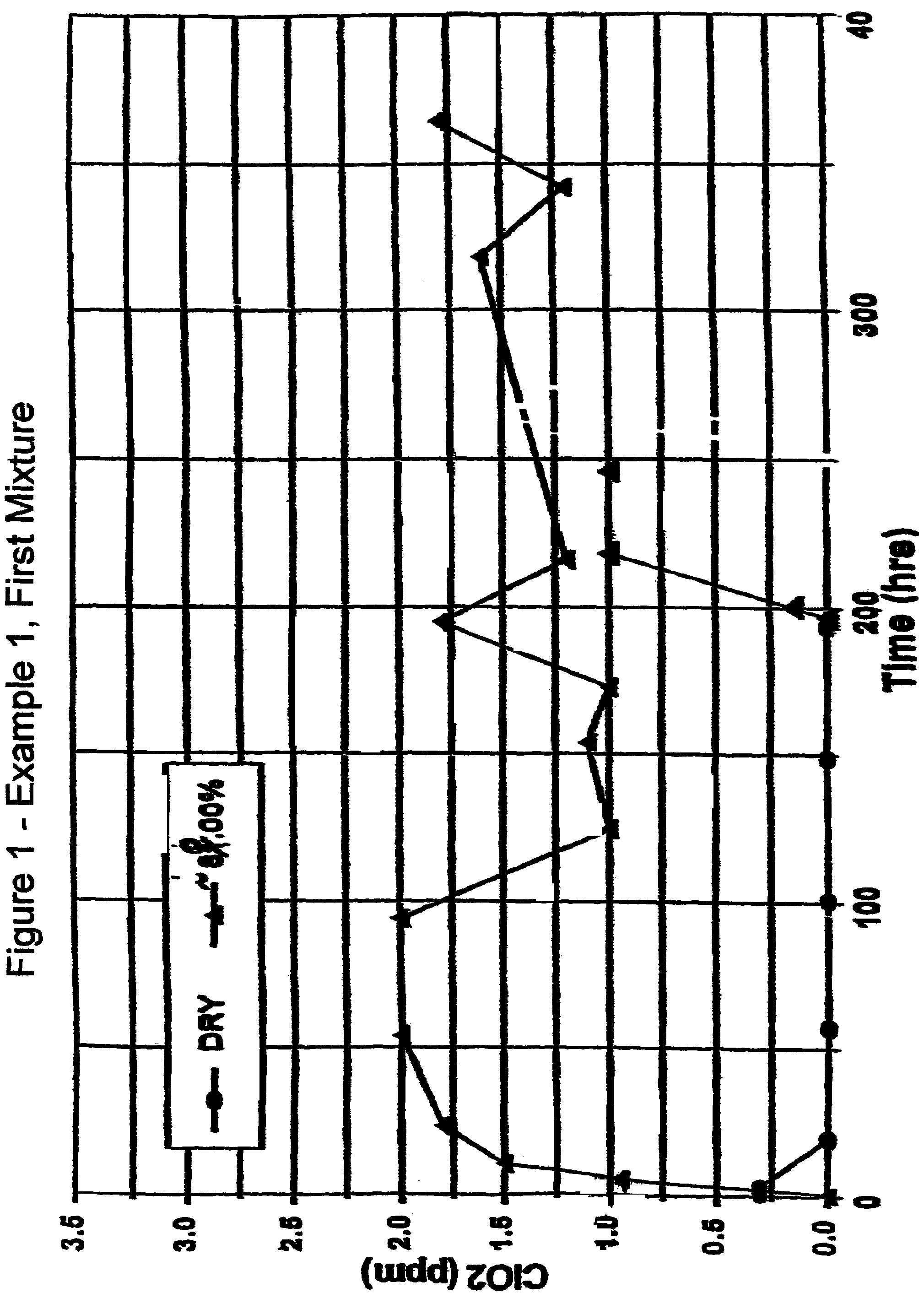
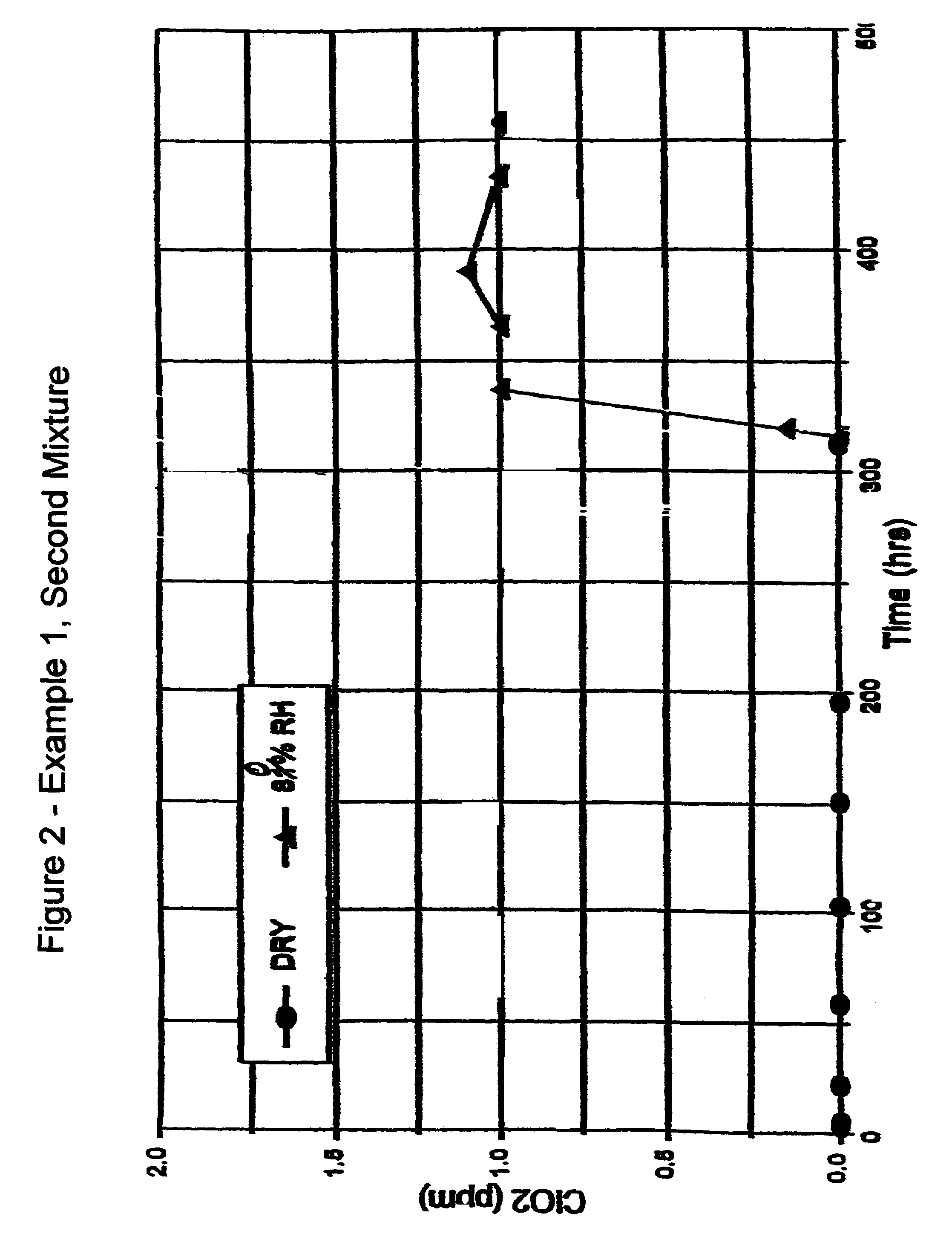
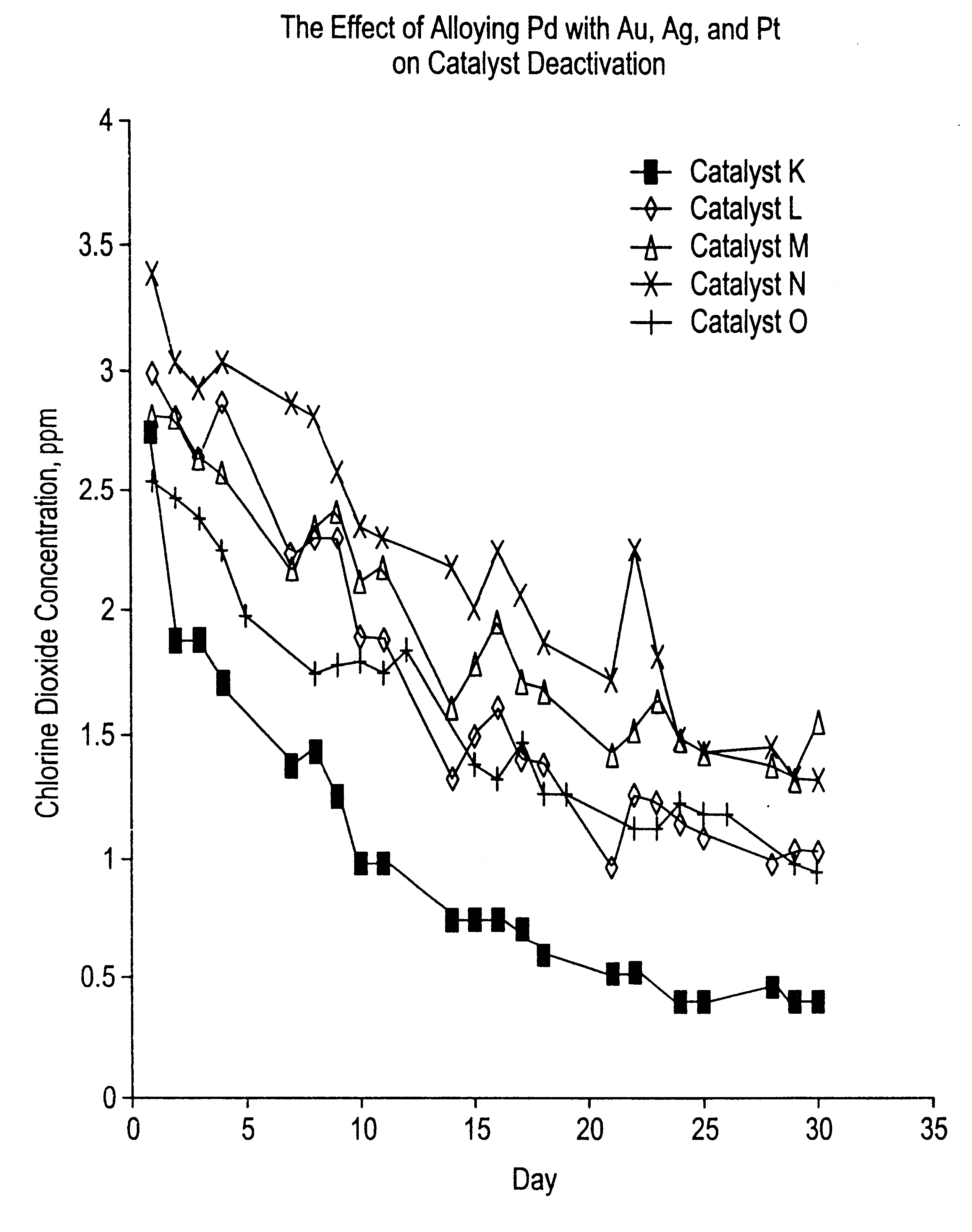
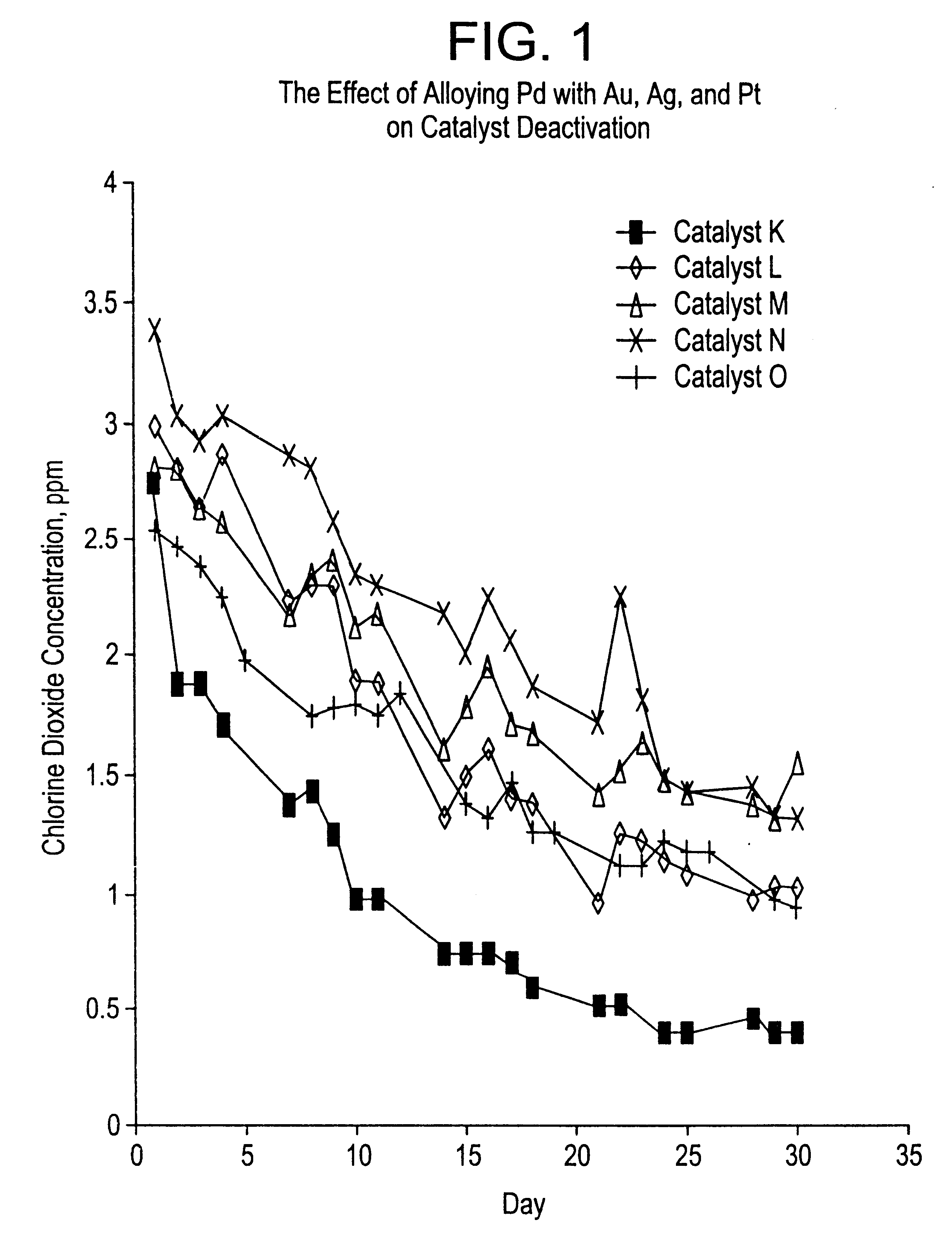
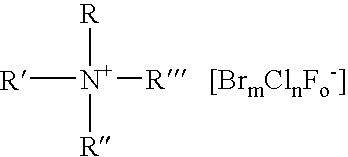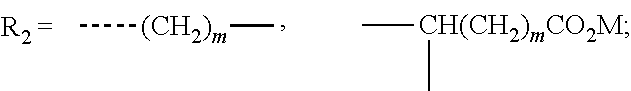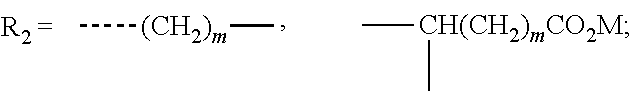Patents
Literature
60results about "Chlorous acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Thickened fluid composition comprising chlorine dioxide
This invention relates to a stable composition and method of making a thickened fluid composition comprising chlorine dioxide. The stable composition includes a mixture containing a chlorite, an acid source, and a thickener component. At least one of the chlorite, the acid source and the thickener component is in particulate form. In an embodiment, the mixture may be a unitary solid body wherein chlorine dioxide is generated upon interaction with an aqueous medium, such as water. A free halogen source may also be added to the mixture in some cases.
Owner:ENGELHARD CORP
Massive bodies containing free halogen source for producing highly converted thickened solutions of chlorine dioxide
A massive body, e.g., a tablet, for producing a thickened solution of chlorine dioxide when the massive body is added to liquid water is disclosed. The massive body comprises a metal chlorite, an acid source and a thickener (incorporated directly into the massive body or added as a component separate from the massive body) and optionally a source of free halogen. The concentration of free chlorine in the solution will be: (a) less than the concentration of chlorine dioxide in said solution on a weight basis and the ratio of the concentration of chlorine dioxide to the sum of the concentrations of chlorine dioxide and chlorite anion in said solution is at least 0.25:1 by weight; or (b) equal to or greater than the concentration of chlorine dioxide in said solution on a weight basis and the ratio of the concentration of chlorine dioxide to the sum of the concentrations of chlorine dioxide and chlorite anion in said solution is at least 0.50:1 by weight.
Owner:BASF AG
Massive bodies containing free halogen source for producing highly converted solutions of chlorine dioxide
InactiveUS7182883B2Increase conversion rateImprove permeabilityBiocideOrganic chemistryHalogenLiquid water
A massive body, e.g., a tablet, for producing a solution of chlorine dioxide when the massive body is added to liquid water. The massive body comprises a metal chlorite such as sodium chlorite, an acid source such as sodium bisulfate and a source of free halogen such as the sodium salt of dichloroisocyanuric acid or a hydrate thereof. The concentration of free halogen in the solution will be:(a) less than the concentration of chlorine dioxide in said solution on a weight basis and the ratio of the concentration of chlorine dioxide to the sum of the concentrations of chlorine dioxide and chlorite anion in said solution is at least 0.25:1 by weight; or(b) equal to or greater than the concentration of chlorine dioxide in said solution on a weight basis and the ratio of the concentration of chlorine dioxide to the sum of the concentrations of chlorine dioxide and chlorite anion in said solution is at least 0.50:1 by weight.
Owner:ENGELHARD CORP
Composition for the production of chlorine dioxide using non-iodo interhalides or polyhalides and methods of making and using the same
ActiveUS7087190B2Reduce microbial countQuick buildBiocideLiquid degasificationChlorine dioxideCHLORITE ION
A composition for the generation of chlorine dioxide including at least one non-iodo interhalide, polyhalide or salt thereof having the formulaBrmClnFoXpwherein m=0–3, n=0–4, o=0–3, p=0–2, X is a cationic moiety and with the provisos that m+n+o cannot be zero; if m+n+p<2, or mixtures thereof, and at least one source of chlorite ions.
Owner:ECOLAB USA INC
Method and system for the controlled release of chlorine dioxide gas
Method, composition and system for generating chlorine dioxide gas in a controlled release manner by combining at least one metal chlorite and a dry solid hydrophilic material that reacts with the metal chlorite in the presence of water vapor, but does not react with the metal chlorite in the substantial absence of liquid water or water vapor to produce chlorine dioxide gas in a sustained amount of from about 0.001 to 1,000 ppm.
Owner:BASF CATALYSTS LLC
Compositions and methods for storing aqueous chlorine dioxide solutions
InactiveUS6284152B1Little and no diffusional lossLoss and degradationCosmetic preparationsBiocideDiffusionChlorine dioxide
The present invention is directed to both physical and chemical means for maintaining continuous high levels of chlorine dioxide in aqueous solutions for extended periods, with minimum loss through degradation in the solution and dissipation through the walls of the containing vessel within which the solution is stored. The ClO2 may be maintained even in the absence of very high levels of chlorite, from which supplemental, offsetting levels of ClO2 may be generated. The present invention relates to the discovery that certain types of plastic and glass containers have the ability to maintain relatively constant levels of ClO2 in their contained solutions over extended periods, particularly in aqueous compositions comprising chlorite / ClO2 ratios sufficient for a stabilizing complex of the two, presumably Cl204-, to suppress the ClO2 loss. Of particular value is certain high-density polyethylene terephthalate (PETE) plastic bottling and plastic containers which have been initially exposed to a fluorinating environment. Specifically, in the latter regard, it has been discovered that certain plastic containers which ordinarily are incapable of maintaining aqueous ClO2 levels can be treated with fluorine gas to transform their surfaces so as to effectively suppress ClO2 diffusion therethrough.
Owner:PROFRESH PROPERTIES
Chlorine dioxide releasing composite article
A composite article that includes a ClO2-producing material integrated into an organic matrix and methods of using the same are described. The organic matrix of the composite article is formable at a temperature under about 150° C., permits contact between an activating stimulus (e.g., water vapor and / or electromagnetic energy) and the ClO2-producing material when the composite article is exposed to the activating stimulus, and is permeable to ClO2.
Owner:BASF CORP
Method for the synthesis of chlorine dioxide
Owner:DEGUSSA CORP
Acidified chlorite disinfectant compositions with olefin stabilizers
InactiveUS20050184273A1Reducing chlorine dioxide generationReduce drynessBiocideCosmetic preparationsDisinfectantCompound (substance)
A two-part disinfecting systems, as well as disinfecting compositions and methods for making and using the same. The two-part disinfecting system contains a first part and a second part adapted to be mixed to yield an aqueous disinfecting composition, wherein the first part comprises a chlorite and the second part comprises an acid, and wherein the first part, the second part, or both the first and second parts comprise an olefin compound.
Owner:ECOLAB USA INC
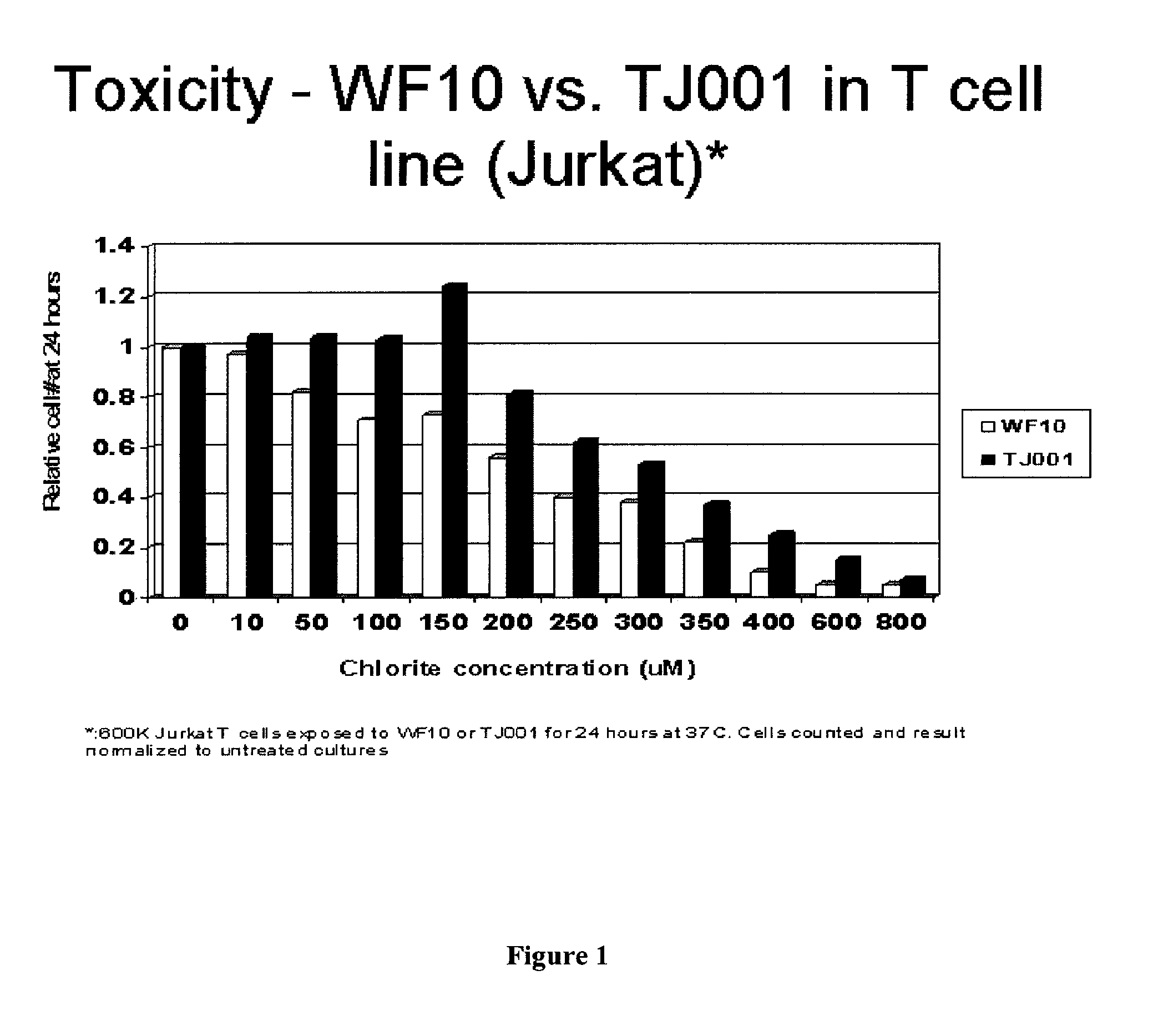
Chlorite Formulations, and Methods of Preparation and Use Thereof
Described herein are chlorite formulations having a pH between about 7 and about 8.5, wherein the chlorite formulations are substantially free of deleterious non-chlorite components. Described herein are chlorite formulations, including pharmaceutical formulations, which are formulated for systemic, parenteral, or intravenous administration. Described herein are methods of preparing and methods of using the chlorite formulations described herein.
Owner:NEUVIVO INC
Composition for generating chlorine dioxide
InactiveUS6602442B1Transportation safetyMade preciselyBiocidePeroxide active ingredientsLithium hypochloriteSodium bisulfate
A dry disinfectant composition for the production of aqueous solutions of chlorine dioxide of predetermined concentration is formulated of a mixture of lithium hypochlorite, sodium bisulfate and sodium chlorite. Special liquid and dry formulations are also contemplated for convenience of use, for quality assurance and for safety.
Owner:OCCIDENTAL CHEM CORP
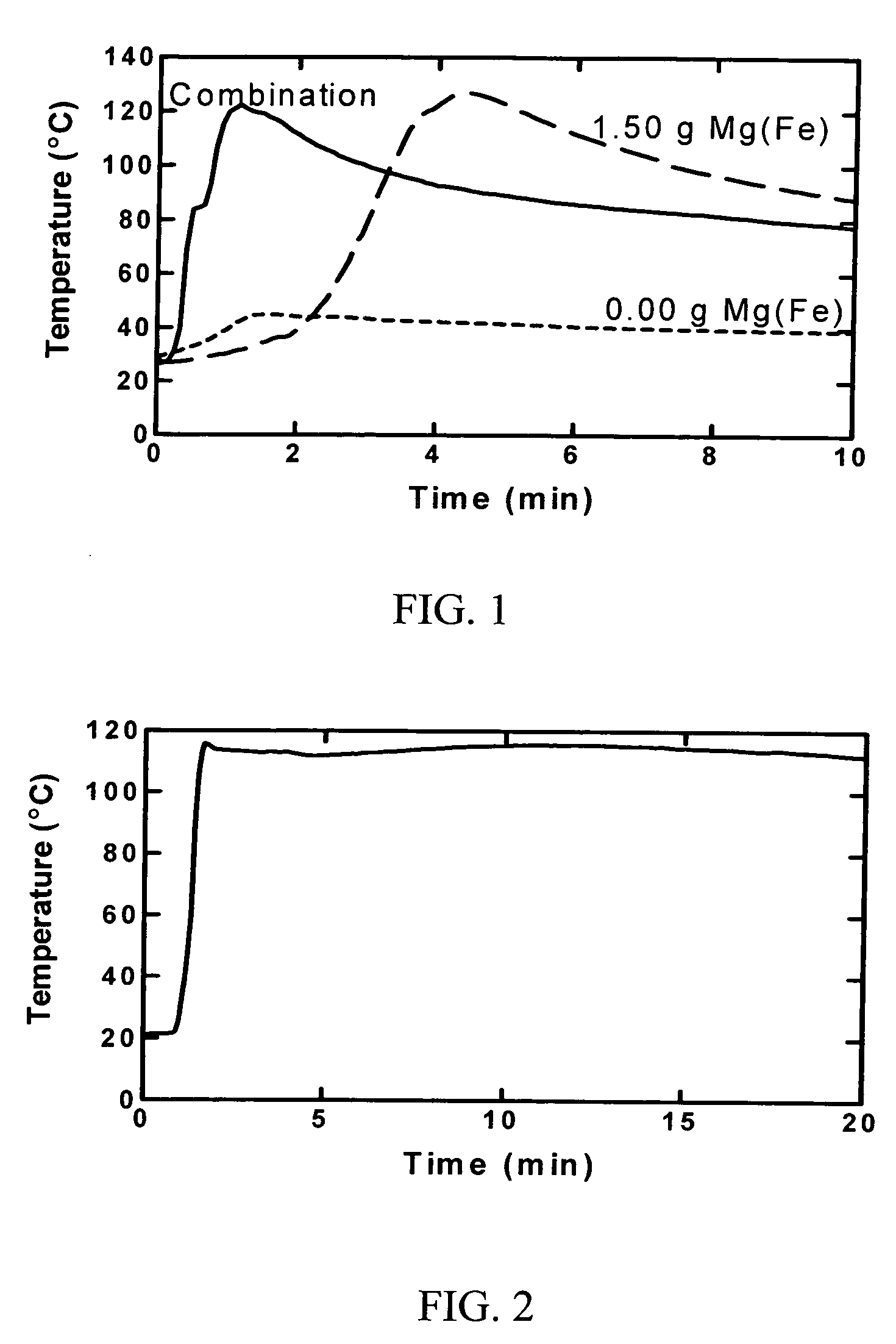
Chemical combination for generation of disinfectant and heat
InactiveUS20060097222A1Eliminate the problemExothermal chemical reaction heat productionLiquid degasificationChlorine dioxideDisinfectant
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Calcium hypochlorite composition
Calcium hypochlorite compositions that are classified as a Packing Group III Division 5.1 oxidizer material or as a non-Division 5.1 oxidizer material are described. In one embodiment, the compositions comprise an admixture of particulate calcium hypochlorite and particulate metaboric acid. The calcium hypochlorite is present in the composition in an amount and is of a concentration such that the composition would be classified as a Packing Group II Division 5.1 oxidizer in the absence of said particulate metaboric acid. Other embodiments described are solid shaped articles, e.g., tablets, comprising the described calcium hypochlorite-metaboric acid composition.
Owner:AXIALL OHIO
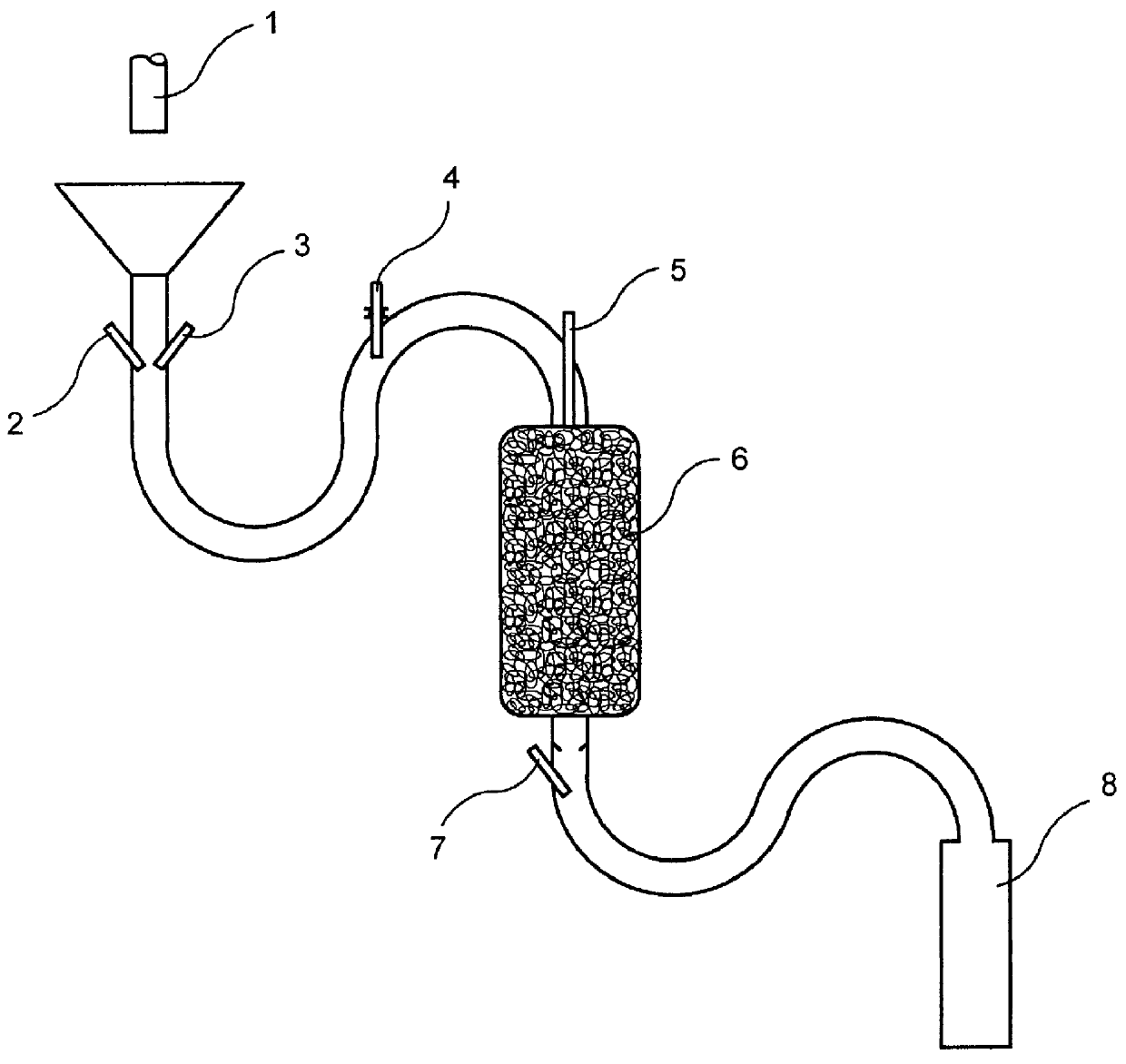
Method and device for the production of an aqueous solution containing chlorine dioxide
InactiveUS7160484B2Improve responseOther chemical processesChlorous acidChlorine dioxideLiquid water
The present invention is generally directed to a method of producing an aqueous solution of chlorine dioxide from the reaction of a metal chlorite and an acid forming component which do not react to produce chlorine dioxide in the substantial absence of water. The reactants are separated from liquid water by a membrane which allows the controlled passage of liquid water and / or water vapor into contact with the reactants. The chlorine dioxide thus generated passes out through the membrane into the liquid water to produce the desired aqueous solution. Aqueous solutions containing chlorine dioxide produced in this manner can be conveniently used for commercial and domestic cleaning operations such as in the food industry.
Owner:ENGELHARD CORP
Process for the production of aqueous chlorine dioxide solutions
InactiveUS6103950ASimple and dependable managementEliminate pollutionOther chemical processesChlorous acidChlorine dioxideRedox
The description relates to a process for the production of aqueous chlorine dioxide solutions through oxidation of chlorite with oxo acids and / or oxo acid anions having a suitable redox potential in a buffered aqueous medium, wherein an acidic aqueous solution A is produced which has a pH of about 5 or less and contains the oxo acids and / or oxo acid anions, and the acidic aqueous solution A is mixed with an aqueous chlorite solution B to form chlorine dioxide, wherein a pH of less than 6.95 is adjusted in the reaction mixture, this pH value being stabilized by a buffering system contained therein.
Owner:RIMPLER MANFRED +2
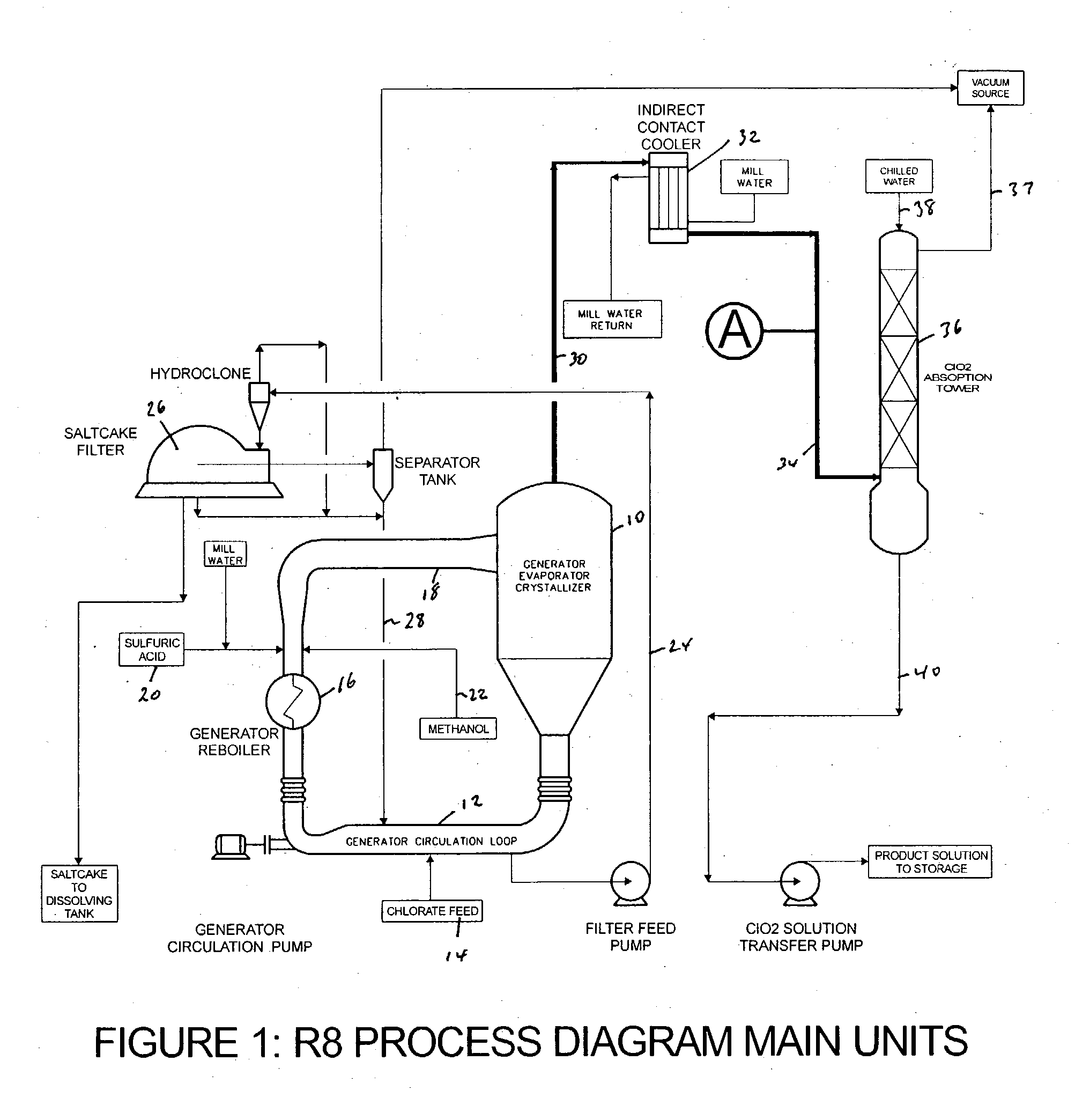
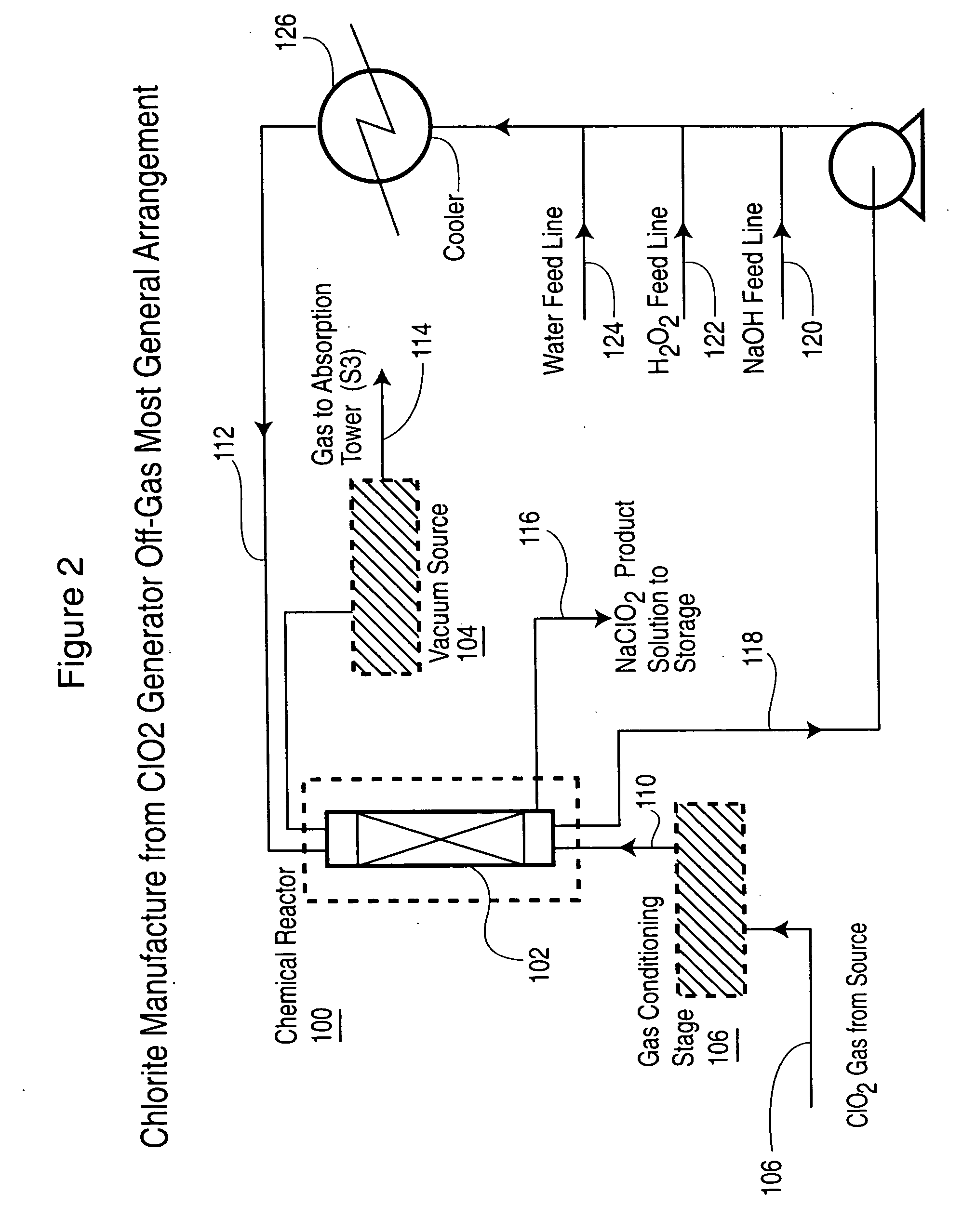
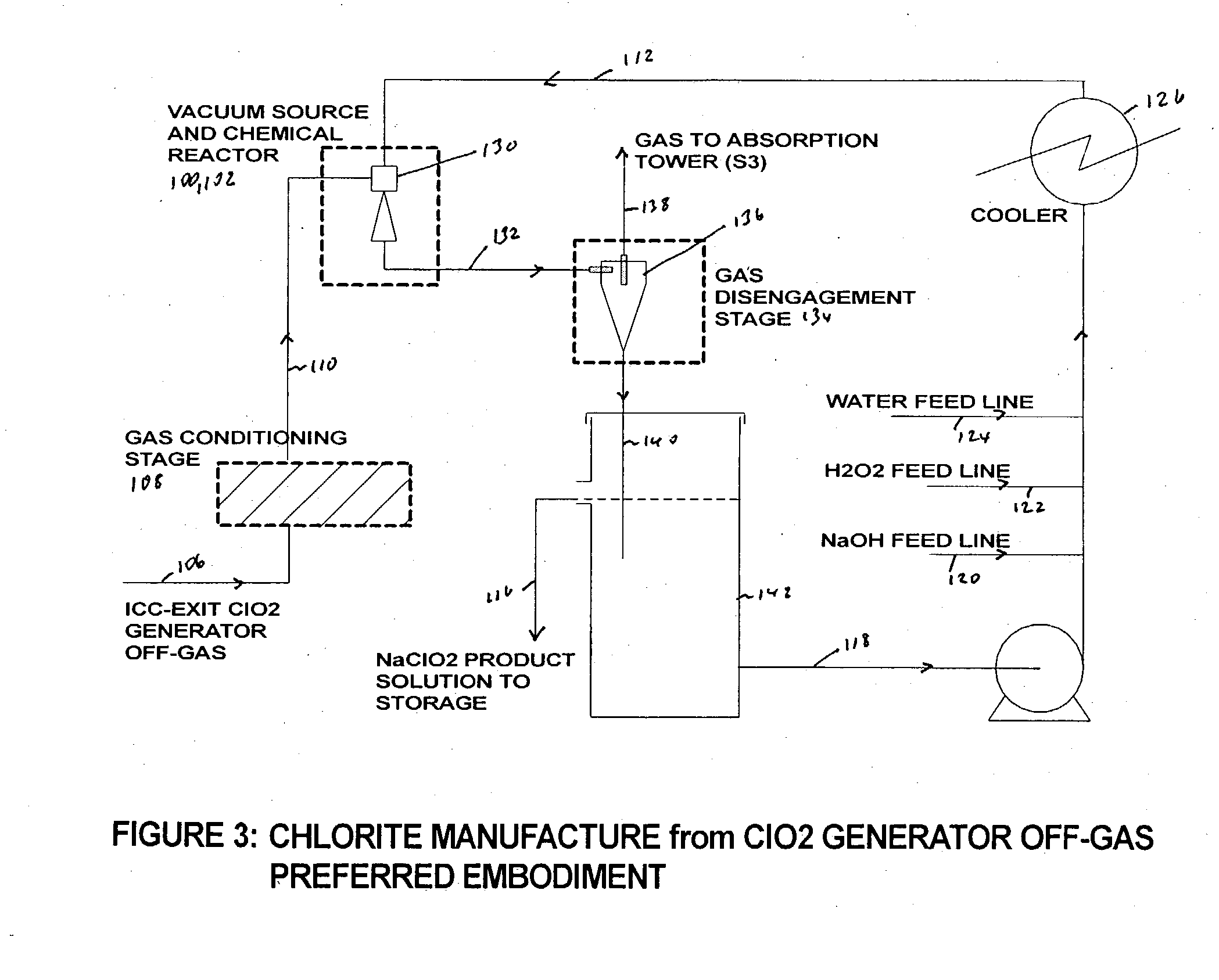
Chlorine dioxide from a methanol-based generating system as a chemical feed in alkali metal chlorite manufacture
InactiveUS20040228790A1Easily substitutedGreat opportunityChlorous acidChloric acidLiquid mediumSodium chlorite
Alkali metal chlorite, particularly sodium chlorite, is produced with a level of purity superior to that expected based on the composition of the chlorine dioxide generator off-gas that is used as a raw chemical feed for chlorite manufacture. The off-gas is preferably drawn before it enters the chlorine dioxide absorption tower and is passed through a conditioning stage to then react in a liquid medium to produce an alkali metal chlorite solution from which the unreacted and produced gases are immediately separated.
Owner:SUPERIOR PLUS INC
Process for preparing sodium chlorite
InactiveCN1378973AGenerate fastHigh Goal Conversion RateChlorous acidHypochloriteSodium chlorateOxygen
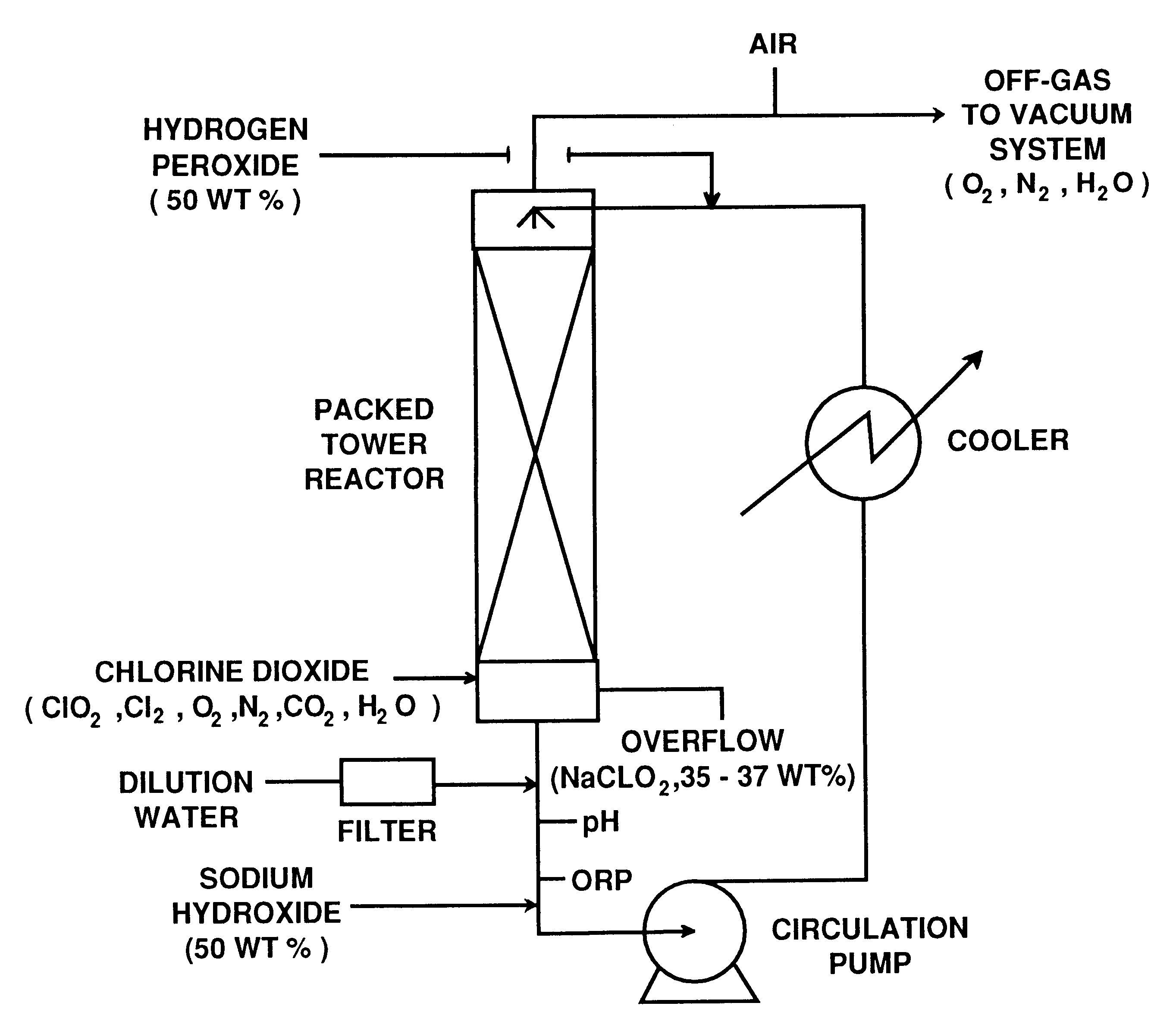
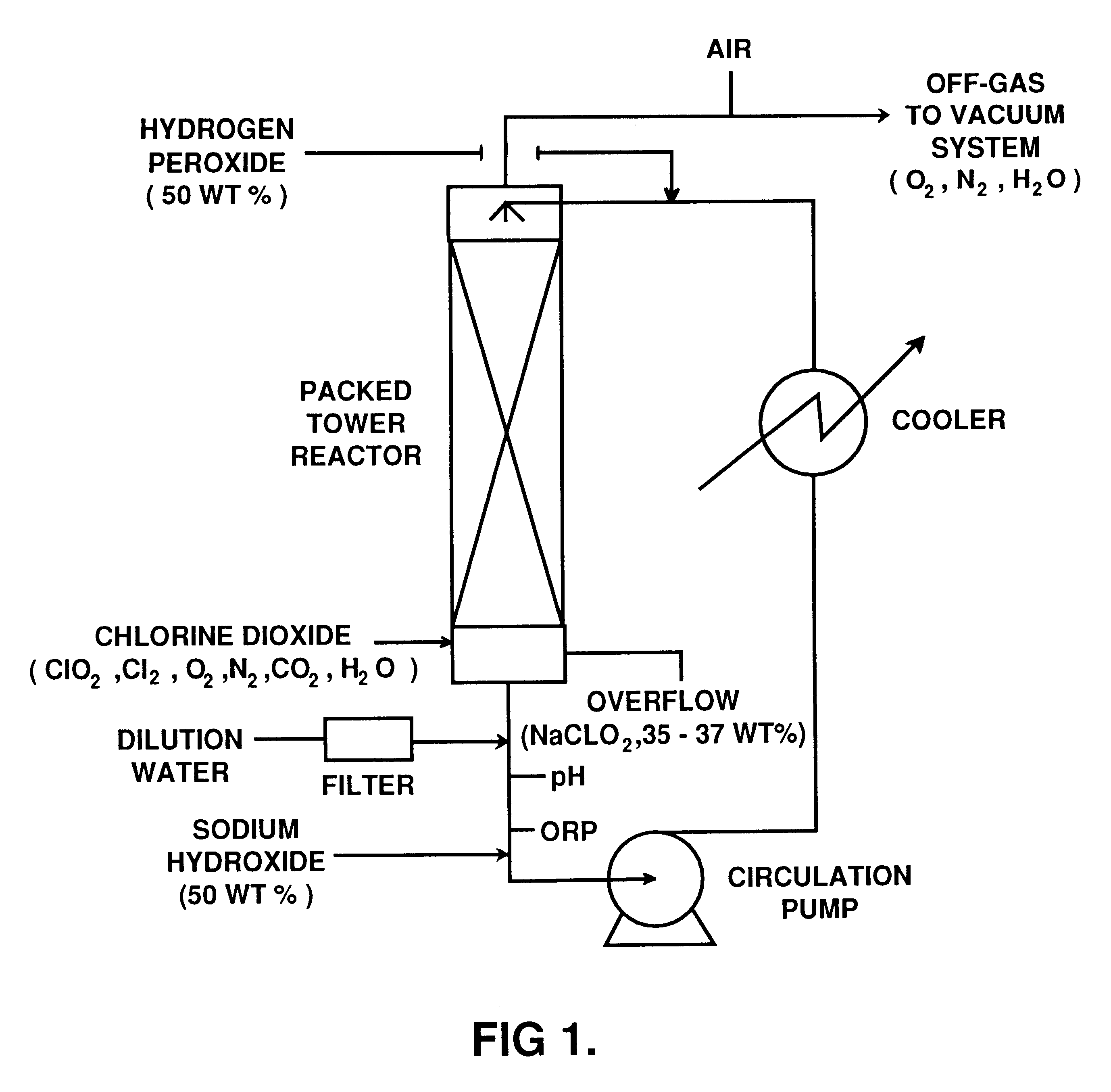
A process for preparing sodium chlorite includes such steps as dissolving hydrogen peroxide and solid sodium chlorate in water, adding the resultant solution along with sulfuric acid to a ClO2 generator to generate the mixture of ClO2 and oxygen, loading the mixed absorbing liquid containing sodium hydroxide and hydrogen peroxide into an absorbing tower, adding ClO2 to the absorbing tower while escaping oxygen, and finally generating sodium chlorite. Its advantages are high output rate (more than 95%) and high purity (more than 95%) of product.
Owner:SOUTH CHINA UNIV OF TECH
Method and device for preparing sodium chlorite through comprehensive method chlorine dioxide process
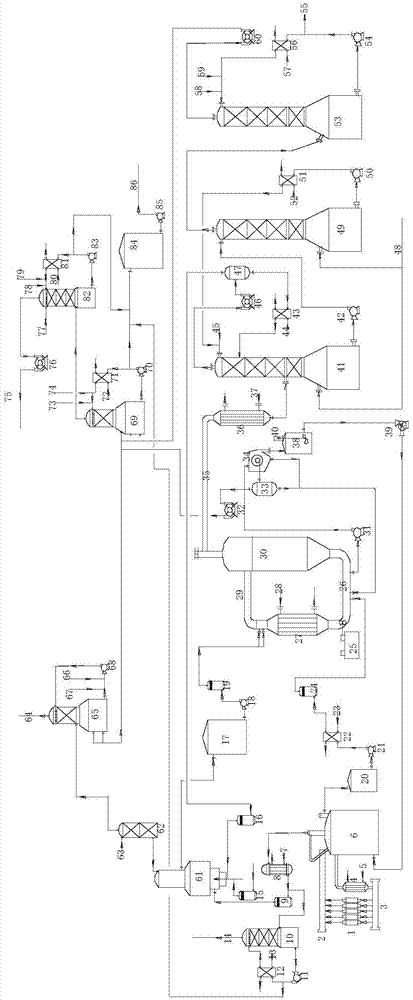
ActiveCN105439095ANo dischargeReduce pollutionChlorine oxidesChlorous acidChemical equationSide product
The invention discloses a method for preparing sodium chlorite through a comprehensive method chlorine dioxide process. According to the reaction principle, the method includes the steps of firstly, conducting sodium chlorate electrolysis according to the chemical equation NaCl+3H2O->NaClO3+3H2; secondly, conducting hydrochloric acid synthesis according to the chemical equation H2+Cl2->2HCl; thirdly, generating chlorine dioxide according to the chemical equation NaClO3+2HCl->NaCl+ClO2+1 / 2Cl2+H2O; fourthly, preparing sodium chlorite according to the chemical equation 2ClO2+2NaOH+H2O2->2NaClO2+2H2O+O2. A preparation device mainly comprises an electrolytic cell, a sodium chlorate reactor, a hydrochloric acid synthesis furnace, a reboiler, a chlorine dioxide generator, a chlorine dioxide absorption stripping tower, a chlorine dioxide stripper and a sodium chlorite preparation tower. When sodium chlorite is prepared through the method and the device, the product cost is low, yield is high, no side products are discharged, no waste acid or solid waste is discharged, environment pollution is reduced, and the advantages of being economical and environmentally friendly are achieved.
Owner:GUANGXI UNIV
High purity alkali metal chlorite and method of manufacture
InactiveUS6251357B1Minimized in sizeImprove water balanceChlorine oxidesChlorous acidAlkali hydroxideHydrogen peroxide
Alkali metal chlorite, particularly sodium chlorite, is produced with a low carbonate level by combining a chlorine dioxide generating system operating at subatmospheric pressure with a chlorite formation reactor in which the chlorine dioxide reacts with hydrogen peroxide in the presence of aqueous alkali metal hydroxide, particularly sodium hydroxide.
Owner:SUPERIOR PLUS INCOME FUND +1
Composition and method for enhanced sanitation and oxidation of aqueous systems
ActiveUS7922933B2Inactivation rate can be enhancedEfficient regenerationHeavy metal active ingredientsBiocideChlorine dioxideNeutral ph
This invention relates to a method for enhanced sanitation and oxidation of aqueous solutions at aquatic facilities. The method provides a means for the in-situ generation of chlorine dioxide from dilute solutions of chlorite anions at near neutral pH, and enhanced inactivation rates of microbiological organisms including cryptosporidium.
Owner:TRUOX
Preparation method of sodium chlorite
InactiveCN103159178AThe reaction process is easy to controlNo reactionChlorous acidSodium chlorateChlorous acid
The invention discloses a preparation method of sodium chlorite. The preparation method comprises the following steps of (1) enabling sodium chlorate solution to react with hydrochloric acid to generate chlorine dioxide gas and chlorine; (2) leading chlorine dioxide gas and chlorine into sodium chlorite solution to further react, so as to generate chlorine dioxide gas; (3) leading the chlorine dioxide gas into sodium hydroxide solution, and adding hydrogen peroxide to react, so as to generate sodium chlorite at the same time; (4) crystallizing and drying sodium chlorite prepared in the step (3) to obtain a final product, wherein the concentration of the sodium chlorate solution in the step (1) is 25-33%, the concentration of hydrochloric acid solution in the step (1) is 31%, and the molar ratio of the hydrochloric acid to chlorous acid in the step (1) is (1-1.5) to 2. The preparation method of sodium chlorite disclosed by the invention does not utilize concentrated sulfuric acid of which the concentration is over 95%, is easy in control of the reaction process, not drastic in reaction, higher in safety coefficient, low in cost, and suitable for industrial large-scale application at the same time.
Owner:常州和方环保科技有限公司
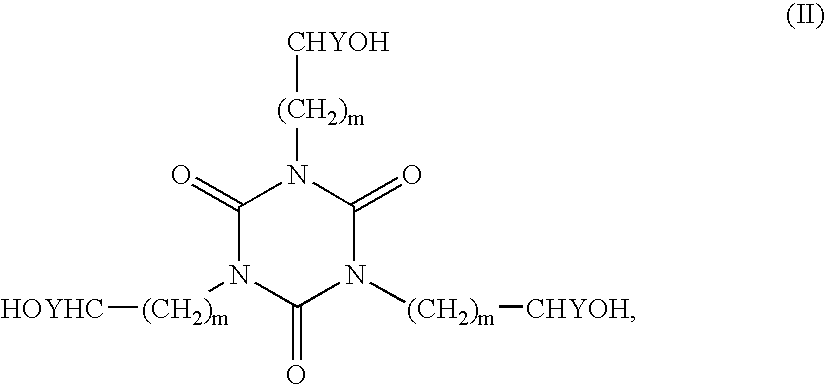
Stabilization composition for halogen-containing polymers
The invention is a stabilizer composition for halogen-containing polymers, comprising a carrier material of the general formula CaxAl2(OH)2(x+2)HPO3.m H2O, wherein x is a number from 2 to 12 and m is a number from 0 to 12, and a salt of a halogen-containing oxy acid or a mixture of two or more such salts, at least one salt of a halogen-containing oxy acid being present in finely distributed form on the carrier material, to a process for the preparation thereof, and to the use thereof.
Owner:BAERLOCHER
Process for producing aqueous chlorine dioxide for surface disinfection and decontamination
The present invention provides for a method of generating an aqueous solution comprising chlorine dioxide using a chlorine-containing chemical oxidant; an effector having the capacity to reduce said chlorine-containing chemical oxidant; a chemical reductant; and water, and operating in either batch or continuous-flow modes. In batch mode, the aqueous chlorine dioxide solution can be generated in a sprayer device, a bottle, or a bucket to disinfect objects by spraying and wiping, by pouring, or by immersion, respectively. In continuous-flow mode, the aqueous chlorine dioxide solution can be generated in flow tubes or continuous-stirred tank reactors, then placed inside a suitable sprayer device, bottle, or bucket.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Composition and process for enhanced sanitation and oxidation of aqueous systems
InactiveUS7927508B2Enhanced sanitizing and oxidizing efficiencyLower potentialOrganic chemistryChlorine oxidesCyclic processChloramine B
An efficient cyclic process and related compositions for the in-situ generation of oxyhalogens from anions of chloride, bromide and chlorite in an aqueous system using in-situ generated sulfate free radicals. The cyclic process and compositions enhance the rate of inactivation of microbiological organisms especially those resistant to inactivation from free halogen based sanitizers, and oxidation of oxidation resistant organic based compounds in aqueous solution. Aquatic facilities susceptible to accumulation of organic N-chloramines and other oxidation resistant compounds, as well as oxidation resistant parasitic organisms such as cryptosporidium and Giardia, obtain dramatic improvements in the rate of oxidation and subsequent inactivation of these undesirable contaminants.
Owner:TRUOX
Solid chlorine dioxide releasing compositions having increased temperature stability and method for delivery of solid chlorine dioxide releasing compositions
InactiveCN1964916AAvoid contactAccurately determineChlorine oxidesChlorous acidChlorine dioxideWater soluble
The creation of chlorine dioxide in a water system can be accomplished by an improved mixture of components that includes a chlorite salt, oxidizing chlorine releasing agent(s) and an acid source and these improved combinations of materials can be pre-measured and delivered into a water system in sealed water soluble containers.
Owner:SAFE SOLID SOLUTIONS
Chlorite formulations, and methods of preparation and use thereof
Described herein are chlorite formulations having a pH between about 7 and about 8.5, wherein the chlorite formulations are substantially free of deleterious non-chlorite components. Described herein are chlorite formulations, including pharmaceutical formulations, which are formulated for systemic, parenteral, or intravenous administration. Described herein are methods of preparing and methods of using the chlorite formulations described herein.
Owner:NEUVIVO INC
Solid Compositions and Methods for Generating Chlorine Dioxide
ActiveUS20110309297A1Simple and convenient and safeHigh yieldOxygen/ozone/oxide/hydroxideOther chemical processesChlorine dioxideSuper absorbent
A composition for generating chlorine dioxide comprises active ingredients, a suitable hydrophobic compound, and a suitable super absorbent compound. A suitable hydrophobic compound will, among other characteristics, repel the solvent for at least the initial 30 seconds of exposure thereto. A suitable super absorbent compound will, among other characteristics, absorb at least 75 times its weight in solvent and will not gel until the chlorine-dioxide generating reaction is substantially complete.
Owner:SIPKA
Method for preparing high-purity chlorine dioxide and sodium chlorite by ferrous sulfide method
InactiveCN102674256AHigh purityGenerate fastChlorine oxidesChlorous acidSodium chlorateChlorous acid
The invention discloses a method for preparing high-purity chlorine dioxide and sodium chlorite by a ferrous sulfate method. The method comprises the following steps of: crushing ferrous sulfide; dissolving sodium chlorate into water to obtain sodium chlorate solution; diluting concentrated sulfuric acid by adding water, adding the diluted sulfuric acid into the sodium chlorate solution and uniformly mixing the solution; adding the mixed solution into ferrous sulfide powder; performing reduction reaction when stirring to produce high-purity chlorine dioxide gas; and inputting the gas into mixed absorption liquid consisting of hydrogen peroxide and sodium hydroxide, and thus obtaining the high-purity sodium chlorite solution, wherein the purity of the high-purity sodium chlorite solution is larger than 98 percent. According to the method, due to the adoption of the ferrous sulfide, the chlorine dioxide is obtained by the one-step reaction, and the sodium chlorite can be obtained by the two-step reaction; the conversion rate is up to more than 97 percent, the purity of the obtained chlorine dioxide and sodium chlorite is high, and the method has a simple production process and low cost, and is easy to industrialize.
Owner:SOUTH CHINA UNIV OF TECH
Acidified chlorite disinfectant compositions with olefin stabilizers
A two-part disinfecting systems, as well as disinfecting compositions and methods for making and using the same. The two-part disinfecting system contains a first part and a second part adapted to be mixed to yield an aqueous disinfecting composition, wherein the first part comprises a chlorite and the second part comprises an acid, and wherein the first part, the second part, or both the first and second parts comprise an olefin compound.
Owner:ECOLAB USA INC
Method of preparing, storing, transporting and testing chlorine dioxide solutions
InactiveUS20120214248A1Easy to storeEasy to transportChloratesChlorous acidChlorine dioxidePhysical chemistry
A method for determining the concentration of chlorine dioxide in a chlorine dioxide solution having the following steps:(1) isolating two samples, Sample 1 and Sample 2, from the chlorine dioxide solution;(2) stripping the chlorine dioxide from Sample 1;(3) completely converting the chlorine dioxide in Sample 2 to other chlorine species;(4) transporting the chlorine dioxide samples either within the facility or outside the facility to a testing site;(5) separately determining the concentration of the chlorine containing species in each of Samples 1 and 2; and(6) calculating the concentration of chlorine dioxide in said chlorine dioxide solution based upon the information obtained in step (5).
Owner:SAMPSON ALLISON H +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com