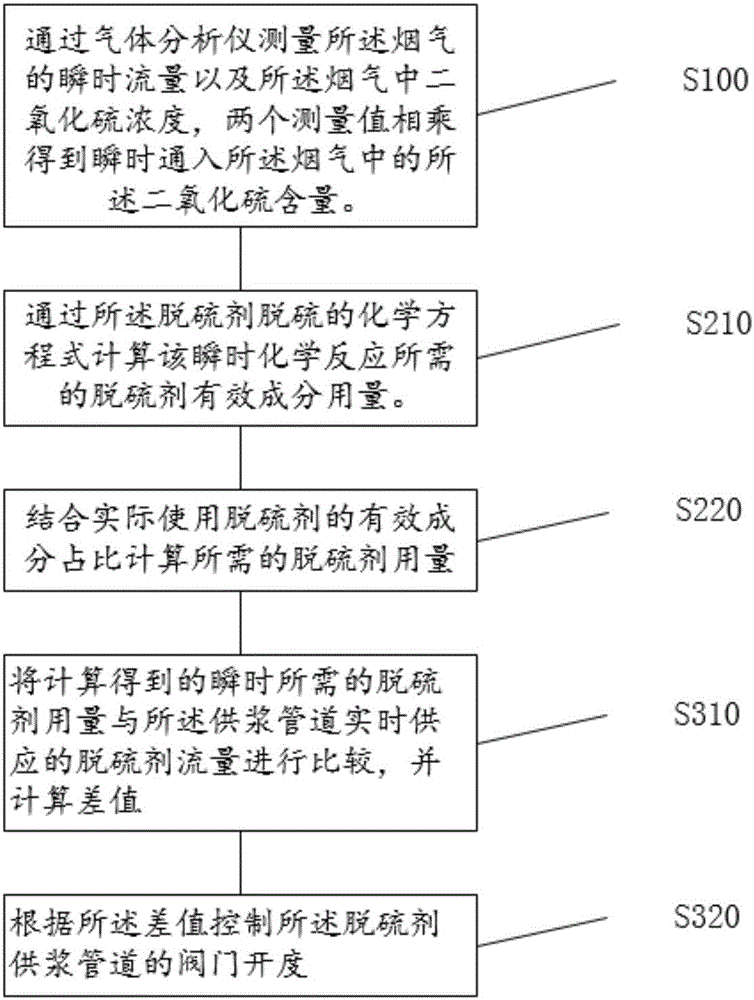
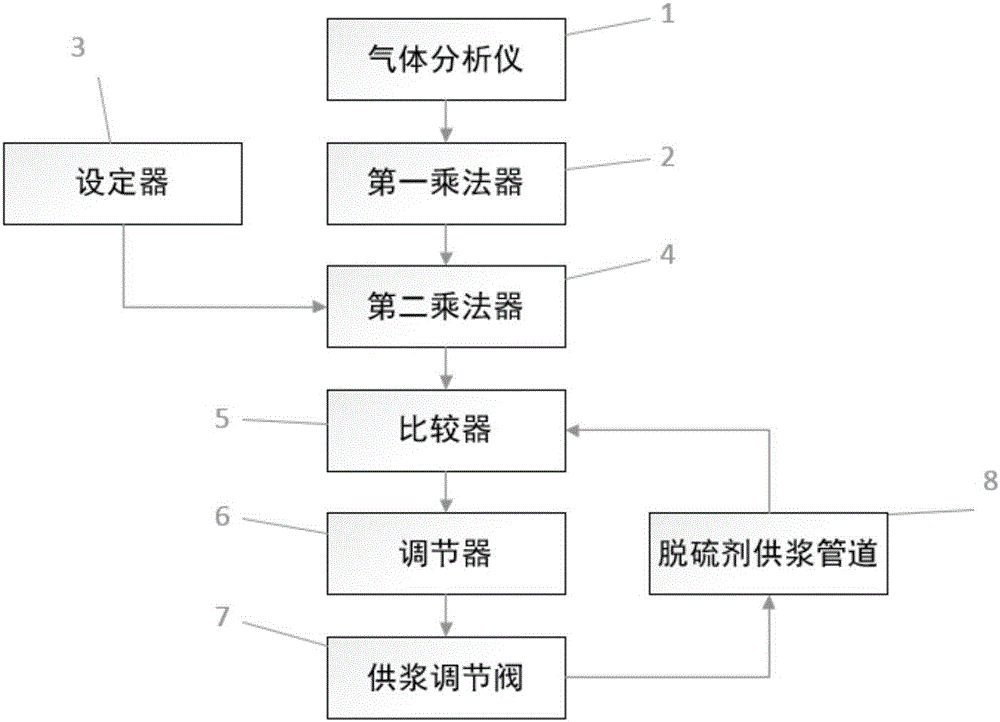
InactiveCN106731635AIncrease profitFast and efficient load adaptabilityDispersed particle separationChemical reactionControl system
The invention discloses a method for controlling the supply of a desulfurizing agent for a flue gas. The desulfurizing agent is used for generating a chemical reaction with sulfur dioxide in the flue gas for desulfurization. The method comprises the following steps of S100, measuring the instantaneous flow rate of the flue gas and the concentration of the sulfur dioxide in the flue gas through a gas analyzer, multiplying two measured values to obtain the content of the sulfur dioxide in an instantaneous flue gas; S210, calculating the use level of an effective component of the desulfurizing agent, which is needed by an instantaneous chemical reaction, through a desulfurization chemical equation of the desulfurizing agent; S220, calculating the needed use level of the actually used desulfurizing agent by combining the proportion of the effective component of the actually used desulfurizing agent; S310, comparing the use level, which is obtained through calculation and is needed instantaneously, of the desulfurizing agent with the flow rate of the desulfurizing agent supplied in real time by a slurry supply pipeline, and calculating a difference value; S320, controlling the opening of a valve on the desulfurizing agent slurry supply pipeline according to the difference value. The accurate and high-efficiency control of the desulfurizing agent for the flue gas is realized. The invention also discloses a system for controlling the supply of the desulfurizing agent for the flue gas.
Owner:SHANGHAI LONGKING ENVIRONMENTAL PROTECTION
Method and device for preparing sodium chlorite through comprehensive method chlorine dioxide process
ActiveCN105439095ANo dischargeReduce pollutionChlorine oxidesChlorous acidChemical equationSide product
The invention discloses a method for preparing sodium chlorite through a comprehensive method chlorine dioxide process. According to the reaction principle, the method includes the steps of firstly, conducting sodium chlorate electrolysis according to the chemical equation NaCl+3H2O->NaClO3+3H2; secondly, conducting hydrochloric acid synthesis according to the chemical equation H2+Cl2->2HCl; thirdly, generating chlorine dioxide according to the chemical equation NaClO3+2HCl->NaCl+ClO2+1 / 2Cl2+H2O; fourthly, preparing sodium chlorite according to the chemical equation 2ClO2+2NaOH+H2O2->2NaClO2+2H2O+O2. A preparation device mainly comprises an electrolytic cell, a sodium chlorate reactor, a hydrochloric acid synthesis furnace, a reboiler, a chlorine dioxide generator, a chlorine dioxide absorption stripping tower, a chlorine dioxide stripper and a sodium chlorite preparation tower. When sodium chlorite is prepared through the method and the device, the product cost is low, yield is high, no side products are discharged, no waste acid or solid waste is discharged, environment pollution is reduced, and the advantages of being economical and environmentally friendly are achieved.
Owner:GUANGXI UNIV
Inductive coupling type high-frequency electrodeless lamp simulation device and method
InactiveCN103235517AReduce development costsLow costSimulator controlChemical reactionPhysical field
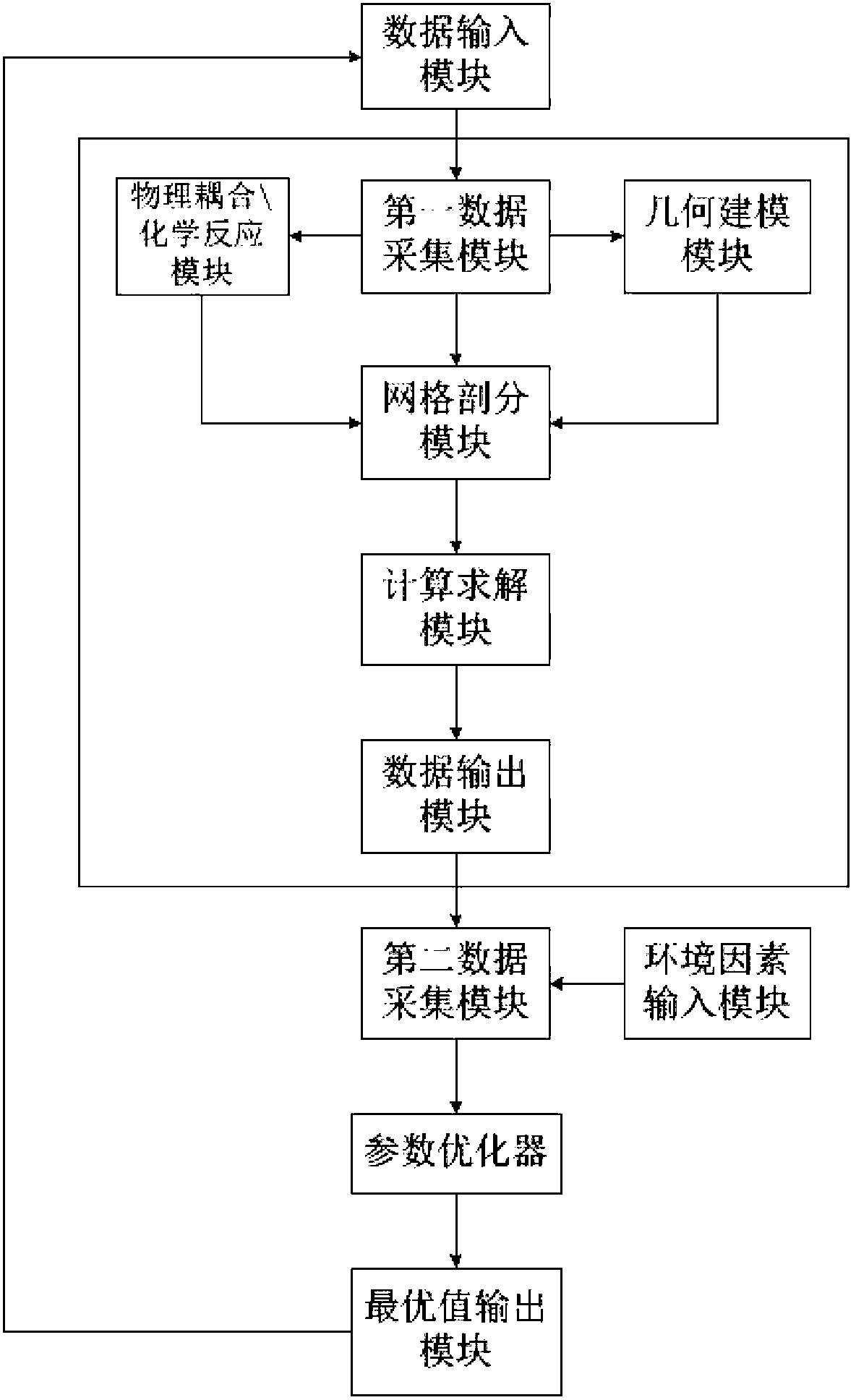
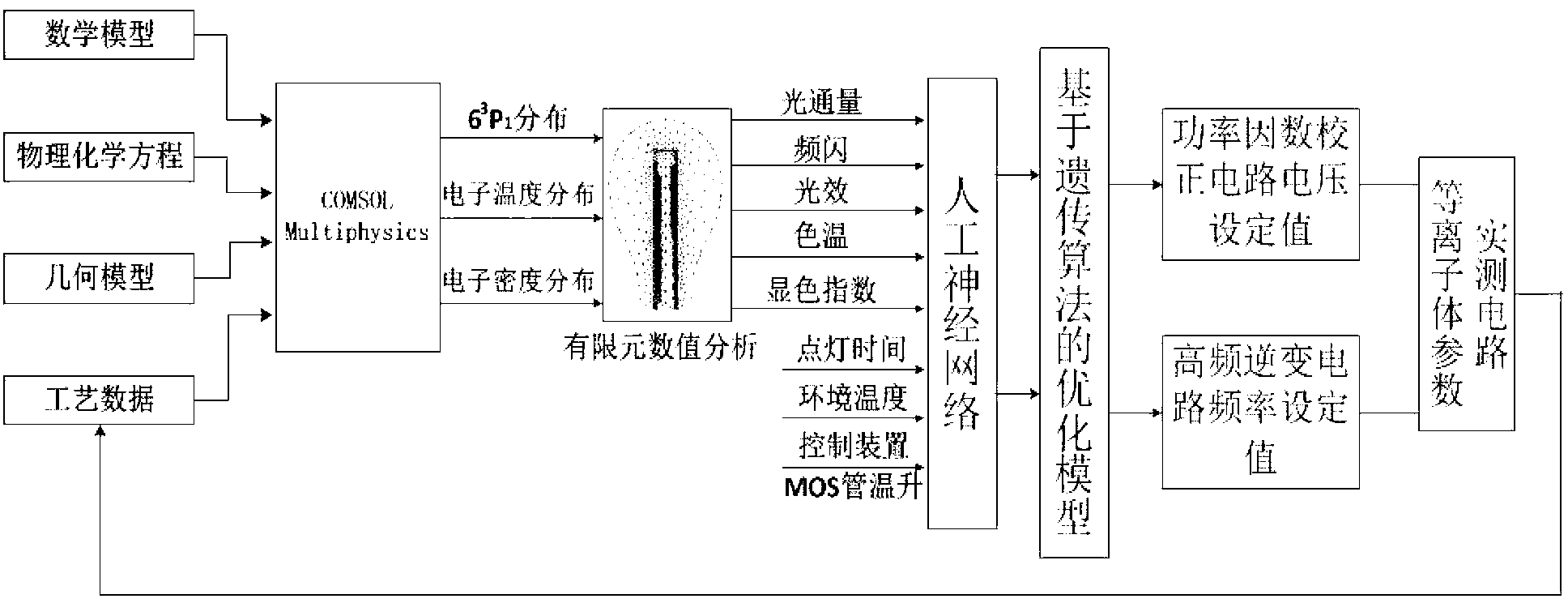
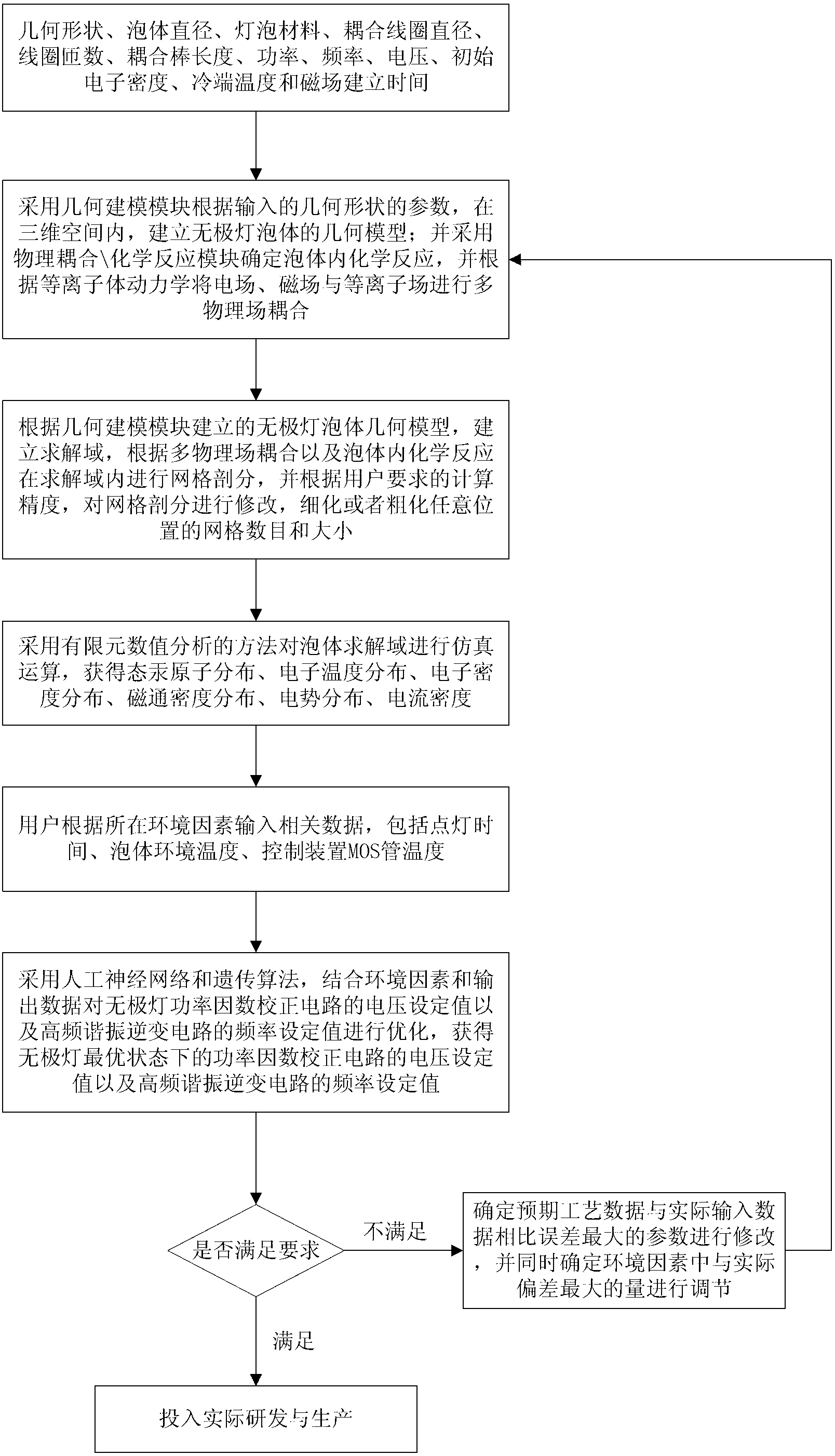
The invention discloses an inductive coupling type high-frequency electrodeless lamp simulation device and method, which belong to the field of lighting engineering. The device comprises a data input module, a first data acquisition module, a geometric modeling module, a physical coupling\chemical reaction module, a mesh generation module, a calculating and solving module, a data output module, an environmental factor input module, a second data acquisition module, a parameter optimization device and an optimal value output module. The device is based on mathematical physics, chemical equations and finite element numerical analysis models, multiple physical fields such as electrical, magnetic and plasma fields of a high-frequency electrodeless lamp are built, realized and optimized so as to guide the electrodeless lamp research and development; a mechanism model and a computer are applied to simulate, so that the development cost is lowered, the development period is shortened, the development quality is improved, the whole electrodeless lamp development process is assisted by the computer, the electrodeless lamp development process is improved from imitation, practicality experiment and data accumulation processes, and reliable technical guarantee is provided for the batch production of the electrodeless lamp.
Owner:NORTHEASTERN UNIV
Method of producing a nickel salt solution
A method for converting nickel into a nickel salt solution. Nickel is dissolved and reacted in an oxygen-enriched acidic solution to produce a nickel salt solution as illustrated in the following chemical equation, wherein X is a conjugate base: Ni+H2X+½O2->NiX+H2O.
Owner:CHEVRON TEXACO TECH VENTURES
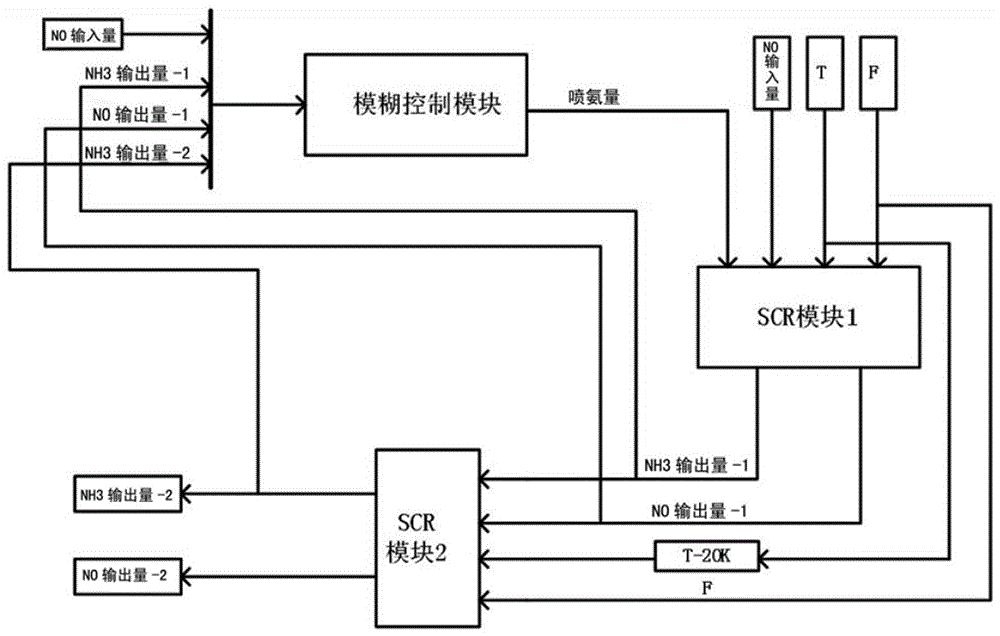
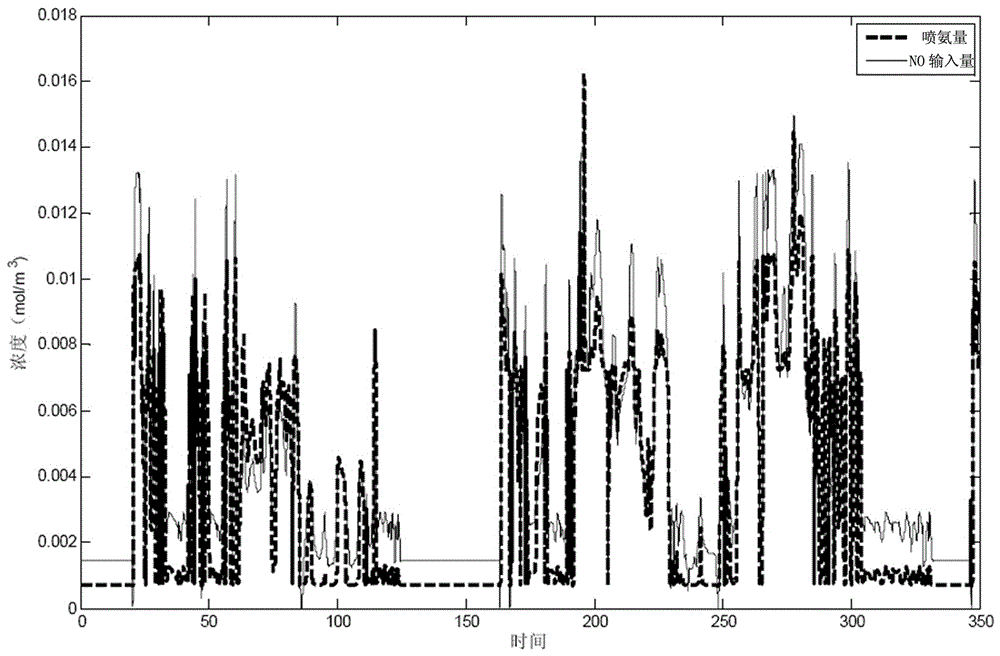
Fuzzy control method for series selective catalytic reduction system
InactiveCN106437956AEliminate effectivePrevent leakageInternal combustion piston enginesExhaust apparatusChemical reactionMathematical model
The invention discloses a fuzzy control method for a series selective catalytic reduction system. The method comprises the following steps: step 1, expressing selective catalytic reduction by a state equation according to a chemical equation of the selective catalytic reduction, and establishing a mathematical model; step 2, establishing a control model by virtue of Simulink through adopting the mathematical model established in the step 1; step 3, obtaining a membership function graph, and establishing a fuzzy control method for controlling an ammonia injection amount of a selective catalytic reduction system; and step 4, setting the fuzzy control method established in the step 3 in a fuzzy control module, and running the control model to obtain a simulation result. The method disclosed by the invention is capable of strictly controlling the NH3 injection amount through adopting series SCR and the fuzzy control method to avoid secondary pollution caused by ammonia leakage, and capable of greatly realizing conversion efficiency with high NOX.
Owner:SHANGHAI MARITIME UNIVERSITY
Rare earth complex heat stabilizer for PVC and method for preparing same
The invention provides a PVC rare-earth composite heat stabilizer and a method for making the same. The heat stabilizer is a wool acid metal rare-earth soap stabilizer and has a general chemical equation of xRE.yCa.zZn.(OOCR)n or x'RE.y'Ba.z'Zn.(OOCR)n. The method for making the heat stabilizer comprises the following steps that: sodium hydroxide is dissolved into an ethanol water solution and is stirred as well as is condensed and flowed back, the melting common and braid wool acid is added to adjust the pH value and decrease the temperature; water, chlorination rare-earth salt solution and salt are added in sequence to be pumped, filtered, washed by ethanol and water, dried and grinded into a product. The stabilizer adopts the common and braid wool acid as the raw material, thereby having wide resources, low cost, reasonable and scientific preparation method and great maneuverability; the product is straw yellow powder and has good primary and long-term heat stabilization performance of PVC and good effects in the aspects of material process and mechanical performance, is nontoxic and protects the environment, and can be applied to the processing and forming aspects of PVC water supply and drainage pipes and have evident social and economical benefits.
Owner:FUZHOU UNIV
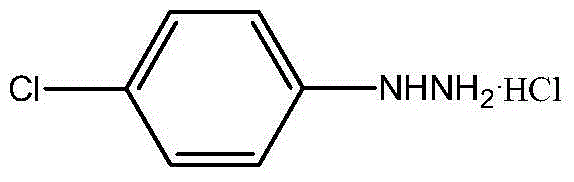
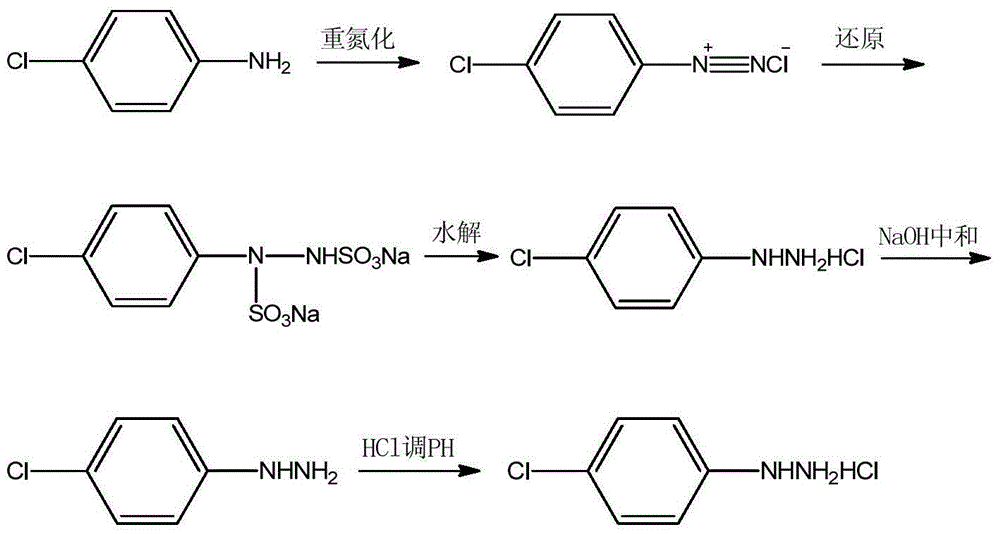
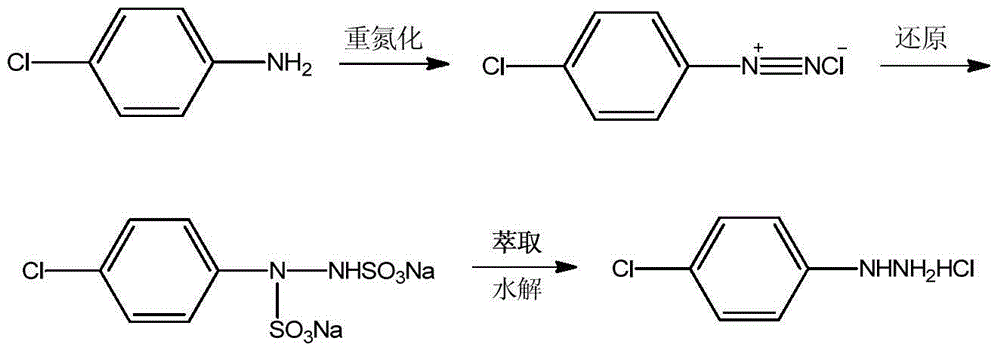
P-chlorophenylu hydrazine hydrochloride preparation method
The invention discloses a p-chlorophenylu hydrazine hydrochloride preparation method. P-chloroaniline serves as a raw material, after diazotization and reduction, non-polar solvent toluene or benzene, dichloromethane, trichloromethane and dichloroethane are used for extracting an aqueous phase to remove impurities, and then hydrochloric acid is added into the aqueous phase for hydrolysis to obtain p-chlorophenylu hydrazine hydrochloride through cooling, filtering and during. The chemical equation is shown as the following (please see the formula in the specification). Compared with the prior art, the non-polar solvents are used for extraction and impurity removal, the yield and the purity of an obtained product are high, the purity of the p-chlorophenylu hydrazine hydrochloride is larger than 99%, the yield is larger than 86%, filtering only needs to be carried out once, and operation is easy. Waste water amount is reduced by half compared with the prior art, and environmental protection pressure is reduced.
Owner:HUNAN HAILI CHEM IND
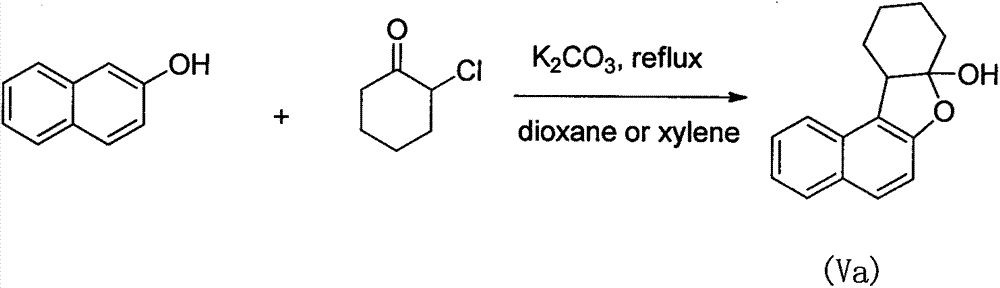
Preparation method of naphthonone and analogues thereof
InactiveCN103613568AHigh yieldMild reaction conditionsOrganic chemistryChemical reaction2,2,2-Trifluoroethanol
The invention provides a preparation method of naphthonone and analogues thereof, which is simple in reaction condition, simple in after-treatment, high in yield and low in production cost. According to the preparation method, in the presence of alkali, a compound shown by a general formula (I) reacts with a compound shown by a general formula (II) or (III) at room temperature by taking multi-fluorine alcohol as a solvent to prepare a compound shown by a general formula (IV) or (V), and the chemical equations are shown in the specification, wherein R is H, C1-C4 alkyl, chlorine or bromine, R' is H or C1-C4 alkyl, X is chlorine or bromine, the multi-fluorine alcohol is trifluoro-ethanol or hexafluoro-isopropanol, and the alkali is sodium carbonate, potassium carbonate, trifluoro-ethanol sodium or triethylamine.
Owner:CHONGQING MEDICAL UNIVERSITY
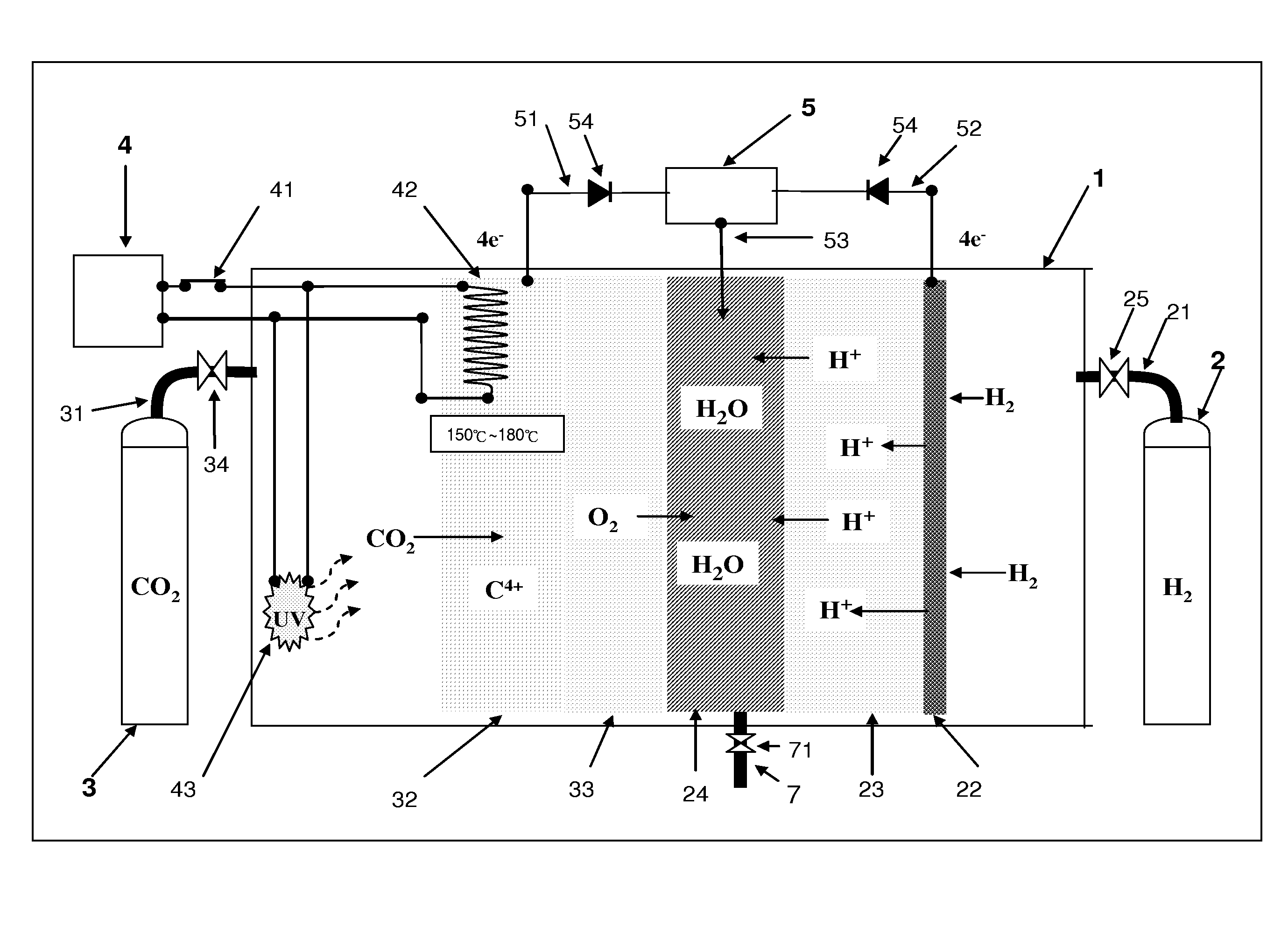
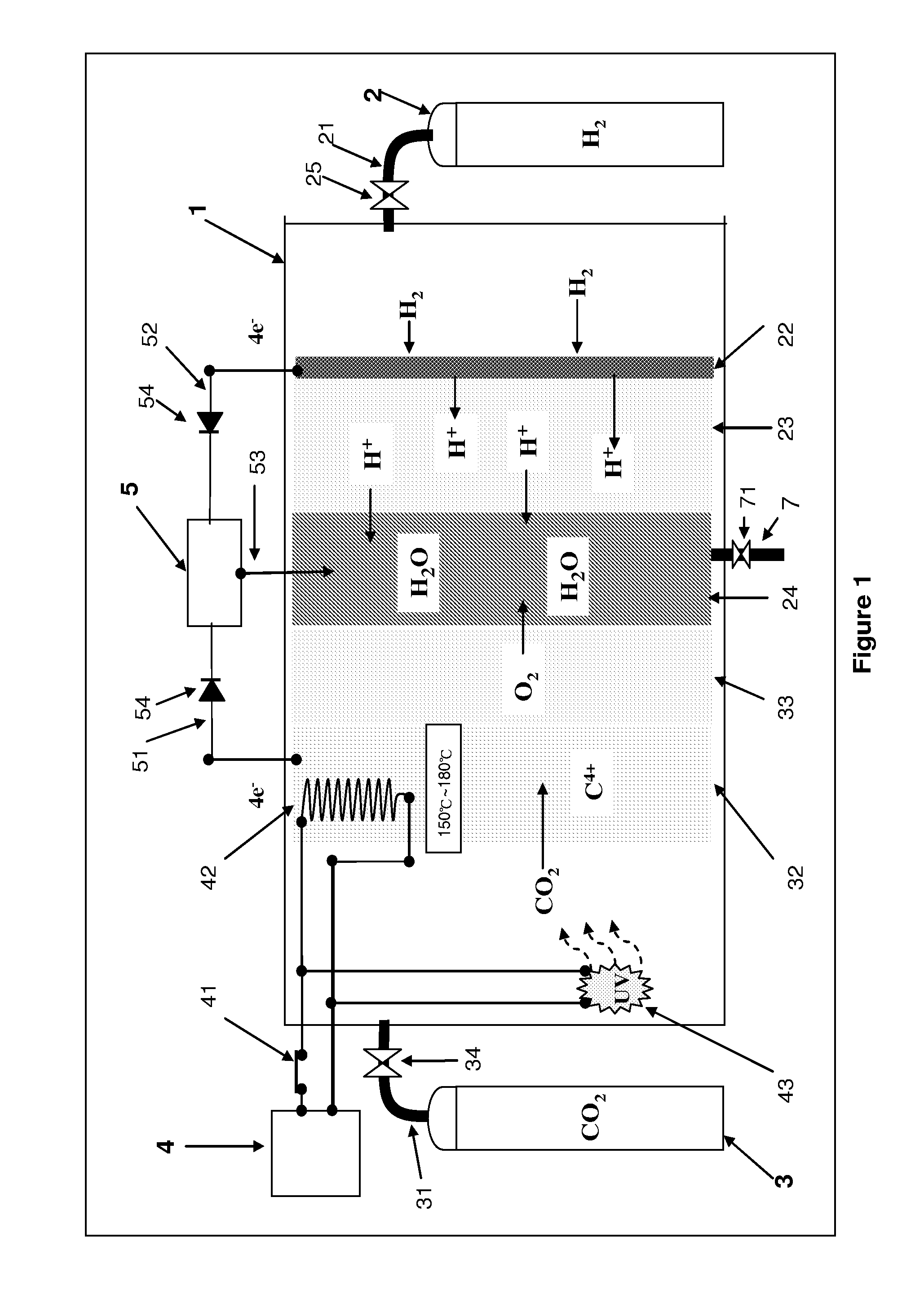
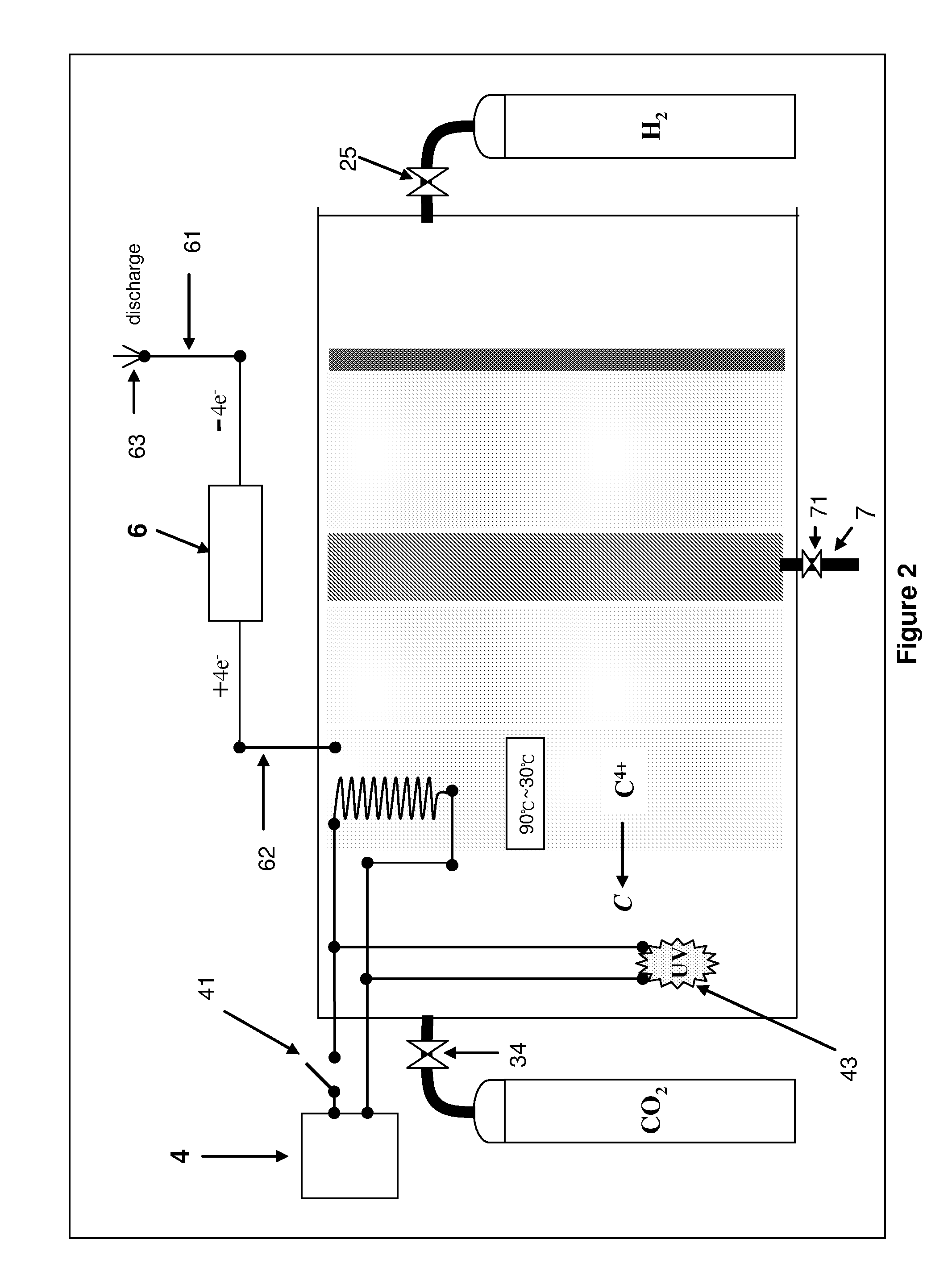
CARBON DIOXIDE DISSOLUTION AND C4+nM STATE CARBON RECYCLING DEVICE AND METHOD
With this technology, we build a nano-material which is structured as a tree with the branches and leaves being tentacles. When the temperature is between 150° C. and 180° C., those tentacles will shape themselves into nanometer holes to catch ion.The chemical equation: uv+CO2+ΔnM+2H2+4e−↑→C+2H2O+∇nM+4e−↓.Those two chemical equations have one common subject, that's they both take high energy light particles to break the electron bond between carbon and oxygen, thus, the invertor can reduce the greenhouse gases (CO2) in the atmosphere or recycle carbon from industrial emissions.
Owner:CHEN SHU CHIN +2
Preparation of cupric nitrate solution
InactiveCN101481133AReduce consumptionAvoid it happening againCopper nitratesChemical reactionReaction temperature
The invention provides a method for preparing a cupric nitrate solution, which comprises the following steps: putting metal copper inside a pressure-proof and nitric acid corrosion-resistant container, and introducing industrial pure oxygen to perform the dissolving reaction of metal copper at a reaction temperature of between 20 100 DEG C and 100 DEG C with a reaction pressure between 0.05 MPa and 0.5 MPa. The nitric acid for reaction is between 1 mol / L and 14 mol / L. The amount of copper for reaction is larger than the amount calculated by the chemical equation and the reaction lasts for 1 to 10 hours.
Owner:SICHUAN NORMAL UNIVERSITY
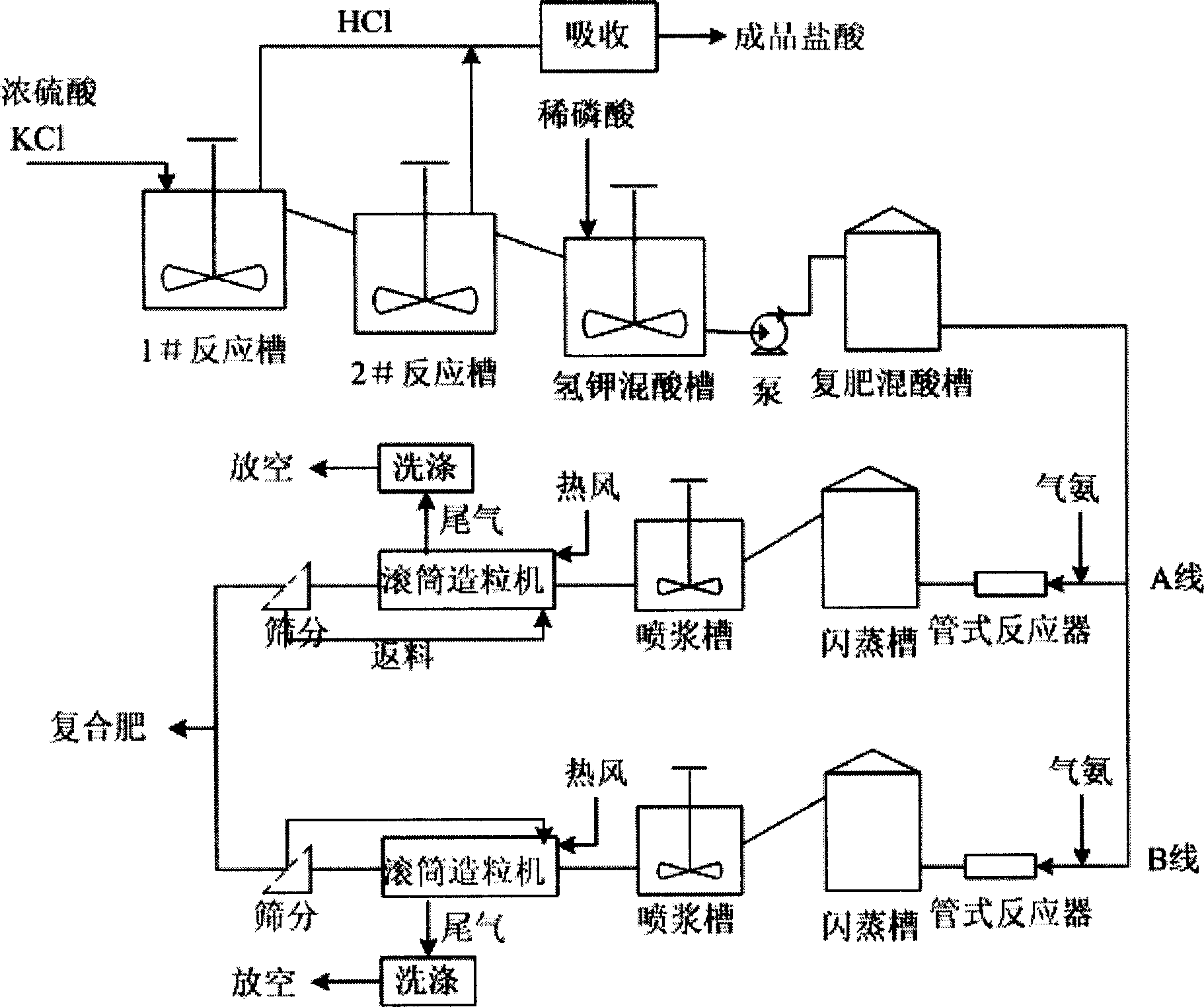
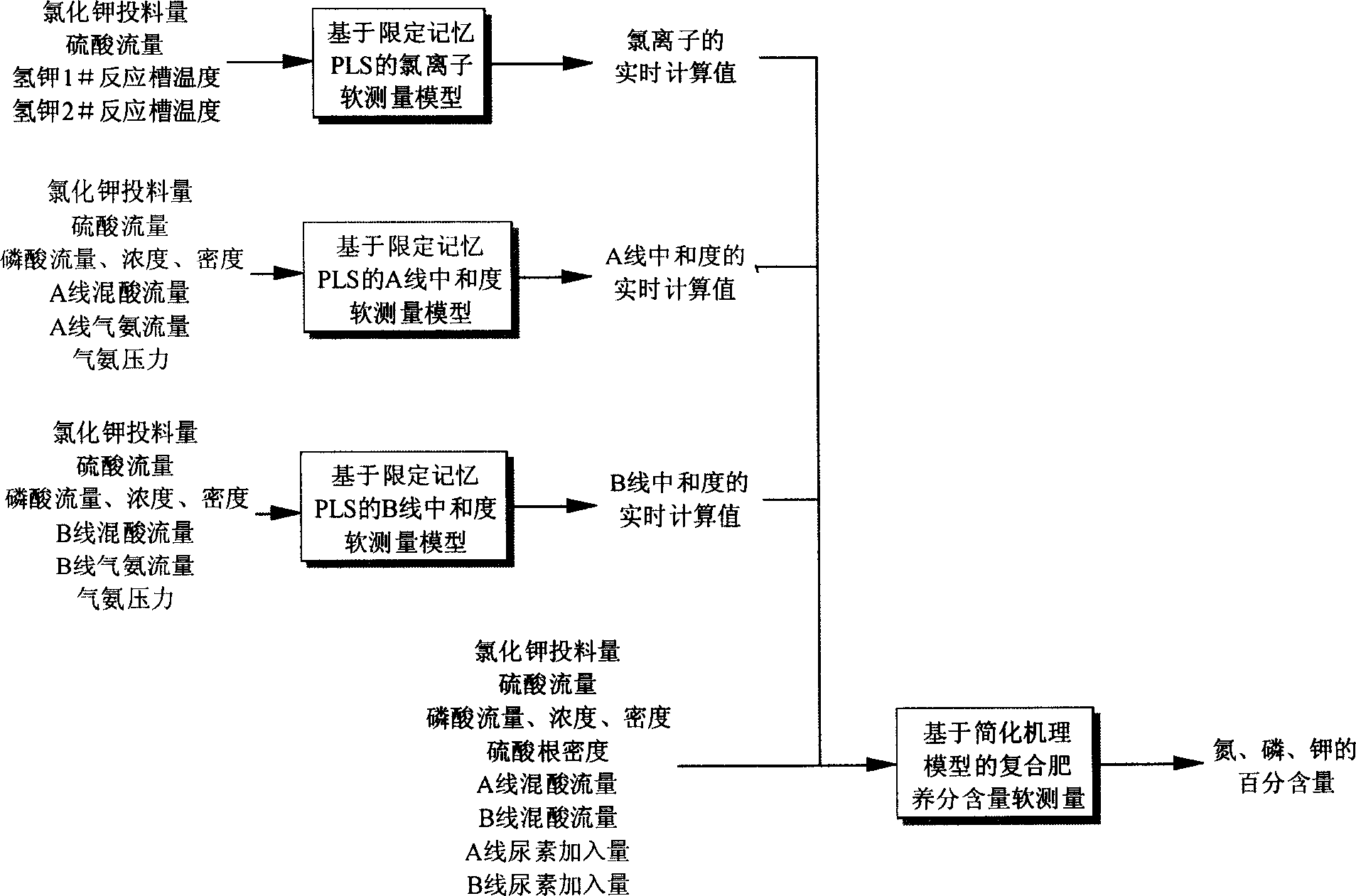
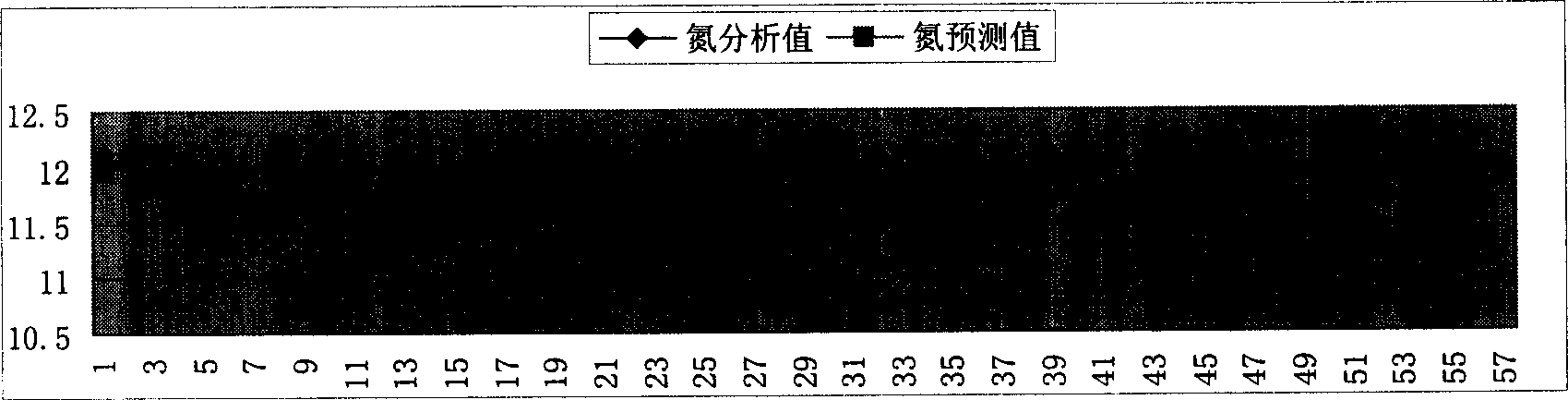
Compound-fertilizer nutrient-content soft-measuring modeling method beased on hybrid multiple models
InactiveCN1815225AImprove reliabilityWith adaptive functionSpecial data processing applicationsChemical methods analysisIon contentChemical reaction
Present invention discloses a modeling method for nutrient content soft measuring based on mix multiple mold type compound fertilizer. Modeling method features 1, whole pattern is mixed model consisting of based on data driven 3 submodel and based on simplify mechanical models 1 submodel, wherein the outputting of data driven submodel used as inputting of based on simplify mechanical models submodel, 2, simplifying mechanical models related model parameter determining method, determining potassium chloride and concentrated sulfuric acid extent of reaction parameter according to chloride ion content and material balance in finished product, 3, method determining (NH4)2HPO4 quality, according to neutralization degree value and measuring method, combining concerned chemical equation to calculate said quality. Said invention has advantages of Said invention has advantages of simple model building, strong explicable ability, high reliability, and fine extrapolate ability.
Owner:ZHEJIANG UNIV
Chemical treatment of aging oil
ActiveCN102295950AEasy to dehydrateRelieve pressureDewatering/demulsification with chemical meansOxalateChemical treatment
The invention discloses a chemical treatment method for aging oil. The method specifically comprises the following steps: heating the aging oil to a temperature of 65 DEG C; adding the heated aging oil to a settling tank, and synchronously adding an emulsion breaker, sodium carbonate and oxalic acid to the settling tank, wherein the total weight of the sodium carbonate and the oxalic acid is 3 per mill of the weight of the aging oil, the odium carbonate reacts with the oxalic acid in the settling tank, the chemical equation of the reaction is as the following: Na2CO3+H2C2O4=Na2C2O2+CO2(gas)+H2O. According to the reaction, the stable emulsifying system is destroyed, such that the oil, the water and the impurities in the aging oil can be effectively separated, the mobility of the aging oil can be increased through the generated carbon dioxide. With the present invention, the aging oil having the water content of 40-50% can be dewatered into the aging oil having the water content less than 1.5%, such that the aging oil meets the transportation standard.
Owner:LIAONING HUAFU ENVIRONMENTAL ENG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com