Method for preparing sodium bicarbonate and co-producing ammonium sulfate and calcium carbonate from glauberite
A sodium bicarbonate and glauberite technology, applied in calcium carbonate/strontium/barium, chemical instruments and methods, ammonium sulfate, etc., can solve problems such as sales difficulties, low utilization rate of sodium ions and sulfate radicals, waste of resources, etc. , achieve significant economic and environmental benefits, realize resource utilization, and clean the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
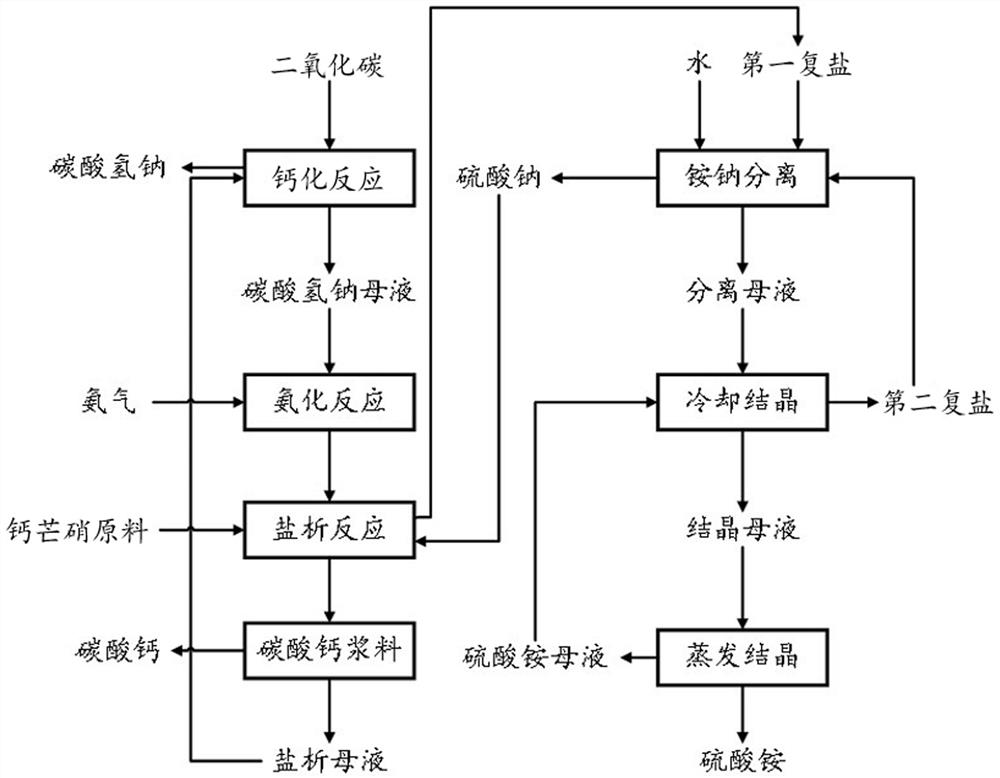
[0050] The present embodiment provides a method for preparing calcium-produced ammonium sulfate and sodium bicarbonate, mirabilite calcium carbonate, such as figure 1 As shown, the method includes the following steps:
[0051] (1) Analysis of the mother liquor into a salt concentration of 90vol% carbon dioxide, the reaction carried calcification 75min, filtered, the mother liquor obtained sodium bicarbonate and sodium bicarbonate at 32 deg.] C;
[0052] (2) step (1) of sodium bicarbonate in the mother liquor obtained through the ammonia gas for amination 45min at 50 ℃, ammoniated to give a mother liquor;
[0053] (3) a mass ratio of 2.4: 1: 3.6 mixture Glauberite material, sulfate, and step (2) the resulting amide mother liquor for salting out the reaction for 90min at 32 ℃, salting the resulting solids slurry introduced into the reaction separation cyclone, the first complex salt concentration slurry flow out from the lower cyclone, calcium carbonate from the hydrocyclone underfl...
Embodiment 2
[0060] The present embodiment provides a method for preparing calcium-produced ammonium sulfate and sodium bicarbonate, mirabilite calcium carbonate, such as figure 1 As shown, the method includes the following steps:
[0061] (1) Analysis of the mother liquor into a salt concentration of 80vol% carbon dioxide, the reaction carried calcification 90min, filtered, the mother liquor obtained sodium bicarbonate and sodium bicarbonate at 30 deg.] C;
[0062] (2) step (1) of sodium bicarbonate in the mother liquor obtained through the ammonia gas for amination 60min at 40 ℃, ammoniated to give a mother liquor;
[0063] (3) a mass ratio of 2.2: 1: 3.5 mixture Glauberite material, sulfate, and step (2) the resulting amide mother liquor for salting out the reaction at 120min 30 ℃, salting the resulting solids slurry introduced into the reaction separation cyclone, the first complex salt concentration slurry flow out from the lower cyclone, calcium carbonate from the hydrocyclone underflow ...
Embodiment 3
[0070] The present embodiment provides a method for preparing calcium-produced ammonium sulfate and sodium bicarbonate, mirabilite calcium carbonate, such as figure 1 As shown, the method includes the following steps:
[0071] (1) Analysis of the mother liquor into a salt concentration of 70vol% carbon dioxide, the reaction for 60min calcification, filtered, sodium bicarbonate and sodium bicarbonate to give a mother liquor at 35 ℃;
[0072] (2) step (1) of sodium bicarbonate in the mother liquor obtained ammonia gas, ammoniated for 30min at 60 ℃, ammoniated to give a mother liquor;
[0073] (3) a mass ratio of 2.5: 1: 3.8 mixture Glauberite material, sulfate, and step (2) the resulting amide mother liquor for salting out the reaction 60min at 35 ℃, salting the resulting solids slurry introduced into the reaction separation cyclone, the first complex salt concentration slurry flow out from the lower cyclone, calcium carbonate from the hydrocyclone underflow stream flows were then f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com