Patents
Literature
377 results about "Lithium hypochlorite" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lithium hypochlorite is the colorless, crystalline lithium salt of hypochlorous acid with the chemical formula of LiClO. It is used as a disinfectant for pools and a reagent for some chemical reactions.
Dual layer tablet, method of making and use thereof
InactiveUS6863830B1Easy to optimizeComprehensive treatmentOrganic chemistryOther chemical processesPotassium persulfateLithium hypochlorite
A method for treating a recirculating water system which comprises introducing into said water system a multifunctional, multilayer tablet, wherein the multilayer tablet comprises a fast dissolving layer and a slow dissolving layer, wherein said fast dissolving layer releases a combination of active ingredients including a member selected from the group consisting of lithium hypochlorite, calcium hypochlorite, trichloroisocyanuric acid (TCCA), anhydrous sodium dichloroisocyanurate, sodium persulfate, potassium persulfate, potassium monopersulfate, sodium monopersulfate, and mixtures thereof, and at least one of a clarifier, chelating agent, sequesterant, algaestat, water softener, algaecide, corrosion inhibitor, scale inhibitor, flocculent, disintegrant, dispersant, colorant, dissolution control agent, fragrance, or surfactant and, wherein said slow dissolving layer includes a member selected from the group consisting of trichloroisocyanuric acid (TCCA), calcium hypochlorite, 1,3-dichloro-5,5-dimethylhydantoin (DCDMH), 1,3-dibromo-5,5-dimethylhydantoin (DBDMH), 1-bromo-3-chloro-5,5-dimethylhydantoin (BCDMH), 1,3-dichloro-5-ethyl-5-methylhydantoin (DCEMH), 1,3-dibromo-5-ethyl-5-methylhydantoin (DBEMH), 1-bromo-3-chloro-5-methyl-5-ethylhydantoin (BCEMH), and mixtures thereof, and at least one of a clarifier, chelating agent, sequesterant, algaestat, water softener, algaecide, corrosion inhibitor, scale inhibitor, flocculent, disintegrant, dispersant, colorant, dissolution control agent or surfactant.
Owner:BIO LAB
Dual layer tablet, method of making and use thereof
InactiveUS20050040116A1Easy to optimizeComprehensive treatmentOrganic chemistryOther chemical processesPotassium persulfateLithium hypochlorite
A method for treating a recirculating water system which comprises introducing into said water system a multifunctional, multilayer tablet, wherein the multilayer tablet comprises a fast dissolving layer and a slow dissolving layer, wherein said fast dissolving layer releases a combination of active ingredients including a member selected from the group consisting of lithium hypochlorite, calcium hypochlorite, trichloroisocyanuric acid (TCCA), anhydrous sodium dichloroisocyanurate, sodium persulfate, potassium persulfate, potassium monopersulfate, sodium monopersulfate, and mixtures thereof, and at least one of a clarifier, chelating agent, sequesterant, algaestat, water softener, algaecide, corrosion inhibitor, scale inhibitor, flocculent, disintegrant, dispersant, colorant, dissolution control agent, fragrance, or surfactant and, wherein said slow dissolving layer includes a member selected from the group consisting of trichloroisocyanuric acid (TCCA), calcium hypochlorite, 1,3-dichloro-5,5-dimethylhydantoin (DCDMH), 1,3-dibromo-5,5-dimethylhydantoin (DBDMH), 1-bromo-3-chloro-5,5-dimethylhydantoin (BCDMH), 1,3-dichloro-5-ethyl-5-methylhydantoin (DCEMH), 1,3-dibromo-5-ethyl-5-methylhydantoin (DBEMH), 1-bromo-3-chloro-5-methyl-5-ethylhydantoin (BCEMH), and mixtures thereof, and at least one of a clarifier, chelating agent, sequesterant, algaestat, water softener, algaecide, corrosion inhibitor, scale inhibitor, flocculent, disintegrant, dispersant, colorant, dissolution control agent or surfactant.
Owner:BIO LAB
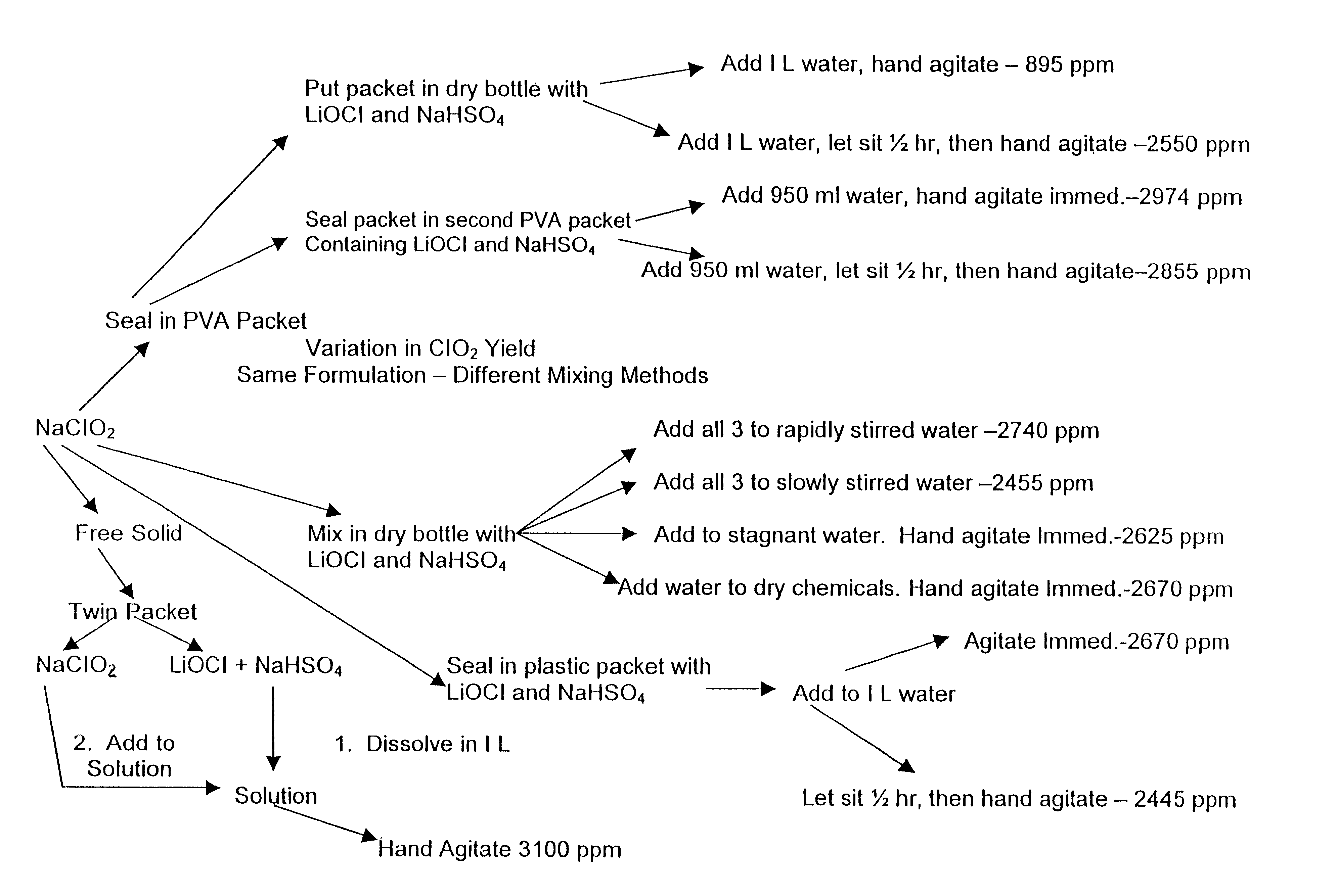
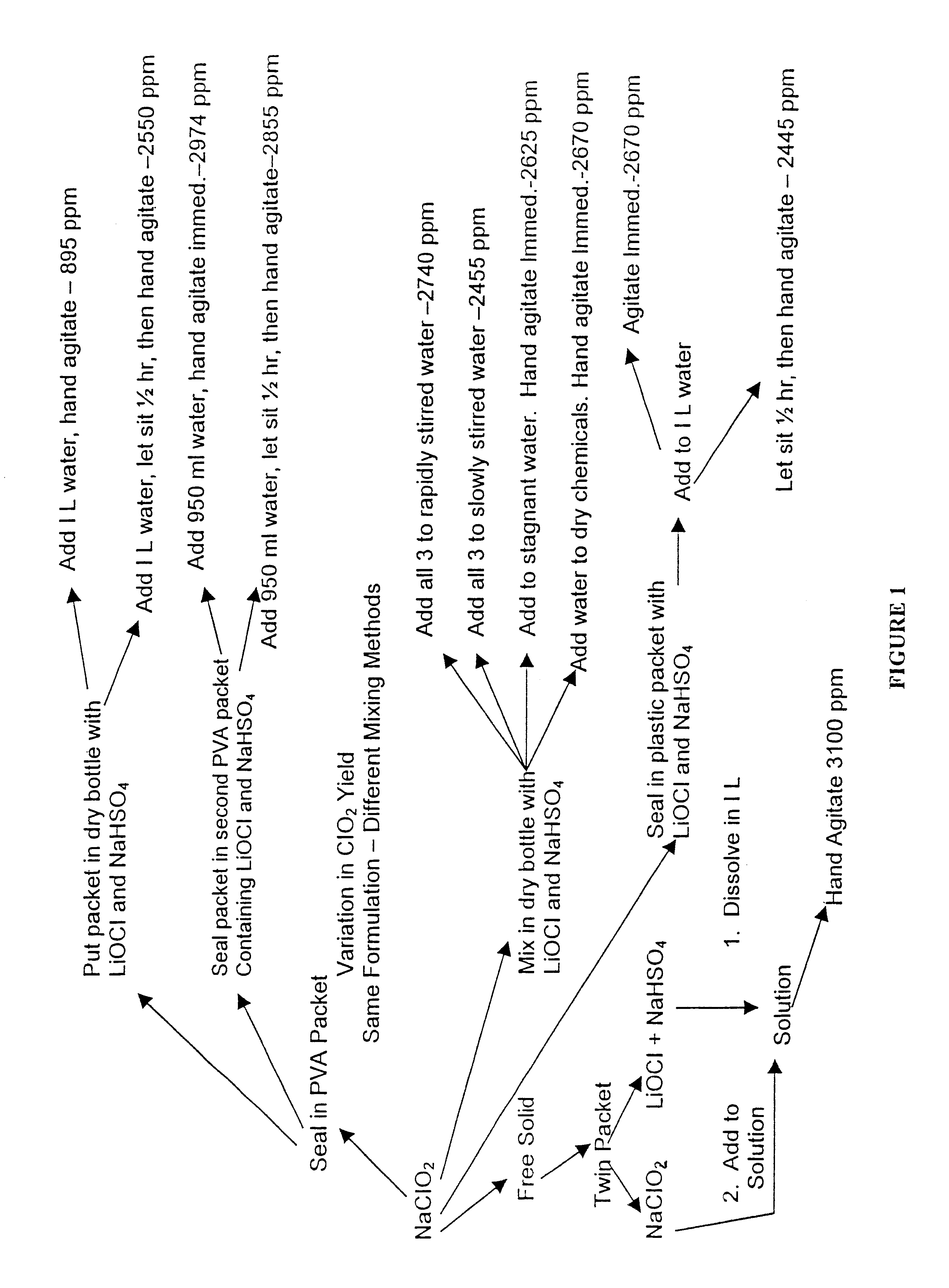
Composition for generating chlorine dioxide
InactiveUS6602442B1Transportation safetyMade preciselyBiocidePeroxide active ingredientsLithium hypochloriteSodium bisulfate
A dry disinfectant composition for the production of aqueous solutions of chlorine dioxide of predetermined concentration is formulated of a mixture of lithium hypochlorite, sodium bisulfate and sodium chlorite. Special liquid and dry formulations are also contemplated for convenience of use, for quality assurance and for safety.
Owner:OCCIDENTAL CHEM CORP
Solid-Layered Bleach Compositions
ActiveUS20100140544A1Inorganic/elemental detergent compounding agentsCosmetic preparationsPotassium hypochloriteBleach
The present invention provides a solid-layered composition having at least two parts. The first part comprises a) calcium hypochlorite, magnesium hypochlorite and mixtures thereof, b) a builder, c) a water-soluble polymer, d) an acid, and e) wherein the first part does not contain sodium hypochlorite, lithium hypochlorite, potassium hypochlorite and mixtures thereof. The second part comprises a) a surfactant, b) a builder, c) an acid, and d) wherein the second part does not contain any oxidant.
Owner:THE CLOROX CO
Solid-layered bleach compositions
ActiveUS8287755B2Inorganic/elemental detergent compounding agentsCosmetic preparationsPotassium hypochloriteBleach
The present invention provides a solid-layered composition having at least two parts. The first part comprises a) calcium hypochlorite, magnesium hypochlorite and mixtures thereof, b) a builder, c) a water-soluble polymer, d) an acid, and e) wherein the first part does not contain sodium hypochlorite, lithium hypochlorite, potassium hypochlorite and mixtures thereof. The second part comprises a) a surfactant, b) a builder, c) an acid, and d) wherein the second part does not contain any oxidant.
Owner:THE CLOROX CO
Solid-layered bleach compositions and methods of use
ActiveUS8361944B2Inorganic/elemental detergent compounding agentsCosmetic preparationsAdditive ingredientBleach
The present invention provides a solid-layered composition having at least two parts. The first part comprises a) calcium hypochlorite, magnesium hypochlorite and mixtures thereof, b) a builder, c) a water-soluble polymer, d) an acid, and e) wherein the first part does not contain sodium hypochlorite, lithium hypochlorite, potassium hypochlorite and mixtures thereof. The second part comprises a) a functional ingredient, b) a builder or filler, and c) wherein the second part does not contain any oxidant.
Owner:THE CLOROX CO
Solid-layered bleach compositions and methods of use
ActiveUS8361945B2Inorganic/elemental detergent compounding agentsCosmetic preparationsAdditive ingredientPotassium hypochlorite
The present invention provides a method for cleaning dentures by contacting the dentures with a solid, multi-layered composition having at least two parts in water. The first part of the composition comprises a) calcium hypochlorite, magnesium hypochlorite and mixtures thereof, b) a builder, c) a water-soluble polymer, d) an acid, and e) wherein the first part does not contain sodium hypochlorite, lithium hypochlorite, potassium hypochlorite and mixtures thereof. The second part comprises a) a functional ingredient, b) a builder or filler, and c) wherein the second part does not contain any oxidant.
Owner:THE CLOROX CO
Solid-layered bleach compositions and methods of use
ActiveUS20110059882A1Inorganic/elemental detergent compounding agentsCosmetic preparationsAdditive ingredientPotassium hypochlorite
The present invention provides a method for cleaning dentures by contacting the dentures with a solid, multi-layered composition having at least two parts in water. The first part of the composition comprises a) calcium hypochlorite, magnesium hypochlorite and mixtures thereof, b) a builder, c) a water-soluble polymer, d) an acid, and e) wherein the first part does not contain sodium hypochlorite, lithium hypochlorite, potassium hypochlorite and mixtures thereof. The second part comprises a) a functional ingredient, b) a builder or filler, and c) wherein the second part does not contain any oxidant.
Owner:THE CLOROX CO
Hypochlorite denture compositions and methods of use
ActiveUS20130109608A1Minimizes and eliminates formation of residueDissolve fastInorganic/elemental detergent compounding agentsCosmetic preparationsDicarbonateHydrotrope
A solid composition including calcium and / or magnesium hypochlorite, a builder (e.g., one or more of carbonate, bicarbonate, sesquicarbonate), an acid, a water-soluble polymer, at least one anionic surfactant, and optionally, a hydrotrope. The composition does not include any potassium hypochlorite, sodium hypochlorite, lithium hypochlorite, N-halogenated compounds, peroxides, persulfates, hydantoins, isocyanurates, or carboxylic acids that also have hydroxyl, amino, amido, imino, or imido groups. Upon dissolution of the composition in water, the calcium and / or magnesium hypochlorite and acid react to form hypochlorous acid. The use of hypochlorous acid, rather than direct use of an alkaline or alkaline earth hypochlorite results in a composition that is typically acidic rather than basic, and that results in improved cleaning. The composition is particularly suited tor cleaning and disinfecting dentures.
Owner:THE CLOROX CO
Hypochlorite denture compositions and methods of use
ActiveUS20110027194A1Minimizes and eliminates formation of residueDissolve fastInorganic/elemental detergent compounding agentsCosmetic preparationsDicarbonateAlkaline earth metal
A solid composition including calcium and / or magnesium hypochlorite, a builder (e.g., one or more of carbonate, bicarbonate, sesquicarbonate), an acid, a water-soluble polymer, at least one anionic surfactant, and optionally, a hydrotrope. The composition does not include any potassium hypochlorite, sodium hypochlorite, lithium hypochlorite, N-halogenated compounds, peroxides, persulfates, hydantoins, isocyanurates, or carboxylic acids that also have hydroxyl, amino, amido, imino, or imido groups. Upon dissolution of the composition in water, the calcium and / or magnesium hypochlorite and acid react to form hypochlorous acid. The use of hypochlorous acid, rather than direct use of an alkaline or alkaline earth hypochlorite results in a composition that is typically acidic rather than basic, and that results in improved cleaning. The composition is particularly suited for cleaning and disinfecting dentures.
Owner:THE CLOROX CO
Solid-layered bleach compositions and methods of use
ActiveUS20110052726A1Inorganic/elemental detergent compounding agentsCosmetic preparationsPotassium hypochloriteBleach
The present invention provides a solid-layered composition having at least two parts. The first part comprises a) calcium hypochlorite, magnesium hypochlorite and mixtures thereof, b) a builder, c) a water-soluble polymer, d) an acid, and e) wherein the first part does not contain sodium hypochlorite, lithium hypochlorite, potassium hypochlorite and mixtures thereof. The second part comprises a) a functional ingredient, b) a builder or filler, and c) wherein the second part does not contain any oxidant.
Owner:THE CLOROX CO
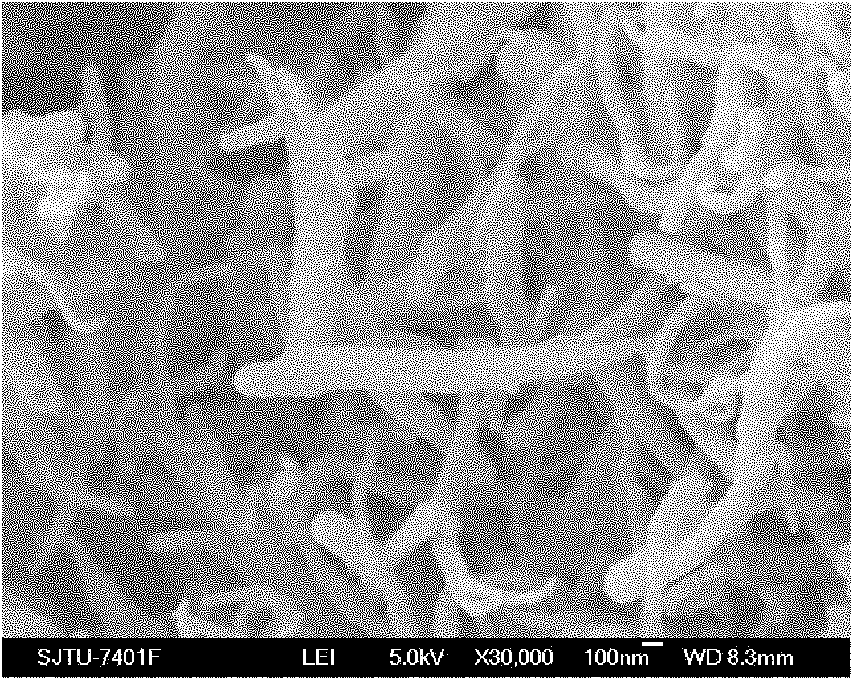
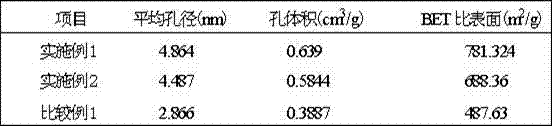
Preparation method of composite solid electrolyte based on polyphosphazene carbide micro-nanometer material
InactiveCN101924246AHigh conductivity at room temperatureImprove mechanical propertiesSecondary cellsLithium hypochloriteLithium-ion battery
The invention relates to a preparation method of composite solid electrolyte based on a polyphosphazene carbide micro-nanometer material, which belongs to the technical field of lithium batteries, the polyphosphazene carbide micro-nanometer material is dispersed in acetonitrile, then polyoxyethylene and lithium hypochlorite are sequentially added and evenly stirred by magnetic force, and then the mixed solution is poured into a polytetrafluoroethylene mould board, thus obtaining the composite solid polymer electrolyte. Compared with the existing electrolyte, the product has higher conductivity and larger lithium ion transference number, good mechanical properties and good electrochemical stability.
Owner:SHANGHAI JIAO TONG UNIV
Hypochlorite denture compositions and methods of use
ActiveUS20110028368A1Minimizes and eliminates formation of residueDissolve fastInorganic/elemental detergent compounding agentsCosmetic preparationsHydrotropeAlkaline earth metal
A solid composition including calcium and / or magnesium hypochlorite, a builder (e.g., one or more of carbonate, bicarbonate, sesquicarbonate), an acid, a water-soluble polymer, at least one anionic surfactant, and optionally, a hydrotrope. The composition does not include any potassium hypochlorite, sodium hypochlorite, lithium hypochlorite, N-halogenated compounds, peroxides, persulfates, hydantoins, isocyanurates, or carboxylic acids that also have hydroxyl, amino, amido, imino, or imido groups. Upon dissolution of the composition in water, the calcium and / or magnesium hypochlorite and acid react to form hypochlorous acid. The use of hypochlorous acid, rather than direct use of an alkaline or alkaline earth hypochlorite results in a composition that is typically acidic rather than basic, and that results in improved cleaning The composition is particularly suited for cleaning and disinfecting dentures.
Owner:THE CLOROX CO
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN106984330AEvenly dopedImprove adsorption capacityCatalyst carriersWater contaminantsUltrasound - actionLithium hypochlorite
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst and belongs to the technical fields of environment protection and chemical engineering catalysts. The preparation method comprises the following steps: by taking porous materials attapulgite, diopside, talc, trona, aluminum hydroxide and celestite as a carrier, after chambering and modifying the carrier through lithium hypochlorite and di(acetylacetone) beryllium, adding a surfactant methyl trioctyl ammonium chloride for surface activating treatment under the action of ultrasonic waves; then performing a hydrothermal reaction on the ultrasonic surface activated carrier in a hydrothermal reaction kettle with a compound mineralizer borax and potassium sulfate, catalytic active auxiliary agent precursors of tri(3-trifluoroacetyl-D-camphor) praseodymium (III), tricyclopentadiene promethium, terbium triacetate hydrate and holmium oxalate decahydrate rare earth metal organic compounds catalytic active central compound precursor common traditional metal organic compounds of ferrous fumarate and nickel citrate, and noble metal compounds of potassium argentite dithiocyanide (I) and a terpyridyl ruthenium chloride hexahydrate under the action of an emulsifier trimethylaminoglycerol glycerol stearate; and after drying a reaction product to remove water, firing the reaction product in a muffle furnace at a certain temperature to obtain the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Hypochlorite denture compositions and methods of use
ActiveUS8361942B2Minimizes and eliminates formation of residueDissolve fastInorganic/elemental detergent compounding agentsCosmetic preparationsDicarbonateAlkaline earth metal
Owner:THE CLOROX CO
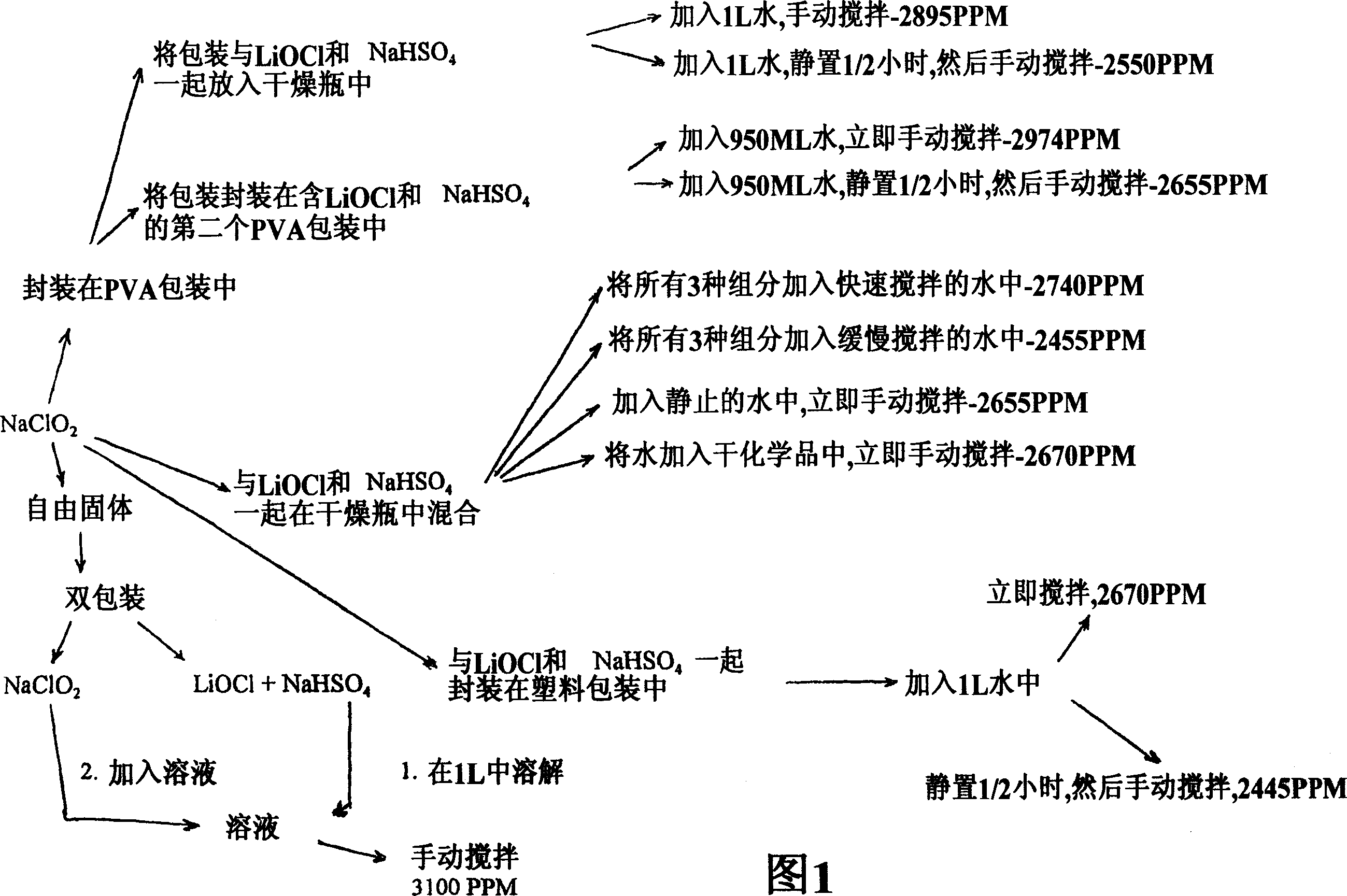
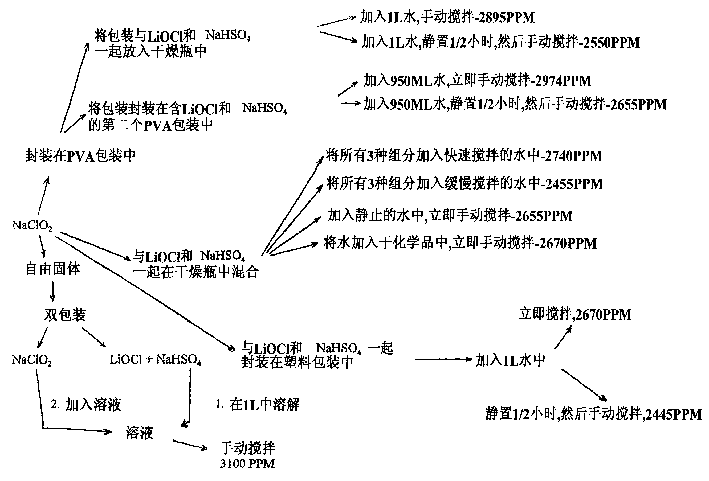
Preparation method of lithium hypochlorite
InactiveCN108557768AReduce concentrationHigh purityHypochloriteLithium hydroxideLithium hypochlorite
The invention relates to a preparation method of lithium hypochlorite. The preparation method of lithium hypochlorite includes the following steps: S1, chlorine is introduced into alkali metal hydroxide solution to react to form a first solution, wherein the molar ratio of chlorine to alkali metal hydroxide is not less than 20:1; S2, the first solution is distilled; the distillate is condensed atthe temperature of between -5 and 10 DEG C to obtain hypochlorous acid solution with the concentration of hypochlorous acid not less than 35wt%; S3, the prepared hypochlorous acid solution is mixed with the prepared lithium hydroxide suspension at the temperature of between 0 and 20 DEG C to react to obtain a lithium hypochlorite mixed solution; S4, the lithium hypochlorite mixed solution is evaporated and crystallized under the vacuum state and at the temperature of between 30 and 60 DEG C; the lithium hypochlorite crystal and the mother liquid are separated; the lithium hypochlorite crystalis dried to obtain the lithium hypochlorite. By the method, the concentration of various impurities can be reduced to a maximum extent, the purity and the yield of the product are improved, the production cost is reduced, the production process is simplified, and the method is an environment-friendly and economical preparation method of lithium hypochlorite.
Owner:FIRST RARE MATERIALS CO LTD
Hypochlorite denture compositions and methods of use
ActiveUS8361943B2Minimizes and eliminates formation of residueDissolve fastInorganic/elemental detergent compounding agentsCosmetic preparationsDicarbonateAlkaline earth metal
A solid composition including calcium and / or magnesium hypochlorite, a builder (e.g., one or more of carbonate, bicarbonate, sesquicarbonate), an acid, a water-soluble polymer, at least one anionic surfactant, and optionally, a hydrotrope. The composition does not include any potassium hypochlorite, sodium hypochlorite, lithium hypochlorite, N-halogenated compounds, peroxides, persulfates, hydantoins, isocyanurates, or carboxylic acids that also have hydroxyl, amino, amido, imino, or imido groups. Upon dissolution of the composition in water, the calcium and / or magnesium hypochlorite and acid react to form hypochlorous acid. The use of hypochlorous acid, rather than direct use of an alkaline or alkaline earth hypochlorite results in a composition that is typically acidic rather than basic, and that results in improved cleaning. The composition is particularly suited for cleaning and disinfecting dentures.
Owner:THE CLOROX CO
Composition for generating chlorine dioxide
Owner:OCCIDENTAL CHEM CORP +1
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107051480AEvenly dopedImprove adsorption capacityCatalyst carriersWater contaminantsHydration reactionPtru catalyst
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst and belongs to the technical field of environment protection and chemical catalysts. The preparation method includes: using activated carbon, carnallite, dolomite, calcite, fluorite and glauberite as the carriers, using lithium hypochlorite and bis(acetylacetone) beryllium to perform pore expansion on the carriers, adding surfactant beta-hydroxyethyl dimethyl dodecyl ammonium sulfate to activate the carriers under ultrasonic action, allowing the carriers to have hydrothermal reaction with compound mineralizer including borax and potassium sulfate, catalytic activity promoter precursors including promethium tricyclopentadienide, tri(2, 2, 6, 6-tetramethyl-3, 5-heptanedionato) gadolinium, terbium acetate hydrate and tri[N, N-bis(trimethyl silane) amine] erbium, and catalytic activity central precursors including cobalt gluconate, cupric glutamate, tetraammine dichloropalladium(II) and diamineplatinum(II) dichloride in a hydrothermal reaction kettle under the effect of an emulsifier chlorinated methyl acryloyloxy ethyl trimethyl ammonium, drying to remove moisture, and burning in a muffle furnace to obtain the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIV
Preparation method for solid catalyst for ozone heterogeneous oxidization
InactiveCN107008389AImprove adsorption capacityBurning at high temperature makes the organic matter completely carbonized and strong adsorptionOther chemical processesWater treatment compoundsUltrasound - actionLithium hypochlorite
The invention relates to a preparation method for a solid catalyst for ozone heterogeneous oxidization and belongs to the technical field of an environment-friendly and chemical catalyst. The preparation method comprises the following steps: taking a perlite, albite, montmorillonite, sylvine, illite and ulexite porous material as a carrier; modifying the carrier by broaching with lithium hypochlorite and bis(acetylacetone) beryllium, and then adding surfactant nonylphenol diquaternary ammonium salt and performing surface activating treatment under the effect of ultrasonic wave; performing hydrothermal reaction on an ultrasonic surface activated carrier, a compound mineralizer including borax and potassium sulphate, catalytic activated assistant precursors, including 1,1,1-trifluoroacetylacetone neodymium, terbium acetate hydrate, holmium oxalate hydrate and tri[N,N-bi(trimethyl silyl)amide] erbium rare-earth metal organic compound and catalytic active core component precursors, including common transition metal organic compound cobalt gluconate, nickel citrate, ammonium zirconium carbonate and precious metal chemical complex tetraammine dichloropalladium in a hydrothermal reaction kettle under the effect of octadecyl dimethyl hydroxypropyl ammonium chloride used as an emulsifier; drying and dewatering the reaction product; burning in a muffle furnace under a certain temperature, thereby acquiring the solid catalyst for ozone heterogeneous oxidization.
Owner:SICHUAN NORMAL UNIVERSITY
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN106994358AInhibition of segregationHigh adsorption selectivityCatalyst carriersWater contaminantsUltrasound - actionRare earth metal compounds
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst, and belongs to the technical field of environmental protection and chemical catalysts. The preparation method comprises the steps of taking activated carbon, carnallite, potassium feldspar, szaibelyite, pulverized fuel ash and a coal gangue porous mineral material as carriers; after bearizing and modifying the carriers through lithium hypochlorite and bis(acetylacetone) beryllium, adding a surface active agent dimethyl hexadecyl ethyl ammonium ethyl sulfate for carrying out surface activation treatment under an ultrasonic action; then carrying out hydrothermal reaction on the ultrasonic surface activated carriers, a compound mineralizing agent prepared from borax and potassium sulphate, samarium acetylacetonate, tri(4,4,4-trifluoro-1-(2-thiophene)-1,3-butanedione) europium, holmium oxalate decahydrate, thulium trifluoromethane sulfonate (III) rare-earth metal compound catalytic active auxiliary, cobalt gluconate, L-aspartic acid molybdenum, potassium dicyanoargentate (I) and a terpyridyl ruthenium chloride hexahydrate transition metal catalytic activity center component in a hydrothermal reaction kettle under the action of an emulgator hexadecyl dimethylamine ethyl sulfate; drying a reaction product to remove moisture, and firing in a muffle furnace at a certain temperature to obtain the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107159199AEvenly dopedImprove adsorption capacityOther chemical processesCatalyst activation/preparationUltrasound - actionCalcite
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst, and belongs to the technical field of environmentally-friendly and chemical catalysts. The preparation method comprises the following steps: by taking medical stone, wollastonite, dolomite, calcite, hydrotalcite and blodite, reaming the carriers with lithium hypochlorite and bis(acetylacetone) beryllium, then adding myristyl tributyl ammonia chloride serving as a surfactant for activation treatment under ultrasonic action, putting the carriers into a hydrothermal reaction kettle for hydrothermal reaction together with borax and potassium sulfate which serve as composite mineralizers, isopropyl scandium oxide (III), tri(4,4,4-trifluoro-1-(2-thiophene)-1,3-butanedione) europium, tri[N,N-bis(trimethylsilane)amine] erbium and thulium trifluoromethane sulfonate which serve as catalytic active auxiliary precursors and a dicyclopentadienyl titanium cyclo-replaced salicylic acid complex, zinc lactate, a catechol ethanediamine tungsten complex and terpyridyl ruthenium chloride hexahydrate which serve as catalytic active center precursors under the action of N-dodecyl dimethyl-N'-trimethyl-2-hydroxypropyl ammonia dichloride serving as an emulsifier, drying a reaction product to remove water, and firing the reaction product in a muffle furnace, thus obtaining the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107008414AEvenly dopedImprove adsorption capacityOther chemical processesWater treatment compoundsUltrasound - actionPtru catalyst
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst, belonging to the technical field of an environment-friendly and chemical catalyst. The preparation method comprises the following steps: by taking attapulgite, diopside, montmorillonite, sylvite, amazonite and lithium limestone porous materials as carriers, performing pore expansion and modification to the carriers through lithium hypochlorite and beryllium bis(acetylacetonate), adding a surfactant dioctadecyl-2-methyl hydroxyethyl ammonium chloride and performing surface activation treatment under ultrasonic wave effect, then leading the ultrasonically surface-activated carriers to have hydrothermal reaction with a complex mineralizer (borax and potassium sulfate), catalytic activity assistant precursors, namely praseodymium(III) tris[3-(trifluoromethylhydroxymethylene)-D-camphorate], 1,1,1-neodymium trifluoroacetylacetonate, tris(6,6,7,7,8,8,8-heptafluoro-2,2-dimethyloctane-3,5-dionato-O,O')dysprosium (III) and holmium oxalate rare earth metal organic compound, and catalytic active site component precursors, namely common transitional metal organic compound ferrous fumarate, nickel citrate and copper glutamate and precious metal compound gold potassium tetrachloride in a hydrothermal reactor under the action of an emulsifier dimethylaminoacrylate palmitate ammonium chloroacetate, drying reactive products to remove moisture, and firing in a muffle furnace at certain temperature, to obtain the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Ozone heterogeneous oxidation solid catalyst preparation method
InactiveCN107008395AImprove adsorption capacityBurning at high temperature makes the organic matter completely carbonized and strong adsorptionCatalyst carriersOther chemical processesUltrasound - actionCalcite
The invention relates to an ozone heterogeneous oxidation solid catalyst preparation method, belonging to the technical field of environmental protection and chemical catalysts. The ozone heterogeneous oxidation solid catalyst preparation method comprises the following steps: carrying out pore-expanded modification on porous materials such as gamma-aluminum oxide, barite, activated carbon, carnallite, dolomite and calcite which serve as carriers by using lithium hypochlorite and bis(acetylacetone) beryllium, then adding ditetradecyl dimethyl ammonium chloride serving as a surface active agent to carry out surface activating treatment under the action of ultrasonic waves, and causing ultrasound surface activated carriers to generate hydrothermal reaction with borax and potassium sulfate which serve as composite mineralizers, rare earth organo metallic compounds such as cerium(IV)-2,2,6,6-tetramethylheptanedionate, tris(3-trifluoroacetyl-D-camphor)praseodymium(III), thulium(III) trifluoromethanesulfonate, and tris(trifluoromethane sulfimide) ytterbium which serve as catalytic activity assistant precursors, common transition organometallic compounds such as manganese lysine and cupric glutamate and precious metal compounds such as dithiocyanide potassium silver (I) and hexanitroso rhodium trisodium which serve as catalytic activity center components under the action of 2, 6-bis(diethylamino methyl)-4-nonylphenol-epichlorohydrin quaternary ammonium salt serving as an emulsifying agent in a hydrothermal reaction kettle, drying a reaction product to remove moisture, and firing in a muffle furnace at a certain temperature to obtain the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Method for preparing ozone heterogeneous phase oxidized solid catalyst
InactiveCN107042115AEvenly dopedImprove adsorption capacityCatalyst carriersOther chemical processesUltrasound - actionBrucite
The invention relates to a method for preparing an ozone heterogeneous phase oxidized solid catalyst, which belongs to the technical fields of environmental protection and a chemical catalyst. The preparation method comprises the following steps: diatom pure, kyanite, brucite, serpentine, aluminium hydroxide and a celestine porous material are taken as carriers, the carriers are subjected to hole-reaming modification through lithium hypochlorite and dis(acetylacetone)beryllium, a surfactant dodecyl dimethyl hydroxyethyl ammonium chloride is added, surface activation treatment is carried out under supersonic wave effect, then the carriers after ultrasonic surface activation are subjected to a hydro-thermal reaction with a composite mineralizer borax and potassium sulfate, a catalytic activity auxiliary agent predecessor tri(hexafluoroacetylacetone)yttrium (III)dehydrate, tricyclopentadiene promethium, holmium nitrate decahydrate, a tri(strifluoromethanesulfonimidate)ytterbium rare earth metallorganic compound, a catalytic activity central composite predecessor common transition metallorganic compound pyruvate iso-nicotinoylhydrazone vanadium, cobalt gluconate and a precious metal compound tetraammine dichloropalladium, tetrachloro iridium hydrate in a hydro-thermal reaction vessel under effect of an emulsifier dioctadecalkyl ammonium bromide, the reaction products are dried to remove the moisture, in a muffle furnace, and the product is calcinated at certain temperature to obtain the ozone heterogeneous oxidized solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107051486AImprove adsorption capacityBurning at high temperature makes the organic matter completely carbonized and strong adsorptionCatalyst carriersOther chemical processesUltrasound - actionLithium hypochlorite
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst and belongs to the technical field of environment protection and chemical catalysts. The preparation method includes: using a diatom purifier, kyanite, aluminum hydroxide, celestite, fly ash and coal gangue as the carriers, using lithium hypochlorite and bis(acetylacetone) beryllium to perform pore expansion on the carriers, adding chlorinated dodecyl dimethyl hydroxyethyl ammonium to activate the carriers under ultrasonic action, allowing the carriers to have hydrothermal reaction with compound mineralizer including borax and potassium sulfate, catalytic activity promoter precursors including tri(hexafluoroacetylacetone) yttrium(III) dihydrate, acetylacetone samarium, tri(4, 4, 4-trifluoro-1-(2-thiophene)-1, 3-butanedione) europium and tri(6, 6, 7, 7, 8, 8, 8-heptafluoro-2, 2-dimethyl-3, 5-octene diketone) dysprosium(III), and catalytic activity central precursors including pyruvic acid isonicotinoyl hydrazone vanadium, nickel citrate, cupric glutamate and dipotassium hexachloroosmate in a hydrothermal reaction kettle under the effect of N-dodecyl dimethyl-N'-dodecyl-dimethyl-2-hydroxypropyl ammonium dichloride, drying to remove moisture, and burning in a muffle furnace to obtain the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107159198AEvenly dopedImprove adsorption capacityOther chemical processesCatalyst activation/preparationUltrasound - actionLithium hypochlorite
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst, and belongs to the technical field of environmentally-friendly and chemical catalysts. The preparation method comprises the following steps: by taking medical stone, wollastonite, dolomite, calcite, nitratine and dolomite as carriers, reaming the carriers with lithium hypochlorite and bis(acetylacetone) beryllium, then adding myristyl tributyl ammonia chloride serving as a surfactant for activation treatment under ultrasonic action, putting the carriers into a hydrothermal reaction kettle for hydrothermal reaction together with borax and potassium sulfate which serve as composite mineralizers, isopropyl scandium oxide (III), tri(4,4,4-trifluoro-1-(2-thiophene)-1,3-butanedione) europium, thulium trifluoromethane sulfonate (III) and lutetium carbonate hydrate which serve as catalytic active auxiliary precursors, a dicyclopentadienyl titanium cyclo-replaced salicylic acid complex, zinc lactate and a catechol ethanediamine tungsten complex which serve as catalytic active center precursors and iridium tetrachloride under the action of dimethyl hexadecyl ethyl sulfate serving as an emulsifier, drying a reaction product to remove water, and firing the reaction product in a muffle furnace, thus obtaining the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Method for preparing ozone heterogeneous oxidation solid catalysts
InactiveCN107051445AEvenly dopedImprove adsorption capacityCatalyst carriersOther chemical processesUltrasound - actionCerium
The invention relates to a method for preparing ozone heterogeneous oxidation solid catalysts, and belongs to the technical field of environmental protection and chemical catalysts. The method includes carrying out pore expansion and modification on carriers which are attapulgite, diopside, sepiolite, baryta feldspar, perlite and albite porous materials by the aid of lithium hypochlorite and bis (acetylacetone) beryllium; adding chlorinated bis-eicosyl dimethyl ammonium into the carriers and carrying surface activation treatment on the carriers under the effects of ultrasonic waves; carrying out hydrothermal reaction on the carriers which are subjected to ultrasonic surface activation, borax, potassium sulfate, tetra-(2, 2, 6, 6-tetramethyl-3, 5-heptadione acid) cerium (IV), tri-[4, 4, 4-trifluoro-1-(2-thiophene)-1, 3-butanedione] europium, tri-(6, 6, 7, 7, 8, 8, 8-heptafluoro-2, 2-dimethyl-3, 5-octene diketone) dysprosium (III), tri-[N, N-bis (trimethylsilane) amine] erbium rare earth metal organic compounds, manganese lysine, ammonium zirconium carbonate, dichlorotetraaminopalladium and tetrachloro iridium dihydrate in hydrothermal reaction kettles under the effect of nitrophenol benzyldimethyl ammonium bromide which is an emulsifier; drying reaction products to remove moisture; burning the reaction products in muffle furnaces at the certain temperatures to obtain the ozone heterogeneous oxidation solid catalysts. The chlorinated bis-eicosyl dimethyl ammonium is used as a catalyst. The borax and the potassium sulfate are used as composite mineralizers, the tetra-(2, 2, 6, 6-tetramethyl-3, 5-heptadione acid) cerium (IV), the tri-[4, 4, 4-trifluoro-1-(2-thiophene)-1, 3-butanedione] europium, the tri-(6, 6, 7, 7, 8, 8, 8-heptafluoro-2, 2-dimethyl-3, 5-octene diketone) dysprosium (III) and the tri-[N, N-bis (trimethylsilane) amine] erbium rare earth metal organic compounds are used as catalytic active auxiliary precursors, the manganese lysine, the ammonium zirconium carbonate, the dichlorotetraaminopalladium and the tetrachloro iridium dihydrate are used as catalytic active central components, the manganese lysine is a common transition metal organic compound, and the dichlorotetraaminopalladium and tetrachloro iridium dihydrate are precious metal compounds.
Owner:SICHUAN NORMAL UNIVERSITY
Cleansing flushing material for sleet melting and snow removing
InactiveCN1687292AReduce corrosionPromote growthOther chemical processesOrganic non-surface-active detergent compositionsLiquid productAluminium chloride
Owner:杨毅男
Ozone heterogeneous oxidation solid catalyst preparation method
InactiveCN107051476ACarbonization strengthens microporous structureCarbonization strengthens the microporous structure by hydrothermal reactionCatalyst carriersWater contaminantsUltrasound - actionRare earth metal compounds
The invention belongs to the technical field of environment protection and chemical catalysts and relates to an ozone heterogeneous oxidation solid catalyst preparation method. The preparation method includes: taking porous mineral materials including activated carbon, carnallite, potassium feldspar, camsellite, illite and ulexite as carriers; subjecting the carriers to lithium hypochlorite and bis(acetylacetone)beryllium broaching modification; adding surfactant methacryloyloxyethyltrimethyl ammonium chloride for surface activation under the action of ultrasonic waves; subjecting the carriers subjected to ultrasonic surface activation to hydrothermal reaction, with a complex mineralizer composed of borax and potassium sulfate, catalytic activity auxiliary agents including samarium acetylacetonate, tri(4,4,4-trifuloro-1-(2-thiophene)-1,3-butanedione)europium, terbium acetate hydrate and tris[N,N-bis(trimethylsilane)amine]erbium rare earth metal compounds and catalytic activity central components including cobalt gluconate, L-aspartic acid molybdenum, ammonium zirconium carbonate, cis-dichlorodiamineplatinum transition metals, in a hydrothermal reactor under the action of stearamidopropyl dimethylamine lactate serving as an emulsifying agent; drying reaction products to remove moisture, and firing in a muffle furnace at a certain temperature to obtain an ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com