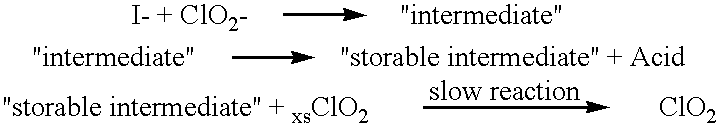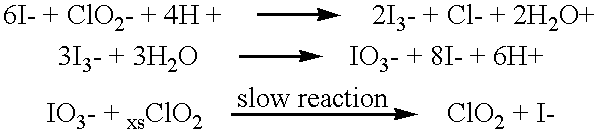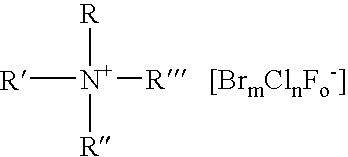Patents
Literature
481results about "Chlorine dioxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
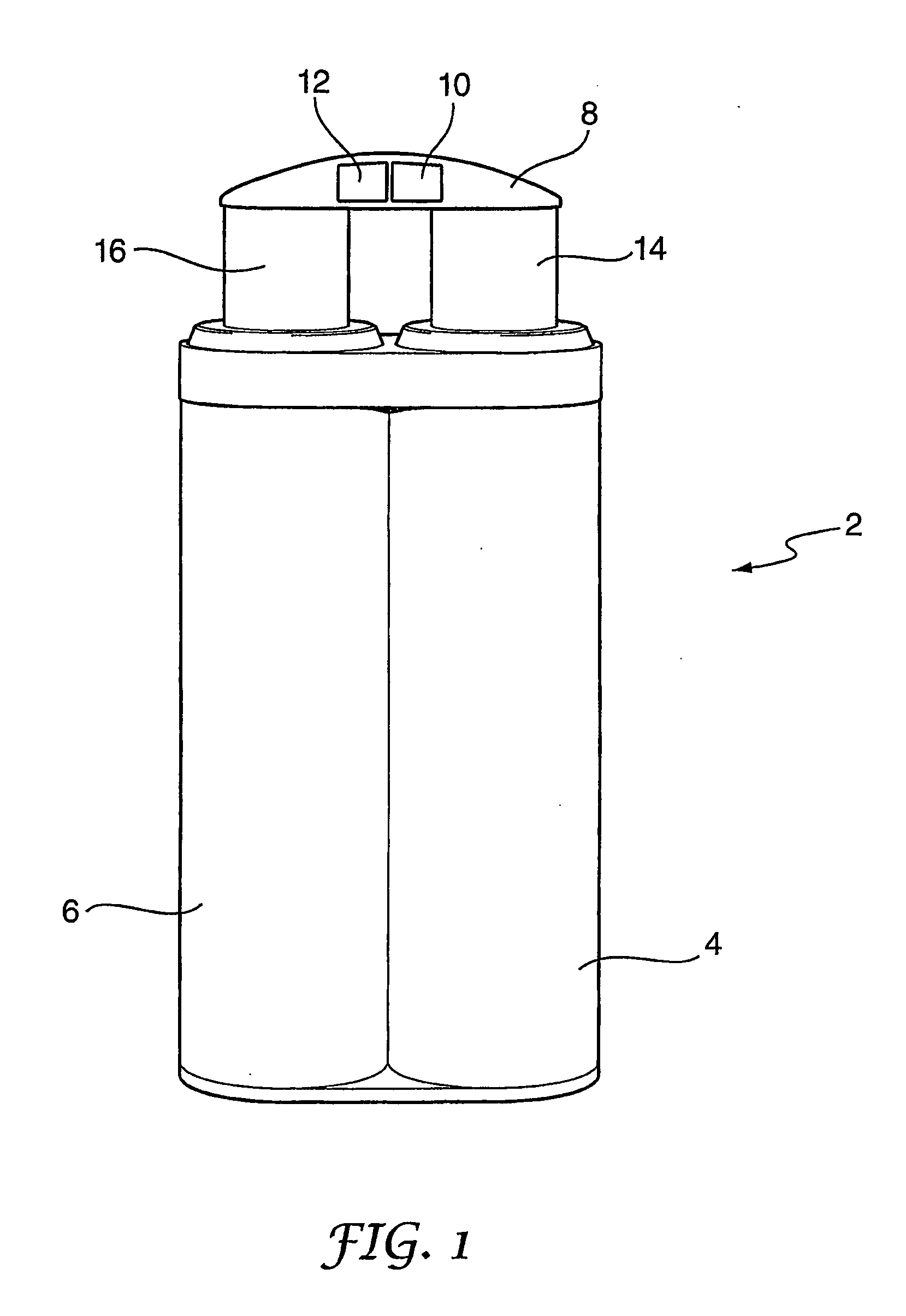
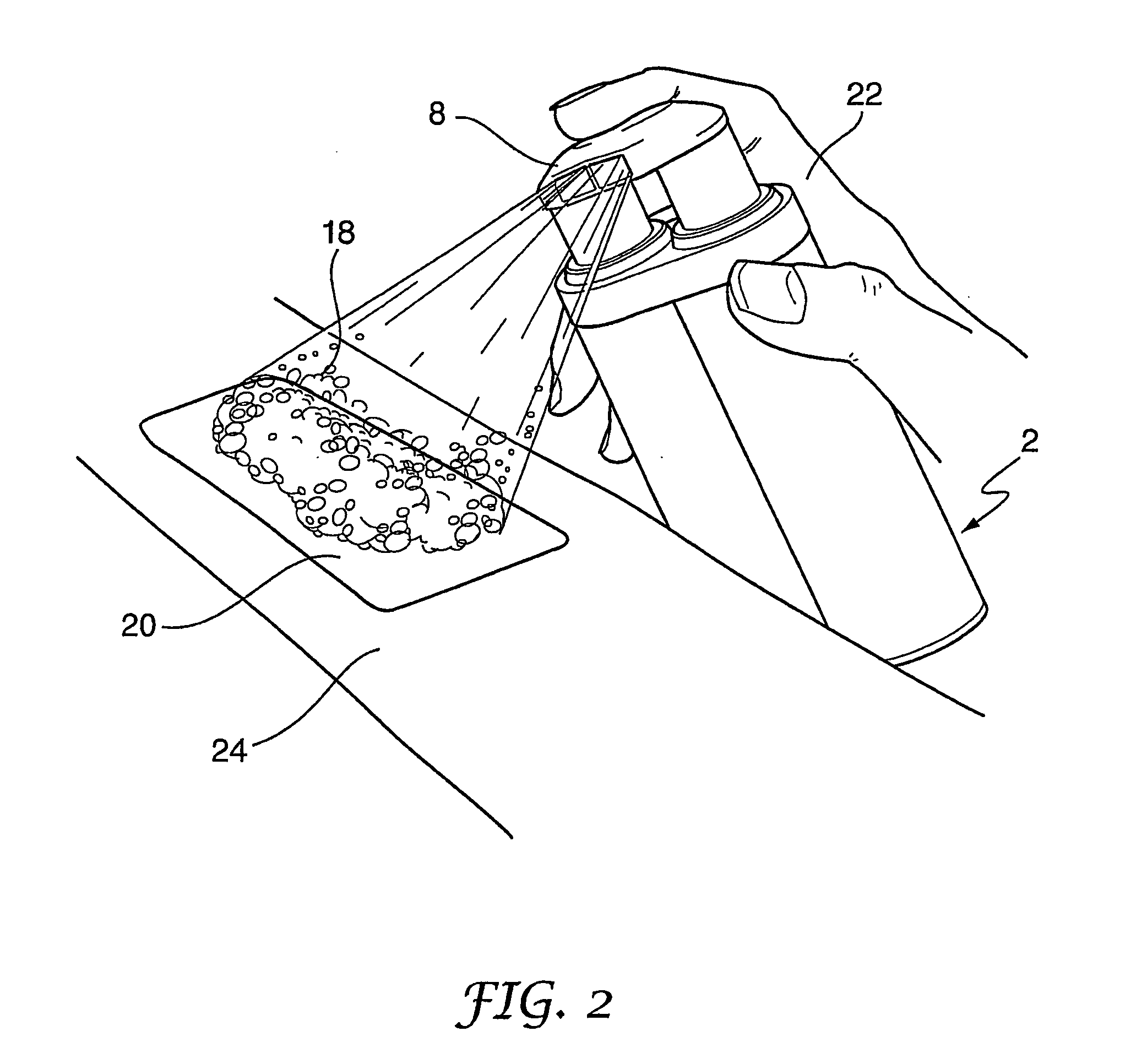
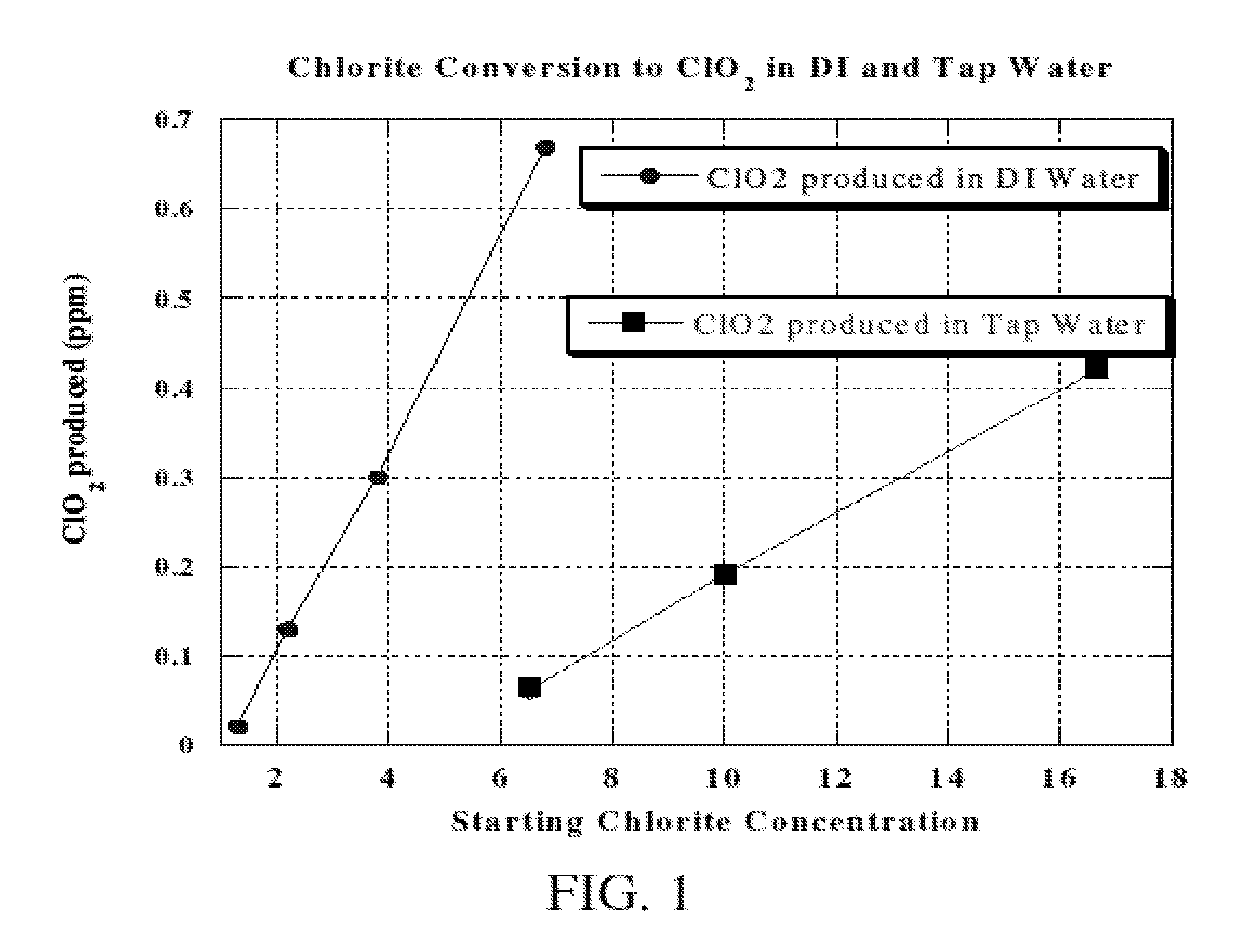
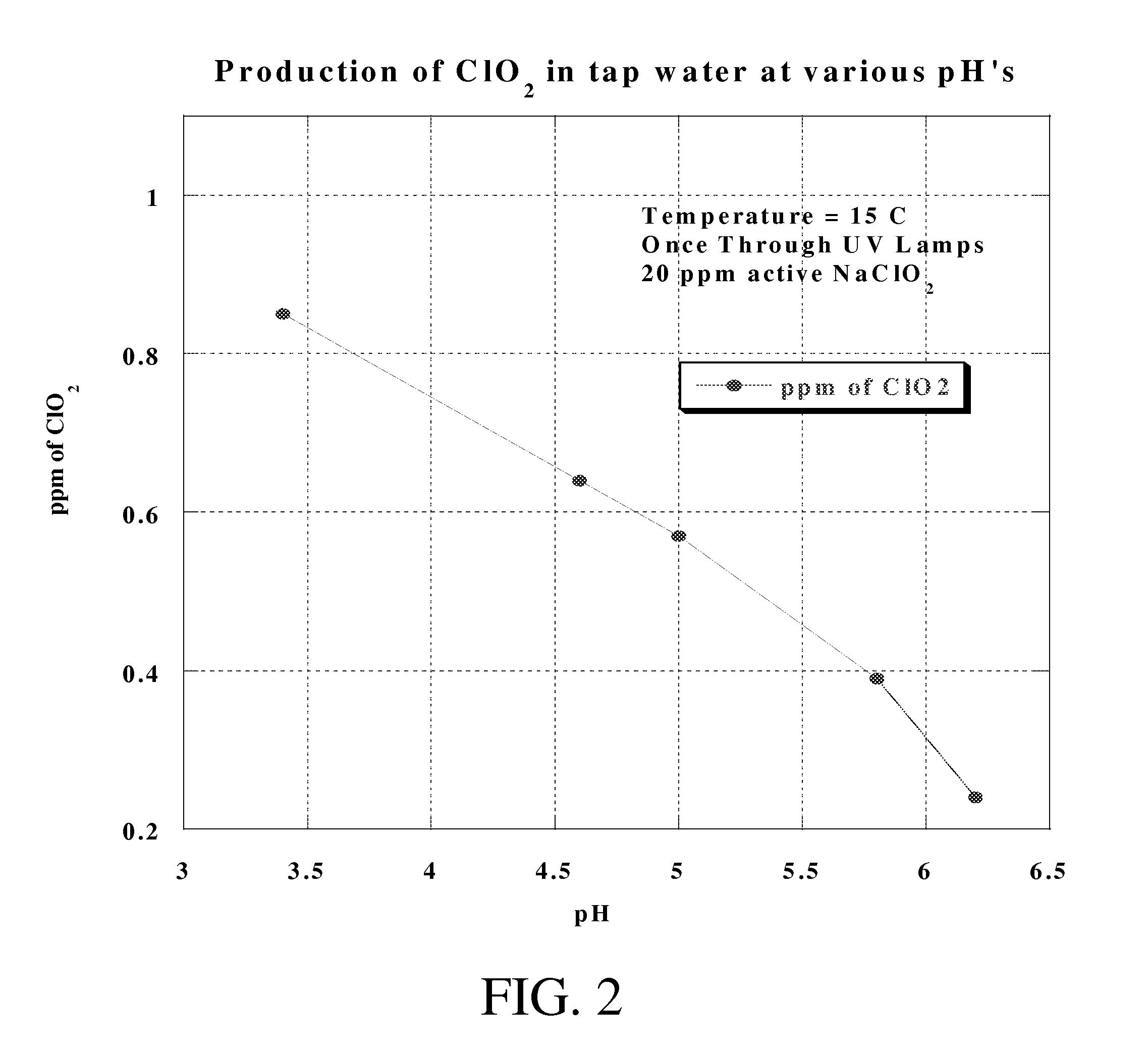
Method of making molecular chlorine dioxide
A method for manufacturing molecular chlorine dioxide, by the addition of potassium iodide to a solution of alkali metal chlorite. The metal chlorite and the potassium iodide are kept separate, until the need for the generation of chlorine dioxide arises-to ensure long-shelf life. After initiation or activation of the chlorite anion to form chlorine dioxide, the beneficial properties of chlorine dioxide can be used, for different health and cosmetic purposes. Such uses include the treatment of herpes, dandruff, acne, skin rashes (e.g. poison ivy), ulcers, bed sores, warts, nail fungus, athletes foot, sun burn and gum disease; and as an antiseptic, disinfectant, and general deodorant form refrigerator sprays to oral mouthrinses.
Owner:MADRAY GEORGE
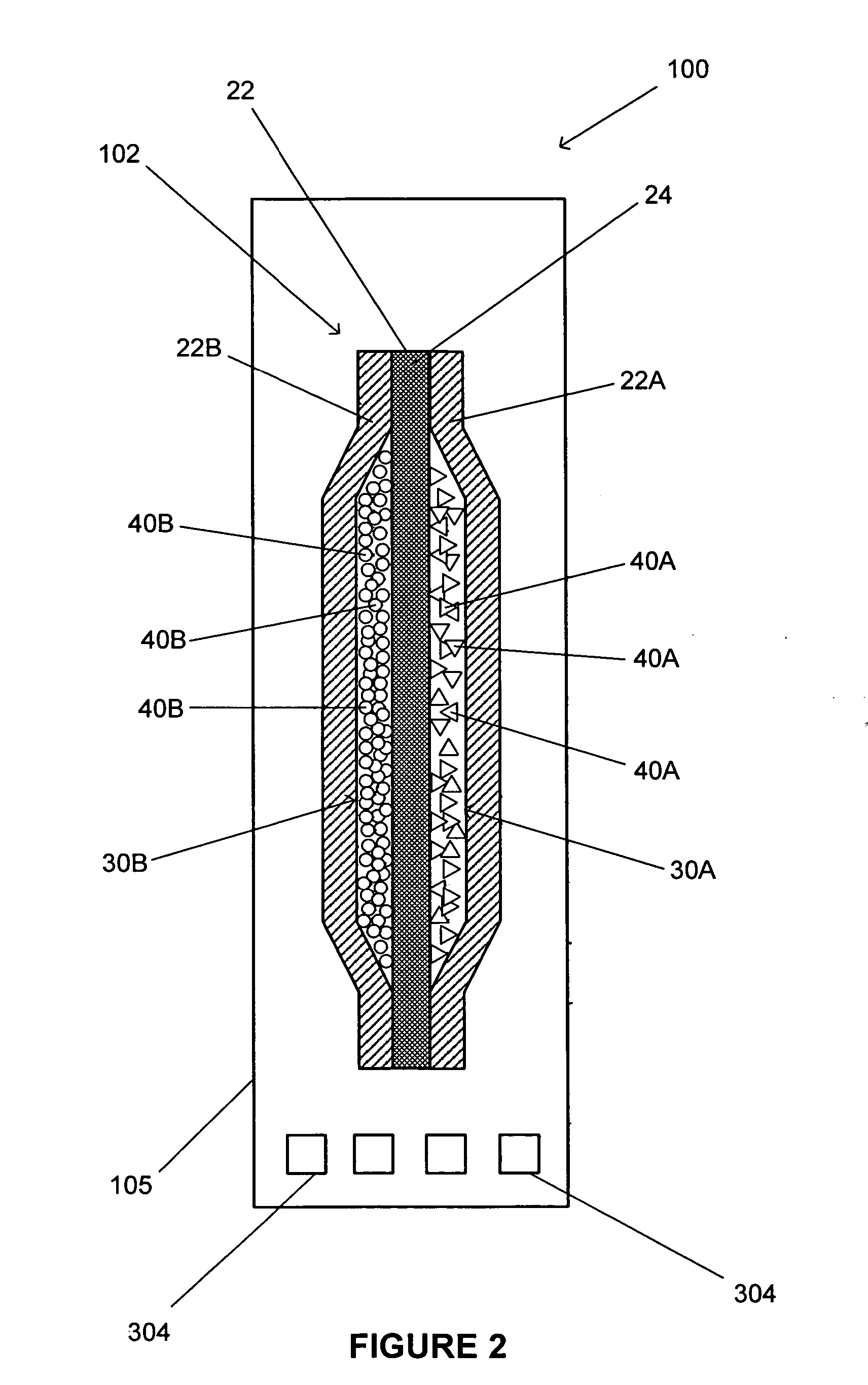
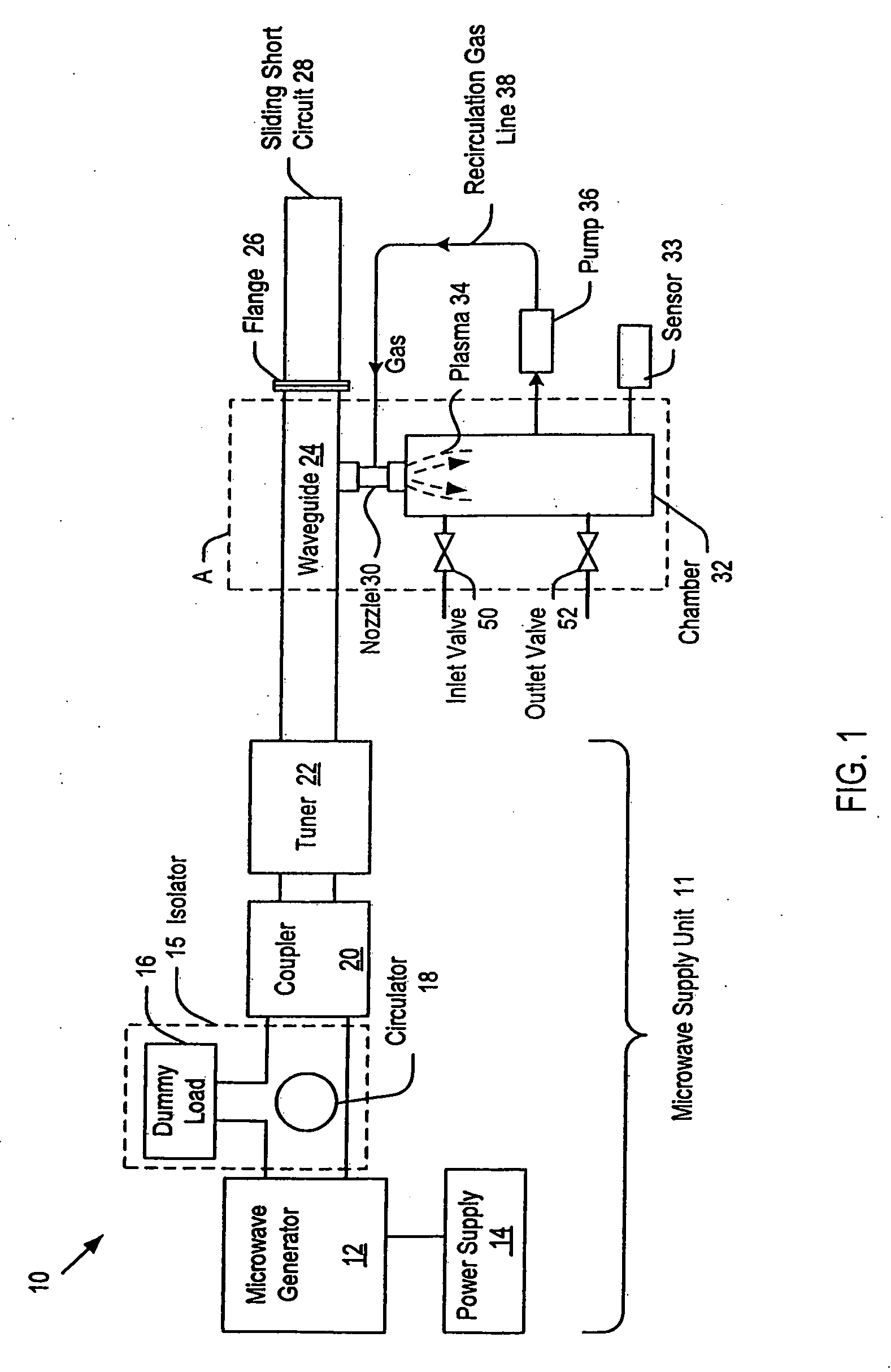
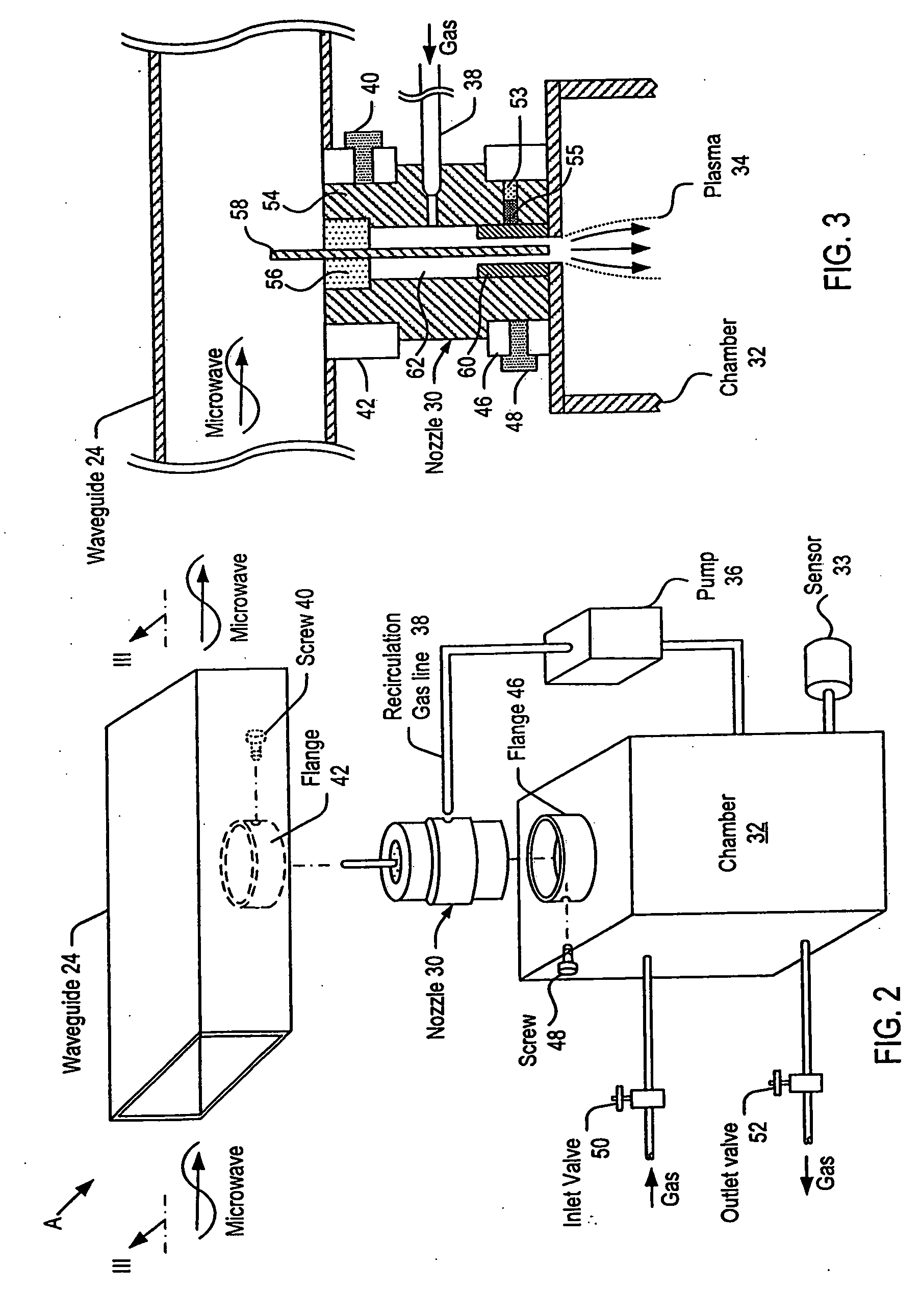
Process and apparatus for the generation of chlorine dioxide using a replenished foam system
InactiveUS20030031621A1Reduce operating costsReadily availableChlorine dioxideChlorine dioxideAlkali metal
An aqueous solution of metal chlorate, mineral acid and a reducing agent are continuously or intermittently sprayed, in a pattern to achieve intimate mixing, into a spherical chamber creating an aqueous foam reaction mixture generating chlorine dioxide which is removed in a direction 90 degrees to the axis of the spray nozzles. A baffle plate may be used to reduce the open cross sectional area of the exit port to increase reaction efficiency. The reactants are a mineral acid and an alkali metal chlorate or chloric acid and a reducing agent such as hydrogen peroxide. The mineral acid is either diluted or concentrated sulfuric acid, hydrochloric acid, acetic acid, nitric acid or a blend thereof. The ratio of acid is greater than one and less than 3 kg acid per kg of ClO2 formed. The chlorine dioxide may be removed with a stripper column.
Owner:GRAVITT ALAN +1
Thickened fluid composition comprising chlorine dioxide
This invention relates to a stable composition and method of making a thickened fluid composition comprising chlorine dioxide. The stable composition includes a mixture containing a chlorite, an acid source, and a thickener component. At least one of the chlorite, the acid source and the thickener component is in particulate form. In an embodiment, the mixture may be a unitary solid body wherein chlorine dioxide is generated upon interaction with an aqueous medium, such as water. A free halogen source may also be added to the mixture in some cases.
Owner:ENGELHARD CORP
Massive bodies containing free halogen source for producing highly converted thickened solutions of chlorine dioxide
A massive body, e.g., a tablet, for producing a thickened solution of chlorine dioxide when the massive body is added to liquid water is disclosed. The massive body comprises a metal chlorite, an acid source and a thickener (incorporated directly into the massive body or added as a component separate from the massive body) and optionally a source of free halogen. The concentration of free chlorine in the solution will be: (a) less than the concentration of chlorine dioxide in said solution on a weight basis and the ratio of the concentration of chlorine dioxide to the sum of the concentrations of chlorine dioxide and chlorite anion in said solution is at least 0.25:1 by weight; or (b) equal to or greater than the concentration of chlorine dioxide in said solution on a weight basis and the ratio of the concentration of chlorine dioxide to the sum of the concentrations of chlorine dioxide and chlorite anion in said solution is at least 0.50:1 by weight.
Owner:BASF AG
Massive bodies containing free halogen source for producing highly converted solutions of chlorine dioxide
InactiveUS7182883B2Increase conversion rateImprove permeabilityBiocideOrganic chemistryHalogenLiquid water
A massive body, e.g., a tablet, for producing a solution of chlorine dioxide when the massive body is added to liquid water. The massive body comprises a metal chlorite such as sodium chlorite, an acid source such as sodium bisulfate and a source of free halogen such as the sodium salt of dichloroisocyanuric acid or a hydrate thereof. The concentration of free halogen in the solution will be:(a) less than the concentration of chlorine dioxide in said solution on a weight basis and the ratio of the concentration of chlorine dioxide to the sum of the concentrations of chlorine dioxide and chlorite anion in said solution is at least 0.25:1 by weight; or(b) equal to or greater than the concentration of chlorine dioxide in said solution on a weight basis and the ratio of the concentration of chlorine dioxide to the sum of the concentrations of chlorine dioxide and chlorite anion in said solution is at least 0.50:1 by weight.
Owner:ENGELHARD CORP
Composition for the production of chlorine dioxide using non-iodo interhalides or polyhalides and methods of making and using the same
ActiveUS7087190B2Reduce microbial countQuick buildBiocideLiquid degasificationChlorine dioxideCHLORITE ION
A composition for the generation of chlorine dioxide including at least one non-iodo interhalide, polyhalide or salt thereof having the formulaBrmClnFoXpwherein m=0–3, n=0–4, o=0–3, p=0–2, X is a cationic moiety and with the provisos that m+n+o cannot be zero; if m+n+p<2, or mixtures thereof, and at least one source of chlorite ions.
Owner:ECOLAB USA INC
Method and system for the controlled release of chlorine dioxide gas
Method, composition and system for generating chlorine dioxide gas in a controlled release manner by combining at least one metal chlorite and a dry solid hydrophilic material that reacts with the metal chlorite in the presence of water vapor, but does not react with the metal chlorite in the substantial absence of liquid water or water vapor to produce chlorine dioxide gas in a sustained amount of from about 0.001 to 1,000 ppm.
Owner:BASF CATALYSTS LLC
Chlorine dioxide based cleaner/sanitizer
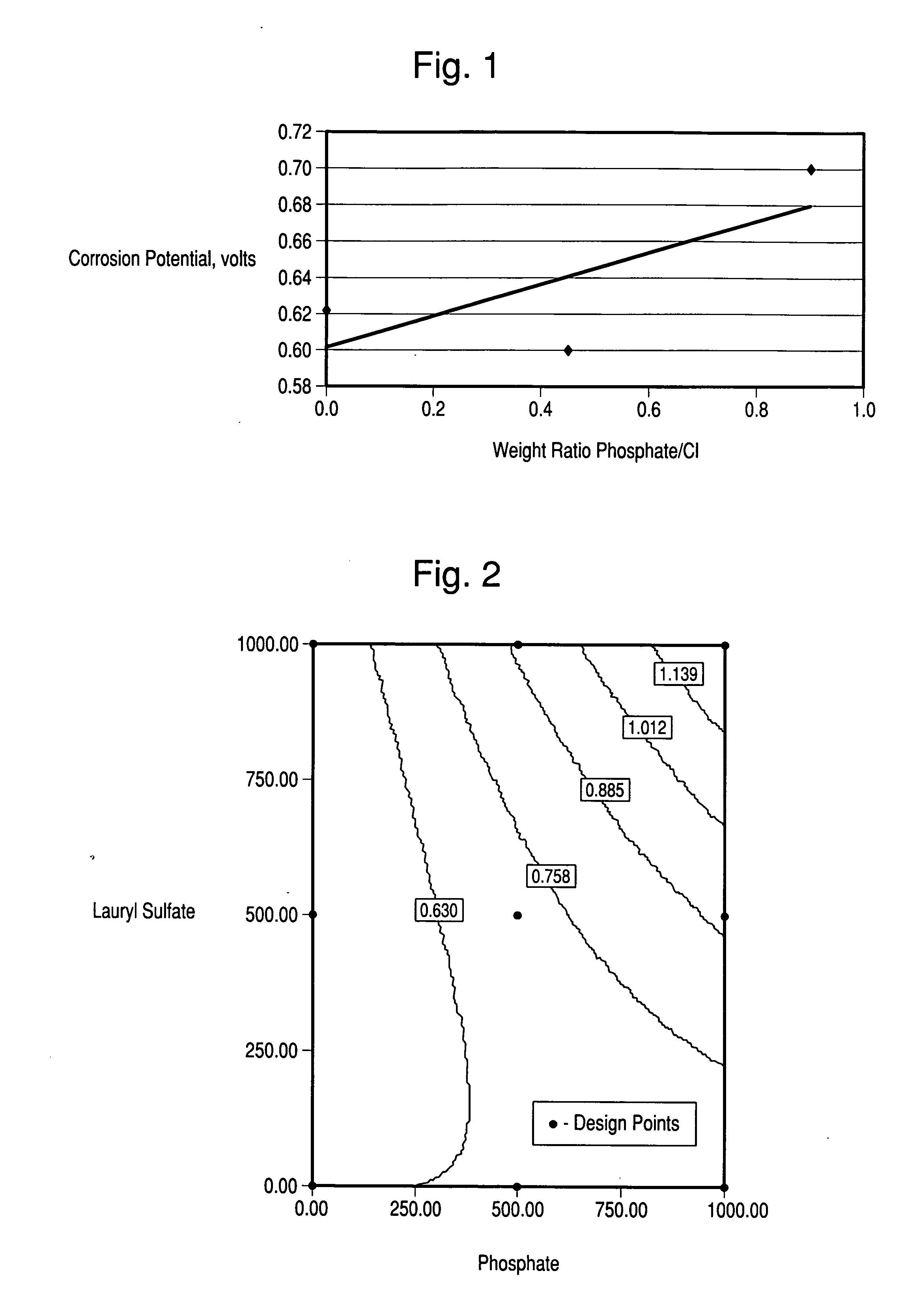
ActiveUS20070202095A1Inhibiting metal corrosionCorrosive nature is lessenedInorganic/elemental detergent compounding agentsBiocideChlorine dioxidePhosphate
This invention relates to an improved chlorine dioxide solution or liquid mixture containing a phosphate and, as well, as to a composition for forming the chlorine dioxide and phosphate liquid mixture. This improved chlorine dioxide solution is used to clean and / or sanitize without causing corrosion. The corrosion nature of the chlorine dioxide solution is lessened due to the addition of phosphate to the composition.
Owner:BASF CORP
Sterilant system
ActiveUS20100036305A1Stable compoundEnhanced surface contactBiocideDead animal preservationAqueous mediumReagent
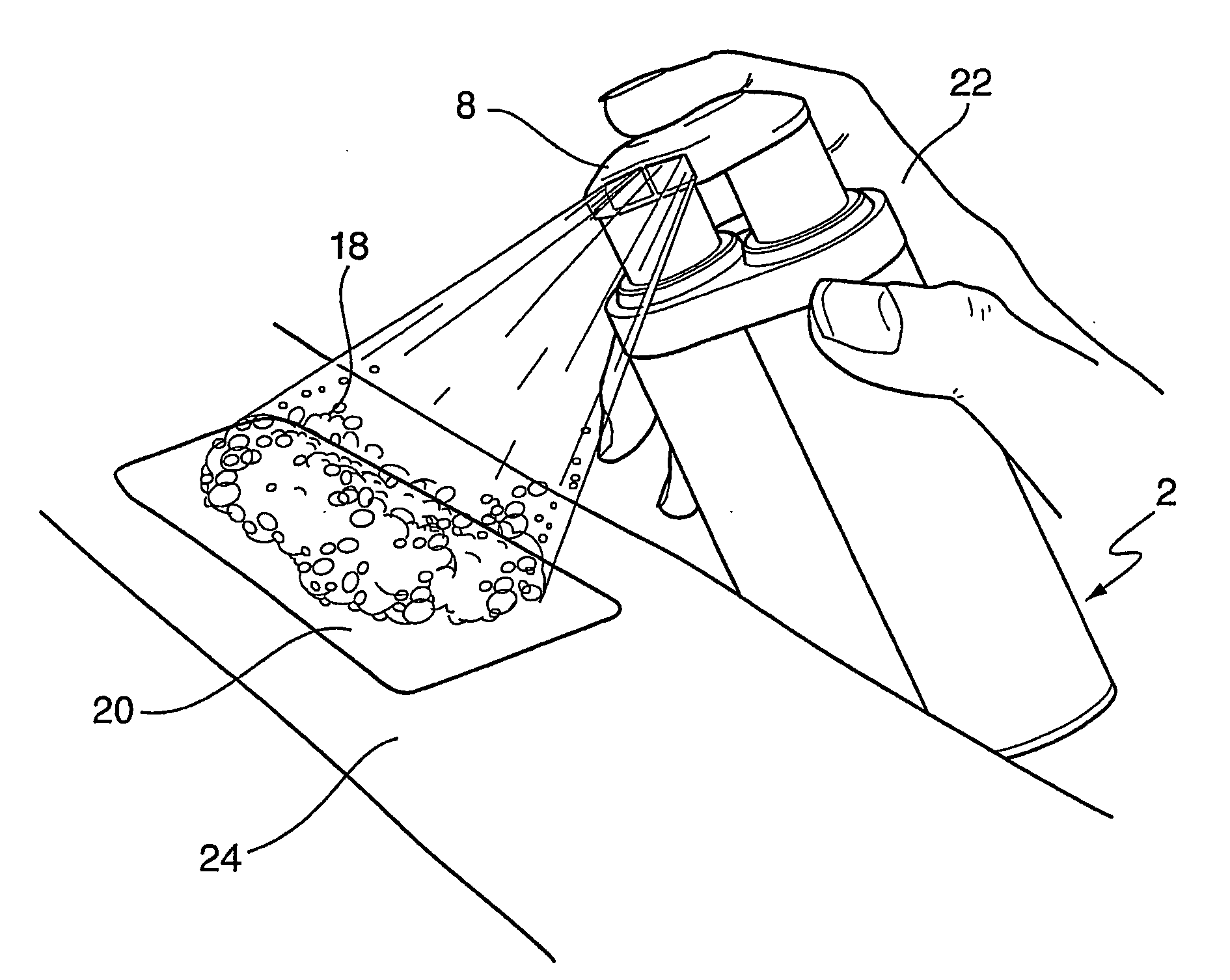
A two-part sterilant system comprises: (a) a first part comprising a first reagent in an aqueous medium having a first foam promoter dissolved therein and contained in a first dispenser whereby it may be dispensed as a first foam; and (b) a second part which comprises a second reagent in an aqueous medium having a second foam promoter dissolved therein and contained in a second dispenser whereby it may be dispensed as a second foam; wherein the first reagent and the second reagent will react to provide a sterilising composition when the first foam is mixed with the second foam.
Owner:TRISTEL PCL
Systems and methods for producing aqueous solutions and gases having disinfecting properties and substantially eliminating impurities
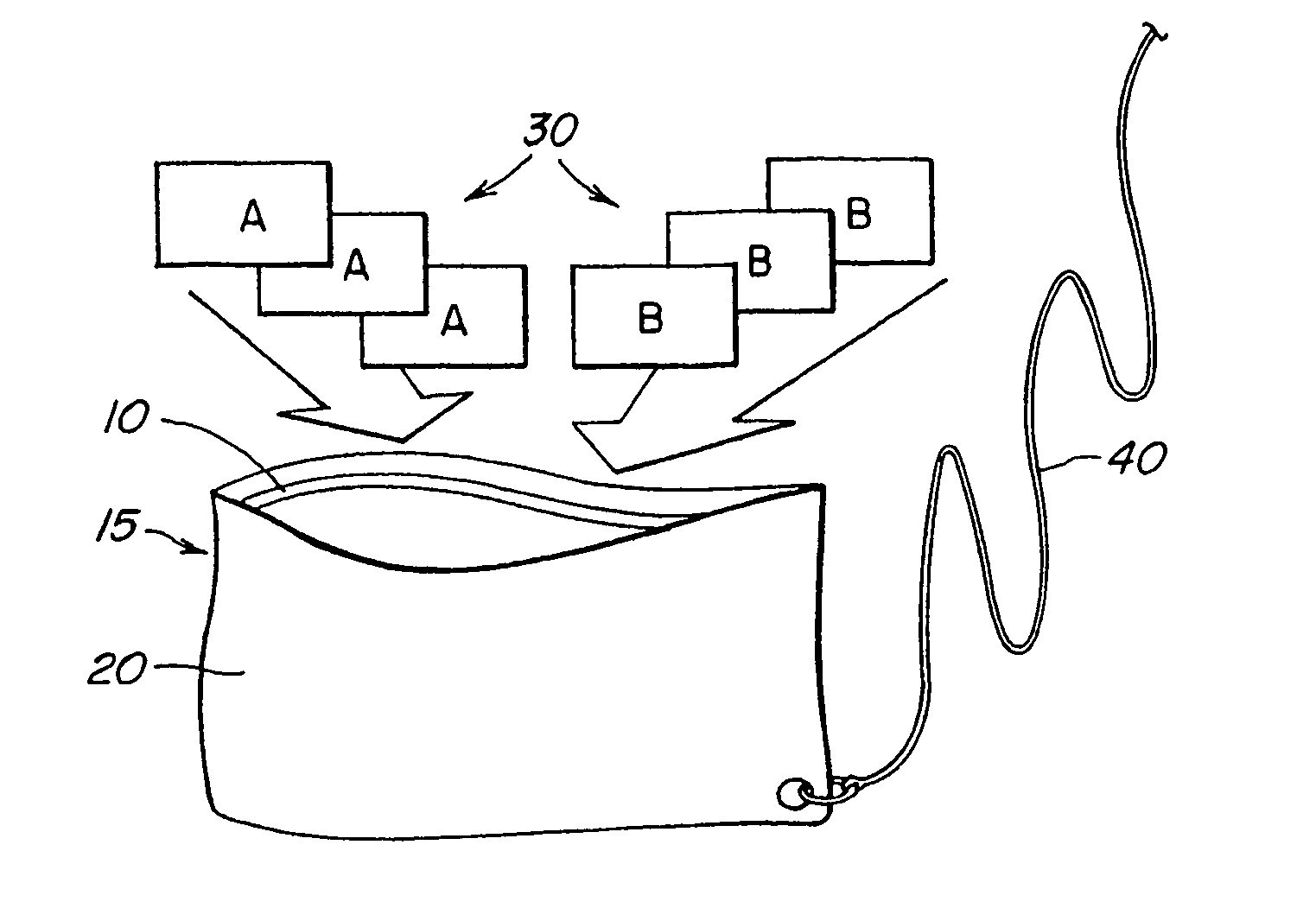
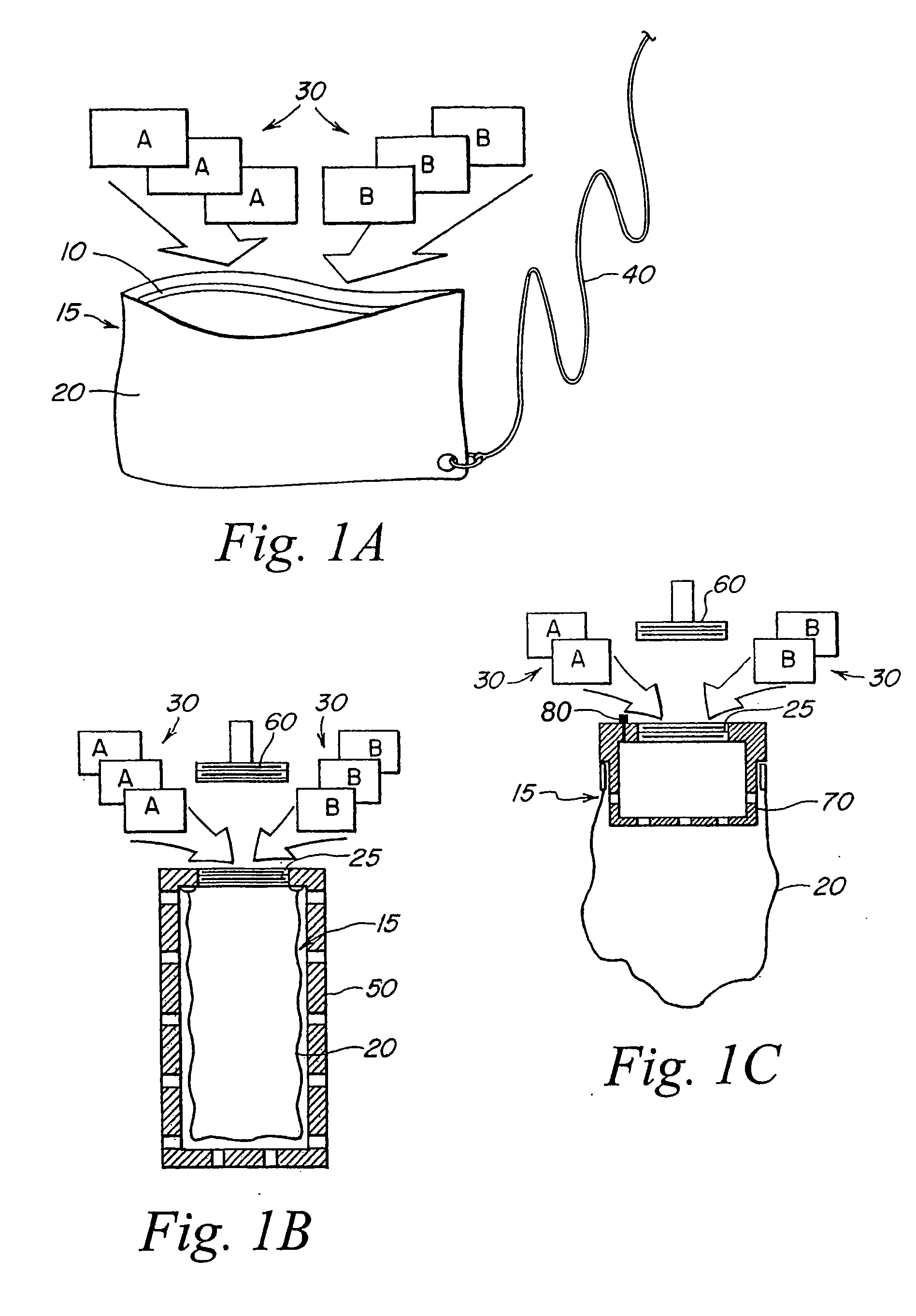
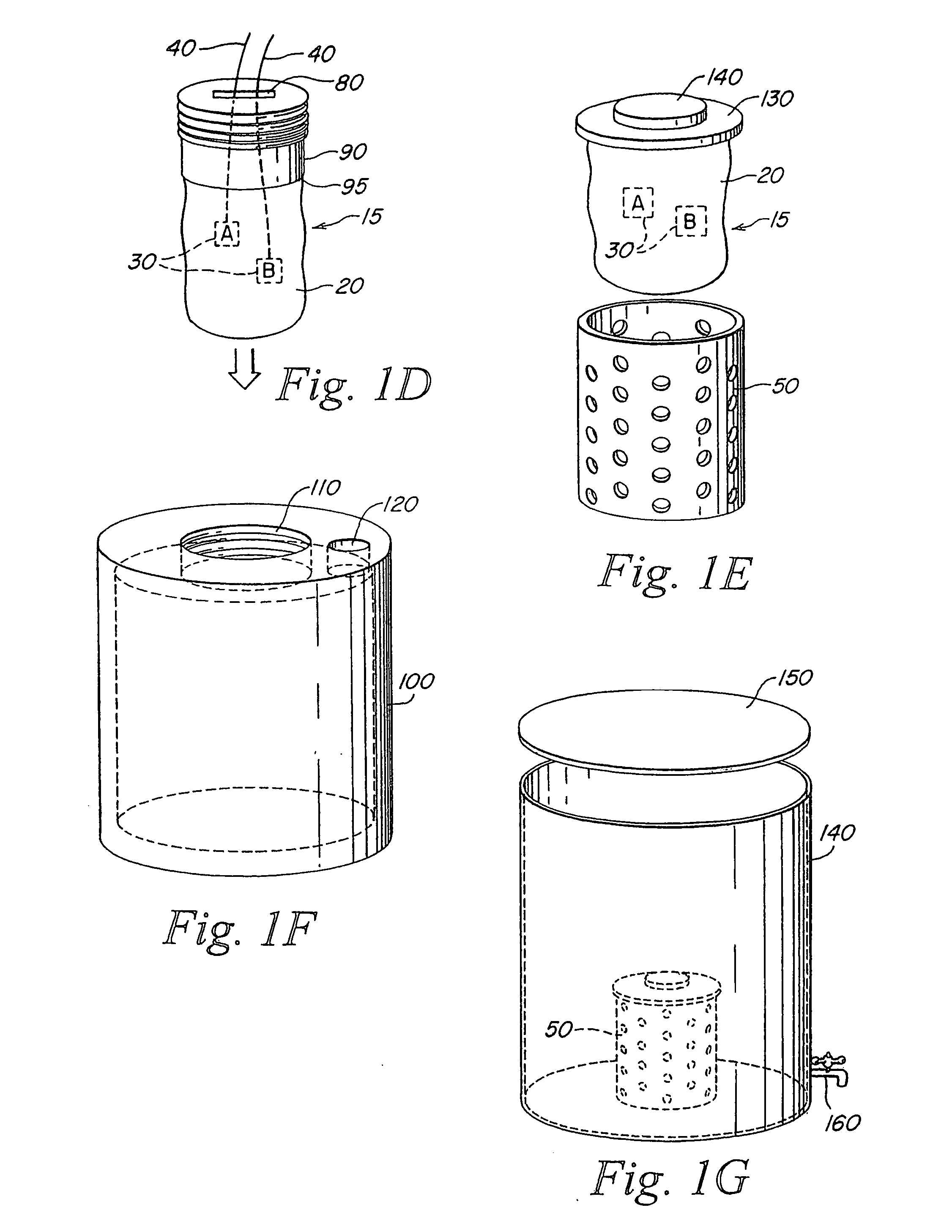
InactiveUS20060039841A1Easy accessIncrease moisture transfer rateBiocideDeodrantsDisinfectantAqueous solution
A system for disinfecting and purifying aqueous solutions and water for drinking purposes comprising an outer pouch, a disinfectant generating device, and an inorganic coagulant. The disinfectant generating device is provided with a membrane shell defining at least two compartments which house a first reactant, a second reactant, and an inorganic coagulant. The disinfectant generating device is capable of producing a disinfectant when exposed to water or moisture and the disinfectant exits the compartment through the membrane shell. The inorganic coagulant aid the formation of flocs of the suspended dispersed particles in the aqueous solution.
Owner:AVANTEC TECH
Compositions and methods for storing aqueous chlorine dioxide solutions
InactiveUS6284152B1Little and no diffusional lossLoss and degradationCosmetic preparationsBiocideDiffusionChlorine dioxide
The present invention is directed to both physical and chemical means for maintaining continuous high levels of chlorine dioxide in aqueous solutions for extended periods, with minimum loss through degradation in the solution and dissipation through the walls of the containing vessel within which the solution is stored. The ClO2 may be maintained even in the absence of very high levels of chlorite, from which supplemental, offsetting levels of ClO2 may be generated. The present invention relates to the discovery that certain types of plastic and glass containers have the ability to maintain relatively constant levels of ClO2 in their contained solutions over extended periods, particularly in aqueous compositions comprising chlorite / ClO2 ratios sufficient for a stabilizing complex of the two, presumably Cl204-, to suppress the ClO2 loss. Of particular value is certain high-density polyethylene terephthalate (PETE) plastic bottling and plastic containers which have been initially exposed to a fluorinating environment. Specifically, in the latter regard, it has been discovered that certain plastic containers which ordinarily are incapable of maintaining aqueous ClO2 levels can be treated with fluorine gas to transform their surfaces so as to effectively suppress ClO2 diffusion therethrough.
Owner:PROFRESH PROPERTIES
Chemical method
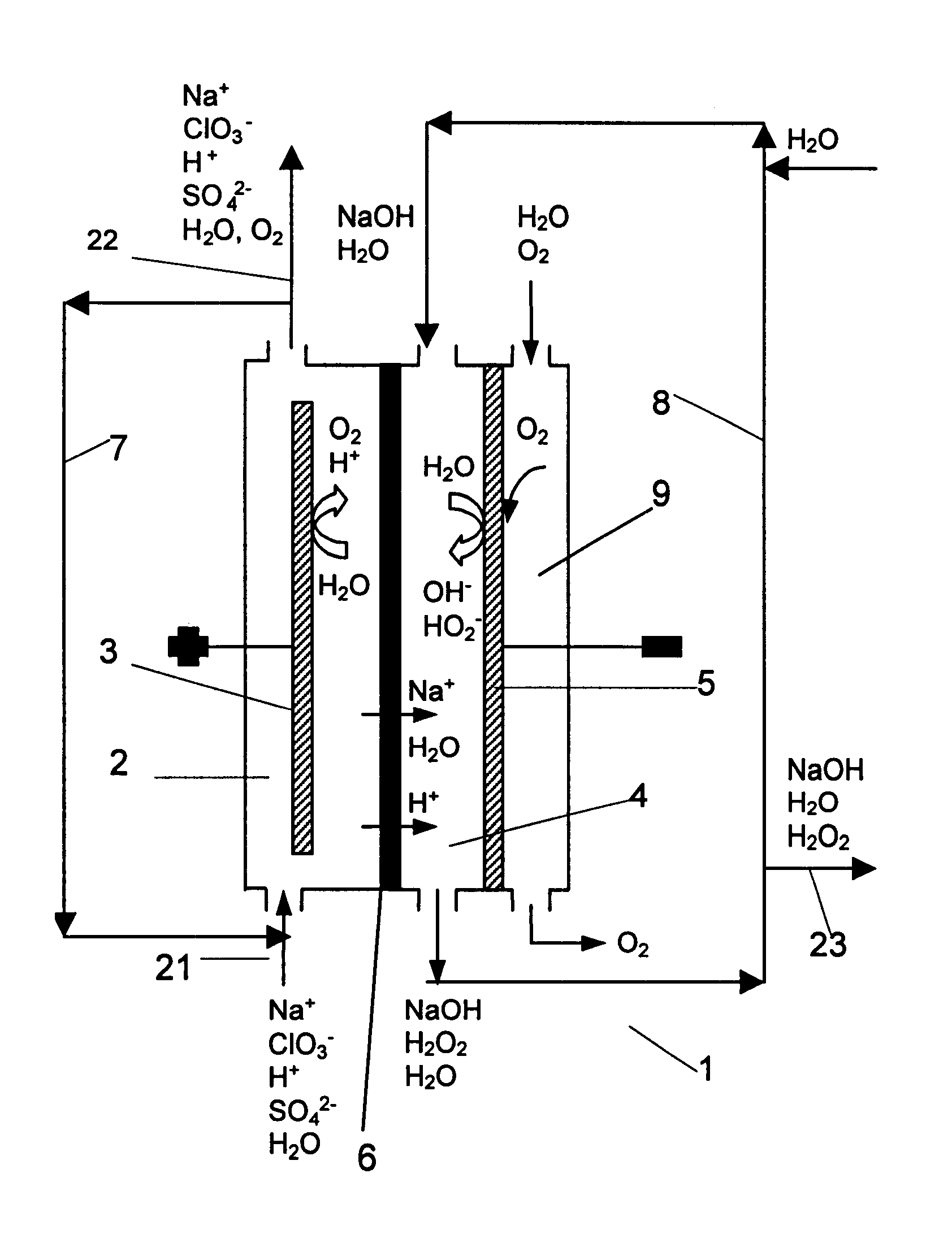
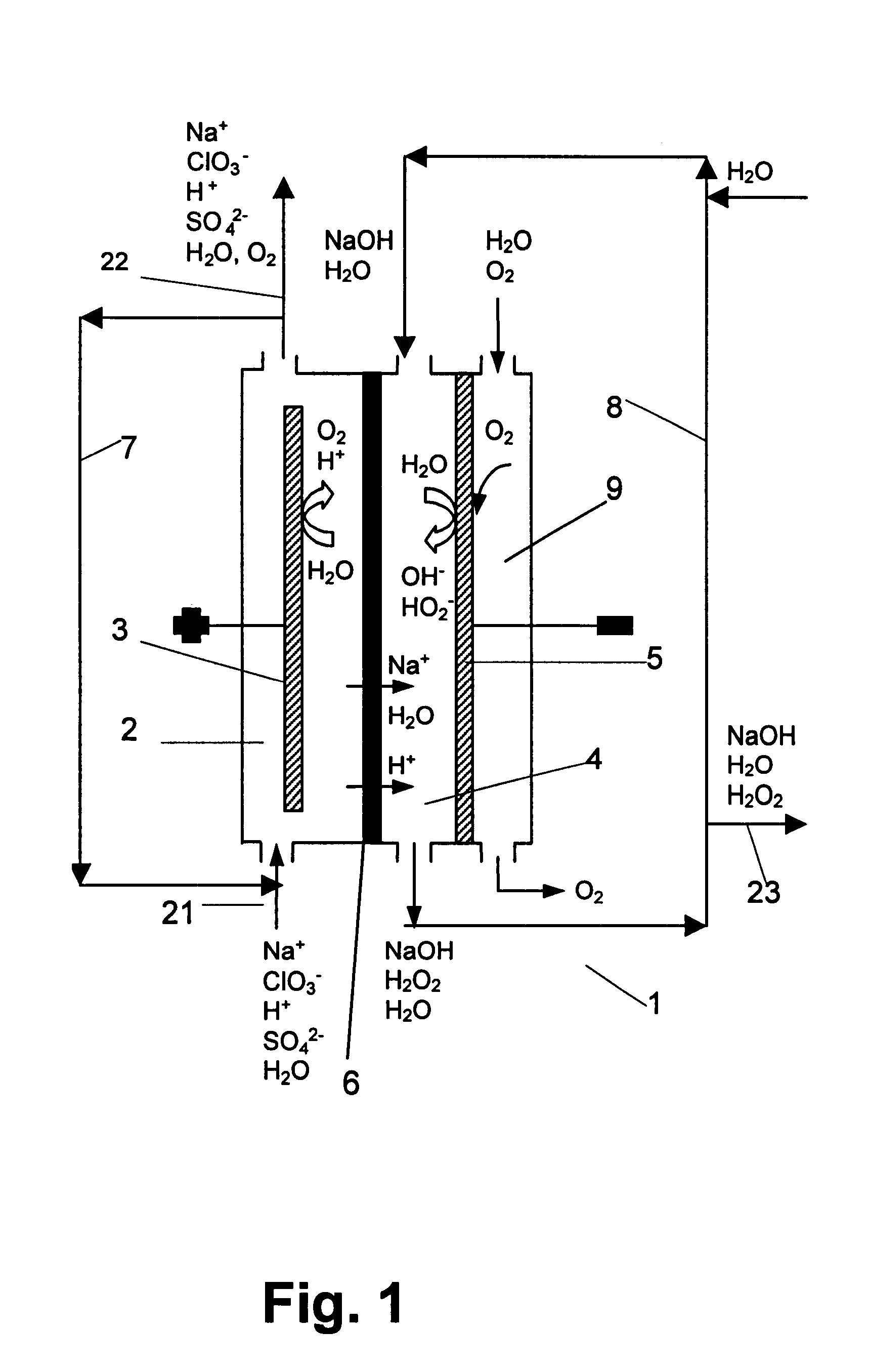
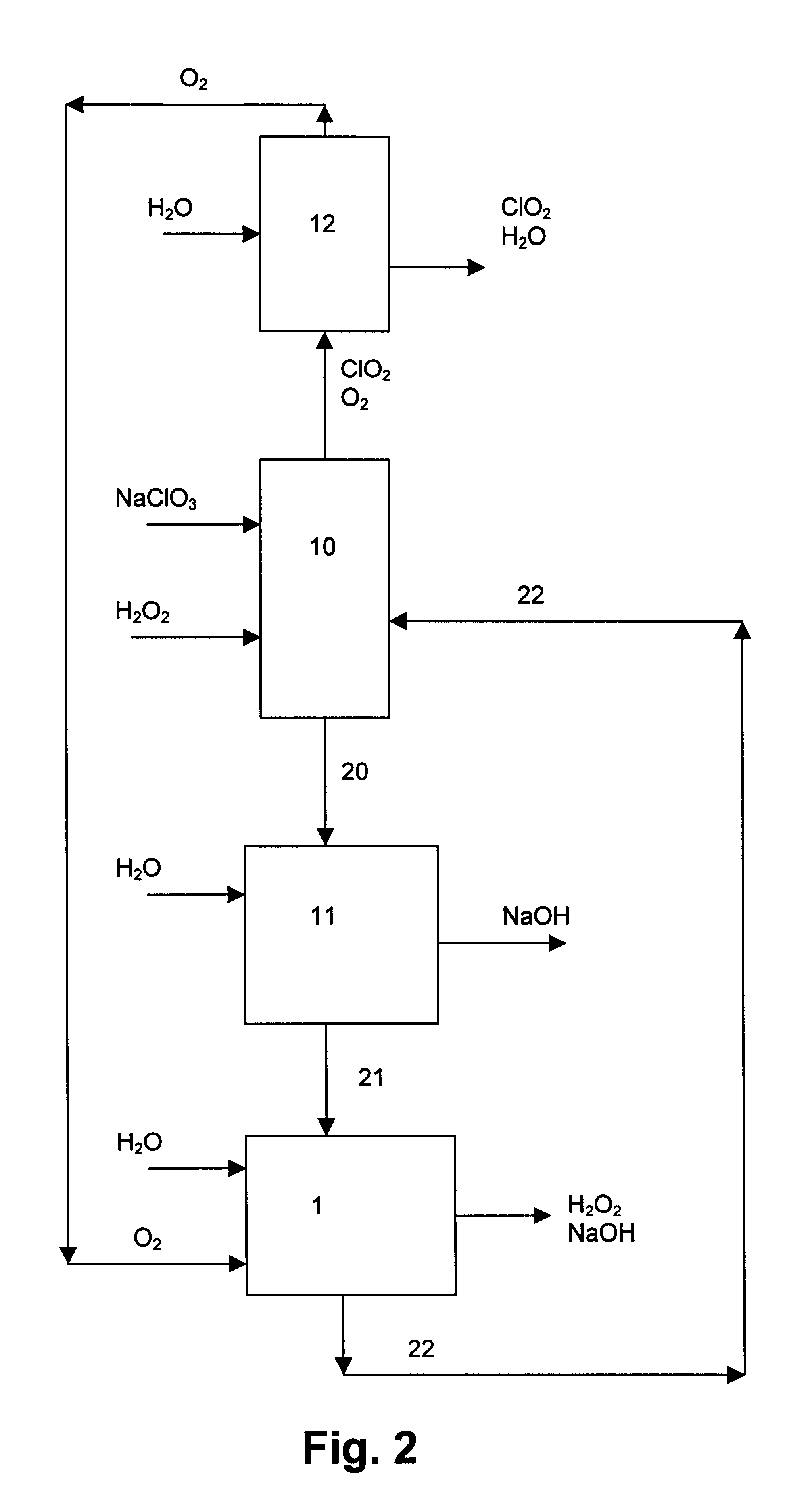
InactiveUS6322690B1Avoiding net productionEliminate needElectrolysis componentsChlorine dioxideChlorate ionChlorine dioxide
The invention relates to a process for production of an alkaline hydrogen peroxide solution and an acidified alkali metal salt solution containing chlorate in an electrochemical cell including an anode compartment provided with an anode and a cathode compartment provided with an oxygen reducing cathode. In the cathode compartment an aqueous alkali metal hydroxide and hydrogen peroxide is formed, wherein the molar ratio MOH:H2O2 for the net production thereof is maintained from about 0.1:1 to about 2:1. Also a process for simultaneous production of chlorine dioxide is disclosed.
Owner:AKZO NOBEL NV
Chlorine dioxide releasing composite article
A composite article that includes a ClO2-producing material integrated into an organic matrix and methods of using the same are described. The organic matrix of the composite article is formable at a temperature under about 150° C., permits contact between an activating stimulus (e.g., water vapor and / or electromagnetic energy) and the ClO2-producing material when the composite article is exposed to the activating stimulus, and is permeable to ClO2.
Owner:BASF CORP
Device and methods for the production of chlorine dioxide vapor
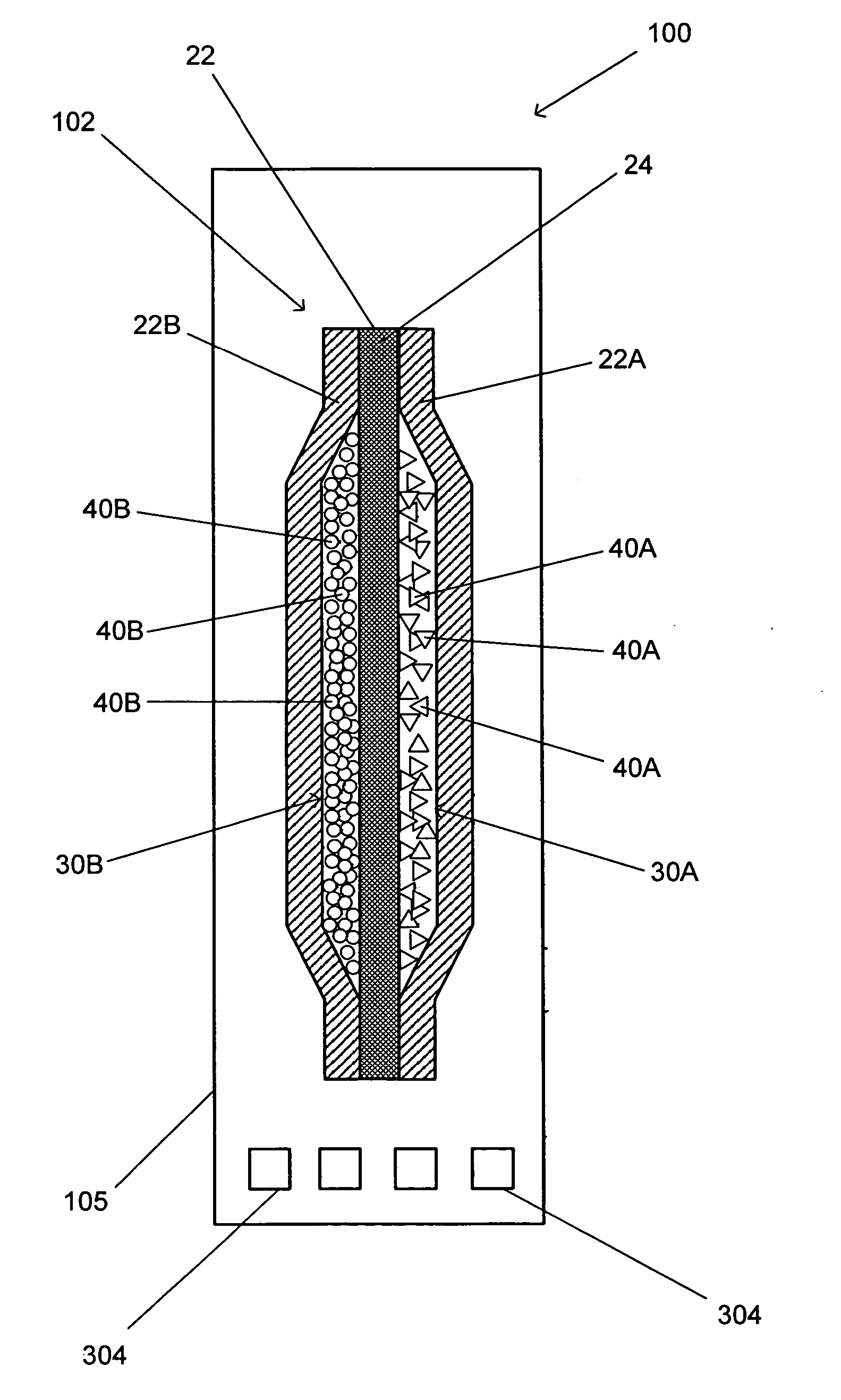
InactiveUS20060039840A1High moisture levelRapid productionDeodrantsGas generation devicesChlorine dioxideLiquid water
A device for producing chlorine dioxide vapor in a time release manner when exposed to ambient moisture is provided. The device comprises two outer membranes and an inner membrane. The outer and inner membranes are sealed together along the edges of the device. The outer and inner membranes together form a pouch comprising two separate compartments separated by the inner membrane. Each compartment is provided with a dry reactant (e.g. an acid component and a metal chlorite component) for producing chlorine dioxide vapor. The outer membranes are permeable to moisture and chlorine dioxide vapor, and impervious to liquid water and the dry reactants. The acid component in one of the compartments is hydrated by the moisture penetrated through the outer membranes, and the hydrated acid component is absorbed by the inner membrane and transported across the inner membrane to come into contact with the metal chlorite component in the other compartment to produce chlorine dioxide vapor. The chlorine dioxide vapor eventually slowly diffuses out of outer membranes into the surrounding environment.
Owner:AVANTEC TECH
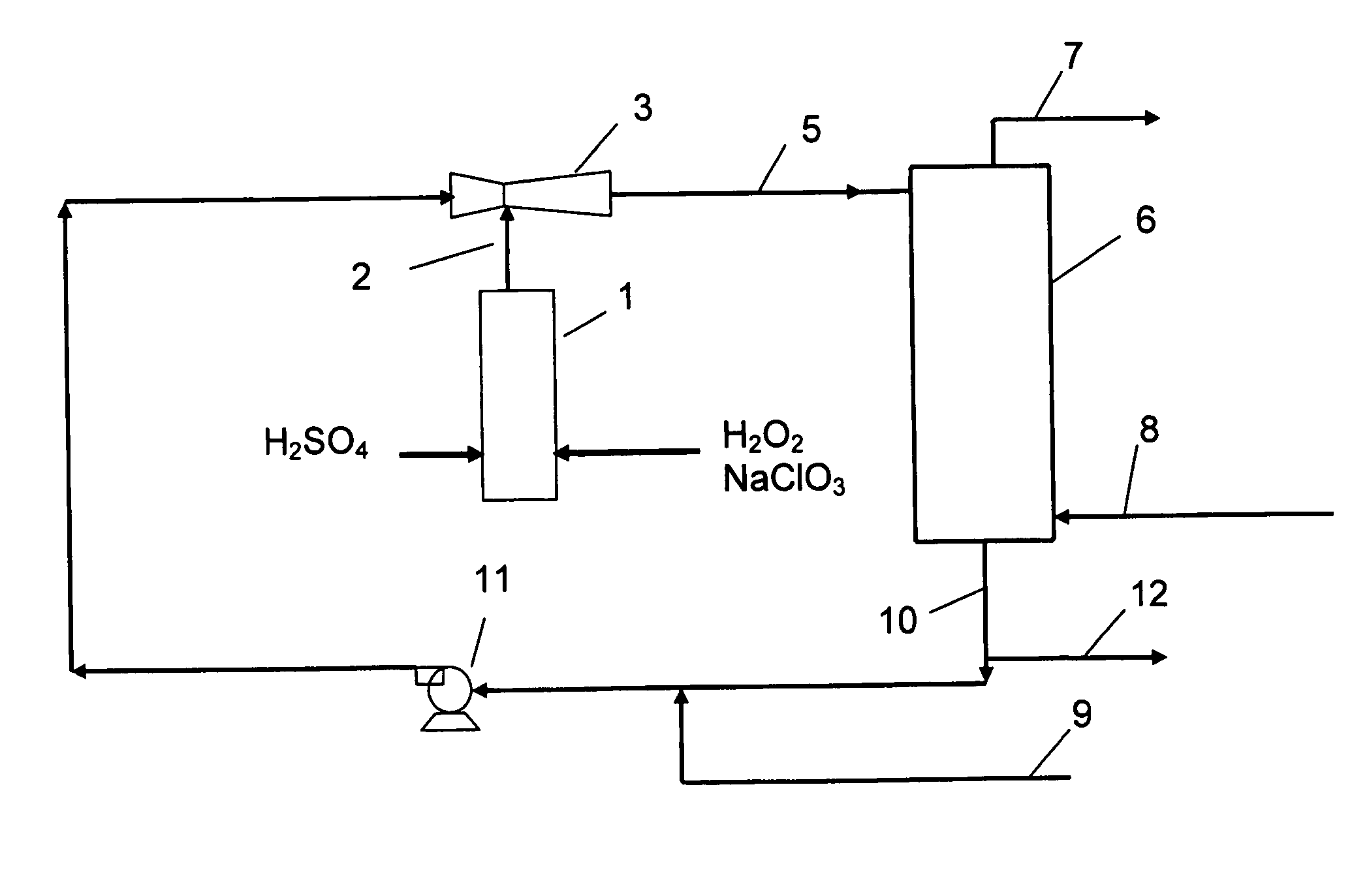
Process for production of chlorine dioxide
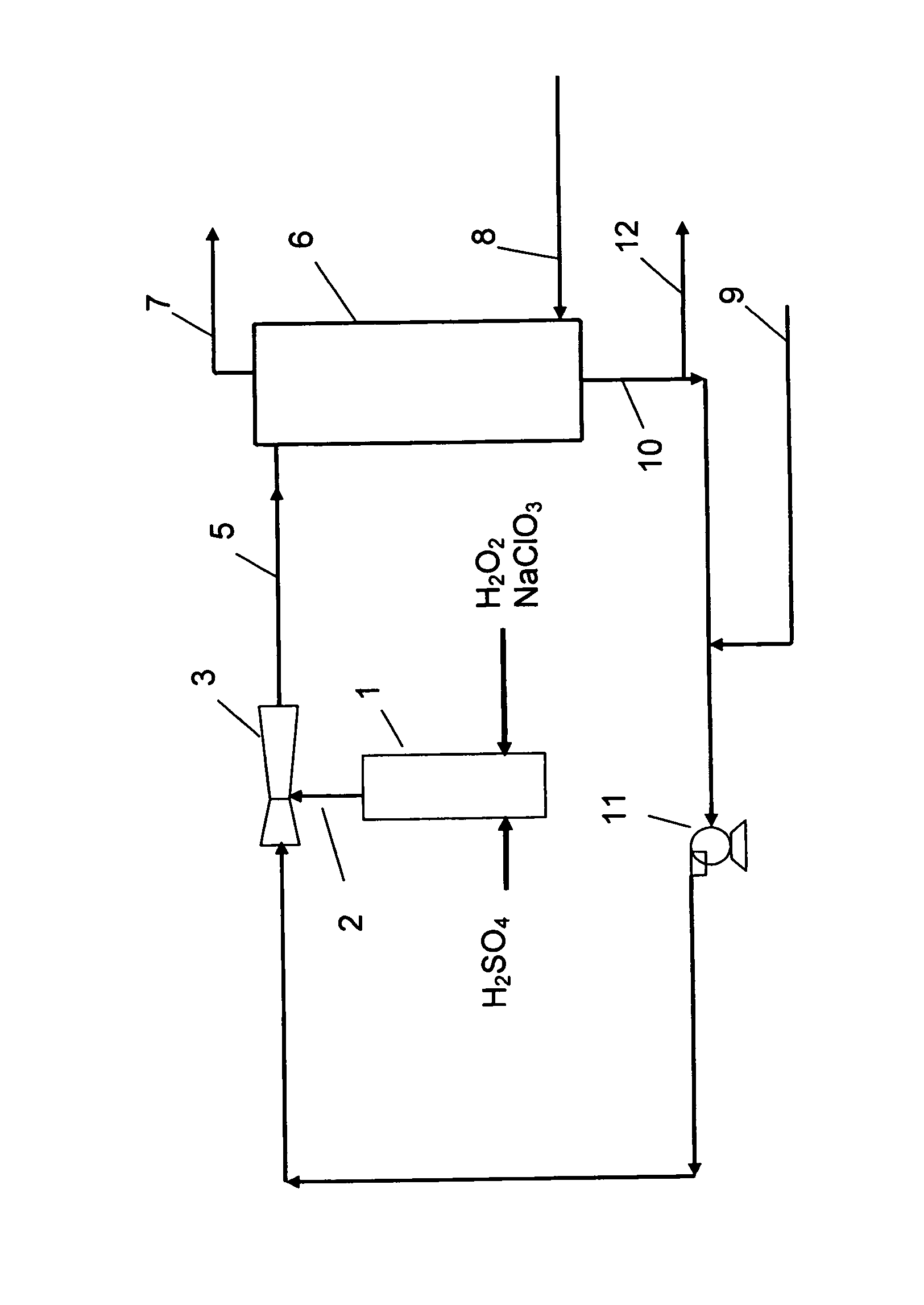
InactiveUS20070116637A1Easy to useEasy to manufactureGaseous chemical processesLiquid-gas reaction of thin-film typeChlorate ionVapor–liquid separator
The present invention relates to a process for the production of chlorine dioxide, said process comprising the steps of continuously: (a) feeding to a reactor an acid, alkali metal chlorate and a reducing agent; (b) reacting the alkali metal chlorate with the acid and the reducing agent to form a product stream comprising chlorine dioxide and alkali metal salt of the acid; (c) bringing the product stream from the reactor to an eductor and mixing it with motive fluid fed to the eductor and thereby forming a diluted product stream; (d) bringing the diluted product stream to a gas-liquid separator where gas is separated from liquid therein; (e) withdrawing a gaseous product stream comprising chlorine dioxide and inert gas from said gas-liquid separator; and, (f) withdrawing a liquid phase from the gas-liquid separator. The invention also relates to a production unit to produce chlorine dioxide.
Owner:ECOLAB USA INC +1
Methods and Solid Compositions for Generating Soapy and Non-Soapy Aqueous Solutions Containing Free Chlorine Dioxide
InactiveUS20080067470A1Improve stabilityThe result is stableOther chemical processesChlorine dioxideChlorine dioxideSolid acid
Some embodiments of the invention provide solid compositions that, when exposed to or otherwise placed in an aqueous solution, will release chlorine dioxide and surfactants, producing a soapy, aqueous solution containing chlorine dioxide. These solid compositions comprise an alkali chlorite salt, a solid acid source, and a surfactant. In some other embodiments, the solid compositions produce a non-soapy aqueous solution containing chlorine dioxide. These solid compositions comprise an alkali chlorite salt and a solid acid source.
Owner:SIPKA
Method for the synthesis of chlorine dioxide
Owner:DEGUSSA CORP
Paper product impregnated with chemical material
The present invention provides a porous paper product impregnated with at least one chemical species. The porous paper product can be in the form of sheets, or compressed pellets. The porous paper can be prepared from a variety of sources, including wood pulp, kenaf, flax, or hemp. The chemical species impregnating the paper react and / or diffuse out of the paper to accomplish a variety of desired results. For example, diffusion of a volatile biocidal chemical out of pores in the paper create a no-growth zone on and immediately surrounding the impregnated paper. In this manner the impregnated paper can provide a sterile environment for activities such as food packaging / storage, the treatment of illness / injury, or waste disposal.
Owner:CATHM LLC D B A PURE PAPER
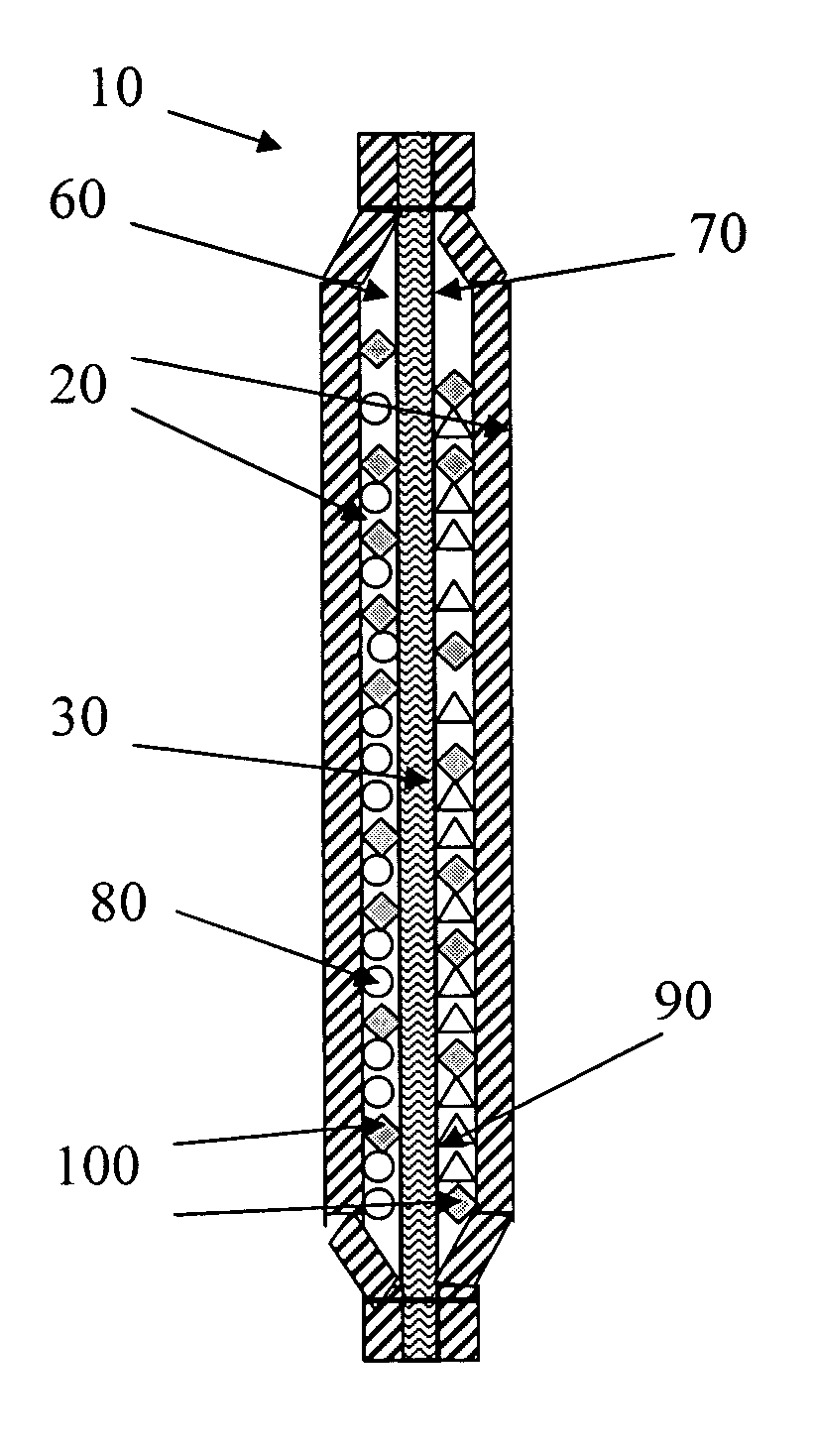
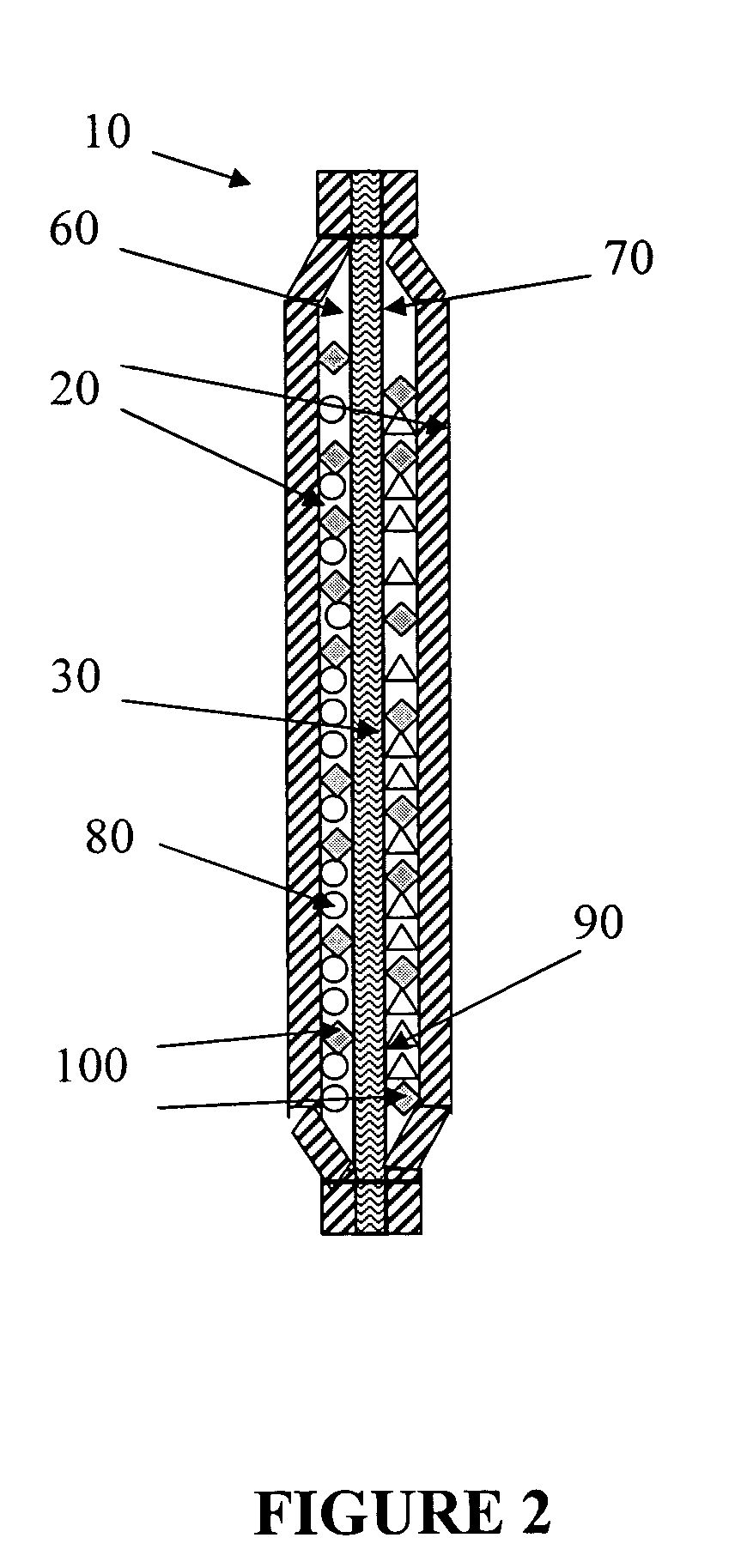
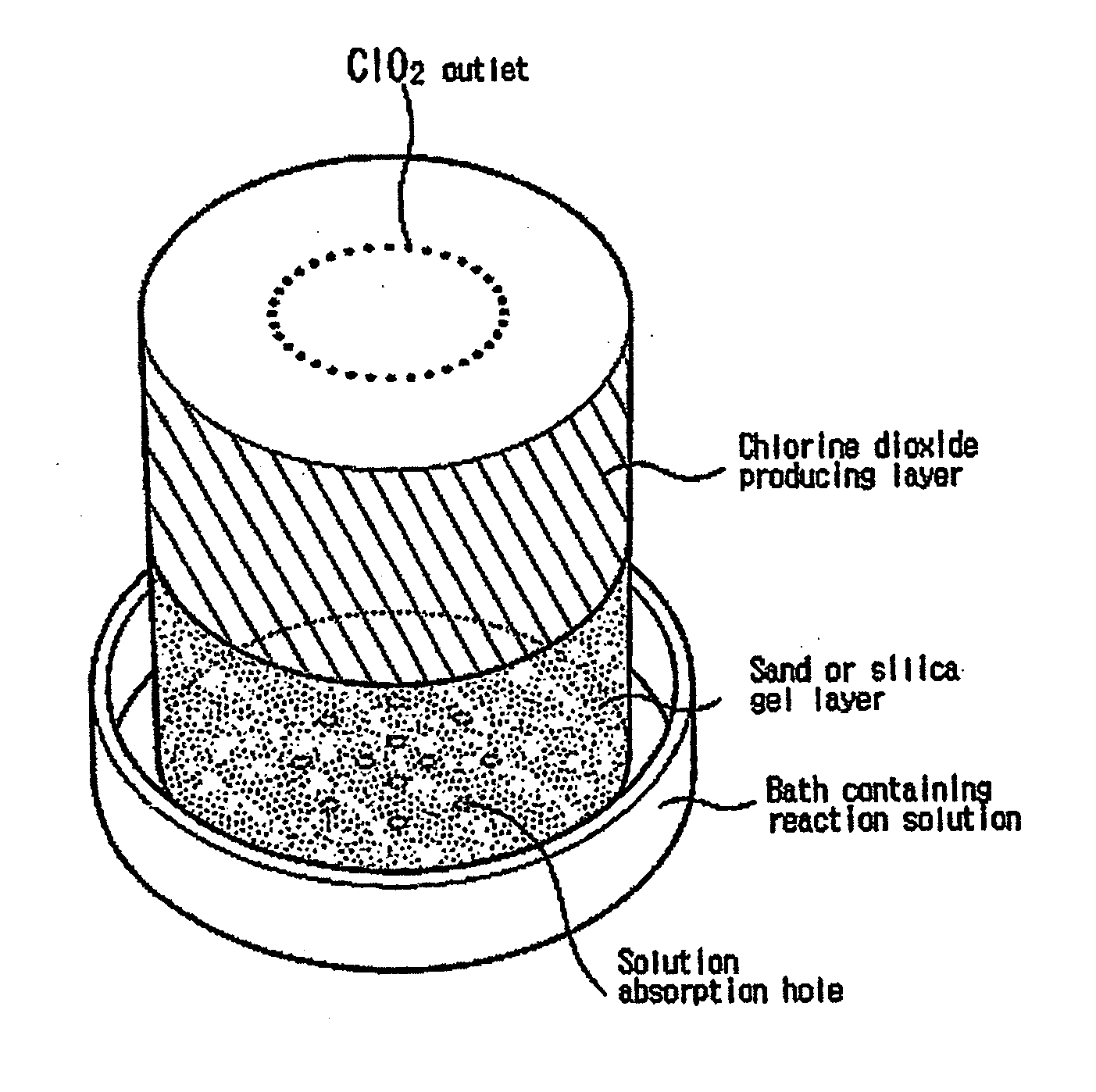
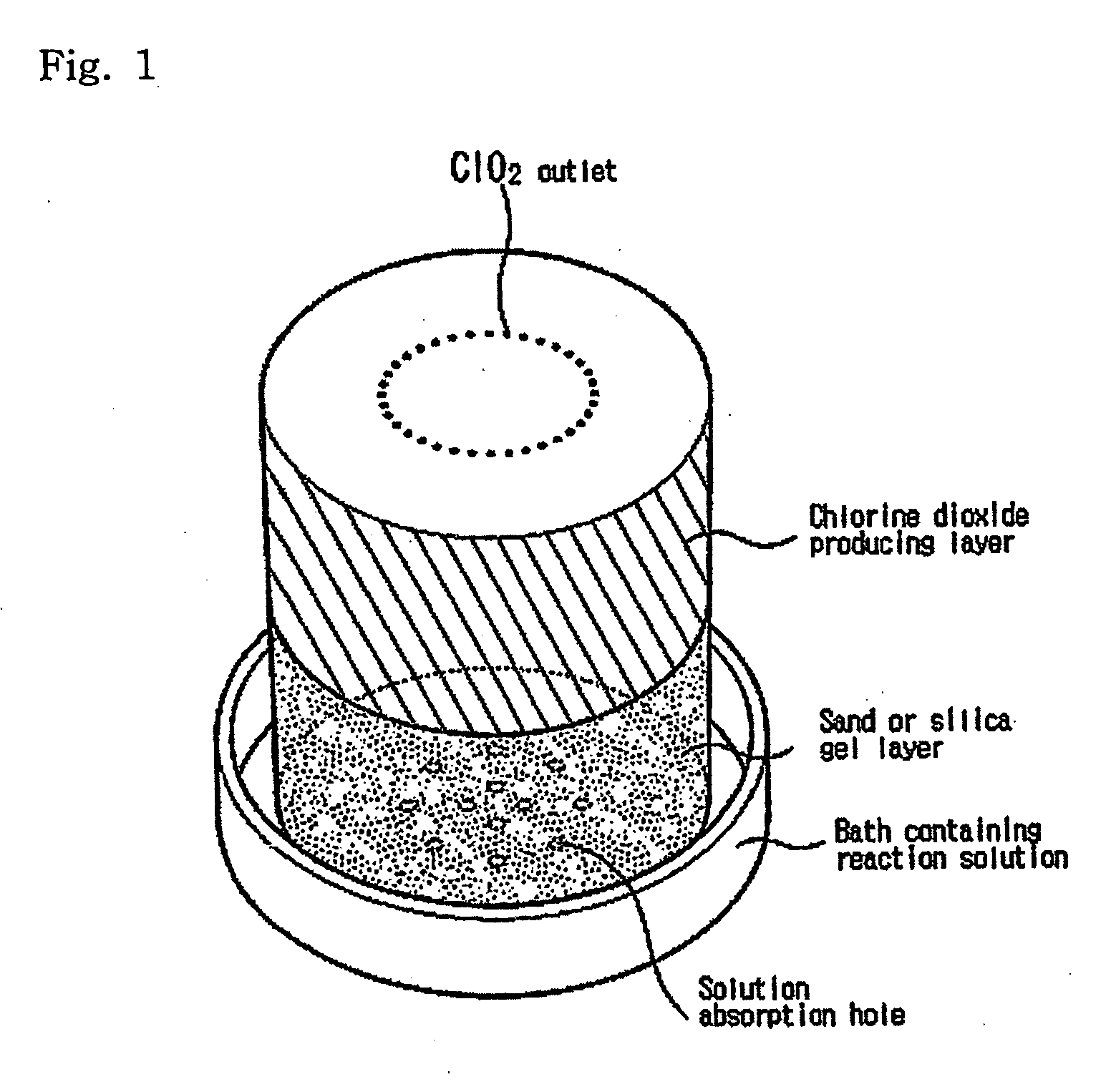
Simple apparatus for producing chlorine dioxide gas
InactiveUS20050224750A1Infeasible production costInstantaneous production of chlorine dioxideOther chemical processesDead animal preservationChlorine dioxidePhysical chemistry
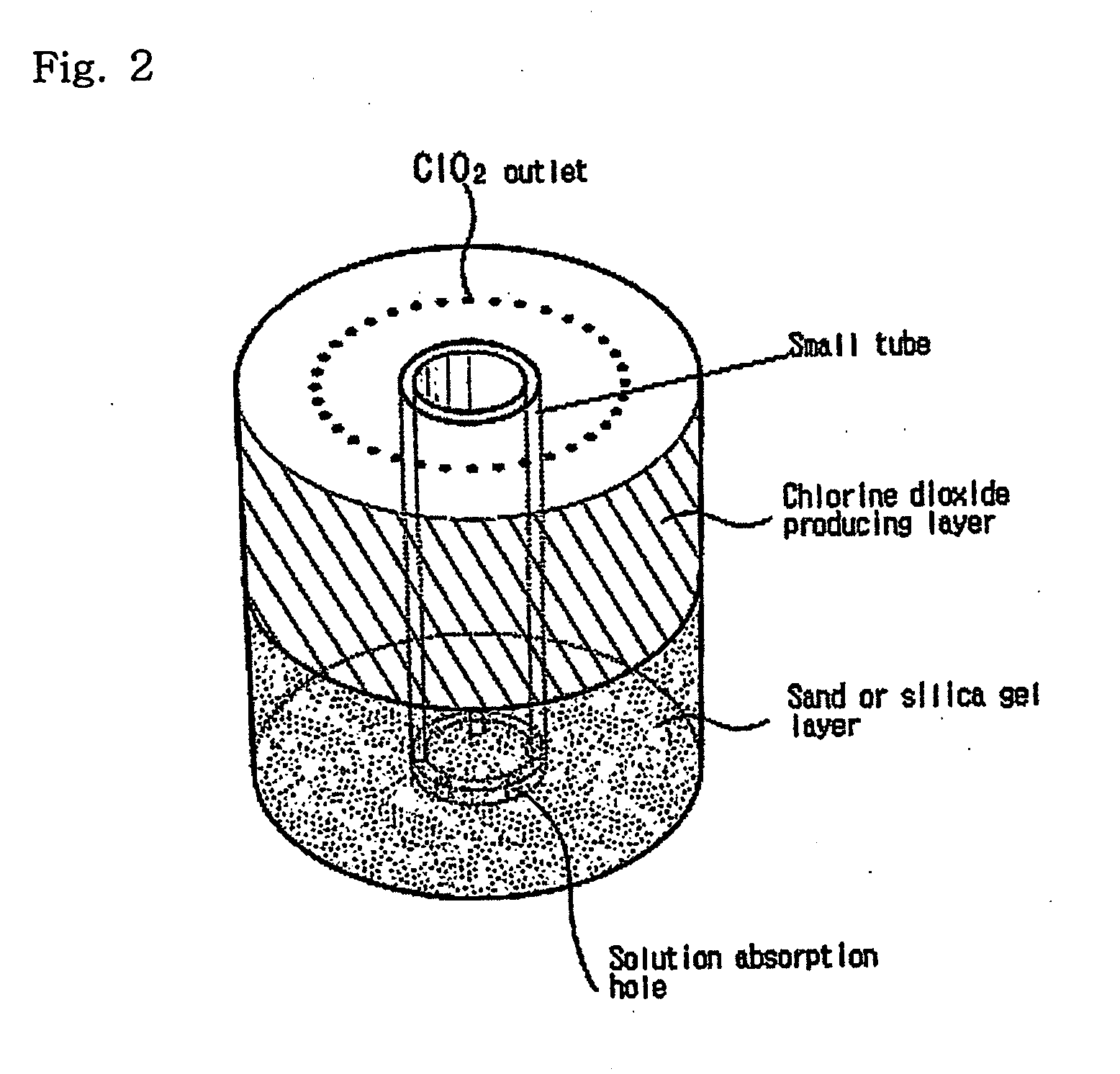
The present invention relates to a simple apparatus for producing chlorine dioxide gas, and more specifically, a simple apparatus for producing chlorine dioxide gas in which a sand or silica gel layer, a chlorine dioxide producing layer and, if necessary, a coarse sand layer and a silica gel or zeolite layer sequentially fill the bottom of a tube, a number of solution-absorption holes are formed at the bottom of the tube, and a number of ClO2 outlets are formed at the top of the tube. According to the apparatus of the invention, it is possible to instantly produce a small amount of chlorine dioxide in simple and safe manner or possible to safely preserve or provide a small amount of chlorine dioxide for a long time.
Owner:AQUA TECH CO LTD +1
Reusable apparatus for gas generation
InactiveUS20060120945A1Low costSave resourcesCarbon compoundsSpecific water treatment objectivesRefill KitEngineering
Owner:SELECTIVE MICRO TECH
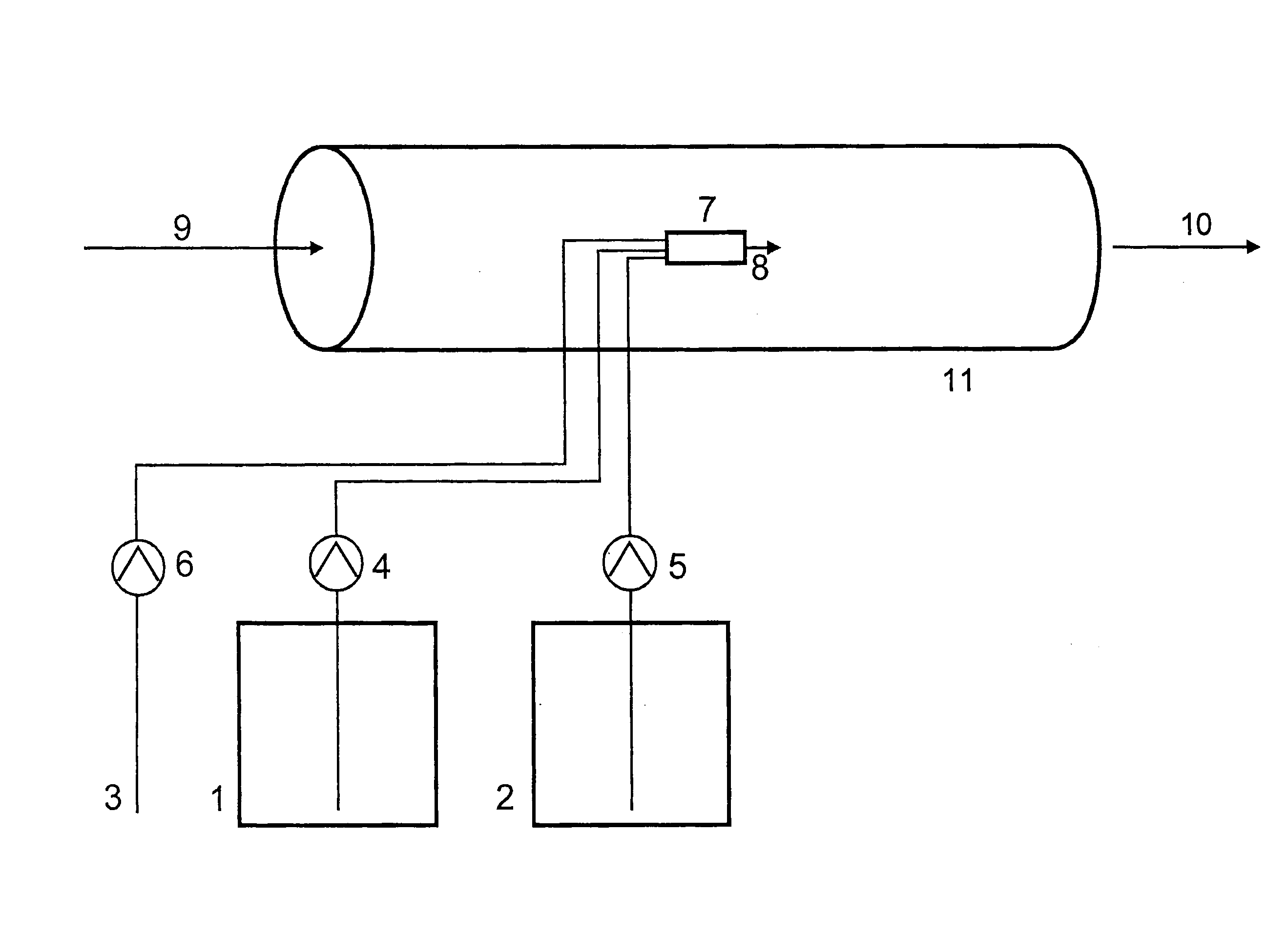
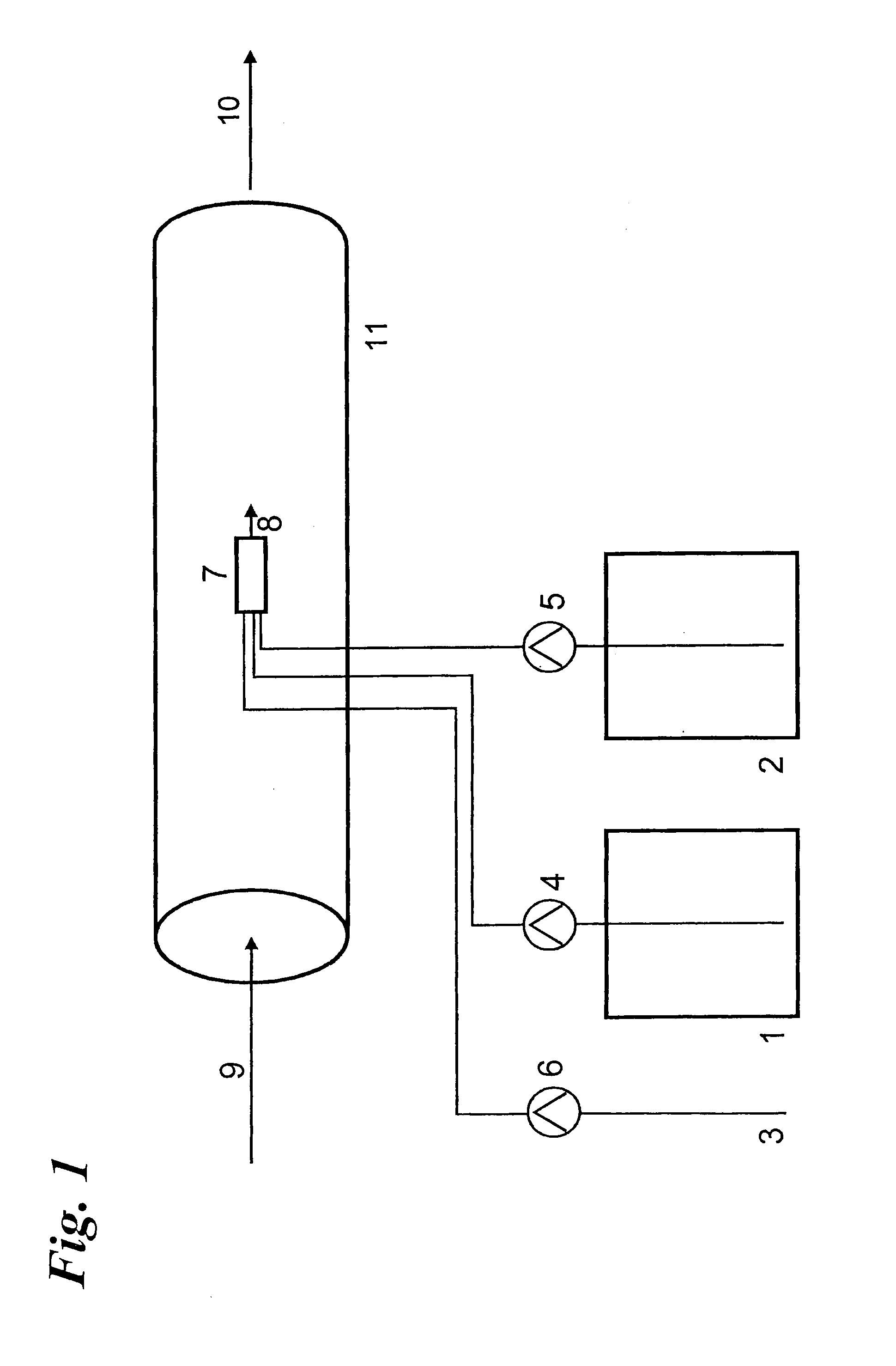
Chlorine dioxide generation
InactiveUS20060051285A1Accurate measurementAppropriate concentrationDeodrantsGas generation devicesChlorine dioxideMedical treatment
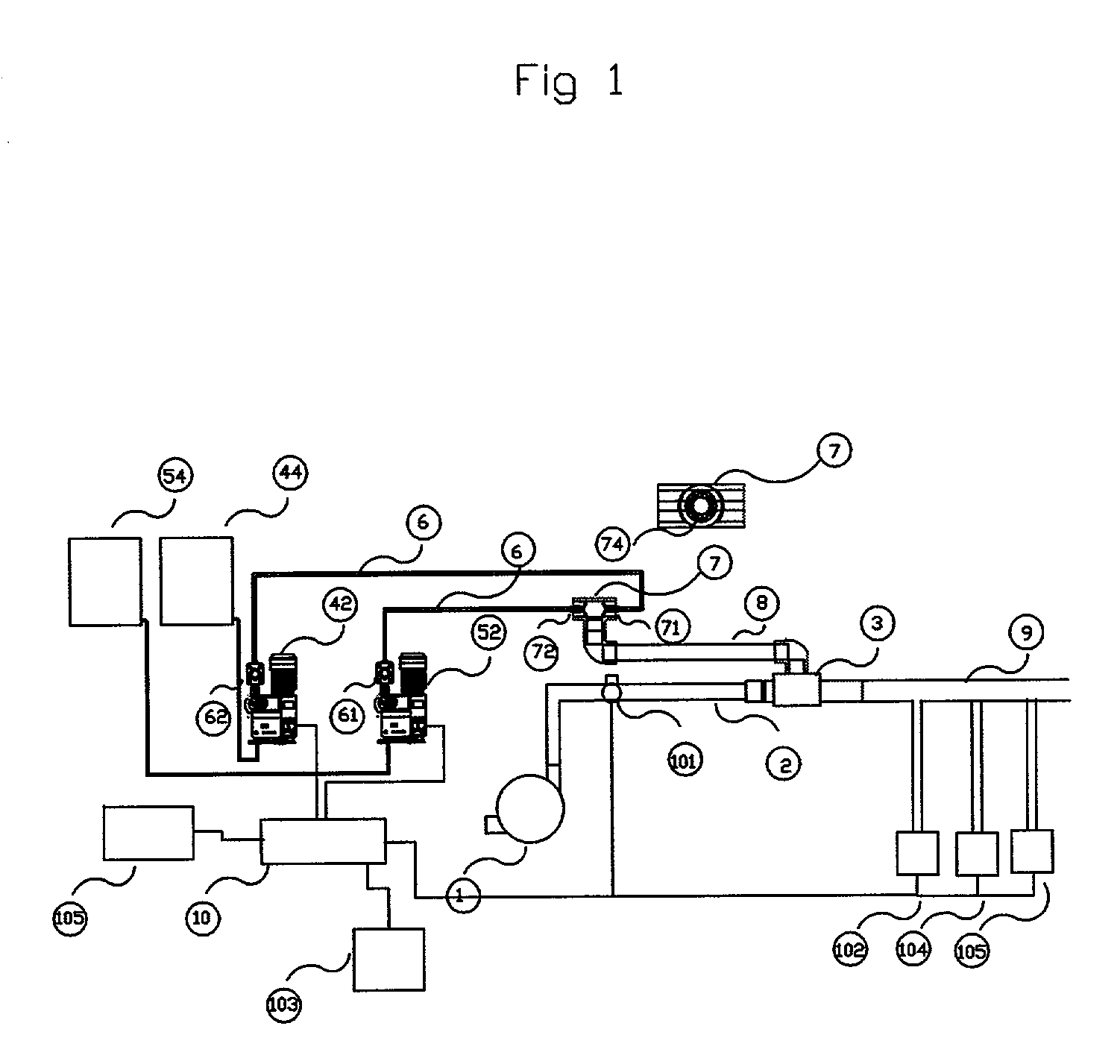
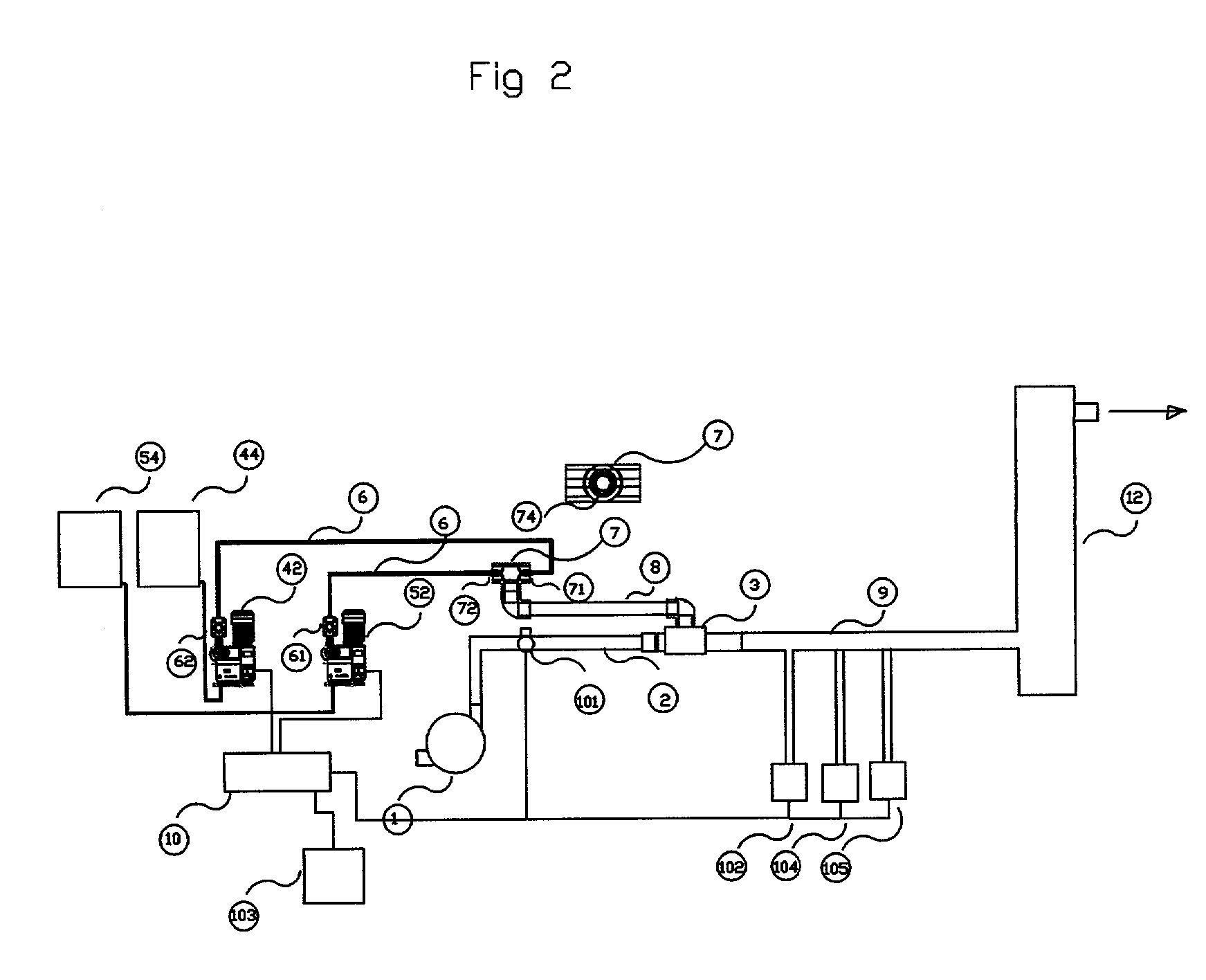
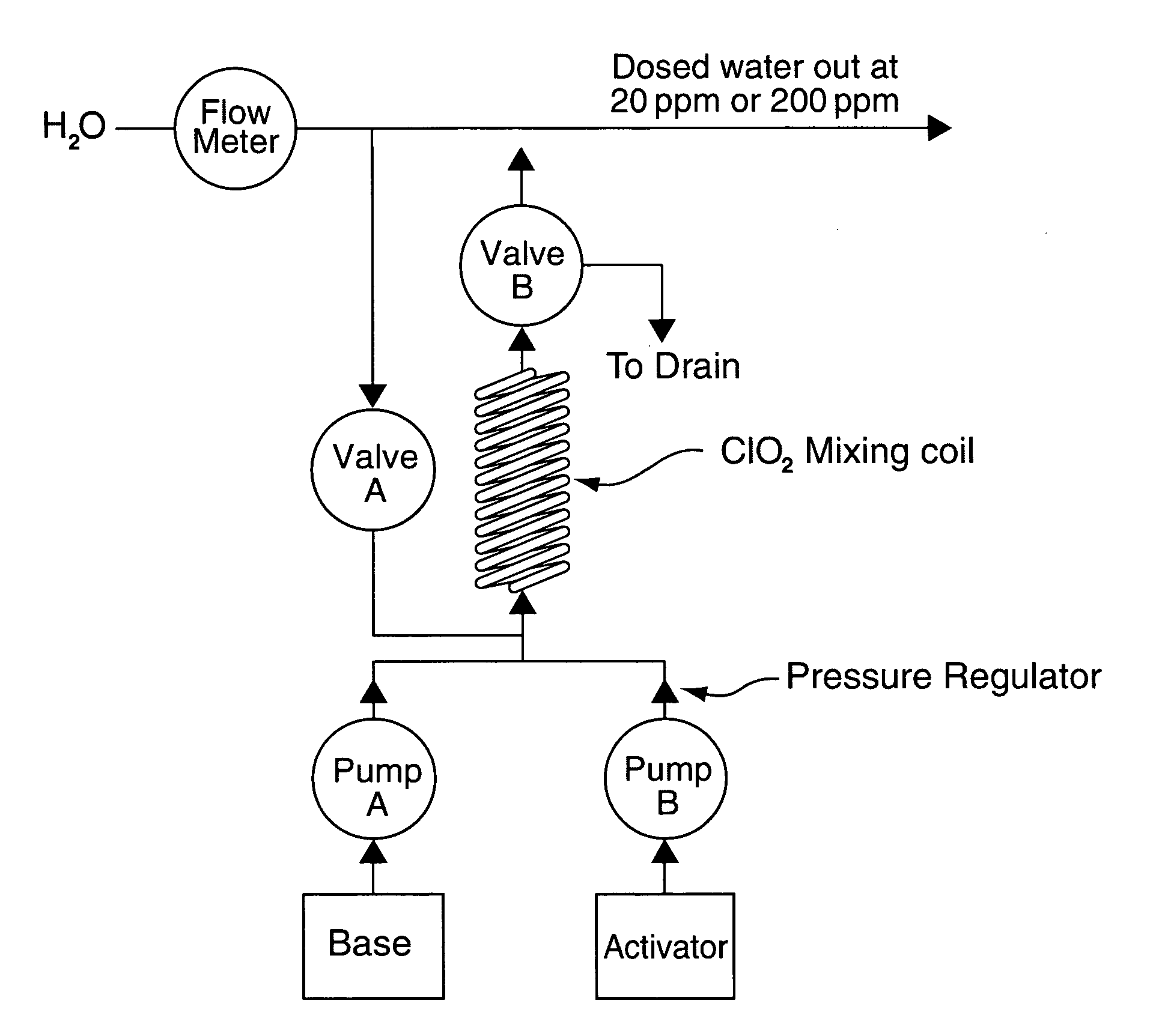
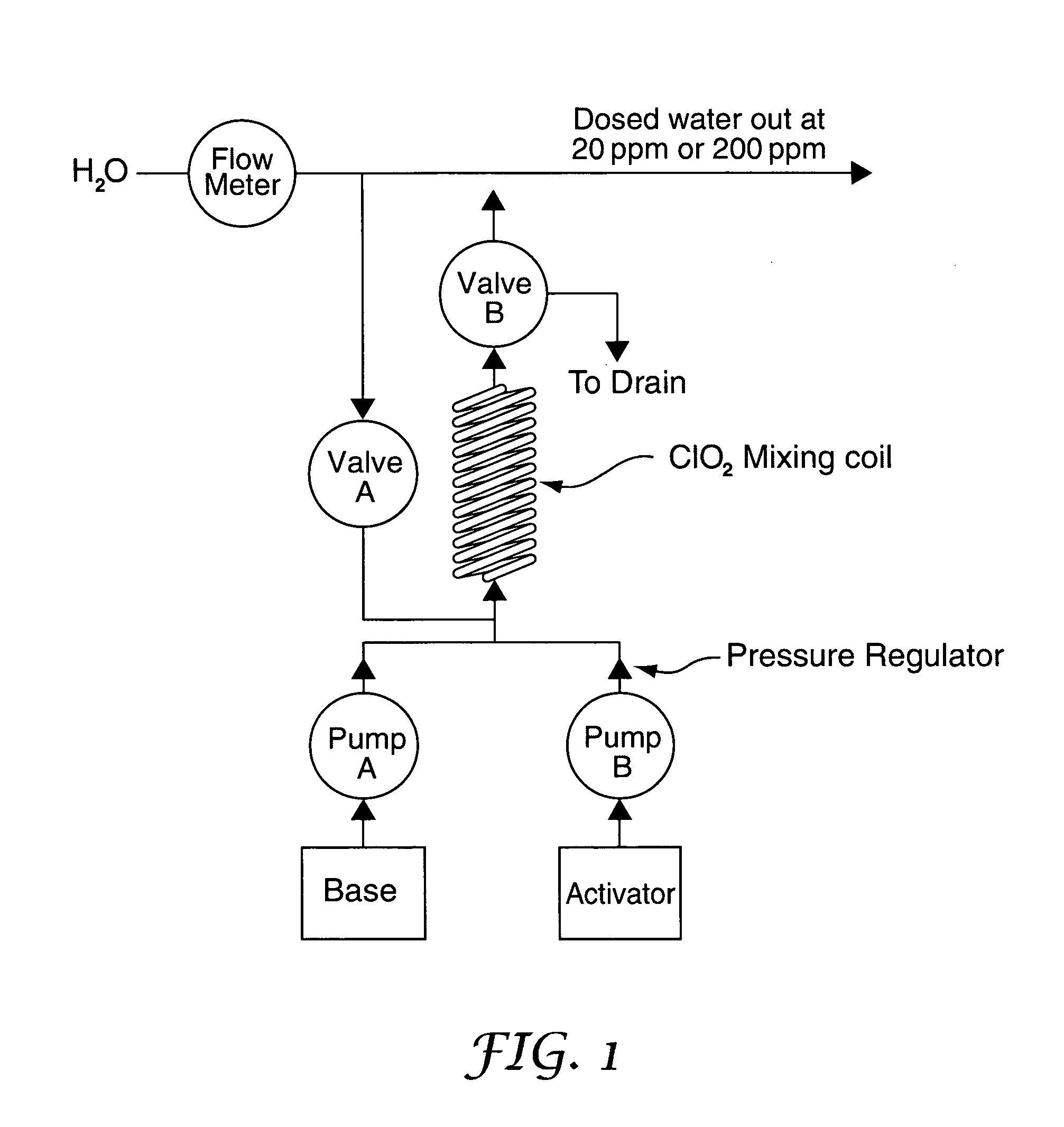
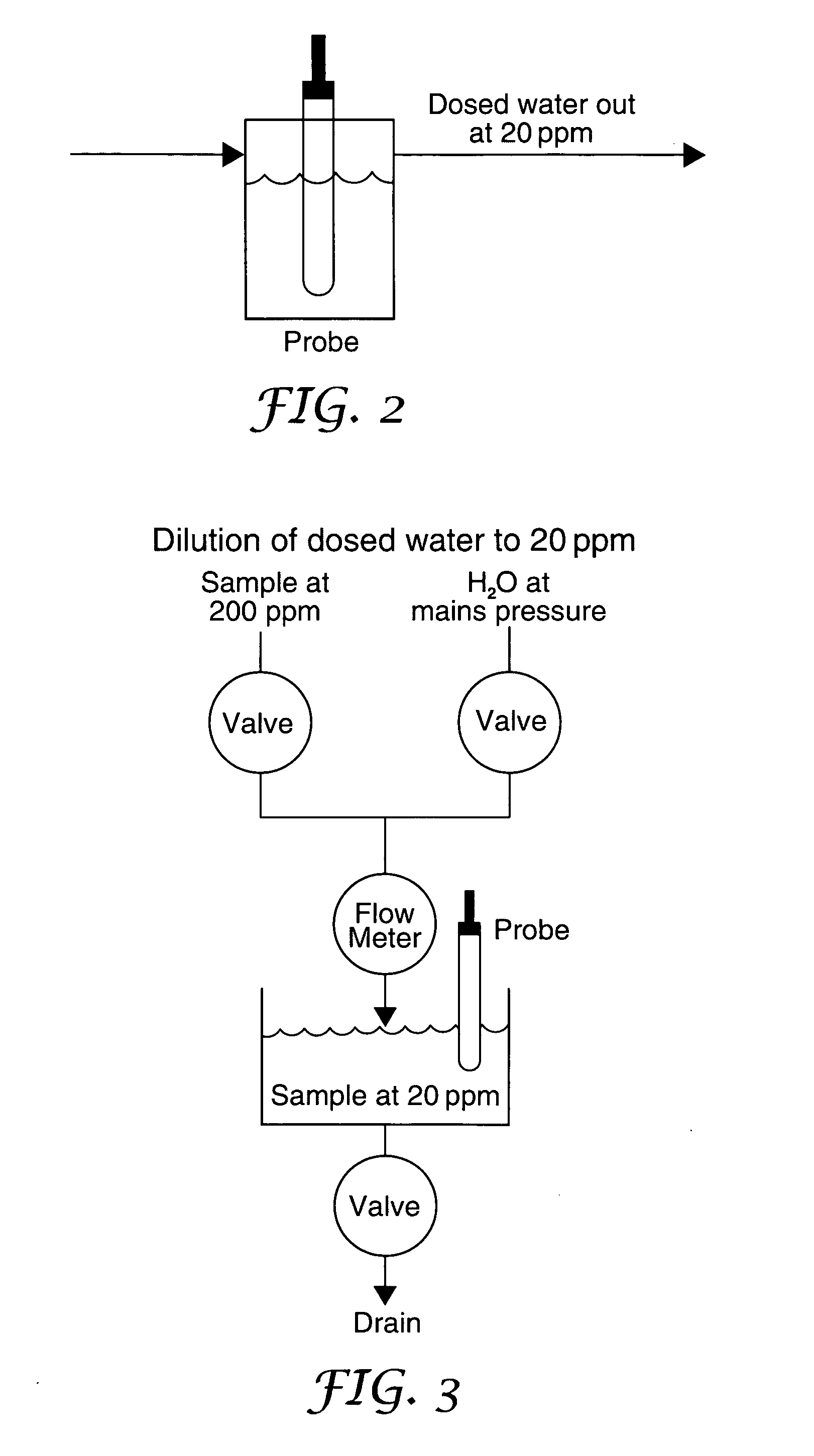
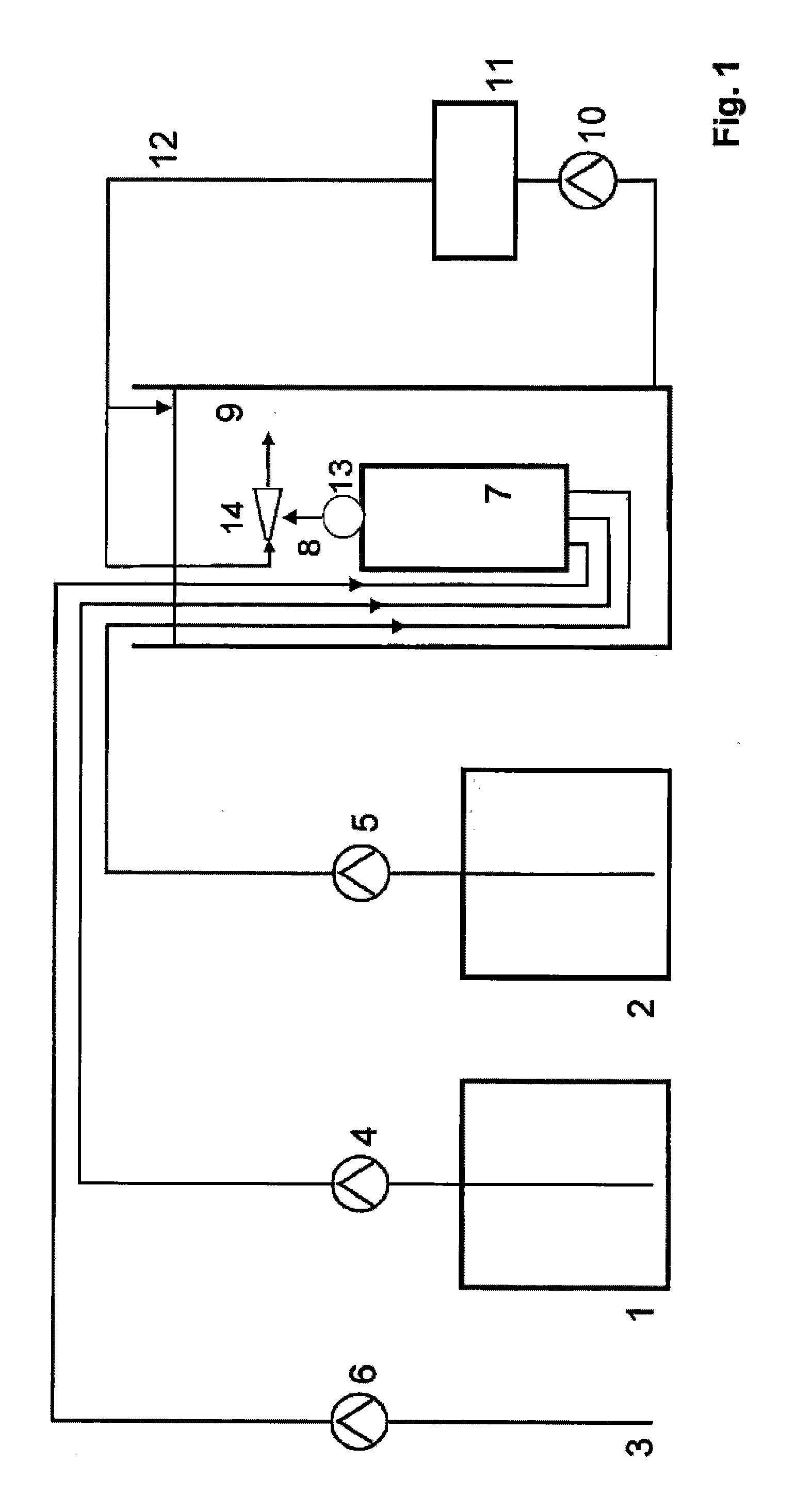
A method of producing a stream of aqueous chlorine dioxide sterilizing fluid suitable for use in sterilizing medical and dental equipment or for wound irrigation or skin asepsis is described. Steps are: pumping fluid portions of a first reagent and a second reagent into the proximal end of an elongate reaction chamber (24) to form a reaction mixture, the reagents being selected to react together to form aqueous chlorine dioxide; providing a stream of water around or adjacent to the distal end of the reaction chamber (24); continuing to pump fluid portions of said reagents so as to cause the reagent mixture to travel through the reaction chamber and then into the stream of water to form a stream of aqueous chlorine dioxide sterilizing fluid; wherein the pumping rates and the internal dimensions of the elongate reaction chamber (24) are selected so that the reaction to form chlorine dioxide is substantially complete when the reagent mixture exits the reaction chamber (24); and continuing pumping fluid portions of said reagents until a desired quantity of said sterilizing fluid has been produced; wherein the pumping of each reagent is carried out by means of a diaphragm pump (90, 92) or a piston pump via a pressure-control valve (32, 34) so that each fluid portion is pumped at substantially constant pressure.
Owner:TRISTEL SOLUTIONS
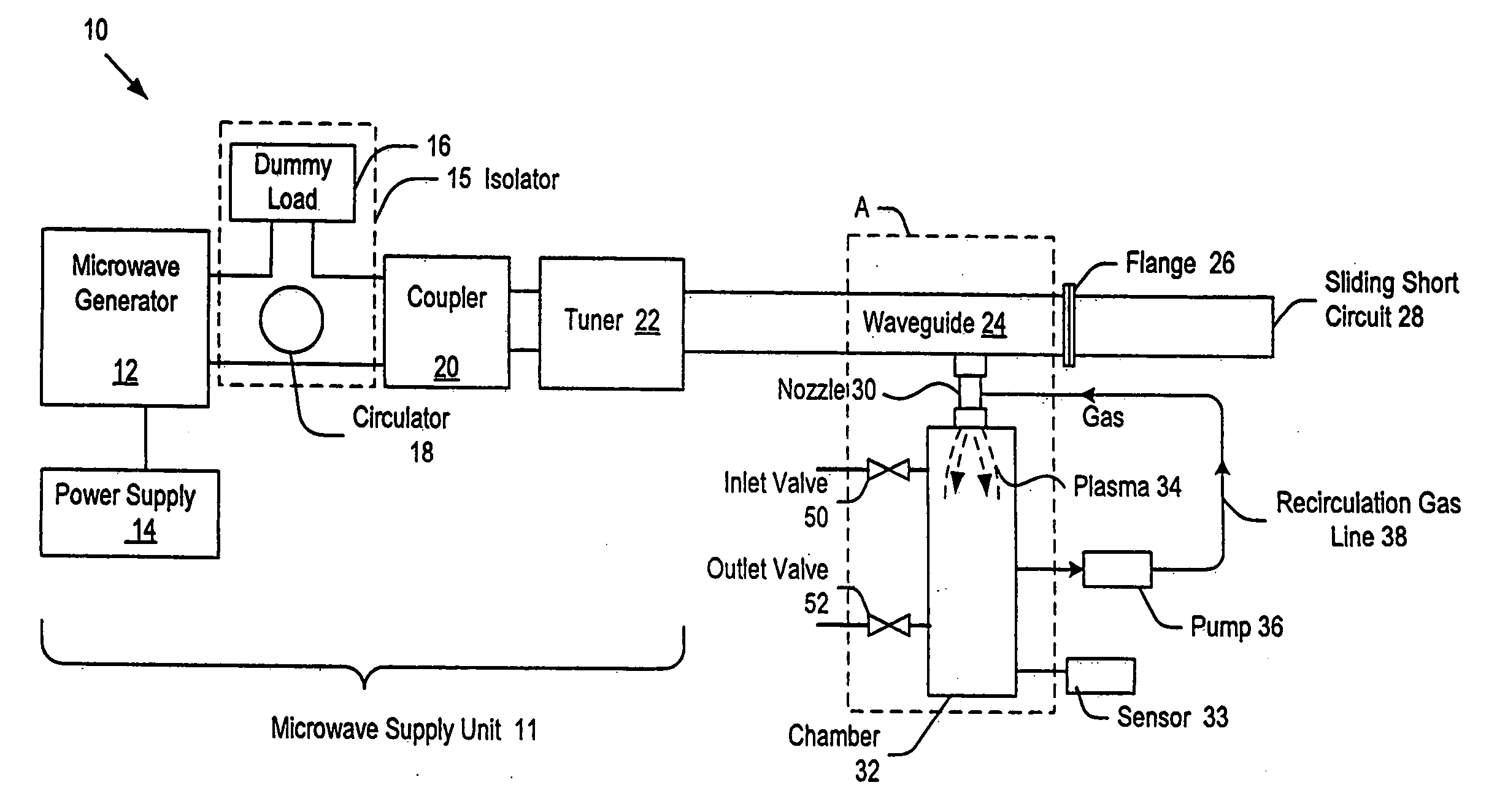
Method of sterilization using plasma generated sterilant gas
A sterilizing apparatus and method provide a sterilizing system having a sterilization chamber and a method for sterilizing an item in the sterilization chamber. The method includes the steps of: loading the item into the sterilization chamber; evacuating gas from the sterilization chamber; preparing sterilant gas by use of plasma; and filling the sterilization chamber with the sterilant gas to a preset pressure. The method further includes the steps of waiting a preset time interval to thereby accomplish an intended sterilization and evacuating the sterilant gas from the sterilization chamber.
Owner:SAIAN CORP +1
Method of treating water and aqueous systems in pipes with chlorine dioxide
The present invention relates to a method of treating water and an aqueous system, situated in a pipe, with chlorine dioxide (ClO2), the method including generating ClO2 in a reaction space such that the generated ClO2 is completely surrounded by a system to be treated, and delivering the ClO2 generated in the reaction space to the system to be treated which is situated in the pipe, wherein the system surrounding the reaction space is the system to be treated, the reaction space is a component of a mobile device and the mobile device can be introduced into the pipe, in which the system to be treated is situated, and removed again independently of the pressure state of the pipe containing the system to be treated, and the reaction space is situated in the pipe containing the system to be treated.
Owner:EVONIK OPERATIONS GMBH
Solid chlorine dioxide releasing compositions having increased temperature stability and method for delivery of solid chlorine dioxide releasing compositions
InactiveUS20050249658A1Improve temperature stabilityReduce deliveryChlorine dioxideChlorine dioxideWater soluble
The creation of chlorine dioxide in a water system can be accomplished by an improved mixture of components that includes a chlorite salt, oxidizing chlorine releasing agent(s) and an acid source and these improved combinations of materials can be pre-measured and delivered into a water system in sealed water soluble containers.
Owner:SAFE SOLID SOLUTIONS
Composition for generating chlorine dioxide
InactiveUS6602442B1Transportation safetyMade preciselyBiocidePeroxide active ingredientsLithium hypochloriteSodium bisulfate
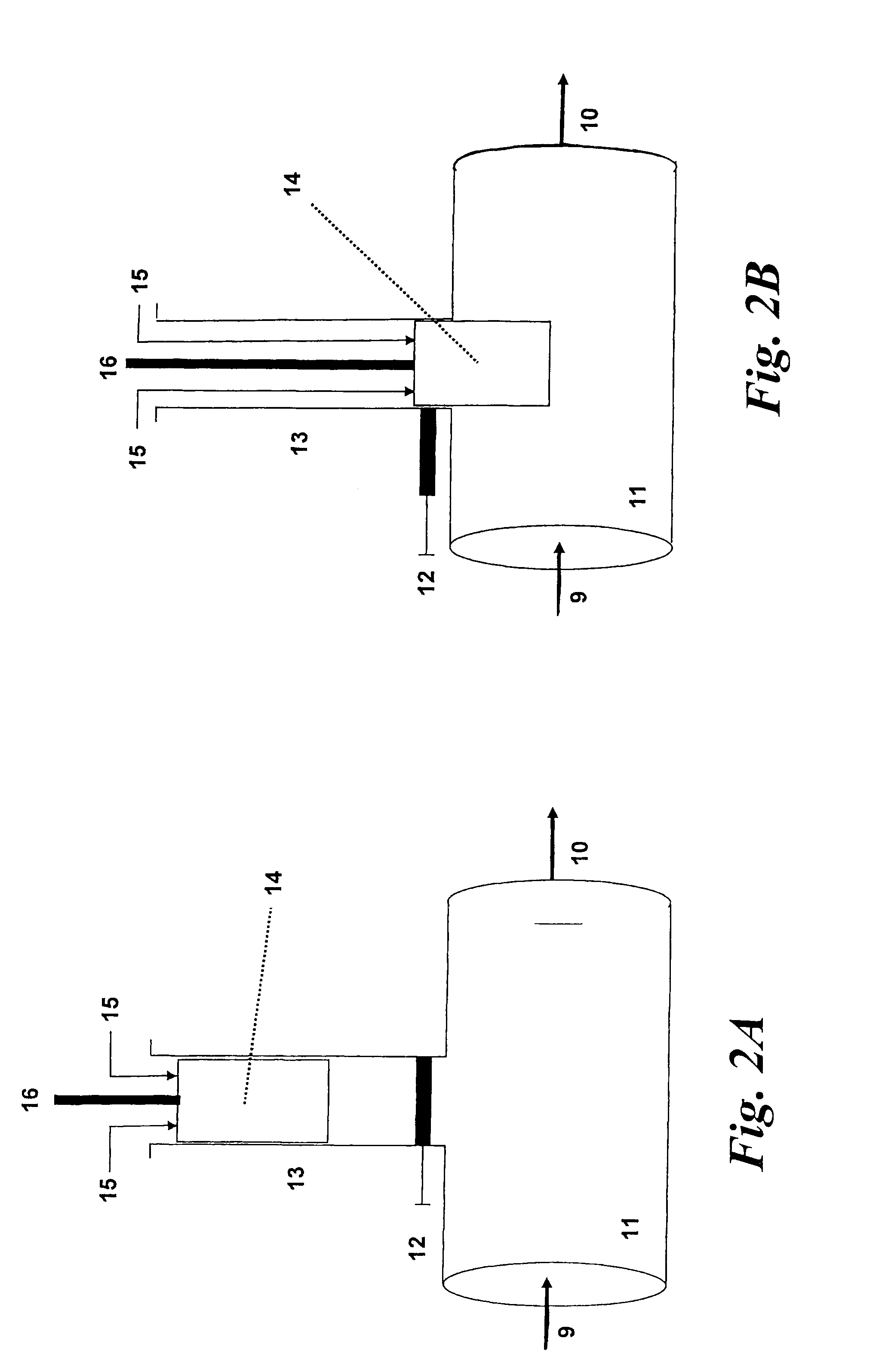
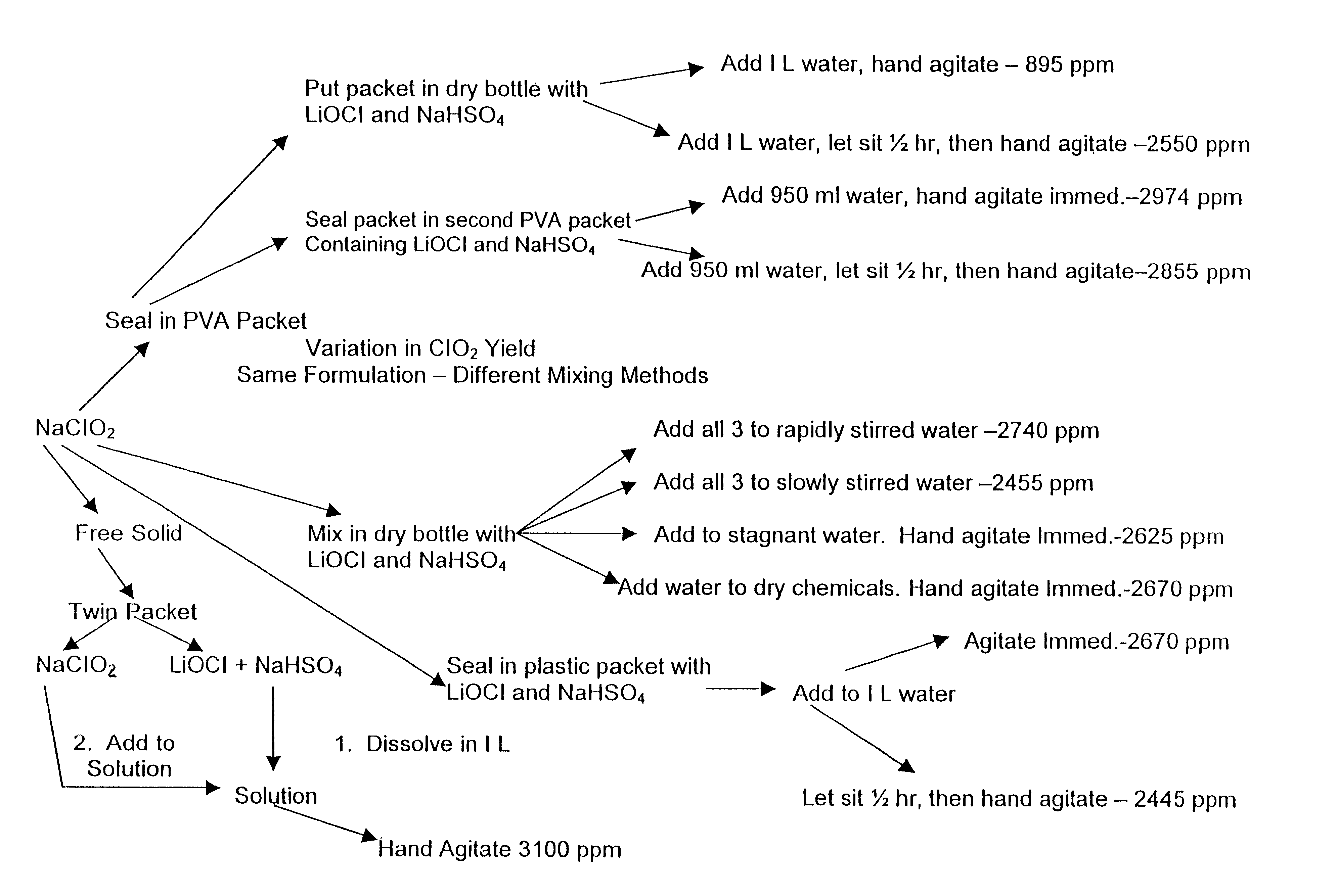
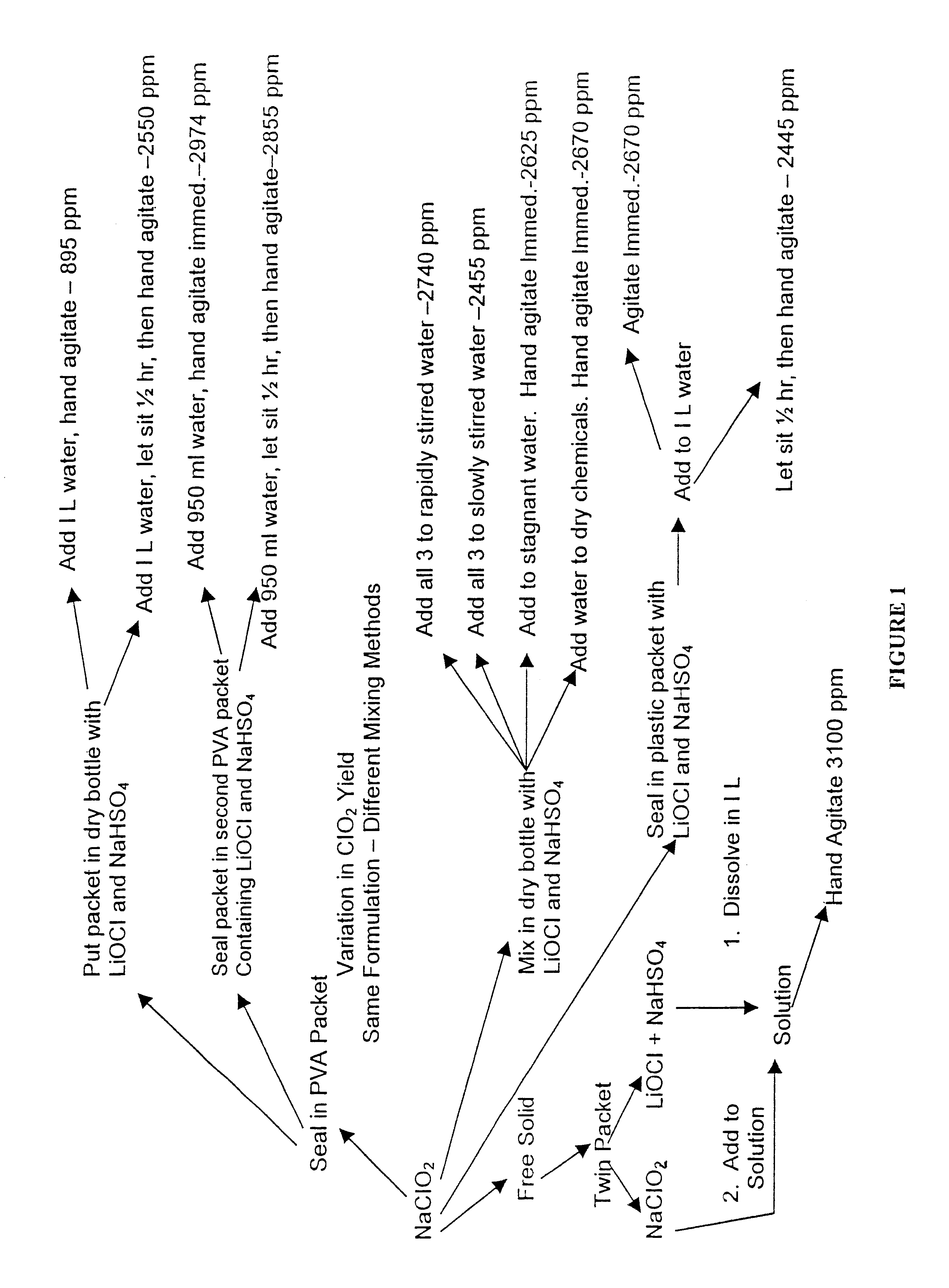
A dry disinfectant composition for the production of aqueous solutions of chlorine dioxide of predetermined concentration is formulated of a mixture of lithium hypochlorite, sodium bisulfate and sodium chlorite. Special liquid and dry formulations are also contemplated for convenience of use, for quality assurance and for safety.
Owner:OCCIDENTAL CHEM CORP
Method of treating water with chlorine dioxide
InactiveUS20090159538A1Safe and efficientMinimize hazard potentialBiocideWater treatment parameter controlChlorine dioxideReaction chamber
A method of treating water with chlorine dioxide (ClO2), by:surrounding a reaction chamber, in which the ClO2 is generated, with water, wherein the water surrounding the reaction chamber is simultaneously the water to be treated; and passing a reaction solution comprising the ClO2 formed in the reaction chamber out of the reaction chamber through an outlet directly into the water, thus treating the water.
Owner:INFRACOR
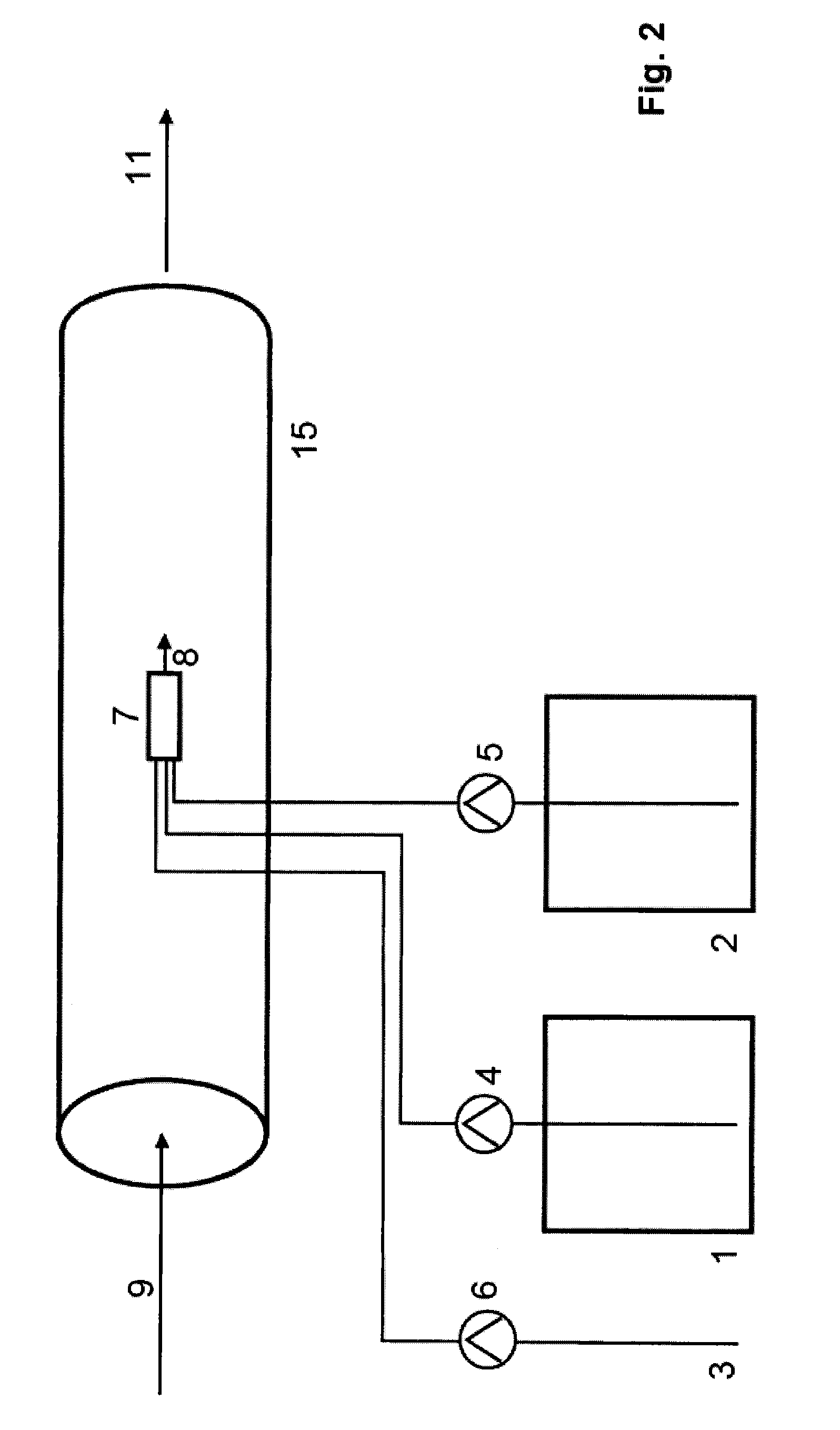
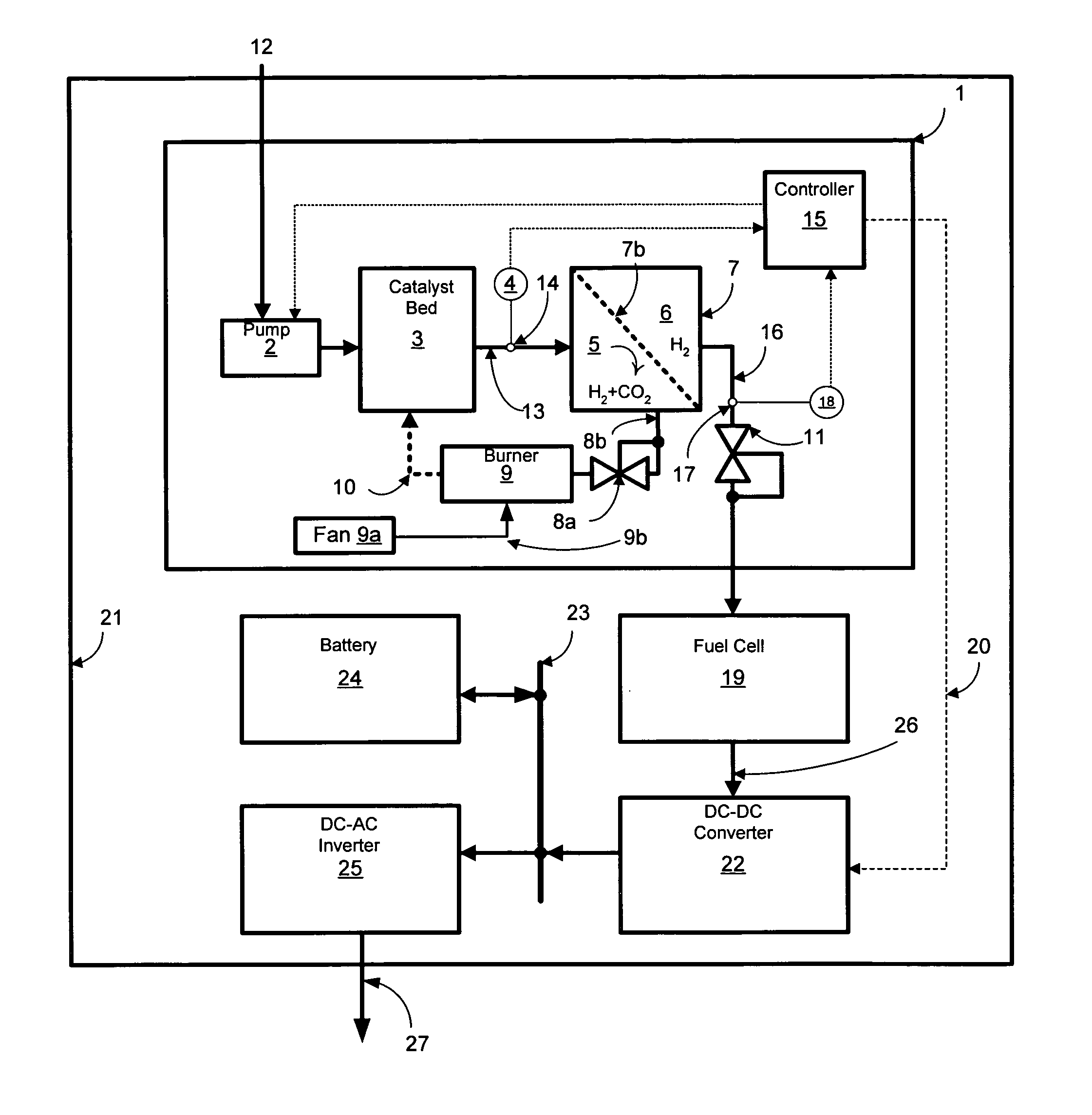
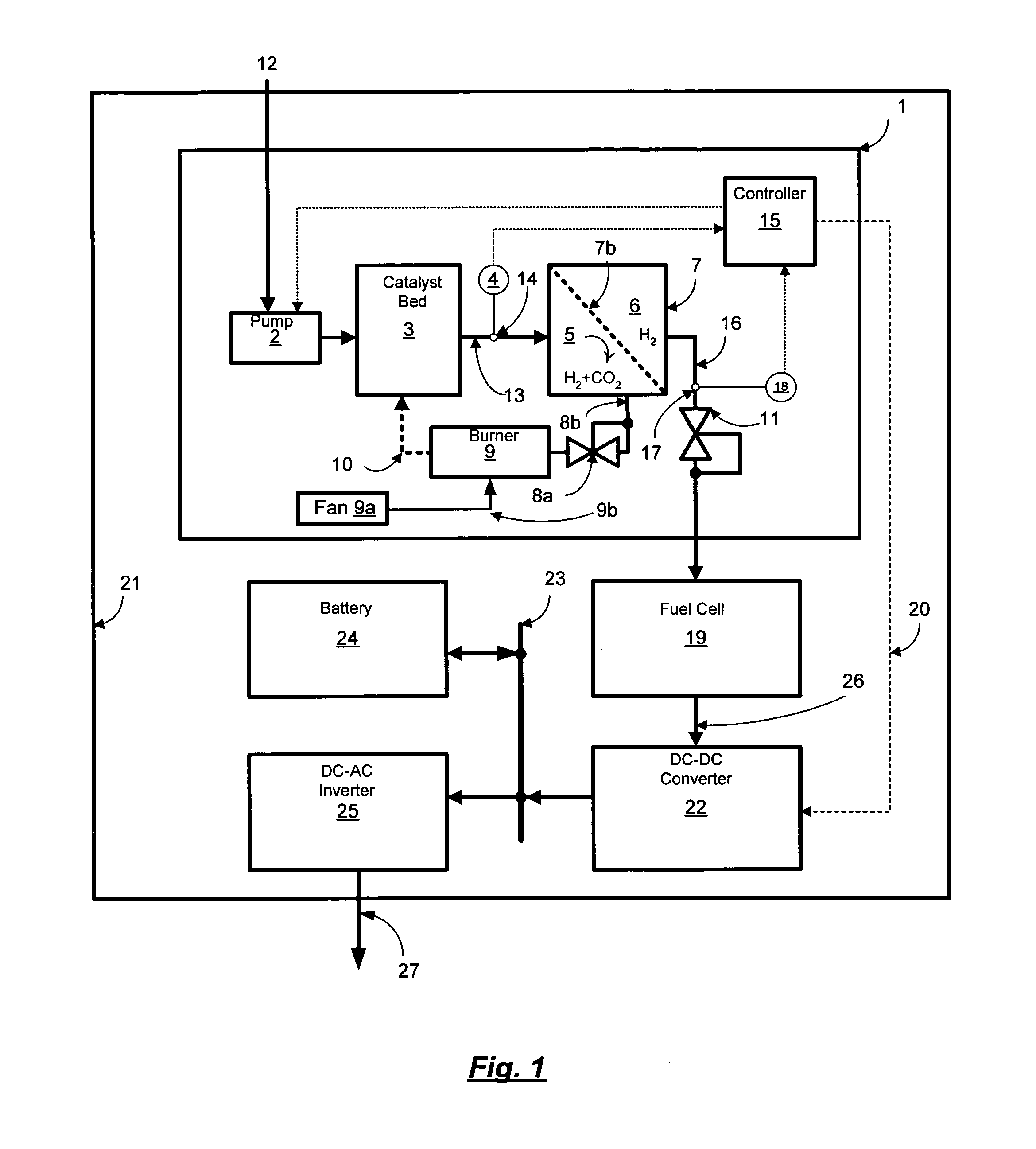
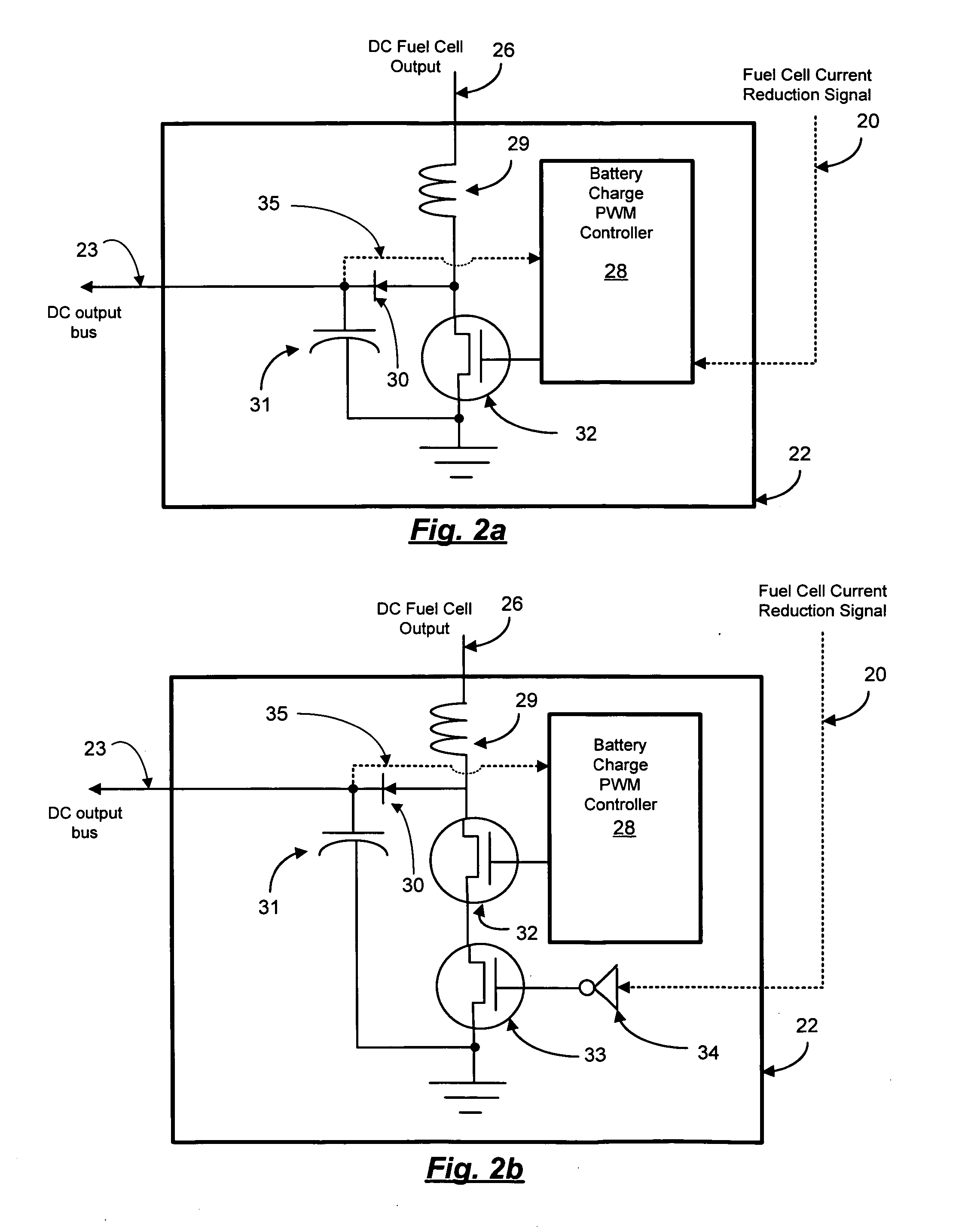
Reformer and fuel cell system control and method of operation
InactiveUS20070190380A1Reduce current outputEasy to integratePhysical/chemical process catalystsReactant parameters controlFuel cellsHydrogen pressure
A fuel cell system includes a reformer producing hydrogen from fuel, a regulator for regulating the output pressure of the produced hydrogen, a fuel cell utilizing the produced hydrogen, a pressure sensor for monitoring the pressure of the hydrogen upstream of the pressure regulator, a temperature sensor for monitoring at least one temperature within the reformer, a pump for introducing the fuel into the reformer, a first controller for controlling the output current of the fuel cell, and a second controller for controlling at least the fuel introduction rate into the reformer. The introduction rate of the fuel is responsive to output hydrogen pressure from the reformer in order to maintain at least one temperature within the reformer above a minimum level, as well as maintaining the pressure of the delivered purified hydrogen above a set pressure. Further, the output current of the fuel cell is reduced responsive to the pressure of the purified hydrogen in order to maintain a minimum hydrogen pressure to the fuel cell.
Owner:GENESIS FUEL TECH INC
Composition and method for reducing demineralization of teeth
InactiveUS20110318282A1Reducing demineralizationPromote remineralizationAntibacterial agentsCosmetic preparationsChlorine dioxideMedicine
A human oral care composition, includes safe and effective amounts of the fluoride ion and stabilized chlorine dioxide, that may take the form of a paste, gel, rinse, spray, powder, varnish or similar that reduces demineralization and promotes remineralization of teeth. The method includes the topical application of the composition to the human oral cavity (including but not limited to the teeth, gingiva, and tongue), preferably at least once daily, to enhance the anti-caries effect of fluoride by released chlorine dioxido compromising any biofilm present.
Owner:MICROPURE
Method and apparatus for the continous production of low concentrations of chlorine dioxide from low concentrations of aqueous chlorite
ActiveUS20110000860A1Reduce riskSpecific water qualityWater treatment parameter controlWater/sewage treatment by irradiationSodium chloriteSodium nitrite
Provided are methods and systems for continuously producing low concentrations of chlorine dioxide from dilute solutions of sodium chlorite. The low concentrations of chlorine dioxide produced allow for reduced exposure risk with direct application of the chlorine dioxide stream. The incorporation of a suitable chlorine dioxide detector permits continuous monitoring and control of chlorine dioxide production ensuring that the process stays within regulatory guidelines. Pretreatment of reaction water is preferred for achieving suitable conversion rates of the low concentrations of chlorite to chlorine dioxide.
Owner:CHEMTREAT
Method for treating reverse osmosis membranes with chlorine dioxide
InactiveUS20050061741A1Effective treatmentMinimize biofilm formationMembranesWater treatment parameter controlBillionthChlorine dioxide
Disclosed is a method for preventing osmotic membrane fouling comprising treating reverse osmotic feed water and membranes with chlorine dioxide at an extremely low concentration, e.g., as low as one part per billion in the feed water. The effective range may be in the range of 1-900 parts per billion. Also disclosed is a membrane separation system in which fouling is prevented using ultraviolet, pH acid adjustment or an electrochemical generator to produce the chlorine dioxide.
Owner:OCCIDENTAL CHEM CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com