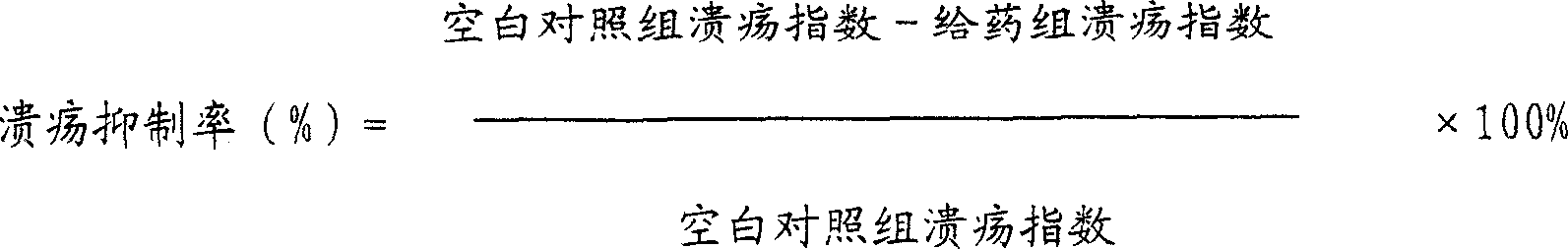
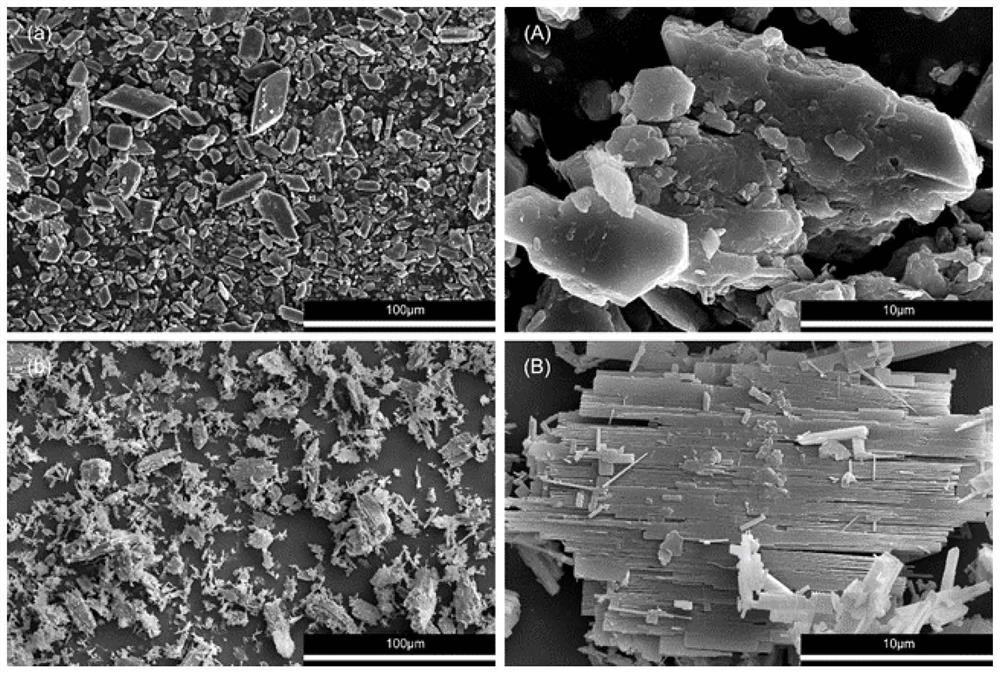
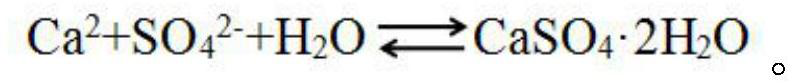Patents
Literature
49 results about "Glauberite" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Glauberite is a monoclinic sodium calcium sulfate mineral with the formula Na₂Ca(SO₄)₂. It was first described in 1808 for material from the El Castellar Mine, Villarrubia de Santiago, Toledo, Castile-La Mancha, Spain. It was named for the extracted Glauber's salts after the German alchemist Johann Rudolf Glauber (1604–1668).
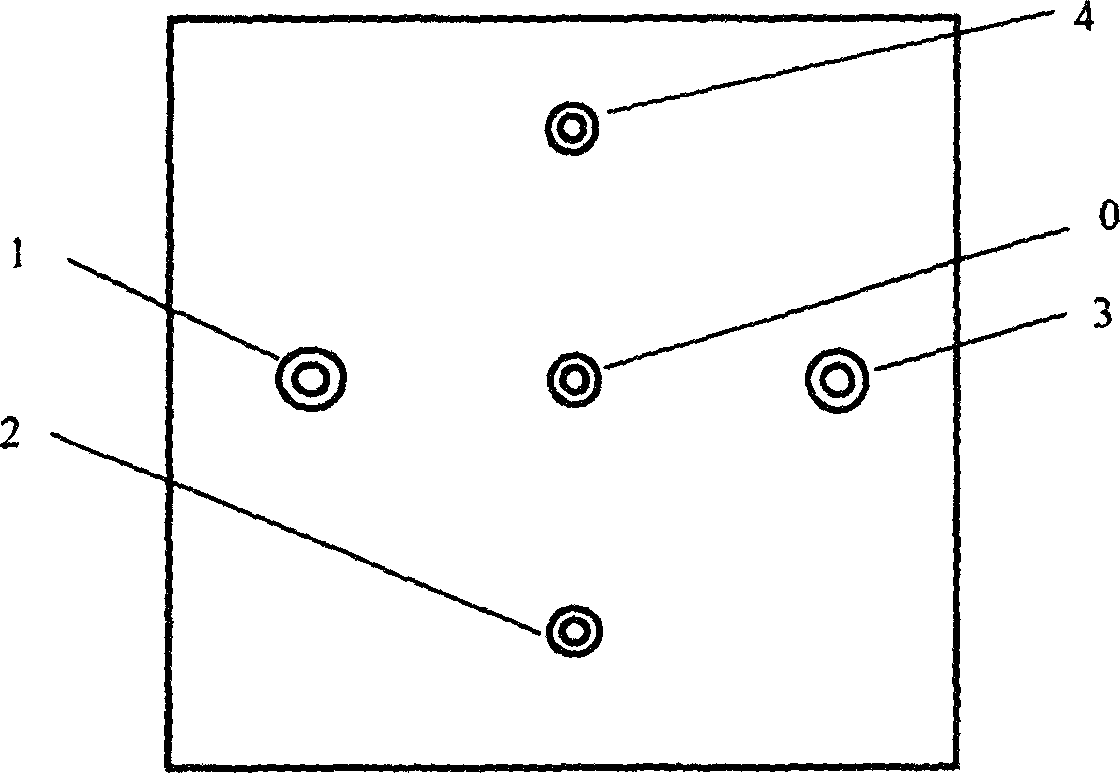
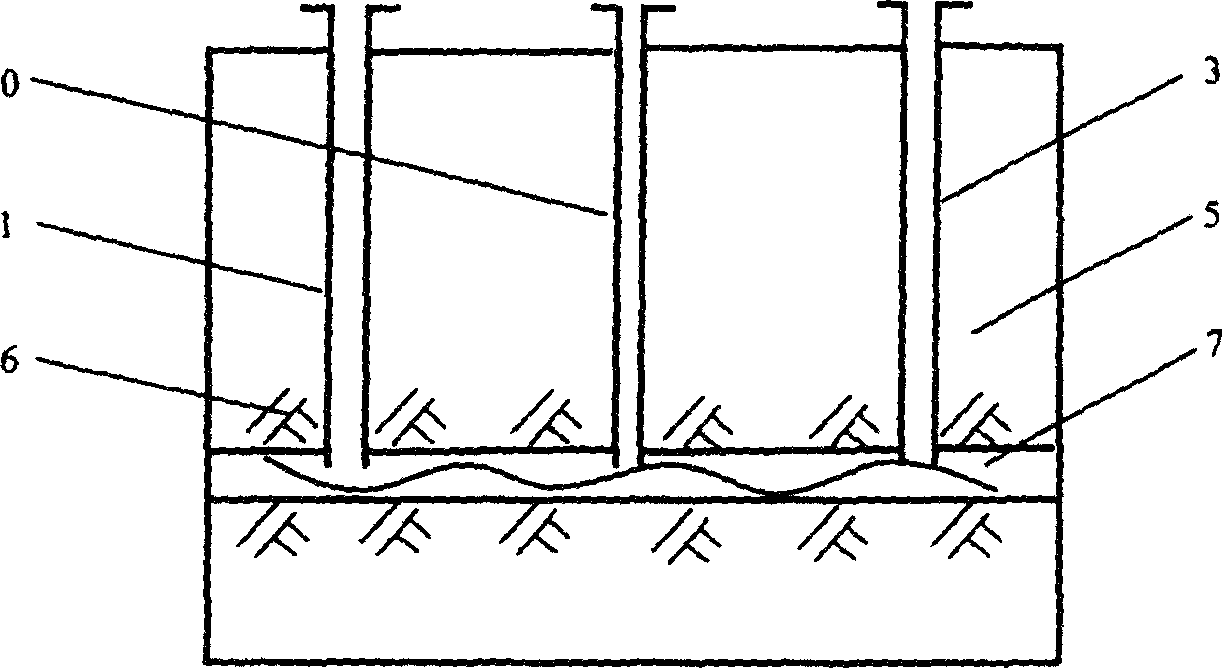
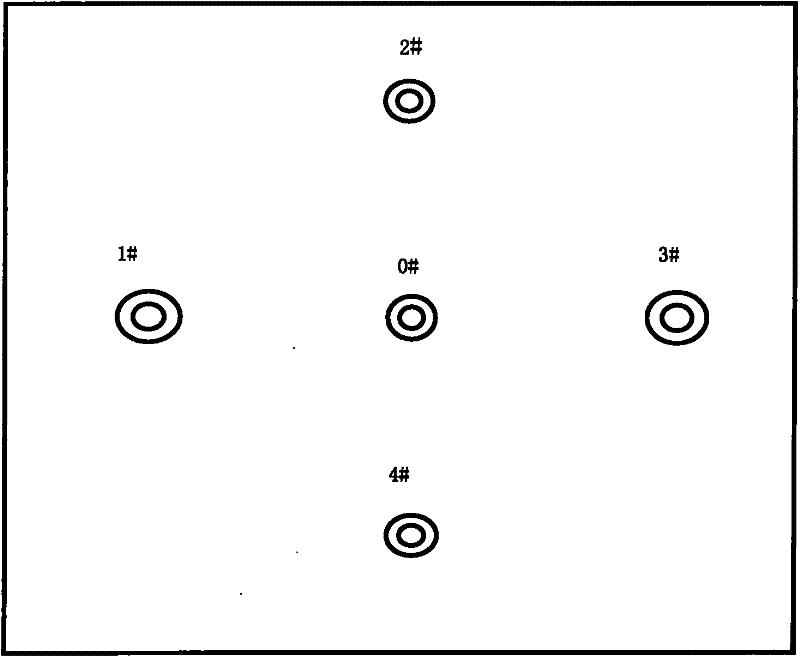
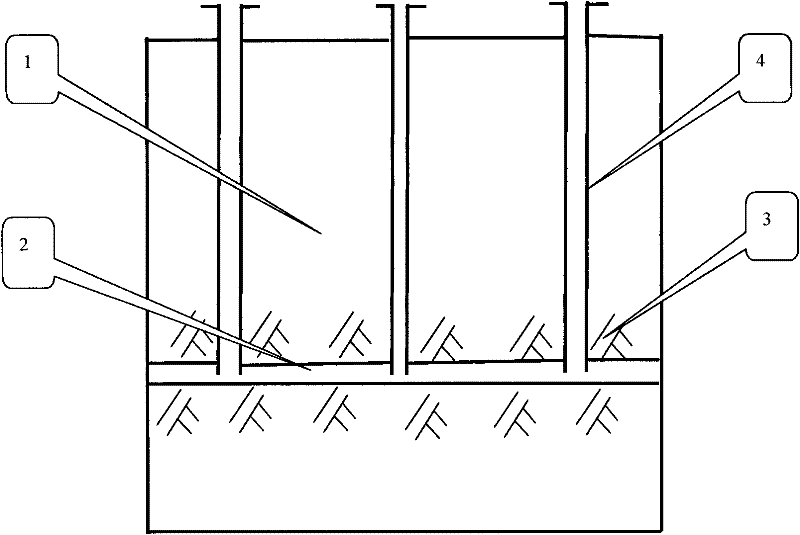
Group-well fracture pressure steeping control aqueous fusion exploitation method of glauberite bal
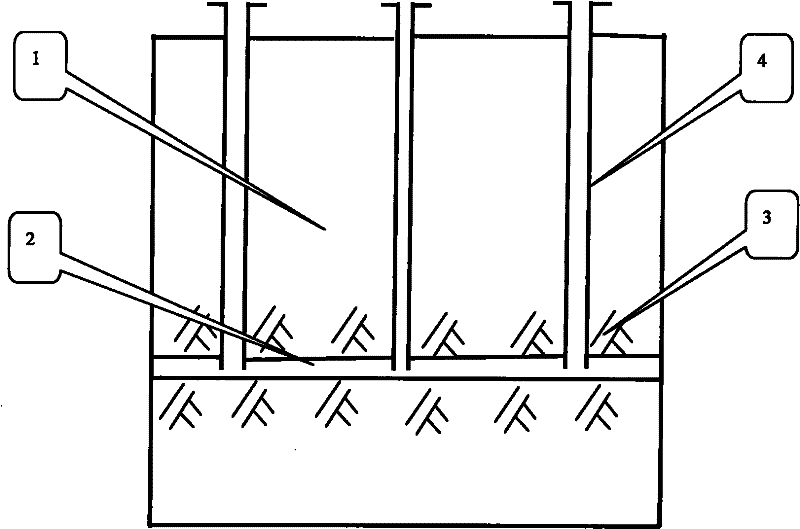
The well group cracking, pressure soaking and water dissolution controlling process for exploiting glauberite ore belongs to the field of exploiting refractory salt mineral deposit. Available water dissolution process has demerits of great labor strength, high cost, dangerous operation environment, low recovery ratio and environment pollution. The exploiting process of the present invention includes arranging several communicated wells, injecting high pressure water to soak for long period, recovering the brine water of certain concentration, re-injecting water and recovering brine water, and so on. The present invention has the features of well group cracking, pressure soaking and controlled water dissolution, and is one ideal scheme of exploiting refractory salt mineral deposit.
Owner:TAIYUAN UNIV OF TECH
Application of calcium-chloride-containing aqueous solution in conversion exploitation of glauberite resource and conversion exploitation method of glauberite resource
ActiveCN102936021AIncrease profitBreak the status quo that cannot be mined and utilizedCalcium/strontium/barium sulfatesAlkali metal sulfites/sulfatesSulfateLower grade
The invention relates to application of calcium-chloride-containing aqueous solution in conversion exploitation of a glauberite resource and a conversion exploitation method of the glauberite resource. The conversion exploitation method comprises the following steps: pouring the calcium-chloride-containing aqueous solution into a rock salt exploitation mine or a low-grade glauberite mine; dissolving and decomposing the glauberite into sparingly-soluble calcium sulfate and soluble sodium sulfate under a calcium chloride solution environment, wherein the calcium sulfate is precipitated in the mine, and the sodium sulfate is dissolved in the water; reacting all dissolved sodium sulfates react with calcium chloride in the calcium-chloride-containing aqueous solution, so as to generate the sparingly-soluble calcium sulfate, precipitate sparingly-soluble calcium sulfate in the bottom of the mine, and synchronously generate soluble sodium chloride and dissolve soluble sodium chloride in the water; and finally exploiting the mineral halide after dissolution for a period of time. According to the conversion exploitation method, the calcium-chloride-containing aqueous solution is adopted in the rock salt exploitation based on a conventional rock salt solution exploitation method, and all the associate glauberite resources in the rock salt can be exploited and utilized, so that the comprehensive utilization ratio of the rock salt resource can be increased.
Owner:江苏苏盐井神股份有限公司 +1
In-situ heat injection steeping control aqueous dissolution exploitation method of glauberite ore
Owner:TAIYUAN UNIV OF TECH
Chinese medicine preparation and ointment for treating scald and burn
InactiveCN1850203AGood curative effectNo pigmentationHydroxy compound active ingredientsDermatological disorderDiseaseExternal application
The present invention relates to a Chinese medicine ointment preparation for external application for effectively curing burn and scald. It is made up by using Chinese medicinal materials of borneol, glauberite, talcum powder, phellodendron bark and sesame oil through a certain preparation process.
Owner:贾曼红
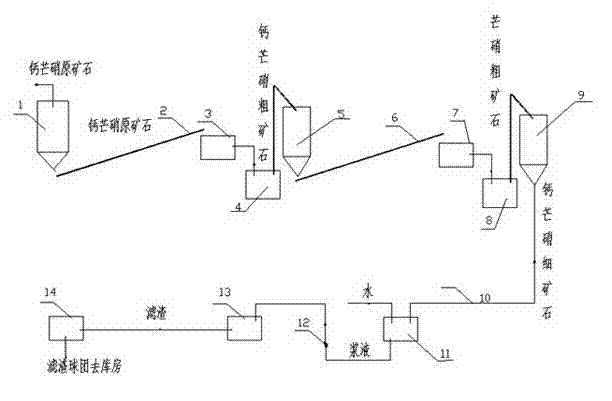
Method for purifying glauberite to prepare dihydrate gypsum
ActiveCN102963920AFull recoveryReduce mining costsCalcium/strontium/barium sulfatesCalcium Sulfate DihydrateMineralogy
The invention discloses a method for purifying glauberite to prepare dihydrate gypsum, which comprises the following steps of: 1) breaking glauberite by breaking equipment to obtain glauberite powder; 2) feeding the glauberite powder into a leaching stirring barrel and mixing with infiltration water; 3) feeding the mixture into mechanical filtering equipment capable of realizing quantitative washing to obtain filter residues of which the sodium sulfate content is lower than 2% and the water content is lower than 30%; and 4) making the filter residues into a pellet material by a pelletizer, wherein the material is a finished product of dihydrate gypsum. According to the invention, the infiltrated suspension is processed by a filter with a washing function, and the filtrate after filtration can be directly used as a raw material for producing anhydrous sodium sulfate since the concentration of sodium sulfate meets the requirements of the production technology of anhydrous sodium sulfate; the washing liquid containing sodium sulfate can be recovered as infiltration water; calcium sulphate dihydrate products reaching the national standard can be prepared by the method; and the method has strong operability and good market prospects, and the production cost is low.
Owner:SICHUAN TONGQING NANFENG
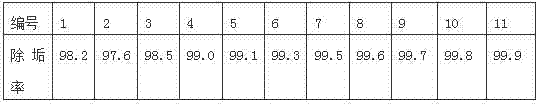
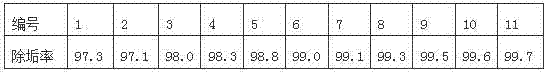
Glauberite pipeline scale descaling agent and application thereof
ActiveCN102505122AReduce corrosionLittle impact on mechanical propertiesEthylene diamineDissolution reaction
The invention discloses a glauberite pipeline scale descaling agent and application thereof. The descaling agent comprises the following components in percentage by weight: 10-25% of NaOH, 5-15% of Na2S2O35H2O, and 0.05-0.5% of sodium polyacrylate. The descaling agent provided by the invention can be used for transforming insoluble CaSO4 into soluble Ca(OH)2 and Na2SO4; a proper amount of Na2S2O35H2O serving as a complexing agent is added and complexed with Ca<2+> in Ca(OH)2 to form a more stable complex, a new precipitate is prevented from generating, and a dissolution reaction is promoted to happen; a proper amount of EDTA (ethylene diamine tetraacetic acid) and a proper amount of polyacrylamide are added to realize a more remarkable scale inhibition effect; and acid is not contained in the components of the descaling agent, and no acid is generated in the descaling process, so that the descaling agent has small corrosive influence on a salt manufacturing pipeline and small influence on the mechanical performance of the salt manufacturing pipeline; and after being subjected to simple sediment treatment, a residual solution obtained after filtering can be returned to a bittern pre-treatment system, so that the alkaline solution in the residual solution can be utilized, so that sewage is hardly drained, and the descaling agent is environmentally-friendly.
Owner:SICHUAN JIUDA SALT MFG CO LTD +2
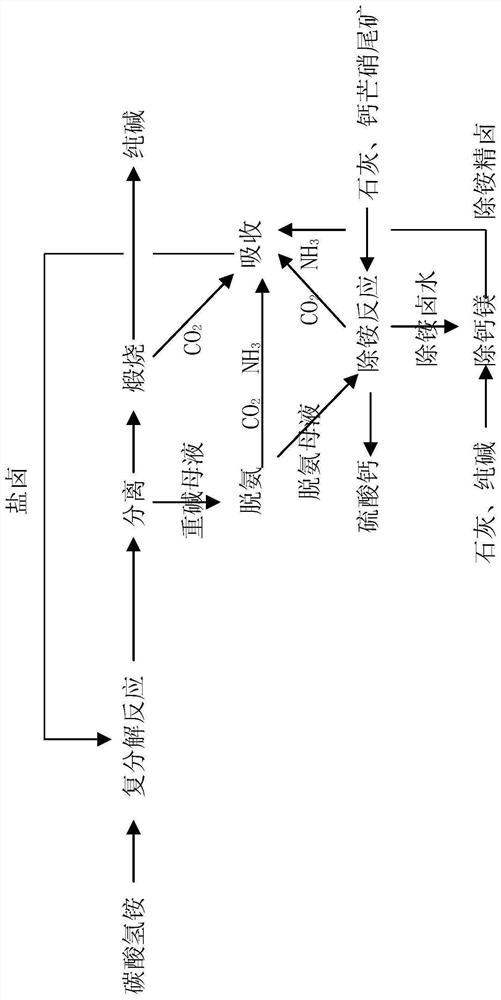
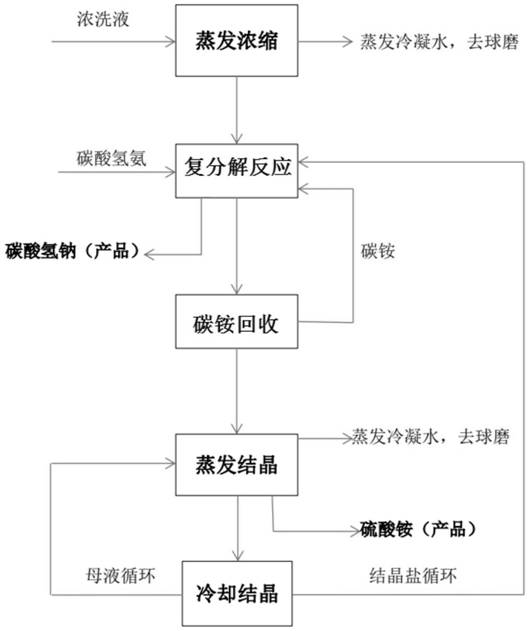
Process for co-production of sodium carbonate and calcium sulfate from ammonium bicarbonate and glauberite tailings
ActiveCN112723391AAvoid pollutionReasonable processCalcium/strontium/barium sulfatesCarbonate preparationSulfateEnvironmental engineering
The invention provides a process for co-production of sodium carbonate and calcium sulfate from ammonium bicarbonate and glauberite tailings. The process comprises the following steps: 1) taking ammonium bicarbonate and bittern in the step 7) as raw materials, carrying out double decomposition reaction, and separating to obtain solid sodium bicarbonate, a sodium bicarbonate mother liquor and a small amount of sodium sulfate; 2) calcining the solid sodium bicarbonate to obtain a sodium carbonate product and carbon dioxide; 3) preheating the heavy alkali mother liquor to remove ammonium bicarbonate and ammonium carbonate at high temperature to obtain ammonia, carbon dioxide and deamination mother liquor; 4) reacting the deamination mother liquor with lime and glauberite tailings to obtain ammonia, carbon dioxide and ammonium-removed brine; 5) reacting the ammonium-removed brine with lime and sodium carbonate to obtain ammonium-removed refined brine and calcium-magnesium mud; 6) reacting the ammonium-removed refined brine with the carbon dioxide in the step 2), the ammonia and carbon dioxide in the step 3) and the ammonia and carbon dioxide in the step 4) to obtain bittern; and 7) circulating the bittern to the metathesis reaction process in the step 1).
Owner:彭赛军 +5
Chinese medicinal embrocation for treating acnes
InactiveCN102416065AEasy dischargeProlonged local efficacyHydroxy compound active ingredientsAerosol deliverySide effectIrritation
The invention provides a Chinese medicinal embrocation for treating acnes. The Chinese medicinal embrocation is prepared from the following raw materials in part by weight: 80 parts of weeping forsythia, 80 parts of baical skullcap root, 150 parts of honeysuckle flower, 70 parts of red-rooted salvia, 65 parts of rhubarb, 120 parts of glauberite, 130 parts of catechu, 70 parts of calamine, 60 parts of divaricate saposhnikovia root, 60 parts of angelica dahurica, 120 parts of phellodendron, 70 parts of dried alum, 15 parts of borneol and 15 parts of peppermint oil. A preparation method comprises the following steps of: grinding the first 12 Chinese medicines into fine powder, sieving with a 200-mesh sieve, grinding the fine powder with the borneol and the peppermint oil uniformly, and packaging in separate bags to obtain a finished product. The Chinese medicinal embrocation is prepared from pure Chinese medicines, does not have toxic or side effect, meets chemical characters of skin, is favorable for exerting the physiological function of the skin, has the effects of treatment and skin protection, is low in irritation and easy to accept by patients and has a wide application prospect, and the drying and tension feelings of the skin are reduced in the using process.
Owner:曹莹莹
Industrial synthetic sulfur gypsum and preparation method thereof
ActiveCN107352824APromote crystallizationEasy to GrindCalcium/strontium/barium sulfatesAluminium oxide/hydroxide preparationWater productionSodium sulfate
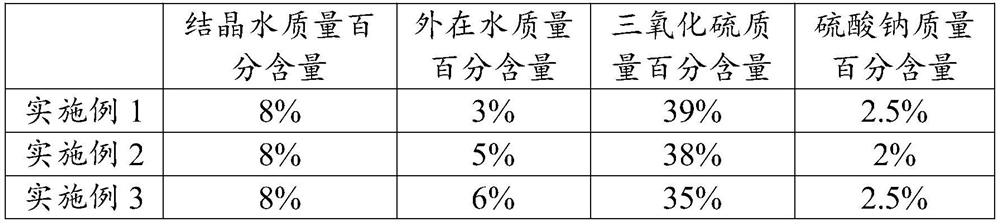
The invention discloses industrial synthetic sulfur gypsum and a preparation method thereof. The industrial synthetic sulfur gypsum is prepared from the following ingredients including 95 to 99 percent of industrial dihydrate gypsum and 1 to 5 percent of accelerated crystallizing agents. The preparation method comprises the following steps of S1, taking and mixing fluorgypsum, titanium gypsum and other industrial gypsum; performing uniform stirring; S2, in the mixed industrial gypsum, adding sodium sulfate in glauberite gypsum for reaction; controlling the temperature to be lower than 60 DEG C; performing crystallization reaction on the industrial gypsum; S3, taking crystallization gypsum in the S2 for secondary crushing stirring; crushing the gypsum into powder. The fluorgypsum and other industrial dihydrate gypsum are used and mixed to be produced into the sulfur gypsum; the sodium sulfate in glauberite gypsum is matched, so that the crystallization of the dihydrate gypsum can be accelerated; the external water production cost is reduced, so that the industrial production effect is achieved; the technical indexes of the obtained gypsum are shown as follows: the sulfur trioxide content can reach 35 percent or higher; the crystallization water content reaches 10 percent or higher; the external water content is less than 10 percent, so that good economic benefits and social benefits are obtained.
Owner:湖南仁义环保建材科技有限公司
Stomach-power Chinese medicine preparation and preparing method
InactiveCN1742820ARaise quality standardsPromote environmental protectionDigestive systemCapsule deliveryDiseaseHelenium
The present invention relates to a gastric power Chinese medicine preparation ''Liuwei Anxiao preparation'' for effectively curing the diseases of epigastralgia, stomach distention, dyspepsia, constipation and dysmenorrhea. It is made up by using inula helenium, rhubarb, kaempferia root, calcined glauberite, chebule and jianhua flower as raw material through a certain preparation process.
Owner:JIANGXI YINTAO PHARMACEUTICAL CO LTD
Downward parallel middle-deep hole sublevel fill stoping mining method for steep and medium-thick hard-to-mine ore body
PendingCN110985114AImprove early strengthHigh compressive strengthMaterial fill-upSlagMining engineering
The invention provides a downward parallel middle-deep hole sublevel fill stoping mining method for a steep and medium-thick hard-to-mine ore body, and belongs to the technical field of mining. The ore body is divided into a plurality of sublevels according to the middle sublevel, stope rooms and stope pillars are divided in the ore body direction, the middle sublevel is divided in the inclined direction of the ore body, sublevel rock drilling roadways are arranged along veins, artificial false roofs and false bottoms are constructed in the sublevel rock drilling roadways in advance, and middle-deep hole stoping ore blocks are adopted from top to bottom. After the stope rooms are mined, the stope rooms are filled with filling materials. After the stope pillars are mined, the stope pillarsare filled with full tailings and / or barren rock powder. The content of particles with the particle size being less than or equal to 30[mu]m in the full tailings for the filling materials accounts for75-77%; the content of the particles with the particle size being less than or equal to 35[mu]m in slag accounts for 86-88%; and an activator is prepared from glauberite and green vitriol with a weight ratio of 1 to (0.5-0.7). According to the downward parallel middle-deep hole sublevel fill stoping mining method, safe, efficient and low-cost mining of the ore body can be realized, continuous mining in an area can be realized, and the sublevel fill stoping mining method is mainly suitable for a gold mine with an inclination of 50-90 degrees and a horizontal thickness of 4-10 m.
Owner:中国黄金集团石湖矿业有限公司
Circulating green production method for salt, alkali and calcium on and underneath ground
PendingCN111115674AImprove recovery rateLow in calciumCalcium/strontium/barium sulfatesAlkali metal chloridesResource recoveryWastewater
The invention relates to the technical field of salt chemical engineering resource recycling, and particularly discloses a circulating green production method for salt, alkali and calcium on and underneath the ground. According to the method, calcium chloride in ammonia-alkali waste liquid and sodium sulfate and glauberite in a mirabilite type rock salt resource are mixed; waste liquid is treatedon the ground in stages, meanwhile, waste resources are reasonably utilized, and all resources such as Ca<2+>, Cl<->, SO4<2-> and Na<+> are recycled; and the problem of pollution caused by discharge of wastewater from a traditional ammonia-alkali process is solved, and cyclic utilization and green production of the resources are achieved.
Owner:JIANGXI JINGHAO SALINIZATION
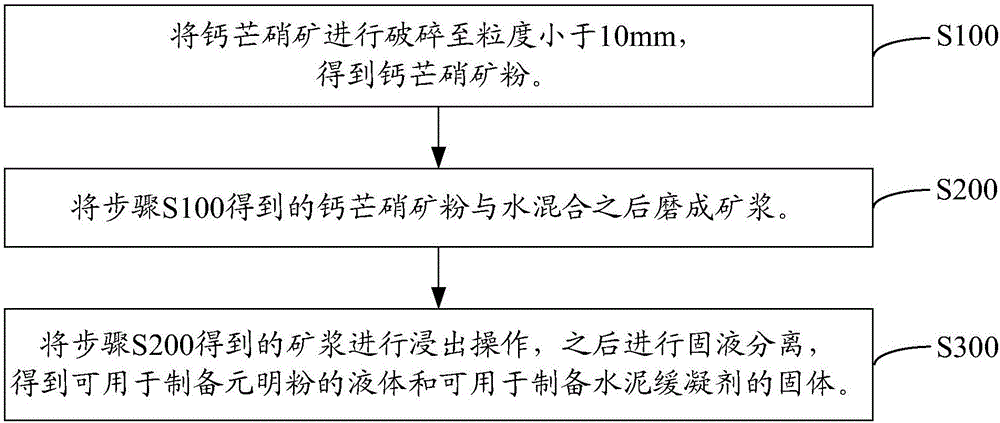
Utilization method of glauberite ore
InactiveCN106745084ARealize resource utilizationSuitable for industrial applicationsCalcium/strontium/barium sulfatesAlkali metal sulfites/sulfatesResource utilizationSulfate
The invention relates to a utilization method of glauberite ore. The method includes the steps of: crushing glauberite ore to a particle size of less than 10mm to obtain glauberite ore powder; mixing the glauberite ore powder with water, and then performing grinding into ore pulp; and subjecting the ore pulp to leaching, and then conducting solid-liquid separation, thus obtaining a liquid for preparation of anhydrous sodium sulphate and a solid for preparation of a cement retarder. The utilization method of glauberite ore provided by the invention finally can acquire the liquid for preparation of anhydrous sodium sulphate and the solid for preparation of a cement retarder, recovers sodium sulfate in glauberite ore, and also achieves comprehensive utilization of calcium sulfate in glauberite ore. Therefore, the method can realize overall resource utilization of glauberite ore, and also can solve land occupation of glauberite ore and waste residue formed thereby, and the possibly caused debris flow, landslides and other secondary geological disasters and environmental pollution problems, thus being suitable for industrial application.
Owner:ZHENGZHOU MINERALS COMPOSITIVE UTILIZATION RES INST CHINESE GEOLOGICAL ACAD
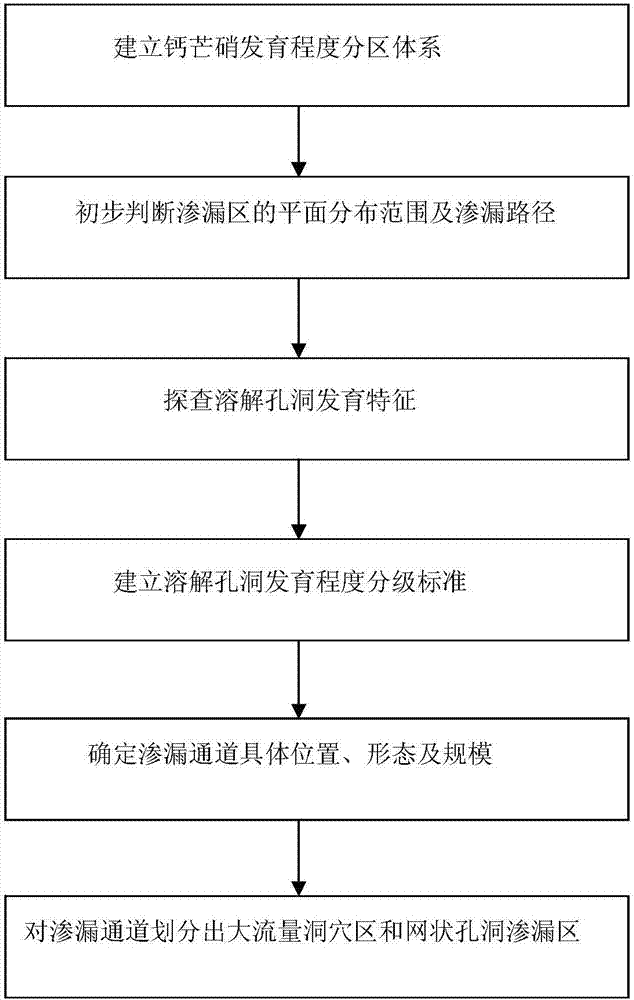
Fast evaluation method of dissolution and leakage passages in strata rich in glauberite
ActiveCN107390290AQuickly find outPinpointGeological measurementsHorizontal distributionGeological survey
The invention relates to a technique of geological engineering exploration, and discloses a fast evaluation method of dissolution and leakage passages in strata rich in glauberite. The method is capable of quickly and precisely determining the horizontal distribution ranges, developing depth, scale and the like of the glauberite dissolution and leakage passages and providing reliable basic information for engineering processing. The method includes the steps of a, constructing a glauberite developing degree partition system; b, primarily judging the horizontal distribution ranges and leakage paths of leakage areas; c, detecting developing features of dissolution holes; d, setting a developing degree grading standard for the dissolution holes; e, determining the specific positions, shapes and scale of the leakage passages; f, dividing the leakage passages into a mass-flow hole area and a net-shaped hole leakage area. The fast evaluation method of the dissolution and leakage passages in the strata rich in glauberite is applicable to fast and precise reconnaissance and evaluation of glauberite leakage passages.
Owner:POWERCHINA CHENGDU ENG
Low-carbon active ligand and low-carbon slag powder cementitious material
InactiveCN101698575ASolve air pollutionExcellent engineering technical indicatorsCement productionAluminiumGlauberite
The invention provides a low-carbon active ligand and a slag powder cementitious material thereof. The invention is characterized in that bauxite, glauberite, fluorite, gypsum, garnets and a plurality of ores are ground and prepared into low-carbon negative and positive ligands respectively; the negative and positive ligands are ground, mixed and prepared into the low-carbon active ligand; the low-carbon active ligand is added to slag; and a low-carbon slag powder cementitious material used to replace cement is obtained after homogenization. As no calcination process is adopted, the problem of atmospheric pollution during cement production is solved (because calcination is not needed); the slag (steel slag, water granulated slag, fly ash and the like) can be utilized well; mineral resources (without limestone) can be protected; and energy can be saved (only 'two-grinding and one-calcination' needs to be changed into 'one grinding'). Moreover, the slag powder cementitious material has good physical performance indexes and can be widely applied in roads, bridges, buildings, cement precast parts and other concrete construction.
Owner:崔传鹏 +1
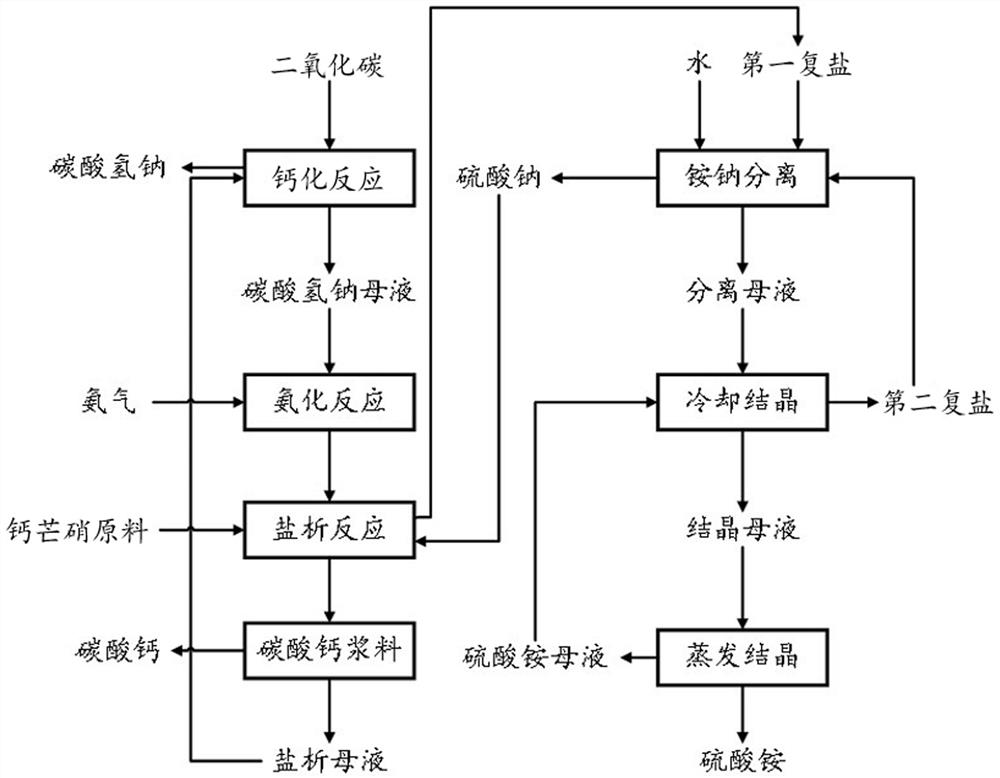
Method for preparing sodium bicarbonate and co-producing ammonium sulfate and calcium carbonate from glauberite
ActiveCN113896219AReduce production processRealize high-value conversionCalcium/strontium/barium carbonatesAmmonium sulfatesSodium bicarbonateSodium sulfate
The invention provides a method for preparing sodium bicarbonate and co-producing ammonium sulfate and calcium carbonate from glauberite, which specifically comprises the following steps: (1) introducing carbon dioxide into salting-out mother liquor to carry out calcification reaction, and carrying out solid-liquid separation to obtain sodium bicarbonate and sodium bicarbonate mother liquor; (2) introducing ammonia gas into the sodium bicarbonate mother liquor for ammoniation reaction to obtain ammoniated mother liquor; (3) mixing the glauberite raw material, sodium sulfate and the ammoniated mother liquor, carrying out salting-out reaction, and carrying out solid-liquid separation to obtain first double salt, calcium carbonate and salting-out mother liquor; (4) carrying out reaction on the mixed double salt and water, and carrying out solid-liquid separation to obtain sodium sulfate and separated mother liquor; (5) carrying out cooling crystallization on the mixed ammonium sulfate mother liquor and the separation mother liquor, and carrying out solid-liquid separation to obtain second double salt and crystallization mother liquor; and (6) carrying out evaporative crystallization on the crystallization mother liquor, and carrying out solid-liquid separation to obtain ammonium sulfate and ammonium sulfate mother liquor. According to the method, sodium bicarbonate is prepared from glauberite as a raw material, high-valued conversion of sodium sulfate and calcium sulfate is achieved, and the problem of stockpiling of glauberite tailings is solved.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI +1
Calcium sulfate gypsum and preparation method thereof
PendingCN111875269AShort reaction timeControl prevents switchingCement productionSulfateEnvironmental engineering
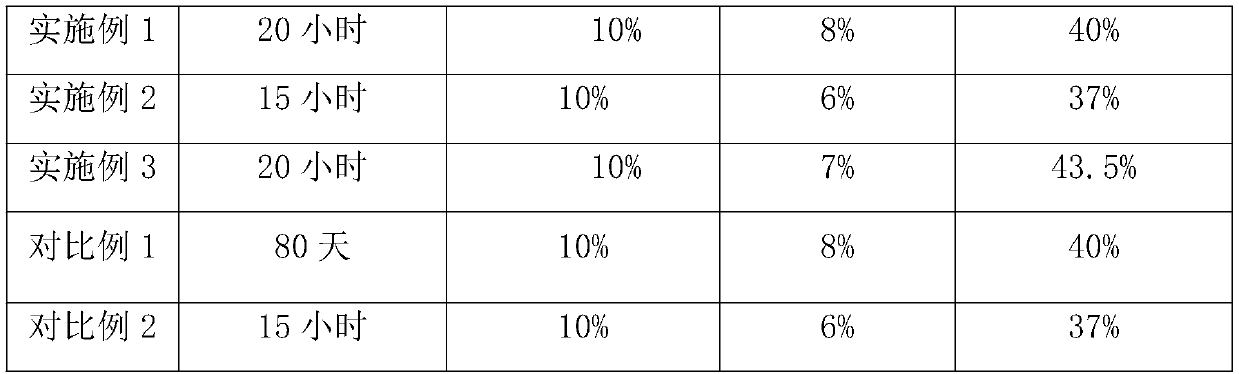
The invention provides calcium sulfate gypsum and a preparation method thereof, and belongs to the technical field of waste recycling and cement. The calcium sulfate gypsum provided by the invention is prepared from the following raw materials: glauberite waste residues and dihydrate gypsum. According to the invention, the glauberite waste residue and dihydrate gypsum are used as raw materials toprepare the raw material calcium sulfate gypsum for industrial cement, so that the resource reutilization of the glauberite waste residue is realized. Meanwhile, the invention further provides a preparation method of the glauberite waste residues, a small amount of sodium sulfate in the glauberite waste residues is utilized, the reaction time of crystal water is greatly saved, the working efficiency is improved, the temperature is controlled to be 60 DEG C or below, and the crystal water can be effectively prevented from being converted into external water; therefore, the mass percentage of sulfur trioxide in the final calcium sulfate gypsum is more than 35%, the mass percentage of crystal water is more than 8%, and the mass percentage of external water is less than 10%. According to the method, good economic benefits and social benefits are brought while resource recycling is achieved.
Owner:汪文杰 +1
Efficient mirabilite gypsum flotation purification process
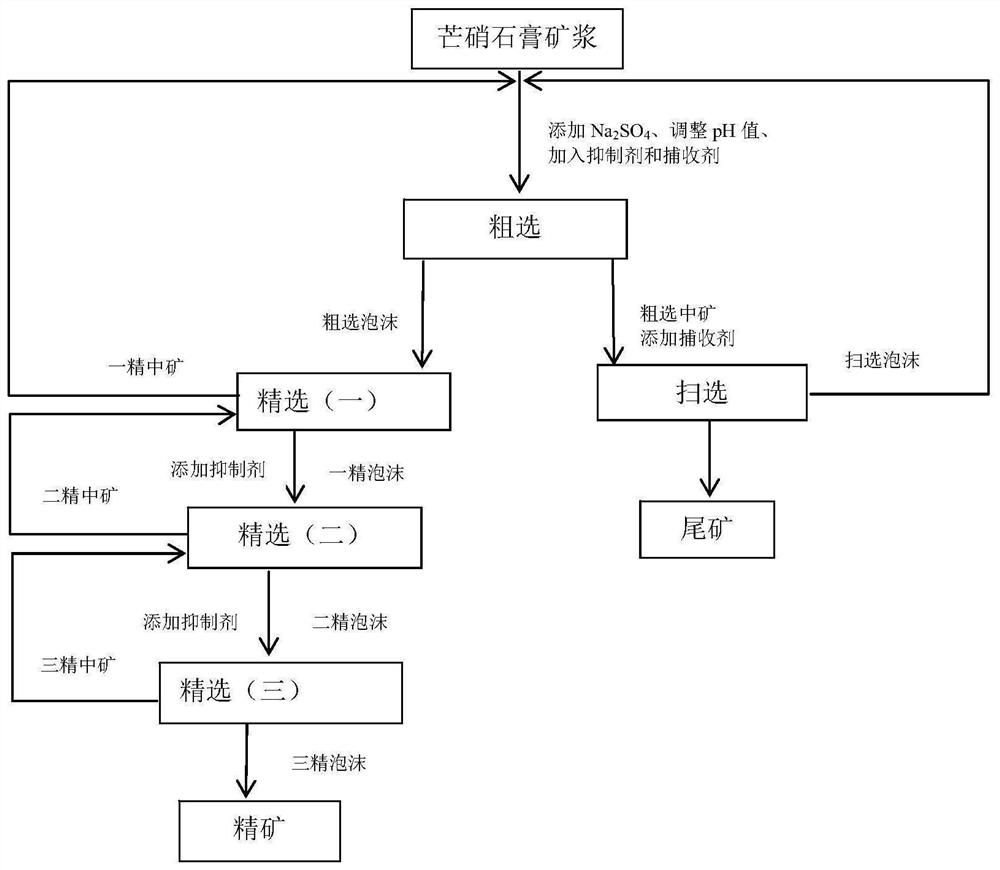
The invention relates to the technical field of mirabilite gypsum purification, and in particular, relates to an efficient mirabilite gypsum flotation purification process, wherein the process comprises the steps: firstly, preparing mirabilite gypsum slurry from glauberite, then sequentially carrying out roughing, scavenging and three times of concentration, and finally obtaining final concentrate from finally obtained foam through a dehydration system. The purity of CaSO4.2H2O in the concentrate product obtained through the process is high, the recovery rate is high, and maximum utilization of glauberite ore resources is achieved.
Owner:SICHUAN TONGQING NANFENG +1
Method for preparing ozone heterogeneous oxidation solid catalysts
InactiveCN107051511AImprove adsorption capacityImprove bindingCatalyst carriersWater contaminantsUltrasound - actionBorax
The invention relates to a method for preparing ozone heterogeneous oxidation solid catalysts, and belongs to the technical field of environmental protection and chemical catalysts. The method includes carrying out pore expansion and modification on carriers which are attapulgite, diopside, fluorite, glauberite, magnesia spinel and peridotite porous materials by the aid of lithium hypochlorite and bis (acetylacetone) beryllium; adding tri-lauryl ammonium chloride into the carriers and carrying surface activation treatment on the carriers under the effects of ultrasonic waves; carrying out hydrothermal reaction on the carriers which are subjected to ultrasonic surface activation, borax, potassium sulfate, tri (3-trifluoroacetyl-D-camphor) praseodymium (III), promethium tricyclic pentadiene, tri-(6, 6, 7, 7, 8, 8, 8-heptafluoro-2, 2-dimethyl-3, 5-octene diketone) dysprosium (III), thulium trifluoromethanesulfonate (III) rare earth metal organic compounds, ferrous fumarate, nickel citrate, gold potassium chloride and hexa-nitroso rhodium trisodium in hydrothermal reaction kettles under the effect of N-dimethyl dodecyl-N'-dodecyl-dimethyl-2-hydroxypropyl ammonium dichloride which is an emulsifier; drying reaction products to remove moisture; burning the reaction products in muffle furnaces at the certain temperatures to obtain the ozone heterogeneous oxidation solid catalysts. The tri-lauryl ammonium chloride is used as a surfactant. The borax and the potassium sulfate are used as composite mineralizers, the tri (3-trifluoroacetyl-D-camphor) praseodymium (III), the promethium tricyclic pentadiene, the tri-(6, 6, 7, 7, 8, 8, 8-heptafluoro-2, 2-dimethyl-3, 5-octene diketone) dysprosium (III) and the thulium trifluoromethanesulfonate (III) rare earth metal organic compounds are used as catalytic active auxiliary precursors, the ferrous fumarate, the nickel citrate, the gold potassium chloride and the hexa-nitroso rhodium trisodium are used as catalytic active central components, the ferrous fumarate is a common transition metal organic compound, and the gold potassium chloride and the hexa-nitroso rhodium trisodium are precious metal compounds.
Owner:SICHUAN NORMAL UNIVERSITY
A kind of industrial synthetic sulfur gypsum and preparation method thereof
ActiveCN107352824BPromote crystallizationEasy to GrindCalcium/strontium/barium sulfatesAluminium oxide/hydroxide preparationWater productionSodium sulfate
The invention discloses industrial synthetic sulfur gypsum and a preparation method thereof. The industrial synthetic sulfur gypsum is prepared from the following ingredients including 95 to 99 percent of industrial dihydrate gypsum and 1 to 5 percent of accelerated crystallizing agents. The preparation method comprises the following steps of S1, taking and mixing fluorgypsum, titanium gypsum and other industrial gypsum; performing uniform stirring; S2, in the mixed industrial gypsum, adding sodium sulfate in glauberite gypsum for reaction; controlling the temperature to be lower than 60 DEG C; performing crystallization reaction on the industrial gypsum; S3, taking crystallization gypsum in the S2 for secondary crushing stirring; crushing the gypsum into powder. The fluorgypsum and other industrial dihydrate gypsum are used and mixed to be produced into the sulfur gypsum; the sodium sulfate in glauberite gypsum is matched, so that the crystallization of the dihydrate gypsum can be accelerated; the external water production cost is reduced, so that the industrial production effect is achieved; the technical indexes of the obtained gypsum are shown as follows: the sulfur trioxide content can reach 35 percent or higher; the crystallization water content reaches 10 percent or higher; the external water content is less than 10 percent, so that good economic benefits and social benefits are obtained.
Owner:湖南仁义环保建材科技有限公司
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107008294AEvenly dopedImprove adsorption capacityCatalyst carriersOther chemical processesUltrasound - actionCerium
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst, belonging to the technical field of environment-friendly and chemical catalysts. The preparation method comprises the following steps: by taking gamma-aluminum oxide, baryte, fluorite, glauberite, brucite and serpentinite porous materials as carriers, performing pore expansion and modification to the carriers through lithium hypochlorite and beryllium bis(acetylacetonate), adding a surfactant dimethyl distearylammonium chloride and performing surface activation treatment under ultrasonic wave effect, then leading the ultrasonically surface-activated carriers to have hydrothermal reaction with a complex mineralizer: borax and potassium sulfate, catalytic activity assistant precursors, namely tetra(2,2,6,6-tetramethyl-3,5-heptanedionato)cerium(IV), samarium(iii) acetylacetonate dehydrate, terbium(III) acetate hydrate, and holmium oxalate rare earth metal organic compound, and catalytic active site component precursors, namely common transitional metal organic compound manganese lysinate and L-aspartic acid molybdenum and precious metal compound potassium hexachloroosmate and tetrachloroiridium dihydrate in a hydrothermal reactor under the action of an emulsifier lauryl bis(octadearyl) methyl ammonium bromide, drying reactive products to remove moisture, and firing in a muffle furnace at certain temperature, to obtain the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Deep development and utilization method for glauberite ore mining waste rocks
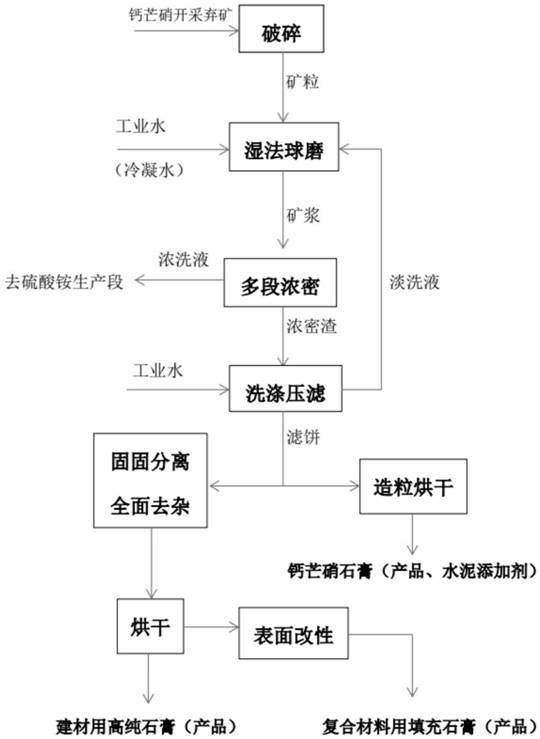
PendingCN113023754ASolve the problem of ineffective resource utilizationImprove conversion utilizationCarbonate preparationAmmonia compoundsMining engineeringSlurry
The invention discloses a deep development and utilization method for glauberite ore mining waste rocks, belongs to the technical field of glauberite ore resource utilization, and aims to provide a deep development and utilization method for glauberite ore mining waste rocks to solve the problem that existing glauberite ore mining waste rocks are not effectively recycled. The method comprises the following steps: carrying out resource deep development and utilization on glauberite ore mining waste rocks, carrying out crushing, wet ball milling and multi-stage dense washing treatment on the waste rocks, performing dense wash solution processing to prepare NaHCO3 and (NH4)2SO4, carrying out washing and filter pressing on dense slurry by a washing type filter press to prepare a filter cake, and carrying out granulation and drying on the filter cake to prepare glauberite gypsum which can be used as a building raw material and a cement additive, carrying out solid-solid separation, comprehensive impurity removal and drying on the filter cake to obtain high-purity gypsum for building materials, and carrying out surface modification treatment on the high-purity gypsum for building materials to obtain filling gypsum for composite materials. The method is suitable for deep development and utilization of the glauberite mining waste rocks.
Owner:四川省洪雅县青工科技有限公司
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107020090AImprove bindingEnhanced anti-toxicityCatalyst carriersWater contaminantsChemical industryUltrasound - action
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst, and belongs to the technical fields of environment protection and catalysts for chemical industry. The preparation method comprises the following steps of using gamma-aluminum oxide, barite, fluorite, glauberite, magnesia spinel and peridotite as carriers, expanding pores of the carriers by lithium hypochlorite and bis(acetylacetone)beryllium, and adding a surfactant (dimethyl distearylammonium chloride) for activating treatment under the action of ultrasonic wave; then, performing hydrothermal reaction on the carriers, composite mineralizing agents (borax and potassium sulfate), catalytic activity additive precursors (cerium(IV)-2,2,6,6-tetramethylheptanedionate, samarium acetylacetonate, terbium(III) acetate hydrate, and erbium tris[bis(trimethylsilyl)amide), catalytic activity center precursors (manganese lysine, L-aspartic acid molybdenum, dipotassium hexachloroosmate and diamminedichloroplatinate (II)) in a hydrothermal reaction kettle under the action of an emulsifier (N-dimethyl dodecyl-N'-dodecyl-dimethyl-2-hydroxypropyl ammonia dichloride), drying to remove water, and firing in a muffle furnace, so as to obtain the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Medicine for treating vertigo
InactiveCN102228639AHigh cure rateLow recurrence rateHeavy metal active ingredientsSenses disorderSide effectCure rate
The invention relates to a medicine for treating vertigo. A preparation method for the medicine comprises the following steps of: adding szechuan tangshen root, astragalus, magnetite, nacre, sea-ear shell, glauberite, medicinal evodia fruit, pinellia tuber, tabasheer, grassleaf sweelflag rhizome, polygalae, gardenia jasminoides, golden thread, bamboo leaf and liquoric root into water, and decocting to obtain decoction; concentrating, adding conventional auxiliary materials and purified water until 1 gram of crude medicines is contained in 1 milliliter of water, filling and sterilizing to prepare an oral liquid preparation. The medicine for treating the vertigo has the effects of tonifying middle-jiao and qi, clearing away heart fire, arresting convulsion, calming nerves and promoting mentality, is high in cure rate and low in relapse rate, does not have side effect of western medicinal preparations on injury of livers, kidneys and the like and also dose not have the tolerance of antibiotics and the like; and by clinical observation, the effective rate is 100 percent.
Owner:崔凤玉
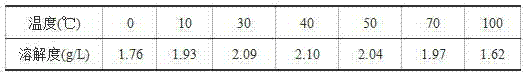
Deep purification method of glauberite gypsum
PendingCN114436311ALess active ingredientsReduce whitenessCalcium/strontium/barium sulfatesHydration reactionAnhydrous Calcium Sulfate
The invention discloses a deep purification method of glauberite gypsum, which specifically comprises the following steps: (1) mixing glauberite gypsum with an acid solution, and pickling for a certain time at a certain temperature; and (2) carrying out hydration reaction on the acid-washed glauberite gypsum at a certain temperature to obtain purified gypsum. By adopting the deep purification method disclosed by the invention, the purity of the purified glauberite gypsum can reach 97% or above, the whiteness is obviously improved, and the purified glauberite gypsum can be used as a high-quality raw material for producing high-added-value gypsum products such as high-strength gypsum, alpha-semi-hydrated gypsum whiskers and anhydrous calcium sulfate whiskers; the deep purification method disclosed by the invention also has the advantages of low production cost, mild reaction conditions, high slurry ratio, large treatment capacity and the like, and has a good industrial application prospect.
Owner:SICHUAN UNIV
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107051478AImprove adsorption capacityBurning at high temperature makes the organic matter completely carbonized and strong adsorptionCatalyst carriersWater contaminantsUltrasound - actionLithium hypochlorite
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst and belongs to the technical field of environment protection and chemical catalysts. The preparation method includes: using a diatom purifier, kyanite, fluorite, glauberite, basalt and blodite as the carriers, using lithium hypochlorite and bis(acetylacetone) beryllium to perform pore expansion on the carriers, adding surfactant monoalkyl ether trimethyl ammonium chloride to activate the carriers under ultrasonic action, allowing the carriers to have hydrothermal reaction with compound mineralizer including borax and potassium sulfate, catalytic activity promoter precursors including tri(hexafluoroacetylacetone) yttrium(III) dihydrate, promethium tricyclopentadienide, tri(4, 4, 4-trifluoro-1-(2-thiophene)-1, 3-butanedione) europium and tri(trifluoromethane sulfonimide) ytterbium, and catalytic activity central precursors including pyruvic acid isonicotinoyl hydrazone vanadium, cobalt gluconate, K[Ag(SCN)2] and terpyridyl ruthenium chloride hexahydrate in a hydrothermal reaction kettle under the effect of an emulsifier lauramide propyl trimethyl ammonium methyl sulfate, drying to remove moisture, and burning in a muffle furnace to obtain the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Ozone heterogeneous oxidation solid catalyst preparation method
InactiveCN107051447AImprove bindingImprove adsorption capacityCatalyst carriersOther chemical processesUltrasound - actionCerium
Owner:SICHUAN NORMAL UNIVERSITY
Method for purifying glauberite to prepare dihydrate gypsum
ActiveCN102963920BFull recoveryReduce mining costsCalcium/strontium/barium sulfatesPhysical chemistryCalcium Sulfate Dihydrate
The invention discloses a method for purifying glauberite ore to prepare dihydrate gypsum, which comprises the following steps: 1) crushing glauberite ore by crushing equipment to obtain glauberite ore powder; 2) making glauberite ore powder Send it into the leaching mixing tank and mix it with the immersion water; 3) Send the mixture into a mechanical filtration device capable of quantitative washing to obtain a filter residue with a sodium sulfate content of less than 2% and a water content of less than 30%. ;4) The filter residue is made into a spherical material through a ball forming mechanism. This material is the finished dihydrate gypsum. In this application, the leached suspension is passed through a filter with washing function. According to the requirements of the sodium sulfate production process, it can be directly used as the raw material for the production of anhydrous sodium sulfate. Because the washing liquid contains sodium sulfate, it can be recycled as immersion water. Through this method, calcium sulfate dihydrate products that meet the national standards can be produced, and the operability Strong, low production cost, good market prospect.
Owner:SICHUAN TONGQING NANFENG
Method of preparation of solid catalyst by ozone heterogeneous oxidation
InactiveCN107088418AEvenly dopedImprove adsorption capacityCatalyst carriersOther chemical processesUltrasound - actionCoal
The invention relates to a method of preparation of solid catalyst by ozone heterogeneous oxidation, belongs to the technical field of environmental protection and chemical engineering catalysts. Diatom pure, kyanite, fluorite, glauberite, pulverized coal ash and coal gangue are adopted as carriers after subjecting to bore-reaming by lithium hypochlorite and di-(acetylacetone) beryllium, activating treatment is conducted by the subjoining of surfactant mono alkyl ether trimethyl ammonium chloride under the action of ultrasonic waves, then the carriers are subject to hydrothermal reaction in a hydrothermal reaction kettle with a composite mineralizer borax and potassium sulfate, precursors of catalytic coagent tri-(acetylacetonate hexafluoride) yttrium (III) dihydrate, tricyclic cyclopentadienyl promethium, tri-(2, 2, 6, 6-tetramethyl-3, 5-pimelic keto acid) gadolinium, tri-(trifluoro methane sulfonyl imine) ytterbium, catalytic active core precursor of pyruvic acid isonicotinoyl hydrazone vanadium, cobalt gluconate, and tetra-chlorine potassium, hexa-nitroso rhodium tri-sodium under the action of emulsifying agent stearic acid trimethylamine ethanol ammonium chloride, after stoving, the moisture content is removed, and a solid catalyst by ozone heterogeneous oxidation is obtained by firing in a muffle furnace.
Owner:SICHUAN NORMAL UNIVERSITY
A kind of method for preparing gypsum-based self-leveling material from glauberite tailings
The invention provides a method for preparing a gypsum-based self-leveling material from glauberite. The gypsum-based self-leveling material is produced from the glauberite with a pressurized aqueoussolution method. Few additives are used, the raw materials are widely sourced, and the cost is low. The method is implemented as follows: 450-600 parts of the glauberite are put in an autoclave, 0.75-1.5 parts of a crystal modifier and 0.64-1.5 parts of a surfactant are evenly mixed with water, a mixture is poured into the autoclave, pH is regulated to 6-8 by a pH regulating agent, the autoclave is tightly closed, a reaction is conducted at 115-130 DEG C for 1-2 h, a solution is taken out, boiling water and absolute ethanol are used for filtering, dehydrating and reagent removal in a vacuum filter, and drying is performed in a drying oven. The gypsum-based self-leveling material prepared from the glauberite has the advantages that the performance exceeds standard requirements in the national building material industry, cheap mirabilite gypsum is used, and accordingly, popularization and application are better facilitated.
Owner:ZHENGZHOU MINERALS COMPOSITIVE UTILIZATION RES INST CHINESE GEOLOGICAL ACAD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com